Abstract
The respiratory system, which includes the trachea, airways, and distal alveoli, is a complex multi-cellular organ that intimately links with the cardiovascular system to accomplish gas exchange. In this review and as members of the NIH/NHLBI-supported Progenitor Cell Translational Consortium, we discuss key aspects of lung repair and regeneration. We focus on the cellular compositions within functional niches, cell-cell signaling in homeostatic health, the responses to injury, and new methods to study lung repair and regeneration. We also provide future directions for an improved understanding of the cell biology of the respiratory system, as well as new therapeutic avenues.
In this review, Basil et al. discuss the complex architecture of the mammalian respiratory system and elaborate on the key aspects of lung repair and regeneration. They also discuss current and future directions for basic research of the respiratory system and the development of new therapeutic avenues.
Main Text
Introduction
The respiratory system is organized into multiple integrated compartments comprising multiple tissues that perform gas exchange between the blood and the external environment. The various anatomical regions of the respiratory tract are populated by numerous types of unique epithelial, vascular, mesenchymal, and immune cells critical for the functioning of each particular compartment.
Historically, the development of the respiratory system has been thought to involve several discrete morphogenetic steps including lineage specification, branching morphogenesis, sacculation, and alveologenesis (Morrisey and Hogan, 2010). While these steps were previously conceived of in terms of distinct temporal stages of development, more recent evidence has suggested that there is overlap between these stages and particular events such as cell specification and commitment, which are now thought to occur very early and coincident with the basic patterning of the respiratory airway tree (Frank et al., 2019).
The branched network of airways and gas exchange surfaces co-develops with the cardiovascular system to bring both organ systems into intimate proximity for full functionality. More details on these important developmental events can be found in several recent reviews (Herriges and Morrisey, 2014, Hines and Sun, 2014, Morrisey and Hogan, 2010, Nikolić et al., 2018, Whitsett et al., 2019, Zepp and Morrisey, 2019). The culmination of these events is the generation of an extensive surface area for efficient gas exchange that in the human lung comprises approximately 70 m2.
This review will focus on how the mature respiratory system maintains its normal homeostatic structure and function and how it responds to injury and regenerates itself. We will explore the cellular constituents of the two major compartments in the lungs—the gas exchange alveoli and the conducting airways including the trachea—and describe established and emerging techniques to explore human lung regeneration.
Compartment-Specific Regeneration in the Respiratory System
Alveolar Regeneration
The lung alveolus is composed of multiple epithelial, endothelial, and mesenchymal cell types (Figure 1 ). In addition to these resident cell types, the alveolus also is inhabited by several immune cell lineages, including alveolar macrophages, interstitial macrophages, and dendritic cells and several recent datasets have shown this diversity of cells at single-cell resolution in both animals and humans (Guo et al., 2019, Travaglini et al., 2019, Vieira Braga et al., 2019). Emerging data suggest there is some degree of inter-cellular communication between the lineages in this niche, but our understanding of the crosstalk among alveolar cell lineages during homeostasis or regeneration remains poor. The alveolar compartment remains largely quiescent in the uninjured lung, and most cells within this niche exhibit a relatively slow turnover. After lung injury, multiple alveolar cell types are able to proliferate, and when repair is effective both alveolar structure and function are restored. This ability to react to injury involves both activation of self-renewal as well as differentiation into more mature cell lineages. The self-renewal and differentiation of various lung epithelial cells are modulated by a growing list of cell types that includes neighboring epithelial cells, mesenchymal cells, airway smooth muscle, neurons and neuroendocrine cells, endothelium, and various leukocyte populations (Barkauskas et al., 2013, Cao et al., 2017, Lechner et al., 2017, Lee et al., 2017, Rafii et al., 2015, Zepp et al., 2017). These studies have highlighted recurrent themes regarding the signals that can drive alveolar epithelial regeneration, including Wnt signaling.
Figure 1.
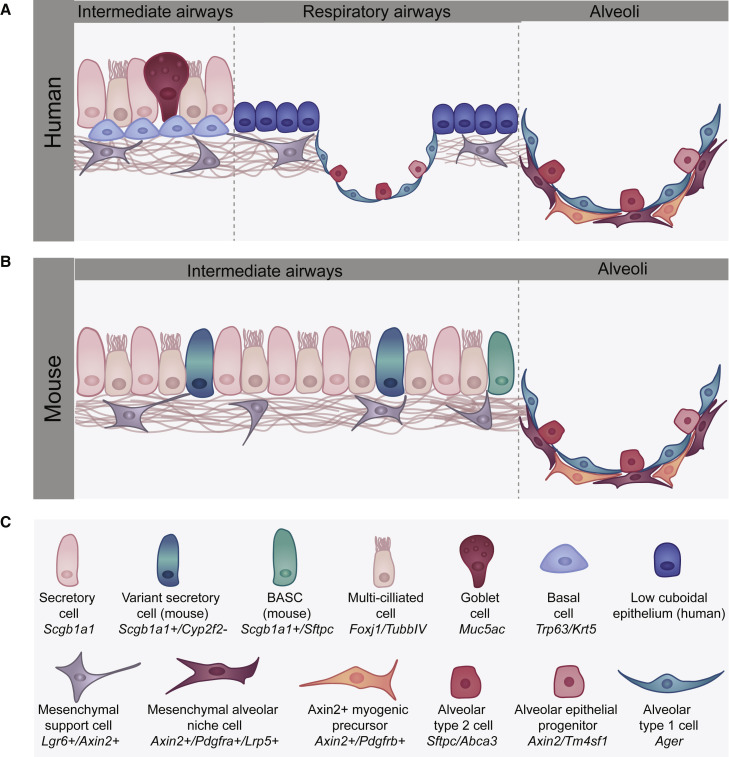
Alveolar Cell Lineages Involved in Lung Repair and Regeneration
(A) The human distal airways connect with the alveolar niche through a transitional respiratory airway (also called the respiratory bronchiole or RB) region. The RB is lined with a simple but poorly characterized cuboidal epithelium while the more intermediate airways exhibit a pseudostratified epithelium containing secretory, goblet, and ciliated cells that may exhibit as yet distinct heterogeneity. Of note, basal cells are found in human intermediate and respiratory airways.
(B) Mice do not have respiratory bronchioles and transition from the intermediate airways, which exhibit a pseudostratified nature but lack basal cells, into the alveolar region. The distal BADJ region in the mouse lung, which is not found in the human lung, contains the BASC population. The architecture and cell lineages found in both the mouse and human lungs are very similar and contain both AT1 and AT2 epithelial lineages as well as various mesenchymal lineages and vascular endothelial cells.
(C) The various cell types found in the distal airways and alveolus of the human and mouse lung.
Alveolar Epithelial Response to Injury. There are two primary lineages in the alveolar epithelium: the alveolar epithelial type 1 (AT1) and type 2 (AT2) cells (Figure 1). AT1 cells cover 95% of the alveolar surface area and are closely juxtaposed with the capillary plexus. AT2s are responsible for generating pulmonary surfactant, which is essential for reducing the surface tension of the alveolar surface area to prevent the lungs from collapsing upon every breath. AT1 and AT2 cells are specified very early in lung development and AT2 cells do not appreciably generate AT1 cells during early postnatal lung growth (Frank et al., 2016, Frank et al., 2019). However, in adult mice, AT2 cells can act as both a self-renewing stem cell like population and regenerating AT1 cells after injury (Barkauskas et al., 2013). A sublineage within the AT2 cell population that expresses the transcriptional target of Wnt signaling, Axin2, has been shown to play a dominant role in repairing the lung alveolus after acute injury (Nabhan et al., 2018, Zacharias et al., 2018). These cells, which have been called alveolar epithelial progenitors or AEPs, preferentially re-enter the cell cycle after injury, self-renew, and regenerate mature AT1 and AT2 cells. AEPs appear primed to enter the cell cycle and they respond robustly to Wnt and Fgf7 signaling (Zacharias et al., 2018). In alveolar development, Wnt-responsive AT2 cells self-renew in the presence of Wnt signaling and differentiate in its absence (Frank et al., 2016), but it is not yet known whether these same signals drive renewal versus differentiation during regeneration. A recent report also shows that AEPs promote metastasis in models of lung cancer (Laughney et al., 2020). AEPs have been identified in the human lung and are responsible for generating the majority of AT2 cell growth in human alveolar organoids (Zacharias et al., 2018). In contrast to AT2s and AEPs, the AT1 cell contribution to alveolar epithelial regeneration is thought to be very limited (Jain et al., 2015). Following the surgical removal of lung tissue in adult mice and other model organisms, new alveoli are formed to compensate for lost alveolar surface area (Buhain and Brody, 1973). This compensatory lung growth after partial pneumonectomy is commonly used as a “sterile” model of lung regeneration. Not surprisingly, this regenerative response involves the coordinated actions of nearly every cell type in the lung including epithelial cells, endothelial cells, mesenchymal cells, and leukocytes (Chen et al., 2012, Jain et al., 2015, Lechner et al., 2017, Rafii et al., 2015). In this model, a small number of cells expressing Hopx+, a marker for the AT1 lineage, were found to both proliferate and, in rare instances, give rise to Sftpc+ AT2 cells (Jain et al., 2015). A recent study revealed heterogeneity within the AT1 cell population where expression of Igfbp2 marked the most mature AT1 cell subtype that lacks differentiation capacity following pneumonectomy (Wang et al., 2018). Although cells expressing AT1 markers proliferate during compensatory lung growth after partial pneumonectomy, whether bona fide AT1 cells are able to contribute to repair after acute lung injury is largely unknown.
Alveolar Endothelial Response to Injury. Effective gas exchange is dependent upon AT1 cell and pulmonary capillary endothelial cell (PCEC) proximity, and successful alveolar regeneration requires re-establishment of this spatial relationship. Following lung injury in rodents, there is rapid proliferation in the microvasculature, with expansion of resident microvascular endothelial progenitor cells (Alvarez et al., 2008). These cells are marked by Cd34 and Cd309, and in organoid culture demonstrate significant vasculogenic capacity. While both the mechanisms of PCEC regeneration and cellular identities within this compartment are incompletely understood, endothelial cells expressing Sox17 have recently been shown to play a role in endothelial regeneration after endotoxic-induced vascular injury (Liu et al., 2019a). Additionally, PCECs enhance alveologenesis following injury, with PCEC-derived Vegfr2 and Fgfr1 mediating epithelial proliferation (Ding et al., 2011). This signaling is thought to be through MMP14, and possibly co-mediated through platelet activation of endothelial SDF-1 receptors (Rafii et al., 2015). Significant additional heterogeneity is thought to exist within the distal lung endothelium, with differential vasculogenic capacities and crosstalk with the epithelium (Stevens et al., 2008), and this has recently been revealed in single-cell transcriptomic studies of the homeostatic lung and its response to acute injury (Niethamer et al., 2020). This study demonstrated the presence of multiple microvascular endothelial subsets including one which expresses high levels of Car4 and Cd34. This study also showed that a population of proliferating endothelial cells emerges soon after influenza injury and single-cell informatic trajectory analysis suggests that these cells arise from multiple endothelial subsets. Future work is needed to define the functional role for these endothelial subsets in both normal alveolar homeostasis and the response to injury.
Alveolar Mesenchymal Response to Injury. The lung alveolus is also home to a complex mixture of mesenchymal cell types, many of which are in close physical association with both alveolar epithelial and endothelial cells and play an active role in alveolar epithelial regeneration. Pioneering electron microscopy studies demonstrated direct and extensive contacts between lung fibroblasts and AT2 cells (Sirianni et al., 2003, Walker et al., 1995). Mesenchymal cells expressing the platelet-derived growth factor alpha (Pdgfra) are often found in close association with AT2 cells (Barkauskas et al., 2013, Green et al., 2016, Zepp et al., 2017). The first functional evidence of trophic interactions between these populations was the observation that Pdgfra+ fibroblasts support the growth and differentiation of AT2s in an alveolar organoid co-culture assay (Barkauskas et al., 2013). Alveolar organoids have been recently used to provide functional evidence that multiple signaling pathways originate in Pdgfra+ cells to influence AT2 cell self-renewal and differentiation into AT1 cells including Fgf7, Bmp, and Il6 (Chung et al., 2018, Green et al., 2016, Zepp et al., 2017). As molecular techniques have evolved, an emerging literature has revealed molecular and functional heterogeneity of lung alveolar mesenchymal cells. In particular, accumulating data demonstrate the previously underappreciated heterogeneity of cells within the Pdgfra+ population of fibroblasts in the adult (Green et al., 2016, Zepp et al., 2017). Single-cell transcriptome analysis combined with spatial distance mapping recently demonstrated that one subpopulation of fibroblasts, defined by the co-expression of Axin2 and Pdgfra, is localized particularly close to AT2 cells and provides signals including Il6, Fgfs, and Bmp antagonists that promote the self-renewal and differentiation of AT2s (Zepp et al., 2017). This fibroblast population has been named the mesenchymal alveolar niche cell or MANC and these cells are thought to have a critical role in homeostatic alveolar regeneration following injury (Zepp et al., 2017).
Immune Response to Alveolar Injury. Resident and circulating leukocytes are thought to also play a critical role in alveolar repair and regeneration, with elegant in vivo and in vitro studies demonstrating that “inflammatory” cytokines have direct effects on the proliferation and differentiation of both airway and alveolar epithelial cells (Danahay et al., 2015, Katsura et al., 2019, Kuperman et al., 2002, Tadokoro et al., 2014, Xie et al., 2018). However, our understanding of the interactions between alveolar epithelial cells and resident or circulating leukocytes is in its infancy. Macrophages, the primary resident immune cell of the alveolus, begin to populate the lung during embryonic lung development (Tan and Krasnow, 2016). LPS stimulation of these early resident macrophages leads to impaired branching morphogenesis, attributed to changes in integrin, Bmp, and Wnt signaling (Blackwell et al., 2011). Whether unstimulated resident macrophages have a normal role in branching morphogenesis or the differentiation of alveolar epithelial cells remains to be elucidated. However, there are multiple lines of evidence indicating that leukocytes play important roles in adult alveolar regeneration, mediated at least in part through bidirectional intercellular communication with alveolar epithelial cells. For example, following either chemical or infectious lung injury, resident alveolar macrophages can stimulate epithelial proliferation through the production of Wnt ligands (Hung et al., 2019). A subset of alveolar macrophages can also act as memory macrophages and help guide the rapid activation of multiple chemokines including those that stimulate neutrophils (Yao et al., 2018).
Compensatory lung growth after partial pneumonectomy provides a relatively simple model to study pro-regenerative epithelial-immune interactions without the confounding effects of infection and inflammation. This model was used to demonstrate that platelets can initiate a regenerative cascade by secreting SDF1 after pneumonectomy. This stimulates capillary endothelial cells to express MMP14, releasing EGF ligands from the extracellular matrix and subsequently promoting AT2 cell proliferation and differentiation (Rafii et al., 2015).
Following partial pneumonectomy, local production of the chemokine CCL2 leads to the recruitment of CCR2+ monocytes to the lung (Lechner et al., 2017). These monocytes and resident macrophages can be polarized by IL13 that is secreted by type 2 innate lymphoid cells, and loss of this axis impairs optimal compensatory lung growth after pneumonectomy. Data from an in vitro co-culture assay suggest that macrophages can directly modulate AT2 cell survival and self-renewal (Lechner et al., 2017). Recruited CCR2+ monocytes have also been implicated in dysplastic alveolar repair following both bleomycin-induced lung injury and other models of lung fibrosis (Misharin et al., 2017, Venosa et al., 2019). These studies highlight the context-dependent roles of CCR2+ monocytes in both normal and abnormal lung regeneration.
In infectious and more destructive lung injury models, it can be difficult to discern the inflammatory roles of leukocytes from regenerative roles, if such a distinction exists. Nevertheless, data from a growing number of contexts have shown that resident and recruited immune cells are essential for resolution of lung injury. For example, the depletion of macrophages during the resolution phase of bleomycin-induced lung injury prolonged the fibrotic response and impaired resolution (Gibbons et al., 2011). This effect was attributed to a decrease in the clearance of accumulated extracellular matrix but could also involve disrupted communication with epithelial progenitor cells or other populations.
Other immune populations are activated or recruited to the alveolar niche following lung injury. We have nascent understandings of the inflammatory cellular diversity and intercellular communications that determine normal versus abnormal lung regeneration in response to lung injury. Understanding this diversity and improving model systems, including more precise animal models and organoid and lung-on-a-chip models that incorporate immune cells, will allow us to study the contributions of immune cell communications that drive lung repair (Gkatzis et al., 2018).
Airway Regeneration
Maintenance and Regeneration of the Proximal Airway Epithelium. The proximal airways in mice and humans are exposed to frequent insults from the environment and serve as the first line of immune and toxin defense in the lung, warming and filtering the air as it passes to more distal regions. Much of what is understood about airway regeneration comes from studies of the mouse trachea, which most closely resembles the structure of human proximal airways. There are many parallels between the murine trachea and the human intrapulmonary airways, with the most relevant for this discussion being the presence of basal cells. In mice, basal cells reside in the trachea and proximal main stem bronchi; however, in humans this population extends for several airway generations (Figures 1 and 2 ). Murine intrapulmonary airways are not pseudostratified and do not contain basal cells which are the primary stem cell population in human airways. Thus, intrapulmonary mouse airways should not be used as a model system for the study of human airways. The pseudostratified upper airway and tracheal epithelium exhibits very slow turnover during health but several of the mature lineages are capable of re-entering the cell cycle to replenish loss of neighboring cells and maintain an epithelial barrier. Basal cells in the proximal airways are the major stem cell population that self-renew and when necessary give rise to multiple cell types such as secretory, goblet, and multi-ciliated cells (Figure 2; Hegab et al., 2012, Hong et al., 2004, Rock et al., 2009, Rock et al., 2011). This process is critical for both maintenance and cellular regeneration after significant injury and is controlled by Notch signaling (Mori et al., 2015, Rock et al., 2011, Ruiz García et al., 2019, Stupnikov et al., 2019). Although it was once thought that basal cells were a rather homogeneous population, recent findings reveal more complexity and have demonstrated their early origins in development (Yang et al., 2018). Careful lineage tracing of cytokeratin 5 (Krt5) basal cells over time indicated that at least two populations of basal cells exist in the upper airway, with one acting more as a self-renewing stem cell, and the other committed to luminal differentiation (Watson et al., 2015). Consistent with this paradigm, depending on enhanced Notch2 signaling or c-myb expression, basal stem cells directly give rise to secretory cells or multi-ciliated cells, respectively (Pardo-Saganta et al., 2015).
Figure 2.
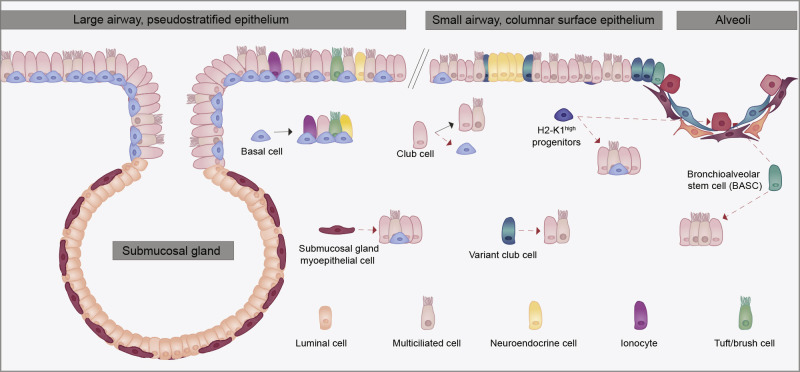
Airway Cell Lineages Involved in Lung Repair and Regeneration
Structure of the pseudostratified large airways and trachea of the mouse lung showing submucosal glands which are lined with both myoepithelial cells and other luminal secretory lineages. The smaller airways of the mouse lung contain various luminal secretory and ciliated epithelial lineages as shown. Some of these including the BASCs and the H2-K1high secretory cell subtypes have been proposed to generate alveolar epithelium after severe injury.
Recent studies have uncovered additional complexity within the pseudostratified airway epithelium of mouse trachea and human large airway, with the description of new, rare cell types including CFTR-rich ionocytes (Montoro et al., 2018, Plasschaert et al., 2018). Multiple studies using lineage tracing analysis combined with single-cell transcriptomic work confirmed that the Krt5 basal cell population was capable of giving rise to all the observed cell types within the airway, including the newly identified ionocyte, and other rare epithelial cell subsets such as the tuft cell, which is not normally present in uninjured mouse airways (Montoro et al., 2018, Mori et al., 2015, Rane et al., 2019, Rock et al., 2011, Ruiz García et al., 2019). Moreover, tuft cells, also known as solitary chemosensory cells, ectopically emerge after influenza injury in the mouse and may play a role in post-injury dysplastic remodeling of the lung (Rane et al., 2019). Some of these lineage relationships in mice can be modeled in vitro with primary human cells in culture, highlighting the shared regenerative potential across species (Rock et al., 2009).
While basal cells are a main driver of regeneration after airway injury, other cell types have been shown to contribute as facultative progenitors. Lineage tracing analysis of airway Scgb1a1+ cells revealed that secretory cells proliferate to help maintain the club cell population (Figure 2; Rawlins et al., 2009, Van Keymeulen and Blanpain, 2012) and are a major source of multi-ciliated cells in normal airways, particularly in the more distal murine airways where basal cells are not typically found. In addition, subsets of secretory cells expressing Upk3a in mice, so-called variant-club cells, have been shown to be localized near neuroendocrine bodies, suggesting a possible niche, and are capable of giving rise to both secretory and ciliated cells in development; however, a role in the response to injury is not yet clear (Guha et al., 2012, Guha et al., 2017). Additionally, neuroendocrine (NE) cells also can function as facultative progenitors after airway injury, interact with immune lineages during expansion, and may harbor sublineages with enhanced progenitor capacity in NE bodies (Branchfield et al., 2016, Garg et al., 2019, Ouadah et al., 2019). In extreme situations, mature secretory/club cells (defined by Scgb1a1 expression) have been reported to dedifferentiate in the setting of marked basal cell loss, and contribute to the basal stem cell pool, although the homeostatic or physiologic role of this in injury is not clear (Tata et al., 2013). In addition, a novel pathway of basal cell repletion from the submucosal gland was also reported by two different groups using lineage tracing and multiple airway injury models (Lynch et al., 2018, Tata et al., 2018). These investigators found migration of glandular myoepithelial cells into the airways with subsequent differentiation to mature airway lineages including basal cells.
Regeneration of Distal Airway Epithelium after Injury. A number of distinct, small populations of progenitor cell types have been reported to contribute to regeneration of distal airway epithelia after injury in mice, which lacks basal cells (Barkauskas et al., 2013, Bertoncello and McQualter, 2010, Chen, 2017, Perl et al., 2005). One of the first was a variant club/secretory cell (v-club cells) defined by its location near neuroendocrine bodies and low cytochrome Cyp2f2 expression, making these cells resistant to naphthalene injury and thus a source of regenerative cells after this injury (Giangreco et al., 2009, Hong et al., 2001). More recently Upk3a was identified as a unique marker for v-club cells. A Upk3acreER lineage trace (Guha et al., 2017) demonstrated that these cells give rise to club cells and ciliated cells during homeostasis and after naphthalene airway injury, as predicted from prior studies (Giangreco et al., 2009, Hong et al., 2001, Volckaert et al., 2011). Although seemingly limited, in these studies some v-club/secretory cells were also noted to differentiate into AT2 cells during bleomycin-induced alveolar injury, implying a capacity for mobilization and distal migration.
A second small population of stem/progenitor cells was identified at the branch point between distal murine airways and alveolus and was termed bronchoalveolar stem cells (BASCs). BASCs were initially characterized by dual expression of the secretory cell marker Scgb1a1 and the AT2 marker Sftpc by immunostaining and their localization at the bronchioalveolar duct junction (BADJ) (Kim et al., 2005). Stem cell antigen-1 (Sca1) was also proposed as a marker for these cells and has been used in flow cytometry to purify these cells for in vitro culture studies (Raiser and Kim, 2009). Recently, intersectional-genetic lineage tracing techniques allowed for the specific tracing of BASCs and confirmed that BASCs can generate distal airway club cells after naphthalene injury and peri-junctional AT2 alveolar cells after bleomycin injury or influenza injury (Liu et al., 2019b, Salwig et al., 2019). One of the limitations of these studies is the use of Scgb1a1 expression to mark BASCs, as this gene and protein are known to be expressed in a subset of AT2 cells, which could confound such lineage tracing (Rawlins et al., 2009). While the genetic depletion of the BASC population resulted in a delay of the murine regenerative response to injury, full regeneration was eventually observed, suggesting that BASCs are not absolutely required for lung regeneration.
An additional distal airway stem/progenitor cell population with regenerative potential was identified descriptively as lineage-negative epithelial progenitors (LNEPs), also referred to as distal airway stem cells (DASCs). These cells are distinct from v-club/secretory cells and BASCs. LNEPs were originally defined as distal airway integrin β4+/CD200+ cells without discernible mature lineage markers by protein immunostaining (Vaughan et al., 2015). These cells are also Sox2+, as expected for an airway epithelial cell. Subsequently it became apparent that LNEPs/DASCs are composed of both Trp63+ cells and Trp63− cells. The Trp63+ cells appear to be holdovers from embryonic Trp63+ basal cells and some 20% of these cells could be traced with the Scgb1a1creER mouse line (Yang et al., 2018). Although true basal cells are not normally found in the distal mouse airway, the rare Krt5+/Trp63+ LNEPs/DASCs expand and mobilize after influenza injury to generate collections (or pods) of Krt5+ basal-like cells throughout the heavily damaged areas of influenza-injured mice (Vaughan et al., 2015, Xi et al., 2017, Zuo et al., 2015). This mobilization is initially protective to the mouse but ultimately is a dysplastic response as Krt5+ basal cells have limited potential to differentiate into AT2 cells and the mouse becomes permanently burdened with airway-like cystic structures throughout the alveolar compartment. Appearance of these permanent cystic structures was linked with activated Notch signaling, and blockade of hypoxemia response via deletion of hypoxia inducible factor 1 (HIF1α) in airway cells promotes the contribution of airway cells to regeneration of AT2s and subsequent improvement in oxygen saturation (Xi et al., 2017).
Contribution of Distal Airway Progenitors to Alveolar Repair. Although endogenous AT2s represent the primary regenerative responders to various alveolar insults and regenerate the overwhelming number of new AT2 and AT1 cells after limited alveolar damage, several lines of evidence implicate activation of distal airway epithelial cells as an alternative source of alveolar epithelial cells following certain types of severe lung injury (Barkauskas et al., 2013, Chapman et al., 2011, Xi et al., 2017). Recently, rare MHChigh (H2-K1high) club cell-like progenitors have been described within the larger Scgb1a1 lineage-traced population of all lung cells. These cells have proliferative capacity, appear to give rise to AT2 and AT1 cells following bleomycin-induced lung injury, and can be purified by flow cytometry using anti-H2-K1 antibodies (Kathiriya et al., 2020). H2-K1high cells are a subpopulation of β4/CD200 cells, express low or no mature lung epithelial lineage markers (e.g., Scgb1a1 or Sftpc) at the protein level, and represent ∼5% of all Scgb1a1CreER-labeled lung cells, but exhibit clonogenic potential to generate these broader populations in cell culture outgrowth assays (Kathiriya et al., 2020). H2-K1high cells were found to survive and differentiate into AT2 and AT1 cells in vivo after intra-airway transplantation into bleomycin-injured mice. These cells were also found to be specifically targeted by H1N1 PR8 influenza virus, consistent with a prior report showing β4+/CD200+ cells as a primary target of the viral injury (Quantius et al., 2016). The alveolar epithelial differentiation capacity of H2-K1high progenitors after bleomycin injury contrasts sharply with the differentiation repertoire of the rare Trp63+ cells which predominantly gives rise to dysplastic Krt5+ pods, but virtually no alveolar epithelial cells (Ray et al., 2016, Vaughan et al., 2015, Xi et al., 2017). Additional studies combining injury models with modern cell lineage tracking techniques and single-cell analysis are needed to clarify the injury-specific activation of airway cells and their relative contributions toward alveolar regeneration. Importantly, whether BASCs, LNEPs, and H2-K1high progenitors represent similar or overlapping populations of cells remains unclear. Future studies will need to directly compare these distal airway cells to the resident AT2 and AEP cell populations, to more fully assess their ability to repair and regenerate the alveolar niche after acute injury and in chronic diseases.
Contribution of the Mesenchyme to Airway Regeneration. A series of complex and interconnected interactions are employed to maintain distal airway epithelium during quiescence and after injury within the local niche. The local niche is comprised of the extracellular matrix (ECM) and several mesenchymal cell types. Specifically, the mesenchyme plays a critical role in function of distal airway epithelium by providing a number of signaling cues that ultimately determines the stem cell response during injury. Much of what is known about the interplay in adults between the mesenchyme and the epithelium has been derived from studying lung development. These developmental signaling pathways include Wnt/β-Catenin, fibroblast growth factor 10 and its receptor Fgfr2 (Fgf10/Fgfr), retinoic acid, and sonic hedgehog (Shh) members of transforming growth factor β (Tgfβ) superfamily including bone morphogenetic proteins (Bmp), Hippo/Yes-associated protein (YAP), and Notch signaling. For example, airway smooth muscle cells promote epithelial repair through the production of Fgf10 (Lee et al., 2017, Volckaert et al., 2011) and a population of Pdgfra+ fibroblasts promote the differentiation of multi-ciliated cells through the production of Il6 (Tadokoro et al., 2014). In addition to signaling to the airway epithelium during regeneration, the airway mesenchyme can undergo a phenotypic change following injury that drives abnormal regeneration. A recently identified mesenchymal subpopulation identified by Axin2 expression but lacking Pdgfra expression, the Axin2+ Myogenic Progenitor or AMP, becomes activated after naphthalene airway injury, begins to express Acta2, and contributes the airway fibrosis in the naphthalene model (Zepp et al., 2017). A more thorough review on these pathways and their cell-specific functions can be found elsewhere (Kotton and Morrisey, 2014, Leach and Morrisey, 2018, Lee and Rawlins, 2018, Zepp and Morrisey, 2019).
Human versus Mouse Lung Repair and Regeneration
The human lung contains many structural and anatomic differences which make it unique from its murine counterpart. The murine lung has a 6,000-fold smaller tidal volume, an 8,000 times smaller surface area, and roughly half the generations of airways compared to its human counterpart (Irvin and Bates, 2003, Knust et al., 2009, Thurlbeck, 1967). This significant difference in organ size poses distinct challenges for gas distribution, immune function, and gas exchange. These differences highlight the critical variances that need to be accounted for as the field moves from bench to bedside.
The alveolus is one of the most architecturally conserved regions between the murine and human lung (Figure 1). Conservation of the cellular populations between mouse and human alveoli have been demonstrated through multiple techniques and include the above-described AT1 and AT2 epithelial lineages and the AEP sublineage. Furthermore, the regenerative capacity of the alveolus appears to be retained across species, as discussed above.
The cellular anatomy of trachea and the most proximal airways in mice and the large airways in humans is very similar. The cells of the murine trachea and their progenitor and regeneration capacity appear to be closely aligned with in vitro models of human proximal airway regeneration. However, the distal conducting airway anatomy of the mouse is quite different from humans. In mice, the intrapulmonary airways are almost completely devoid of cartilaginous rings, bronchial blood support, submucosal glands, and pseudostratified epithelium, all of which is in direct contrast to their human counterparts. Furthermore, murine conducting airways terminate directly into alveolar sacs at the site of the BADJ. Humans do not have a BADJ, and instead have a distinct distal airway compartment which includes the respiratory bronchioles. These distal respiratory bronchioles are interdigitated with alveolar-like structures that leads into the larger alveolar compartment. There is no analogous counterpart to the human respiratory bronchioles in mice. Terminal and respiratory bronchioles in humans contain Krt5+ basal cells that are generally absent in the distal airways of mice. To date, a BASC-like cell population has not been found in humans, perhaps due to the lack of the corresponding BADJ anatomical niche. Thus, the cellular origins of distal airway repair and the origin of airway-based stem/progenitors mobilized during alveolar injury in humans are likely different from that of mice. Given the significant differences between the human and mouse lung, especially in the distal airways, greater focus is needed to study the human lung, both through descriptive assessment using new techniques including single-cell analysis as well as more sophisticated assays including organoids, ex vivo lung explants, and pluripotent stem cell-derived human lung lineages.
Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is the most common adult interstitial lung disease (ILD), a class of pulmonary diseases pathologically defined by interstitial fibrosis, inflammation, or the combination of fibrosis and inflammation (Lederer and Martinez, 2018). A recognized theory of IPF pathogenesis places the initial site of “micro-injury” in the alveolar space with the AT2 cell holding an important position in disease development. Recent transcriptional interrogation of the distal epithelium in IPF identified activation of cell stress and senescence pathways, and murine modeling of AT2 cell dysfunction from expression of either mutant SFTPC, loss of telomere function, and increased mechanical tension have provided in vivo proof of concept that disruption of AT2 cell homeostasis is a driver of lung fibrosis (Katzen et al., 2019, Naikawadi et al., 2016, Nureki et al., 2018, Reyfman et al., 2019, Wu et al., 2020).
An emerging hypothesis of IPF pathogenesis is that the dysfunctional AT2 cell loses its facultative progenitor capacity creating a regenerative void for lung repair. In support of this hypothesis, a cardinal feature of the pathobiology of IPF is bronchiolization, a term coined decades ago to codify the observation by chest pathologists that epithelial cells with airway markers accumulate in the fibrotic regions of human lungs, appearing to extend the junctional region between distal airways and remaining alveoli (Chilosi et al., 2002). Micro-honeycomb cysts, another cardinal feature of fibrotic remodeling in humans, are mainly lined by epithelial cells with airway markers, including basal, goblet, and ciliated markers (Seibold et al., 2013). The realization that distal airway stem/progenitors robustly mobilize in mice to occupy alveolar surfaces suggests a parallel process in humans. While potentially protective against loss of tissue integrity, the progenitors arising from distal airways are also subject to signals that direct their differentiation to airway rather than alveolar phenotypes. These include hypoxia and Notch signaling (Chen, 2017). Once differentiated to airways cells, differentiation to normal AT2 or AT1 cells may be very difficult and inefficient, potentially accounting for the dysplastic structures that dominate the pathobiology of lung fibrosis (Kumar et al., 2011, Vaughan et al., 2015). In this paradigm, the dysplastic epithelial cysts accumulating progressively in fibrotic human lungs represent remnants of failed repair, as discussed above. Future efforts to both better understand the development of AT2 cell progenitor dysfunction and to minimize the pathway of airway differentiation of activated progenitors within alveoli of humans could be therapeutic.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide, and prevalence continues to increase across the globe with the continued rise in cigarette use and toxic biomasses (Burney et al., 2015). In contrast to IPF, which is characterized by robust cellular production at the site of injury resulting in a dysplastic, fibrotic pulmonary parenchyma, the cardinal feature of COPD in the alveolar space is cellular loss and alveolar simplification, known as emphysema. Mechanistic studies in COPD, however, have failed to generate novel therapeutics aimed at actual lung regeneration.
While much of the work on the deleterious effects of toxin exposures on airway cells has been done in bronchial or upper airway cells, careful anatomic studies have suggested that the site of obstruction is localized to the distal airways and in particular the respiratory airways (McDonough et al., 2011, Koo et al., 2018). The cellular structure and composition of the distal airway region of the human respiratory system, in particular the respiratory bronchioles, is poorly understood. The final endpoint of emphysema, however, is marked by loss of the alveolar epithelium, which can be studied in murine models. Studies have pointed to an increase in senescence in AT2 cells and the associated endothelium as a mechanism for the development of COPD in certain patient populations and the increased prevalence of the disease with aging (Gao et al., 2017). Together with the presence of increased senescence in lung fibroblasts from patients with COPD, these studies may suggest an exhausted phenotype in a final common pathway downstream of repeated alveolar repair in the setting of recurring toxic exposure (Müller et al., 2006). Furthermore, defective alveolar epithelial repair has been associated with reduced Wnt signaling in the COPD microenvironment, and this defective response has been suggested to be downstream of a shift in canonical to noncanonical Wnt signaling in the presence of toxic stimuli such as cigarette smoke (Baarsma et al., 2017). Whether this change in Wnt signaling is associated with an aberrant or absent response by the Wnt-responsive AEP sublineage in the AT2 cell population, remains unclear. More work is needed to understand how toxic injuries incite distal epithelial cell responses in COPD, in order to be able to develop therapies aimed at bona fide repair of the alveolus, and therefore clinical improvement.
Tools and Techniques to Study Human Lung Regeneration
Deriving Lung Epithelium De Novo via Directed Differentiation of ESCs/iPSCs
The fact that most types of primary lung epithelial cells have been difficult to propagate as stable phenotypes in cell culture has raised significant hurdles for performing basic mechanistic studies in vitro for human lung lineages. A potential solution to these challenges has emerged with the discovery of techniques to differentiate pluripotent stem cells (PSCs) in vitro into a diversity of lung lineages. Embryonic stem cells (ESCs) and their engineered equivalents—induced pluripotent stem cells (iPSCs)—represent the gold standards of PSCs, and a rapidly emerging literature has gradually led to successful methods for controlling their differentiation in cell culture.
Directed Differentiation of Pluripotent Stem Cells. The in vitro differentiation of either ESCs or iPSCs into specific tissue lineages can be guided by adding combinations of growth factors or small molecules to media at specific times during culture (Figure 3 ), in order to recapitulate the signaling pathways that regulate in vivo organ development (Murry and Keller, 2008). The process of recapitulating development in vitro to sequentially pattern pluripotent stem cells toward desired fates is termed “directed differentiation” and has been successfully applied for deriving multiple cell types from ESCs/iPSCs, including lung epithelia (Huang et al., 2015). To date most of the cell types produced from ESC/iPSC have an immature phenotype and are not yet ready for clinical applications.
Figure 3.
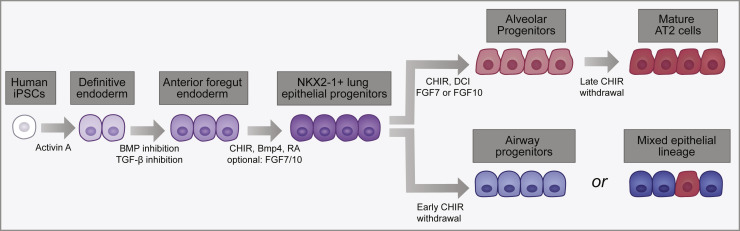
A Schematic of the Directed Differentiation of hiPSCs to Lung Epithelial Cells
The key signaling factors and the main stages of the in vitro derivation of lung epithelial cells are provided. Following lung specification, late withdrawal of CHIR gives rise to mature AT2 cells (dark red). Early withdrawal of CHIR gives rise to airway progenitors (light blue) or a mixture of mature airway cells (dark blue) and AT2 cells, depending on the protocol used.
Initial attempts at deriving lung epithelium from PSCs were inefficient, stochastic, used incompletely defined media, or relied on the presence of drug-resistance genes (Coraux et al., 2005, Van Haute et al., 2009, Wang et al., 2007). The difficulty in part was due to a lack of information regarding normal lung development in vivo. As basic mechanisms were discovered that regulate the formation of definitive endoderm, an ability to derive lung epithelia from PSCs in vitro via sequential differentiation into definitive endoderm and subsequent patterning into foregut endoderm followed (Green et al., 2011, Kubo et al., 2004). The most successful of these strategies to date has involved the exogenous addition of growth factors and inhibitors at specific times and concentrations to mimic the progressive cell signaling between the endoderm and mesoderm that specify definitive endoderm, pattern the endoderm anteriorly and then ventrally, ultimately inducing Nkx2-1+ respiratory progenitors that differentiate into lung epithelium (Green et al., 2011, Longmire et al., 2012, Mou et al., 2012, Rankin et al., 2016). A variety of studies to date indicate that the key step of initial specification of the lung epithelial lineage from foregut endodermal precursors generated from PSCs in vitro can be monitored by assessing the kinetics and efficiency of expression of Nkx2-1 or reporters for this locus (Gotoh et al., 2014, Green et al., 2011, Longmire et al., 2012, Mou et al., 2012, Rankin et al., 2016). These studies indicate that lineage specification requires the precise temporal activation of Wnt, BMP, and RA signaling (Huang et al., 2014, Rankin et al., 2016, Serra et al., 2017).
Although a high degree of agreement exists on strategies to generate lung-specified anterior foregut endoderm (AFE), the generation of mature lineages is less straightforward. Similar to other organs and tissues, the generation of fully mature cells remains challenging and is a major limitation to the application of human PSC-derived cells for disease modeling and regenerative medicine (Lancaster and Knoblich, 2014). Nevertheless, substantial progress has been made in recent years and some guiding principles have emerged. In initial studies, differentiation of PSC-derived lung progenitors was performed in adherent 2D cultures, both in mouse (Longmire et al., 2012, Mou et al., 2012) and human (Firth et al., 2014, Huang et al., 2014, Huang et al., 2015, Mou et al., 2012). This led to cultures containing a mixture of cells of undetermined maturity, but including cells expressing markers of AT2 cells. However, expression of SFTPC, one of the most specific markers of AT2 cells, was sparse. Culture of the same cells on thin slices of human decellularized lung matrix induced strong expression of SFTPC, suggesting that SFPTC expression and hence emergence of AT2 cells was facilitated by a 3D environment (Huang et al., 2014). Furthermore, cultures in 3D conditions, typically Matrigel, induced SFTPC expression accompanied by emergence of a mature AT2 program that includes production of surfactant proteins and phospholipid (Gotoh et al., 2014, Jacob et al., 2017, Yamamoto et al., 2017). Culture systems have now been developed where AT2 cells can be propagated as spheroids in 3D Matrigel cultures in presence and absence of feeders consisting of pulmonary fibroblasts (Jacob et al., 2017, Yamamoto et al., 2017). Similarly, airway progenitors generated from developmental lung progenitors specified to an anterior fate can be grown as epithelial spheres containing ciliated and secretory cells in 3D cultures (Konishi et al., 2016, McCauley et al., 2017). Single-cell RNA sequencing profiles of these spheres (McCauley et al., 2018) has confirmed the derivation of airway lineages, but has also raised a cautionary note as non-lung endodermal lineages (e.g., hepatic or gut epithelia) also appear within these spheres over extensive culture periods, representing an ongoing challenge to the field.
Further efforts have resulted in the generation of more complex lung organoids from PSCs. Lung organoids grown in Matrigel droplets can generate airway structures after xenografting into mice, while the bud tip organoids could only generate small pockets of ciliated cells after transplantation into naphthalene-injured lungs of immunodeficient mice (Dye et al., 2015, Dye et al., 2016, Miller et al., 2018, Miller et al., 2019). In a second model, PSC-derived anterior foregut endoderm cells were grown in suspension culture and as spheres containing respiratory endoderm, and the endodermal cells expressed sonic hedgehog (SHH), while the mesenchymal component expressed SHH targets. These organoids were termed lung bud organoids (Chen et al., 2017). Most of the cell types in these PSC lung organoid assays have been shown to consist of fetal stage cells, again illustrating the challenges associated with achieving full maturation (Chen et al., 2017, Dye et al., 2015, Dye et al., 2016, Miller et al., 2018, Miller et al., 2019). The origin of the mesenchymal cells that appear to co-develop along with endoderm in these PSC lung organoid cultures to date remains unclear.
The second emerging principle arising from experience with iPSC models is a need to better understand the temporal developmental signaling pathways required for lung lineage specification. For example, BMP inhibition is required for AFE specification (Green et al., 2011). However, consistent with mouse genetic models, subsequent BMP agonism promotes lung fate from AFE (Huang et al., 2014, Longmire et al., 2012). An important component for the differentiation of AT2 cells is dexamethasone, 8-bromo-cAMP, and isobutylmethylxanthine (DCI), FGF7 or 10, and Gsk3β agonism using the small molecule CHIR9902 (Huang et al., 2014, Huang et al., 2015, Longmire et al., 2012, Serra et al., 2017, Yamamoto et al., 2017, Miller et al., 2018). Withdrawal of Gsk3β inhibition, which results in reduced Wnt signaling, appears to induce a proximal fate in lung progenitors (McCauley et al., 2017). Conversely, maintenance of CHIR9902 in 2D cultures and subsequent plating in 3D cultures in the presence of CHIR9902 yielded AT2 spheroids that could be replated and expanded for many passages (Jacob et al., 2017). Interestingly, full morphological maturation accompanied by reduced proliferation of AT2 cells could be accomplished by withdrawal of Gsk3β inhibition in the 3D cultures (Jacob et al., 2017). In some 3D models, withdrawal of CHIR9902 led to both proximal and distal maturation, with the generation of NGFR+ postnatal basal cells, AT2 cells, and cells expressing markers of AT1 cells (de Carvalho et al., 2019). Gsk3β inhibition was also required to generate renewing distal tip progenitors (Miller et al., 2018). Together, these studies indicate that further analysis of the temporal activation and repression of Wnt signaling in driving lung endoderm fate is needed. Importantly, Gsk3β integrates multiple inputs and affects a wide variety of signaling pathways and cellular process, and some of its effects may be independent of Wnt signaling (Patel and Woodgett, 2017). Additional signaling pathways including Notch (Chen et al., 2017, Chen et al., 2019, Firth et al., 2014, McCauley et al., 2017, Konishi et al., 2016) and retinoic acid are important for promoting lung endoderm cell fate (Miller et al., 2018).
A third principle guiding effective directed differentiation of PSCs into lung epithelium is the proper initial establishment of lung progenitor fate. The purity of the PSC-derived lung progenitors, which can vary from line to line, remains a problem (Huang et al., 2015). One solution is the use of reporters, such as fluorochrome constructs targeted to NKX2.1, SFTPC, or SCGB3A2 loci, which allow flow cytometric purification of desired cell types (Gotoh et al., 2014, Hawkins et al., 2017, McCauley et al., 2017, McCauley et al., 2018, Serra et al., 2017). These cell lineage-specific reporters have been useful in defining the differentiation strategies of various cell types in the lung. However, a drawback of reporter lines is that this strategy is unlikely to be approved for the purification of human cells for clinical therapies. A second solution is the use of surface markers to enrich for lung endoderm progenitors such as carboxypeptidase M or CD47hiCD26lo cells (Gotoh et al., 2014, Hawkins et al., 2017, Korogi et al., 2019).
Lung Disease Modeling via Pluripotent Stem Cells. One of the major applications for iPSC-derived lung epithelial lineages is providing an in vitro platform for studying human pulmonary diseases. Early applications of iPSCs attempted to model cystic fibrosis (CF), a disease caused by mutations in the anion channel protein, CFTR (cystic fibrosis transmembrane conductance regulator), which leads to progressive lung damage due to impaired mucociliary clearance, inflammation, and recurrent respiratory infections. Wong et al. (2012) developed the first iPSC-derived model of CF using a 2D system in which CF-iPSC-derived epithelial cells exhibited lower CFTR expression and reduced anion transport in response to forskolin, a known CFTR activator. A subsequent 2D airway model was published by Firth et al. (2014), in which the authors demonstrated the presence of functional CFTR channels, with anion currents that could be modulated by both forskolin and a CFTR inhibitor. CRISPR correction of a CFTR mutation in iPSCs by two separate groups then demonstrated an in vitro rescue of channel function (Crane et al., 2015, Firth et al., 2015). Finally, recent work from McCauley et al. (2017) used iPSC-derived airway epithelial spheres to demonstrate that CRISPR correction of CFTR mutants can rescue defects in forskolin-induced organoid swelling, providing an easily visualized read-out for future high throughput screens of airway CFTR function.
More recent applications of iPSC-derived lung models have focused on a broad diversity of genetic lung diseases, lung cancer, or platforms for the study of viral respiratory infections. For example, 2D differentiation of hPSC-derived lung progenitors allowed modeling influenza susceptibility in iPSCs from a patient with a genetic deficiency in IRF7 (Ciancanelli et al., 2015) and from a patient with TLR3 mutation (Lim et al., 2019). The importance of these findings lies in the fact that they showed that not only interferon production by immune cells was relevant to the increased susceptibility of these patients to lethal influenza infection, but that interferon production within distal lung epithelial cells is likely involved as well. In branching 3D organoids, the pathological changes associated with respiratory-syncytial virus-associated bronchiolitis could be reproduced (Chen et al., 2017) and infection with clinical isolates of parainfluenza virus could be modeled (Porotto et al., 2019). In 2D cultures, through genetic manipulation of Rb and p53 expression in Notch-inhibited epithelium enriched in neuroendocrine cells, a human in vitro model for small cell lung cancer was developed (Chen et al., 2019). For modeling genetic diseases affecting the distal lung, distal specification and generation of expandable AT2 cells in 3D allowed modeling of SFTPB deficiency, which was corrected by CRISPR/Cas9-mediated gene editing (Jacob et al., 2017). Recessive mutations in some genes implicated in Hermansky-Pudlak syndrome (HPS) cause HPS-associated interstitial pneumonia (HPSIP), a clinical entity similar to idiopathic pulmonary fibrosis (Lederer and Martinez, 2018). Introduction of HPS mutations associated with HPSIP in ESCs promoted fibrotic changes in branching lung organoids (Chen et al., 2017), while deletion of HPS8, which is not associated with HPSIP, did not (Strikoudis et al., 2019). Further studies revealed an essential role for IL11 in the fibrotic process, suggesting that IL11 is a potential therapeutic target in this intractable disease (Strikoudis et al., 2019). Additional work using AT2 cells generated from patient-specific iPSCs profiled the AT2 cell dysfunction that results from HPS1 mutations (Korogi et al., 2019).
Cell-Based Therapy for the Respiratory System
Many false starts and claims of engraftment of exogenous cells into injured murine lung tissue have confused the field for the past 20 years, with an extensive literature emerging during the controversial claims of bone marrow plasticity during the 1990s (reviewed in Kotton, 2012). More recently a few groups have begun to publish more convincing evidence in support of the capacity of epithelial stem/progenitor cells, of both mouse and human origin, to survive in injured mouse lungs after intratracheal or intravenous delivery (Farrow et al., 2018, Miller et al., 2018, Nichane et al., 2017, Rosen et al., 2015, Vaughan et al., 2015). There is also recent evidence of more mature AT2 cells of both mouse and human origin surviving transplantation into injured mouse lungs (Hillel-Karniel et al., 2020, Weiner et al., 2019). Whether these cells are functionally integrated into host lung tissue, are long-lived, and retain expression of a complete lung transcriptomic program are still open questions that will need to be addressed before these cells can be referred to as “engrafted.” For example, only one study to date has attempted to profile the transcriptomes of grafted cells at single-cell resolution (Nichane et al., 2017). The use of lineage tracing techniques and clonality studies are notably absent from most prior reports, raising uncertainty about the cellular origins of the source cells and preventing assessment of the stem cell properties of transplanted cells. Importantly, physiologic improvement of injured mouse lungs after cell transplantation as described in some of the above reports might be explained by paracrine effects or transient secondary reparative effects on recipient lung tissue rather than by direct, durable replacement of the epithelium with functional cells. In addition to alveolar cell therapy, a recent study demonstrated that it is possible to correct CF mutations using primary airway basal cells, which could be used for engraftment into patients (Vaidyanathan et al., 2020).
Many additional challenges remain in the translation of experimental cell therapy in mice to humans with major lung injury, including the issue of allogeneic rejection, possibly circumvented by the development of autologous, syngeneic iPSC-derived stem/progenitors if these were to have engraftable potential (Huang et al., 2015, McCauley et al., 2017, Miller et al., 2018). The limitations listed above have also resulted in a dearth of information regarding the mechanisms or microenvironmental niche interactions that might regulate homing, survival, proliferation, or functional activation of potentially engrafted cells. More sophisticated methods are needed to quantify the contributions of exogenously delivered cells, relative to either endogenous cells or competing alternative source cells. Additional studies are needed to resolve the ongoing debate over which of many candidate lung cell populations, from resident progenitors or iPSC derivatives to common mature airway or alveolar cells, might have the best relative potential to reconstitute an injured epithelium after transplantation.
Single-Cell Genomic Analysis in Lung Biology
The extensive cellular heterogeneity within the respiratory system and the lack of insight into the phenotype differences in these purported cell lineages make single-cell genomics a very useful tool in the study of lung repair and regeneration. This spatial diversity in architecture and cellular heterogeneity reinforces the importance of sampling at multiple regions along the respiratory airway axis to obtain a true cell atlas. Advances in scRNA-seq techniques allow for prediction of cell state, origins, and trajectories without requiring permanent genetic labeling of cells. New tools enabling data analysis, visualization, and interpretation are being developed at a rapid pace and are readily available as “open source” tools. Likewise, data web portals provide the research community with open access to rich diversity of RNA, protein, and lipidomic and imaging data related to the lung. Examples of this include the Lung Gene Expression Atlas and BREATH databases (https://research.cchmc.org/pbge/lunggens/mainportal.html and https://www.lungmap.net) (Du et al., 2017). While time series of single-cell transcriptomic data are most useful in identifying progenitor cells and their trajectories, these processes can be identified in pseudotime using data derived from a single time point when large numbers of cells are in transition; for example, during organ formation, tissue repair, or during active disease. Likewise, single-cell transcriptomic data generated with embryonic stem cells or induced pluripotent stem cells as they differentiate into distinct lung cell types have provided new insights into the identity of lung cell progenitors and the regulatory networks controlling cell fate decisions (Chen et al., 2019, Hawkins et al., 2017, McCauley et al., 2018, Nikolić et al., 2018).
Trajectory inference algorithms, or pseudotime analysis, reconstruct continuous cell state transitions from “snapshots” of single-cell transcriptomic data, providing insights into gene expression kinetics and regulatory dynamics of biological processes (Gerber et al., 2018, Guo et al., 2017, Hawkins et al., 2017). Wanderlust (Bendall et al., 2014), Monocle 1 (Trapnell et al., 2014), Waterfall (Shin et al., 2015), and TSCAN (Ji and Ji, 2016) are among the early cell trajectory inference algorithms using scRNA-seq data. The general analytical workflow of a pseudotime analysis algorithms includes (1) modeling individual cells as data points in high-dimensional gene expression space, where each dimension is defined by the expression of a gene; (2) projecting cells onto a lower-dimensional space using a dimension reduction method, e.g., principal component analysis, which finds a smaller set of new dimensions, each being a combination of original input genes, to represent major variances in the original gene expression space, reduce noise, and enable data visualization; (3) determining cell trajectories in the reduced dimensional space; (4) ordering single cells by progress along the cell trajectories; and (5) identifying gene expression patterns along cell orderings.
With the evolution of single-cell technologies and exponential growth in numbers of cells collected from single-cell experiments (Svensson et al., 2018), scRNA-seq datasets have captured complex cell trajectories that often contain multiple cell type decision branches. Monocle 2 (Qiu et al., 2017), Wishbone (Setty et al., 2016), DPT (Haghverdi et al., 2016), SLICE (Guo et al., 2017), Slingshot (Street et al., 2018), and URD (Farrell et al., 2018) can be used to infer tree-structured cell trajectories from scRNA-seq data, reconstructing branched cell transitional paths from a progenitor toward multiple cell fates. To infer a robust tree-structured cell lineage model, Slingshot (Street et al., 2018) combines the use of multiple techniques recently developed for processing highly noisy scRNA-seq data and allows users to specify initial and terminal cells to supervise parts of the tree construction, which might lead to more accurate data-specific lineage tree inference. Monocle 2 (Qiu et al., 2017) is a comprehensive computational pipeline for scRNA-seq analysis, including gene expression modeling, preprocessing, cell clustering, differentiation expression analysis, and cell trajectory inference analysis. Recently developed methods, including PAGA (Wolf et al., 2019) and Monocle 3 (Cao et al., 2019), can be used to infer more complex, non-tree-based cell trajectories. Together with a number of implementation improvements in computational efficiency and memory usage, PAGA and Monocle 3 can be applied to infer complex lineage relations from large scRNA-seq datasets with more than 1 million cells, for example inferring the lineage relations of cells from a whole adult animal (Plass et al., 2018) or the reconstruction of 10 disjointed major cell trajectories from ∼1.5 million mouse embryonic cells during organogenesis (Cao et al., 2019).
Most of the trajectory inference methods pseudo-temporally order cells purely based on transcriptomic similarity without estimating cell states (e.g., differentiation states). They require the use of external knowledge, such as time information, cell identity, marker gene expression, or user inputs, to determine the start and end points and the directions of inferred cell trajectories. StemID (Grün et al., 2016), SLICE (Guo et al., 2017), and RNA Velocity (La Manno et al., 2018) provide the capability to estimate cell differentiation states, predicting progenitor cells and directions of cell differentiation transitions. StemID (Grün et al., 2016) and SLICE (Guo et al., 2017) both exploit the concept of entropy for predicting cell differentiation states from single-cell transcriptomic profiles. RNA Velocity showed that scRNA-seq data can provide not only static information on mRNA abundance of each gene in a single cell but also dynamic information on how the expression of each gene in a single cell is changing through annotating the ratio of unspliced and spliced transcripts of each gene in each cell, predicting the future state of each cell (La Manno et al., 2018).
Single-cell experiments can be performed at multiple time points relevant to biological processes, such as lung development, regeneration, or disease progression, generating single-cell time-course data. Trajectory inference methods, including STITCH (Wagner et al., 2018) and Waddington-OT (Schiebinger et al., 2019), utilize the time of collection to supervise the analysis and improve the accuracy of pseudotime analysis using time-course scRNA-seq data (Wagner et al., 2018). Since data collected at multiple time points can be influenced by technical variations in cell isolation, library generation, and sequencing, STITCH (Wagner et al., 2018) analyzed time-course scRNA-seq data by first constructing a single-cell graph from each time point and then joining pairs of graphs from adjacent time points by connecting similar cells (Wagner et al., 2018). Waddington-OT, on the other hand, directly estimates a transitional probability between any two cells from consecutive time points in a time-course scRNA-seq data without explicitly inferring a lineage topology (Schiebinger et al., 2019). Given a set of cells at a time point, Waddington-OT predicts the most likely ancestor cells in earlier time points and descent cells in later time points based on the derived cell-cell transition probabilities, reconstructing a cell transitional path across time points. Importantly, these cellular trajectory models require experimental validation either in the form of cell-type-specific genetic lineage tracing in mice or the use of cellular barcoding strategies in non-murine systems such as was performed in a recent study to predict the differentiation of lung epithelial progenitors from pluripotent stem cells (Hurley et al., 2020).
Single-cell analysis has been used extensively to assess both mouse and human lung development and disease. Improved scRNA-seq methods carefully coupled with cell-type-specific lineage tracing has uncovered new developmental and regenerative origins for multiple lung cell lineages. The early specification of AT1 and AT2 cells, coincident with such early and basic tissue patterning processes such as branching morphogenesis, and the heterogeneity of embryonic alveolar epithelial progenitors, was recently uncovered using scRNA-seq methods (Frank et al., 2019, Guo et al., 2019). The heterogeneity within the adult lung mesenchyme was recently described and revealed the importance of the mesenchymal niche in alveolar homeostasis and regeneration (Guo et al., 2019, Lee et al., 2017, Zepp et al., 2017). Human lung scRNA-seq has been reported in both “normal” lungs and in diseased lungs such as idiopathic pulmonary fibrosis (Reyfman et al., 2019, Xu et al., 2016). scRNA-seq was also used to identify the ionocyte, which is thought to play a key role in cystic fibrosis (Montoro et al., 2018, Plasschaert et al., 2018).
There are multiple consortia that are working to establish a human lung cell atlas including the NIH-supported LungMAP and the Chan-Zuckerberg-supported Human Cell Atlas. One of the major hurdles in using single-cell techniques to map the human lung is the high level of cell heterogeneity that exists in the organ, which means that isolation procedures including both upstream and downstream processing are going to be highly variable depending on the site and research group involved. These issues are revealed in many experiments that lack significant representation of fragile cells such as AT1 cells and over-representation of immune cells. In the absence of procedures and protocols that generate highly reproducible isolation of all known cell types, both qualitatively and quantitatively, across most if not all lung samples, the resulting datasets are unlikely to provide an accurate cell atlas. Moreover, the respiratory system is highly complex and contains many spatially restricted compartments and niches which need to be uniquely sampled, possibly using different techniques for each region. Thus, much work remains to be done to ensure that such single-cell mapping efforts can reproducibly isolate and characterize all resident and immune cells within the human respiratory system.
Bioengineering of the Whole Lung
Bioengineering of the lung has made remarkable progress in the recent years, motivated by strong clinical needs and enabled by advances in tissue engineering and stem cell biology. As many as 25 million people suffer from end-stage lung disease in the United States alone, with ∼400,000 patients dying each year, a third of these from nonmalignant diseases (OPTN, 2012, Morrisey and Hogan, 2010, Petersen et al., 2010). Worldwide, lung disease remains the third leading cause of death (Murphy et al., 2018, Rabe et al., 2007). Notably, most lung diseases affect epithelium, including the acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), emphysema, cystic fibrosis (CF), and pulmonary fibrosis.
Lung transplantation, the only definitive treatment option for end-stage lung disease, remains hampered by a severe shortage of donor organs. In addition, a majority of donor lungs are deemed unacceptable for transplantation at the time of receipt (Ware et al., 2002), making lung the least utilized solid organ and necessitating the use of extracorporeal membrane oxygenation (ECMO) as a bridge to transplant for critically ill patients (Fuehner et al., 2012, Javidfar and Bacchetta, 2012, Klein et al., 2010, Pomfret et al., 2008, Ware et al., 2002).
To overcome this crisis, there are major efforts to increase the number of transplantable lungs, including (1) criteria expansion, i.e., accepting older donors, lungs donated following cardiac death (Bittle et al., 2013, Elgharably et al., 2015, Van Raemdonck et al., 2009), (2) ex vivo lung perfusion (EVLP) to recover marginally unacceptable donor lungs (Cypel and Keshavjee, 2015), (3) bioengineering of functional lungs by populating decellularized lungs or scaffolds with epithelial and vascular cells (Ott et al., 2010, Petersen et al., 2010, Rosen et al., 2015, Wagner et al., 2013, Wobma and Vunjak-Novakovic, 2016), and (4) utilization of xenogeneic lungs from swine or non-human primates (Cooper et al., 2012, Laird et al., 2016). Thus far, the number of lung transplantations remains steady, resulting in the increasing waitlist mortality (Valapour et al., 2015). New strategies are being developed to increase the numbers of lungs for transplantation by recovering marginal quality donor lungs and developing physiological ex vivo platforms for modeling lung disease. The enormous complexity of the lung, with its hierarchical architecture, more than 40 cell types, and a very large area (∼70 m2) for gas exchange, presents major challenges to lung recovery (Crapo et al., 1982, Massaro and Massaro, 1996, Weibel, 1973). Finally, generation of humanized whole lungs through stem cell complementation in other species could lead to a new source of transplantable lungs (Mori et al., 2019).
Ex vivo Lung Perfusion (EVLP) and Cross-circulation with a Living Host. Among current approaches, EVLP holds the strongest potential for immediate clinical impact by recovering marginally unacceptable donor lungs, which has been already implemented and is being further evaluated in clinical trials (Popov et al., 2015). Notably, many of the conditions that render donor lungs unacceptable for transplantation (e.g., aspiration, infection, pulmonary contusions) could be reversible. However, conventional methods of donor lung preservation that involve cold static ischemia preclude endogenous repair and recovery (Guibert et al., 2011, Pinezich and Vunjak-Novakovic, 2019). The field of ex vivo lung perfusion (EVLP) is now addressing this limitation by providing initially unacceptable donor lungs with physiologic conditions of normothermia, perfusion, and ventilation, to recover function outside the body to a level acceptable for transplantation (Makdisi et al., 2017, Tane et al., 2017). Since the introduction of EVLP by Steen and colleagues in 2001 (Steen et al., 2001), EVLP platforms have successfully demonstrated short-term support and recovery of marginal quality donor lungs in pre-clinical and clinical settings (Cypel et al., 2011, Warnecke et al., 2018). A major limitation of EVLP is the lack of whole-body homeostasis that requires renal, hepatic, pancreatic, and neurohormonal functions. The current durations of clinical usage of EVLP systems are too short for advanced therapeutic interventions (e.g., immunomodulation, cell therapy) (Warnecke et al., 2012). Moreover, current EVLP systems cannot recover the majority of unusable donor lungs, due to the lack of appropriate physiologic milieu for endogenous repair.
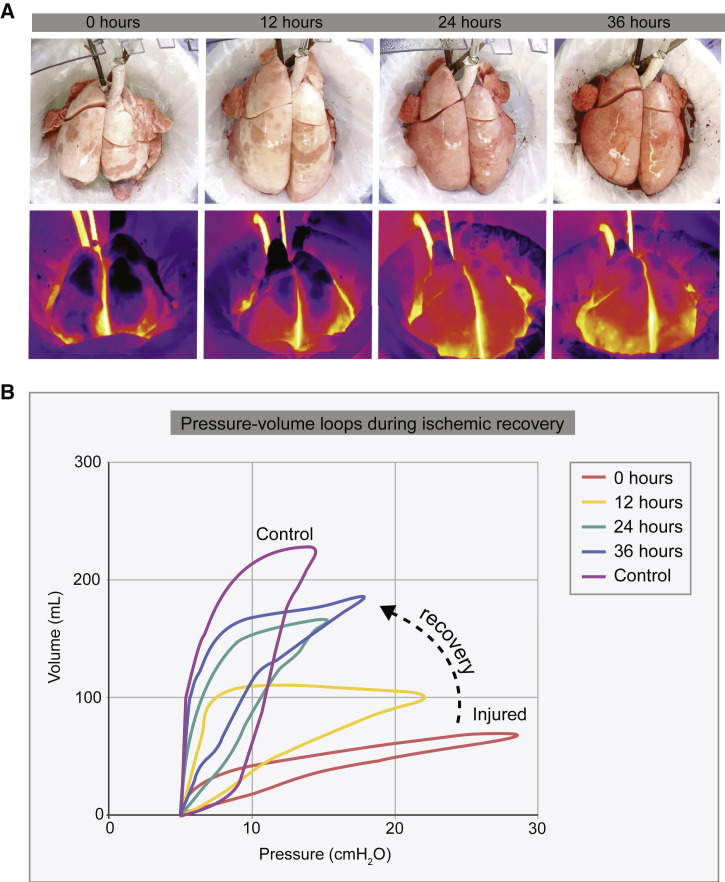
Using a clinically relevant swine model, a cross-circulation platform has been established with the recipient support enabling 36 h of normothermic perfusion for the maintenance and recovery of injured lungs (O’Neill et al., 2017). In recent studies, the duration of lung support on cross-circulation has been extended to 4 days (Hozain et al., 2019). Cross-circulation is actually an old technique that has been used in the past to support patients suffering from a critical but potentially reversible illness given sufficient time for recovery (e.g., hepatic insufficiency, uremia, eclampsia) (Burnell et al., 1965, Burnell et al., 1967, Eschbach et al., 1964) by a healthy individual.
For extracorporeal lungs, cross-circulation was used to extend the duration of lung maintenance ex vivo from hours to days, by providing metabolic clearance and systemic factors to the perfused and ventilated lung (Figure 4 ; O’Neill et al., 2017). This method allowed time for multiscale therapeutic interventions, with the aid of real-time theranostic (therapeutic + diagnostic) imaging (Kim et al., 2015b, Kim et al., 2017). By the end of cross-circulation support, the lungs that were severely damaged by ischemia or gastric aspiration exceeded transplantation criteria, and the recipients tolerated the procedure without significant changes in physiologic parameters. These findings suggest that cross-circulation could enable extended support necessary for gene and cell therapies of the extracorporeal lungs.
Figure 4.
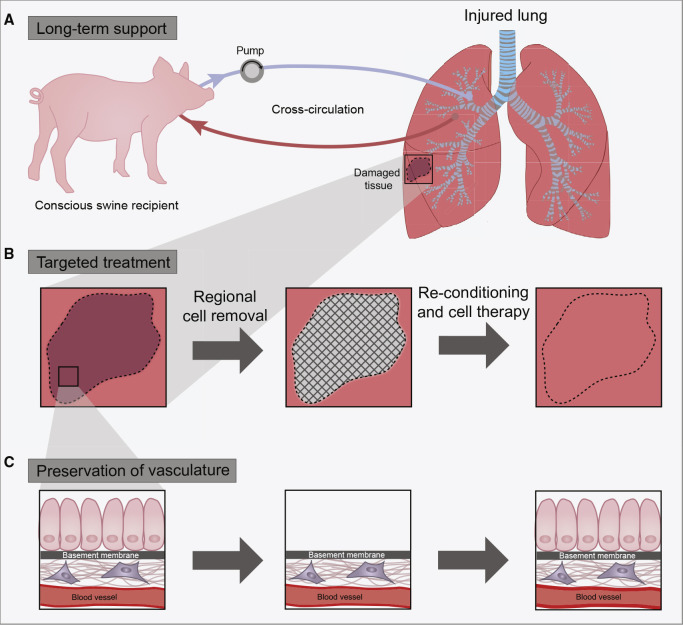
Bioengineering Approach to the Recovery of Injured Lungs
Three methodological advances have been combined to enable functional recovery of lungs damaged by cold ischemia and gastric aspiration.
(A) Multiday normothermic support of extracorporeal lungs using cross-circulation with a recipient in a clinically relevant swine model.
(B) Targeted treatment of the most injured lung regions, with the preservation of the surrounding lung tissue, through the local removal of damaged cells and their replacement with healthy therapeutic cells.
(C) Removal of injured lung epithelium from the targeted regions of the lung, with preservation of the basement membranes and the vascular compartment.
Lung Bioengineering by Complete Decellularization and Recellularization of the Lung Parenchyma and Vasculature. The Niklason and Ott laboratories have pioneered a methodology that involves removal of all cells from the lung and subsequent infusion of epithelial cells into parenchymal region and endothelial cells into the vascular region of the lung (Ott et al., 2010, Petersen et al., 2010). However, these bioengineered lungs fail shortly upon transplant, due to incomplete regeneration and leaky vasculature causing alveolar edema and thrombosis (Ott et al., 2010, Petersen et al., 2010, Petersen et al., 2011, Petersen et al., 2012). While this highly innovative approach has advanced through many meritorious studies, three limitations remain: (1) the inability to restore gas exchange, (2) the inability to maintain vascular function upon transplantation, and (3) the need for very high cell numbers (billions) to repopulate the lung (Petersen et al., 2010, Song et al., 2011).
Targeted Treatment of Lung Epithelium with the Preservation of Lung Vasculature. In principle, the limitations of full decellularization/recellularization of the lung could be overcome if acutely injured donor lungs are treated to replace only the epithelium in the most injured regions of the lung, while preserving the integrity of the lung vasculature. Because acute injury tends to affect some regions of the lung and not the entire lung (Raghavendran et al., 2011), a targeted treatment could help preserve much of the existing lung function and facilitate regeneration of the injured regions. An alternate approach to lung bioengineering has been developed that is based on removing damaged epithelial cells from distal lungs (Dorrello et al., 2017) while maintaining the integrity of the basement membrane, surrounding lung cells and matrix, and the functionality of lung vasculature. Denuded regions in the lung airway are repopulated with epithelial progenitors. The maintenance of an intact vascular network was considered critical for maintaining the blood-gas barrier as well as for supporting survival of newly delivered cells. This airway-specific approach was first demonstrated in the rat model, resulting in vascularized lung grafts that supported the attachment and growth of human adult pulmonary cells and stem-cell-derived alveolar progenitor cells (Dorrello et al., 2017).
Targeted decellularization/recellularization of the lung epithelium was achieved by introducing soluble reagents (such as the solutions for cell removal, suspensions of therapeutic cells) in micro-volume liquid plugs to the targeted branches of the pulmonary airway: upper airways, small airways (bronchioles), or the most distal lung (alveoli). Liquid plugs (only <1 mL in volume) were instilled into the upper airway and pushed into a specific more distal airway to form liquid film covering lung epithelium, using programmed ventilation of the lung in conjunction with radiation-free transpleural imaging (Kim et al., 2015a).
Combination of Long-Term Lung Support Ex Vivo with the Targeted Treatment of Lung Epithelium. Cell replacement therapy offers compelling prospects for the treatment of injured lungs, if the extracellular matrix (including the basement membranes) could be preserved. A new methodology for lung regeneration is now under development through an integrated use of three components: (1) extended duration of lung support ex vivo (several days) by cross-circulation with a living host, (2) targeted treatment of the injured regions of the lung (from conducting airways to distal regions), with the preservation of the surrounding lung parenchyma, and (3) selective removal and replacement of lung epithelium with the preservation of lung vasculature (for immediate blood supply for lung survival and function) (Figure 4). The same issues regarding whether newly delivered or replaced cells merit use of the controversial term, “engraftment,” (indicating true, durable integration of functional cells) apply to this novel approach as discussed above (see Cell-Based Therapy for the Respiratory System) and have not yet been addressed.
The utility of this approach was demonstrated in clinical-scale swine models for recovering lungs unsuitable for transplant due to acute injury by ischemia (O’Neill et al., 2017) and gastric aspiration (Guenthart et al., 2019) (Figure 5 ). In both cases, the lung epithelium was removed from injured regions while preserving the surrounding cells, matrix, and intact lung vasculature (Dorrello et al., 2017), and the denuded epithelial regions were repopulated with pulmonary progenitor cells. In one study, the injured lungs were maintained on cross-circulation support without decline in lung function and were subjected to therapeutic interventions that enabled cellular regeneration and improved function over the course of 36 h (Guenthart et al., 2019). Regeneration of severely damaged lungs to the levels necessary for meeting the transplantation criteria would help significantly increase the pool of donor lungs available for transplant, for example by recovering lungs rejected because of gastric aspiration damage.
Figure 5.
Macroscopic Appearance of Injured Lungs over 36 h on Cross-circulation
(A) Gross appearance of ischemic lungs and the corresponding thermal images for ischemic lungs damaged by gastric aspiration. The increase in heat exchange indicates improved vascular perfusion.
(B) Pressure-volume loops during ischemic recovery. The increase volume and decreased pressure indicates improved pulmonary function to the level necessary for lung transplant (reproduced with permission from O’Neill et al., 2017).
On cross-circulation, the injured lungs recovered their mechanical compliance to 68% of that of uninjured control lungs, corresponding to a 6-fold improvement relatively to the levels measured post-injury. A lung injury scoring rubric that has been developed in these studies (Guenthart et al., 2019, O’Neill et al., 2017) was also used to assess the extent of lung injury over 4 days of lung maintenance and recovery (Hozain et al., 2019). The lung injury scores decreased across all categories, with the greatest improvements observed in alveolar cells functionality, lung edema, and cell apoptosis. The recovered lungs displayed regeneration of cell surface markers in the pulmonary epithelium, endothelium, tight and gap junctions, recovery of subcellular structures (e.g., intracellular vesicles), and cellular function and integrity (Guenthart et al., 2019). Throughout the duration of extracorporeal support, the recipient tolerated cross-circulation with no significant changes in physiologic parameters. After 36 h of the procedure, the lungs injured by ischemia or gastric aspiration exceeded transplantation criteria, as evidenced by the gross appearance, thermography, and P-V loops that were consistent with recovered lungs (Figure 5). Future studies may lead to interventional cross-circulation being used for the expansion of donor lung pools through the salvage of severely damaged lungs.
Future Directions
The field of lung regeneration has advanced immensely in the last decade. The emergence of cell-type-specific lineage tracing coupled with better injury models has allowed investigators to begin to directly link the behavior among the various cell lineages in the lung and their responses to acute injury. New methods for generating human lung cells using PSC technologies, coupled with better imaging modalities and evolving assays such as human lung organoids and precision cut lung slices, are allowing for the direct use of human lung tissue to experimentally and mechanistically assess the response to acute injuries and chronic diseases. The recent advent of single-cell genomics and advanced bioengineering techniques allow not only for the exquisite definition of all cell types within the human and mouse lung but provides clinically relevant approaches to regenerate unusable human lungs for transplant. Finally, the ongoing COVID-19 pandemic highlights the need for a better understanding of lung regeneration in the face of overwhelming acute viral injury. Given that lung disease remains the third leading cause of morbidity and mortality in the world, all of these advances and many more will be needed to develop better therapeutic approaches for the many patients in need.
Acknowledgments
The following authors were supported by funds from the National Institutes of Health: M.J.H. (HL149263) and J.J.K. (HL143931). All of the PIs listed as authors received funding from the NIH/NHLBI-supported Progenitor Cell Translational Consortium (PCTC): H.A.C. (HL134766), D.N.K. (HL134766, HL134745), H.-W.S. (HL134760), G.V.-N. (HL134760), and E.E.M. (HL134745).
References
- Alvarez D.F., Huang L., King J.A., ElZarrad M.K., Yoder M.C., Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L419–L430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- Baarsma H.A., Skronska-Wasek W., Mutze K., Ciolek F., Wagner D.E., John-Schuster G., Heinzelmann K., Günther A., Bracke K.R., Dagouassat M. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 2017;214:143–163. doi: 10.1084/jem.20160675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall S.C., Davis K.L., Amir A.D., Tadmor M.D., Simonds E.F., Chen T.J., Shenfeld D.K., Nolan G.P., Pe’er D. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014;157:714–725. doi: 10.1016/j.cell.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncello I., McQualter J.L. Endogenous lung stem cells: what is their potential for use in regenerative medicine? Expert Rev. Respir. Med. 2010;4:349–362. doi: 10.1586/ers.10.21. [DOI] [PubMed] [Google Scholar]
- Bittle G.J., Sanchez P.G., Kon Z.N., Claire Watkins A., Rajagopal K., Pierson R.N., 3rd, Gammie J.S., Griffith B.P. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J. Heart Lung Transplant. 2013;32:760–768. doi: 10.1016/j.healun.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Blackwell T.S., Hipps A.N., Yamamoto Y., Han W., Barham W.J., Ostrowski M.C., Yull F.E., Prince L.S. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J. Immunol. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchfield K., Nantie L., Verheyden J.M., Sui P., Wienhold M.D., Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhain W.J., Brody J.S. Compensatory growth of the lung following pneumonectomy. J. Appl. Physiol. 1973;35:898–902. doi: 10.1152/jappl.1973.35.6.898. [DOI] [PubMed] [Google Scholar]
- Burnell J.M., Thomas E.D., Ansell J.S., Cross H.E., Dillard D.H., Epstein R.B., Eschbach J.W., Jr., Hogan R., Hutchings R.H., Motulsky A. Observations on cross circulation in man. Am. J. Med. 1965;38:832–841. doi: 10.1016/0002-9343(65)90002-1. [DOI] [PubMed] [Google Scholar]
- Burnell J.M., Dawborn J.K., Epstein R.B., Gutman R.A., Leinbach G.E., Thomas E.D., Volwiler W. Acute hepatic coma treated by cross-circulation or exchange transfusion. N. Engl. J. Med. 1967;276:935–943. doi: 10.1056/NEJM196704272761701. [DOI] [PubMed] [Google Scholar]
- Burney P.G., Patel J., Newson R., Minelli C., Naghavi M. Global and regional trends in COPD mortality, 1990-2010. Eur. Respir. J. 2015;45:1239–1247. doi: 10.1183/09031936.00142414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Ye T., Sun Y., Ji G., Shido K., Chen Y., Luo L., Na F., Li X., Huang Z. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci. Transl. Med. 2017;9:9. doi: 10.1126/scitranslmed.aai8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Spielmann M., Qiu X., Huang X., Ibrahim D.M., Hill A.J., Zhang F., Mundlos S., Christiansen L., Steemers F.J. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566:496–502. doi: 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.A., Li X., Alexander J.P., Brumwell A., Lorizio W., Tan K., Sonnenberg A., Wei Y., Vu T.H. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J. Clin. Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Origin and regulation of a lung repair kit. Nat. Cell Biol. 2017;19:885–886. doi: 10.1038/ncb3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Acciani T., Le Cras T., Lutzko C., Perl A.K. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell Mol. Biol. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.W., Huang S.X., de Carvalho A.L.R.T., Ho S.H., Islam M.N., Volpi S., Notarangelo L.D., Ciancanelli M., Casanova J.L., Bhattacharya J. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 2017;19:542–549. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.J., Poran A., Unni A.M., Huang S.X., Elemento O., Snoeck H.W., Varmus H. Generation of pulmonary neuroendocrine cells and SCLC-like tumors from human embryonic stem cells. J. Exp. Med. 2019;216:674–687. doi: 10.1084/jem.20181155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Poletti V., Murer B., Lestani M., Cancellieri A., Montagna L., Piccoli P., Cangi G., Semenzato G., Doglioni C. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab. Invest. 2002;82:1335–1345. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- Chung M.I., Bujnis M., Barkauskas C.E., Kobayashi Y., Hogan B.L.M. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145:145. doi: 10.1242/dev.163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciancanelli M.J., Huang S.X., Luthra P., Garner H., Itan Y., Volpi S., Lafaille F.G., Trouillet C., Schmolke M., Albrecht R.A. Infectious disease. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science. 2015;348:448–453. doi: 10.1126/science.aaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D.K., Ekser B., Burlak C., Ezzelarab M., Hara H., Paris L., Tector A.J., Phelps C., Azimzadeh A.M., Ayares D. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19:144–158. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraux C., Nawrocki-Raby B., Hinnrasky J., Kileztky C., Gaillard D., Dani C., Puchelle E. Embryonic stem cells generate airway epithelial tissue. Am. J. Respir. Cell Mol. Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- Crane A.M., Kramer P., Bui J.H., Chung W.J., Li X.S., Gonzalez-Garay M.L., Hawkins F., Liao W., Mora D., Choi S. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports. 2015;4:569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J.D., Barry B.E., Gehr P., Bachofen M., Weibel E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- Cypel M., Keshavjee S. Extending the donor pool: rehabilitation of poor organs. Thorac. Surg. Clin. 2015;25:27–33. doi: 10.1016/j.thorsurg.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Cypel M., Yeung J.C., Liu M., Anraku M., Chen F., Karolak W., Sato M., Laratta J., Azad S., Madonik M. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- Danahay H., Pessotti A.D., Coote J., Montgomery B.E., Xia D., Wilson A., Yang H., Wang Z., Bevan L., Thomas C. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- de Carvalho A.L.R.T., Strikoudis A., Liu H.Y., Chen Y.W., Dantas T.J., Vallee R.B., Correia-Pinto J., Snoeck H.W. Glycogen synthase kinase 3 induces multilineage maturation of human pluripotent stem cell-derived lung progenitors in 3D culture. Development. 2019;146:146. doi: 10.1242/dev.171652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B.S., Nolan D.J., Guo P., Babazadeh A.O., Cao Z., Rosenwaks Z., Crystal R.G., Simons M., Sato T.N., Worgall S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrello N.V., Guenthart B.A., O’Neill J.D., Kim J., Cunningham K., Chen Y.W., Biscotti M., Swayne T., Wobma H.M., Huang S.X.L. Functional vascularized lung grafts for lung bioengineering. Sci. Adv. 2017;3:e1700521. doi: 10.1126/sciadv.1700521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Kitzmiller J.A., Sridharan A., Perl A.K., Bridges J.P., Misra R.S., Pryhuber G.S., Mariani T.J., Bhattacharya S., Guo M. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax. 2017;72:481–484. doi: 10.1136/thoraxjnl-2016-209598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye B.R., Hill D.R., Ferguson M.A., Tsai Y.H., Nagy M.S., Dyal R., Wells J.M., Mayhew C.N., Nattiv R., Klein O.D. In vitro generation of human pluripotent stem cell derived lung organoids. eLife. 2015;4:4. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye B.R., Dedhia P.H., Miller A.J., Nagy M.S., White E.S., Shea L.D., Spence J.R. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife. 2016;5:5. doi: 10.7554/eLife.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgharably H., Shafii A.E., Mason D.P. Expanding the donor pool: donation after cardiac death. Thorac. Surg. Clin. 2015;25:35–46. doi: 10.1016/j.thorsurg.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Eschbach J.W., Jr., Hutchings R.H., Meston B., Burnell J.M., Scribner B.H. A technique for repetitive and long-term human cross circulation. Trans. Am. Soc. Artif. Intern. Organs. 1964;10:280–284. [PubMed] [Google Scholar]
- Farrell J.A., Wang Y., Riesenfeld S.J., Shekhar K., Regev A., Schier A.F. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science. 2018;360:360. doi: 10.1126/science.aar3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow N., Cmielewski P., Donnelley M., Rout-Pitt N., Moodley Y., Bertoncello I., Parsons D. Epithelial disruption: a new paradigm enabling human airway stem cell transplantation. Stem Cell Res. Ther. 2018;9:153. doi: 10.1186/s13287-018-0911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.L., Dargitz C.T., Qualls S.J., Menon T., Wright R., Singer O., Gage F.H., Khanna A., Verma I.M. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:E1723–E1730. doi: 10.1073/pnas.1403470111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.L., Menon T., Parker G.S., Qualls S.J., Lewis B.M., Ke E., Dargitz C.T., Wright R., Khanna A., Gage F.H., Verma I.M. Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs. Cell Rep. 2015;12:1385–1390. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.B., Peng T., Zepp J.A., Snitow M., Vincent T.L., Penkala I.J., Cui Z., Herriges M.J., Morley M.P., Zhou S. Emergence of a Wave of Wnt Signaling that Regulates Lung Alveologenesis by Controlling Epithelial Self-Renewal and Differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.B., Penkala I.J., Zepp J.A., Sivakumar A., Linares-Saldana R., Zacharias W.J., Stolz K.G., Pankin J., Lu M., Wang Q. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc. Natl. Acad. Sci. USA. 2019;116:4362–4371. doi: 10.1073/pnas.1813952116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehner T., Kuehn C., Hadem J., Wiesner O., Gottlieb J., Tudorache I., Olsson K.M., Greer M., Sommer W., Welte T. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am. J. Respir. Crit. Care Med. 2012;185:763–768. doi: 10.1164/rccm.201109-1599OC. [DOI] [PubMed] [Google Scholar]
- Gao N., Wang Y., Zheng C.-M.M., Gao Y.-L.L., Li H., Li Y., Fu T.-T.T., Xu L.-L.L., Wang W., Ying S. β2-Microglobulin participates in development of lung emphysema by inducing lung epithelial cell senescence. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L669–L677. doi: 10.1152/ajplung.00516.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A., Sui P., Verheyden J.M., Young L.R., Sun X. Consider the lung as a sensory organ: A tip from pulmonary neuroendocrine cells. Curr. Top. Dev. Biol. 2019;132:67–89. doi: 10.1016/bs.ctdb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Gerber T., Murawala P., Knapp D., Masselink W., Schuez M., Hermann S., Gac-Santel M., Nowoshilow S., Kageyama J., Khattak S. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science. 2018;362:eaaq0681. doi: 10.1126/science.aaq0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Arwert E.N., Rosewell I.R., Snyder J., Watt F.M., Stripp B.R. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc. Natl. Acad. Sci. USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons M.A., MacKinnon A.C., Ramachandran P., Dhaliwal K., Duffin R., Phythian-Adams A.T., van Rooijen N., Haslett C., Howie S.E., Simpson A.J. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:569–581. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- Gkatzis K., Taghizadeh S., Huh D., Stainier D.Y.R., Bellusci S. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur. Respir. J. 2018;52:1800876. doi: 10.1183/13993003.00876-2018. [DOI] [PubMed] [Google Scholar]
- Gotoh S., Ito I., Nagasaki T., Yamamoto Y., Konishi S., Korogi Y., Matsumoto H., Muro S., Hirai T., Funato M. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports. 2014;3:394–403. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.D., Chen A., Nostro M.C., d’Souza S.L., Schaniel C., Lemischka I.R., Gouon-Evans V., Keller G., Snoeck H.W. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat. Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Endale M., Auer H., Perl A.K. Diversity of Interstitial Lung Fibroblasts Is Regulated by Platelet-Derived Growth Factor Receptor α Kinase Activity. Am. J. Respir. Cell Mol. Biol. 2016;54:532–545. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün D., Muraro M.J., Boisset J.C., Wiebrands K., Lyubimova A., Dharmadhikari G., van den Born M., van Es J., Jansen E., Clevers H. De Novo Prediction of Stem Cell Identity using Single-Cell Transcriptome Data. Cell Stem Cell. 2016;19:266–277. doi: 10.1016/j.stem.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthart B.A., O’Neill J.D., Kim J., Queen D., Chicotka S., Fung K., Simpson M., Donocoff R., Salna M., Marboe C.C. Regeneration of severely damaged lungs using an interventional cross-circulation platform. Nat. Commun. 2019;10:1985. doi: 10.1038/s41467-019-09908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Vasconcelos M., Cai Y., Yoneda M., Hinds A., Qian J., Li G., Dickel L., Johnson J.E., Kimura S. Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl. Acad. Sci. USA. 2012;109:12592–12597. doi: 10.1073/pnas.1204710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Deshpande A., Jain A., Sebastiani P., Cardoso W.V. Uroplakin 3a+ Cells Are a Distinctive Population of Epithelial Progenitors that Contribute to Airway Maintenance and Post-injury Repair. Cell Rep. 2017;19:246–254. doi: 10.1016/j.celrep.2017.03.051. [DOI] [PubMed] [Google Scholar]
- Guibert E.E., Petrenko A.Y., Balaban C.L., Somov A.Y., Rodriguez J.V., Fuller B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011;38:125–142. doi: 10.1159/000327033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Bao E.L., Wagner M., Whitsett J.A., Xu Y. SLICE: determining cell differentiation and lineage based on single cell entropy. Nucleic Acids Res. 2017;45:e54. doi: 10.1093/nar/gkw1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Du Y., Gokey J.J., Ray S., Bell S.M., Adam M., Sudha P., Perl A.K., Deshmukh H., Potter S.S. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat. Commun. 2019;10:37. doi: 10.1038/s41467-018-07770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghverdi L., Büttner M., Wolf F.A., Buettner F., Theis F.J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods. 2016;13:845–848. doi: 10.1038/nmeth.3971. [DOI] [PubMed] [Google Scholar]
- Hawkins F., Kramer P., Jacob A., Driver I., Thomas D.C., McCauley K.B., Skvir N., Crane A.M., Kurmann A.A., Hollenberg A.N. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Invest. 2017;127:2277–2294. doi: 10.1172/JCI89950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab A.E., Nickerson D.W., Ha V.L., Darmawan D.O., Gomperts B.N. Repair and regeneration of tracheal surface epithelium and submucosal glands in a mouse model of hypoxic-ischemic injury. Respirology. 2012;17:1101–1113. doi: 10.1111/j.1440-1843.2012.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M., Morrisey E.E. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillel-Karniel C., Rosen C., Milman-Krentsis I., Orgad R., Bachar-Lustig E., Shezen E., Reisner Y. Multi-lineage Lung Regeneration by Stem Cell Transplantation across Major Genetic Barriers. Cell Rep. 2020;30:807–819.e4. doi: 10.1016/j.celrep.2019.12.058. [DOI] [PubMed] [Google Scholar]
- Hines E.A., Sun X. Tissue crosstalk in lung development. J. Cell. Biochem. 2014;115:1469–1477. doi: 10.1002/jcb.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Giangreco A., Hurley C.M., Stripp B.R. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Hozain A.E., Tipograf Y., Pinezich M.R., Cunningham K.M., Donocoff R., Queen D., Fung K., Marboe C.C., Guenthart B.A., O’Neill J.D. Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine. J. Thorac. Cardiovasc. Surg. 2019 doi: 10.1016/j.jtcvs.2019.09.121. S0022-5223(19)32146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.X., Islam M.N., O’Neill J., Hu Z., Yang Y.G., Chen Y.W., Mumau M., Green M.D., Vunjak-Novakovic G., Bhattacharya J., Snoeck H.W. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.X., Green M.D., de Carvalho A.T., Mumau M., Chen Y.W., D’Souza S.L., Snoeck H.W. The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc. 2015;10:413–425. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L.Y., Sen D., Oniskey T.K., Katzen J., Cohen N.A., Vaughan A.E., Nieves W., Urisman A., Beers M.F., Krummel M.F., Herbert D.R. Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol. 2019;12:64–76. doi: 10.1038/s41385-018-0096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley K., Ding J., Villacorta-Martin C., Herriges M.J., Jacob A., Vedaie M., Alysandratos K.D., Sun Y.L., Lin C., Werder R.B. Reconstructed Single-Cell Fate Trajectories Define Lineage Plasticity Windows during Differentiation of Human PSC-Derived Distal Lung Progenitors. Cell Stem Cell. 2020 doi: 10.1016/j.stem.2019.12.009. S1934-5909(19)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin C.G., Bates J.H. Measuring the lung function in the mouse: the challenge of size. Respir. Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A., Morley M., Hawkins F., McCauley K.B., Jean J.C., Heins H., Na C.L., Weaver T.E., Vedaie M., Hurley K. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell. 2017;21:472–488.e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R., Barkauskas C.E., Takeda N., Bowie E.J., Aghajanian H., Wang Q., Padmanabhan A., Manderfield L.J., Gupta M., Li D. Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nat. Commun. 2015;6:6727. doi: 10.1038/ncomms7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javidfar J., Bacchetta M. Bridge to lung transplantation with extracorporeal membrane oxygenation support. Curr. Opin. Organ Transplant. 2012;17:496–502. doi: 10.1097/MOT.0b013e328357fa4f. [DOI] [PubMed] [Google Scholar]
- Ji Z., Ji H. TSCAN: Pseudo-time reconstruction and evaluation in single-cell RNA-seq analysis. Nucleic Acids Res. 2016;44:e117. doi: 10.1093/nar/gkw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiriya J.J., Brumwell A.N., Jackson J.R., Tang X., Chapman H.A. Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration. Cell Stem Cell. 2020;26:346–358.e4. doi: 10.1016/j.stem.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H., Kobayashi Y., Tata P.R., Hogan B.L.M. IL-1 and TNFα Contribute to the Inflammatory Niche to Enhance Alveolar Regeneration. Stem Cell Reports. 2019;12:657–666. doi: 10.1016/j.stemcr.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen J., Wagner B.D., Venosa A., Kopp M., Tomer Y., Russo S.J., Headen A.C., Basil M.C., Stark J.M., Mulugeta S. An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. 2019;4:4. doi: 10.1172/jci.insight.126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kim J., O’Neill J.D., Dorrello N.V., Bacchetta M., Vunjak-Novakovic G. Targeted delivery of liquid microvolumes into the lung. Proc. Natl. Acad. Sci. USA. 2015;112:11530–11535. doi: 10.1073/pnas.1512613112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., O’Neill J.D., Vunjak-Novakovic G. Rapid retraction of microvolume aqueous plugs traveling in a wettable capillary. Appl. Phys. Lett. 2015;107:144101. doi: 10.1063/1.4932956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Guenthart B., O’Neill J.D., Dorrello N.V., Bacchetta M., Vunjak-Novakovic G. Controlled delivery and minimally invasive imaging of stem cells in the lung. Sci. Rep. 2017;7:13082. doi: 10.1038/s41598-017-13280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A.S., Messersmith E.E., Ratner L.E., Kochik R., Baliga P.K., Ojo A.O. Organ donation and utilization in the United States, 1999-2008. Am. J. Transplant. 2010;10:973–986. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- Knust J., Ochs M., Gundersen H.J., Nyengaard J.R. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat. Rec. (Hoboken) 2009;292:113–122. doi: 10.1002/ar.20747. [DOI] [PubMed] [Google Scholar]
- Konishi S., Gotoh S., Tateishi K., Yamamoto Y., Korogi Y., Nagasaki T., Matsumoto H., Muro S., Hirai T., Ito I. Directed Induction of Functional Multi-ciliated Cells in Proximal Airway Epithelial Spheroids from Human Pluripotent Stem Cells. Stem Cell Reports. 2016;6:18–25. doi: 10.1016/j.stemcr.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H.-K.K., Vasilescu D.M.M., Booth S., Hsieh A., Katsamenis O.L., Fishbane N., Elliott W.M., Kirby M., Lackie P., Sinclair I. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir. Med. 2018;6:591–602. doi: 10.1016/S2213-2600(18)30196-6. [DOI] [PubMed] [Google Scholar]
- Korogi Y., Gotoh S., Ikeo S., Yamamoto Y., Sone N., Tamai K., Konishi S., Nagasaki T., Matsumoto H., Ito I. In Vitro Disease Modeling of Hermansky-Pudlak Syndrome Type 2 Using Human Induced Pluripotent Stem Cell-Derived Alveolar Organoids. Stem Cell Reports. 2019;12:431–440. doi: 10.1016/j.stemcr.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton D.N. Next-generation regeneration: the hope and hype of lung stem cell research. Am. J. Respir. Crit. Care Med. 2012;185:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- Kotton D.N., Morrisey E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat. Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J.M., Kouskoff V., Kennedy M., Woo S., Fehling H.J., Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D., Sun Y., Joo L.S., Dagher R., Zielonka E.M. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D.A., Huang X., Koth L.L., Chang G.H., Dolganov G.M., Zhu Z., Elias J.A., Sheppard D., Erle D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- La Manno G., Soldatov R., Zeisel A., Braun E., Hochgerner H., Petukhov V., Lidschreiber K., Kastriti M.E., Lönnerberg P., Furlan A. RNA velocity of single cells. Nature. 2018;560:494–498. doi: 10.1038/s41586-018-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird C., Burdorf L., Pierson R.N., 3rd Lung xenotransplantation: a review. Curr. Opin. Organ Transplant. 2016;21:272–278. doi: 10.1097/MOT.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- Laughney A.M., Hu J., Campbell N.R., Bakhoum S.F., Setty M., Lavallée V.P., Xie Y., Masilionis I., Carr A.J., Kottapalli S. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat. Med. 2020;26:259–269. doi: 10.1038/s41591-019-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J.P., Morrisey E.E. Repairing the lungs one breath at a time: How dedicated or facultative are you? Genes Dev. 2018;32:1461–1471. doi: 10.1101/gad.319418.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A.J., Driver I.H., Lee J., Conroy C.M., Nagle A., Locksley R.M., Rock J.R. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration following Pneumonectomy. Cell Stem Cell. 2017;21:120–134.e7. doi: 10.1016/j.stem.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer D.J., Martinez F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Rawlins E.L. Developmental mechanisms and adult stem cells for therapeutic lung regeneration. Dev. Biol. 2018;433:166–176. doi: 10.1016/j.ydbio.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Tammela T., Hofree M., Choi J., Marjanovic N.D., Han S., Canner D., Wu K., Paschini M., Bhang D.H. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell. 2017;170:1149–1163.e12. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.K., Huang S.X.L., Chen J., Kerner G., Gilliaux O., Bastard P., Dobbs K., Hernandez N., Goudin N., Hasek M.L. Severe influenza pneumonitis in children with inherited TLR3 deficiency. J. Exp. Med. 2019;216:2038–2056. doi: 10.1084/jem.20181621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhang L., Marsboom G., Jambusaria A., Xiong S., Toth P.T., Benevolenskaya E.V., Rehman J., Malik A.B. Sox17 is required for endothelial regeneration following inflammation-induced vascular injury. Nat. Commun. 2019;10:2126. doi: 10.1038/s41467-019-10134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu K., Cui G., Huang X., Yao S., Guo W., Qin Z., Li Y., Yang R., Pu W. Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat. Genet. 2019;51:728–738. doi: 10.1038/s41588-019-0346-6. [DOI] [PubMed] [Google Scholar]
- Longmire T.A., Ikonomou L., Hawkins F., Christodoulou C., Cao Y., Jean J.C., Kwok L.W., Mou H., Rajagopal J., Shen S.S. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T.J., Anderson P.J., Rotti P.G., Tyler S.R., Crooke A.K., Choi S.H., Montoro D.T., Silverman C.L., Shahin W., Zhao R. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell. 2018;22:653–667.e5. doi: 10.1016/j.stem.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makdisi G., Makdisi T., Jarmi T., Caldeira C.C. Ex vivo lung perfusion review of a revolutionary technology. Ann. Transl. Med. 2017;5:343. doi: 10.21037/atm.2017.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro G.D., Massaro D. Formation of pulmonary alveoli and gas-exchange surface area: quantitation and regulation. Annu. Rev. Physiol. 1996;58:73–92. doi: 10.1146/annurev.ph.58.030196.000445. [DOI] [PubMed] [Google Scholar]
- McCauley K.B., Hawkins F., Serra M., Thomas D.C., Jacob A., Kotton D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell. 2017;20:844–857.e6. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley K.B., Alysandratos K.D., Jacob A., Hawkins F., Caballero I.S., Vedaie M., Yang W., Slovik K.J., Morley M., Carraro G. Single-Cell Transcriptomic Profiling of Pluripotent Stem Cell-Derived SCGB3A2+ Airway Epithelium. Stem Cell Reports. 2018;10:1579–1595. doi: 10.1016/j.stemcr.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough J.E., Yuan R., Suzuki M., Seyednejad N., Elliott W.M., Sanchez P.G., Wright A.C., Gefter W.B., Litzky L., Coxson H.O. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.J., Hill D.R., Nagy M.S., Aoki Y., Dye B.R., Chin A.M., Huang S., Zhu F., White E.S., Lama V., Spence J.R. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Reports. 2018;10:101–119. doi: 10.1016/j.stemcr.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.J., Dye B.R., Ferrer-Torres D., Hill D.R., Overeem A.W., Shea L.D., Spence J.R. Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 2019;14:518–540. doi: 10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K.J.N. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro D.T., Haber A.L., Biton M., Vinarsky V., Lin B., Birket S.E., Yuan F., Chen S., Leung H.M., Villoria J. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Mahoney J.E., Stupnikov M.R., Paez-Cortez J.R., Szymaniak A.D., Varelas X., Herrick D.B., Schwob J., Zhang H., Cardoso W.V. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142:258–267. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Furuhashi K., Danielsson J.A., Hirata Y., Kakiuchi M., Lin C.S., Ohta M., Riccio P., Takahashi Y., Xu X. Generation of functional lungs via conditional blastocyst complementation using pluripotent stem cells. Nat. Med. 2019;25:1691–1698. doi: 10.1038/s41591-019-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E.E., Hogan B.L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Zhao R., Sherwood R., Ahfeldt T., Lapey A., Wain J., Sicilian L., Izvolsky K., Musunuru K., Cowan C., Rajagopal J. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K.C.C., Welker L., Paasch K., Feindt B., Erpenbeck V.J., Hohlfeld J.M., Krug N., Nakashima M., Branscheid D., Magnussen H. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir. Res. 2006;7:32. doi: 10.1186/1465-9921-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.L., Xu J., Kochanek K.D., Arias E. Mortality in the United States, 2017. NCHS Data Brief. 2018;328:1–8. [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naikawadi R.P., Disayabutr S., Mallavia B., Donne M.L., Green G., La J.L., Rock J.R., Looney M.R., Wolters P.J. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016;1:e86704. doi: 10.1172/jci.insight.86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichane M., Javed A., Sivakamasundari V., Ganesan M., Ang L.T., Kraus P., Lufkin T., Loh K.M., Lim B. Isolation and 3D expansion of multipotent Sox9+ mouse lung progenitors. Nat. Methods. 2017;14:1205–1212. doi: 10.1038/nmeth.4498. [DOI] [PubMed] [Google Scholar]
- Niethamer T.K., Stabler C.T., Leach J.P., Zepp J.A., Morley M.P., Babu A., Zhou S., Morrisey E.E. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife. 2020;9:9. doi: 10.7554/eLife.53072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolić M.Z., Sun D., Rawlins E.L. Human lung development: recent progress and new challenges. Development. 2018;145:145. doi: 10.1242/dev.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nureki S.I., Tomer Y., Venosa A., Katzen J., Russo S.J., Jamil S., Barrett M., Nguyen V., Kopp M., Mulugeta S., Beers M.F. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J. Clin. Invest. 2018;128:4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J.D., Guenthart B.A., Kim J., Chicotka S., Queen D., Fung K., Marboe C., Romanov A., Huang S.X.L., Chen Y.-W. Cross-circulation for extracorporeal support and recovery of the lung. Nat. Biomed. Eng. 2017;1:0037. [Google Scholar]
- OPTN Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am. J. Transplant. 2012;12(Suppl 1):1–156. doi: 10.1111/j.1600-6143.2011.03886.x. [DOI] [PubMed] [Google Scholar]
- Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Ouadah Y., Rojas E.R., Riordan D.P., Capostagno S., Kuo C.S., Krasnow M.A. Rare Pulmonary Neuroendocrine Cells Are Stem Cells Regulated by Rb, p53, and Notch. Cell. 2019;179:403–416.e23. doi: 10.1016/j.cell.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Saganta A., Law B.M., Tata P.R., Villoria J., Saez B., Mou H., Zhao R., Rajagopal J. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell. 2015;16:184–197. doi: 10.1016/j.stem.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Woodgett J.R. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr. Top. Dev. Biol. 2017;123:277–302. doi: 10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Perl A.K., Wert S.E., Loudy D.E., Shan Z., Blair P.A., Whitsett J.A. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am. J. Respir. Cell Mol. Biol. 2005;33:455–462. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T.H., Calle E.A., Colehour M.B., Niklason L.E. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20:1117–1126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T.H., Calle E.A., Colehour M.B., Niklason L.E. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs (Print) 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinezich M., Vunjak-Novakovic G. Bioengineering approaches to organ preservation ex vivo. Exp. Biol. Med. (Maywood) 2019;244:630–645. doi: 10.1177/1535370219834498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass M., Solana J., Wolf F.A., Ayoub S., Misios A., Glažar P., Obermayer B., Theis F.J., Kocks C., Rajewsky N. Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science. 2018;360:360. doi: 10.1126/science.aaq1723. [DOI] [PubMed] [Google Scholar]
- Plasschaert L.W., Žilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., Klein A.M., Jaffe A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomfret E.A., Sung R.S., Allan J., Kinkhabwala M., Melancon J.K., Roberts J.P. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons’ State-of-the-Art Winter Symposium. Am. J. Transplant. 2008;8:745–752. doi: 10.1111/j.1600-6143.2007.02146.x. [DOI] [PubMed] [Google Scholar]
- Popov A.-F., Sabashnikov A., Patil N.P., Zeriouh M., Mohite P.N., Zych B., Saez D.G., Schmack B., Ruhparwar A., Dohmen P.M. Ex vivo lung perfusion - state of the art in lung donor pool expansion. Med. Sci. Monit. Basic Res. 2015;21:9–14. doi: 10.12659/MSMBR.893674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M., Ferren M., Chen Y.W., Siu Y., Makhsous N., Rima B., Briese T., Greninger A.L., Snoeck H.W., Moscona A. Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids. MBio. 2019;10:10. doi: 10.1128/mBio.00723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantius J., Schmoldt C., Vazquez-Armendariz A.I., Becker C., El Agha E., Wilhelm J., Morty R.E., Vadász I., Mayer K., Gattenloehner S. Influenza Virus Infects Epithelial Stem/Progenitor Cells of the Distal Lung: Impact on Fgfr2b-Driven Epithelial Repair. PLoS Pathog. 2016;12:e1005544. doi: 10.1371/journal.ppat.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K.F., Hurd S., Anzueto A., Barnes P.J., Buist S.A., Calverley P., Fukuchi Y., Jenkins C., Rodriguez-Roisin R., van Weel C., Zielinski J., Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Rafii S., Cao Z., Lis R., Siempos I.I., Chavez D., Shido K., Rabbany S.Y., Ding B.S. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat. Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K., Nemzek J., Napolitano L.M., Knight P.R. Aspiration-induced lung injury. Crit. Care Med. 2011;39:818–826. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiser D.M., Kim C.F. Commentary: Sca-1 and Cells of the Lung: A matter of Different Sorts. Stem Cells. 2009;27:606–611. doi: 10.1002/stem.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane C.K., Jackson S.R., Pastore C.F., Zhao G., Weiner A.I., Patel N.N., Herbert D.R., Cohen N.A., Vaughan A.E. Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L1141–L1149. doi: 10.1152/ajplung.00032.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S.A., Han L., McCracken K.W., Kenny A.P., Anglin C.T., Grigg E.A., Crawford C.M., Wells J.M., Shannon J.M., Zorn A.M. A Retinoic Acid-Hedgehog Cascade Coordinates Mesoderm-Inducing Signals and Endoderm Competence during Lung Specification. Cell Rep. 2016;16:66–78. doi: 10.1016/j.celrep.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H., Wang F., Hogan B.L. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S., Chiba N., Yao C., Guan X., McConnell A.M., Brockway B., Que L., McQualter J.L., Stripp B.R. Rare SOX2+ Airway Progenitor Cells Generate KRT5+ Cells that Repopulate Damaged Alveolar Parenchyma following Influenza Virus Infection. Stem Cell Reports. 2016;7:817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyfman P.A., Walter J.M., Joshi N., Anekalla K.R., McQuattie-Pimentel A.C., Chiu S., Fernandez R., Akbarpour M., Chen C.I., Ren Z. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., Randell S.H., Hogan B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Gao X., Xue Y., Randell S.H., Kong Y.Y., Hogan B.L. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C., Shezen E., Aronovich A., Klionsky Y.Z., Yaakov Y., Assayag M., Biton I.E., Tal O., Shakhar G., Ben-Hur H. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat. Med. 2015;21:869–879. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- Ruiz García S., Deprez M., Lebrigand K., Cavard A., Paquet A., Arguel M.J., Magnone V., Truchi M., Caballero I., Leroy S. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development. 2019;146:146. doi: 10.1242/dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salwig I., Spitznagel B., Vazquez-Armendariz A.I., Khalooghi K., Guenther S., Herold S., Szibor M., Braun T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38:e102099. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebinger G., Shu J., Tabaka M., Cleary B., Subramanian V., Solomon A., Gould J., Liu S., Lin S., Berube P. Optimal-Transport Analysis of Single-Cell Gene Expression Identifies Developmental Trajectories in Reprogramming. Cell. 2019;176:928–943.e22. doi: 10.1016/j.cell.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold M.A., Smith R.W., Urbanek C., Groshong S.D., Cosgrove G.P., Brown K.K., Schwarz M.I., Schwartz D.A., Reynolds S.D. The idiopathic pulmonary fibrosis honeycomb cyst contains a mucocilary pseudostratified epithelium. PLoS ONE. 2013;8:e58658. doi: 10.1371/journal.pone.0058658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M., Alysandratos K.D., Hawkins F., McCauley K.B., Jacob A., Choi J., Caballero I.S., Vedaie M., Kurmann A.A., Ikonomou L. Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung- versus thyroid-lineage specification. Development. 2017;144:3879–3893. doi: 10.1242/dev.150193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty M., Tadmor M.D., Reich-Zeliger S., Angel O., Salame T.M., Kathail P., Choi K., Bendall S., Friedman N., Pe’er D. Wishbone identifies bifurcating developmental trajectories from single-cell data. Nat. Biotechnol. 2016;34:637–645. doi: 10.1038/nbt.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Berg D.A., Zhu Y., Shin J.Y., Song J., Bonaguidi M.A., Enikolopov G., Nauen D.W., Christian K.M., Ming G.L., Song H. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015;17:360–372. doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni F.E., Chu F.S., Walker D.C. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am. J. Respir. Crit. Care Med. 2003;168:1532–1537. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- Song J.J., Kim S.S., Liu Z., Madsen J.C., Mathisen D.J., Vacanti J.P., Ott H.C. Enhanced in vivo function of bioartificial lungs in rats. Ann. Thorac. Surg. 2011;92:998–1005, discussion 1005–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Steen S., Sjöberg T., Pierre L., Liao Q., Eriksson L., Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–829. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- Stevens T., Phan S., Frid M.G., Alvarez D., Herzog E., Stenmark K.R. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc. Am. Thorac. Soc. 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street K., Risso D., Fletcher R.B., Das D., Ngai J., Yosef N., Purdom E., Dudoit S. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics. 2018;19:477. doi: 10.1186/s12864-018-4772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikoudis A., Cieślak A., Loffredo L., Chen Y.W., Patel N., Saqi A., Lederer D.J., Snoeck H.W. Modeling of Fibrotic Lung Disease Using 3D Organoids Derived from Human Pluripotent Stem Cells. Cell Rep. 2019;27:3709–3723.e5. doi: 10.1016/j.celrep.2019.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupnikov M.R., Yang Y., Mori M., Lu J., Cardoso W.V. Jagged and Delta-like ligands control distinct events during airway progenitor cell differentiation. eLife. 2019;8:8. doi: 10.7554/eLife.50487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson V., Vento-Tormo R., Teichmann S.A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 2018;13:599–604. doi: 10.1038/nprot.2017.149. [DOI] [PubMed] [Google Scholar]
- Tadokoro T., Wang Y., Barak L.S., Bai Y., Randell S.H., Hogan B.L. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc. Natl. Acad. Sci. USA. 2014;111:E3641–E3649. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.Y., Krasnow M.A. Developmental origin of lung macrophage diversity. Development. 2016;143:1318–1327. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tane S., Noda K., Shigemura N. Ex Vivo Lung Perfusion: A Key Tool for Translational Science in the Lungs. Chest. 2017;151:1220–1228. doi: 10.1016/j.chest.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Tata P.R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B.M., Vinarsky V., Cho J.L., Breton S., Sahay A. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata A., Kobayashi Y., Chow R.D., Tran J., Desai A., Massri A.J., McCord T.J., Gunn M.D., Tata P.R. Myoepithelial Cells of Submucosal Glands Can Function as Reserve Stem Cells to Regenerate Airways after Injury. Cell Stem Cell. 2018;22:668–683.e6. doi: 10.1016/j.stem.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck W.M. The internal surface area of nonemphysematous lungs. Am. Rev. Respir. Dis. 1967;95:765–773. doi: 10.1164/arrd.1967.95.5.765. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Cacchiarelli D., Grimsby J., Pokharel P., Li S., Morse M., Lennon N.J., Livak K.J., Mikkelsen T.S., Rinn J.L. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglini K.J., Nabhan A.N., Penland L., Sinha R., Gillich A., Sit R.V., Chang S., Conley S.D., Mori Y., Seita J. A molecular cell atlas of the human lung from single cell RNA sequencing. bioRxiv. 2019 doi: 10.1101/742320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan S., Salahudeen A.A., Sellers Z.M., Bravo D.T., Choi S.S., Batish A., Le W., Baik R., de la O S., Kaushik M.P. High-Efficiency, Selection-free Gene Repair in Airway Stem Cells from Cystic Fibrosis Patients Rescues CFTR Function in Differentiated Epithelia. Cell Stem Cell. 2020;26:161–171.e4. doi: 10.1016/j.stem.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapour M., Skeans M.A., Heubner B.M., Smith J.M., Hertz M.I., Edwards L.B., Cherikh W.S., Callahan E.R., Snyder J.J., Israni A.K., Kasiske B.L. OPTN/SRTR 2013 annual data report: lung. Am. J. Transplant. 2015;15(Suppl 2):1–28. doi: 10.1111/ajt.13200. [DOI] [PubMed] [Google Scholar]
- Van Haute L., De Block G., Liebaers I., Sermon K., De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir. Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A., Blanpain C. Tracing epithelial stem cells during development, homeostasis, and repair. J. Cell Biol. 2012;197:575–584. doi: 10.1083/jcb.201201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raemdonck D., Neyrinck A., Verleden G.M., Dupont L., Coosemans W., Decaluwé H., Decker G., De Leyn P., Nafteux P., Lerut T. Lung donor selection and management. Proc. Am. Thorac. Soc. 2009;6:28–38. doi: 10.1513/pats.200808-098GO. [DOI] [PubMed] [Google Scholar]
- Vaughan A.E., Brumwell A.N., Xi Y., Gotts J.E., Brownfield D.G., Treutlein B., Tan K., Tan V., Liu F.C., Looney M.R. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa A., Katzen J., Tomer Y., Kopp M., Jamil S., Russo S.J., Mulugeta S., Beers M.F. Epithelial Expression of an Interstitial Lung Disease-Associated Mutation in Surfactant Protein-C Modulates Recruitment and Activation of Key Myeloid Cell Populations in Mice. J. Immunol. 2019;202:2760–2771. doi: 10.4049/jimmunol.1900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Braga F.A., Kar G., Berg M., Carpaij O.A., Polanski K., Simon L.M., Brouwer S., Gomes T., Hesse L., Jiang J. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- Volckaert T., Dill E., Campbell A., Tiozzo C., Majka S., Bellusci S., De Langhe S.P. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J. Clin. Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.E., Bonvillain R.W., Jensen T., Girard E.D., Bunnell B.A., Finck C.M., Hoffman A.M., Weiss D.J. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18:895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.E., Weinreb C., Collins Z.M., Briggs J.A., Megason S.G., Klein A.M. Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science. 2018;360:981–987. doi: 10.1126/science.aar4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.C., Behzad A.R., Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc. Res. 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- Wang D., Haviland D.L., Burns A.R., Zsigmond E., Wetsel R.A. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tang Z., Huang H., Li J., Wang Z., Yu Y., Zhang C., Li J., Dai H., Wang F. Pulmonary alveolar type I cell population consists of two distinct subtypes that differ in cell fate. Proc. Natl. Acad. Sci. USA. 2018;115:2407–2412. doi: 10.1073/pnas.1719474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L.B., Wang Y., Fang X., Warnock M., Sakuma T., Hall T.S., Matthay M. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]
- Warnecke G., Moradiellos J., Tudorache I., Kühn C., Avsar M., Wiegmann B., Sommer W., Ius F., Kunze C., Gottlieb J. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012;380:1851–1858. doi: 10.1016/S0140-6736(12)61344-0. [DOI] [PubMed] [Google Scholar]
- Warnecke G., Van Raemdonck D., Smith M.A., Massard G., Kukreja J., Rea F., Loor G., De Robertis F., Nagendran J., Dhital K.K. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 2018;6:357–367. doi: 10.1016/S2213-2600(18)30136-X. [DOI] [PubMed] [Google Scholar]
- Watson J.K., Rulands S., Wilkinson A.C., Wuidart A., Ousset M., Van Keymeulen A., Göttgens B., Blanpain C., Simons B.D., Rawlins E.L. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep. 2015;12:90–101. doi: 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E.R. Morphological basis of alveolar-capillary gas exchange. Physiol. Rev. 1973;53:419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Weiner A.I., Jackson S.R., Zhao G., Quansah K.K., Farshchian J.N., Neupauer K.M., Littauer E.Q., Paris A.J., Liberti D.C., Scott Worthen G. Mesenchyme-free expansion and transplantation of adult alveolar progenitor cells: steps toward cell-based regenerative therapies. NPJ Regen. Med. 2019;4:17. doi: 10.1038/s41536-019-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J.A., Kalin T.V., Xu Y., Kalinichenko V.V. Building and Regenerating the Lung Cell by Cell. Physiol. Rev. 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobma H., Vunjak-Novakovic G. Tissue engineering and regenerative medicine 2015: a year in review. Tissue Eng. Part B Rev. 2016;22:101–113. doi: 10.1089/ten.teb.2015.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf F.A., Hamey F.K., Plass M., Solana J., Dahlin J.S., Göttgens B., Rajewsky N., Simon L., Theis F.J. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 2019;20:59. doi: 10.1186/s13059-019-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.P., Bear C.E., Chin S., Pasceri P., Thompson T.O., Huan L.J., Ratjen F., Ellis J., Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Yu Y., Huang H., Hu Y., Fu S., Wang Z., Shi M., Zhao X., Yuan J., Li J. Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. Cell. 2020;180:107–121.e17. doi: 10.1016/j.cell.2019.11.027. [DOI] [PubMed] [Google Scholar]
- Xi Y., Kim T., Brumwell A.N., Driver I.H., Wei Y., Tan V., Jackson J.R., Xu J., Lee D.K., Gotts J.E. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Wang Y., Deng N., Huang G., Taghavifar F., Geng Y., Liu N., Kulur V., Yao C., Chen P. Single-Cell Deconvolution of Fibroblast Heterogeneity in Mouse Pulmonary Fibrosis. Cell Rep. 2018;22:3625–3640. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Mizuno T., Sridharan A., Du Y., Guo M., Tang J., Wikenheiser-Brokamp K.A., Perl A.T., Funari V.A., Gokey J.J. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Gotoh S., Korogi Y., Seki M., Konishi S., Ikeo S., Sone N., Nagasaki T., Matsumoto H., Muro S. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat. Methods. 2017;14:1097–1106. doi: 10.1038/nmeth.4448. [DOI] [PubMed] [Google Scholar]
- Yang Y., Riccio P., Schotsaert M., Mori M., Lu J., Lee D.K., García-Sastre A., Xu J., Cardoso W.V. Spatial-Temporal Lineage Restrictions of Embryonic p63+ Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Dev. Cell. 2018;44:752–761.e4. doi: 10.1016/j.devcel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., Lai R., Afkhami S., Chen Y., Dvorkin-Gheva A. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 2018;175:1634–1650.e17. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., Zhou S., Cantu E., Morrisey E.E. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J.A., Morrisey E.E. Cellular crosstalk in the development and regeneration of the respiratory system. Nat. Rev. Mol. Cell Biol. 2019;20:551–566. doi: 10.1038/s41580-019-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp J.A., Zacharias W.J., Frank D.B., Cavanaugh C.A., Zhou S., Morley M.P., Morrisey E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell. 2017;170:1134–1148.e10. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W., Zhang T., Wu D.Z., Guan S.P., Liew A.A., Yamamoto Y., Wang X., Lim S.J., Vincent M., Lessard M. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]