Abstract
Background
The outbreak of 2019 novel coronavirus disease (COVID-19) in Wuhan, China, has spread rapidly worldwide. In the early stage, we encountered a small but meaningful number of patients who were unintentionally scheduled for elective surgeries during the incubation period of COVID-19. We intended to describe their clinical characteristics and outcomes.
Methods
We retrospectively analyzed the clinical data of 34 patients underwent elective surgeries during the incubation period of COVID-19 at Renmin Hospital, Zhongnan Hospital, Tongji Hospital and Central Hospital in Wuhan, from January 1 to February 5, 2020.
Findings
Of the 34 operative patients, the median age was 55 years (IQR, 43–63), and 20 (58·8%) patients were women. All patients developed COVID-19 pneumonia shortly after surgery with abnormal findings on chest computed tomographic scans. Common symptoms included fever (31 [91·2%]), fatigue (25 [73·5%]) and dry cough (18 [52·9%]). 15 (44·1%) patients required admission to intensive care unit (ICU) during disease progression, and 7 patients (20·5%) died after admission to ICU. Compared with non-ICU patients, ICU patients were older, were more likely to have underlying comorbidities, underwent more difficult surgeries, as well as more severe laboratory abnormalities (eg, hyperleukocytemia, lymphopenia). The most common complications in non-survivors included ARDS, shock, arrhythmia and acute cardiac injury.
Interpretation
In this retrospective cohort study of 34 operative patients with confirmed COVID-19, 15 (44·1%) patients needed ICU care, and the mortality rate was 20·5%.
Funding
National Natural Science Foundation of China.
Keywords: COVID-19, SARS-cov-2, Surgery, Incubation period
Research in context.
Evidence before this study
We searched PubMed and the China National Knowledge Infrastructure database for articles up to March 10, 2020, using the keywords “2019 novel coronavirus”, “2019-nCoV”, “SARS-CoV-2″, “coronavirus”, “Wuhan coronavirus”, “Wuhan seafood market pneumonia virus”, “COVID-19”, “pneumonia”, “Wuhan”, AND “incubation period” “latent”, AND “surgery”, "operation" for articles published without any language restrictions. We found two articles in Chinese: one titled “Preliminary Recommendations for Lung Surgery during 2019 Novel Coronavirus Disease (COVID-19) Epidemic Period” in the Zhongguo Fei Ai Za Zhi, and another titled “Several suggestion of operation for colorectal cancer under the outbreak of Corona Virus Disease 19 in China” in the Zhonghua Wei Chang Wai Ke Za Zhi. We did not find any published studies about the surgical patients with latent COVID-19 infection.
Added value of this study
We described the epidemiological, clinical, and laboratory characteristics, treatment, and clinical outcomes of 34 patients who underwent surgery during the incubation period of COVID-19 infection. This report, to the best of our knowledge, is the first retrospective study to describe the effects of surgery on COVID-19 progression and its prognosis. Our findings also suggest potential risk factors for the poor outcomes of operative patients with COVID-19 infection, including age, comorbidities, and surgical complexity.
Implications of all the available evidence
In this retrospective cohort study of 34 operative patients with confirmed COVID-19, 15 (44·1%) patients needed to receive ICU care, and mortality rate was 20·5%.
Alt-text: Unlabelled box
1. Introduction
In December 2019, an outbreak of the 2019 novel coronavirus disease (COVID-19) caused by the SARS coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China [1,2]. It has spread rapidly to other areas in China and worldwide. [3,4] The most common manifestations of COVID-19 included fever, dry cough, dyspnea, myalgia, fatigue, hypolymphaemia, and radiographic evidence of pneumonia. Complications (eg, acute respiratory distress syndrome [ARDS], arrhythmia, shock, acute cardiac injury, secondary infection, and acute kidney injury) and death may occur in severe cases. [2,[5], [6], [7]] The course of the COVID-19 is long, and COVID-19 is highly contagious even during the incubation period. [8] Furthermore, asymptomatic carrier of SARS-CoV-2, accounting for 1% of the laboratory confirmed cases of SARS-CoV-infection, [9] may potentially transmit the virus during incubation time, [10] which makes the identification and prevention of COVID-19 infection highly challenging. During the early phase of the COVID-19 outbreak, we encountered a small number of asymptomatic patients who underwent elective surgeries during the incubation period of COVID-19 infection, but the clinical manifestations and prognosis of these patients were beyond our expectation. It is our belief that these represent a specific surgical patient population that deserves our attention.
Currently, the data on the clinical characteristics and outcomes of patients with COVID-19 infection undergoing surgeries are rare. A study by Chen and colleagues reported nine pregnant women who had COVID-19 infection and whose babies needed to be delivered via cesarean section surgery. [11] However, this report mainly focused on the general clinical characteristics of pregnant women and whether or not intrauterine vertical transmission may occur. The data on the impact of surgery performed unintentionally during the incubation period of COVID-19 infection on the outcomes of patients are lacking, but are of utmost importance to reduce the morbidity and mortality, given that surgery may cause an immediate impairment of cell-mediated immunity, [12] one of the major mechanisms that bring viral infections under control. [13] In this study, we aim to present the epidemiological, clinical, and laboratory characteristics, treatment, and outcomes of patients undergoing elective surgeries during the incubation period of COVID-19 infection and to compare severe patients who received ICU care during disease progression and those who did not receive ICU care. We hope that our findings of the SARS-CoV-2 associated postoperative morbidity and mortality will benefit the global community in the battle against COVID-19 infection.
2. Methods
2.1. Study design and participants
This is a multicenter, retrospective study, which was done at Renmin Hospital, Zhongnan Hospital, Tongji Hospital and Central Hospital in Wuhan, the most serious area of COVID-19 epidemic in China. We retrospectively reviewed patients who had undergone elective surgeries admitted from January 1 to February 5, 2020, the early stage of COVID-19 epidemic in Wuhan, China. 37 asymptomatic patients developed symptoms after operation and were diagnosed with COVID-19 according to WHO interim guidance. [14] Laboratory confirmation of SAR-CoV-2 was done by quantitative RT-PCR on samples from the respiratory tract, which was performed by the local health authority as described. [2,5] Of these patients, 3 patients were visited shortly after surgery by persons who were thereafter confirmed for COVID-19 infection, which made us difficult to judge whether or not the patients’ infections were due to their exposures prior to surgery. Thus, these 3 patients were excluded, and finally 34 patients were included in this study.
This study was reviewed and approved by the Medical Ethical Committee of participating institutes (approval number WDRY2020-K067). Oral consent was obtained from the patients. The clinical outcomes of these operative patients were monitored up to March 10, 2020, the final date of follow-up, when all the patients were discharged.
2.2. Surgical difficulty category
The patients included in this study underwent various surgical procedures as shown in Table 1, and were categorized into four levels based on the degree of technical difficulty, complexity and risk according to the measures for the hierarchical management of surgical procedures published by the National Heath Commission of China. [15] Briefly, level-1, various operations with low risks, simple procedures and low technical difficulty; level-2, various operations with mild risks, general complexity of procedures and general technical difficulty; level-3, various operations with moderate risks, complex procedures, and moderate technical difficulty; level-4, various operations with high risks, highly complex procedures and high technical difficulty.
Table 1.
Types of surgery and grading of surgical difficulty.
| Surgical procedures | Surgical risk category | Number of case |
|---|---|---|
| Anterior decompression of cervical spinal canal | Level-3 | 1 |
| Artificial femoral head replacement | Level-3 | 2 |
| Clipping of cerebral aneurysm | Level-4 | 1 |
| Cesarean section | Level-2 | 5 |
| Debridement of lower extremity | Level-2 | 1 |
| Debridement of eyeball | Level-2 | 1 |
| Excision of breast mass | Level-1 | 1 |
| Excision of intracranial lesions | Level-3 | 1 |
| Excision of lower extremity muscle lesions | Level-2 | 1 |
| Laparoscopic appendectomy | Level-2 | 2 |
| Laparoscopic partial colectomy | Level-3 | 4 |
| Laparoscopic radical gastrectomy | Level-4 | 1 |
| Pancreatoduodenectomy | Level-3 | 1 |
| Radical operation of ovarian cancer | Level-3 | 1 |
| Radical operation of laryngeal cancer | Level-3 | 1 |
| Radical resection of rectal cancer | Level-3 | 1 |
| Radical resection of esophageal cancer | Level-3 | 2 |
| Radical unilateral mastectomy | Level-3 | 1 |
| Removal of tibial internal fixation plate | Level-2 | 1 |
| Renal transplant | Level-3 | 1 |
| Thoracoscopic lobectomy | Level-3 | 3 |
| Total hip replacement | Level-3 | 1 |
2.3. Data collection
We reviewed clinical records, nursing records, laboratory findings, and chest computed tomographic (CT) scans for all 34 operative patients. The admission date of these patients was from January 1 to February 5, 2020. Epidemiological, clinical, laboratory, and radiological characteristics and treatment and outcomes data were obtained with data collection forms from electronic medical records. Information included demographic, exposure history, underlying comorbidities, chest CT image, surgical type, surgical time, signs and symptoms, time of surgery to first symptoms, time of first symptom to dyspnea, vita signs and laboratory values on hospital admission, COVID-19 onset and ICU admission, treatments, complications, and prognosis. The durations from hospital admission to surgery, first symptom, dyspnea, ARDS, and ICU admission were also recorded. Two research investigators independently reviewed the data collection forms to verify data accuracy. Any missing or uncertain records were collected and clarified by direct communication with patients and their families.
2.4. Definitions
The time of COVID-19 onset was defined as the date when the first sign or symptom was noticed. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition. [16] Acute cardiac injury was identified if the cardiac biomarkers (eg, hypersensitive troponin I, Creatine kinase–MB) were above the 99% upper reference limit or new abnormalities were shown in electrocardiography and echocardiography. [5] Acute kidney injury was defined according to the KDIGO clinical practice guidelines. [17] Patients were admitted and transferred to intensive care unit (ICU) based on the progression of organ dysfunction or the need of mechanical ventilation. [2,18] For patients admitted to ICU, myocardial enzymes, inflammatory stress, and blood gas analysis were determined on the day of ICU admission.
2.5. Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) and compared by using independent group t tests when the data were normally distributed; otherwise, the Mann–Whitney test was used. Categorical variables were expressed as frequencies and percentages and compared by Pearson's chi-square or Fisher's exact test between ICU and non-ICU groups or survival and death groups. A two-sided α of less than 0.05 was considered statistically significant. All statistical analyses were performed with the SPSS (version 25·0) software.
2.6. Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, and the writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
All 34 patients included in this study were residents of Wuhan City. The age range was 21 to 84 years, and median age was 55 years (IQR 43–63) (Table 2). 20 (58·8%) patients were woman, and 15 (44·1%) patients were admitted to ICU because of the progression of organ dysfunction or the need for mechanical ventilation. 20 (58·8%) patients had 1 or more comorbidities. Hypertension (13 [38·2%]), malignancy (9 [26·5%]), diabetes (8 [23·5%]), and cardiovascular disease (7 [20·6%]) were the most common comorbidities.
Table 2.
Baseline characteristics of operative patients with COVID-19 infection.
| No.(%) |
|||||
|---|---|---|---|---|---|
| 95% CI | Total (N = 34) | ICU (n = 15) | Non-ICU (n = 19) | P Value a | |
| Age, median (IQR), y | 46–57 | 55 (43–63) | 55 (44–74) | 47 (29–58) | 0·03 |
| Sex | |||||
| Female | NA | 20 (58·8) | 10 (66·7) | 10 (52·6) | 0·50 |
| Male | NA | 14 (41·2) | 5 (33·3) | 9 (47·4) | 0·50 |
| Any comorbidity | NA | 20 (58·8) | 12 (80·0) | 8 (42·1) | 0·04 |
| Hypertension | NA | 13 (38·2) | 9 (60·0) | 4 (21·1) | 0·03 |
| Malignancy | NA | 9 (26·5) | 5 (33·3) | 4 (21·1) | 0·46 |
| Diabetes | NA | 8 (23·5) | 6 (40·0) | 2 (10·5) | 0·10 |
| Cardiovascular disease | NA | 7 (20·6) | 6 (40·0) | 1 (5·3) | 0·03 |
| Cerebrovascular disease | NA | 2 (5·9) | 2 (13·3) | 0 | 0·19 |
| COPD | NA | 1 (2·9) | 1 (6·7) | 0 | 0·44 |
| Chronic kidney disease | NA | 1 (2·9) | 1 (6·7) | 0 | 0·44 |
| Surgical difficulty category | |||||
| Level-1 | NA | 1 (2·9) | 0 | 1 (5·3) | > 0.99 |
| Level-2 | NA | 11 (32·4) | 1 (6·7) | 10 (52·6) | 0·008 |
| Level-3 | NA | 20 (58·8) | 13 (86·7) | 7 (36·8) | 0·005 |
| Level-4 | NA | 2 (5·9) | 1 (6·7) | 1 (5·3) | > 0·99 |
| Surgical time, median (IQR), minutes | 142–230 | 178 (70–249) | 200 (125–240) | 70 (53–215) | 0·04 |
| Signs and symptoms | |||||
| Fever | NA | 31 (91·2) | 15 (100) | 16 (84·2) | > 0·99 |
| Fatigue | NA | 25 (73·5) | 12 (80·0) | 13 (68·4) | 0·70 |
| Dry cough | NA | 18 (52·9) | 9 (60·0) | 9 (47·4) | 0·51 |
| Dyspnea | NA | 15 (44·1) | 9 (60·0) | 6 (31·6) | 0·16 |
| Myalgia or arthralgia | NA | 11 (32·4) | 6 (40·0) | 5 (26·3) | 0·47 |
| Expectoration | NA | 11 (32·4) | 7 (46·7) | 4 (21·1) | 0·15 |
| Dizziness or Headache | NA | 8 (23·5) | 5 (33·3) | 3 (15·8) | 0·41 |
| Pharyngalgia | NA | 7 (20·6) | 3 (20·0) | 4 (21·1) | > 0·99 |
| Anorexia | NA | 5 (14·7) | 3 (20·0) | 2 (10·5) | 0·63 |
| nausea | na | 3 (8·8) | 1 (6·7) | 2 (10·5) | > 0·99 |
| diarrhea | na | 2 (5·9) | 1 (6·7) | 1 (5·3) | > 0·99 |
| Abdominal pain | NA | 1 (2·9) | 1 (6·7) | 0 | 0·44 |
| Time of surgery to first symptom, median (IQR), days | 2.0–3.5 | 2·0 (1·0–4·0) | 2·5 (1·0–5·0) | 2·0 (1·0–3·3) | 0·61 |
| First symptom to dyspnea, median (IQR), days | 2.6–5.0 | 3·5 (2·0–5·3) | 2·0 (1·0–4·0) | 5·0 (2·0–6·5) | 0·02 |
| Bilateral distribution of patchy shadows or ground glass opacity, No. (%) | NA | 34 (100) | 15 (100) | 19 (100) | > 0·99 |
Abbreviations: ARDS, acute respiratory distress syndrome; COVID-19: 2019 novel coronavirus disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; CI, confidence interval; NA, not applicable.
P < 0·05 was considered statistically significant.
P values indicate differences between non-ICU and ICU patients.
In the present study, most patients underwent surgeries with the surgical difficulty category at level-2 (11 [32·4%]) and level-3 (20 [58·8%]), only 2 (5·9%) patients underwent surgeries with the surgical difficulty category at level-4, the highest surgical difficulty category (Table 2). 13 of 15 patients admitted to ICU underwent level-3 surgeries. By contrast, the surgical difficulty category was level-2 for the majority of non-ICU patients. The overall median surgical time was 178 min (IQR, 70–249). The patients in ICU had longer surgical time (median time, 200 min [IQR, 125–240] vs 70 min [IQR, 53–215]; P = 0·04) and shorter time from surgery to first symptom (median time, 2·0 days [IQR, 1·0–4·0] vs 5·0 days [IQR, 2·0–6·5]; P = 0.02) than that of non-ICU patients.
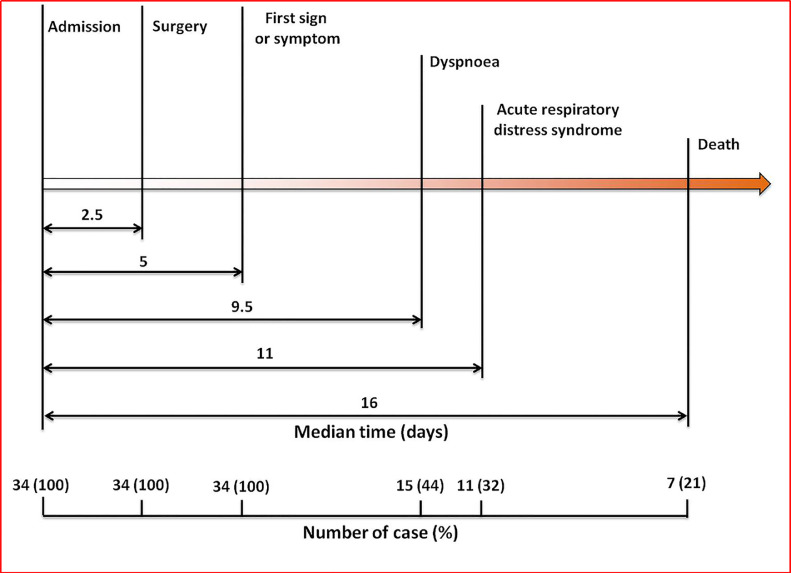
The most common symptoms at COVID-19 onset were fever (31 [91·2%]), fatigue (25 [73·5%]), dry cough (18 [52·9%]), dyspnea (15 [44·1%]), myalgia or arthralgia (11 [32·4%]), and expectoration (11 [32·4%]). Less common symptoms were dizziness, headache, pharyngalgia, nausea, diarrhea, and abdominal pain. The median time from surgery to first symptom was 2·0 days (IQR, 1·0–4·0) and 3.0 days (IQR, 2.0–4.5) to diagnosis of pneumonia. The median time from first symptoms to dyspnea was 3·5 days (IQR, 2·0–5·3) (Table 2). The median duration from hospital admission to surgery was 2·5 days (IQR, 1·0–4·0), to first sign or symptoms was 5·0 days (IQR, 3·3–8·8), to dyspnea was 9·5 days (IQR 6·0–14·5), to ARDS was 10·0 days (IQR, 8·0–15·0). For the non-survival patients, the median time from hospital admission to death was 16·0 days (IQR, 11·0–25·0) (Fig. 1).
Fig. 1.
Timeline of the operative patients with COVID-19 infection after hospital admission.
Compared with patients who did not receive ICU care, patients who required ICU care were significantly older (median age, 55 years [IQR, 44–74] vs 47 years [IQR, 29–58]; P = 0·03), and were more likely to have underlying comorbidities (12 [80·0%] vs 8 [42·1%; P = 0.04), including hypertension (9 [60·0%] vs 4 [21·1%]; P = 0·03) and cardiovascular disease (6 [40·0%] vs 1 [5·3%]); P = 0·03) (Table 2).
There were numerous differences in laboratory findings between ICU and non-ICU patients (Table 3), including higher white blood cell (median count, 8·5 [IQR, 4·7–13·1] × 109/L vs 5·7 [IQR, 4·6–8·1] × 109/L; P = 0·049) and neutrophil counts (median count, 7·9 [IQR, 4·1–10·7] × 109/L vs 4·1 [IQR, 3·1–5·8] × 109/L; P = 0·02), as well as higher levels of total bilirubin (median concentration, 13·0 μmol/L [IQR, 9·1–19·2] vs 8·1μmol/L [IQR, 6·5–12·9]; P = 0·04), blood urea nitrogen (median concentration, 5·8 mmol/L [IQR, 4·9–10·3] vs 4·0 mmol/L [IQR, 3·3–4·2]; P = 0·03) and creatinine (median concentration, 63·0 μmol/L [IQR, 60·0–76·0] vs 46·8 μmol/L [IQR, 42·0–57·0]; P = 0·049). The number of patients with increased concentration of procalcitonin (≥ 0·1 ng/mL) in ICU was more than non-ICU patients (12 [80·0%] vs 7 [36·8%]; P = 0·02). These measures were recorded on the day of COVID-19 onset for all patients. All the 34 patients demonstrated bilateral distribution of patchy shadows or ground glass opacity on chest CT scan (Table 3).
Table 3.
Laboratory findings of postoperative patients on COVID-19 onset.
| Median (IQR) |
|||||
|---|---|---|---|---|---|
| 95% CI | Total (N = 34) | ICU (n = 15) | Non-ICU (n = 19) | P Valuea | |
| White blood cell count, × 109/L | 6.5–9.5 | 7·4 (4·7–10·0) | 8·5 (4·7–13·1) | 5·7 (4·6–8·1) | 0·049 |
| Neutrophil count, × 109/L | 4.8–7.8 | 5·1 (3·3–8·3) | 7·9 (4·1–10·7) | 4·1 (3·1–5·8) | 0·02 |
| Lymphocyte count, × 109/L | 0.6–1.2 | 0·7 (0·6–0·9) | 0·7 (0·5–0·9) | 0·7 (0·6–1·2) | 0·98 |
| <1.1, No. (%) | NA | 29 (85.3) | 14 (93.3) | 15 (78.9) | 0·35 |
| Monocyte count, × 109/L | −2.8–10.9 | 0·5 (0·4–0·8) | 0·6 (0·3–1·1) | 0·5 (0·4–0·7) | 0·32 |
| Platelet count, × 109/L | 162.7–226.6 | 190 (128–238) | 204 (123–237) | 187 (140–239) | 0·81 |
| Prothrombin time, s | 11.2–12.2 | 11·5 (10·6–12·4) | 11·4 (10·6–12·7) | 11.5 (10·6–12·3) | 0·62 |
| Activated partial thromboplastin time, s | 27.3–32.4 | 27·9 (25·8–31·9) | 28·0 (26·3–30·6) | 27·7 (25·4–32·2) | 0·75 |
| D-dimer, mg/L | −16.0–59.9 | 1·8 (0·6–2·8) | 1·9 (1·2–3·1) | 1·5 (0·4–2·9) | > 0·99 |
| Alanine aminotransferase, U/L | 22.2–37.2 | 21·5 (13·0–46·3) | 14·6 (13·0–41·9) | 23·0 (13·0–51·0) | 0·54 |
| Aspartate aminotransferase, U/L | 27.5–41.4 | 26·0 (20·3–55·7) | 23·0 (20·0–54·9) | 30·0 (20·4–61·0) | 0·49 |
| Total bilirubin, μmol/L | 1.9–43.0 | 9·9 (7·3–16·8) | 13·0 (9·1–19·2) | 8·1 (6·5–12·9) | 0·04 |
| Blood urea nitrogen, mmol/L | 4.5–10.0 | 4·5 (3·8–7·5) | 5·8 (4·9–10·3) | 4·0 (3·3–4·2) | 0·03 |
| Creatinine, μmol/L | 46.5–107.1 | 57·4 (46·6–64·0) | 63·0 (60·0–76·0) | 46·8 (42·0–57·0) | 0·049 |
| Lactate dehydrogenase, U/L | 194–221 | 209 (191–230) | 218 (188–230) | 207 (192–231) | 0·51 |
| Creatine kinase, U/L | 49–100 | 61(43–94) | 70 (47–163) | 61 (31–89) | 0·35 |
| C-reactive protein, mg/L | 29–69 | 30·3 (8·4–74·3) | 29·6 (12·5–86·6) | 24·8 (7·7–72·3) | 0·55 |
| Hypersensitive C-reactive protein, mg/L | 17.3–61.7 | 14·6 (5·3–47·8) | 16·0 (5·3–75·0) | 9·2 (4·7–21·3) | 0·72 |
| Procalcitonin, ng/mL, ≥ 0.1, No. (%) | NA | 19 (55·9) | 12 (80·0) | 7 (36·8) | 0·02 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; bpm, beats per minute; MAP, mean arterial pressure; CI, confidence interval; NA, not applicable.
P < 0·05 was considered statistically significant.
P values indicate differences between non-ICU and ICU patients.
All patients had developed pneumonia after surgery. Common complications among the 34 patients included ARDS (11 [32·4%]), shock (10 [29·4%]), secondary infection (10 [29·4%]), arrhythmia (8 [23·5%]), acute cardiac injury (5 [14·7%]), and acute kidney injury (2 [5·9%]; Table 3). ICU patients were more likely to have ARDS, shock, second infection and acute cardiac injury than non-ICU patients. All patients received antiviral therapy (lopinavir/ritonavir) and antibiotic therapy. Part of the patients received glucocorticoid therapy (16 [47·1%]) and immunoglobulin therapy (14 [41·2%]), and 1 (2·9%) patient received kidney replacement therapy. In ICU, 7 (46·7%) patients received high-flow oxygen or noninvasive ventilation, and 5 (33·3%) required invasive mechanical ventilation, 1 (6·7%) of whom received extracorporeal membrane oxygenation as rescue therapy (Table 4).
Table 4.
Treatments and outcomes of postoperative patients infected with COVID-19.
| No.(%) |
||||
|---|---|---|---|---|
| Total (N = 34) | ICU (n = 15) | Non-ICU (n = 19) | P Valuea | |
| Complications | ||||
| ARDS | 11 (32·4) | 9 (60·0) | 2 (10·5) | 0·003 |
| Shock | 10 (29·4) | 8 (53·3) | 2 (10·5) | 0·01 |
| Secondary infection | 10 (29·4) | 7 (46·7) | 2 (10·5) | 0·03 |
| Arrhythmia | 8 (23·5) | 5 (33·3) | 3 (15·8) | 0·42 |
| Acute cardiac injury | 5 (14·7) | 5(33·3) | 0 | 0·01 |
| Acute kidney injury | 2 (5·9) | 2 (13·3) | 0 | 0·19 |
| Treatment | ||||
| Antiviral therapy | 34 (100) | 15 (100) | 19 (100) | > 0·09 |
| Antibiotic therapy | 34 (100) | 15 (100) | 19 (100) | > 0·09 |
| Glucocorticoid therapy | 16 (47·1) | 9 (60·0) | 7 (36·8) | 0·30 |
| Immunoglobulin | 14 (41·2) | 8 (53·3) | 6 (31·6) | 0·30 |
| CKRT | 1 (2·9) | 1 (6·7) | 0 | 0·44 |
| Oxygen support | ||||
| Nasal cannula | 19 (55·9) | 3 (20·0) | 16 (84·2) | 0·003 |
| Noninvasive ventilation or | ||||
| high-flow nasal cannula | 10 (29·4) | 7 (46·7) | 3 (15·8) | 0·07 |
| Invasive mechanical ventilation | 5(14·7) | 5 (33·3) | 0 | 0·01 |
| ECOMA | 1 (2·9) | 1 (6·7) | 0 | 0·44 |
| Prognosis | ||||
| Discharge | 27 (79·4) | 8 (53·3) | 19 (100) | 0·001 |
| Death | 7 (20·6) | 7 (46·7) | 0 | 0·001 |
Abbreviations: ARDS, acute respiratory distress syndrome; CKRT, continuous kidney replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
P < 0·05 was considered statistically significant.
P values indicate differences between non-ICU and ICU patients.
Seven patients died after admission to the ICU, they all underwent surgeries at the surgical difficulty category level-3. The age range was 34 to 83 years old, and 4 were women (Table 5). The surgery duration ranged from 110 to 379 min. All these patients had 1 or more coexisting medical conditions. The most common comorbidities were cardiovascular disease (4 [57·1%]), malignancy (4 [57·1%]) and hypertension (3 [42·9%]). 4 of these 7 patients presented with fever as first symptom. The median duration from first symptom to death was 9 days (IQR, 6–11). All these patients developed respiratory failure and had three or more complications. The most common complications among the 7 patients included ARDS (7 [100%]), shock (4 [57·1%]), arrhythmia (4 [57·1%]) and acute cardiac injury (4 [57·1%]).
Table 5.
Clinical characteristics of seven non-survival operative patients with COVID-19 infection.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | No. (%) | |
|---|---|---|---|---|---|---|---|---|
| Date of admission | Jan 12 | Jan 13 | Jan 14 | Jan 15 | Jan 15 | Jan 20 | Jan 27 | NA |
| Age, years | 34 | 55 | 63 | 48 | 55 | 83 | 77 | NA |
| Sex, Female/male | Female | Male | Male | Female | Female | Male | Female | NA |
| Epidemiological history* | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 (100) |
| Comorbidities | ||||||||
| Cardiovascular disease | No | Yes | No | No | Yes | Yes | Yes | 4 (57·1) |
| Malignancy | Yes | Yes | Yes | Yes | No | No | No | 4 (57·1) |
| Hypertension | No | Yes | No | No | No | Yes | Yes | 3 (42·9) |
| Diabetes | No | No | No | Yes | No | No | No | 1 (14·3) |
| Cerebrovascular disease | No | No | No | No | No | Yes | No | 1 (14·3) |
| COPD | No | Yes | No | No | No | No | No | 1 (14·3) |
| Date of surgery | Jan 16 | Jan 23 | Jan 17 | Jan 19 | Jan 17 | Jan 22 | Jan 30 | NA |
| Surgical type | Pancreatoduo-denectomy | Total esophagectomy | Thoracoscopic lobectomy | Radical resection of rectal cancer | Thoracoscopic lobectomy | Artificial femoral head replacement | Total hip replacement | NA |
| Surgical time, min | 377 | 240 | 379 | 200 | 195 | 150 | 110 | NA |
| Date of first symptom or sign | Jan 19 | Jan 28 | Jan 18 | Jan 20 | Jan 19 | Jan 25 | Feb 2 | NA |
| First symptom or sign | Fever | Fatigue | Cough | Fever | Fever | Fever | Cough | NA |
| Date of Confirmatory test done (SARS-CoV-2 quantitative RT-PCR) | Jan 25 | Feb 4 | Jan 22 | Jan 26 | Jan 21 | Jan 29 | Feb 5 | NA |
| Complications | ||||||||
| Respiratory failure | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 (100) |
| ARDS | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 (100) |
| Shock | Yes | Yes | Yes | Yes | No | No | No | 4 (57·1) |
| Arrhythmia | No | Yes | No | Yes | Yes | Yes | No | 4 (57·1) |
| Acute cardiac injury | No | Yes | Yes | Yes | No | No | Yes | 4 (57·1) |
| Secondary infection | Yes | Yes | No | No | No | Yes | No | 3 (42·9) |
| Acute kidney injury | Yes | Yes | No | No | No | No | No | 2 (28·6) |
| Date of death | Jan 30 | Feb 6 | Jan 29 | Jan 27 | Jan 25 | Feb 7 | Feb 6 | NA |
| First symptom to death, days | 11 | 9 | 11 | 7 | 6 | 13 | 4 | NA |
Abbreviations: ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; NA, not applicable. *Exposure to Wuhan city where the epidemic is very serious in China.
4. Discussion
This report, to the best of our knowledge, is the first retrospective cohort study to describe the clinical characteristics and outcomes of operative patients with SARS-CoV-2 infection. All the 34 patients involved in this study had a history of direct exposure to Wuhan City before hospital admission, and none of them had any sign or symptom of COVID-19 before surgery. Of note, symptoms of COVID-19 manifested quickly after the completion of surgery, and SARS-CoV-2 infection was laboratory-confirmed soon thereafter. The length of time from hospital admission to surgery (median time, 2·5 days [IQR, 1·0–4·0]) is shorter than the median incubation time of 5·2 days obtained from a study of patients with confirmed SARS-CoV-2 infections in Wuhan, [19] and also shorter than the overall incubation time (median time, 4·0 days [IQR, 2·0–7·0]) derived from a study of patients with COVID-19 from 552 hospitals in China. [6] These evidences collectively support our belief that the patients included in the current study are in their incubation period of COVID-19 infection before undergoing surgeries.
During the disease progression, 15 of 34 postoperative patients received ICU care. This proportion (44·1%) is much higher than the reported 26·1% in hospitalized COVID-19 patients without surgery. [2] Most patients in ICU were older, had more underlying comorbidities and longer surgical time, and undergone more difficult surgery than patients not admitted to ICU. This suggests that old age, comorbidities, surgical time, and difficulty of operation may be risk factors for poor outcome. Compared with the symptoms in non-ICU patients, dyspnea occurred earlier in critically ill patients. The time of first symptom to dyspnea in ICU patients was much shorter than non-ICU patients. This symptom characteristic may help physicians identify patients with potential poor prognosis.
The data in this study suggest that surgery may accelerate and exacerbate disease progression of COVID-19. This is derived from the following findings. The patients developed COVID-19 symptoms very shortly (average 2·6 days) after surgery completion. The median time of COVID-19 onset (which was defined as the date when the first sign or symptom was noticed) to dyspnea in the current study was 3·5 days (IQR, 2·0–5·3), which is shorter than the reported time of 8·0 days (IQR, 5·0–13·0) from the onset of first symptom to dyspnea in a study of 41 laboratory-confirmed cases infected with SARS-CoV-2, [5] and also shorter than the reported 5·0 days (IQR, 1·0–10·0) in another study of 138 hospitalized patients with confirmed SARS-CoV-2 in Wuhan. [2] Seven of the 34 operative patients (20·6%) died of COVID-19 associated complications. This mortality rate is much higher than the reported overall case-fatality rate of 2·3% in COVID-19 patients without surgery, [9] and also higher than the case-fatality rate of 7·9% in noncardiac surgical patients without COVID-19 infection who were admitted to multidisciplinary ICU. [20] Furthermore, the average duration from the time of first symptom to death of the 7 non-survivors (8·7 days, range 4 to 13 days) is apparently shorter than that reported previously. In the report of Huang and colleagues, [5] the median time from onset of symptoms to first hospital admission was 7.0 days (4.0–8.0) and to ICU admission was 10.5 days (8.0–17.0) and 6 patients (15% of the total of 41 cases studied) did not survive ICU. The study did not report the exact dates of patient death, but it is safe for us to say that the average time from the onset of symptoms to death was longer than 10.5 days and thus longer than that of the non-survivors in our study. In other words, it is highly likely that surgical stress occurred during the incubation period of SARS-CoV-2 infection exacerbated disease progression and severity.
Currently, no specialized medication is available for the treatment of SARS-CoV-2 infection, and supportive measures remain the mainstay of COVID-19 treatment. Thus, the patient's immune function is a major determinant of the disease severity, and populations with low immune function, such as older people are more vulnerable and have high mortality after COVID-19 infection. [7] Surgery may not only cause immediate impairment immune function, [12] but also induce early systemic inflammatory response. [21] Similar to the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection, [22] the SARS-CoV infected lung could induce and increase the amount of macrophage and neutrophil infiltration, and increase the levels of pro-inflammatory cytokines and chemokines. [23,24] And, the high levels of circulating inflammatory cytokines has been reported to be correlated with the severity of illness in patients infected with the 2019 novel coronavirus (SARS-CoV-2). [5] Consistent with these findings, the most common laboratory abnormalities in this study were lymphopenia (29 [85·3%] of 34 patients) and increased hypersensitive C-reactive protein. In addition, the patients admitted to ICU showed higher counts of white blood cell and neutrophil.
Our study has some similarity to a case reported during MERS-CoV outbreak. [25] In the report, a 61-year-old man who was electively admitted for cardiac bypass surgery and the patient's laboratory examinations were normal despite the history of hypertension and diabetes, and MERS-CoV test was negative before the surgery. However, this patient developed MERS-CoV infection-related symptom in the 2nd day after surgery, and was tested positive for MERS-CoV on post-operative day 6, and the patient passed away on post-operative day 9. Possibility exists that this patient could be a healthy carrier of MERS-CoV who then had the virus activated by the stress of surgery. For the patients included in our current study, we performed routine laboratory tests but did not specifically test for SARS-CoV-2 before surgery by use of quantitative RT-PCR with the CDC (Chinese Center for Disease Control and Prevention) recommended kit based on the considerations that these patients had no any sign of COVID-19 related symptoms and the CDC recommended kit was very limited and could only be used for highly suspected case of SARS-CoV-2 infection at that time. However, a thorough review of the patients' profile made us confident to believe that the 34 patients included in this study got infected before their hospital admission. We now retrospectively understand that it might be necessary and very important for a patient to be isolated for a certain period (such as the WHO recommended 14-days quarantine period) or the possibility of the new coronavirus infection being excluded before being considered for an elective surgery during the COVID-19 epidemic.
Our study has several notable limitations. The sample size of the present study was small, as it was a rare situation and patients were unintentionally scheduled for elective surgeries during the incubation period of COVID-19 infection. Additionally, the patients were not performed the specific SARS-CoV-2 confirmation test before surgery, due to the limited understanding of the epidemic situation and the shortage of SARS-CoV-2 kits at that time. Thus, our assumption of the intubation periods of the patients included the current study was mainly based on clinical profiles and routine laboratory tests. Nevertheless, it is our hope that the findings of the SARS-CoV-2 associated postoperative morbidity and mortality will alert the global community to be better prepared in the battle against COVID-19 infection.
In summary, we described the clinical characteristics and outcomes of the patients who were unintentionally scheduled for elective surgeries during the incubation period of COVID-19 infection. This retrospective cohort study showed 44·1% patients needed ICU care, and mortality was 20·5%. Risk factors for the poor prognosis of operative patients with COVID-19 need to be further study in larger sample size.
5. Contributors
Z-YX and SL had the idea for the study. Z-YX and ZX designed the study and have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SL, WS, CC, JC, WM, l-YZ and YJ collected the data. SL, FJ, DL and LZ performed data analysis. SL, FJ and WS drafted the manuscript. ZX and Z-YX revised the final manuscript.
Conflict of Interest
None.
Acknowledgements
The authors' work was supported by the grants from National Natural Science Foundation of China (NSFC 81772049, 81970247). We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Wuhan. We thank the patients and their families for providing requested data and information.
Contributor Information
Zhong-Yuan Xia, Email: xiazhongyuan2005@aliyun.com.
Zhengyuan Xia, Email: zyxia@hkucc.hku.hk.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in china. N Engl J Med. 2019;382(8) doi: 10.1056/NEJMoa2001017. 2020727-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein W.K., Stroud L., Cleghorn G.E., Leis J.A. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020 doi: 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu P., Zhu J., Zhang Z., Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amodeo G., Bugada D., Franchi S. Immune function after major surgical interventions: the effect of postoperative pain treatment. J Pain Res. 2018;11:1297–1305. doi: 10.2147/JPR.S158230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermesh T., Moltedo B., Lopez C.B., Moran T.M. Buying time-the immune system determinants of the incubation period to respiratory viruses. Viruses. 2010;2(11) doi: 10.3390/v2112541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. WHO. 2020;28 Jan(accessed Feb 23, 2020) [Google Scholar]
- 15.Measures for the hierarchical management of surgical procedures in medical institutions. China NHCo. 2012;3 Aug(accessed Feb 20, 2020) [Google Scholar]
- 16.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23) doi: 10.1001/jama.2012.5669. 2526-33. [DOI] [PubMed] [Google Scholar]
- 17.Kellum J.A., Lameire N., Group K.A.G.W. Diagnosis, evaluation, and management of acute kidney injury: a Kdigo summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler R.A., Lapinsky S.E., Hallett D. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 19.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P., Renuka M.K., Kalaiselvan M.S., Arunkumar A.S. Outcome of noncardiac surgical patients admitted to a multidisciplinary intensive care unit. Indian J Crit Care Med. 2017;21(1):17–22. doi: 10.4103/0972-5229.198321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni Choileain N., Redmond H.P. Cell response to surgery. Arch Surg. 2006;141(11) doi: 10.1001/archsurg.141.11.1132. 1132-40. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Chu H., Li C. Active replication of middle east respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis. 2014;209(9) doi: 10.1093/infdis/jit504. 1331-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien J.Y., Hsueh P.R., Cheng W.C., Yu C.J., Yang P.C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11(6) doi: 10.1111/j.1440-1843.2006.00942.x. 715-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law H.K., Cheung C.Y., Ng H.Y. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7) doi: 10.1182/blood-2004-10-4166. 2366-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seddiq N., Al-Qahtani M., Al-Tawfiq J.A., Bukamal N. First confirmed case of middle east respiratory syndrome coronavirus infection in the Kingdom of Bahrain: in a Saudi gentleman after cardiac bypass surgery. Case Rep Infect Dis. 2017 doi: 10.1155/2017/1262838. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]