Vaccines have had a positive impact on society, but their safety is often questioned; this article addresses the real and unsubstantiated claims of vaccine safety.
Keywords: Therapeutic vaccines, Infectious disease, Immunization policy, Vaccine adjuvants, Vaccine controversies, Pediatric vaccines
Abstract
Vaccines have had a considerable impact on society by eliminating the threat from several infectious diseases. Although vaccines have generally proven to be safe, safety issues have arisen that have resulted in some members of the public having a poor perception of vaccines. However, the technological advances made in recent years make the development of even safer vaccines a possibility. The new generation of vaccines will be based on pure recombinant proteins, conjugates and killed viruses. In addition, studies can be conducted in large numbers of individuals to allay unjustified fears of vaccine safety. These data will increase public confidence and ensure that vaccines become better appreciated as valuable products. Widespread confidence will be inspired by the effective communication of the realities of the benefit-to-risk ratios for each vaccine.
The introduction of vaccines into medical practice at the beginning of the last century has had an extraordinary impact on human health, and represents an unparalleled success story in modern medicine. Vaccines are broadly considered to be the safest and most effective medical intervention strategy. In conjunction with the introduction of antibiotics and modern hygiene, vaccines have significantly contributed to a steady decline in the mortality and morbidity that is caused by infectious diseases (Table 1). Vaccines have been responsible for the eradication of the virus that causes smallpox, and significant efforts are underway to eradicate poliovirus. The eradication of naturally occurring smallpox also resulted in the elimination of the need for continued vaccination, the ultimate success for a vaccine. However, the termination of vaccination has opened up the possibility for the deliberate reintroduction of the pathogen through bioterrorism. A recent review highlighted the value of vaccination, but also emphasized that vaccines are often underused and undervalued [1].
Table 1.
The impact of vaccines on disease burden in the USA
| Disease | Maximum number of cases identified (year) | Cases reported in 2001 | Reduction in disease(%) |
| Smallpox | 48,164 (1901) | 0 | 100.00 |
| Diphtheria | 206,939 (1921) | 2 | 99.99 |
| Pertussis | 265,269 (1934) | 4788 | 98.20 |
| Tetanus | 1560 (1923) | 26 | 98.34 |
| Polio | 21,269 (1952) | 0 | 100.00 |
| Measles | 894,134 (1941) | 96 | 99.99 |
| Rubella | 57,686 (1969) | 19 | 99.97 |
| Mumps | 152,209 (1968) | 216 | 99.86 |
| Hemophilus influenzae | 20,000 (1992) | 51 | 99.75 |
Adapted from Ref. [48].
Each year, pediatric vaccines prevent up to three million deaths worldwide and protect >750,000 children from serious disability. Nevertheless, the issue of vaccine safety has been with us since vaccination was first established in the 19th century (Figure 1) and continues to attract intense scrutiny [2]. Although pediatric vaccines have been used safely for decades, there has been a relatively recent shift in the public perception of their safety. As a result of vaccination policy, the incidence of several vaccine-preventable diseases in Western societies is now low, for example, diphtheria and tetanus (Table 1). This has encouraged some parents to suggest that their children no longer need to be vaccinated. However, this approach ignores societal responsibilities and fails to appreciate the crucial role of ‘herd immunity’, which prevents the circulation of pathogens and protects the whole population. Vaccines are not 100% effective, and so there will always be a susceptible population who are more prone to adverse consequences of infection, including immunocompromised individuals or people with a genetic susceptibility. Moreover, infectious diseases disproportionately affect the young, old and infirm because these people often have impaired immunity. The population of susceptible people will increase as health care improvements extend the life expectancy of people suffering from chronic diseases. Vaccines are often mandatory to ensure protection for all, not just the individual who might choose not to be vaccinated. Here, the safety of vaccines is described and real safety problems are highlighted. In addition, safety issues that have been widely publicized without supporting evidence are discussed.
Figure 1.

Vaccines have been met with skepticism since they were first introduced. Reproduced with kind permission from the Wellcome Library (London, UK; http://library.wellcome.ac.uk).
Current vaccines and safety concerns
Immunization policy in the USA is predominantly determined by the Advisory Committee on Immunization Practices (ACIP; http://www.cdc.gov/nip/acip), which is an advisory group to the US Public Health Service and the Centers for Disease Control and Prevention (CDC; http://www.cdc.gov). Infants that are vaccinated according to the current guidelines will receive up to 18 separate injections for protection against 12 different infectious diseases by the time they are two years of age. Although recommendations in other Western societies differ, policy in the USA is broadly representative of industrialized societies. Given the number of injections required and the close temporal relationship between vaccinations and the onset of many childhood diseases, it is not surprising that there has been considerable speculation about the links between the onset of disease and childhood vaccinations (Table 2), even though the value of vaccination is clear (Table 1). Nevertheless, when detailed studies have been undertaken, the possibility of a causative link with vaccination has mostly been eliminated or not proven, including claims of a link between hepatitis B vaccine and multiple sclerosis (MS) [3, 4]. Many approaches are currently being evaluated to facilitate vaccination without the use of needles, including mucosal delivery of vaccines and other technologies and devices such as topical immunization. However, none of these strategies is as yet close to being established for routine use [5].
Table 2.
Some unsubstantiated claims of safety issues with commonly used vaccines
| Vaccines | Safety issues |
| Measles | Autisma |
| Hepatitis B | Multiple sclerosisa |
| Multiple vaccines | Diabetesa |
| Multiple vaccines | ‘Antigen overload’a |
| Inactivated polio | Cancer (SV40)a |
| Multiple vaccines | Autoimmunitya |
| Diphtheria, tetanus and pertussis | Sudden infant death syndromea |
| Whole-cell pertussis | Chronic neurological disordersb |
Abbreviation: SV40, simian virus 40.
No supporting evidence;
Evidence exists, but connection has not been proven.
Although a claim of complete safety is impossible for any medical intervention, with almost 100 years of accumulated evidence involving many billions of doses, it can be stated with confidence that vaccines have an enviable and exemplary safety profile. However, some vaccines have been shown to cause safety problems in a minority of individuals (Table 3). Vaccines are administered annually to hundreds of millions of infants and therefore the level of scrutiny will continue to be intense. Consequently, the safety hurdles for the approval of new vaccines will be high, with rigorous and detailed evaluations being routine. When vaccines have shown safety problems (Table 3), important lessons have been learned and approaches have been modified to avoid repeating the problem. Although serious adverse events have been rare, their occurrence can have a powerful impact on the affected individuals and those that are closest to them. Hence, even rare adverse events can have a negative impact on vaccines if they are broadly reported and not put into the context of their relative occurrence versus the benefit afforded by the vaccine. To prevent this, and to support vaccination policy, public health agencies, industry, academia and responsible journalists all have a role to play in ensuring that the benefits of vaccines are explained while acknowledging that adverse events do occur. Although this article will focus principally on the developed world, the global perspective of the World Health Organization (http://www.who.int) on the safety of vaccines was recently discussed [6]. Reviews of technologies that can improve the safety of vaccine administration [7] and a report on the first international symposium on vaccine safety [8] have also been recently published.
Table 3.
Safety issues associated with the use of vaccines
| Vaccine | Problem | Outcome | Refs |
| Killed respiratory syncytial virus | Enhanced disease after exposure to virus | Vaccine was never introduced onto the market | [51] |
| Oral polio virus | Reversion to virulence | Live vaccines now require larger and more stable deletions before approval | [52] |
| Live oral rotavirus | Intussusception | Vaccine no longer recommended and the required safety database has been expanded for future products | [9] |
| Inactivated measles | Atypical measles | Vaccine removed from the market | [53] |
| Intranasal flu with bacterial toxin adjuvant | Bell's palsy (facial paralysis) | Vaccine removed from the market | [54] |
| Whole-cell pertussis vaccine | Reactogenic | Removed from market in the majority of the developed world | [17] |
| Anthrax – cell-free filtrate product anthrax vaccine adsorbed | Reactogenic | Purified recombinant protein will be available in the near future | [55] |
| Swine flu | Guillain-Barré syndrome | Vaccination discontinued | [56] |
A recent example can be used to highlight vaccine safety and to illustrate how the vaccine community responded to an apparent problem with a marketed product. In 1998, a live, oral rotavirus vaccine was introduced onto the market for immunization of infants, but a year later this product was withdrawn because of an association with intussusception, a rare but serious obstruction of the intestine. Before licensure, only five cases of intussusception were reported in 10,000 vaccine recipients versus one case in 4633 placebo recipients [9]. However, nine months after licensure, the CDC recommended that vaccination should be suspended and the existing data evaluated further to investigate the relationship between the vaccine and intussusception [9]. Subsequent epidemiological studies appeared to confirm an excess of cases of intussusception after administration of the first dose [10] and the recommendation for use of the vaccine was withdrawn by the ACIP. Hence, it can be argued that postlicensure surveillance in the USA was effective at identifying a safety problem that appeared at low levels. Moreover, the safety database required to allow licensure for subsequent rotavirus vaccines has been expanded and studies are now underway in 60,000 infants for second-generation products. The story of the rotavirus vaccine in the USA brings up an important issue in relation to safety and/or benefit analysis. The vaccine was removed from the list of recommended vaccines because of the low level of incidence of a potentially serious adverse event, but rotavirus infection is not normally fatal in the USA. By contrast, rotavirus causes up to 600,000 deaths per annum in the developing world. Although the administration of the rotavirus vaccine could have prevented many of these deaths, once the recommendation had been withdrawn in the USA, it was considered unethical to promote the vaccine in the developing world despite the fact that it would have saved many lives. Fortunately, second-generation rotavirus vaccines are now moving through late stages of development and testing, including safety evaluations in large numbers of infants. Moreover, it appears that the original vaccine might be reintroduced onto the market (http://www2.niaid.nih.gov/Newsroom/Releases/rotavirus04.htm), following a re-evaluation of the existing safety data [11].
A historical perspective on vaccine safety
Long before the development of molecular methods that enable the specific engineering of recombinant viruses, a live, attenuated oral poliovirus vaccine (OPV) was developed using empirical methods involving the passage of virus in cell culture. However, shortly after the introduction of OPV, sporadic cases of vaccine-associated paralytic polio were reported at a level of one per 750,000 recipients [12]. In rare situations, the few attenuating mutations that are present in one vaccine strain could potentially cause the live OPV to revert to virulence. Nevertheless, the OPV vaccine has continued to be used worldwide and could be responsible for eradicating polio infection in the next few years. However, as the level of polio falls, OPV is gradually being replaced with an alternative killed, inactivated polio vaccine (IPV), which cannot cause polio even in the most susceptible individuals. An important lesson is that future live vaccines will not be acceptable unless there is a clear understanding of their molecular attenuation and the potential for reversion to virulence has been eliminated, either as a result of the size or the number of attenuating deletions. Molecular biology techniques have now made it possible to prevent live viruses from reverting to virulence.
The history of the development of IPV also taught the vaccine community a second important lesson. Long after the introduction of IPV into clinical use, it was discovered that early IPV was contaminated with simian virus 40 (SV40) from the cell line used to produce the vaccine. Although SV40 is oncogenic in several species, extensive investigations have shown that the use of contaminated IPV did not result in an increase in the rate of cancers in vaccine recipients [13]. Nevertheless, as a result of this problem, the rules and regulations that control the use of cell-culture systems in vaccines were extensively improved and they continue to evolve today to meet additional challenges, including the need to use media and reagents from animals that have been proven to be free of transmissible spongiform encephalitis (TSE).
Vaccines against whooping cough were first developed in the 1940s by formalin inactivation of bacterial cells and were later combined with tetanus and diphtheria toxoids to create the first combination vaccines. Although the whole-cell vaccines were effective at protecting against whooping cough, concerns about adverse reactions began to accumulate in the 1970s, particularly with regard to seizures and infantile spasms [14, 15]. Public opinion turned against whole-cell vaccines and their use declined, which predictably resulted in an upsurge in the incidence of disease and mortality from whooping cough [16]. It was finally established that in extreme cases the whole-cell pertussis vaccine could cause acute transient fever, hypotonic-hyporesponsive episodes, inconsolable crying and seizures, but there was no evidence that the vaccine caused chronic neurological problems [17]. However, the negative publicity and poor acceptance of the whole-cell vaccine resulted in extensive research to identify the protective antigens against pertussis. In the early 1980s, several companies [including GlaxoSmithKline (http://www.gsk.com), Aventis Pasteur (http://www.aventispasteur.com), Wyeth Lederle (http://www.whale.to/v/wyeth1.htm) and Chiron Vaccines (http://www.chiron.com)] initiated programs to evaluate the potency of subunit vaccines, which could be combined with tetanus and diphtheria vaccines. The development of subunit pertussis vaccines can be considered the start of the ‘minimalist’ approach to vaccine development, in which only the necessary antigens are included in the vaccine [18]. Several of these products were shown to be effective in protecting against disease and have now replaced whole-cell vaccines in most of the developed world. However, because of the increased cost, subunit pertussis vaccines are not yet available in the developing world.
Can vaccines cause autoimmune disease?
Predominantly as a consequence of the success of vaccines, the morbidity and mortality associated with many acute childhood infectious diseases has been replaced by an increasing incidence of more chronic disorders, for example, diabetes, MS, autism, asthma and allergy. Because the cause of these chronic diseases is often unknown and there is a temporal relationship between their onset and childhood vaccinations, there is considerable speculation regarding a causal link. A recent study involving more than 739,000 children born in Denmark between 1990 and 2000 showed that there was no support for a causal relationship between childhood vaccines and diabetes [19]. This was consistent with a previous study that had found no association between type I diabetes and any of the recommended childhood vaccines in the USA [20]. A possible relationship between hepatitis B vaccine and MS was also raised and investigated, but the results from two large-scale studies established that there is no significant association between hepatitis B vaccination and MS [3, 4]. Moreover, there is no evidence that routine vaccines, including hepatitis B, can increase the risk of exacerbation of MS [21]. Indeed, accumulated data have established that the hepatitis B vaccine, which is a purified recombinant protein, is among the safest vaccines developed to date. Wraith et al. [22] recently reviewed the evidence that vaccines could cause autoimmune disease and concluded that autoimmunity is a feature of a healthy immune system, which can occasionally be triggered by infection. Although it is possible that similar phenomena might also be triggered by vaccination, there is little evidence to suggest that this results in autoimmune disease. Fortunately, the immune system has numerous ‘fail-safe’ mechanisms, and naturally re-establishes the normal balance of adequate response versus over response.
Can vaccines cause anaphylaxis?
Anaphylaxis is a serious and potentially fatal hypersensitivity reaction that leads to a variety of distressing symptoms, often in children. Common causes for anaphylaxis include food types (e.g. peanuts), medications (e.g. penicillin) and bee stings. Although the problem can be caused by exposure to a range of materials in everyday life, vaccine-associated anaphylaxis has also been reported [23]. However, anaphylaxis is a rare occurrence and, after administration of 7,644,049 vaccine doses, only five potential cases of vaccine-associated anaphylaxis have been reported (giving a risk of 0.65 cases per million doses), none of which resulted in death [23]. In addition to antigens, vaccines contain several other components, including preservatives, adjuvants and manufacturing residuals. Although it is not always clear which components might be responsible for anaphylaxis, gelatin and egg proteins are present in some vaccines at sufficient levels to induce hypersensitivity reactions. Vaccines are not the only source of such materials and susceptible individuals are likely to encounter these agents at some point in their lives, even if they are not vaccinated. Exposure in a physician's office, where the reaction can be treated, is preferable to an uncontrolled situation in which the exposure could occur elsewhere. The levels of mercury, aluminium, formaldehyde, albumin, antibiotics and yeast proteins that are present in vaccines have not been shown to be harmful to humans or experimental animals [24].
Current controversies in vaccination
The removal of thiomersal (thimerosal), a mercury-containing preservative, from childhood vaccines was recommended by the American Academy of Pediatrics in 1999 (http://www.cdc.gov/nip/vacsafe/concerns/thimerosal). The recommendation to remove thiomersal caused significant problems and heightened public fears that children had already been damaged by the use of vaccines containing this component. However, there was no evidence to indicate that the low levels of mercury contained in vaccines could cause harmful effects (http://www.who.int/vaccine_safety/topics/thiomersal/en). A subsequent study confirmed that ethylmercury (thiomersal) was eliminated from infants rapidly and that concentrations reached in blood after vaccination were well below the level associated with toxic effects [25]. Unfortunately, the publicity over this issue resulted in the perception that all vaccines containing mercury were ‘unsafe’ for children and as a consequence some children at high risk for developing serious complications of influenza infection did not receive vaccines.
Adjuvants are added to vaccines to improve their immunogenicity [26] and were first introduced in the 1920s in combination with tetanus and diphtheria toxoid vaccines. The only adjuvants that are licensed for use in the USA are insoluble aluminium salts, which are generically called alum, such as the phosphate and hydroxide salts. Although the safety of vaccines adsorbed to alum has been established over 70 years of use [27], questions are still asked concerning safety. Studies have shown that at all time points after vaccination, the calculated body burden of aluminium remains below the minimal risk level [28]. A recent meta-analysis of the available safety data on alum could find no evidence of any serious long-lasting adverse effects [29]. Alum-adsorbed vaccines can induce local reactions (e.g. redness, pain and hardening of the injection site) in a significant number of recipients, but these are usually light to moderate and of short duration. Systemic reactions can also occasionally occur, including malaise, fever and aches. Although alum-adsorbed vaccines have been linked to a previously unknown inflammatory muscle disorder called macrophagic myofascitis [30], there is no evidence to support an association of this minor local lesion with any systemic disease (http://www.who.int/vaccine_safety/topics/aluminium/en). Because alum favors the induction of T-helper cell (Th) type 2 immune responses, including immunoglobulin E antibody responses, it has been suggested that alum could contribute to the development of allergic reactions (Th2-mediated) in predisposed individuals, but there is no evidence to support this. A recent study used a birth cohort of 30,000 in the UK to investigate the possibility that vaccination could be responsible for the increase in the prevalence of allergy in children. The article concluded that routine vaccinations are not a risk factor for asthma or eczema [31].
The highest profile vaccine controversy in recent years has been the unsubstantiated link between measles, mumps and rubella (MMR) vaccine and autism in the UK. Wakefield et al. [32] suggested that the measles vaccine was a possible contributory factor to the development of autism in predisposed children. The media storm created in the UK and the USA by this hypothesis led to a drop in the number of children immunized, which therefore enabled measles infection to recirculate, with adverse consequences for non-immunized children. In 2004, the journal that published the article by Wakefield et al. [32], in conjunction with the majority of the authors of the report, printed a partial retraction of the paper, largely because the possible association between measles and autism had been significantly over-interpreted [33]. This incident has become a salutary lesson in how important it is to ensure that scientific information is conveyed to the public in an appropriate manner that precludes interpretations that are not supported by the facts. On a global scale, measles caused the deaths of 869,000 people in 1999, mostly children. However, the number of deaths is declining (30% drop between 1999 and 2002) with the broader use of the MMR vaccine.
It has been conjectured that too many vaccines are administered simultaneously and that the concomitant administration of multiple vaccines results in the immune system being ‘overloaded’. There is no evidence to support this proposal and infants have an enormous capacity to respond safely and effectively to multiple vaccines [34]. For example, the whole-cell pertussis vaccine alone contains many more antigens than are currently administered to children in routine vaccination. A recent review article directly addressed the concerns of parents about the possibility that multiple vaccines can weaken the immune system of an infant and concluded that there was no evidence to support this [35]. The success of childhood vaccines has been partially blamed for the ‘hygiene hypothesis’, which suggests that because infants do not contract infections early in life their immune systems are tilted (Th2 responses) towards generating atopic disease later in life, including allergy and asthma. Thus, even when vaccines are accepted as being useful and effective, they are still highlighted as a potential cause of long-term problems. However, vaccines only prevent a few of the many infections that can be contracted by children and thus even fully vaccinated children will still be infected with pathogens that can have an impact on their developing immune systems. There is some evidence that the established tuberculosis (TB) vaccine Mycobacterium bovis Bacillus Calmette-Guérin (BCG) might result in a ‘protective’ effect against the development of allergy [36]; this is probably because this whole-cell vaccine induces a potent Th1 response that might affect the response to some other vaccines administered at similar times [37]. However, BCG vaccination is no longer routine in the developed world.
The balance of safety versus benefit for vaccines
There are several recent examples that demonstrate the impact of vaccines in reducing mortality and morbidity. The introduction of protein-polysaccharide conjugate vaccines against Neisseria meningitidis serogroup C in the UK showed a clear reduction in the number of deaths [38] (Figure 2), as did the recent introduction of the Pneumococcus conjugate vaccine [39]. A similar outcome had been achieved in the 1980s with the introduction of the first conjugate vaccine against Hemophilus influenzae type b (Hib) [40]. The economic impact of universal vaccination against Hib was recently reviewed and the substantial cost benefits were highlighted [41]. All of these vaccines are safe and well tolerated, with the occurrence of adverse events a rarity. Hence, the safety of vaccines needs to be considered in light of their impact in reducing death and disease.
Figure 2.
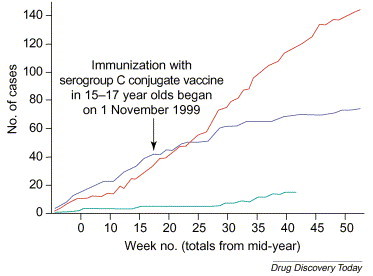
Impact of the introduction of a protein-polysaccharide conjugate vaccine against Neisseria meningitidis serogroup C in the UK in 1999. The colored lines indicate cumulative cases of N. meningitidis serogroup C over a particular period of time: red line represents 1998–1999; blue line represents 1999–2000; and green line represents 2000–2001 (to week 16 of 2001). Adapted from Ref. [38].
In addition to the positive impact of vaccines, there have also been incidents of adverse consequences as a result of a failure to vaccinate. This has sometimes been a consequence of the perception of the public of a ‘problem’ with vaccine safety, including measles or hepatitis B, but is also the result of a change in vaccination policy. From 1962 to 1987, the routine vaccination of Japanese schoolchildren against influenza was mandatory, but the laws were relaxed in 1987 and repealed in 1994, which resulted in a considerable drop in the level of vaccination [42]. A subsequent study showed that the change in policy had a significant adverse effect on the elderly population in Japan and resulted in increased numbers of deaths [43]. The change in policy was largely caused by sensationalized reports of lawsuits that alleged adverse effects, which were unsubstantiated but resulted in the public losing confidence in the vaccine [42]. The experience in Japan enabled a clear correlation to be made between vaccination policy, the establishment of herd immunity and a positive impact of vaccines in preventing death and disability in a susceptible population [43].
Technologies to improve vaccine safety
Several approaches have been developed recently that have the potential to produce safer vaccines. The use of the genomic information that is available on important microorganisms and the approach termed ‘reverse vaccinology’ enables the identification of novel recombinant antigens that have the potential to be excellent vaccine candidates [44]. Based on the experience with the recombinant hepatitis B vaccine, it is expected that these highly purified recombinant antigens will prove to be much safer than traditional vaccines. Several novel vaccine adjuvants with improvements over alum are currently undergoing clinical and preclinical testing, including ‘delivery systems’ that are designed to promote the uptake of antigens into key cells of the immune system and potent immunopotentiators that are designed to have a higher specificity for the activation of these cells [45]. The most significant barrier to the development of new adjuvants has been the requirement for safety, but an emulsion-based adjuvant (MF59) was introduced onto the European market in 1997 [46] and additional approaches are expected to follow in the coming years. Advances in understanding the mechanisms of how adjuvants work has already resulted in the development of safer candidates, with more specific effects on limited cell types, and this trend will continue [45]. Improvements in genetic engineering approaches, including the approach of ‘reverse genetics’, now enable the production of viral vectors with predetermined genetic defects, which should improve their safety. In addition, the development of non-replicating viral vectors (e.g. alphaviruses) will result in improvements in safety over the traditional approaches [47]. At present, the biggest problem for vaccine safety worldwide is the inappropriate reuse of needles in developing countries, which results in infection with blood-borne pathogens such as HIV and hepatitis C virus (HCV). However, important developments in the use of auto-disposable syringes and in needle-free vaccine-delivery systems will minimize these problems [5]. Overall, the increasing use of postlicensure safety studies (often called Phase IV studies) for new vaccine products will ensure that safety issues that arise even at low levels will be identified quickly, as has already been demonstrated with the oral rotavirus vaccine.
The need for new and improved vaccines
There is a clear need to develop new and improved vaccines against; (i) infectious diseases for which vaccines are not yet available, or are currently inadequate, for example, HIV, HCV, N. meningitidis type B, group B Streptococcus, TB and malaria; (ii) to protect against the threat of pandemic strains of influenza virus and the continued growth and spread of antimicrobial resistant organisms, including vancomycin-resistant Staphylococcus infections, multidrug-resistant TB and drug-resistant strains of HIV; (iii) to protect against several emerging or re-emerging infectious diseases, such as West Nile virus, severe acute respiratory syndrome (SARS), Ebola, Hanta and Dengue viruses; and (iv) to protect against the threats of bioterrorism. Furthermore, there is an increasing awareness that infectious agents often cause chronic diseases, and many of these might be prevented or even treated with novel vaccines (e.g. hepatitis B and C). Therapeutic vaccines would be able to sustain a higher level of adverse events than traditional vaccines, particularly if used in an oncology setting or to treat a life threatening infectious disease, because they would be used in people who were already sick or infected. Hence, the benefit-to-risk ratio would be significantly shifted in favor of the vaccine and the level of scrutiny in relation to safety would be changed.
Conclusions
Rappuoli et al. [48] highlighted the ‘intangible value of vaccination’, which is the immeasurable benefit to all of us of being free from the impact of several childhood diseases that might otherwise have led to premature death or resulted in irreparable physical or mental damage. However, society does not currently value ‘prevention’ as high as it values therapy. Unfortunately, the economic value of vaccines is undervalued when compared with pharmaceutical drugs and this has resulted in many companies choosing not to invest in vaccines. The UK government recently expressed concern that the small number of companies producing vaccines signifies that the supply of vaccines is in danger [49]. The economic disincentive to invest in vaccines is heightened by the risks of liability. We live in an increasingly litigious society, in which lawyers are eager to take up the cases of individuals who believe that they have been negatively affected by a medical procedure, including vaccination. Although a claim of complete safety is impossible and irresponsible for any medical product, the benefits of vaccination far outweigh the risks. The public needs to be educated to ensure that vaccination is recognized as a means for individuals to establish themselves in society as responsible citizens. To promote the use of vaccines and to encourage active participation, attempts to understand the negative perceptions of some for vaccines must be made [50]. Although the vast majority of people receive only benefit from vaccines, these people are silent. By contrast, the few who suffer adverse events, or the perception of adverse events, might become passionate and vociferous opponents of vaccination. If these are the only people who voice an opinion, the overall perception of vaccines could be that they do more harm than good.
Although the medical community has generally been successful at convincing people about the benefit of childhood vaccines, education of the population about the benefit of vaccines in later life must be improved. In the coming years, the availability of vaccines that will be targeted at adolescent and elderly individuals will increase. All of us in the vaccine community have a role to play in educating the public to have a greater appreciation of the value of vaccines to ensure that lives are not lost prematurely to preventable infectious diseases. The numbers of available vaccines will increase as a consequence of the growing awareness that chronic diseases can be caused by infectious agents.
Overall, the field of vaccinology has evolved considerably over the past century and much has been learned, although sometimes through unfortunate mistakes. Today, there is the potential to develop highly purified and safe vaccines that are predominantly based on recombinant proteins or protein-polysaccharide conjugates. These vaccines will eliminate the problems previously encountered with whole-cell and live-attenuated products. Moreover, mechanisms are now in place to enable evaluations in large numbers of people that directly address some of the concerns about the potential for vaccines to induce diabetes, autism and MS, among others. It is hoped that confidence in vaccination will improve as safety reaches unprecedented levels and irrational fears will continue to be allayed by convincing scientific data. However, the key to enhancing confidence in vaccination is total transparency about the real and unsubstantiated issues in vaccine safety.
Acknowledgements
We are grateful to Clem Lewin for several helpful suggestions and to Nelle Cronen for excellent assistance in manuscript formatting.
References
References
- 1.Ehreth J. The value of vaccination: a global perspective. Vaccine. 2003;21:4105–4117. doi: 10.1016/s0264-410x(03)00377-3. [DOI] [PubMed] [Google Scholar]
- 2.Jodar L. Ensuring vaccine safety in immunization programmes – a WHO perspective. Vaccine. 2001;19:1594–1605. doi: 10.1016/s0264-410x(00)00358-3. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in multiple sclerosis study group. N. Engl. J. Med. 2001;344:319–326. doi: 10.1056/NEJM200102013440501. [DOI] [PubMed] [Google Scholar]
- 4.Ascherio A. Hepatitis B vaccination and the risk of multiple sclerosis. N. Engl. J. Med. 2001;344:327–332. doi: 10.1056/NEJM200102013440502. [DOI] [PubMed] [Google Scholar]
- 5.O'Hagan D., Rappuoli R. Novel approaches to vaccine delivery. Pharm. Res. 2004;21:1519–1530. doi: 10.1023/B:PHAM.0000041443.17935.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duclos P. A global perspective on vaccine safety. Vaccine. 2004;22:2059–2063. doi: 10.1016/j.vaccine.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Clements C.J. Technologies that make administration of vaccines safer. Vaccine. 2004;22:2054–2058. doi: 10.1016/j.vaccine.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Demicheli V., Jefferson T. The first international symposium on vaccine safety. Vaccine. 2004;22:2042–2043. [Google Scholar]
- 9.Murphy T.V. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 10.Chang H.G. Intussusception, rotavirus diarrhea, and rotavirus vaccine use among children in New York state. Pediatrics. 2001;108:54–60. doi: 10.1542/peds.108.1.54. [DOI] [PubMed] [Google Scholar]
- 11.Murphy B.R. Reappraisal of the association of intussusception with the licensed live rotavirus vaccine challenges initial conclusions. J. Infect. Dis. 2003;187:1301–1308. doi: 10.1086/367895. [DOI] [PubMed] [Google Scholar]
- 12.Strebel P.M. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin. Infect. Dis. 1992;14:568–579. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 13.Strickler H.D. Contamination of poliovirus vaccines with simian virus 40 (1955–1963) and subsequent cancer rates. J. Am. Med. Assoc. 1998;279:292–295. doi: 10.1001/jama.279.4.292. [DOI] [PubMed] [Google Scholar]
- 14.Strom J. Further experience of reactions, especially of a cerebral nature, in conjunction with triple vaccination: a study based on vaccinations in Sweden 1959–65. Br. Med. J. 1967;4:320–323. doi: 10.1136/bmj.4.5575.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart G.T. Toxicity of pertussis vaccine. Lancet. 1977;2:1130. doi: 10.1016/s0140-6736(77)90573-6. [DOI] [PubMed] [Google Scholar]
- 16.Gangarosa E.J. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. 1998;351:356–361. doi: 10.1016/s0140-6736(97)04334-1. [DOI] [PubMed] [Google Scholar]
- 17.Miller D. Pertussis immunization and serious acute neurological illnesses in children. Br. Med. J. 1993;307:1171–1176. doi: 10.1136/bmj.307.6913.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rappuoli R. Acellular pertussis vaccines: a turning point in infant and adolescent vaccination. Infect. Agents Dis. 1996;5:21–28. [PubMed] [Google Scholar]
- 19.Hviid A. Childhood vaccination and type 1 diabetes. N. Engl. J. Med. 2004;350:1398–1404. doi: 10.1056/NEJMoa032665. [DOI] [PubMed] [Google Scholar]
- 20.DeStefano F. Childhood vaccinations, vaccination timing, and risk of type 1 diabetes mellitus. Pediatrics. 2001;108:E112. doi: 10.1542/peds.108.6.e112. [DOI] [PubMed] [Google Scholar]
- 21.Rutschmann O.T. Immunization and MS: a summary of published evidence and recommendations. Neurology. 2002;59:1837–1843. doi: 10.1212/wnl.59.12.1837. [DOI] [PubMed] [Google Scholar]
- 22.Wraith D.C. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]
- 23.Bohlke K. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112:815–820. doi: 10.1542/peds.112.4.815. [DOI] [PubMed] [Google Scholar]
- 24.Offit P.A., Jew R.K. Addressing parents' concerns: do vaccines contain harmful preservatives, adjuvants, additives, or residuals? Pediatrics. 2003;112:1394–1397. doi: 10.1542/peds.112.6.1394. [DOI] [PubMed] [Google Scholar]
- 25.Pichichero M.E. Mercury concentrations and metabolism in infants receiving vaccines containing thiomersal: a descriptive study. Lancet. 2002;360:1737–1741. doi: 10.1016/S0140-6736(02)11682-5. [DOI] [PubMed] [Google Scholar]
- 26.Singh M., O'Hagan D.T. Recent advances in vaccine adjuvants. Pharm. Res. 2002;19:715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 27.Clements C.J., Griffiths E. The global impact of vaccines containing aluminium adjuvants. Vaccine. 2002;20(Suppl. 3):S24–S33. doi: 10.1016/s0264-410x(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 28.Keith L.S. Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine. 2002;20 (Suppl. 3):S13–S17. doi: 10.1016/s0264-410x(02)00165-2. [DOI] [PubMed] [Google Scholar]
- 29.Jefferson T. Adverse events after immunisation with aluminium-containing DTP vaccines: systematic review of the evidence. Lancet Infect. Dis. 2004;4:84–90. doi: 10.1016/S1473-3099(04)00927-2. [DOI] [PubMed] [Google Scholar]
- 30.Gherardi R.K. Macrophagic myofascitis: an emerging entity. Groupe d'Etudes et Recherche sur les Maladies Musculaires Acquises et Dysimmunitaires (GERMMAD) de l'Association Francaise contre les Myopathies (AFM) Lancet. 1998;352:347–352. doi: 10.1016/s0140-6736(98)02326-5. [DOI] [PubMed] [Google Scholar]
- 31.McKeever T.M. Vaccination and allergic disease: a birth cohort study. Am. J. Public Health. 2004;94:985–989. doi: 10.2105/ajph.94.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakefield A.J. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–641. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 33.Murch S.H. Retraction of an interpretation. Lancet. 2004;363:750. doi: 10.1016/S0140-6736(04)15715-2. [DOI] [PubMed] [Google Scholar]
- 34.Gregson A.L., Edelman R. Does antigenic overload exist? The role of multiple immunizations in infants. Immunol. Allergy Clin. North Am. 2003;23:649–664. doi: 10.1016/s0889-8561(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 35.Offit P.A. Addressing parents' concerns: do multiple vaccines overwhelm or weaken the infant's immune system? Pediatrics. 2002;109:124–129. doi: 10.1542/peds.109.1.124. [DOI] [PubMed] [Google Scholar]
- 36.Townley R.G. The effect of BCG vaccine at birth on the development of atopy or allergic disease in young children. Ann. Allergy Asthma Immunlo. 2004;92:350–355. doi: 10.1016/S1081-1206(10)61574-8. [DOI] [PubMed] [Google Scholar]
- 37.Ota M.O. Influence of Mycobacterium bovis bacillus Calmette-Guérin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 2002;168:919–925. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 38.Balmer P. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 2002;51:717–722. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 39.Klugman K.P. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect. Dis. 2001;1:85–91. doi: 10.1016/S1473-3099(01)00063-9. [DOI] [PubMed] [Google Scholar]
- 40.Adams W.G. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. J. Am. Med. Assoc. 1993;269:221–226. [PubMed] [Google Scholar]
- 41.Zhou F. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics. 2002;110:653–661. doi: 10.1542/peds.110.4.653. [DOI] [PubMed] [Google Scholar]
- 42.Hirota Y. Japan lagging in influenza jabs. Nature. 1996;380:18. doi: 10.1038/380018a0. [DOI] [PubMed] [Google Scholar]
- 43.Reichert T.A. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 2001;344:889–896. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 44.Pizza M. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 45.O'Hagan D.T., Valiante N.M. Recent advances in the discovery and delivery of vaccines and adjuvants. Nat. Rev. Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott G. The Adjuvant MF59: A 10-year Perspective. In: O'Hagan D., editor. Vaccine Adjuvants: Preparation Methods and Research Protocols. Humana Press; 2000. pp. 211–228. [Google Scholar]
- 47.Polo J.M., Dubensky T.W. Virus-based vectors for human vaccine applications. Drug Discov. Today. 2002;7:719–727. doi: 10.1016/s1359-6446(02)02324-3. [DOI] [PubMed] [Google Scholar]
- 48.Rappuoli R. Medicine. The intangible value of vaccination. Science. 2002;297:937–939. doi: 10.1126/science.1075173. [DOI] [PubMed] [Google Scholar]
- 49.House of Commons (2004) Procurement of vaccines by the Department of Health (HC 429). The House of Commons (http://www.publications.parliament.uk/pa/cm200304/cmselect/cmpubacc/429/429.pdf)
- 50.Poland G.A., Jacobson R.M. Understanding those who do not understand: a brief review of the anti-vaccine movement. Vaccine. 2001;19:2440–2445. doi: 10.1016/s0264-410x(00)00469-2. [DOI] [PubMed] [Google Scholar]
- 51.Kapikian A.Z. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 52.Minor P.D. Attenuation and reversion of the Sabin vaccine strains of poliovirus. Dev. Biol. Stand. 1993;78:17–26. [PubMed] [Google Scholar]
- 53.Fulginiti V.A. Altered reactivity to measles virus: local reactions following attenuated measles virus immunization in children who previously received a combination of inactivated and attenuated vaccines. Am. J. Dis. Child. 1968;115:671–676. doi: 10.1001/archpedi.1968.02100010673006. [DOI] [PubMed] [Google Scholar]
- 54.Mutsch M. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 2004;50:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 55.Pittman P.R. Anthrax vaccine: short-term safety experience in humans. Vaccine. 2001;20:972–978. doi: 10.1016/s0264-410x(01)00387-5. [DOI] [PubMed] [Google Scholar]
- 56.Langmuir A.D. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am. J. Epidemiol. 1984;119:841–879. doi: 10.1093/oxfordjournals.aje.a113809. [DOI] [PubMed] [Google Scholar]


