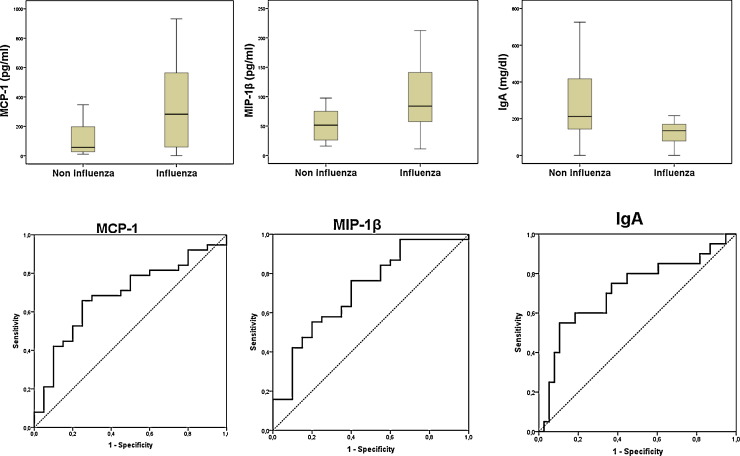
We have recently reported the protective role of IgM in a cohort of patients with severe disease caused by influenza A(H1N1)pdm09 virus [1]. Here we compared levels of 17 cytokines and 7 immunoglobulin isotypes in the plasma of these patients against those of a cohort of patients suffering from pneumonia caused by other viruses. Cytokines and immunoglobulins were measured by using Biorad multiplex kits. Respiratory samples were screened for the presence of viruses by using the A(H1N1)pdm09 Detection Set (Roche®), the Respiratory Viral Panel-XTAG RVP (Abott®) or culturing them on Hep2/Vero/MDCK-SIAT cells. In the non-influenza group (n = 20), the viral aetiology was as follows: Entero/Rhinovirus (n = 7), Metapneumovirus (n = 6), Respiratory Syncytial Virus (n = 5), Coronavirus NL63 (n = 1) and Herpesvirus (n = 1). Patients with influenza (n = 38) were younger (median (yrs), [interquartile rank, IQR]): influenza: (54 [19]; other viruses: 64 [19]) (p = 0.005 Mann–Whitney U test). The frequency of prior immunosuppression was similar: 12 out of 38 (31.6%) for influenza; 5 out of 20 (25.0%) for non-influenza, (p = 0.601, Chi squared Test). APACHE II score at admission was also similar in both groups: (median [IQR]): influenza: (18.5 [10]); other viruses: (16.5 [25]) (p = 0.761). Nevertheless, patients with influenza needed more frequently of invasive mechanical ventilation: influenza: 29 out of 38 (76.3%); other viruses: 10 out of 20 (50.0%), p = 0.042. Frequency of bacterial co-infection at admission was also comparable: influenza: 7 out of 38 (18.4%); other viruses: 3 out of 20 (15.0%) p = 0.772. The percentage of fatal cases was higher in the influenza group, although difference was not significant: influenza: 14 out of 38 (36.8%); other viruses: 3 out of 20 (15.0%) p = 0.080. Patients with influenza showed higher levels of MCP-1 (282.6 [521.1]; 56.7[203.1]) and MIP-1β (83.8 [86.1]; 51.4 [49.7]), median (pg/ml), [IQR], indicating probably a worst control of the infection [2]. Conversely, influenza patients showed lower levels of IgA (133.9 [92.8]; 212.1 [308.9]), median (mg/dl), [IQR]) (Fig. 1 ). The area under the receiver operating characteristic (AUROC) curves for MCP-1, MIP-1β and IgA levels allowed distinguishing between influenza and non influenza infection (AUROC, [CI 95%], p): MCP-1 (0.69, [0.54–0.82] 0.021); MIP-1β (0.71, [0.57–0.85] 0.007); IgA (0.71, [0.56–0.86] 0.008) (Fig. 1).
Fig. 1.
Up: Box plots showing levels of chemokines and IgA in both groups (p < 0.05). Down: AUROC for influenza (MCP-1, MIP-1β)/non-influenza diagnosis (IgA).
Local IgA in the respiratory mucosa has been shown to play an important protective role in the infection caused by respiratory viruses [3], [4]. However the role-played by systemic IgA in this context is not well known. This work evidences for the first time the existence of lower levels of plasma IgA in patients with viral pneumonia caused by influenza. The potential of IgA in determining viral aetiology along with its participation in the pathogenic events in this disease merits further investigation.
Funding
The authors acknowledge the support of “Instituto de Salud Carlos III – Fondo de Investigaciones Sanitarias, FIS” and Health Council, Junta de Castilla y León (JCYL-IECSCYL-SACYL): Programa de Investigación Comisionada en Gripe, GR09/0021, GR09/0022.
Competing interest
The authors declare no conflict of interest.
Ethical approval
Approval of the study protocol for both the scientific and the ethical aspects was obtained from the Scientific Committee for Clinical Research of our hospital. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Acknowledgements
The authors thank Lucia Rico and Verónica Iglesias for their support with laboratory work and David Banner for language reviewing of the manuscript.
References
- 1.Justel M., Socias L., Almansa R., Ramírez P., Gallegos M.C., Fernandez V. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2013;58(de november de (3)):564–567. doi: 10.1016/j.jcv.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Almansa R., Anton A., Ramirez P., Martin-Loeches I., Banner D., Pumarola T. Direct association between pharyngeal viral secretion and host cytokine response in severe pandemic influenza. BMC Infect Dis. 2011;11:232. doi: 10.1186/1471-2334-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Riet E., Ainai A., Suzuki T., Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30(de agosto de (40)):5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 4.Dubois M.E., Yoshihara P., Slifka M.K. Antibody-mediated protection against respiratory viral infection. Semin Respir Crit Care Med. 2005;26(diciembre de (6)):635–642. doi: 10.1055/s-2005-925527. [DOI] [PubMed] [Google Scholar]