Abstract
A 12-year-old female neutered cat presented with acute onset unilateral vestibular syndrome, a spontaneous cutaneous wound, polyuria, polydipsia, and diabetes mellitus. Hyperadrenocorticism was demonstrated by means of hyper-responsiveness to adrenocorticotropic hormone stimulation, elevated urine cortisol-to-creatinine ratio, bilaterally enlarged adrenal glands on abdominal ultrasound, and pituitary enlargement on computed tomography imaging. The cat was euthanased and post-mortem histological examination revealed feline skin fragility syndrome; confirmed a pituitary cromophobe macroadenoma; and generalised toxoplasmosis with tachyzoites in the pancreas, bowel and brain. This report is the first to describe the concurrence of macroadenoma pituitary-dependent hyperadrenocorticism and generalised toxoplasmosis in a cat with central vestibular syndrome.
Feline spontaneous hyperadrenocorticism (HAC) is rare and in approximately 80% of cases caused by a functional pituitary adenoma (pituitary-dependent hyperadrenocorticism; PDH). About 50% of pituitary tumours are microscopic in size; the remaining tumours are large (usually greater than 3–4 mm in greatest diameter). 1 In rare cases pituitary macroadenomas have been associated with specific central nervous system (CNS) neurological signs, 2,3 but in the majority of reports lethargy was the only potential neurological sign. 4–7
Seroprevalence studies suggest that Toxoplasma gondii infection of cats is common worldwide. Most healthy cats develop immunity that prevents clinical disease, but does not destroy tissue cysts and the cats remain latently infected. 8 Feline tissues affected by T gondii include the liver, kidney, spleen, muscle, lung, heart, eye, brain and spinal cord. 9 In the brain, T gondii may cause meningoencephalitis with involvement of central vestibular structures.10
Feline spontaneous HAC has never been described in association with generalised toxoplasmosis, although it has been associated with T gondii oocysts excretion. 11 The cat in this case report was diagnosed with PDH associated with a macroadenoma, and with concomitant generalised toxoplasmosis and neurological manifestations of a central vestibular syndrome.
A 12-year-old spayed female domestic shorthair cat weighing 3.8 kg, which lived indoors and was fed a commercial diet for the last year (previously was an outdoor cat with possibility to hunt) was referred to Milan University Medical Teaching Hospital with an acute onset of ataxia, a spontaneous skin wound and polyuria and polydipsia (PU/PD). Physical examination revealed an enlarged abdomen, dehydration (∼10%), diffuse muscle wasting, the presence of a 5 cm×5 cm skin tear on the right hind limb (Fig 1) and severe, generalised thinning of the skin with prominent dermal blood vessels (Fig 2). Neurological examination revealed abnormal mentation with heavy obtunded mental status, unilateral head tilt, imbalance, ataxia, dysmetria, circling to right side, and ipsilateral proprioceptive deficits and weakness. Head tilt, imbalance, ataxia and circling indicated a vestibular system dysfunction. Obtunded mental status, dysmetria and ipsilateral proprioceptive deficits and weakness could be explained by a CNS central diffuse or multifocal lesion with a major component involving the cerebellum, pons and medulla.12
Fig 1.
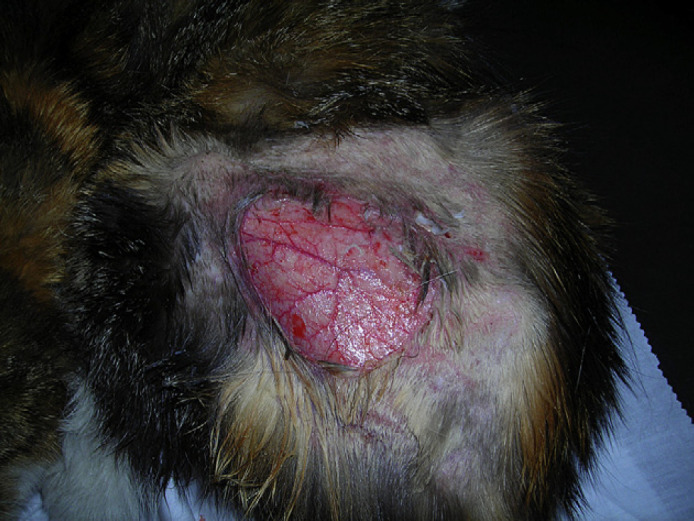
12-year-old neutered female domestic shorthair cat with PDH. Note the spontaneous skin tear on the left caudal limb.
Fig 2.
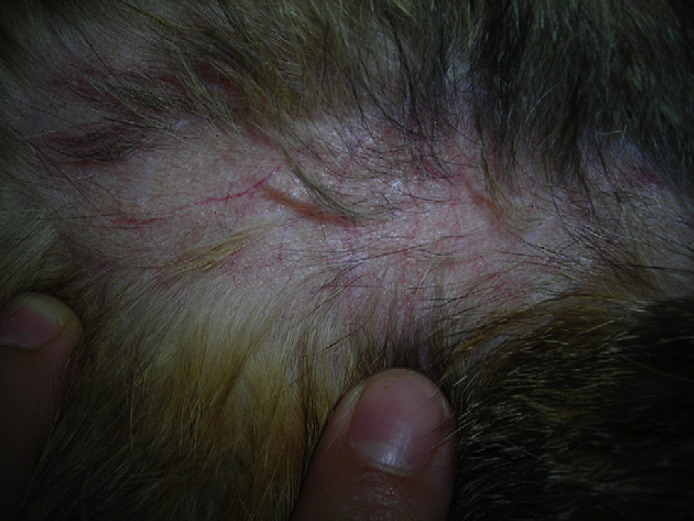
12-year-old neutered female domestic shorthair cat with PDH. Note the thin skin with prominent dermal blood vessels.
Blood examinations revealed neutrophilia (15.0; range 2.5–12.5×109/l), lymphopenia (0.3; range, 1.5–7.0×109/l), and hyperglycaemia (20.1 nmol/l; range, 4.4–6.1 nmol/l). The urine had a specific gravity of 1.020 with glucose presence of 3+. Urine was negative for bacterial and fungal growth. A combined enzyme-linked immunosorbent assay (ELISA) for feline leukaemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody were negative (Snap Combo Plus FeLV Ag/FIV Ab, Idexx Laboratories). Indirect fluorescent antibody (IFA) technique for feline coronavirus (FCoV) antibody was negative and indirect haemagglutination test (IHA) for T gondii IgG was 1:20.480 (Toxo-Hai Fumouze, Fumouze Diagnostics, Levallois, Perret Cedex, France). IgM serology for T gondii was not undertaken because IHA test primarily measures IgG and proves the active toxoplasmosis documenting a fourfold rise of the IgG titre over 2–3 weeks. 13,14
The pituitary-adrenal axis was assessed using an adrenocorticotropin (ACTH) stimulation test with synthetic ACTH (0.125 mg intramuscularly; Synacthen; Novartis Farma). This revealed hyper-responsiveness with baseline serum cortisol of 726 nmol/l (range 20–270 nmol/l), 30 min post-stimulation cortisol of 985 nmol/l (range<400 nmol/l), and 90 min post-stimulation cortisol of 226 nmol/l (range<400 nmol/l). The urinary cortisol-to-creatinine ratio (UC:CR) measured on an home-collected urine sample was 11 (range 2–3.6×10−5). 15 No further blood tests were undertaken. Abdominal ultrasonography revealed the liver was enlarged with normal echogenicity and an homogeneous parenchyma. The adrenal glands were hypoechoic, homogeneous, and enlarged (width of left adrenal 0.66 cm, right adrenal 0.46 cm; range<0.39 cm). 16 These data were consistent with a diagnosis of PDH. To correct the dehydration, hyperglycaemia and to prevent a systemic infection from the skin tear the cat was initially treated with intravenous fluids, porcine lente insulin (0.5 U/kg subcutaneously q 12 h; Caninsulin; Intervet), and cefazolin (20 mg/kg intravenously q 12 h; Dorom; Teva Pharma), respectively.
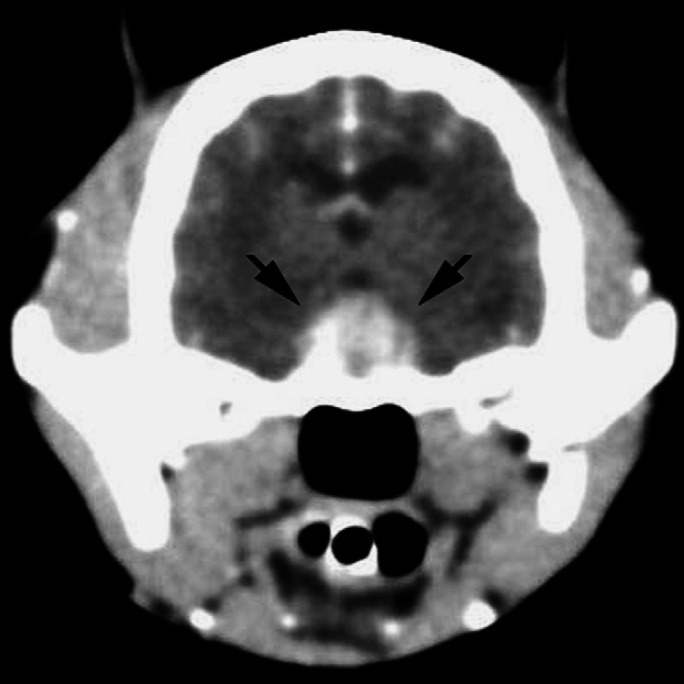
A computed tomography (CT) scan of the head was performed 3 days after the admission of the cat. Pre-contrast images showed a large mass (10 mm× 10 mm× 6 mm) with well-defined margins within the sella turcica. The mass appeared slightly hyper-dense compared to the surrounding parenchyma, and showed high homogeneous contrast enhancement in post-contrast images (Fig 3). There was no evidence of mass effect or compression of the ventricular system. The tomographic findings were consistent with a pituitary macroadenoma associated with a thin surrounding hypo-dense lesion consistent with a mild perilesional oedema. There was also a hypo-dense area in the caudal portion of the cerebellum.
Fig 3.
CT scan of the head of a 12-year-old neutered female domestic shorthair cat with PDH: post-contrast transverse slice at the level of the diencephalon. A well-defined space-occupying lesion (10 mm high, 10 mm wide, and 6 mm long) is evident in the hypothalamic-pituitary region (arrows).
Due to the cat's poor clinical condition the owners requested she be euthanased when the cat was still under anaesthesia for CT, and gave their permission for an autopsy. The main gross autopsy findings were bilateral adrenal enlargement, a multi-nodular firm pancreas and a severely enlarged (7 mm in greatest diameter) pituitary gland. A large, poorly defined malacic area in the cerebellar white matter was also detected.
Examination of coronal sections of the brain, performed a few days thereafter on formalin fixed tissue, showed a large (1×0.9 cm diameter), poorly defined malacic area within the cerebellum and brainstem. Specifically, the lesion comprised the area of right medial, rostral and lateral vestibular nuclei, and caudal cerebellar peduncle, extending cranially and dorsally to the flocculus into the cerebellar folia.
Histologically, the skin was characterised by diffuse, severe, epidermal orthokeratotic hyperkeratosis, dermal–epidermal atrophy, and large areas of epidermal detachment, consistent with feline skin fragility syndrome (FSFS). 17 Diffuse adrenal cortical hyperplasia and severe, multifocal to coalescing, necrotising, neutrophilic and macrophagic, subacute pancreatitis with intense fibrosis were also present. Innumerable intralesional protozoal tachyzoites, 3–4 μm, with small basophilic nuclei and clear cytoplasmic halo were recognisable within pancreatic necrotic areas, both free and within macrophage or pancreatic acinar cell cytoplasm (Fig 4). A small area of intramural necrosis with neutrophilic infiltration was detected within duodenal wall muscle layer, in close proximity to pancreatic duct. Pituitary mass was consistent with a chromophobe adenoma, composed of well differentiated small chromophobe cells, supported by a thin fibrovascular stroma (Fig 5). The malacic area grossly detected within the cerebellum was consistent with necrotising and purulent encephalitis with intralesional free tachyzoites, occasional protozoal cysts and peripheral lymphoplasmacytic perivascular cuffing. Similar, smaller lesions were multifocally detected scattered within brain parenchyma.
Fig 4.
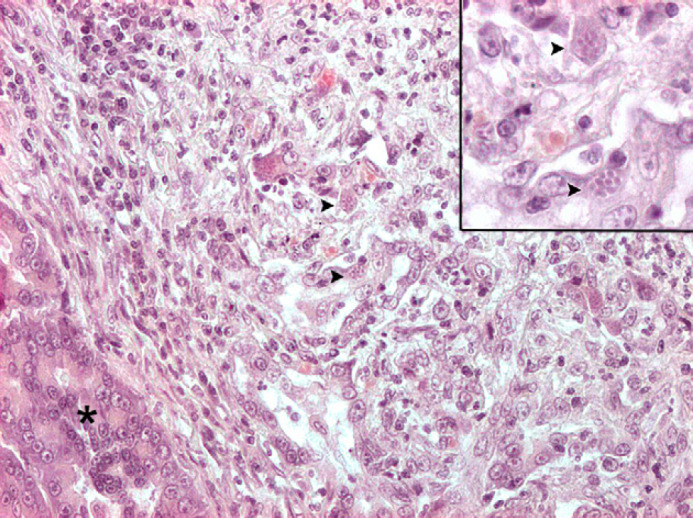
Exocrine pancreas (asterisk): a large area of necrosis, neutrophilic and lymphoplasmacytic infiltration and fibrosis effaces normal architecture. Numerous tachyzoites and cysts are scattered throughout the lesions (arrowheads). Haematoxylin and eosin, 200×. The small insert at the upper right shows higher magnification of the same protozoal cysts (haematoxylin and eosin 400×).
Fig 5.
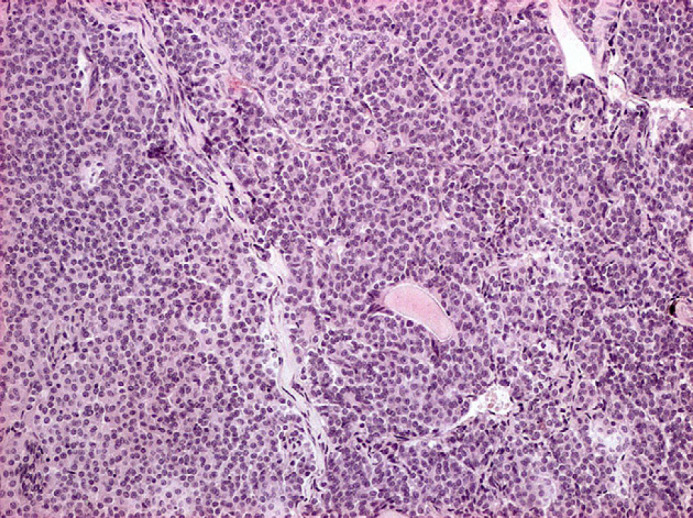
Pituitary gland chromophobe adenoma: well differentiated small chromophobe cells, organised in small packets supported by a delicate fibrovascular stroma. Haematoxylin and eosin, 100×.
Immunohistochemistry was performed on deparaffinised sections of all tissues sampled at necropsy. The standard avidin–biotin–peroxidase complex (ABC) technique 18 was used. Primary antibody, a rabbit antiserum directed against T gondii (Medicago AB, Uppsala, Sweden, 1:10.000 diluted) was incubated overnight at 4°C in a humid chamber. AEC (amino-ethyl-carbazole) served as the chromogen and sections were counterstained with Mayer haematoxylin. Negative controls were performed under identical conditions, incubating the sections with normal goat serum. Appropriate positive controls were also included. Protozoal tachyzoites within brain and pancreatic necrotic foci immunostained positive for T gondii (Figs 6, 7 ).
Fig 6.
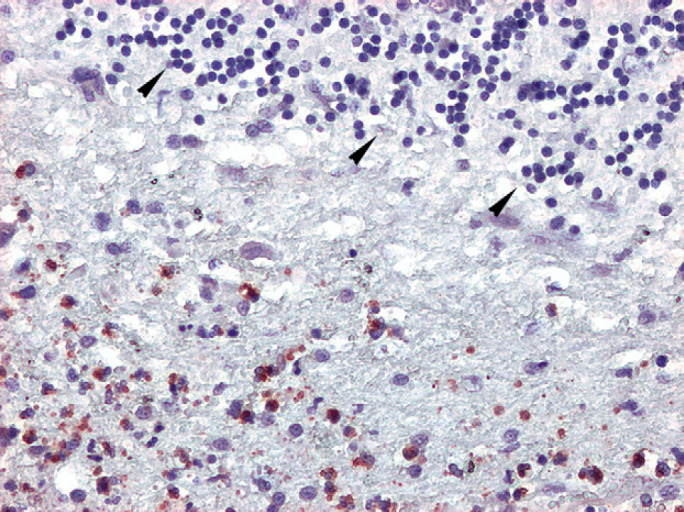
Cerebellum, numerous T gondii tachyzoites, immunolabelled with anti-T gondii antibody (red stained), are visible scattered throughout necrotic debris. Thinned and disorganised cerebellar granular cell layer is recognisable on the upper right of the picture (arrowheads). Standard ABC immunohistochemical method, chromogen amino-ethyl-carbazole, 200×.
Fig 7.
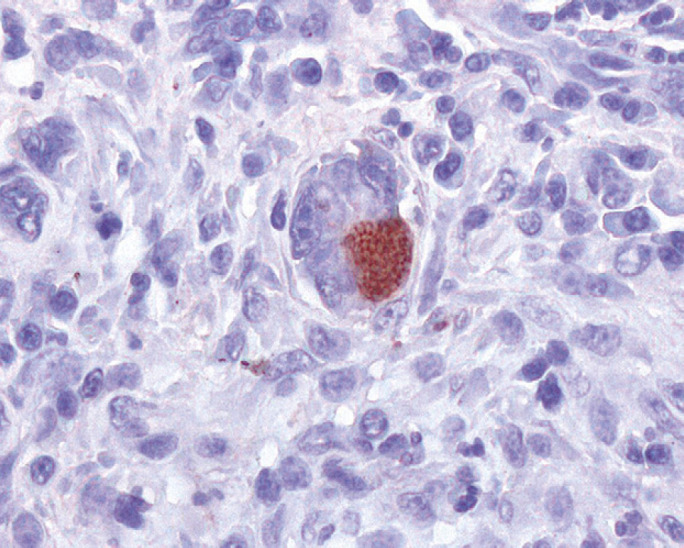
Exocrine pancreas: numerous T gondii tachyzoites, within pancreatic acinar cell cytoplasm, are immunolabelled with anti-T gondii antibody (red stained). Standard ABC immunohistochemical method, chromogen amino-ethyl-carbazole, 400×.
In feline HAC the most common clinical signs include PU/PD, polyphagia, weight loss, abdominal enlargement, thin skin, skin tears, and hair loss. 1 In the case described here the predominant signs were PU/PD, cutaneous lesions due to FSFS, and neurological signs of central vestibular disease.
PU/PD are often the first overt signs of HAC and are usually associated with marked hyperglycaemia because 80–100% of cats with HAC are diabetic. 19 FSFS is a rare disorder characterised by markedly fragile, thin skin which is easily damaged by minor trauma. Moreover, FSFS has been associated with several underlying disease processes, most commonly spontaneous or iatrogenic HAC, and the use of progestagens.20
In feline medicine, disorders that may cause a central vestibular syndrome are meningoencephalitis due to infectious-inflammatory disorders of CNS (eg, toxoplasmosis, feline infectious peritonitis (FIP), FIV), aberrant parasitic migration, neoplasm (eg, meningioma, lymphoma), or thiamine deficiency. 10,12 In the cat described in this report, neurological examination revealed the presence of head tilt, imbalance, ataxia and circling to right side indicated a vestibular system dysfunction, and obtunded mental status, dysmetria and ipsilateral proprioceptive deficits and weakness which could have been explained by a CNS central diffuse or multifocal lesion with a major component involving the cerebellum, pons and medulla. 12 Even if the serology for T gondii IgG was positive, we didn't have the opportunity to verify a fourfold increase in the IgG titre after 2–4 weeks which could be supportive of an active infection, 14 and the definitive diagnosis of toxoplasmosis was reached by histological and immunohistochemical analysis of the lesions. Feline CNS toxoplasmosis typically causes an encephalitis or meningoencephalitis with a lack of gross lesions. Histological findings are microscopic granulomas with intracellular tachyzoites and mononuclear perivascular cuffing. 9 Neurological signs are related to the parasite localisation in the brain. 14 In this case histological examination revealed a poorly defined malacic area within the cerebellum and brainstem. Specifically, the lesion comprised all right vestibular nuclei, caudal cerebellar peduncle, extending to the flocculus into the cerebellar folia, and was consistent with necrotising and purulent encephalitis with intralesional free T gondii tachyzoites, and occasional protozoal cysts. These lesions could have been related to the neurological signs of a central vestibular syndrome, specifically for obtunded mental status, dysmetria, circling to the right side and ipsilateral proprioceptive deficit and weakness.
In cats immunosuppression caused by high doses of drugs such as cyclosporine and prednisolone 21 or a lymphotropic virus such as FIV 22 may reactivate a latent infection result in clinical toxoplasmosis.
In this cat, lack of recent exposure history to T gondii (in the past the cat was an outdoor cat with the possibility to hunt mammals or birds, but in the last year was exclusively an indoor cat which was fed only with commercial diet and never received raw meat) suggests reactivation of a latent infection was the most likely scenario. The cat was FIV and FeLV negative and received no immunosuppressive drugs. Immunosuppression could have been related to the endogenous increase of cortisol that finally may have been associated with the recrudescent toxoplasmosis. It is demonstrated that glucorticoids at clinical dosages (5 mg/kg body weight of methylprednisolone acetate administered intramuscularly weekly) for 4 weeks did not induce oocysts shedding in cats recently or chronically experimentally infected with T gondii. 23 Conversely, a high dose of glucorticoids (10–80 mg/kg body weight of methylprednisolone acetate administered intramuscularly weekly) prolonged oocysts shedding and may lead to reactivation of toxoplasmosis in naturally infected cats.24
Many cats with pituitary gland adenomas die from complications relating to endocrine disease before neurological signs manifest as the tumour size increases. Pituitary macroadenomas are responsible for CNS signs when the enlarged hypophysis compresses the surrounding structures in the brain. 25 Neurological deficits commonly involve diencephalic signs such as mental changes, circling, visual and pupillary light reflex deficits (mydriasis), and seizures.25
In this case of macroadenoma PDH in association with generalised toxoplasmosis and neurological signs, it is difficult to establish if the neurological signs were caused by the pituitary macroadenoma and/or the CNS toxoplasmosis, because both diseases may cause similar clinical signs. The majority of the reports of macroadenoma PDH in cats in the veterinary literature showed no obvious central neurological signs. 4–7 One case has been described as macroadenoma with central neurological signs, but the pituitary gland was larger than the case reported here. 3 In our case there was no evidence of mass effect or compression by the pituitary adenoma detectable at CT scan, but there were encephalic lesions (cerebellar malacia, multiple necrosis foci in the brain) due to T gondii tachizoites in the vestibular nuclei and cerebellum. In the opinion of the authors it is most probable that the neurological signs of a central vestibular syndrome were due to CNS toxoplasmosis. The authors suggest that in feline HAC associated with central neurological signs, it is important to include in the differential diagnosis infective diseases such as CNS toxoplasmosis.
References
- 1.Feldman E.C., Nelson R.W. Hyperadrenocorticism in cats (Cushing's syndrome). Feldman E.C., Nelson R.W. Canine and feline endocrinology and reproduction, 3rd edn, 2004, Saunders Elsevier: St Louis, 358–393. [Google Scholar]
- 2.Mayer M.N., Greco D.S., LaRue S.M. Outcomes of pituitary tumour irradiation in cats, J Vet Inter Med 20, 2006, 1151–1154. [DOI] [PubMed] [Google Scholar]
- 3.Fracassi F., Mandrioli L., Diana A., Hilbe M., Grinwis G., Gandini G. Pituitary macroadenoma in a cat with diabetes mellitus, hypercortisolism and neurological signs, J Vet Med A Physiol Pathol Clin Med 54, 2007, 359–363. [DOI] [PubMed] [Google Scholar]
- 4.Peterson M.E., Steele P. Pituitary-dependent hyperadrenocorticism in a cat, J Am Vet Med Assoc 189, 1986, 680–683. [PubMed] [Google Scholar]
- 5.Kipperman B.S., Nelson R.W., Griffey S.M., Feldman E.C. Diabetes mellitus and exocrine pancreatic neoplasia in two cats with hyperadrenocorticism, J Am Anim Hosp Assoc 28, 1992, 415–418. [Google Scholar]
- 6.Furuzawa Y., Une Y., Nomura Y. Pituitary dependent hyperadrenocorticism in a cat, J Vet Med Sci 54, 1992, 1201–1203. [DOI] [PubMed] [Google Scholar]
- 7.Skelly B.J., Petrus D., Nicholls P.K. Use of trilostane for the treatment of pituitary-dependent hyperadrenocorticism in a cat, J Small Anim Pract 44, 2003, 269–272. [DOI] [PubMed] [Google Scholar]
- 8.Lappin M.R. Polysystemic protozoal infections: feline toxoplasmosis. Nelson R.W., Couto C.G. Small animal internal medicine, 3rd edn, 2003, Mosby: St Louis, 1296–1299. [Google Scholar]
- 9.Dubey J.P., Carpenter J.L. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990), J Am Vet Med Assoc 203, 1993, 1556–1566. [PubMed] [Google Scholar]
- 10.LeCounteur R.A. Feline vestibular diseases – new developments. Proceedings of the ESFM Congress, Stockholm, 2002, J Feline Med Surg 5, 2003, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerding P.A., Morton L.D., Dye J.A. Ocular and disseminated candidiasis in an immunosuppressed cat, J Am Vet Med Assoc 204, 1994, 1635–1638. [PubMed] [Google Scholar]
- 12.De Lahunta A., Glass E. Veterinary neuroanatomy and clinical neurology. Chapter 12 vestibular system: special proprioception, and Chapter 13 cerebellum. 3rd edn., 2009, WB Saunders: Philadelphia, 319–47, 348–88. [Google Scholar]
- 13.Lappin M.R., Powell C. Comparison of latex agglutination, indirect hemagglutination, and ELISA techniques for the detection of Toxoplasma gondii-specific antibodies in the serum of cats, J Vet Inter Med 5, 1991, 299–301. [DOI] [PubMed] [Google Scholar]
- 14.Dubey J.P., Lappin M.R. Toxoplasmosis and neosporosis. Greene C.E. Infectious diseases of the dog and cat, 3rd edn, 2006, Elsevier Saunders: Missouri, 754–775. [Google Scholar]
- 15.Goossens M.M.C., Meyer H.P., Voorhout G., Sprang E.P. Urinary excretion of glucocorticoids in the diagnosis of hyperadrenocorticism in cats, Domest Anim Endocrinol 12, 1995, 355–362. [DOI] [PubMed] [Google Scholar]
- 16.Zimmer C., Horauf A., Reusch C. Ultrasonographic examination of the adrenal gland and evaluation of the hypophyseal-adrenal axis in 20 cats, J Small Anim Pract 41, 2000, 156–160. [DOI] [PubMed] [Google Scholar]
- 17.Ginn E., Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie M., ed. Jubb, Kennedy and Palmer's pathology of domestic animals vol 1. 5th edn. Philadelphia: Elsevier Saunders, 2007: 634–5. [Google Scholar]
- 18.Hsu S.M., Raine L., Fanger H. Use of avidin-biotin peroxidase complex (ABC) in the immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures, J Histochem Cytochem 29, 1981, 577–580. [DOI] [PubMed] [Google Scholar]
- 19.Nelson R.W., Feldman E.C., Smith M.C. Hyperadrenocorticism in cats: seven cases (1978–1987), J Am Vet Med Assoc 193, 1988, 245–250. [PubMed] [Google Scholar]
- 20.Helton-Rhodes K. Cutaneous manifestations of hyperadrenocorticism. August J.R. Consultations in feline internal medicine 3, 1997, WB Saunders: Philadelphia, 191–198. [Google Scholar]
- 21.Bernsteen L., Gregory C.R., Aronson L.R., Lirztman L.A., Brummer D.G. Acute toxoplasmosis following renal transplantation in three cats and a dog, J Am Vet Med Assoc 215, 1999, 1123–1126. [PubMed] [Google Scholar]
- 22.Davidson M.G., Rottman J.B., English R.V., Lappin R.M., Tompkins M.B. Feline immunodeficiency virus predisposes cats to acute generalized toxoplasmosis, Am J Pathol 143, 1993, 1486–1497. [PMC free article] [PubMed] [Google Scholar]
- 23.Lappin M.R., Dawe D.L., Lindl P.A., Greene C.E., Prestwood A.K. The effect of glucocorticoid administration on oocyst shedding, serology, and cell-mediated immune responses of cats with acute or chronic toxoplasmosis, J Am Animal Hosp Assoc 27, 1992, 625–632. [Google Scholar]
- 24.Dubey J.P., Frenkel J.K. Immunity to feline toxoplasmosis: modification by administration of corticosteroids, Vet Pathol 11, 1974, 350–379. [DOI] [PubMed] [Google Scholar]
- 25.Vernau K.M., Dickinson P.J. Brain tumors. August J.R. Consultations in feline internal medicine 5, 2006, Elsevier Saunders: Philadelphia, 505–516. [Google Scholar]