Abstract
Most studies exploring the role of upper airway viruses and bacteria in paediatric acute respiratory infections (ARI) focus on specific clinical diagnoses and/or do not account for virus–bacteria interactions. We aimed to describe the frequency and predictors of virus and bacteria codetection in children with ARI and cough, irrespective of clinical diagnosis. Bilateral nasal swabs, demographic, clinical and risk factor data were collected at enrollment in children aged <15 years presenting to an emergency department with an ARI and where cough was a symptom. Swabs were tested by polymerase chain reaction for 17 respiratory viruses and seven respiratory bacteria. Logistic regression was used to investigate associations between child characteristics and codetection of the organisms of interest. Between December 2011 and August 2014, swabs were collected from 817 (93.3%) of 876 enrolled children, median age 27.7 months (interquartile range 13.9–60.3 months). Overall, 740 (90.6%) of 817 specimens were positive for any organism. Both viruses and bacteria were detected in 423 specimens (51.8%). Factors associated with codetection were age (adjusted odds ratio (aOR) for age <12 months = 4.9, 95% confidence interval (CI) 3.0, 7.9; age 12 to <24 months = 6.0, 95% CI 3.7, 9.8; age 24 to <60 months = 2.4, 95% CI 1.5, 3.9), male gender (aOR 1.46; 95% CI 1.1, 2.0), child care attendance (aOR 2.0; 95% CI 1.4, 2.8) and winter enrollment (aOR 2.0; 95% CI 1.3, 3.0). Haemophilus influenzae dominated the virus–bacteria pairs. Virus–H. influenzae interactions in ARI should be investigated further, especially as the contribution of nontypeable H. influenzae to acute and chronic respiratory diseases is being increasingly recognized.
Keywords: Acute respiratory illness, bacteria, children, cough, viruses
Introduction
The importance of virus–bacteria interactions in childhood acute respiratory infections (ARI) remains uncertain [1]. Molecular methods enabling simultaneous detection of bacteria and viruses from a single specimen [2] allow these relationships to be investigated. Until recently, however, studies have focused mainly on either viruses [3] or bacteria alone [4]. Studies that have examined both viruses and bacteria during an ARI have typically been limited in their scope, including short duration (e.g. 1 year during the 2009 influenza A/H1N1 pandemic [5]), linkage to specific diagnostic criteria [6] and small sample sizes [7].
There is a substantial body of work in the literature that has examined aetiologic associations between respiratory microbes and ARI that has used upper airway specimens, particularly lower ARI [8]. While upper airway specimens (nasopharyngeal swabs) are controversial because they cannot reliably distinguish between carriage and disease [8], they continue to be widely used in observational and experimental studies of ARI in children, including those attempting to identify associations between organisms and clinical symptoms and/or severity. Notably, the association between viral ARI and the later development of asthma has been based on upper airway specimens [9]. While a causal association between human rhinovirus (hRV) and respiratory syncytial virus (RSV) infections in early life with future asthma has been proposed by cohort studies [9], these studies did not report on upper airway bacteria that are likely to be important in early immune development and viral infections [10], [11]. Indeed recent larger studies which examined for both bacteria and viruses found that after adjustment for potential confounding factors it was the number of respiratory episodes rather than hRV or RSV infections in early life that were associated with future asthma [12].
In the context of the above limitations, we focused on relating the virus and bacteria detections with epidemiologic data instead of attempting to assign causal associations between upper airway microbes and clinical disease. We describe the upper airway bacteria and viruses in 817 children presenting to a tertiary paediatric emergency department (ED) with an ARI that included cough as a symptom. We sought to describe the frequency and child-specific predictors of virus and bacteria codetection in this population and to describe the seasonal distribution of each organism and its codetections.
Methods
Setting
The Royal Children's Hospital (RCH), Brisbane, Australia (now the Lady Cilento Children's Hospital), is the largest tertiary pediatric hospital in the state. Its ED annually services over 25 000 children. Brisbane has a subtropical climate with maximum temperatures averaging 30°C in summer and 17°C in winter. The average monthly rainfall is almost 100 mm, with summer the wettest season.
Design
We conducted a prospective study of children aged <15 years presenting to the RCH ED with an ARI including cough as a symptom between 11 December 2011 and 30 August 2014. The primary objective of the overall cohort study was to determine the prevalence and predictors of chronic cough after ARI in children; its full study protocol has been published previously [13]. Here we focus on the microbiologic aspects of that primary study. The Children's Health Queensland (HREC/11/QRCH/83) and Queensland University of Technology Research ethics committee (2012000700) approved the study.
Children were excluded if they had known chronic medical conditions (excluding asthma); were immunocompromised or receiving immunomodulating drugs (other than short-course (<2 weeks) oral or inhaled steroids) in the preceding 30 days, or had insufficient English. Written informed consent was obtained from parents/guardians of the child and from adolescents (aged >12 years).
Bilateral anterior nasal swabs were obtained using the Virocult specimen collection system (Medical Wire and Equipment, Wiltshire, England, UK). Protocol-specific criteria with respect to the adequacy of collection technique helped assess sampling quality [13]. Swabs were stored at −80°C within 24 hours of collection and were transferred to the research laboratory for virus and bacteria identification by validated PCR assays, as described previously [14], [15]. Viruses of interest included hRV, RSV A and B, influenza A and B, parainfluenza 1–3, adenovirus, human metapneumovirus, human coronaviruses (OC43, 229E, NL63 + HKU1), human bocavirus, enterovirus and human polyomaviruses KI and WU. Respiratory bacterial pathogens of interest included Streptococcus pneumoniae, nontypeable Haemophilus influenzae (NTHi), Moraxella catarrhalis, Staphylococcus aureus, Bordetella pertussis, Mycoplasma pneumoniae and Chlamydia pneumoniae.
Analyses
Descriptive analyses were performed with data expressed as proportions and/or means of the selected characteristics with the corresponding 95% confidence intervals (CIs). Where continuous data were not normally distributed, medians with accompanying interquartile ranges are presented.
Logistic regression was used to assess the relationship between codetection of virus and bacteria and specimen quality, age, gender, length of illness (days), antibiotics in the past 7 days, oral steroids in the previous 30 days, household tobacco smoke exposure, child care attendance, siblings, household pets, breastfeeding history and season of enrollment. Validated vaccination histories were not available and hence are not included in the analysis, although parent-reported influenza vaccination in the preceding 12 months was included. Factors in univariable analyses with p <0.1 were entered into a backwards selection regression model to identify characteristics independently associated with virus and bacteria codetection; adjusted odds ratios (aOR) and their corresponding 95% CIs were calculated; p <0.05 was considered statistically significant. Model goodness of fit was assessed by the Pearson chi-square likelihood ratio test. All analyses were performed in Stata V12SE (StataCorp, College Station, TX, USA).
Results
Of the 2594 children screened for participation, 876 (33.8%) were enrolled. Reasons for nonenrollment included ineligibility (24.8%), refusal (33.2%) and other reasons (42.0%) (e.g. ED staff workload, discharged before consent obtained, critically ill children). Nasal swabs were collected from 827 (94.4%) enrolled (60.2% male) children; median age was 27.7 months (interquartile range 13.9–60.3 months). Parent-reported receipt of an influenza vaccine in the preceding 12 months occurred in 8% of children in the study. Ten swabs were not tested because of poor sample quality; hence, 817 children were included in this analysis.
Overall, 740 (90.6%) of 817 specimens were positive for any organism. Four hundred ninety-seven (60.8%) of the 817 swabs had at least one virus; 411 (50.3%) of 817 had only one virus and 86 (10.5%) of 817 had two or more viruses detected. The most commonly detected viruses were hRV (27.0%) and RSV (16.5%) (Supplementary Table 1). Six hundred sixteen (75.4%) of the 817 swabs had at least one bacterial pathogen identified; 232 (28.4%) of 817 tested positive for only one, while 384 (47.0%) of 817 had two or more bacteria detected. The most commonly detected bacteria were M. catarrhalis (53.4%), S. pneumoniae (46.5%) and NTHi (29.6%) (Supplementary Table 1). In contrast, only ten specimens were positive for M. pneumoniae and two for C. pneumoniae; these were not considered further in the analysis. Both viruses and bacteria were codetected in 423 (51.8%) of 817 specimens, and of these, 67 swabs (15.8%) tested positive for two or more bacteria and two viruses together.
Univariate analyses of associations between child characteristics and virus–bacteria codetections identified age, gender, child care attendance, siblings and enrolling season for inclusion in regression models (Table 1 ). Factors that remained significantly associated with codetection in the final model (likelihood ratio χ2 = 0.32, p 0.569) were age (aOR for age <12 months = 4.9, 95% CI 3.0, 7.9; age 12 to <24 months = 6.0, 95% CI 3.7, 9.8; age 24 to <60 months = 2.4, 95% CI 1.5, 3.9), male gender (aOR 1.46, 95% CI 1.1, 2.0), child care attendance (aOR 2.0, 95% CI 1.4, 2.8) and winter enrollment (aOR 2.0, 95% CI 1.3, 3.0).
Table 1.
Univariate associations between child-specific characteristics and detection of upper airway viruses and bacteria in children seeking care at emergency department with acute respiratory infection with cough as symptom
| Characteristic | Any organism positive |
Any bacteria positive (no virus) |
Any virus positive (no bacteria) |
Both virus and bacteria positive |
||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | |
| Specimen quality | ||||||||
| Good | 437/522 (83.7) | 0.43 (0.15–1.22) | 129/522 (24.7) | 0.53 (0.29–0.95) | 64/522 (12.3) | 2.28 (0.69–7.53) | 238/522 (45.7) | 0.91 (0.51–1.61) |
| Fair | 184/207 (88.9) | 0.67 (0.22–2.02) | 59/207 (28.5) | 0.64 (0.34–1.20) | 22/207 (10.6) | 1.94 (0.56–6.76) | 101/207 (49.0) | 1.04 (0.57–1.91) |
| Poor | 48/52 (92.3) | Ref | 20/52 (38.5) | Ref | 3/52 (5.8) | Ref | 25/52 (48.1) | Ref |
| Age group | ||||||||
| <6 months | 49/53 (92.5) | 7.51 (2.33–24.2) | 13/53 (24.5) | 1.15 (0.46–2.88) | 11/53 (20.8) | 0.93 (0.36–2.38) | 23/53 (43.4) | 3.41 (1.38–8.42) |
| 6 to <12 months | 107/112 (95.5) | 13.12 (4.52–38.0) | 28/112 (25.0) | 1.18 (0.53–2.61) | 8/112 (7.1) | 0.27 (0.10–0.73) | 71/112 (63.4) | 7.70 (3.39–17.46) |
| 12 to <24 months | 193/203 (95.1) | 11.83 (5.02–27.8) | 39/203 (19.2) | 0.84 (0.40–1.79) | 15/203 (7.4) | 0.28 (0.13–0.66) | 137/202 (67.8) | 9.37 (4.29–20.46) |
| 24 to <60 months | 226/250 (90.4) | 5.77 (2.84–11.73) | 71/250 (28.4) | 1.41 (0.68–2.89) | 29/250 (11.6) | 0.47 (0.21–1.00) | 122/250 (48.8) | 4.24 (1.97–9.10) |
| 60 to <120 months | 105/157 (66.9) | 1.24 (0.64–2.40) | 49/157 (31.2) | 1.61 (0.76–3.40) | 20/157 (12.7) | 0.52 (0.23–1.17) | 35/157 (22.3) | 1.28 (0.56–2.88) |
| 120 to <180 months | 31/50 (62.0) | Ref | 11/50 (22.0) | Ref | 11/50 (22.0) | Ref | 9/49 (18.4) | Ref |
| Gender | ||||||||
| Male | 434/498 (87.2) | 1.24 (0.83–1.85) | 124/498 (24.9) | 1.24 (0.83–1.84) | 50/498 (10.0) | 0.71 (0.46–1.09) | 255/498 (51.2) | 1.35 (1.02–1.79) |
| Female | 274/324 (84.6) | Ref | 85/324 (26.2) | Ref | 44/324 (13.6) | Ref | 141/322 (43.8) | Ref |
| Length of illness | ||||||||
| <3 days | 293/344 (85.2) | 1.35 (0.69–2.63) | 86/344 (25.0) | 0.96 (0.58–1.60) | 49/344 (14.2) | 1.42 (0.71–2.84) | 153/344 (44.5) | 0.71 (0.46–1.10) |
| 3–7 days | 264/303 (87.1) | 0.97 (0.47–2.03) | 74/303 (24.4) | 0.93 (0.56–1.55) | 29/303 (9.6) | 0.90 (0.44–1.88) | 158/302 (52.3) | 0.98 (0.63–1.53) |
| 8–14 days | 56/66 (84.9) | 1.18 (0.75–1.84) | 24/66 (36.4) | 1.65 (0.84–3.21) | 4/66 (6.1) | 0.55 (0.17–1.81) | 27/66 (40.9) | 0.62 (0.33–1.15) |
| 15+ days | 93/105 (88.6) | Ref | 27/105 (25.7) | Ref | 11/105 (10.5) | Ref | 55/104 (52.9) | Ref |
| Antibiotics in past 7 days | ||||||||
| Yes | 120 (81.1) | 0.62 (0.39–0.99) | 36/148 (24.3) | 0.91 (0.61–1.38) | 16/148 (10.8) | 0.93 (0.53–1.65) | 68/147 (46.3) | 0.91 (0.64–1.31) |
| No | 591 (87.3) | Ref | 176/677 (26.0) | Ref | 78/677 (11.5) | Ref | 328/676 (48.5) | Ref |
| Oral steroids in past 30 days | ||||||||
| Yes | 163/199 (82.0) | 0.66 (0.43–1.00) | 43/199 (21.6) | 0.76 (0.52–1.12) | 23/199 (11.6) | 1.00 (0.61–1.65) | 95/199 (47.7) | 0.98 (0.71–1.35) |
| No | 530/607 (87.3) | Ref | 161/607 (26.5) | Ref | 70/607 (11.5) | Ref | 292/605 (48.3) | Ref |
| Exposure to household tobacco smoke | ||||||||
| Yes | 145/159 (91.2) | 1.83 (1.02–3.30) | 48/159 (30.2) | 1.32 (0.90–1.94) | 20/159 (12.6) | 1.13 (0.67–1.92) | 77/159 (48.4) | 1.02 (0.72–1.45) |
| No | 559/658 (85.0) | Ref | 162/658 (24.6) | Ref | 74/658 (11.3) | Ref | 314/656 (47.9) | Ref |
| Child care | ||||||||
| Yes | 380/404 (94.1) | 4.22 (2.62–6.81) | 105/404 (26.0) | 1.04 (0.76–1.42) | 29/404 (7.2) | 0.42 (0.27–0.67) | 241/404 (59.7) | 2.47 (1.86–3.28) |
| No | 322/408 (78.9) | Ref | 103/408 (25.3) | Ref | 63/408 (15.4) | Ref | 152/406 (37.4) | Ref |
| Siblings in household | ||||||||
| Yes | 478/570 (83.9) | 0.49 (0.30–0.79) | 151/570 (26.5) | 1.16 (0.82–1.63) | 57/570 (10.0) | 0.66 (0.42–1.03) | 263/568 (46.3) | 0.78 (0.58–1.04) |
| No | 235/257 (91.4) | Ref | 61/257 (23.7) | Ref | 37/257 (14.4) | Ref | 135/257 (52.5) | Ref |
| Breastfeeding history | ||||||||
| Ever breastfed | 553/641 (86.3) | 1.46 (0.81–2.62) | 166/641 (25.9) | 1.07 (0.63–1.80) | 72/641 (11.2) | 0.85 (0.43–1.68) | 306/640 (47.8) | 1.16 (0.74–1.84) |
| Never breastfed | 69/85 (81.2) | Ref | 21/85 (24.7) | Ref | 11/85 (12.9) | Ref | 37/84 (44.1) | Ref |
| Currently breastfed (if age <12 months) | ||||||||
| Yes | 68/74 (91.9) | 0.39 (0.09–1.60) | 24/74 (32.4) | 2.09 (1.02–4.28) | 8/74 (10.8) | 0.88 (0.34–2.32) | 36/74 (48.7) | 0.54 (0.29–1.00) |
| No | 88/91 (96.7) | Ref | 17/91 (18.7) | Ref | 11/91 (12.1) | Ref | 58/91 (63.7) | Ref |
| Household pets | ||||||||
| Yes | 369/438 (84.3) | 0.69 (0.46–1.03) | 111/438 (25.3) | 0.96 (0.70–1.31) | 50/438 (11.4) | 1.00 (0.65–1.54) | 205/436 (47.0) | 0.91 (0.69–1.19) |
| No | 342/386 (88.6) | 101/386 (26.2) | Ref | 44/386 (11.4) | Ref | 191/386 (49.5) | Ref | |
| Season of enrollment | ||||||||
| Summer | 138/167 (82.6) | Ref | 47/167 (28.1) | Ref | 18/167 (10.8) | Ref | 73/167 (43.7) | Ref |
| Autumn | 248/290 (85.5) | 1.24 (0.74–2.08) | 73/290 (25.2) | 0.86 (0.56–1.32) | 44/290 (15.2) | 1.48 (0.82–2.66) | 129/288 (44.8) | 1.04 (0.71–1.53) |
| Winter | 232/257 (90.3) | 1.95 (1.10–3.47) | 61/257 (23.7) | 0.79 (0.51–1.24) | 19/257 (7.4) | 0.66 (0.34–1.30) | 145/257 (56.4) | 1.67 (1.12–2.47) |
| Spring | 95/113 (84.1) | 1.11 (0.58–2.11) | 31/113 (27.4) | 0.97 (0.57–1.64) | 13/113 (11.5) | 1.08 (0.50–2.29) | 51/113 (45.1) | 1.06 (0.66–1.71) |
CI, confidence interval; OR, odds ratio; Ref, reference.
Table 2 presents the unadjusted and adjusted (for age, season and antibiotics in the past 7 days) associations between individual viruses and codetection with respiratory bacterial pathogens. Of note was that RSV was significantly associated with NTHi, S. pneumoniae, M. catarrhalis and S. aureus (Table 2). Given the associations between RSV and each bacterium, we constructed a model to identify predictors of RSV that included all four bacteria of interest, age, season and antibiotics in the past 7 days. In the final model, NTHi (aOR 1.9 (95% CI 1.2, 2.8), age (<12 months: aOR 7.5, 95% CI 3.5, 16.3; 12 to <24 months = 5.9, 95% CI 2.7, 12.8; 24 to <60 months = 3.6, 95% CI 1.7, 7.7) and autumn enrollment (aOR 4.3, 95% CI 2.4, 7.7) remained significantly associated with RSV detection.
Table 2.
Crude and adjusteda odds ratios for codetection of respiratory viruses with Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis and Staphylococcus aureus in nasal swabs from children with cough
| Virus | n |
Haemophilus influenzae |
Streptococcus pneumoniae |
Moraxella catarrhalis |
Staphylococcus aureus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||
| RSV | |||||||||||||
| Yes | 137 | 56 (40.9) | 1 84 (1.26–2.69) | 1.84 (1.21–2.81) | 79 (57.7) | 1.72 (1.19– 2.49) | 1.54 (1.03–2.32) | 90 (65.7) | 1.90 (1.29–2.78) | 1 58 (1.03–2.43) | 12 (8.8) | 049 (0 26–0.92) | 0.59 (0.30–1.16) |
| No | 679 | 186 (27.4) | Ref | Ref | 300 (44.2) | Ref | Ref | 341 (50.4) | Ref | Ref | 111 (16.4) | Ref | Ref |
| HRV | |||||||||||||
| Yes | 223 | 67 (30.0) | 1.05 (0.75– 1.48) | 0.91 (0.64 –1.30) | 111 (50.7) | 1.24 (0.91–1.70) | 1.03 (0.75–1.43) | 132 (60.0) | 1.47 (1.07–2 00) | 1.17 (0.83 1.64) | 31 (13.9) | 0.88 (0 57–1.36) | 0.93 (0.59 1.47) |
| No | 593 | 175 (29.5) | Ref | Ref | 268 (45.2) | Ref | Ref | 300 (50.6) | Ref | Ref | 92 (15.5) | Ref | Ref |
| Adenovirus | |||||||||||||
| Yes | 39 | 19 (48.7) | 2.36 (1.24 4 51) | 1.83 (0.92–3.61) | 20 (51.3) | 1.21 (0.64–2.31) | 107 (0.55–2.10) | 24 (61.5) | 1.44 (0.74–2.78) | 1.31 (0.65–2.66) | 3 (7.7) | — | — |
| No | 777 | 223 (28.7) | Ref | Ref | 359 (46.2) | Ref | Ref | 407 (52.4) | Ref | Ref | 120 (15.4) | — | — |
| Influenza | |||||||||||||
| Yes | 32 | 8 (25.0) | 0.78 (0.34 1.76) | 0.86 (0.36 2.04) | 22 (68.8) | 2.61 (1.22–5.58) | 3 08 (1.38–6.86) | 21 (65.6) | 1.72 (0.82 –3.61) | 1.88 (0.82 4.32) | 5 (15.6) | — | — |
| No | 784 | 234 (29.9) | Ref | Ref | 357 (45.5) | Ref | Ref | 410 (52.3) | Ref | Ref | 118 (15.1) | — | — |
| MPV | |||||||||||||
| Yes | 18 | 6 (33.3) | 1.18 (0.44–3.19) | 1.02 (0.37–2.86) | 12 (66.7) | 2.33 (0.86–6.26) | 2.22 (0.80–6.20) | 12 (66.7) | 1.79 (0.66–4 80) | 1.66 (0.57–4.82) | 3 (16.7) | — | — |
| No | 798 | 236 (29.6) | Ref | Ref | 367 (46.0) | Ref | Ref | 419 (52.5) | Ref | Ref | 120 (15.0) | — | — |
| Polyoma virus | |||||||||||||
| Yes | 36 | 21 (58.3) | 3 51 (1.78–6.94) | 2 87 (1.04–5 83) | 21 (58.3) | 1.63(0 83–3.211) | 142 (0 71 –2.85) | 24 (66.7) | 1.81 (0.89–3 66) | 155 (0.73–3.29) | 0 | — | — |
| No | 780 | 221 (28.3) | Ref | Ref | 358 (46.1) | Ref | Ref | 408 (52.5) | Ref | Ref | 123 (15.8) | — | — |
| Corona virus | |||||||||||||
| Yes | 25 | 11 (45.8) | 2 04 (0.91 –4.62) | 1.53 (0 65–3.61) | 14 (58.3) | 1.62 (0.71–3.69) | 1.13 (0.49–2.61) | 15 (62.5) | 1.49 (0.64–3.43) | 0.84 (0.35–2.03) | 4 (16.0) | — | — |
| No | 791 | 231 (29.3) | Ref | Ref | 365 (46.3) | Ref | Ref | 417 (52.9) | Ref | Ref | 119 (15.0) | — | — |
| Para influenza | |||||||||||||
| Yes | 53 | 22 (41.5) | 1.74 (0.99–3.07) | 1.15 (0.63–2.09) | 27 (50.9) | 1.20 (0.69–2.10) | 0.89 (0 49–1.60) | 33 (62.3) | 1.49 (0.84–2 64) | 1.19 (0.64–2.21) | 4 (7.6) | — | — |
| No | 763 | 220 (28.3) | Ref | Ref | 352 (46.4) | Ref | Ref | 399 (52.5) | Ref | Ref | 119 (15.6) | — | — |
| Boca virus | |||||||||||||
| Yes | 30 | 14 (46.7) | 2 13(1.03–4.431) | 1.96 (0.91 –4.22) | 16 (53.3) | 1.32 (0.64–2.74) | 1.04 (0 49–2.21) | 23 (76.7) | 3.00(1.27–7 08 | 2.04 (0.83–5.03) | 0 | — | — |
| No | 787 | 228 (29.0) | Rei | Ref | 363 (46.4) | Ref | Ref | 409 (52.2) | Ref | Ref | 123 (15.6) | — | — |
| Enterovirus | |||||||||||||
| Yes | 26 | 10 (38.5) | 148 (0.66–3.32) | 138 (0.59–3.22) | 17 (65.4) | 2.23(0.98–5.071) | 2.22 (0.95–5.20) | 17 (65.4) | 1.72 (0.76–3.89) | 1.47 (0.62–3.56) | 4 (15.4) | — | — |
| No | 790 | 232 (29.4) | Ref | — | 362 (45.8) | Ref | Ref | 414 (52.4) | Ref | Ref | 119 (15.1) | — | — |
CI, confidence interval; OR, odds ratio; Ref, reference.
Adjusted for age, season of enrolment and antibiotic use in past 7 days.
Associations with season are presented in Fig. 1 and Supplementary Table 1. In brief, RSV was associated with autumn (odds ratio (OR) 3.7, 95% CI 2.1, 6.5), whereas influenza (OR 5.5, 95% CI 1.6, 18.6), human bocavirus (OR 11.2, 95% CI 1.5, 85.3) and the coronaviruses (OR 5.2, 95% CI 1.2, 23.1) prevailed in winter. There were no clear associations between season and S. pneumoniae. NTHi codetection with any virus was less likely in autumn (OR 0.3, 95% CI 0.1, 0.8) and winter (OR 0.25, 95% CI 0.1, 0.8), while M. catarrhalis codetections with viruses was associated with the winter months (OR 2.5, 95% CI 1.2, 5.4).
Fig. 1.
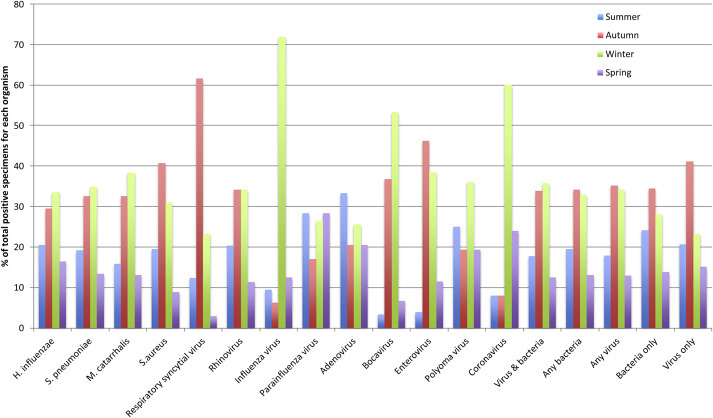
Seasonal distribution of upper airway viruses and bacteria identified in children aged 15 years presenting to emergency department with acute respiratory infection including cough as a symptom.
Discussion
We investigated the child characteristics associated with nasal codetection of viruses and bacteria in children with ARI with cough using molecular methods. In 817 children presenting to a tertiary paediatric ED with an ARI and cough, at least one virus or bacterium was detected in nasal swab specimens from 90.6% of cases, while viruses and bacteria were codetected in 51.8%. Identification of organisms in isolation was uncommon. Factors significantly associated with virus–bacteria codetections were young age, male gender, child care attendance and winter season. Differences emerged between clinically important viruses [16]. For example, RSV was associated with age, the autumn months and NTHi, while influenza was associated with winter and S. pneumoniae.
Other than for S. pneumoniae with influenza or RSV, little published data exist describing the frequency of virus–bacteria coinfections in nasal specimens during an ARI [17]. H. influenzae dominated the virus–bacteria pairs in our study despite not being the most common bacteria detected. Indeed, the role of NTHi in the pathogenesis of ARI may be underestimated [18]. There is a growing body of evidence suggesting both beneficial and detrimental synergies [19] that may play important roles in regulating host responses, clinical severity [20] and treatment responses [21]. Further, the availability of vaccines, now and in the future, that may affect H. influenzae necessitates the need for it to be a focus of ARI research.
Risk factors associated with virus–bacteria codetections are similar to those observed for ARI elsewhere [22]. A study of 3181 hospitalized children with ARI in China found those aged 1 to 4 years and boys were more likely to have virus–bacteria codetection [23]. However, the prevalence of any bacteria identified in that study was only 22%, and codetection was just 17%. It also did not include B. pertussis, S. aureus or M. catarrhalis and relied solely on culture for identifying bacteria.
While our overall detection rate of at least one organism in >90% of subjects was substantially higher than some studies reporting on upper airways organisms in association with disease, our high detection rate is similar to other studies. A community-acquired pneumonia study [21] and an American Indian child cohort of ARI [5] described detection rates of 97% in the former [21] and 88% in the latter [5]. The seasonal distribution of most organisms correlated with the patterns of childhood ARI in subtropical climates, particularly over autumn and winter months, although all organisms were identified year round. Of note in our study is the relatively low frequency of RSV (16.5%) and influenza (3.9%) detected despite significant RSV and influenza seasons reported by state surveillance systems over the study period (https://www.health.qld.gov.au/ph/cdb/sru_data.asp). Similarly, only seven children (0.8%) were found to be positive for B. pertussis despite the presence of a waning pertussis epidemic in the first year of the study [24]. The low prevalence of RSV may partly be related to the relatively low number of enrolled children aged <12 months, in whom RSV is a dominant ARI pathogen [25], and those who were critically ill.
The prevalence of influenza virus in our study is consistent with other Australian paediatric studies that included both low and high influenza activity seasons [26], [27]. An Australian observational study of influenza vaccine effectiveness in children aged 6 months to <3 years in 2010 (a low influenza activity season) found 5 (4.3%) of 117 influenza-like illnesses with specimens were influenza virus positive [26]. Similarly, in an Australian cohort study of ARIs in children aged <5 years followed for 12 months that coincided with increased influenza activity, influenza virus was detected in 4% of 543 ARIs where parent-collected nasal specimens were obtained [27]. However, a study from the United States of paediatric ED visits over a 10-year period found the proportion of ARI/fever visits involving confirmed influenza infections ranged from 10 to 48% (median 15%) [28], while a French study of a rapid influenza diagnostic tests in a paediatric ED setting reported 57% of children presenting with fever without source were positive for influenza virus during an epidemic period [29]. The difference between these and our study are likely to reflect differences in case definitions and that the study was not conducted during an influenza pandemic, during which parents may be more likely to present to an ED if their child is ill. With respect to B. pertussis, the ratios of notifications of pertussis cases against the 5-year means in Queensland for 2012, 2013 and 2014 were 1.4, 0.6 and 0.2 respectively (https://www.health.qld.gov.au/ph/cdb/sru_data.asp), and the declines were evident in all age groups (Lisa McHugh, personal communication, 2015); hence, it is likely that the study period incorporated an interepidemic period, consistent with pertussis trends over time.
A strength of our study is the detail of potential factors associated with detection rates. We accounted for prior antibiotic and oral steroid use and illness duration at presentation and did not limit recruitment to children with specific clinical entities, such as pneumonia or wheezing illnesses. The lack of an effect of prior antibiotic exposure likely reflects our reliance on PCR assays rather than culture for bacteria identification. The major limitation of the study is the proportion of children with cough who were screened but not enrolled, particularly those in the younger age groups and those with mild or severe disease, and our results may thus not reflect the population of children with ARI attending an ED. Further limitations include the inability to investigate causality, given the absence of controls and the cross-sectional design, meaning that temporal relationships between codetected organisms could not be examined. However, that was not the intent of our study. The use of anterior nasal rather than nasopharyngeal swabs may have led to an underestimation of bacteria species. However, anterior nasal swabs are less traumatic in young children and facilitate bilateral sampling; a loss in sensitivity for bacteria was considered acceptable for the purposes of the overall study [13] for which the samples were collected. The infrequency of detecting just a single bacterium with a virus precluded investigating the interactions between organisms in greater detail.
Our study in children with cough highlights several things. Firstly, relating the upper airway microbial epidemiology in relation to ARI in children is complex and suggests that reports focusing solely on either bacteria or virus should be interpreted cautiously. Secondly, studies that relate upper airway pathogens to clinical data should take into account factors that influence bacteria and virus detection, such as age, gender, season, child care attendance, duration of illness, specimen quality and prior antibiotic and steroid use. Thirdly, our finding of the significant association of NTHi with RSV should be further investigated in the context of the increasing appreciation that the contribution of NTHi to acute and chronic respiratory diseases is receiving [8]. We are now in an era of recognizing these complexities and the interactions of individual constituents and how this might influence host-specific factors, including immune responses and clinical severity [19]. Making substantial inroads into reducing the ARI burden in children therefore requires high-quality studies in several different population settings.
Acknowledgements
We thank the following for their support with study implementation and recruitment: M. Lang, P. Hetherington, E. Casey, M. Price, B. Saul and J. O'Shea from the RCH Paediatric Emergency Research Unit; S. Rablin, B. Drescher, K. Hall, C. Shevell, D. Arnold and B. Arnold from the Queensland Children's Medical Research Institute's Respiratory infection Outreach and Research team; and J. Gaydon from the Queensland Paediatric Infectious Diseases Laboratory.
Editor: F. Allerberger
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cmi.2016.02.004.
Transparency Declaration
Financial support was received from a Queensland Children's Health Foundation Program Grant awarded to ABC and JA. KFO is supported by a Queensland Government Smart Futures Fellowship (51008) and an Australian National Health and Medical Research Council (NHMRC) Career Development Award (1045157). VG is supported by a NHMRC Post-Graduate Training Fellowship (1075119). ABC is supported by a NHMRC Practitioner Fellowship (545216). All authors report no conflicts of interest relevant to this article.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Brealey J.C., Sly P.D., Young P.R., Chappell K.J. Viral bacterial co-infection of the respiratory tract during early childhood. FEMS Microbiol Lett. 2015;362 doi: 10.1093/femsle/fnv062. [DOI] [PubMed] [Google Scholar]
- 2.O’Grady K.F., Mackay I.M., Chang A.B. Cough formation in viral infections in children. In: Singh S.K., editor. Respiratory viral infections. Taylor & Francis Group/CRC Press; Hyderabad: 2013. [Google Scholar]
- 3.Asner S.A., Rose W., Petrich A., Richardson S., Tran D.J. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect. 2015;21 doi: 10.1016/j.cmi.2014.08.024. 264.e261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis M.F., Peng R.D., McCormack M.C., Matsui E.C. Staphylococcus aureus colonization is associated with wheeze and asthma among US children and young adults. J Allergy Clin Immunol. 2015;135 doi: 10.1016/j.jaci.2014.10.052. 811–3.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat N., Tokarz R., Jain K., Haq S., Weatherholtz R., Chandran A. A prospective study of agents associated with acute respiratory infection among young American Indian children. Pediatr Infect Dis J. 2013;32:e324–e333. doi: 10.1097/INF.0b013e31828ff4bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum G.B., Morris P.S., Grimwood K., Maclennan C., White A.V., Chatfield M.D. Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: a multicentre, randomized, placebo-controlled trial. Front Pediatr. 2015;3:32. doi: 10.3389/fped.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A.B., Smith-Vaughan H., Sloots T., Valery P.C., Whiley D., Beissbarth J. Upper airway viruses and bacteria detection in clinical pneumonia in a population with high nasal colonisation do not relate to clinical signs. Pneumonia. 2015;21:48–56. doi: 10.15172/pneu.2015.6/636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudan I., O’Brien K.L., Nair H., Liu L., Theodoratou E., Qazi S. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P., Hartert T.V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noverr M.C., Huffnagle G.B. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Li F., Sun R., Gao X., Wei H., Li L.J. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun. 2013;4:2106. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnelykke K., Vissing N.H., Sevelsted A., Johnston S.L., Bisgaard H. Association between respiratory infections in early life and later asthma is independent of virus type. J Allergy Clin Immunol. 2015;136 doi: 10.1016/j.jaci.2015.02.024. 81–6.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drescher B.J., Chang A.B., Phillips N., Acworth J., Marchant J., Sloots T.P. The development of chronic cough in children following presentation to a tertiary paediatric emergency department with acute respiratory illness: study protocol for a prospective cohort study. BMC Pediatr. 2013;13:125. doi: 10.1186/1471-2431-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Grady K.A., Torzillo P.J., Rockett R.J., Whiley D.M., Nissen M.D., Sloots T.P. Successful application of a simple specimen transport method for the conduct of respiratory virus surveillance in remote Indigenous communities in Australia. Trop Med Int Health. 2011;16:766–772. doi: 10.1111/j.1365-3156.2011.02757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Grady K.A., Whiley D.M., Torzillo P.J., Sloots T.P., Lambert S.B. Mailed versus frozen transport of nasal swabs for surveillance of respiratory bacteria in remote Indigenous communities in Australia. BMC Infect Dis. 2013;13:543. doi: 10.1186/1471-2334-13-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J Glob Health. 2015;5:010408. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 18.Van Eldere J., Slack M.P., Ladhani S., Cripps A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis. 2014;14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa K., Camargo C.A., Jr. Airway microbiota and acute respiratory infection in children. Expert Rev Clin Immunol. 2015;11:789–792. doi: 10.1586/1744666X.2015.1045417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakwinska O., Bastic Schmid V., Berger B., Bruttin A., Keitel K., Lepage M. Nasopharyngeal microbiota in healthy children and pneumonia patients. J Clin Microbiol. 2014;52:1590–1594. doi: 10.1128/JCM.03280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honkinen M., Lahti E., Osterback R., Ruuskanen O., Waris M. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect. 2012;18:300–307. doi: 10.1111/j.1469-0691.2011.03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinzon-Rondon A.M., Aguilera-Otalvaro P., Zarate-Ardila C., Hoyos-Martinez A. Acute respiratory infection in children from developing nations: a multi-level study. Paediatr Int Child Health. 2015 doi: 10.1179/2046905515Y.0000000021. 2046905515Y0000000021. [DOI] [PubMed] [Google Scholar]
- 23.Wei L., Liu W., Zhang X.A., Liu E.M., Wo Y., Cowling B.J. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009–2013. Medicine. 2015;94:e742. doi: 10.1097/MD.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillsbury A., Quinn H.E., McIntyre P.B. Australian vaccine preventable disease epidemiological review series: pertussis, 2006–2012. Commun Dis Intell Quarterly Report. 2014;38:E179–E194. doi: 10.33321/cdi.2014.38.34. [DOI] [PubMed] [Google Scholar]
- 25.Mazur N.I., Martinon-Torres F., Baraldi E., Fauroux B., Greenough A., Heikkinen T. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3:888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 26.Dierig A., Heron L.G., Lambert S.B., Yin J.K., Leask J., Chow M.Y. Epidemiology of respiratory viral infections in children enrolled in a study of influenza vaccine effectiveness. Influenza Other Respir Viruses. 2014;8:293–301. doi: 10.1111/irv.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert S.B., Allen K.M., Druce J.D., Birch C.J., Mackay I.M., Carlin J.B. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics. 2007;120:e929–e937. doi: 10.1542/peds.2006-3703. [DOI] [PubMed] [Google Scholar]
- 28.Jules A., Grijalva C.G., Zhu Y., Talbot H.K., Williams J.V., Poehling K.A. Influenza-related hospitalization and ED visits in children less than 5 years: 2000–2011. Pediatrics. 2015;135:e66–e74. doi: 10.1542/peds.2014-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroix S., Vrignaud B., Avril E., Moreau-Klein A., Coste M., Launay E. Impact of rapid influenza diagnostic test on physician estimation of viral infection probability in paediatric emergency department during epidemic period. J Clin Virol. 2015;72:141–145. doi: 10.1016/j.jcv.2015.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


