Traditional knowledge-driven bioprospecting offers access to multiple targets that can provide a safer and more cost-effective platform for newer scaffolds to facilitate drug discovery.
Keywords: Systems biology, Traditional medicine, Bioprospecting, Immunomodulation, Immunostasis, Ayurveda
Abstract
Modulation of the immune system can be addressed through a variety of specific and non-specific approaches. Many agents of synthetic and natural origin have stimulatory, suppressive or regulatory activity. There is growing evidence that drugs or biological agents capable of modulating single pathways or targets are of limited value as immune-related therapies. Systems biology approaches are now gaining more interest compared with monovalent approaches, which can be of limited benefits with complications. This has stimulated interest in the use of ‘cocktails’ of immunodrugs to restore immunostasis. Botanicals are chemically complex and diverse and could therefore provide appropriate combinations of synergistic moieties useful in drug discovery. Here, the importance of traditional medicine in natural product drug discovery related to immunodrugs is reviewed.
The immune response requires the timely interplay of multiple cell types within specific microenvironments to maintain immune homeostasis. The selectivity and flexibility that is necessary to regulate cell traffic under homeostatic and diseased conditions is provided by the differential distribution and regulated expression of cytokines and their receptors. As a consequence, cytokines are responsible for the development of phenotypes and are, therefore, logical targets for therapeutic immune modulation [1]. Immunodrugs include synthetic organics, biologicals, such as cytokines and antibodies, and microbial and botanical natural products, which influence immunoregulatory cascades to bring about their specific stimulatory, suppressive or regulatory effect. Immune suppression has been widely studied for clinical applications [2]. Historically, botanicals have been a mainstay of drug treatments and are currently receiving attention as sources of synergistic combinations. Here, recent developments in botanical immunodrug discovery are reviewed.
Immunodrugs in cancer
Although the treatment of cancer using active immunotherapy has had limited success, passive immunotherapy with antibody and cytokine therapies brings new hope. The most widely studied approaches consist of whole-cell vaccines, dendritic cell-based immunotherapy and peptide vaccines. Many clinical studies have demonstrated safety but not necessarily the efficacy of such strategies [3]. Moreover, there is emerging consensus that the most efficacious therapy should activate several components of the immune system [4]. Cytokine therapy in cancer is another attractive approach; however, balancing optimal doses to avoid toxic reactions remains challenging [5]. Several cytotoxic drugs have immunomodulatory effects at relatively low doses and can exert immunity-dependent curative effects in animal models of cancer. Combination therapies involving low-dose anticancer agents and cytokines have demonstrated some benefits. For example, combinations of appropriate regimens of doxorubicin plus interleukin (IL)-2 or tumour necrosis factor (TNF) could be curative and produced a life-long immunological memory in an EL4 lymphoma C57BL/6 mouse model. Many researchers have demonstrated that the induction of T-helper (Th) 1-promoting cytokines, using specific adjuvants, can enhance antitumour immunity and can prevent or reduce tumour growth. These trends substantiate the possibility of establishing combination regimens based on low-dose anticancer drugs, specific cytokines and immunological adjuvants [6].
Immunodrugs in infection
Immunomodulators could also have beneficial roles in the prevention and treatment of infectious disease. A diverse array of synthetic, natural and recombinant compounds are available. Of the synthetic immunomodulators, Levamisol, Isoprinosine, Pentoxifilline and Thalidomide are some of the more significant [7]. Microbial immunomodulators, such as bacille Canette-Guérin, have been in use for years for non-specific activation of the immune system in some forms of cancer (bladder) and infectious disease [8].
Targeting cytokines is now considered to be one of the logical approaches for the prevention and treatment of infectious disease. Some of these substances, such as granulocyte colony-stimulating factor (G-CSF), interferons, IL-12, various chemokines, synthetic cytosine phosphate-guanosine (CpG) oligodeoxynucleotides and glucans are being investigated in preclinical and clinical studies [9]. Interferons are widely used for the treatment of chronic infections, particularly hepatitis B, D and C viruses [10]. Pegylated interferon is a recently developed pharmaceutical preparation that has proven beneficial in non-responsive patients, particularly in those with cirrhosis or hepatitis C virus genotype 1 [11]. Other cytokines, such as IL-1, IL-2 and IL-17, have shown potential in augmenting immune responses in various infectious conditions and malignancies. The therapeutic effect of cytokine blockers is also reported in septic shock [12]. A recent study on hepatitis C has shown that interferon-γ (IFN-γ), in combination with ribavirin, induces a higher percentage of lymphocyte activation [13] than for IFN-γ alone. Such approaches are currently being examined for their potential to boost host immune response to fight infection.
Immunomodulation and inflammation
Many immune targets have been identified as having potential for the central control of inflammation. Targeting activated T-cell subsets was considered to be one of the most rational approaches and biologicals, such as monoclonal antibodies (mAbs) against CD4, CD5, CD7, CD25 and CD52, were evaluated in patients with autoimmune disorders. Limited clinical benefit and complications, such as the prevalence of opportunistic infection and/or malignancies, were observed and the hope of reprogramming the host immune response remained unfulfilled [14, 15].
Studies on targets related to leukocyte infiltration, such as leukocyte-function associated antigen-1 (LFA-1), CD11α/CD18 (adhesion-receptor-counter-receptor pair) and intercellular adhesion molecule-1 (ICAM-1) CD54, are in progress; however, the development of humanized antibodies and their long-term safety evaluation have yet to be established [16]. Cytokine therapy, such as anti-TNF-α or IL-1, has been an attractive treatment option; however, an optimal treatment regimen with respect to dosage, interval and particularly long-term safety needs to be explored [17, 18, 19]. The use of the effector functions of Th cells is now considered to be one of the more promising innovative therapeutic strategies. Thus, the idea of switching Th1-dominated responses into Th2-mediated responses appears intriguing. This approach has been studied in various animal models of autoimmune diseases but clinical validation has not been achieved [20, 21, 22]. Newer targets central to innate and adaptive immunity, such as Toll-like receptors (TLR) and the complement system and nuclear factor-κB (NF-κB) activation, are being studied [23, 24, 25]. Combination approaches to the treatment of inflammatory diseases appear promising; in particular, combining methotrexate with TNF-α inhibitors has provided some encouraging results [26].
Immunodrugs and vaccine adjuvants
The combined use of vaccines and immunomodulators are innovative strategies in vaccine design and development. Many synthetic, biological and natural immunomodulators are under evaluation as vaccine adjuvants. Administration of cytokine genes along with DNA vaccines has been shown to achieve selective modulation of T-cell responses [27]. Moreover, innate immunity targets, such as TLR, and their modulation are currently being researched for their ability to provide effective adjuvant action. QS-21 and glucans are experimental adjuvants currently under clinical evaluation with different vaccines [28, 29, 30].
Immunostasis: Targets and regulation
Th lymphocytes are divided into distinct phenotype subsets of Th1 (e.g. IFN-g, IL-2 and TNF-a) and Th2 (e.g. IL-3, IL-4, IL-5, and so on) effector cells. This classification is based on their functional capabilities and cytokine profiles. Th1 cells drive the cellular immunity to fight intracellular organisms, eliminate cancerous cells and stimulate delayed-type hypersensitivity reactions. By contrast, Th2 cells drive humoural immunity and upregulate antibody productions to fight extracellular organisms. T-cell homeostasis or immunostasis requires a fine balance between Th1-Th2 response and such agents could exhibit stimulatory, suppressive or regulatory activity [31]. Currently, much of the literature supports the view that Th1-Th2 is essential to immunostasis and many of the T-cell-directed therapies have provided modest clinical benefits [32]. Although this view of signal conversion looks relatively simplistic, mediators of signal transduction do not interact in a linear manner but within a biochemical matrix. As a consequence, each cytokine has multiple functions and their modulation would be expected to result in diverse therapeutic functions. Such complex crosstalk between signalling networks imposes challenges for the discovery of optimal therapeutic interventions [33]. In cancer, researchers have arrived at a consensus that reconstitution of function is important, rather than reconstitution of cells or cytokines, and the most efficacious therapy will be one in which multiple responses of the immune system are activated. Trends indicate that future immunotherapy should involve cocktails of drugs that concurrently and/or simultaneously address vital components of the immune matrix [34]. This will require synergistic combinations for homeostatic regulation of the immune system. Historically, botanicals have facilitated and enriched the drug discovery process and, we propose, should be explored as sources for synergistic combinations.
Botanical immunodrugs
Many herbal preparations alter immune function and display an array of immunomodulatory effects. In various in vitro and in vivo studies, herbal medicines have been reported to modulate cytokine secretion, histamine release, immunoglobulin secretion, class switching, cellular co-receptor expression, lymphocyte expression, phagocytosis, and so on [35]. A recent study, using a transgenic mouse model of melanoma, showed that the anticancer effects of popular Kampo medicine were mediated via an enhanced antigen-specific antitumour cytotoxic T-lymphocyte response [36]. Botanicals produce a diverse range of natural products with antimicrobial and immunomodulating potential, including isoflavonoids, indoles, phytosterols, polysaccharides, sesquiterpenes, alkaloids, glucans and tannins. There are many immune-related conditions with a high unmet clinical need and this is particularly true in the case of new viruses and the phenomenon of increasing antibiotic resistance. Botanical immunomodulating agents might be able to provide an alternative to costly immunotherapeutics.
Natural product drug discovery
There are two major ways of bioprospecting natural products for investigation. First, the classical method that relies on phytochemical factors, serendipity and random screening approaches. Second, the use of traditional knowledge and practices as a drug discovery engine: this is also known as an ethnopharmacology approach, which is time and cost effective and could lead to better success than routine random screening [37, 38]. Traditional methods, for example, Chinese medicine, Japanese Kampo and Indian Ayurveda, are becoming important bioprospecting tools [39]. Ayurveda gives a separate class of immunomodulatory botanicals named Rasayanas. Several botanicals from these texts have been studied for their immunomodulatory properties and have the potential to provide new scaffolds for safer, synergistic, cocktail immunodrugs [40]. In subsequent sections, examples are provided of important botanicals based on traditional knowledge that have been studied for the bioprospecting of potential immunodrugs. These botanicals have an array of compounds with diverse activities directed at various targets of the immune matrix, in cancer and in infection and inflammation.
Glycyrrhiza glabra
The root Glycyrrhiza glabra, commonly known as liquorice, has been used since ancient times in Indian, Chinese, Egyptian, Greek and Roman medicine. It is prominent in Ayurveda as Rasayana with cytoprotective and demulcent effects and is a popular home remedy for minor throat infections. Biologically active substances in liquorice roots include glycyrrhizic acid (GL; Figure 1) and its aglycone (GA), phenolic compounds, oligosaccharides and polysaccharides, lipids, sterine, and so on. Recently, GL and GA were shown to exert a hepatoprotective effect via modulation of immune-mediated hepatocyte toxicity, NF-κB and IL-10, which explains how their administration resulted in a downregulation of inflammation in the liver [41]. GL has been reported to increase the resistance to Candida albicans and herpes simplex virus-1 infection in animal models [42, 43]. Many researchers have suggested that effects on the production of IFN and Th2 cytokines might be one of the mechanisms involved in the anti-infective process. Recently, glycyrrhizin was found to be active in inhibiting replication of the severe acute respiratory syndrome (SARS)-associated virus (FFM-1 and FFM-2). In the study, GA was found more effective compared with ribavirin, 6-azauridine, pyrazofurin or mycophenolic acid [44]. GL is also reported to have modulatory effects on the complement system. Reports indicate that GL blocks C5 or a more distal stage of the complement cascade, suggesting that it might have a role in preventing tissue injury not only in chronic hepatitis but also in autoimmune and inflammatory diseases [45].
FIGURE 1.
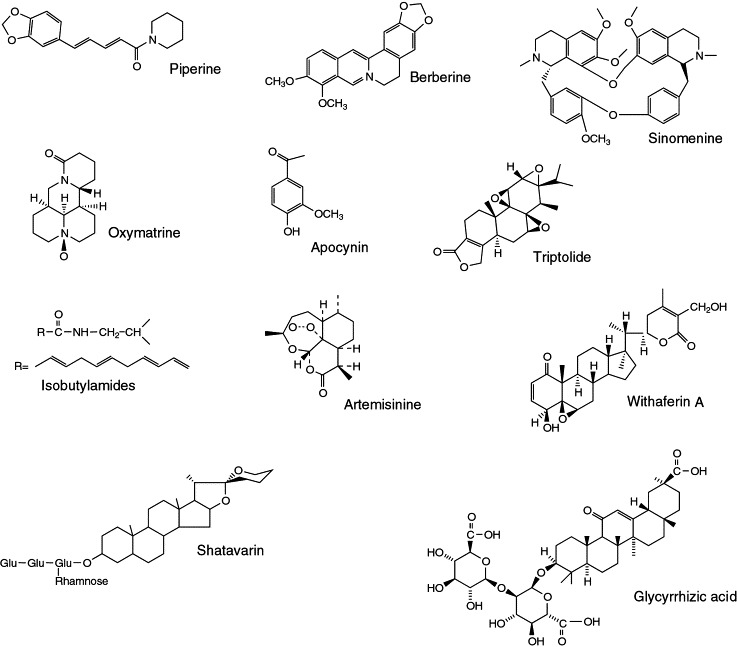
Chemical structures of immunoactive leads from botanicals.
Many researchers have reported response-modifying activity in flavonoids and chalcones isolated from the root extract. Modulation of the Bcl-2/Bax family of apoptotic regulatory factors, by components of the root, has been suggested as a possible mechanism for its reported cytoprotective activity [46]. These activities include antioxidant, chemopreventive and antimicrobial activities [47].
Chemical modification of GL and GA has been tried and significant improvement in anti-inflammatory, antiallergic, and antiulcer activities was observed [48]. These observations indicate immune-modulating and biological response-modifier activities associated with GL [49].
Uncaria tomentosa
Uncaria tomentosa, also known as cat's claw, is from the highlands of the Peruvian Amazon and has been used by natives for hundreds of years to treat immunologic and digestive disorders. It was found that two chemotypes of this plant occur in nature, each with different alkaloid patterns [50]: the roots of one type contain pentacyclic oxindoles and the other contains tetracyclic oxindoles, reported to have antagonistic activities. Tetracyclic oxindole alkaloids dose-dependently reduce the activity of pentacyclic oxindole alkaloids on human endothelial cells [51].
Aqueous extracts and mixtures of oxindole alkaloids have shown positive influence on IL-1, IL-6 and IFN-γ production, suggesting immunoregulatory activity. In one clinical study, extract exhibited immune adjuvant activity with pneumococcal vaccine resulting in enhanced lymphocyte:neutrophil ratio and persistent antibody titre responses towards 12 pneumococcal serotypes [52].
In vitro, in vivo and gene expression studies on extracts of this plant indicated that anti-inflammatory activity is mediated through negation of NF-κB activation and suppression of TNF-α synthesis [53, 54]. Randomized clinical studies on a purified extract, rich in pentacyclic alkaloids, demonstrated safer and moderate benefit in patients with active rheumatoid arthritis compared with those taking sulfasalazine or hydroxychloroquinine [55].
Cytoprotection was observed in extracts devoid of alkaloids. The oxindole alkaloid-free fraction modulated apoptosis, tumour cell proliferation and DNA repair processes, leading to cytoprotection in chemically induced leucopoenia rat model [56]. Extracts have also shown promising antitumour activity mediated via selective induction of apoptosis [57].
Echinacea spp
The Echinacea plant is a member of the Compositae family; the three species of medicinal interest being Echinacea angustifolia, Echinacea purpurea and Echinacea pallida. Most uses of E. purpurea are based on its reported immunological properties. There are four types of constituents purported to be pharmacologically active molecules: phenolic caffeic acid derivatives, alkylamides and isobutylamides, polysaccharides and glycoproteins. Limited experimental evidence of immunostimulatory activity exists for caffeic acid derivatives and alkylamides [58]. By contrast, Echinacea polysaccharides were found to directly activate non-specific immune cell types, such as monocytes, macrophages and natural killer (NK) cells [59]. This characterization of Echinacea polysaccharides is the best demonstration of in vitro bioassay activity yielding reproducible in vivo pharmacological effects [60, 57]. Several randomized trials have reported health benefits of Echinacea extracts in upper respiratory tract infections [61].
Withania somnifera
Withania somnifera (WS) - known as ashwagandha, Indian ginseng and winter cherry - is also classified as Rasayana in Ayurveda. The major biochemical constituents of WS root are steroidal alkaloids and steroidal lactones known as withanolides. Much of WS pharmacological activity has been attributed to withaferin A and withanolide D. WS is reported to have immunomodulatory, antitumour, cytoprotective and antioxidant properties [62]. All these activities are thought to be involved in the overall immunoregulatory properties of WS.
Several preclinical studies have examined the cytoprotective potential of WS. In one study, WS exhibited myeloprotection in tumour model without compromising antitumour efficacy of cyclophosphamide, azathioprin or prednisolone [63]. In another instance, cytoprotection against experimental skin cancer was observed, where reduced levels of glutathione, superoxide dismutase, catalase and glutathione peroxidase returned to normal following WS administration [64].
WS exhibited modulatory effects on cytotoxic lymphocyte production leading to reduced tumour growth. Withaferin A was found to be better than doxorubicin in inhibiting growth of breast and colon cancer cell lines [65]. A recent study suggested that the increased production of inducible nitric oxide synthase was one of the possible mechanisms for the increased cytotoxic effect of macrophages exposed to WS extracts. Moreover, withaferin A in combination with radiotherapy increased the response to radio-resistant tumours [66].
WS treatment in normal and tumour-bearing mice showed a positive influence on NK cell activity resulting in enhanced cell killing [67]. Many studies, including our own, have demonstrated the immunomodulatory potential of WS, resulting in increased haemolytic titres, inhibition of delayed type sensitivities and an increase in phagocytic activity of macrophages [68, 69]. We also observed that animals receiving WS showed immunoprotection to Bordetella pertussis infection, as evident by increased antibody titres and higher survival percentage [70]. In a recent study, Immun-21, a polyherbal formulation containing WS, exhibited immunomodulatory activity leading to modest clinical benefits in groups of HIV patients [71]. These observations suggest that WS could be used as an immunological adjuvant with multiple therapeutic benefits in cancer, infection and AIDS. In a comparative pharmacological investigation of WS and ginseng, the WS-treated group showed better anabolic and antistress activity than ginseng with additional anti-inflammatory activity [72].
WS has also been reported to be effective on acute-phase reactants, nitric oxide synthase and glycosaminoglycan synthesis in addition to immunoregulation [73]. Clinical studies on WS have shown moderate analgesic, anti-inflammatory and disease-modifying activity in arthritis patients [74, 75].
Tinospora cordifolia
Another Ayurvedic Rasayana known for immunomodulatory and cytoprotective activities is Tinospora cordifolia(TC), the chemistry of which has been studied extensively and its chemical constituents can be broadly divided into alkaloids, diterpenoids, steroids, flavonoids and lignans. Reviews have appeared on quaternary alkaloids and biotherapeutic diterpene glucosides of TC: syringin, cordiol, cordioside and coriofolioside were found to possess immunopotentiating activity [76].
Much of the work has been conducted on berberine, jatrorrhizine, tinosporaside and columbin. The possible mechanism of immunomodulatory activity was elucidated as activation of macrophages, leading to increases in granulocyte-macrophage colony-stimulating factor (GM-CSF), leading to leukocytosis and improved neutrophil function. TC is also reported to inhibit C3-convertase of the classical complement pathway [77]. In a recent study, a polysaccharide (α-D-glucan) derived from TC resulted in activation of NK cells, complement system, Th1-pathway cytokines, coupled with low nitric oxide synthesis [78]. Antitumour activity of TC was evaluated in cultured HeLa cells and revealed that the effect of extract was comparable and better than doxorubicin treatment. Hepatoprotective activity of TC against carbon tetrachloride-induced liver damage has also been reported [79].
Other immunoactive leads from botanicals sources, which have shown potential as cytoprotectives, antitumour, anti-infective and anti-inflammatory agents are given in Table 1 and Figure 1. These are only a fraction of botanical immunodrugs and the scope of these agents remains quite vast.
TABLE 1.
Immunoactive leads from botanicals
| Compound name | Source | Chemical class | Activity | Refs |
|---|---|---|---|---|
| Ginsan | Panax ginseng | Polysaccharide | Anticancer | [85] |
| Triptolide | Tripterygium wilfordii | Diterpenoid triepoxide | Rheumatoid arthritis and leprosy | [86] |
| Mistletoe lectin | Viscum album | Lectin | Cytostatic and apoptotic | [87] |
| Piperine | Piper longum | Alkaloid | Antitumour and bioavailability enhancer | [88] |
| Matrine | Sophora alopecuoides | Alkaloid | Antiviral | [89] |
| Sinomenine | Sinomenium acutum | Alkaloid | Arthritis and rheumatoid arthritis | [89] |
| Artemisinin | Artemisia annua | Sesquiterpene lactone | Psoriasis and autoimmune disorders | [90] |
| ASP | Acanthopanax senticosus | Polysaccharide | Antitumour | [91, 92] |
| Apocynin | Picrorhiza kurroa | Iridoid glycoside | Cytoprotective and antitumor | [93] |
| Shatavarin | Asparagus racemosus | Triterpenoid saponin | Immunostimulant and vaccine adjuvant | [94] |
| KRN7000™ (Kirin Brewery) | Sponge (Agelas mauritianus) | α-Galactosylceramide | Anticancer | [80] |
Abbreviation: ASP, Acanthopanax senticosus polysaccharide.
Conclusion
Analysis of current immunomodulating strategies indicates that monovalent approaches in isolation are unlikely to restore immunostasis or attain status of complete therapy (Box 1). This complements emerging systems biology approaches, which holistically monitor operating biological processes as an integrated system. It is likely that multiple immune-modulating strategies will be necessary to achieve clinical success owing to complex interplay between pathways. We need designer drugs, which involve safer, curative and synergistic combinations. Bioprospecting will have a major role in identifying such combinations. The botanical immunodrugs discussed here have such potential because they offer additional therapeutic benefits to address associated conditions such as infection, inflammation and cancer. Botanical extracts provide cytoprotective, anticancer, anti-inflammatory and anti-infective activities in addition to immunoregulatory activity. For example, crude extract of TC has immunostimulant, anticancer and cytoprotective activities, where cytoprotective activities are attributed to polysaccharides and immunostimulant activity is attributed to diterpenoids [76]. Recently, researchers observed antimicrobial activity coupled with immune modulation [80]. Bryostatins isolated from the marine organism Bugula neritina have antineoplastic activity along with mimicking the effect of recombinant human GM-CSF [81]. Three polysaccharides (krestin, lentinan and scihzophyllum) developed by Japanese scientists from mushrooms are in clinical use as adjuvants in chemotherapy. These polysaccharides have now been developed into multipurpose medicines with cytostatic, anti-inflammatory and antithrombotic activities [82]. Similarly, polysaccharides and oxygenated triterpenoids derived from Ganoderma lucidum (a fungus from traditional Chinese medicine) showed broad spectrum pharmacological functions [83]. These examples underline the importance of bioprospecting for newer synergistic combinations and pharmacological agents. Although many researchers have studied synergism and antagonism in botanicals, systematic scientific investigations on pharmacodynamics, kinetics, dosing and interactions are required. Traditional knowledge, particularly from the great traditions of, for example, Ayurveda and China, will have an important role in bioprospecting. Rasayanas are a selected class of botanicals from Ayurveda with putative capabilities to rejuvenate, promote longevity and health (Box 2). A golden triangle1 , with the integration of modern medicine, traditional knowledge and the robust use of science and technologies with a systems biology approach, could open up new opportunities for immunodrugs and therapy (Figure 2) [84].
BOX 1. Immunotherapy scenario.
-
(i)
Host immune response can be modulated for clinical benefit.
-
(ii)
Monovalent therapeutic approaches might not be effective.
-
(iii)
Combination approaches offer synergistic efficacy and safety.
-
(iv)
Cytokine therapy remains to be optimized.
BOX 2. Ayurveda and Rasayana.
Ayurveda means science of life in Sanskrit (Ayur means life; veda means science) and aims at the holistic management of health and disease. It remains one of the most ancient medical systems widely practiced in the Indian subcontinent and has a sound philosophical, experiential and experimental basis. Charak samhita and Sushrut Samhita (100-500 BC) are main Ayurvedic classics, which describe over 700 botanicals along with their classification, pharmacological and therapeutic properties.
Rasayana therapy is one of the eight branches of Ayurveda and generally means nourishing and rejuvenating drugs with multiple applications for longevity, memory enhancement, immunomodulation and adaptogenic. Many researchers have supposed neuroendocrine immune axis theory to explain Rasayana action.
FIGURE 2.
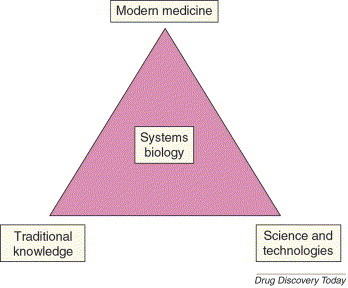
Golden triangle approach. The golden triangle approach promotes effective integration of traditional wisdom, the power of contemporary science and technology, and the evidence base of modern medicine, where the holistic strategies are reflected from the principles of systems biology. This provides an holistic or whole-person healing viewpoint alongside a reverse pharmacology-based platform for drug discovery.
In short, botanical immunodrugs could provide a unique opportunity to bioprospect diverse and synergistic chemicals moieties, which in combination might act on multiple targets and improve the therapeutic spectrum. Traditional knowledge and practices bring experiential wisdom to provide a safer and more cost effective platform for newer scaffolds and immunodrugs discovery.
Acknowledgements
We thank New Millennium Indian Technology Initiative of Council for Scientific and Industrial Research, Government of India. Thanks also go to Kalpana Joshi, Sham Diwanay and Girish Tillu for their suggestions and comments.
Footnotes
Mashelkar, R.A. (2003) Chitrakoot Declaration, National Workshop on Ayurveda Research Scenario - Challenges, Opportunities and Prospects for Excellence. National Botanical Research Institute, 24–26 May 2003, Lucknow, India.
References
References
- 1.Oberholzer A. Cytokine signaling-regulation of the immune response in normal and critically ill states. Crit. Care Med. 2000;28:N3–N12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Nelson R.P., Jr, Ballow M. Immunomodulation and immunotherapy: drugs, cytokines, cytokine receptors, and antibodies. J. Allergy Clin. Immunol. 2003;111:S720–S743. doi: 10.1067/mai.2003.146. [DOI] [PubMed] [Google Scholar]
- 3.Geissler M., Weth R. Immunotherapy: new insights. Schweiz. Rundsch. Med. Prax. 2002;91:2236–2246. doi: 10.1024/0369-8394.91.51.2236. [DOI] [PubMed] [Google Scholar]
- 4.Whelan M. Cancer immunotherapy: an embarrassment of riches. Drug Discov. Today. 2003;8:253–258. doi: 10.1016/s1359-6446(03)02633-3. [DOI] [PubMed] [Google Scholar]
- 5.Chada S. Cytokine- and chemokine-based gene therapy for cancer. Curr. Opin. Mol. Ther. 2003;5:463–474. [PubMed] [Google Scholar]
- 6.Mihich E., Ehrke M.J. Anticancer drugs plus cytokines: immunodulation based therapies of mouse tumors. Int. J. Immunopharmacol. 2000;22:1077–1081. doi: 10.1016/s0192-0561(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 7.Marriot J.B. Thalidomide as an emerging immunotherapeutic agent. Immunol. Today. 1999;20:538–540. doi: 10.1016/s0167-5699(99)01531-5. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Meijden A.P.M. Non-specific immunotherapy with bacille Canette-Guérin (BCG) Clin. Exp. Immunol. 2001;123:179–180. doi: 10.1046/j.1365-2249.2001.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald U.K., Pirofski L. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 2003;9:945–968. doi: 10.2174/1381612033455189. [DOI] [PubMed] [Google Scholar]
- 10.Kuhen K.L., Samuel C.E. Mechanism of interferon action: functional characterization of positive and negative domains that modulate transcriptional activation of the human RNA-dependent protein kinase PKR promoter. Virology. 1999;254:182–195. doi: 10.1006/viro.1998.9536. [DOI] [PubMed] [Google Scholar]
- 11.Glue P. Pegylated interferon- alpha-2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Clin. Pharmacol. Ther. 2000;68:556–567. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell T.S., Christman J.W. Sepsis and cytokines: current status. Br. J. Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine H. Recovery from chronic hepatitis C in long-term responders to ribavirin plus interferon alfa. Lancet. 2000;356:41. doi: 10.1016/S0140-6736(00)02434-X. [DOI] [PubMed] [Google Scholar]
- 14.Schulze-Koops H. What we have learned from trials of immunomodulatory agents in rheumatoid arthritis: Future directions. Drugs Today (Barc) 1999;35:327–351. doi: 10.1358/dot.1999.35.4-5.552208. [DOI] [PubMed] [Google Scholar]
- 15.Cobbold S.P. Reprogramming the immune system for tolerance with monoclonal antibodies. Semin. Immunol. 1990;2:377–387. [PubMed] [Google Scholar]
- 16.Kavanaugh A.F. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J. Rheumatol. 1996;23:1338–1344. [PubMed] [Google Scholar]
- 17.Sfikakis P.P., Kollias G. Tumor necrosis factor biology in experimental and clinical arthritis. Curr. Opin. Rheumatol. 2003;15:380–386. doi: 10.1097/00002281-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Paleolog E. The therapeutic potential of TNF-alpha blockade in rheumatoid arthritis. Expert Opin. Investig. Drugs. 2003;12:1087–1095. doi: 10.1517/13543784.12.7.1087. [DOI] [PubMed] [Google Scholar]
- 19.Palladino M.A. Anti-TNF-alpha therapies: the next generation. Nat. Rev. Drug Discov. 2003;2:736–746. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 20.Cope A.P. Studies of T-cell activation in chronic inflammation. Arthritis Res. 2002;4(Suppl. 3):S197–S211. doi: 10.1186/ar557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis L.S. Rheumatoid synovial CD4+ T cells exhibit a reduced capacity to differentiate into IL-4-producing T-helper-2 effector cells. Arthritis Res. 2001;3:54–64. doi: 10.1186/ar140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adorini L. Manipulation of the Th1/Th2 cells balance: an approach to treat human autoimmune diseases? Autoimmunity. 1996;23:53–68. doi: 10.3109/08916939608995329. [DOI] [PubMed] [Google Scholar]
- 23.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 24.Ghebrehiwet B. CC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol. Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Burke J.R. Targeting I kappa B kinase for the treatment of inflammatory and other disorders. Curr. Opin. Drug Discov. Devel. 2003;6:720–728. [PubMed] [Google Scholar]
- 26.Maini R. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354:1932–1999. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.J. Modulation of antigen-specific cellular immune responses to DNA vaccination in rhesus macaques through the use of IL-2, IFN-g, or IL-4 gene adjuvants. Vaccine. 2001;19:2496–2505. doi: 10.1016/s0264-410x(00)00479-5. [DOI] [PubMed] [Google Scholar]
- 28.Marciani D.J. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today. 2003;8:934–943. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 29.Burdin N. Immunological foundations to the quest for new vaccine adjuvants. BioDrugs. 2004;18:79–93. doi: 10.2165/00063030-200418020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Brown G.D., Gordon S. Fungal B glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 31.Mossman T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 32.Singh V.K. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol. Res. 1999;20:147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 33.Giese K. Unravelling novel intracellular pathways in cell-based assays. Drug Discov. Today. 2002;7:179–186. doi: 10.1016/s1359-6446(01)02126-2. [DOI] [PubMed] [Google Scholar]
- 34.Roger P.M. Revival of the regulatory T cell: New targets for drug development. Drug Discov. Today. 2004;9:310–316. doi: 10.1016/S1359-6446(03)03021-6. [DOI] [PubMed] [Google Scholar]
- 35.Plaeger S.F. Clinical immunology and traditional herbal medicines. Clin. Diagn. Lab. Immunol. 2003;10:337–338. doi: 10.1128/CDLI.10.3.337-338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Y. T-cell-immunity-based inhibitory effects of orally administered herbal medicine juzen-taiho-to on the growth of primarily developed melanocytic tumors in RET-transgenic mice. J. Invest. Dermatol. 2001;117:694–701. doi: 10.1046/j.0022-202x.2001.01457.x. [DOI] [PubMed] [Google Scholar]
- 37.Patwardhan B. Ayurveda and natural products drug discovery. Curr. Sci. 2004;86:789–799. [Google Scholar]
- 38.Rollinger J.M. Combining ethnopharmacology and virtual screening for lead structure discovery: COX-inhibitors as application example. J. Chem. Inf. Comput. Sci. 2004;44:480–488. doi: 10.1021/ci030031o. [DOI] [PubMed] [Google Scholar]
- 39.Patwardhan B. Ayurveda: the ‘designer’ medicine: a review of ethnopharmacology and bioprospecting research. Indian drugs. 2000;37:213–227. [Google Scholar]
- 40.Rege N.N. Adaptogenic properties of six rasayana herbs in ayurvedic medicine. Phytother. Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa M. Effects of glycyrrhizin on immune-mediated cytotoxicity. J. Gastroenterol. Hepatol. 1997;12:243–248. doi: 10.1111/j.1440-1746.1997.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 42.Sekizawa T. Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virol. 2001;45:51–54. [PubMed] [Google Scholar]
- 43.Utsunomiya T. Glycyrrhizin improves the resistance of MAIDS mice to opportunistic infection of Candida albicans through the modulation of MAIDS-associated type 2 T cell responses. Clin. Immunol. 2000;95:145–155. doi: 10.1006/clim.2000.4854. [DOI] [PubMed] [Google Scholar]
- 44.Cinatl J. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujisawa Y. Glycyrrhizin inhibits the lytic pathway of complement-possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol. Immunol. 2000;44:799–804. doi: 10.1111/j.1348-0421.2000.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 46.Jo E.H. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J. Agric. Food Chem. 2004;52:1715–1719. doi: 10.1021/jf035012t. [DOI] [PubMed] [Google Scholar]
- 47.Barfod L. Chalcones from Chinese liquorice inhibit proliferation of T cells and production of cytokines. Int. Immunopharmacol. 2002;2:545–555. doi: 10.1016/s1567-5769(01)00202-8. [DOI] [PubMed] [Google Scholar]
- 48.Baltina L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003;10:155–171. doi: 10.2174/0929867033368538. [DOI] [PubMed] [Google Scholar]
- 49.Abe M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J. Gastroenterol. 2003;38:962–967. doi: 10.1007/s00535-003-1179-7. [DOI] [PubMed] [Google Scholar]
- 50.Reinhard K.H. Uncaria tomentosa (Willd.) D.C.: cat's claw, una de gato, or saventaro. J. Altern. Complement. Med. 1999;5:143–151. doi: 10.1089/acm.1999.5.143. [DOI] [PubMed] [Google Scholar]
- 51.Wurm M. Pentacyclic oxindole alkaloids from Uncaria tomentosa induce human endothelial cells to release a lymphocyte-proliferation-regulating factor. Planta Med. 1998;64:701–704. doi: 10.1055/s-2006-957561. [DOI] [PubMed] [Google Scholar]
- 52.Winkler C. In vitro effects of two extracts and two pure alkaloid preparations of Uncaria tomentosa on peripheral blood mononuclear cells. Planta Med. 2004;70:205–210. doi: 10.1055/s-2004-815536. [DOI] [PubMed] [Google Scholar]
- 53.Sandoval-Chacon M. Anti-inflammatory actions of cat's claw: the role of NF-kappa B. Aliment. Pharmacol. Ther. 1998;12:1279–1289. doi: 10.1046/j.1365-2036.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- 54.Sandoval M. Cat's claw inhibits TNF-α production and scavenges free radicals: role in cytoprotection. Free Radic. Biol. Med. 2000;29:71–78. doi: 10.1016/s0891-5849(00)00327-0. [DOI] [PubMed] [Google Scholar]
- 55.Mur E. Randomized double-blind trial of an extract from the pentacyclic alkaloid-chemotype of Uncaria tomentosa for the treatment of rheumatoid arthritis. J. Rheumatol. 2002;29:678–681. [PubMed] [Google Scholar]
- 56.Sheng Y. Treatment of chemotherapy-induced leucopoenia in a rat model with aqueous extract from Uncaria tomentosa. Phytomedicine. 2000;7:137–143. doi: 10.1016/S0944-7113(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 57.Sheng Y. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998;18:3363–3368. [PubMed] [Google Scholar]
- 58.Stimpel M. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect. Immun. 1984;46:845–849. doi: 10.1128/iai.46.3.845-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anonymous Echinacea monographM. Altern. Med. Rev. 2001;6:411–414. [PubMed] [Google Scholar]
- 60.Melchart D. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J. Altern. Complement. Med. 1995;1:145–160. doi: 10.1089/acm.1995.1.145. [DOI] [PubMed] [Google Scholar]
- 61.Henneicke-von Z.H. Efficacy and safety of a fixed combination phytomedicine in the treatment of the common cold (acute viral respiratory tract infection): results of a randomised, double-blind, placebo-controlled, multicentric study. Curr. Med. Res. Opin. 1999;15:214–227. doi: 10.1185/03007999909114094. [DOI] [PubMed] [Google Scholar]
- 62.Mishra L.C. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern. Med. Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 63.Diwanay S. Immunoprotection by botanical drugs in cancer chemotherapy. J. Ethnopharmacol. 2004;90:49–55. doi: 10.1016/j.jep.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Prakash J. Withania somnifera root extract prevents DMBA-induced squamous cell carcinoma of skin in Swiss albino mice. Nutr. Cancer. 2002;42:91–97. doi: 10.1207/S15327914NC421_12. [DOI] [PubMed] [Google Scholar]
- 65.Jayaprakasam B. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74:125–132. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Iuvone T. Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci. 2003;72:1617–1625. doi: 10.1016/s0024-3205(02)02472-4. [DOI] [PubMed] [Google Scholar]
- 67.Davis L., Kuttan G. Effect of Withania somnifera on cell-mediated immune responses in mice. J. Exp. Clin. Cancer Res. 2002;21:585–590. [PubMed] [Google Scholar]
- 68.Davis L., Kuttan G. Immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2000;71:193–200. doi: 10.1016/s0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- 69.Ziauddin M. Studies on the immunomodulatory effects of Ashwagandha. J. Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 70.Gautam M. Immune response modulation to DPT vaccine by aqueous extract of Withania somnifera in experimental system. Int. Immunopharmacol. 2004;4:841–849. doi: 10.1016/j.intimp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Singh, S. et al. (2001) An Indian herbal immunomodulator: highly effective in the treatment of HIV/AIDS. Proceedings of 6th International Conference on AIDS Asia Pacific, 5-10 October 2001, Melbourne, Australia (Abstract No. WePO 1254)
- 72.Grandhi A. A comparative pharmacological investigation of Ashwagandha and Ginseng. J. Ethnopharmacol. 1994;44:131–135. doi: 10.1016/0378-8741(94)01119-2. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal R. Studies on immunoregulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 1999;67:27–35. doi: 10.1016/s0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 74.Kulkarni R.R. Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, crossover study. J. Ethnopharmacol. 1991;33:91–95. doi: 10.1016/0378-8741(91)90167-c. [DOI] [PubMed] [Google Scholar]
- 75.Chopra A. Randomized double-blind trial of an Ayurvedic plant derived formulation for treatment of rheumatoid arthritis. J. Rheumatol. 2000;27:365–372. [PubMed] [Google Scholar]
- 76.Kapil A., Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 77.Thatte U.M. Tinospora cordifolia induces colony-stimulating activity in serum. J. Postgrad. Med. 1994;40:202–203. [PubMed] [Google Scholar]
- 78.Nair R.P.K. Immune-stimulating properties of a novel polysaccharide from the medicinal plant Tinospora cordifolia. Int. Immunopharmacol. 2004;4:1645–1659. doi: 10.1016/j.intimp.2004.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diwanay S.S. Cytoprotection and immunomodulation in cancer therapy. Curr. Med. Chem. Anti-Canc. Agents. 2004;4:479–490. doi: 10.2174/1568011043352704. [DOI] [PubMed] [Google Scholar]
- 80.Tan B.K., Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. Curr. Med. Chem. 2004;11:1423–1430. doi: 10.2174/0929867043365161. [DOI] [PubMed] [Google Scholar]
- 81.Burkhard H. Drugs from the deep: marine natural products as drug candidates. Drug Discov. Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 82.Waser S.P. Medicinal mushrooms as source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 83.Shao B.M. Immune receptors for polysaccharides from Ganoderma lucidum. Biochem. Biophys. Res. Commun. 2004;323:133–141. doi: 10.1016/j.bbrc.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 84.Kim T.K. Chemical genomics and medicinal systems biology: chemical control of genomic networks in human systems biology for innovative medicine. J. Biochem. Mol. Biol. 2004;37:53–58. doi: 10.5483/bmbrep.2004.37.1.053. [DOI] [PubMed] [Google Scholar]
- 85.Song J.Y. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int. Immunopharmacol. 2002;2:857–865. doi: 10.1016/s1567-5769(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 86.Chen B.J. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leukemia and Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 87.Fritz P. Impact of mistletoe lectin binding in breast cancer. Anticancer Res. 2004;24:1187–1192. [PubMed] [Google Scholar]
- 88.Sunila E.S., Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn and piperine. J. Ethnopharmacol. 2004;90:339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 89.Liu M. Advance in the pharmacological research on matrine. Zhongguo. Zhong Yao ZaY. 2003;28:801–804. [PubMed] [Google Scholar]
- 90.Zhang L.H. Several compounds from Chinese traditional and herbal medicine as immunomodulators. Phytotherapy Res. 1995;9:315–322. [Google Scholar]
- 91.Yoon T.J. Anti-metastatic activity of Acanthopanax senticosus extract and its possible immunological mechanism of action. J. Ethnopharmacol. 2004;93:247–253. doi: 10.1016/j.jep.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 92.Han S.B. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int. Immunopharmacol. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 93.Vanden W.E. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 94.Gautam M. Immunoadjuvant potential of Asparagus racemosus in experimental system. J. Ethnopharmacol. 2004;91:251–255. doi: 10.1016/j.jep.2003.12.023. [DOI] [PubMed] [Google Scholar]


