Highlights
-
•
We report a respiratory infection outbreak caused by HAdV-B55 in physical training facility.
-
•
Severe lower respiratory infection, such as pneumonia, young cases were observed in this outbreak.
-
•
We used whole genome sequencing to confirm the type of HAdV-B55.
Keywords: Human adenovirus-B55, Respiratory infection outbreak, Whole genome sequence, Phylogenetic analysis
Summary
Objectives
To investigate the cause of an acute respiratory tract infection (ARTI) outbreak.
Methods
Thirty-eight clinical samples were collected from 19 patients in an ARTI outbreak that occurred in a physical training facility in January 2013; patient demographic information was also collected. In addition, 60 influenza virus-negative samples from febrile respiratory patients were collected from the same community at the same time to determine whether these were the same infections. Multiplex PCR (multi-PCR) was used to detect the possible pathogen in these samples. All human adenovirus (HAdV)-positive samples were inoculated onto Hep-2 cells for isolation. HAdV isolates were typed by hexon gene, fiber gene, and whole genome sequencing using primers designed in-house and compared to different type/serotype HAdVs downloaded from GenBank. Phylogenetic analysis was used to determine the type of the HAdV.
Results
Of the 38 samples, 34 from 17 cases were HAdV-positive; two of them were co-infected, one with respiratory syncytial virus A and the other with human rhinovirus. The hexon gene open reading frame (ORF; 2841 nucleotides (nt)) and fiber gene ORF (978 nt) were obtained from four HAdV strains (TJ-2013-92, TJ-2013-94, TJ-2013-100, TJ-2013-122) from three upper respiratory infection cases and one pneumonia case. They were all completely identical. One HAdV isolate, TJ-2013-90, was selected for whole genome sequencing; 34 238 nt were obtained. Phylogenetic analysis showed the whole genome of TJ-2013-90 to be clustered together with HAdV-B55/HAdV-B11a. Three of 60 influenza virus-negative specimens were HAdV-positive, but hexon and fiber gene analysis showed that they were grouped in different branches to the HAdV isolates from this outbreak.
Conclusions
The cause of this ARTI outbreak was HAdV-B55. This was another outbreak caused by this re-emerging virus. Continuous surveillance of respiratory adenovirus is necessary for disease control.
1. Introduction
Human adenoviruses (HAdVs) are responsible for a variety of illnesses of the gastrointestinal, urinary, and respiratory tracts. So far, 67 types, including candidates from HAdV-55 to HAdV-67, have been classified into seven species (A–G) according to their biophysical and biochemical properties and a new paradigm based on genomics.1, 2, 3, 4, 5 Specific types have been linked to distinct clinical syndromes. Species B includes two genetic clusters, subspecies B1 and B2. B1 subspecies (including HAdV-3, HAdV-7, HAdV-16, HAdV-21, and HAdV-50) usually cause acute respiratory tract infections (ARTIs), while B2 subspecies (including HAdV-11, HAdV-14, HAdV-34, and HAdV-35) are more often associated with urinary tract infections and opportunistic infections in immune-compromised hosts, except HAdV-14 (which is a respiratory pathogen).2
Although HAdVs primarily cause diseases in young children, it has been reported repeatedly that these viruses also cause acute respiratory disease in adults. These infections usually occur as outbreaks in training facilities and are characterized by respiratory tract disease with extensive morbidity and occasional mortality.
As the serological type definition is being replaced by genomic criteria, several or more HAdV types have been recognized by the International Committee on Taxonomy of Viruses (ICTV) and registered as HAdV-52 to HAdV-67.3, 4, 5 HAdV-B55 is one of these, and used to be recognized as HAdV-B11a; it has been associated with acute respiratory disease since 1969, in Europe and especially in Asia.6, 7, 8, 9
We report an outbreak of ARTI in a physical training facility in Tianjin, China caused by HAdV-B55.
2. Materials and methods
2.1. Patients and samples
In January 2013, an ARTI outbreak occurred in a physical training facility in Tianjin, China. Thirty-eight respiratory samples were collected from 19 patients with an ARTI (both a throat swab and a nasopharyngeal aspirate from each patient). Patient information is given in Table 1 . Sixty influenza virus-negative throat swabs from patients with an influenza-like illness (ILI) in the same community during the same period were investigated for HAdV. All samples were collected into tubes containing 3 ml transport medium (pH 7.4–7.6 minimum essential medium (MEM) containing 1 mg/ml gentamicin and 2 μg/ml amphotericin B) and transported rapidly on ice to the laboratory; they were maintained at −70 °C until further examination.
Table 1.
Information for the patients in this study
| Case | Isolate designation | Date of onset | Date of clinical sample collection | Age, years | Diagnosis | Temperature (°C) | Other symptoms |
|---|---|---|---|---|---|---|---|
| 1 | TJ-2013-90 | Jan 1, 2013 | Jan 10, 2013 | 18 | URI | 40.3 | - |
| 2 | TJ-2013-92 | Dec 20, 2012 | Jan 10, 2013 | 22 | URI | 38 | - |
| 3 | TJ-2013-94 | Jan 4, 2013 | Jan 10, 2013 | 23 | URI | 38 | Diarrhea |
| 4 | TJ-2013-96 | Jan 5, 2013 | Jan 10, 2013 | 19 | URI | - | Diarrhea |
| 5 | TJ-2013-98 | Jan 3, 2013 | Jan 10, 2013 | 19 | URI | 41 | - |
| 6 | TJ-2013-100 | Jan 10, 2013 | Jan 14, 2013 | 19 | URI | 40.3 | - |
| 7 | TJ-2013-102 | Jan 12, 2013 | Jan 13, 2013 | 20 | URI | 39.6 | - |
| 8 | TJ-2013-104 | Jan 12, 2013 | Jan 14, 2013 | 19 | URI | 40.5 | Diarrhea |
| 9 | TJ-2013-106 | Jan 11, 2013 | Jan 13, 2013 | 20 | URI | 39.5 | Diarrhea |
| 10 | TJ-2013-108 | Jan 10, 2013 | Jan 14, 2013 | 18 | URI | 39.9 | - |
| 11 | TJ-2013-110 | Jan 10, 2013 | Jan 13, 2013 | 22 | URI | 39.9 | - |
| 12 | TJ-2013-112 | Jan 8, 2013 | Jan 13, 2013 | 22 | Pneumonia | 39.5 | - |
| 13 | TJ-2013-114 | Jan 10, 2013 | Jan 13, 2013 | 18 | URI | 38 | - |
| 14 | TJ-2013-116 | Jan 11, 2013 | Jan 13, 2013 | 19 | Pneumonia | 39.8 | - |
| 15 | TJ-2013-118 | Jan 11, 2013 | Jan 13, 2013 | 19 | Pneumonia | 39.5 | - |
| 16 | TJ-2013-120 | Jan 10, 2013 | Jan 13, 2013 | 18 | URI | 37.9 | - |
| 17 | TJ-2013-122 | Jan 10, 2013 | Jan 13, 2013 | 18 | Pneumonia | 39.3 | - |
| 18 | TJ-2013-124 | Jan 8, 2013 | Jan 13, 2013 | 19 | URI | 39.5 | - |
| 19 | TJ-2013-126 | Jan 10, 2013 | Jan 13, 2013 | 20 | Pneumonia | 39.3 | - |
URI, upper respiratory infection.
2.2. Respiratory virus detection and HAdV isolation
Nucleic acid was extracted from 500 μl of the clinical specimen or from 100 μl tissue cultured viruses using NucliSENS easyMAG (BioMetrix, France) in accordance with the manufacturer's instructions.
All clinical samples were subjected to multiplex PCR (multi-PCR) using Seeplex RV Detection Kit 1 (Seegene, Korea) for the detection of common respiratory viruses, as per the manufacturer's instructions: HAdV, parainfluenza viruses 1, 2, and 3 (PIV1, 2, 3), influenza viruses A and B (Flu A, B), human rhinovirus (HRhV), respiratory syncytial viruses A and B (RSV A, B), coronavirus OC43/HKU1, 229E/NL63, human metapneumovirus (hMPV), and human bocavirus (HBoV). First strand cDNA was produced using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA). HAdV 3, 4, 7, and 11 were detected by PCR, as described previously.10
All HAdV PCR-positive samples were inoculated onto Hep-2 cells and cultured with MEM (Gibcol, USA) in the presence of 2% serum. The cytopathic effect (CPE) was checked every day. Medium was collected when the CPE was up to 75–100% cells. Universal HAdV primers were then used to check whether HAdV was present, as described previously.11
2.3. HAdV hexon gene, fiber gene, and whole genome sequencing
The hexon and fiber genes of HAdV from tissue culture were amplified as described previously.12 As the use of whole genome sequencing is advised to confirm the type of HAdV,2, 13 the whole genome of one isolate in this study was sequenced using 41 sets of primers designed in-house. In brief, 41 pairs of M13F/M13R-tailed primers for the whole genome were used to perform separate PCRs. The product of each primer had overlapping sequences of 200–300 bp. PCR amplification of the hexon and fiber genes and whole genome sequencing segments was performed with HotStar HiFidelity Polymerase Kit (Qiagen, Germany); 0.8 μM of each primer was used. PCRs were performed in a GeneAmp PCR-9700 thermocycler (Applied Biosystems, USA) as follows: for the hexon gene, 95 °C for 15 min, followed by 30 cycles of 45 s at 94 °C, 45 s at 60 °C, 90 s at 72 °C, and a final extension step of 72 °C for 10 min; for the fiber gene and whole genome sequencing fragments, 95 °C for 15 min, followed by 30 cycles of 45 s at 94 °C, 45 s at 46 °C, 60 s at 72 °C, and a final extension step of 72 °C for 10 min.
The PCR products were analyzed with a QIAxcel Nucleic Acid Analyzer (Qiagen, Germany), purified using the QIAquick PCR Purification Kit (Qiagen, Germany), and then sequenced by Invitrogen Biotechnology Co. Ltd in Shanghai, China using an ABI 3730 DNA Analyzer (Applied Biosystems, USA).
2.4. Sequence alignment and phylogenetic analysis
Sequence assembly was conducted with SeqMan in Lasergene 7 software package. Nucleotide sequence alignments were generated using the ClustalW algorithm of MEGA 4.0.1. The nucleotide identities were calculated using MegAlign in Lasergene 7. Phylogenetic trees were constructed by MEGA 4.0.1 using the neighbor-joining method. Bootstrap proportions were plotted at the main internal branches of the phylogram to show support values. Other HAdV whole genome sequences and hexon and fiber gene sequences from different groups and different types were downloaded from GenBank. The accession numbers of HAdV strains used in this study are shown in Figure 1, Figure 2, Figure 3 .
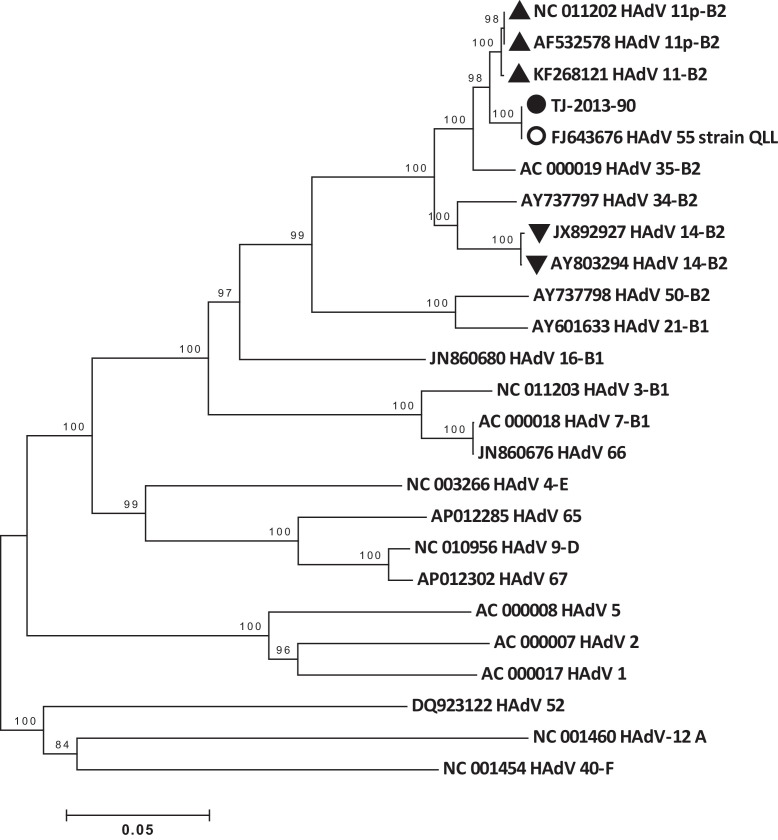
Figure 1.
Bootstrap neighbor-joining hexon gene phylogenetic tree of human adenoviruses (HAdV) designed with MEGA 4.0. The species of adenovirus is shown at the end of the strain information. Filled circle (●): isolate from this outbreak; empty circle (○): QS-DLL strain; triangles (▴): HAdV 11 isolates; inverted triangles (▾): HAdV 14 isolates.
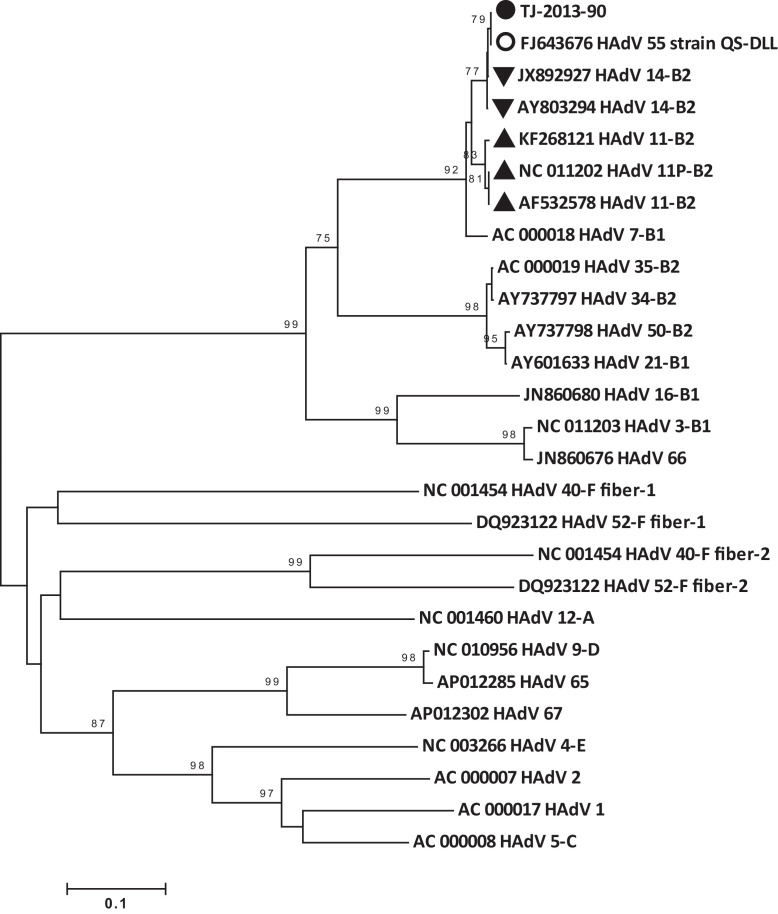
Figure 2.
Bootstrap neighbor-joining fiber gene phylogenetic tree of human adenoviruses (HAdV) designed with MEGA 4.0. The species of adenovirus is shown at the end of the strain information. Filled circle (●): isolate from this outbreak; empty circle (○): QS-DLL strain; triangles (▴): HAdV 11 isolates; inverted triangles (▾): HAdV 14 isolates.
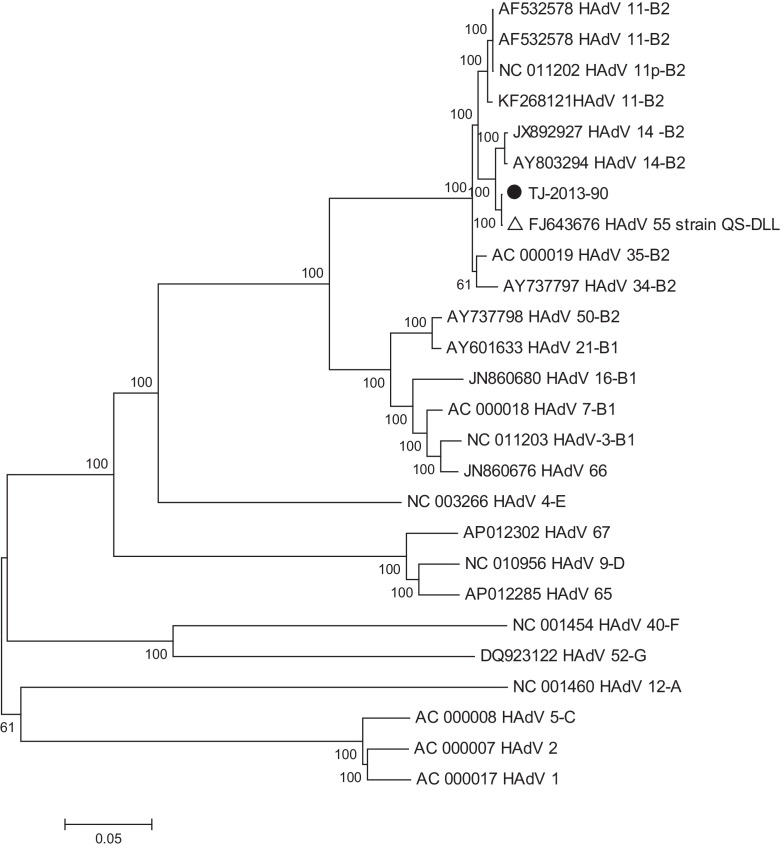
Figure 3.
Bootstrap neighbor-joining whole genome phylogenetic tree of human adenoviruses (HAdV) designed with MEGA 4.0. The species of adenovirus is shown at the end of the strain information. Filled circle (●): isolate from this outbreak.
3. Results
3.1. Epidemiology
In January 2013, an ARTI outbreak occurred in a physical training facility in Tianjin, China. Most of the patients had febrile respiratory symptoms, in some cases accompanied by severe pneumonia; these latter patients had to be hospitalized. Respiratory tract samples were collected from 19 patients. Most patients had upper respiratory infection symptoms, such as fever (most ≥39 °C), cough, expectoration, thoracalgia, rhinorrhea, headache, and sore throat; some experienced tachypnea, dyspnea, and diarrhea. Among these 19 cases, five had pneumonia. Chest X-rays showed single lobar or segment infiltrate/consolidation. Patient details are given in Table 1.
3.2. Etiological detection of the outbreak
3.2.1. Multi-PCR
A total of 38 respiratory samples were collected from 19 patients; 34 specimens from 17 patients were HAdV-positive using multi-PCR (Seegene, Korea) and universal HAdV PCR primers. Among the 17 HAdV-positive patients, two were co-infected with another respiratory virus (one was co-infected with RSV A and the other with HRhV); the remaining four specimens from two cases were influenza A-positive.
3.2.2. Virus isolation
All HAdV-positive specimens were inoculated onto Hep-2 cells for virus isolation. Isolation was successful in all cases.
3.2.3. HAdV typing
All the universal primer-positive HAdVs were amplified using HAdV 3, 4, 7, and 11-specific primers, but they were all negative. To allow for the determination of the viral type, four randomly selected HAdV strains from upper respiratory infection and pneumonia cases were investigated and sequenced for hexon and fiber genes (TJ-2013-92, TJ-2013-94, TJ-2013-100, TJ-2013-122).
3.2.4. Fiber gene, hexon gene, and whole genome amplification
The fiber gene open reading frame (ORF; 978 nucleotides (nt)) and hexon gene ORF (2841 nt) were obtained for the four HAdV strains described above. These sequences were blasted in GenBank. All showed the highest identity with HAdV-B55, which used to be called HAdV-B11a, but they also showed similar identity with HAdV-B11 or HAdV-B14.
In order to confirm the type of HAdV, the whole genome of one HAdV isolate, named TJ-2013-90, was sequenced and deposited in GenBank (accession number KF908851); the whole length was 34 238 nt.
3.3. Genetic characteristics of HAdV-B55
Phylogenetic trees of the whole genome and of the hexon and fiber gene segments were constructed, as shown in Figure 1, Figure 2, Figure 3. All three trees showed that the HAdVs isolated in this outbreak clustered together with HAdV-B55. The hexon segment clustered together with HAdV-B11, while the fiber segment and whole genome clustered with HAdV-B14. The whole genome and fiber and hexon gene identities of TJ-2013-90 compared to HAdV-B11, HAdV-B14, and HAdV-B55 are shown in Table 2 . The hexon and fiber genes of the four HAdV strains (described above) isolated in this outbreak were completely identical.
Table 2.
Sequence identities of TJ-2013-90 compared to different types of HAdV
| Type | GenBank accession number | Hexon | Fiber | Whole genome |
|---|---|---|---|---|
| HAdV55 | FJ643676 (QS-DLL) | 100.0 | 100.0 | Almost 100.0 (only four nucleotides different) |
| HAdV11 | AF532578 | 98.3 | 94.4 | 97.9 |
| KF268121 | 98.3 | 94.6 | 98.0 | |
| NC011202 | 98.3 | 94.4 | 97.9 | |
| HAdV14 | AY803294 | 92.8 | 99.6 | 99.0 |
| JX892927 | 92.7 | 99.7 | 99.0 |
HAdV, human adenovirus.
To elucidate whether the same HAdV infection had occurred in this community, 60 influenza virus-negative throat swabs from ILI cases in the same area during the same period were investigated for HAdV. Three samples were HAdV-positive. They were all typed by HAdV-B3, 4, 7, and 11-specific primers. One was HAdV-B3 and the other two were not HAdV-B3, 4, 7, or 11; the hexon and fiber genes of these two HAdVs were sequenced further. The sequences were different from the HAdV strains isolated in this outbreak. The same HAdV was not detected in the neighborhood community.
4. Discussion
Numerous outbreaks of ARTIs caused by HAdV have been reported during recent decades in many countries, including China.10, 12, 14
HAdV is a double-stranded DNA virus that has a common non-enveloped icosahedral capsid morphology and a similar genome structure. Many different criteria are used to classify HAdVs. According to genomic criteria, HAdVs are grouped primarily into seven species (A–G). The species classification is closely associated with differences in the affected human organs (tropism). Within each species, serotypes are distinguished based on their reactivity in the virus neutralization assay. This reactivity is mainly due to antibodies to the major capsid protein, the hexon, but also to antibodies to the penton base and fiber protein. Presently, 51 HAdV serotypes have been recognized. Within many serotypes, variants have been defined by restriction enzyme analysis of genomic DNA.15
Currently, the serological type definition is being replaced by genomic criteria.2 On this basis, 16 more HAdV types have been registered as HAdV-52 to HAdV-67. Among these, HAdV-B55 is a re-emerging pathogen; it was first identified as HAdV-B11a in an outbreak during a military training exercise in 1974,9 with a second appearance in another military training exercise in 2004.6 Next came the Singapore military recruit outbreak in 2005,7 and an outbreak in a civilian population in Shanxi Province in China in 2006.8 Most of the infections caused by HAdV-B55 in adults are upper respiratory illnesses occurring as outbreaks. These usually arise in military training facilities and crowded population groups. One of the risk factors for this severe illness may be the crowding and increased physical and psychological stress. In the present study, we report another HAdV-B55 outbreak in a physical training facility in Tianjin, China. In this outbreak, five of 19 cases had severe pneumonia and had to be hospitalized. All five cases were young males with no underlying diseases. A recent report has shown that HAdV may lead to community-acquired pneumonia in children less than 10 years of age.16 Surveillance for HAdV in both children and adults should be enhanced.
It has taken several years to arrive at a new name for this re-emerging and re-recognized virus, HAdV-B55. However, disagreements remain.
HAdV-B55 was first identified as HAdV-B11a, albeit by partial characterization of its hexon and fiber genes, in an outbreak during a military training exercise.9 It was first named HAdV-B55 by Michael through computational analysis of the whole genome of the viral strain QS-DLL,17 which was isolated from hydrothorax fluid samples of a patient who died during the HAdV outbreak in Qishan County, Shaanxi Province, China in 2006.8, 18 Complete genome analysis of QS-DLL demonstrated that this HAdV-B55 was a re-emerging virus that contained a genome resulting from a recombination of a renal pathogen, HAdV-B11, and a respiratory pathogen, HAdV-B14.
After that, Seto et al. proposed that HAdV should be identified, characterized, and typed on the basis of complete genome sequence analysis rather than serological approaches.2, 10 They also proposed that HAdV-B11a, which contains a partial hexon recombination of HAdV-B11 and HAdV-B14, should be renamed HAdV-B55. The hexon and fiber genes of the viruses isolated in this outbreak in Tianjin clustered with HAdV-B11 and HAdV-B14, respectively, and also showed highest identity with QS-DLL. The identities of the fiber and hexon genes from HAdV isolated in this outbreak were 100%, and the absence of any other relevant pathogen suggests that this virus was responsible for the outbreak. To allow for the determination of the exact viral type, we sequenced the whole genome of one of the viruses isolated here. The whole genome analysis showed that the virus isolated in this outbreak shared almost 100% homology (only four nucleotides were different) to QS-DLL, and it was also grouped together with QS-DLL in the phylogenetic tree; this suggests HAdV-B55 to be the reason for the outbreak.
Two years later, Kajon et al. stated a different opinion.15 They did not agree to the use of HAdV-B55 to designate the Chinese inter-typic recombinant HAdV H11/F14 virus strain QS-DLL, because this generated confusion regarding the antigenic identity of the virus. They strongly suggested that the Chinese virus strain QS-DLL should be serotyped as HAdV-B11-14. They also proposed that the molecular typing and designation of recombinant viruses be based on the sequences of the hexon and fiber genes, as they confirmed that these genes accommodated the major epitopes involved in virus neutralization (VN). One month later, Seto wrote a letter to the editor of the same journal to dispute the opinions of Kajor. Seto claimed that using serology-based typing and restriction fragment length polymorphism (RFLP) were no longer performed in HAdV research, both of these assays being interpretive, subjective, and error-prone.13 At the Tenth International Adenovirus Meeting (Umea, Sweden, 2012), it was agreed that ‘genotype’ was to refer to HAdV characterized and type-numbered with genome data, ‘molecular type’ was to refer to viruses with limited DNA sequence data, and ‘serotype’ was to be reserved for strains characterized completely with serological methods. The designation of the QS-DLL strain as HAdV-55 was correct.13
Recombination is one of the mechanisms of evolution by which viruses escape from human immunity. Partial genome analysis is not sufficient to investigate virus recombination. Whole genome sequence analysis is necessary for recombination research. China has a large, dense population, which makes the spread of infectious diseases easier. The need to establish and improve both epidemiological and virological surveillance for HAdV infections in China is emphasized. Continuous surveillance and molecular characterization of respiratory adenovirus isolates worldwide will contribute to the elucidation of the natural history and pathogenesis of HAdV respiratory infections.
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation funded project (grant number 2013M541186) and also by Tianjin Municipal Science and Technology Commission (grant number 07SYSYSF05100).
Ethical approval: Not required.
Conflict of interest: No conflict of interest to report.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Contributor Information
Xiaoyan Li, Email: xiaoyanli1291@163.com.
Xu Su, Email: suxu@cdctj.gov.cn.
Qing Gu, Email: gqing_1964@163.com.
References
- 1.Jones M.S., II, Harrach B., Ganac R.D., Gozum M.M., Dela Cruz W.P., Riedel B. New adenovirus species found in a patient presenting with gastroenteritis. J Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seto D., Chodosh J., Brister J.R., Jones M.S. Members of the Adenovirus Research Community. Using the whole-genome sequence to characterize and name human adenoviruses. J Virol. 2011;85:5701–5702. doi: 10.1128/JVI.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushima Y., Shimizu H., Kano A., Nakajima E., Ishimaru Y., Dey S.K. Genome sequence of a novel virus of the species human adenovirus d associated with acute gastroenteritis. Genome Announc. 2013:1. doi: 10.1128/genomeA.00068-12. pii: e00068-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelmann I., Madisch I., Pommer H., Heim A. An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin Infect Dis. 2006;43:e64–e66. doi: 10.1086/507533. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima Y., Shimizu H., Kano A., Nakajima E., Ishimaru Y., Dey S.K. Novel human adenovirus strain. Bangladesh. Emerg Infect Dis. 2012;18:846–848. doi: 10.3201/eid1805.111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chmielewicz B., Benzler J., Pauli G., Krause G., Bergmann F., Schweiger B. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J Med Virol. 2005;77:232–237. doi: 10.1002/jmv.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajon A.E., Dickson L.M., Metzgar D., Houng H.S., Lee V., Tan B.H. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training camp. J Clin Microbiol. 2010;48:1438–1441. doi: 10.1128/JCM.01928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z., Zhang Y., Xu S., Yu P., Tian X., Wang L. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J Clin Microbiol. 2009;47:697–703. doi: 10.1128/JCM.01769-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hierholzer J.C., Pumarola A., Rodriguez-Torres A., Beltran M. Occurrence of respiratory illness due to an atypical strain of adenovirus type 11 during a large outbreak in Spanish military recruits. Am J Epidemiol. 1974;99:434–442. doi: 10.1093/oxfordjournals.aje.a121632. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q., Su X., Gong S., Zeng Q., Zhu B., Wu Z. Comparative genomic analysis of two strains of human adenovirus type 3 isolated from children with acute respiratory infection in southern China. J Gen Virol. 2006;87:1531–1541. doi: 10.1099/vir.0.81515-0. [DOI] [PubMed] [Google Scholar]
- 11.Allard A.K., Girones R., Juto P., Wadell G. Polymerase chain reaction for detection of adenoviruses in stools. J Clin Microbiol. 1990;28:2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W., Cui A., Shi Z., Wang H., Zhang Y., Tang Z. Etiological research of the unknown mild respiratory tract infectious disease in an outbreak in Jiangsu Province. Chinese Journal of Virology. 2005;21:325–331. [Google Scholar]
- 13.Seto D., Jones M.S., Dyer D.W., Chodosh J. Characterizing, typing, and naming human adenovirus type 55 in the era of whole genome data. J Clin Virol. 2013;58:741–742. doi: 10.1016/j.jcv.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Harley D., Harrower B., Lyon M., Dick A. A primary school outbreak of pharyngoconjunctival fever caused by adenovirus type 3. Commun Dis Intell. 2001;25:9–12. doi: 10.33321/cdi.2001.25.2. [DOI] [PubMed] [Google Scholar]
- 15.Kajon A.E., de Jong J.C., Dickson L.M., Arron G., Murtagh P., Viale D. Molecular and serological characterization of species B2 adenovirus strains isolated from children hospitalized with acute respiratory disease in Buenos Aires, Argentina. J Clin Virol. 2013;58:4–10. doi: 10.1016/j.jcv.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Cao B., Huang G.H., Pu Z.H., Qu J.X., Yu X.M., Zhu Z. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. 2014;145:79–86. doi: 10.1378/chest.13-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh M.P., Seto J., Jones M.S., Chodosh J., Xu W., Seto D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol. 2010;48:991–993. doi: 10.1128/JCM.01694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Zhu Z., Tang L., Wang L., Tan X., Yu P. Genomic analyses of recombinant adenovirus type 11a in China. J Clin Microbiol. 2009;47:3082–3090. doi: 10.1128/JCM.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]