Abstract
Severe acute respiratory syndrome (SARS) is caused by a novel coronavirus termed SARS-CoV. No antiviral treatment has been established so far. Interferons are cytokines which induce the synthesis of several antivirally active proteins in the cell. In this study, we demonstrated that multiplication of SARS-CoV in cell culture can be strongly inhibited by pretreatment with interferon-beta. Interferon-alpha and interferon-gamma, by contrast, were less effective. The human MxA protein is one of the most prominent proteins induced by interferon-beta. Nevertheless, no interference with SARS-CoV replication was observed in Vero cells stably expressing MxA. Therefore, other interferon-induced proteins must be responsible for the strong inhibitory effect of interferon-beta against SARS-CoV.
Abbreviations: IFN, interferon; PFU, plaque forming units; PKR, protein kinase R; SARS, severe acute respiratory syndrome; SARS-CoV, SARS-associated coronavirus
Keywords: SARS-Coronavirus, Interferon, MxA protein, Virus inhibition
1. Introduction
Severe acute respiratory syndrome (SARS) is an infectious disease which has recently emerged in China and rapidly spread to other countries (Chan et al., 2003). To date, 8098 cases with 774 deaths are reported (WHO, 2003). Intensive research has led to the identification of a positive-stranded RNA virus, termed SARS-coronavirus (SARS-CoV), as the etiologic agent (Drosten et al., 2003, Fouchier et al., 2003, Ksiazek et al., 2003, Kuiken et al., 2003, Peiris et al., 2003).
Virus infection of mammalian cells prompts the innate immune system to establish a first line of defense. Interferons (IFNs) play a key role in these events, since they activate the innate immune system and help to shape adaptive immunity (Stark et al., 1998). Two types of IFNs are involved in establishing an antiviral state. Type I IFN (IFN-α/β) is synthesized by most cell types as a direct response to virus infection, whereas type II IFN (IFN-γ) is produced by immune cells after contact with antigen-presenting cells. Both type I and type II IFNs induce the synthesis of distinct but partially overlapping sets of mRNAs which encode proteins with antiviral, antiproliferative, and immunomodulatory properties (de Veer et al., 2001). Among the type I IFN-induced proteins, the main antiviral factors are the Mx proteins (Haller and Kochs, 2002), the 2′-5′-oligoadenylate synthetase (2′-5′-OAS)/RNaseL system (Silverman, 1994), and the protein kinase R (PKR; (Williams, 1999). Mx proteins are large GTPases that inhibit the multiplication of several RNA viruses, including representative members of the Bunyaviridae, Paramyxoviridae, Rhabdoviridae, Orthomyxoviridae, and Togaviridae (Haller and Kochs, 2002). Mx proteins inhibit virus replication at early stages of infection by affecting transcription and/or replication of the viral genome (Haller and Kochs, 2002).
In this study, we investigated the potential of different IFNs to inhibit replication of SARS-CoV in cell culture and determined whether the human MxA protein contributes to the antiviral effect of type I IFNs.
2. Methods
2.1. Cells and viruses
African green monkey kidney (Vero) cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. Vero cell clones VA3, VA9, and VA12 which are permanently transfected with MxA expression plasmids (Frese et al., 1995) were grown in the same medium plus 2 mg of G418 per ml. The FFM-1 isolate of SARS-CoV was kindly provided by Stephan Becker, University of Marburg, Germany.
2.2. Interferons
Human IFN-α A/D (BglII), pegylated IFN-α, IFN-β, and IFN-γ were purchased from PBL Biomedical Laboratories, Griffith Micro Science, Schering and Sigma, respectively.
2.3. Plaque assays
Virus plaque assays were performed as described previously (Frese et al., 1995). Briefly, Vero cell monolayers were infected with dilutions of supernatants from infected cells, overlaid with soft agar, and allow to form plaques for 72 h. Then the agar overlay was removed and cells were stained with a solution of 1% crystal violet, 3,6% formaldehyde, 1% methanol, and 20% ethanol.
3. Results and discussion
3.1. Inhibitory effects of interferons
We investigated the inhibitory effect of type I and II IFNs on SARS-CoV multiplication in cell culture. We used universal IFN-α A/D (BglII), pegylated human IFN-α, human IFN-β, and human IFN-γ. These cytokines are known to inhibit the replication of several pathogenic viruses in cell culture or in patients (Samuel, 2001). For all following experiments, Vero cells were chosen because they are unable to synthesize IFN but are fully responsive to IFN treatment (Diaz et al., 1988). Therefore, any additional effects of virus-induced IFN that would bias the results can be excluded. Cells were pretreated with the IFNs, infected with SARS-CoV, and virus in the supernatant was determined after overnight incubation. Fig. 1 shows that administration of IFN-α and IFN-γ led to a 10-fold inhibition of virus growth. IFN-β was even more potent since it resulted in a 1000-fold reduction of virus titers. We also tested tumor necrosis factor α, but found no inhibitory effect (data not shown). These data are in agreement with a recent report by Cinatl et al. (2003) and demonstrate that IFN-β is the most effective antiviral cytokine against SARS-CoV.
Fig. 1.

Effect of IFNs on growth of SARS-CoV. VeroCH cells were pretreated with 1000 units each of the IFNs as indicated and infected with 0.1 PFU of SARS-CoV per cell. At 24 h post-infection, virus in the supernatant was determined by plaque assay. Mean values and standard deviations of three independent experiments are shown.
3.2. Virus replication in MxA-expressing cells
Mx is one of the main IFN-α/β-induced proteins which are antivirally active. Previous gene array analyses have revealed that the mRNA for the human MxA protein is induced 31-fold by IFN-β, 21-fold by IFN-α, and not at all by IFN-γ (Der et al., 1998). Due to the similarity of the antiviral effect of the different IFNs (see Fig. 1) and the pattern of MxA expression, it was suspected that MxA might be the responsible factor (Cinatl et al., 2003). Therefore, we tested growth of SARS-CoV in Vero cell lines which stably express MxA (Frese et al., 1995). These cells are widely used to demonstrate the antiviral effect of MxA against a range of RNA viruses (Haller and Kochs, 2002). The Vero cells expressing MxA (VA3, VA9, VA12) and control cells (VN36, Vero) were infected with the SARS-CoV isolate FFM-1, and viral titers in the supernatants were determined at 48 h post-infection. As depicted in Fig. 2A , no apparent differences in virus growth were detected. In all cases titers of approximately 108 infectious particles per ml were reached. Likewise, when MxA-expressing VA9 and control Vero cells were directly used in a plaque assay, no differences in the efficiency of plaque formation or size were observed (Fig. 2B). These results suggest that MxA is not the critical factor that mediates inhibition of SARS-CoV, despite its high-level induction by IFN-β. Thus, the search for antiviral effects against SARS-CoV should focus on other IFN-induced proteins such as PKR or RNase L.
Fig. 2.
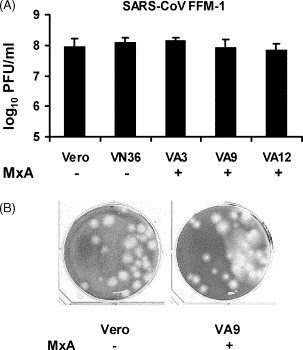
Growth of SARS-CoV in MxA-expressing cells. (A) Viral titers. Vero cell lines expressing MxA (VA3, VA9, VA12) and control cells (Vero, VN36) were infected with SARS-CoV at a multiplicity of infection of 1, and viral titers in the supernatants were determined at 48 h post-infection. Mean values and standard deviations of three independent experiments are shown. The same batches of MxA-expressing cells potently suppress growth of Thogoto-orthomyxovirus, as published previously (Frese et al., 1995). (B) Plaque formation. Monolayers of 106 Vero cells (left) and MxA-expressing VA9 cells (right) were infected with 20 PFU of SARS-CoV and overlaid with soft agar.
Acknowledgements
We thank Stephan Becker for the generous gift of SARS-CoV isolate FFM-1. Work in the authors’ laboratories is supported by grants from the Deutsche Forschungsgemeinschaft.
References
- Chan H.L, Tsui S.K, Sung J.J. Coronavirus in severe acute respiratory syndrome (SARS) Trends Mol. Med. 2003;9:323–325. doi: 10.1016/S1471-4914(03)00135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veer M.J, Holko M, Frevel M, Walker E, Der S, Paranjape J.M, Silverman R.H, Williams B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- Der S.D, Zhou A, Williams B.R, Silverman R.H. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M.O, Ziemin S, Le Beau M.M, Pitha P, Smith S.D, Chilcote R.R, Rowley J.D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt H.R, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier R.A, Berger A, Burguiere A.M, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra J.C, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk H.D, Osterhaus A.D, Schmitz H, Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A, Kuiken T, Schutten M, van Amerongen G, van Doornum G.J, van den Hoogen B.G, Peiris M, Lim W, Stohr K, Osterhaus A.D. Aetiology: Koch’s postulates fulfilled for SARS virus. Nature. 2003;423:240. doi: 10.1038/423240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus. J. Virol. 1995;69:3904–3909. doi: 10.1128/jvi.69.6.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Kochs G. Interferon-induced Mx proteins: dynamin-like GTPases with antiviral activity. Traffic. 2002;3:710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G, Erdman D, Goldsmith C.S, Zaki S.R, Peret T, Emery S, Tong S, Urbani C, Comer J.A, Lim W, Rollin P.E, Dowell S.F, Ling A.E, Humphrey C.D, Shieh W.J, Guarner J, Paddock C.D, Rota P, Fields B, DeRisi J, Yang J.Y, Cox N, Hughes J.M, LeDuc J.W, Bellini W.J, Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Fouchier R.A, Schutten M, Rimmelzwaan G.F, van Amerongen G, van Riel D, Laman J.D, de Jong T, van Doornum G, Lim W, Ling A.E, Chan P.K, Tam J.S, Zambon M.C, Gopal R, Drosten C, van der Werf S, Escriou N, Manuguerra J.C, Stohr K, Peiris J.S, Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S, Lai S.T, Poon L.L, Guan Y, Yam L.Y, Lim W, Nicholls J, Yee W.K, Yan W.W, Cheung M.T, Cheng V.C, Chan K.H, Tsang D.N, Yung R.W, Ng T.K, Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;4:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R.H. Fascination with 2-5A-dependent RNase: a unique enzyme that functions in interferon action. J. Interferon Res. 1994;14:101–104. doi: 10.1089/jir.1994.14.101. [DOI] [PubMed] [Google Scholar]
- Stark G.R, Kerr I.M, Williams B.R, Silverman R.H, Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- WHO, 2003. Summary table of SARS cases by country, 1 November 2002–26 September 2003. http://www.who.int/csr/sars/country/table2003_09_23/en/.
- Williams B.R. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]


