Summary
The coronavirus disease 2019 (COVID-19) pandemic poses an enormous challenge to healthcare systems in affected communities. Older patients and those with pre-existing medical conditions have been identified as populations at risk of a severe disease course. It remains unclear at this point to what extent chronic liver diseases should be considered as risk factors, due to a shortage of appropriate studies. However, patients with advanced liver disease and those after liver transplantation represent vulnerable patient cohorts with an increased risk of infection and/or a severe course of COVID-19. In addition, the current pandemic requires unusual allocation of healthcare resources which may negatively impact the care of patients with chronic liver disease that continue to require medical attention. Thus, the challenge hepatologists are facing is to promote telemedicine in the outpatient setting, prioritise outpatient contacts, avoid nosocomial dissemination of the virus to patients and healthcare providers, and at the same time maintain standard care for patients who require immediate medical attention.
Abbreviations: ACE2, angiotensin-converting enzyme 2; ACE-I, angiotensin-converting enzyme inhibitor; ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; EGD, esophagogastroduodenoscopy; ERC, endoscopic retrograde cholangiography; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NUC, nucleoside analogue; PIs, protease inhibitors; RdRp, RNA-dependent RNA polymerase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ULN, upper limit of normal
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the recently identified coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Symptoms associated with COVID-19 are mostly fever, tiredness and dry cough. Some patients also develop symptoms including shortness of breath, aches, nasal congestion, sore throat, anosmia and ageusia. Nausea, vomiting and diarrhoea occur less frequently.1 While symptoms remain mild throughout the course of infection in the majority of patients, older patients were identified to be at higher risk of a fatal disease course, with hypertension, diabetes and coronary heart disease being the most frequent comorbidities in these cohorts.2,3
COVID-19 and the liver
While pre-existing liver disease is not specifically listed in the published cohort studies, elevated alanine aminotransferase (ALT) levels, reduced platelet counts and reduced albumin levels at the time of admission have been associated with higher mortality,2 although not all of these alterations are independent risk factors. It remains unclear at this time whether these laboratory test alterations are a sign for pre-existing liver diseases in patients with a more severe course of infection, whether they rather reflect liver damage caused by the virus itself, or whether they mirror a severe inflammatory response (hypoalbuminemia) with disseminated intravascular coagulation (thrombocytopenia). Possibly, patients with advanced chronic liver disease are at increased risk of infection due to cirrhosis-associated immune dysfunction.4 The same could be true for patients after liver transplantation and possibly those with autoimmune liver diseases who receive immunosuppressive therapies.
However, currently, there are only limited data available linking underlying liver diseases with the course of SARS-CoV-2 infection and there are many open questions (Box 1). Chronic hepatitis B virus infection for example, which is more prevalent in China than Europe, does not appear to affect the outcome of COVID-19.1 In addition, although there is no proof for this assumption, immunosuppression may even provide some protection from immunopathology, which appears to contribute to lung damage in cases with more severe manifestations of the disease.5,6 These may be associated with a macrophage activation syndrome in the context of a hyperinflammatory syndrome characterised by a cytokine storm with multi-organ failure.7 On the other hand, systemic viral infections are often associated with transient elevations of transaminases which may reflect general immune activation or inflammation caused by circulating cytokines without compromising liver function, a phenomenon called “bystander hepatitis”. This may also be the case in patients with COVID-19 where liver failure has not been specifically reported, even in the most severe and fatal courses of disease.1,2 However, signs of liver dysfunction may occur in critically ill patients with COVID-19,8 as reviewed in.9,10 Whether patients with cirrhosis and COVID-19 are at increased risk of decompensation or development of acute-on-chronic liver failure (ACLF), as has been shown for influenza infection,11 remains to be determined. SARS-CoV-2 may also directly infect liver cells as the receptor of the virus, angiotensin-converting enzyme 2 (ACE2),12 has been shown to be expressed on cholangiocytes,13,14 which may explain cases of viral shedding into the faeces.15,16 How far observations from Asian cohorts can be transferred to European populations remains to be determined, but it appears unlikely that SARS-CoV-2 infection causes liver damage to an amount that substantially contributes to the overall disease burden.
Box 1. Open questions for liver-related basic/translational research regarding COVID-19.
ACLF, acute-on-chronic liver failure; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Aside from the direct implications of liver disease on the course of COVID-19 or vice versa, there are several additional aspects that require attention. The current pandemic and the amount of available information – including misinformation and rumours – result in uncertainties and concerns among patients but also healthcare providers. While the threat that COVID-19 poses should not be underestimated, it remains important to maintain care of patients with chronic liver disease and to identify potential ways to prioritise care of these patients in times of limited healthcare resources.
Considerations for patients with chronic liver disease and patients after liver transplantation
The recommendations provided here address the specific characteristics of patients with liver disease and are meant to provide additional guidance for the care of these patients. General recommendations and guidelines with regards to prevention, diagnosis and treatment of COVID-19 from local authorities should be adhered to.
Outpatient care
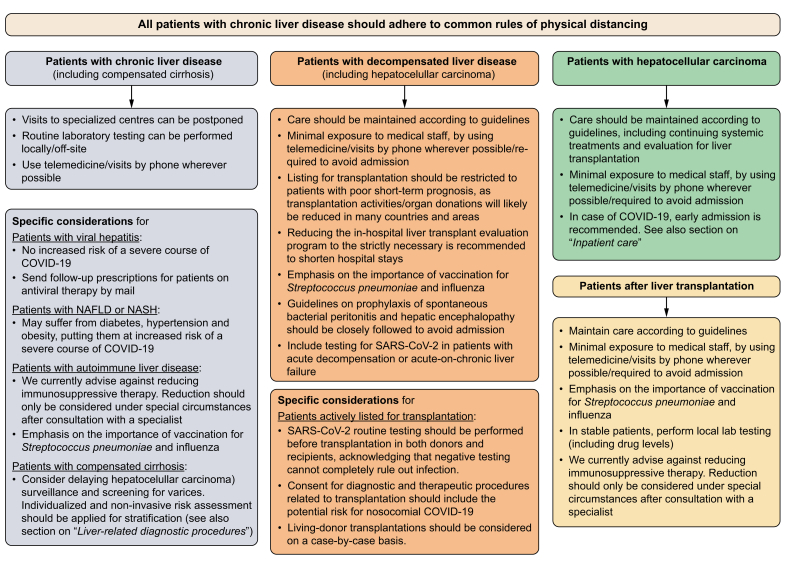
The management and surveillance of patients with advanced liver disease and those receiving immunosuppressive treatment is often performed in larger units or centres. These institutions, however, are currently also COVID-19 hotspots, thus, potentially putting outpatients with chronic liver diseases at risk of nosocomial infections. In addition, hospital staffs face challenges such as long working hours and even reduced staffing because of COVID-19 quarantining. Therefore, several factors have to be considered by hepatologists who provide care for these vulnerable patients. Clearly, prioritisation criteria need to be defined for outpatient contacts. The general and specific recommendations provided here (Fig. 1) cannot comprehensively cover all patient cohorts and are not backed up by datasets. Moreover, the precise management of these patients strongly depends on the local COVID-19 burden. In case of COVID-19 in patients with more advanced chronic liver disease, we recommend admission for inpatient care depending on the presence of certain risk factors, which is discussed in more detail in a separate section.
Fig. 1.
Flow chart for the prioritisation of patient care in patients with chronic liver disease.
The individual management of these patients strongly depends on the local COVID-19 burden and officially implemented rules and regulations. In some countries and areas, maintenance of standard care might not be able and transplantation activities might be reduced. COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Reduction of direct exposure
Physical distancing has been suggested and/or officially implemented in multiple countries across the globe to prevent dissemination of SARS-CoV-2. This measure aims to prevent a rapid increase in SARS-CoV-2 infections in the general population with the overall goal of minimising the number of patients with a severe course of COVID-19 at any one time, enabling healthcare providers to maintain medical/intensive care of these patients. At the same time, vulnerable patient cohorts should be protected from individuals with potential SARS-CoV-2 exposure or infection. Thus, in these patients, the benefits of maintaining patient care have to be weighed against the risk of infection. These considerations require adaption of standard operating procedures for outpatient care, including remodelling of waiting areas to allow sufficient distance between patients, reduction of waiting times and encouraging patients to wait outside wherever possible and eventually be contacted by phone. In addition, exposure to medical staff should be minimised wherever possible.
Face-to-face contact is the basis for the physician-patient relationships and allows physicians to quickly assess the overall condition of the patient. However, due to the amount of individual contacts, physicians are at risk of infection and subsequently also dissemination of the virus. Thus, it seems appropriate to also limit the amount of face-to-face contacts for patients at risk of a more severe course of infection. Technical solutions are available to enable remote physician-patient interactions and their benefits have already been discussed in the context of the COVID-19 pandemic.17 Health authorities should be urged to equip hospitals with such systems not only to attend to COVID-19 patients who stay in quarantine at home but also to care for patients that need to be protected from a potentially harmful infection and guide them through the pandemic, explaining the future treatment/management strategies/plan and provide advice concerning general prevention measures. The current pandemic clearly underlines the importance of making these technical solutions available to health care providers in order to be better prepared for situations like these in the future.
Patients with compensated liver disease
General considerations
-
•
Visits to specialised centres can be postponed.
-
•
Use telemedicine or visits by phone wherever possible.
-
•
Routine laboratory testing can be performed locally, e.g. through primary care physician, and its frequency needs careful individual risk-benefit considerations.
Specific considerations
-
•
Chronic viral hepatitis does not appear to increase the risk of a severe course of COVID-19.1 Use telemedicine/local laboratory testing for follow-up visits in patients under antiviral therapy, send follow-up-prescriptions by mail.
-
•
Patients with non-alcoholic fatty liver disease (NAFLD) or steatohepatitis (NASH) may suffer from metabolic comorbidities such as diabetes, hypertension and obesity putting them at increased risk of a severe course of COVID-19.
-
•
In patients with autoimmune liver disease, we currently advise against reducing immunosuppressive therapy. Reductions should only be considered under special circumstances (e.g. medication-induced lymphopenia, or bacterial/fungal superinfection in case of severe COVID-19) after consultation of a specialist.
-
•
Emphasis on the importance of vaccination for Streptococcus pneumoniae and influenza.
-
•
In patients with compensated cirrhosis, consider delaying hepatocellular carcinoma (HCC) surveillance and screening for varices. Non-invasive risk assessment for the presence of varices should be applied for stratification (thrombocyte count or Baveno VI).18 See also section on “liver-related diagnostic procedures”.
Patients with decompensated liver disease
General considerations
-
•
Care should be maintained according to guidelines but consider minimal exposure to medical staff, by using telemedicine/visits by phone wherever possible/required to avoid admission.
-
•
Listing for transplantation should be restricted to patients with poor short-term prognosis including those with acute/acute-on-chronic liver failure (ALF/ACLF), high model for end-stage liver disease (MELD) score (including exceptional MELDs) and HCC at the upper limits of the Milan criteria, as transplantation activities/organ donations will likely be reduced in many countries and areas.
-
•
Reducing the in-hospital liver transplant evaluation program to the strictly necessary is recommended to shorten the in-hospital stay and also reduce the number of consultations in other departments/clinics (i.e. ophthalmologic, dermatologic, dental, neurologic consultations can be done in local outpatient settings).
-
•
Emphasis on the importance of vaccination for Streptococcus pneumoniae and influenza.
-
•
Guidelines on prophylaxis of spontaneous bacterial peritonitis and hepatic encephalopathy should be closely followed to prevent decompensation and avoid admission.
-
•
Include testing for SARS-CoV-2 in patients with acute decompensation or ACLF.
Specific considerations for patients actively listed for transplantation
-
•
SARS-CoV-2 routine testing should be performed before transplantation in both donors and recipients, acknowledging that negative testing cannot completely rule out infection.
-
•
Consent for diagnostic and therapeutic procedures related to transplantation should include the potential risk of nosocomial COVID-19.
-
•
Living-donor transplantations should be considered on a case-by-case basis.
Patients with hepatocellular carcinoma
-
•
Care should be maintained according to guidelines including continuing systemic treatments and evaluation for liver transplantation, but consider minimal exposure to medical staff, by using telemedicine/visits by phone wherever possible/required to avoid admission.
-
•
In case of COVID-19, early admission is recommended. See also section on “inpatient care”.
Patients after liver transplantation
-
•
Maintain care according to guidelines, but consider minimal exposure to medical staff, by using telemedicine/visits by phone wherever possible/required to avoid admission.
-
•
Emphasis on the importance of vaccination for Streptococcus pneumoniae and influenza.
-
•
In stable patients: perform local laboratory testing (including drug levels).
-
•
We currently advise against reduction of immunosuppressive therapy. Reduction should only be considered under special circumstances (e.g. medication-induced lymphopenia, or bacterial/fungal superinfection in case of severe COVID-19) after consultation of a specialist.19
Liver-related diagnostic procedures
Endoscopy
Patients without COVID-19: Depending on available resources, screening for varices by esophagogastroduodenoscopy (EGD) should be reserved for patients at risk of variceal bleeding, such as patients with a history of variceal bleeding or signs of significant portal hypertension (ascites, thrombocyte count <100,000/μl). Non-invasive risk assessment for the presence of varices should be applied for stratification (thrombocyte count or Baveno VI).
Endoscopic retrograde cholangiography (ERC) for dilatation or stent replacement in patients after liver transplantation or patients with primary sclerosing cholangitis should be performed after careful individual risk-benefit considerations, including risk for nosocomial SARS-CoV-2 infection depending on local COVID-19 burden.
Patients with COVID-19: Endoscopic procedures are associated with an increased risk of disseminating SARS-CoV-2. During EGD or ERC, spreading of virus-containing droplets can occur. In addition, shedding of the virus in the faeces increases the risk of dissemination during colonoscopy. Thus, indications for endoscopic procedures in patients with COVID-19 should be limited to emergencies such as gastrointestinal bleeding, bacterial cholangitis or other life-threatening conditions.
Ultrasound (HCC surveillance)
Patients without COVID-19: HCC surveillance can be deferred based on available resources (including availability of therapeutic options in case of HCC diagnosis) at the centre and the individual risk assessment. Patients with increased risk, such as patients with elevated alpha-fetoprotein levels, advanced cirrhosis, chronic hepatitis B, NASH/diabetes etc., may be prioritised if resources are limited.
Patients with COVID-19: HCC surveillance should be deferred until after recovery.
Liver biopsy
Patients without COVID-19: Recommendations strongly depend on the local COVID-19 burden and the individual indication for histological assessment.
Biopsy should be deferred in patients with
-
•
NAFLD or chronic viral hepatitis (for grading/staging)
-
•
mildly elevated transaminases (e.g. ALT <3× the upper limit of normal [ULN]) of unknown aetiology
Biopsy should be performed in patients with
-
•
strongly elevated transaminases (e.g. ALT >5× ULN) of unknown aetiology (in case of suspected autoimmune liver disease, treatment without histological diagnosis can be considered based on individual risk-benefit considerations)
-
•
liver masses suspicious of malignancy
Patients with COVID-19: Liver biopsy should be deferred in most patients as
-
•
treatment/care for COVID-19 outweighs diagnosis of co-existing liver disease
-
•
systemic inflammation associated with COVID-19 will obscure aetiology-specific histologic characteristics
-
•
liver biopsy may represent a risk for viral transmission (although the virus has so far not been detected in liver tissue, the expression of its receptor on cholangiocytes suggests that the virus might be present in the liver as well).
Collaboration with local health care providers and primary care physicians
The necessity to reduce travel and limit visits to specialised centres is a challenge for liver disease patients and local health care providers alike. In order to facilitate decentralised care for these patients, close collaborations between specialised hepatologists and local health care providers is required. This pertains to several factors such as communication of immunosuppressive target levels in patients after liver transplantation, management of patients with severe ALT elevations of unknown aetiologies and others. Thus, we recommend that specialised centres provide easily accessible contact information for local health care providers to facilitate fast and uncomplicated collaboration for the benefit of patients with liver diseases.
Inpatient care
Many patients with chronic liver disease will continue to require inpatient care during the COVID-19 crisis for decompensation, cholangitis, rejection or other complications. General measures to prevent SARS-CoV-2 exposure and infection will be of utmost importance for these patients. Depending on the local infrastructure, implementation of COVID-19-clean wards or hospitals is warranted. However, based on the local COVID-19-burden, a sharp separation of “clean” and “dirty” wards/hospitals may not be sustainable, although recent evidence provides somewhat reassuring data regarding the absence of environmental contamination with SARS-CoV-2 RNA of inanimate surfaces outside patient rooms.20 Wherever possible, patients with chronic liver disease requiring inpatient care for non-COVID-19 causes should be admitted to COVID-19-clean wards or hospitals. As these institutions may not be able to provide specialised hepatology-care, similar to our recommendations on outpatient care, we recommend that specialised centres provide easily accessible contact information to facilitate immediate hepatology consultations.
However, due to the contagiousness of the virus, patients with underlying liver disease will become infected and will subsequently require inpatient care for COVID-19. With regards to nosocomial-infections, recent observations from Spain that compared patient characteristics between patients with community-acquired influenza and nosocomial-acquired influenza could not detect a significant difference between these cohorts with regards to underlying chronic liver disease.21 Whether these observations will also hold up in the context of the COVID-19 pandemic remains to be proven. Until further evidence emerges, we recommend that patients with chronic liver disease and COVID-19 are admitted for inpatient care if they have additional risk factors for a more severe COVID-19 course like hypertension, diabetes or obesity, cirrhosis, HCC or a post-transplant status.
Treatment considerations for COVID-19
Although there are currently no drugs approved for SARS-CoV-2, several repurposed drugs have been tested in recent weeks and many of them are still under investigation (see Table 1).22 Remdesivir acts as an adenosine-analogue that induces RNA chain termination and was initially developed as an antiviral agent against Ebola.23 It has recently been shown to inhibit a clinical isolate of SARS-CoV-2 in vitro24 and to reduce disease severity of the related MERS-CoV-infection in a non-human primate model in vivo.25 In addition, several reports have suggested clinical efficacy in patients suffering from COVID-19.26,27 Clinical trials are ongoing to further evaluate efficacy in affected patients. Although ritonavir-boosted lopinavir showed some antiviral effect on SARS-CoV-2 in vitro, a recently published clinical trial in patients with severe COVID-19 showed no effect in vivo compared to no treatment.28 Other drugs currently under evaluation include chloroquine phosphate or hydroxychloroquine.29 Chloroquine has shown antiviral efficacy against SARS-CoV-2 in vitro through interference with the ACE2-receptor mediated endocytosis and is widely used in the treatment of patients with severe COVID-19 as monotherapy, but also in combination with azithromycin.24,[30], [31], [32] The administration of any of these compounds to infected patients will remain an individual decision as efficacy and optimal timing remain to be clarified. However, with regards to patients with chronic liver disease, possible adverse events have to be kept in mind. This is particularly important with respect to drug-interactions in patients with certain immunosuppressive therapies where drug levels of cyclosporine, tacrolimus, sirolimus or everolimus will have to be closely monitored. In addition, patients with impaired liver function are at high risk of drug toxicities, i.e. in patients with Child-Pugh B/C cirrhosis. Table 1 summarises some considerations with regards to potential toxicities in these patients. It is also important to stress that all the drugs currently under investigation are not approved for SARS-CoV-2. However, given that early initiation of antiviral therapy is known to blunt the course of influenza, it is reasonable to assume that early treatment initiation might also be beneficial to prevent severe pneumonia in COVID-19. Thus, in patients with liver disease and risk factors for a severe course of the disease, we recommend inclusion into early antiviral treatment programmes or clinical trials that might be active at different centres.
Table 1.
Selected repurposed drugs that have been suggested/discussed for the treatment of COVID-19 and considerations for patients with liver disease or after liver transplantation.
| Drug | Mechanism of action, rationale for COVID-19 | Considerations for patients with liver diseases or after liver transplantation |
|---|---|---|
| Remdesivir |
|
|
| Chloroquine/Hydroxychloroquine35 ± azithromycin |
|
|
| Lopinavir/ritonavir |
|
|
| Tocilizumab |
|
|
| Methylprednisolone (steroids) |
|
|
| Convalescent plasma |
|
|
| Umifenovir (Arbidol)∗ |
|
|
| Favipiravir/favilavir∗ |
|
|
| Sofosbuvir∗ maybe combination with ribavirin |
|
|
| Baricitinib∗ |
|
|
| Camostat∗ |
|
|
| Emapalumab∗ |
|
|
| Anakinra∗ |
|
|
This list is not intended to give treatment recommendations. The evidence to use these drugs is low. Multiple RCTs for the above-mentioned drugs and some other drugs are ongoing in patients with SARS-CoV-2 infection.
ACE2, angiotensin-converting enzyme 2; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; NUC, nucleoside analogue; PIs, protease inhibitors; RdRp, RNA-dependent RNA polymerase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
These drugs are listed to include the most frequently discussed agents for COVID-19. These drugs may lack clinical efficacy, and some have been considered based on in vitro observations or their mode of action.
General considerations for patients with chronic liver disease and COVID-19
-
•
Consider early admission according to the presence of additional risk factors and inclusion in clinical trials and (experimental) antiviral therapy of COVID-19 following local guidelines (see Table 1 for guidance with regards to CLD)
-
•
Prevent acetaminophen overdosing (2–3 g/day is considered safe in patients with cirrhosis without active alcohol consumption33)
-
•
Do not administer non-steroidal anti-inflammatory drugs in patients with cirrhosis and portal hypertension33
-
•
See also section on “liver-related diagnostic procedures” for recommendations on endoscopy, HCC surveillance and liver biopsy
Specific considerations for patients with chronic liver disease and COVID-19
-
•
In patients with decompensated cirrhosis, treatment for cirrhosis-associated complications such as portal hypertension, ascites, hepatic encephalopathy, spontaneous bacterial peritonitis etc. should be continued.
-
•
In patients with HCC, locoregional therapies should be postponed whenever possible and immune-checkpoint inhibitor therapy temporarily withdrawn. The decision on whether to continue (reduced dose) kinase inhibitors in non-severe COVID-19 should be taken on a case-by-case basis.
-
•
In patients after liver transplantation, dose adjustment of calceurin- and/or mTOR- inhibitors might be required depending on the antiviral therapy initiated (Table 1).
Conflicts of interest
Dr Berg reports grants, personal fees and non-financial support from Gilead. Dr Boettler reports consultancy fees from Gilead. Dr Cornberg reports personal fees from Gilead. All other authors report no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgement
We thank Benjamin Maasoumy for critically reviewing the manuscript and providing valuable comments.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100113.
Supplementary data
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., Shu H., Xia Ja, Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020 doi: 10.1111/liv.14435. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schütte A., Ciesek S., Wedemeyer H., Lange C.M. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 [Google Scholar]
- 14.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMp2003539. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Maurice J.B., Brodkin E., Arnold F., Navaratnam A., Paine H., Khawar S. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65:899–905. doi: 10.1016/j.jhep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L., Xu X., Ma K., Yang J., Guan H., Chen S. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020 doi: 10.1111/ajt.15869. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colaneri M., Seminari E., Piralla A., Zuccaro V., Filippo A.D., Baldanti F. Lack of SARS-CoV-2 RNA environmental contamination in a tertiary referral hospital for infectious diseases in Northern Italy. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.03.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godoy P., Torner N., Soldevila N., Rius C., Jane M., Martínez A. Hospital-acquired influenza infections detected by a surveillance system over six seasons, from 2010/2011 to 2015/2016. BMC Infect Dis. 2020;20:80. doi: 10.1186/s12879-020-4792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison C. Coronavirus puts drug repurposing on the fast track. 2020. Available from: https://www.nature.com/articles/d41587-020-00003-1. [Accessed 12 March 2020]. [DOI] [PubMed]
- 23.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. New Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 30.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 31.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 32.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Chandok N., Watt K.D.S. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clinic Proc. 2010;85:451–458. doi: 10.4065/mcp.2009.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.COVID-19 Drug interactions. 2020. http://www.covid19-druginteractions.org/ Available from: [Google Scholar]
- 35.[Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Z. 2020;43:E019. doi: 10.3760/cma.j.issn.1001-0939.2020.0019. [DOI] [PubMed] [Google Scholar]
- 36.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casado J.L., Del Palacio M., Moya J., Rodriguez J.M., Moreno A., Perez-Elías M.J. Safety and pharmacokinetics of lopinavir in HIV/HCV coinfected patients with advanced liver disease. HIV Clin Trials. 2011;12:235–243. doi: 10.1310/hct1205-235. [DOI] [PubMed] [Google Scholar]
- 39.Genovese M.C., Kremer J.M., van Vollenhoven R.F., Alten R., Scali J.J., Kelman A. Transaminase levels and hepatic events during tocilizumab treatment: pooled analysis of long-term clinical trial safety data in rheumatoid arthritis. Arthritis Rheumatol. 2017;69:1751–1761. doi: 10.1002/art.40176. [DOI] [PubMed] [Google Scholar]
- 40.Chen L.-F., Mo Y.-Q., Jing J., Ma J.-D., Zheng D.-H., Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859–869. doi: 10.1111/1756-185X.13010. [DOI] [PubMed] [Google Scholar]
- 41.WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Accessed 18 March 2020].
- 42.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng P., Zhong D., Yu K., Zhang Y., Wang T., Chen X. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HEP Drug interactions. 2020. Available from: https://www.hep-druginteractions.org/. [Accessed 20 March 2020].
- 46.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markham A. Baricitinib: first global approval. Drugs. 2017;77:697–704. doi: 10.1007/s40265-017-0723-3. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsey M.L., Nuttall J., Hart P.A., on behalf of the TIT A phase 1/2 trial to evaluate the pharmacokinetics, safety, and efficacy of NI-03 in patients with chronic pancreatitis: study protocol for a randomized controlled trial on the assessment of camostat treatment in chronic pancreatitis (TACTIC) Trials. 2019;20:501. doi: 10.1186/s13063-019-3606-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Press release on planned clinical trials to evaluate anakinra and emapalumab in COVID-19. 2020. Available from: https://www.sobi.com/en/press-releases/sobi-initiate-clinical-study-evaluate-whether-anakinra-and-emapalumab-may-relieve. [Accessed 30 March 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.