Summary
Background
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan in December 2019 and has rapidly spread across different cities within and outside China. Hong Kong started to prepare for COVID-19 on 31st December 2019 and infection control measures in public hospitals were tightened to limit nosocomial transmission within healthcare facilities. However, the recommendations on the transmission-based precautions required for COVID-19 in hospital settings vary from droplet and contact precautions, to contact and airborne precautions with placement of patients in airborne infection isolation rooms.
Aim
To describe an outbreak investigation of a patient with COVID-19 who was nursed in an open cubicle of a general ward before the diagnosis was made.
Method
Contacts were identified and risk categorized as ‘close’ or ‘casual’ for decisions on quarantine and/or medical surveillance. Respiratory specimens were collected from contacts who developed fever, and/or respiratory symptoms during the surveillance period and were tested for SARS-CoV-2.
Findings
A total of 71 staff and 49 patients were identified from contact tracing, seven staff and 10 patients fulfilled the criteria of ‘close contact’. At the end of 28-day surveillance, 76 tests were performed on 52 contacts and all were negative, including all patient close contacts and six of the seven staff close contacts. The remaining contacts were asymptomatic throughout the surveillance period.
Conclusion
Our findings suggest that SARS-CoV-2 is not spread by an airborne route, and nosocomial transmissions can be prevented through vigilant basic infection control measures, including wearing of surgical masks, hand and environmental hygiene.
Keywords: COVID-19, Coronavirus disease-2019, SARS-CoV-2, Outbreak, Contact tracing, Infection control
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), started in Wuhan since December 2019 but has now spread throughout different provinces in China, and has since been declared a Public Health Emergency of International Concern by the World Health Organization (WHO) on 30th January 2020 [[1], [2], [3]]. Hong Kong, in close vicinity with China and ruthlessly affected by the Severe Acute Respiratory Syndrome (SARS) 17 years ago, started preparation for COVID-19 on 31st December 2019, when an alert of clustered pneumonia of unknown origin was announced by the Wuhan Municipal Health Commission [4]. The first imported case of COVID-19 in Hong Kong was reported on 23rd January 2020, and the first local case (with no known travel history or travel-related contact history) was reported on 31st January 2020. The WHO currently recommended droplet and contact precautions for patients with suspected or known COVID-19, while applying airborne precautions when performing aerosol-generating procedures (AGPs) [5]. While the Australian Government has adopted the WHO recommendations [6], the Centers for Disease Control and Prevention, Public Health England and the Hong Kong Hospital Authority (HA), the statutory body responsible for managing Hong Kong's public hospital service, recommended contact and airborne precautions for patients with suspected or known COVID-19, including placement in an airborne infection isolation room (AIIR) [[7], [8], [9]]. The potential risk of nosocomial transmission of SARS-CoV-2 has posed great stress and anxiety to healthcare workers, a shadow cast by the SARS epidemic in 2003 [10,11]. For COVID-19, current reports from China reveal an attack rate of 2.09–29% among healthcare workers [3,12,13] Although the major routes of transmission of SARS-CoV-2 is believed to be droplet and contact, some healthcare workers remained extremely mindful of the potential ‘super-spreading events’ due to opportunistic airborne transmission through various healthcare-related activities [11,14]. While opening suction of respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation were well accepted as AGPs [5], the extent of infectious aerosols generated by the use of nebulizers, high-flow oxygen (especially through venturi-type masks) and non-invasive positive pressure ventilations were more controversial [15,16]. These activities were avoided during the SARS epidemic in view of the potential risks [15], but a subsequent systematic review found no statistically significant increase in SARS transmission risk with therapeutic activities such as suction before or after intubation, nebulizer treatment, oxygen mask manipulation or chest compression [16]. Here, we report the outbreak investigation and outcome of 49 patients and 71 healthcare workers exposed to a patient with severe pneumonia due to SARS-CoV-2 in a general ward setting before the diagnosis was made.
Material and methods
Setting
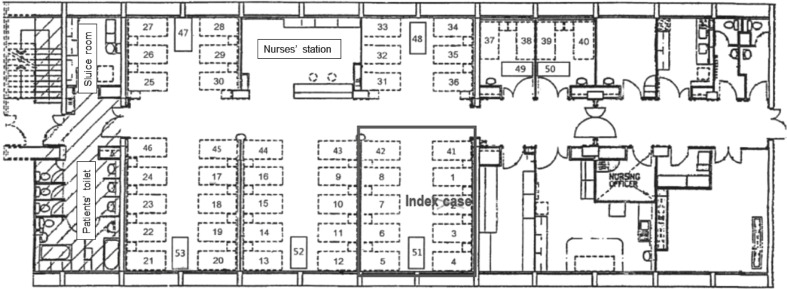
Queen Elizabeth Hospital is a major acute hospital with over 2000 beds with a 24-h Accident and Emergency Service. The Accident and Emergency Service has an attendance of approximately 14,700 patients per month. Ward A is a 53-bedded female medical ward for renal patients, with five open cubicles and two three-bedded rooms (Figure 1 ). Ward A has an average of 260 admissions per month.
Figure 1.
Floor plan of Ward A with index patient staying in bed 2 with 10 other patients.
Epidemiological investigation
On 3rd February 2020, an epidemiological investigation was convened when a patient was diagnosed with COVID-19. The patient had stayed in an open cubicle (bed 2) of ward A with 10 other patients for 35 h (including 18.5 h of oxygen therapy at 8 L/min) before transferring to AIIR (12 air changes per hour (ACH)) for intubation with implementation of contact and airborne precautions. Ethical approval was obtained from the Research Ethics Committee of the Kowloon Central/Kowloon East Cluster, HA. Written consent for publication was obtained from the index patient.
Contact tracing and outbreak management
Immediate infection control measures were implemented, and contact tracing was conducted to search for all staff and patients who had been exposed to the index patient before the diagnosis of COVID-19 was made. A contact case was defined as a patient or staff who stayed or worked in the same ward as the index patient. Patients would be identified through the Patient Administration Contact Tracing System from the index patient admission until the diagnosis of COVID-19 was made, while staff contacts were identified through ward managers. These contacts were interviewed and risk categorized according to the nature of activities, duration of exposure, personal protective equipment (PPE) worn at the time of exposure. ‘Staff close contact’ was defined as staff who had contact within 2 m of the index case for a cumulative time of >15 min, or had performed AGPs, without ‘appropriate’ PPE. ‘Appropriate’ PPE in the above contact episodes referred to the use of N95 respirator, face shield/goggles, gown and gloves. Patients who shared the same cubicle with the index case were considered as ‘patient close contact’. Staff close contacts were subjected to a 14-day work exclusion and quarantined at a designated camp site, followed by medical surveillance for another 14 days. Patient close contacts were quarantined into an AIIR (or quarantine camp if the patient was deemed clinically stable to be discharged from hospital) for 14 days, followed by medical surveillance for 14 days. Other staff and patient contacts (‘casual contacts’) were subjected to medical surveillance for 28 days with no restriction to work or discharge from hospital. Body temperature and respiratory symptoms were monitored daily throughout the 28-day period. Any abnormalities were reported to the medical personnel at the quarantine camp, or to the hospital infection control team, with hospitalization into an AIIR and testing of SARS-CoV-2 where indicated.
Real-time reverse transcription polymerase chain reaction assay for SARS-CoV-2
Upper respiratory tract specimens, e.g., nasopharyngeal aspirates (NPA), nasopharyngeal swabs (NPS) with or without concurrent throat swabs, or lower respiratory tract specimens, e.g., sputum, tracheal aspirate or bronchoalveolar lavage, were all acceptable specimen types for the real-time reverse transcription-polymerase chain reaction (RT-PCR) assay. All specimens were preserved in viral transport medium before further processing. Total nucleic acid extraction was performed using MagNA Pure LC 2.0 (Roche, Switzerland) (from 3rd to 5th February 2020) or MagNA Pure 96 (Roche, Switzerland) (from 6th February 2020), and the RT-PCR was performed using LightMix® Modular SARS and Wuhan CoV E-gene, EAV RNA extract control (TIB-MOLBIOL, Berlin, Germany), and the LightCycle® Multiplex RNA Virus Master (Roche Diagnostics, Mannheim, Germany) on cobas z 480 real-time PCR analyser (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Briefly, each 20-μL reaction mixture contained 4.0 μL of Roche Master, 0.1 μL of RT enzyme, 0.5 μL of reagent mix, 5.4 μl of water and 10 μL of extracted RNA template. RT-PCR was performed under the following conditions: RT step at 55°C for 5 min and 95°C for 5 min, then 45 thermal cycling at 95°C for 3 s, 60°C for 12 s and 72°C for 3 s, followed by cooling at 40°C for 30 s. Specimens with a Cp-value of lower than 39 were be sent to the Public Health Laboratory Service Branch, Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region, for confirmation.
Results
Outbreak investigation
The index patient, a 64-year-old woman, attended the Department of Accident and Emergency at 23:42 h on 1st February 2020 with fever, productive cough and breathlessness for 2 days. She developed flu-like symptoms on 24th January 2020 with transient improvement after taking antibiotics and symptomatic treatment prescribed by a general practitioner. However, her fever relapsed on 30th January 2020 with productive cough and dyspnea. She had no history of travel in the preceding month, but she owned a fashion boutique with many mainland Chinese customers owing to its proximity to the West Kowloon high-speed rail station. On admission, she had a fever of 39.6°C with sinus tachycardia of 126 bpm and blood pressure of 198/92 mmHg. She was tachypnoeic with oxygen saturation of 80% in room air. Bilateral crepitations were heard on auscultation and chest X-ray revealed multi-lobar pulmonary infiltrates (Figure 2 (a)). In the absence of history of travel to China or contact with a confirmed COVID-19 patient, she was admitted to ward A in an open cubicle under standard precautions for community-acquired pneumonia (6 ACH, normal pressure setting) at 01:22 h on 2nd February 2020. She could not wear a surgical mask as she was on oxygen therapy through a simple facemask (Soundway®, Ningbo Shengyurui Medical Appliances Co. Ltd, Ningbo, China; Figure 2(b)). She also had frequent coughs while residing in the ward. She became more hypoxic around 18:00 h and required an increase in oxygen therapy to 8 L/min, delivered through the same facemask. On 3rd February 2020, she was transferred to an AIIR at 12:35 h and was electively intubated at 13:00 h for progressive respiratory failure. Nasopharyngeal swab for multiplex PCR FilmArray® RP2 panel (Biofire Diagnostics, bioMérieux, Marcy-l’Étoile, France), urine for BinaxNOW Legionella and pneumococcal antigen (Abbott, IL, USA) were negative. The patient was tested for COVID-19 as enhanced laboratory surveillance on 3rd February 2020, where both combined NPA with throat swab, and tracheal aspirate showed detectable SARS-CoV-2 RNA, with a Cp value of 22.8 and 26.1, respectively. The patient was subsequently transferred to the Infectious Diseases Centre, Princess Margaret Hospital, for further management.
Figure 2.
(a) Chest-X-ray of index patient on admission showing bilateral pulmonary infiltrates. (b) Simple facemask (Hudson mask) used for high-flow oxygen therapy on the index patient during her stay in ward A.
Contact tracing and outbreak management
Terminal disinfection and changing of all curtains were performed in ward A immediately after the diagnosis of COVID-19 in the index patient, followed by regular environmental disinfection twice daily with 1000 ppm sodium hypochlorite. Also, ward A was closed to new admissions for 14 days. Enhanced infection control measures in accordance with the Emergency Response level in the HA since 26th January 2020 were reinforced, including the wearing of surgical masks (ASTM F2100 level 1) by all staff, patients and visitors in all hospital areas, as well as the minimization of traffic by suspension of visiting hours, volunteer service and clinical attachments.
A total of 71 staff and 49 patients were identified from contact tracing. Seven staff and 10 patients fulfilled the criteria of ‘close contact’ (Table I ). All staff and patients who did not fulfill the pre-defined criteria for close contacts were managed as ‘casual contacts’ (Supplementary Tables S1 and S2). Thirty staff and 22 patients developed fever and/or reported respiratory symptoms during the surveillance period, with 76 respiratory specimens sent for RT-PCR for SARS-CoV-2. All specimens from the 52 contacts were negative for SARS-CoV-2, including all patient close contacts and six of the seven staff close contacts (Table I). Four patients (casual contacts) died from conditions unrelated to COVID-19 during the surveillance period. The remaining contacts were asymptomatic throughout the 28-day surveillance period.
Table I.
Nature of exposure among staff and patient close contacts in ward A
| Type of contact | >15 min contact time (duration of contact if known, hours) | Activity performed (staff)/bed number at time of exposure (patient) | Patient on 8 L/min oxygen | PPE used during contact | SARS-CoV-2 test during surveillance period (day of testing from last exposure date∗) |
|---|---|---|---|---|---|
| Staff (Doctor) | Yes | History taking, chest auscultation | No | N95 | None |
| Staff (Doctor) | Yes | Blood taking | No | Surgical mask | Yes (1) |
| Staff (Nurse) | Yes | Administration of medications | Yes | N95, goggles | Yes (1) |
| Staff (Nurse) | Yes | Obtained nasopharyngeal swab | Yes | N95, gloves, cap | Yes (1) |
| Staff (Nurse) | Yes | Escorted patient from general ward to airborne isolation ward (AIIR) | Yes | N95 | Yes (1) |
| Staff (Nurse) | Yes | Nurse-in-charge for the affected cubicle | Yes | N95 | Yes (1) |
| Staff (Nurse) | Yes | Escorted patient from general ward to AIIR | Yes | N95 | Yes (1) |
| Patient | Yes (35) | 01 | Yes | Consistent use of surgical masks | Yes (1, 4, 8, 11, 13) |
| Patient | Yes (33) | 51 | Yes | — | Yes, (1, 13) |
| Patient | Yes (35) | 03 | Yes | — | Yes (1, 3, 13) |
| Patient | Yes (35) | 04 | Yes | Inconsistent use of surgical masks | Yes (1, 2, 4, 14) |
| Patient | Yes (35) | 05 | Yes | Consistent use of surgical masks | Yes (1, 14) |
| Patient | Yes (35) | 06 | Yes | — | Yes (1, 6, 9, 13) |
| Patient | Yes (35) | 07 | Yes | — | Yes (2, 4, 9, 11, 14) |
| Patient | Yes (35) | 08 | Yes | Inconsistent use of surgical masks | Yes (1, 2, 5, 7, 11, 13) |
| Patient | Yes (35) | 41 | Yes | Consistent use of surgical masks | Yes (13) |
| Patient | Yes (35) | 42 | Yes | Inconsistent use of surgical masks | Yes (14) |
—, not available due to inability of patient to provide information on compliance of surgical mask wearing; AIIR, airborne infection isolation room; PPE, personal protective equipment.
Last exposure date, i.e. Feb 3, 2020, was counted as day 0.
Discussion
More than 100,000 cases of COVID-19 have been reported worldwide at the time of writing, after just over 2 months after the identification of this novel coronavirus [17,18]. While it is believed that person-to-person spread of SARS-CoV-2 occurs predominantly through droplet and contact transmissions, the actual dynamics remained uncertain. The reproduction number of SARS-CoV-2 appears to be higher than SARS, with an initial estimation of 1.4–6.49, and median of 2.79 [[19], [20], [21]]. The high attack rates among household contacts [[22], [23], [24]], and passengers on the Diamond Princess Cruise are most concerning [25]. In the described scenario, our patient coughed frequently and a high viral load (as implicated by the low Cp-value) detected from her respiratory specimens; moreover, she could not put on a surgical mask during her stay in the general medical ward. All these factors could have contributed to a great deal of droplet generation from our index patient, with secondary contamination of her surroundings. Moreover, she received oxygen therapy at 8 L/min through a simple oxygen facemask for a significant amount of time. Oxygen therapy with flow of ≥6 L/min was considered an AGP in the HA hospitals. Despite the aforementioned, none of the neighbouring patients or staff contacts were infected. While there is no consensus on what constitutes ‘close’ and ‘casual’ contacts, our risk assessment approach was similar to the subsequent published guidance for contact tracing [6,[26], [27], [28], [29]] (Table II ). The authors believe that the universal mask wearing of staff, patients and visitors as a component of the Emergency Response in HA hospitals, as well as the heightened alertness to hand hygiene and environmental cleanliness have played a major part in halting further transmissions in this incident. In fact, frequent hand hygiene and appropriate use of surgical masks have previously been shown to be a protective factor against SARS, and influenza [[30], [31], [32], [33]]. In the Shenzhen family cluster of COVID-19, the uninfected family member was the only person reported to have consistent use of surgical masks [22]. Also, the lack of secondary transmission in our experience may imply that oxygen therapy ≥6 L/min with a simple facemask poses a low risk of aerosol generation, consistent with the finding from a previous study [34]. Another local study on aerosol dispersion in various respiratory therapies demonstrated that the air leak through the side vents of the simple oxygen facemask during delivery of oxygen for 6, 8, 10 L/min were limited to 0.22, 0.3 and 0.4 m [35], respectively, hence the neighbouring patients who should be at least 1 m from the index patient were not at increased risk of droplet transmission despite the use of high-flow oxygen.
Table II.
Risk categorization of contacts of COVID-19 patients in a hospital setting
| World Health Organization [26] | Centers for disease control and prevention [27] | Public Health England [28] | European centres for disease prevention and control [29] | Communicable disease network Australia [6] | |
|---|---|---|---|---|---|
| Close/high- to medium-risk contact | (No explicit differentiation between close and casual contact) Contacts include provision of direct care for patients with COVID-19, or visiting patients or staying in the same environment as a patient with COVID-19, or working with HCWs with COVID-19 disease |
Within 2 m of a person with COVID-19 for a prolonged period of time (>1–2 min). Taking into account the clinical syndrome patients (e.g., coughing) and whether patient wore facemask High risks: perform or present in the same room for AGPs when the HCW eyes, nose or mouth were not protected. Medium risks: perform or present in the same room for AGPs without using gown and gloves (but eyes, nose and mouth were protected), or HCWs with unprotected eyes, nose or mouth with prolonged close contact with patients with COVID-19 who was or was not wearing a facemask, or HCW with direct contact with secretion/excretion of COVID-19 patient without wearing gloves and failed to perform immediate hand hygiene |
Face-to-face contact, or spending >15 min within 2 m of an infected person | Direct physical contact, or unprotected direct contact, or face-to-face contact within 2 m for >15 min, or closed environment for 15 min at a distance of <2 m as a COVID-19 case, or laboratory workers handling specimens from a COVID-19 case; AND without recommended PPE or with a possible breach of PPE |
Face-to-face contact for >15 min in any setting with a confirmed case in the period extending from 24 h before onset of symptoms in the confirmed case, or sharing of a closed space with a confirmed case for >2 h in the period extending from 24 h before onset of symptoms in the confirmed case |
| Casual-/low-risk contacts | HCW wearing a facemask or respirator only and have prolonged close contact with a patient who was wearing a facemask, or HCW using all recommended PPE (i.e., a respirator, eye protection, gloves and a gown) while caring for or having contact with the secretions/excretions of a patient, or HCW (not using all recommended PPE) who have brief interactions with a patient regardless of whether patient was wearing a facemask (e.g., brief conversation at a triage desk; briefly entering a patient room but not having direct contact with the patient or their secretions/excretions; entering the patient room immediately after they have been discharged) | Not defined | Person in a closed environment with a COVID-19 case for <15 min, or at a distance of more than 2 m, or a person having had face-to-face contact with a COVID-19 case for <15 min and at within 2 m | <15 min face-to-face contact with a symptomatic confirmed case in any setting, or sharing a closed space with a symptomatic confirmed case for less than 2 h | |
| Management | Monitor health and symptoms for 14 days from the last day of contact | High- and medium-risk: work exclusion of HCWs and medical surveillance for 14 days after last exposure Low-risk: self-monitoring with delegated supervision until 14 days after the last potential exposure |
Work exclusion and home quarantine for 14 days following the last possible contact with the case | High-risk contact: work exclusion and medical surveillance for 14 days from the last possible day of contact; avoid social contact and travel Low risk contact: self-monitoring and self-isolation if respiratory symptoms are experienced; NO suspension from work. |
Work exclusion and home quarantine for 14 days from the last possible day of contact |
| Testing for SARS-CoV-2 | If fever or respiratory symptoms consistent with COVID-19 develop during the 14-day surveillance period | ||||
AGP, aerosol-generating procedures (e.g., open suctioning of respiratory tract, intubation, bronchoscopy, cardiopulmonary resuscitation); COVID-19, coronavirus disease 2019; HCW, healthcare worker; PPE, personal protective equipment.
There are a few limitations to our study. Firstly, our sample size was small with only one index patient. However, the detailed description of contact tracing including nature of exposure and PPE worn involving 120 contacts may still be a useful observation of transmission dynamics in a normal ward environment, because the majority of the subsequent patients were admitted directly into AIIR. Secondly, we only tested contacts with fever or respiratory symptoms with RT-PCR, hence the possibility of asymptomatic infection among the staff close contacts can not be entirely excluded. None the less, all 10 close patient contacts, who had stayed in the same cubicle as the index patient for >20 h had multiple negative SARS-CoV-2 RT-PCR results, up to days 13 and 14 from the last exposure. Thirdly, quantitative RT-PCR and viral culture were not available at our centre, thus the exact viral load and the viability of the virus can not be ascertained.
In conclusion, the route of transmission of SARS-CoV-2 remains to be confirmed, however there is no reason to suspect its physical property would differ greatly from SARS, MERS or any other coronavirus. Our index patient with COVID-19, despite a 35-h stay in an open cubicle in a general ward, did not result in any secondary nosocomial infection in any contacts at the time of writing (37 days after last exposure to the index case). Vigilance with basic infection control measures, including wearing of surgical masks, hand hygiene and environmental hygiene continues to remain fundamental and essential in the prevention of human-to-human transmissions of SARS-CoV-2.
Acknowledgements
The authors thank the Infectious Disease Team in Princess Margaret Hospital for providing care upon the patient's transfer to the Infectious Diseases Centre. We also thank the Molecular Laboratory of Department of Pathology, Queen Elizabeth Hospital for their support in performing SARS-CoV-2 RT-PCR for the index patient and contacts.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.03.036.
Conflict of interest statement
None declared.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) 20 Jan 2020. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) Available at:
- 3.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . China CDC Weekly; 2020. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020.http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51 Available at: [last accessed March 2020] [PMC free article] [PubMed] [Google Scholar]
- 4.Wuhan Municipal Health Commission . Wuhan Municipal Health Commission; Wuhan, China: 2019 Dec 31. Report of clustering pneumonia of unknown etiology in Wuhan City.http://wjw.wh=uhan.gov.cn/front/web/showDetail/2019123108989 Available at: [last accessed February 2020] (In Chinese) [Google Scholar]
- 5.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 20 Jan 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at:
- 6.Communicable Disease Network Australia . The Department of Health. Australian Government; 2019. Coronavirus disease 2019 (COVID-19): CDNA national guidelines for public Health units.https://www1.health.gov.au/internet/main/publishing.nsf/Content/cdna-song-novel-coronavirus.htm Available at: [last accessed February 2020] [Google Scholar]
- 7.Centers for Disease Control and Prevention Interim Infection Prevention and Control Recommendations, for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons under Investigation for COVID-19 in Healthcare Settings. 21 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhcp%2Finfection-control.html Available at:
- 8.Public Health England COVID-19: infection prevention and control guidance. 2020 Feb 19. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/wuhan-novel-coronavirus-wn-cov-infection-prevention-and-control-guidance Available at:
- 9.Central Committee on Infectious Diseases and Emergency Response Interim Recommendation on Clinical Management of Adult Cases with Coronavirus Disease 2019 (COVID-19). Hong Kong Hospital Authority. 13 Feb 2020. https://gateway2.ha.org.hk/ho/cico/,DanaInfo=ha.home+Interim_Recommendation_on_Clinical_Management_of_Adult_Cases_with_COVID-19.pdf Available at:
- 10.Varia M., Wilson S., Sarwal S., McGeer A., Gournis E., Galanis E. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169(4):285–292. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of 2019 novel coronavirus infection in China. New Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [Google Scholar]
- 14.Ho A.S., Sung J.J., Chan-Yeung M. An outbreak of severe acute respiratory syndrome among hospital workers in a community hospital in Hong Kong. Ann Intern Med. 2003;139(7):564–567. doi: 10.7326/0003-4819-139-7-200310070-00008. [DOI] [PubMed] [Google Scholar]
- 15.Li T.S., Buckley T.A., Yap F.H., Sung J.J., Joynt G.M. Severe acute respiratory syndrome (SARS): infection control. Lancet. 2003;361(9366):1386. doi: 10.1016/S0140-6736(03)13052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran K., Cimon K., Severn M., Pessoa-Silva C., Conly J. Aerosol-generating procedures and risk of transmission of acute respiratory infections: a systematic review. CADTH Technol Overv. 2013;3(1) [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Wenjie, Zhao Xiang, Ma Xuejun, Wang Wenling, Niu Peihua, Xu Wenbo. A novel coronavirus genome identified in a cluster of pneumonia cases — Wuhan, China 2019−2020[J] China CDC Weekly. 2020;2(4):61–62. http://weekly.chinacdc.cn/en/article/ccdcw/2020/4/61 Available at: [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020 Jan 30;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020 Feb 13 doi: 10.1093/jtm/taaa021. pii: taaa021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 Feb 15;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centre for Health Protection . Department of Health; Hong Kong Special Administrative Region: 2020. CHP investigates 10 additional cases of novel coronavirus infection.https://www.info.gov.hk/gia/general/202002/09/P2020020900704.htm Available at: [Google Scholar]
- 24.Centre for Health Protection . Department of Health; Hong Kong Special Administrative Region: 2020. Latest situation of cases of novel coronavirus infection.https://www.chp.gov.hk/files/pdf/enhanced_sur_pneumonia_wuhan_eng.pdf Available at: [Google Scholar]
- 25.Wikipedia Coronavirus outbreak in cruise ships. 2020. https://en.wikipedia.org/wiki/2020_coronavirus_outbreak_in_cruise_ships Available at:
- 26.World Health Organization Home care for patients with suspected novel coronavirus (COVID-19) infection presenting with mild symptoms, and management of their contacts. Interim guidance. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts Available at: 4 Feb 2002.
- 27.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19): Healthcare Personnel with Potential Exposure to COVID-19. 2020 Feb 25. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html Available at:
- 28.Phin N. Expert interview: What is contact tracing? Public Health England. https://publichealthmatters.blog.gov.uk/2020/02/13/expert-interview-what-is-contact-tracing/ Available at:
- 29.European Centres for Disease Prevention and Control Public Health management of persons, including healthcare workers, having had contact with COVID-19 cases in the European Union. 25 Feb 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-public-health-management-contact-novel-coronavirus-cases-EU.pdf Available at:
- 30.Lau J.T., Tsui H., Lau M., Yang X. SARS transmission, risk factors, and prevention in Hong Kong. Emerg Infect Dis. 2004;10(4):587–592. doi: 10.3201/eid1004.030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Xu F., Zhou W., Feikin D.R., Lin C.Y., He X. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis. 2004;10(2):210–216. doi: 10.3201/eid1002.030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowling B.J., Chan K.H., Fang V.J., Cheng C.K., Fung R.O., Wai W. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 33.Suess T., Remschmidt C., Schink S.B., Schweiger B., Nitsche A., Schroeder K. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011. BMC Infect Dis. 2012;12:26. doi: 10.1186/1471-2334-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonds A.K., Hanak A., Chatwin M., Morrell M., Hall A., Parker K.H. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14(46):131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 35.Hui D.S., Chan M.T., Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20(Suppl 4):9–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.