Abstract
Background
An autopsy case of a two-month-old male infant who suddenly and unexpectedly died during his sleep, eight days after the onset of benign varicella.
Objectives
To describe post-mortem combined histological and tissue molecular biological techniques for the diagnosis of cytomegalovirus and varicella zoster virus co-infection as a cause of death.
Study design
Real-time quantitative PCR and RT-PCR assays for Herpesviruses, respiratory viruses, Adenovirus, Enterovirus and Parvovirus B19 were performed on multi-organ frozen samples and paraffin-embedded tissues in combination with histology.
Results
Cytomegalovirus and varicella zoster virus were detected by molecular biology with highest viral loads detected in the lungs (4.6 × 107 and 1.9 × 105 genome copies per million of cells, respectively). Pulmonary extensive necrotizing inflammation and immunohistochemistry correlated to virological data. Virological molecular biology was negative on paraffin-embedded tissues.
Conclusions
This case shows that thorough quantitative virological investigations on frozen tissues must be performed in combination with histology and immunohistochemistry for the determination of the cause of a sudden unexplained infant death.
Keywords: Sudden unexpected infant death, Virology, Histopathology, Real-time PCR assay, Cytomegalovirus, Varicella zoster virus
1. Why this case is important
Previous studies have demonstrated the major role of viral diseases in the pathogenesis of sudden unexpected infant death (SUID) [1], [2], [3], [4]. We report on a case of a two-month-old male infant who suddenly and unexpectedly died during his sleep, without premonitory symptoms. Post-mortem virological quantitative molecular analyses performed on frozen tissue samples revealed cytomegalovirus (CMV) and varicella zoster virus (VZV), mainly in the lungs. These results correlated to histological findings. Virological molecular biology was negative on paraffin-embedded tissues.
This case shows that quantitative virological investigations on multi-organ frozen samples must be performed in SUID in combination with histology and immunohistochemistry. Without this diagnostic approach, viral infections can be underestimated as a cause of death in infants.
2. Case description
A two-month-old male infant was found dead in his cot. According to national diagnostic protocol in SUID, an autopsy was performed in our institution.
His mother had positive VZV and CMV serologies with antibodies titers of, respectively, 1800 international units (IU) and 560 arbitrary units (AU) per liter (Enzygnost® assay, Siemens, Erlangen, Germany) associated with high anti-CMV IgG avidity during pregnancy, suggestive of a maternal primary infection before pregnancy. The infant was prematurely delivered at thirty-six weeks. The newborn was hospitalized during the first sixteen days after delivery in a neonatal intensive care unit, because of a severe hypotrophy (weight: 1725 g) associated with thrombopenia-related diffuse petechiae (58,000/mm3 platelets). Platelet count increased spontaneously, up to 148,000/mm3, with no explanation. No perinatal investigations of CMV congenital infection were performed. The newborn weighted 2080 g when he was discharged.
At the age of one month and 21 days, he had varicella contracted from his brother who had been diagnosed with chickenpox 15 days earlier. The newborn's infection was mild, consisting of three successive skin rashes, with no other manifestations. No antiviral treatment was given. Eight days after the onset of the varicella, he was found dead on the back in his cot. In this context of a SUID, an autopsy was performed according to national protocol. His weight was 3725 g. Disseminated skin necrotic lesions were present. The organs were found normal. No cause of death was found. Viral (Herpes Simplex virus type 1 and type 2 (HSV-1 and -2), CMV, Epstein–Barr virus (EBV), VZV, Human Herpes Virus type 6 (HHV-6) and Enterovirus) and bacterial tests were negative in the cerebrospinal fluid (CSF). Interferon alpha was negative in the CSF. Viral cultures performed from skin lesions, throat and anal swabs, as well as from tracheobronchial aspiration were negative. Hemocultures, throat and fecal cultures were negative for main bacterial pathogens.
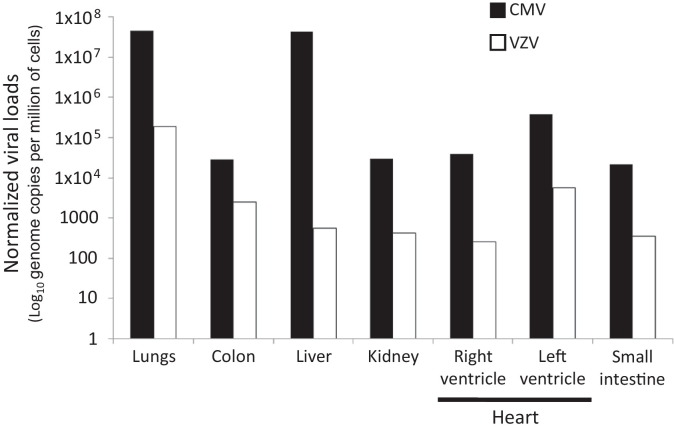
Histology showed extensive necrotizing inflammation in the lungs and moderate mononuclear inflammation in the kidneys (Fig. 1A and B). Other organs were otherwise normal. Immunohistochemistry on paraffin blocks using monoclonal CMV antibody (Argene bioMerieux, Verniolle, France) revealed a few CMV cell inclusions in the lungs whereas numerous CMV inclusions were observed in the kidneys (Fig. 1C and D). Heart, lung, kidney, liver, small intestine and colonic samples were collected for virological analyses and stored at −80 °C. These samples were analyzed with real-time PCR and RT-PCR assays for herpesviruses (HSV-1, HSV-2, VZV, CMV, EBV and HHV-6), respiratory viruses (Influenza A&B viruses, Parainfluenza 1 to 4 viruses, Respiratory Syncytial Virus (RSV), Rhinovirus, human Bocavirus (hBoV), human Coronavirus NL63, OC43, HKU1, 229E and the human Metapneumovirus), Adenovirus, Enterovirus and Parvovirus B19 (HSV1 HSV2 VZV r-gene®, Respiratory Multi Well System r-gene®, Argene bioMerieux, Verniolle, France) [5], [6], [7], [8], [9], [10]. CMV and VZV genomes were detected in all tissue samples (Fig. 2 ). The highest CMV and VZV loads were found in the lungs, 4.6 × 107 and 1.9 × 105 genome copies per million of cells, respectively (Fig. 2). In contrast, CMV and VZV DNA were not detected in paraffin-embedded tissues.
Fig. 1.
Pulmonary (A and C) and renal (B and D) histological and immunohistochemical features. (A) Hematoxylin eosin-stained pulmonary sections showing necrotizing inflammation (×200). (B) Hematoxylin eosin-stained renal inflammation with moderate mononuclear cell infiltrates (×200). (C) A CMV cell inclusion detected in lung by anti-CMV anti-body (brown stained cell; ×200). (D) Numerous CMV inclusions detected in renal tubular cells by anti-CMV antibody (brown stained cells; ×200).
Fig. 2.
CMV and VZV loads, normalized per million of cells, assessed in multi-organ frozen tissues sampled during the autopsy.
3. Other similar and contrasting cases in the literature
Several studies have demonstrated frequent detection of viruses in post-mortem specimens suggesting their involvement in the pathogenesis of SUID [1], [2], [3], [4]. The main viruses reported were respiratory viruses, such as RSV, Adenovirus, Influenza and Parainfluenza viruses, responsible for respiratory tract inflammation, apnoea and hypoxemia, which can exceptionally lead to death [2], [3], [11], [12], [13], [14], [15], [16], [17], [18]. Other viruses involved in common childhood diseases such as HSV, EBV, CMV, HHV-6, Parvovirus B19 and Enterovirus have also been detected in lungs, heart and CSF of deceased infants, but their involvment in the death remained sometimes uncertain [2], [3], [19], [20], [21], [22], [23], [24], [25]. VZV has been reported in only one case of SUID involving a healthy 17-month-old boy who unexpectedly died 3 days after onset of benign varicella. Enzyme-immunoassay with monoclonal antibodies to VZV revealed disseminated infection involving the skin, the lungs, the liver, the spleen and the gastrointestinal tract [26]. To our knowledge, the present case is the first description of a CMV and VZV co-infection in SUID.
4. Discussion
In this case report, the infant's clinical presentation and circumstances of the death were consistent with a SUID syndrome. The term SUID syndrome refers to those SUID cases, in which no cause of death is found despite a complete autopsy, histology of all organs and thorough biological investigations. The varicella diagnosed clinically a few days prior to the death was considered mild with no alarming symptoms. However, pulmonary necrotizing inflammatory lesions, immunohistochemistry, and molecular detection of CMV and VZV in multi-organs samples demonstrated the role of the co-infection in the death.
Varicella zoster virus is responsible for a common childhood disease, which is benign in most cases. The morbidity rate is estimated at 4% and the mortality at 1 per 100,000 children, mainly due to viral or bacterial pulmonary superinfections [27], [28]. Several risk factors to develop a severe form of varicella were evidenced in the present case. The main risk factor was prematurity. Premature newborns tend to be at higher risk to develop a severe infection because of their lymphocyte-related immune system immaturity. Moreover, some studies have shown a link between the pregnancy term and the newborn's antibodies titers against VZV in an immune mother [29], [30]. Specific IgG antibodies are mainly transmitted to the fetus during the third trimester of the pregnancy leading to lower titer in a premature newborn. Furthermore, the antibodies decrease is faster in the first months following the birth of a premature than in an infant born on due term. It is noteworthy that in our case, the severe infection occurred despite maternal protecting antibodies against VZV. The protection level might have not been sufficient because of prematurity.
Another risk factor could be the virulence of the strain, itself. VZV strains that had lost an immunodominant B-cell epitope on the glycoprotein E ectodomain have been reported to have exhibited enhanced cell-to-cell spread in cell culture as well as in the SCID-hu mouse [31], [32]. In the present case, the sequencing of the glycoprotein E coding gene did not reveal any particular virulence regarding the strain isolated (not shown; accession number: KF056799).
The CMV co-infection should also be considered as a risk factor to develop a severe and disseminated varicella. CMV is well known for its immunosuppressive properties through many mechanisms including homologue IL-10 production [24], [33]. Even if the kinetics of viral infections is impossible to prove because of the lack of samples previous to the death, CMV infection should have occurred first, before the infection due to VZV, either during the pregnancy or at the birth by reinfection or reactivation of the mother or during the two months of the infant's life, leading to CMV-related immunosuppression therefore promoted the multivisceral spread of the VZV.
As far as diagnostic methods are concerned, molecular tools were used in several post-mortem protocols to diagnose viral infections in SUID and revealed more viruses than reported with less sensitive methods such as immunofluorescence assay or cell culture [2]. Published studies also showed that qualitative PCR was not sufficient to determine the role of viruses in SUID [2], [3], [25], [34]. In our case, the use for the first time of virus quantitation in combination with histology permitted the assessment of the role of the viruses in the pathogenesis of SUID. Moreover, molecular tests were positive on frozen tissues, but not on paraffin-embedded tissues. This could be explained by modification of nucleic acids caused by formalin fixation. Indeed, this process can cause significant protein–nucleic acid crosslinks as well as fragmentation of nucleic acids reducing the quantity and size of nucleic acids suitable for amplification [35]. Moreover, nucleic acids extract from paraffin-embedded tissues often contains remnants of substances that can inhibit the amplification reaction. These results showed that, without quantitative virology on frozen tissues, viral infection can be underestimated as a cause of death in SUID.
The high viral loads assessed in the lungs associated with necrotizing inflammatory lesions suggest subclinical hypoxia as the possible mechanism of the death [14]. Despite the presence of virus in other organs, histology was normal raising the question of the pathogenic role of viruses in this condition.
In conclusion, this first reported case of CMV and VZV co-infection as a cause of SUID demonstrates that quantitative molecular biology on frozen tissues is mandatory for the diagnosis in combination with histology.
Funding
None.
Competing interests
None of the authors of the present manuscript have a commercial or other association that might pose a conflict of interest (e.g., pharmaceutical stock ownership, consultancy).
Ethical approval
The present study was conducted by the university hospital of Reims (Champagne-Ardenne, France) and was approved by the hospital's ethics committee.
References
- 1.Prtak L., Al-Adnani M., Fenton P., Kudesia G., Cohen M.C. Contribution of bacteriology and virology in sudden unexpected death in infancy. Arch Dis Child. 2010;95:371–376. doi: 10.1136/adc.2009.162792. [DOI] [PubMed] [Google Scholar]
- 2.Weber M.A., Hartley J.C., Ashworth M.T., Malone M., Sebire N.J. Virological investigations in sudden unexpected death in infancy (SUDI) Forensic Sci Med Pathol. 2010;6:261–267. doi: 10.1007/s12024-010-9181-x. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Rodríguez A., Ballesteros S., de Ory F., Echevarría J.E., Alvarez-Lafuente R., Vallejo G. Virological analysis in the diagnosis of sudden children death: a medico-legal approach. Forensic Sci Int. 2006;10:8–14. doi: 10.1016/j.forsciint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Rambaud C., Guibert M., Briand E., Grangeot-Keros L., Coulomb-L’Herminé A., Dehan M. Microbiology in sudden infant death syndrome (SIDS) and other childhood deaths. FEMS Immunol Med Microbiol. 1999;25:59–66. doi: 10.1111/j.1574-695X.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 5.Candotti D., Etiz N., Parsyan A., Allain J.P. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J Virol. 2004;78:12169–12178. doi: 10.1128/JVI.78.22.12169-12178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fafi-Kremer S., Brengel-Pesce K., Barguès G., Bourgeat M.J., Genoulaz O., Seigneurin J.M. Assessment of automated DNA extraction coupled with real-time PCR for measuring Epstein–Barr virus load in whole blood, peripheral mononuclear cells and plasma. J Clin Virol. 2004;30:157–164. doi: 10.1016/j.jcv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Kares S., Lönnrot M., Vuorinen P., Oikarinen S., Taurianen S., Hyöty H. Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using LightCycler. J Clin Virol. 2004;29:99–104. doi: 10.1016/s1386-6532(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 8.Heim A., Ebnet C., Harste G., Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70:228–239. doi: 10.1002/jmv.10382. [DOI] [PubMed] [Google Scholar]
- 9.Gautheret-Dejean A., Manichanh C., Thien-Ah-Koon F., Fillet A.M., Mangeney N., Vidaud M. Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J Virol Meth. 2002;100:27–35. doi: 10.1016/s0166-0934(01)00390-1. [DOI] [PubMed] [Google Scholar]
- 10.Najioullah F., Thouvenot D., Lina B. Development of a real-time PCR procedure including an internal control for the measurement of HCMV viral load. J Virol Meth. 2001;92:55–64. doi: 10.1016/s0166-0934(00)00273-1. [DOI] [PubMed] [Google Scholar]
- 11.Lucas J.R., Haas E.A., Masoumi H., Krous H.F. Sudden death in a toddler with laryngotracheitis caused by human parainfluenza virus-1. Pediatr Dev Pathol. 2009;12:165–168. doi: 10.2350/08-06-0485.1. [DOI] [PubMed] [Google Scholar]
- 12.Krous H.F., Chadwick A.E., Miller D.C., Crandall L., Kinney H.C. Sudden death in toddlers with viral meningitis, massive cerebral edema, and neurogenic pulmonary edema and hemorrhage: report of two cases. Pediatr Dev Pathol. 2007;10:463–469. doi: 10.2350/06-08-0156.1. [DOI] [PubMed] [Google Scholar]
- 13.Greeley C.S. Sudden death from human parainfluenza virus 2. J Infect. 2005;50:366. doi: 10.1016/j.jinf.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Samuels M. Viruses and sudden infant death. Paediatr Respir Rev. 2003;4:178–183. doi: 10.1016/s1526-0542(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 15.Bajanowski T., Rolf B., Jorch G., Brinkmann B. Detection of RNA viruses in sudden infant death (SID) Int J Legal Med. 2003;117:237–240. doi: 10.1007/s00414-003-0367-6. [DOI] [PubMed] [Google Scholar]
- 16.Siani V., Netter J.C., Gorguet B., Carles D., Pellegrin de Villeneuve M. [Detection of respiratory syncytial virus using immunohistochemistry. Report of 53 cases of sudden infant death] Ann Pathol. 1999;19:99–102. [PubMed] [Google Scholar]
- 17.Bajanowski T., Wiegand P., Cecchi R., Pring-Akerblom P., Adrian T., Jorch G. Detection and significance of adenoviruses in cases of sudden infant death. Virchows Arch. 1996;428:113–118. doi: 10.1007/BF00193939. [DOI] [PubMed] [Google Scholar]
- 18.An S.F., Gould S., Keeling J.W., Fleming K.A. Role of respiratory viral infection in SIDS: detection of viral nucleic acid by in situ hybridization. J Pathol. 1993;171:271–278. doi: 10.1002/path.1711710407. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Lafuente R., Aquilera B., Suarez-Mia M.A., Morentui B., Vallejo G., Gomez J. Detection of human herpesvirus-6, Epstein–Barr virus and cytomegalovirus in formalin-fixed tissues from sudden infant death: a study with quantitative real-time PCR. Forensic Sci Int. 2008;178:106–111. doi: 10.1016/j.forsciint.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Dettmeyer R., Sperhake J.P., Müller J., Madea B. Cytomegalovirus-induced pneumonia and myocarditis in three cases of suspected sudden infant death syndrome (SIDS): diagnosis by immunohistochemical techniques and molecularpathologic methods. Forensic Sci Int. 2008;174:229–233. doi: 10.1016/j.forsciint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Baasner A., Dettmeyer R., Graebe M., Rissland J., Madea B. PCR-based diagnosis of enterovirus and parvovirus B19 in paraffin-embedded heart tissue of children with suspected sudden infant death syndrome. Lab Invest. 2003;83:1451–1455. doi: 10.1097/01.lab.0000092232.51370.66. [DOI] [PubMed] [Google Scholar]
- 22.Dettmeyer R., Baasner A., Schlamann M., Haag C., Madea B. Coxsackie B3 myocarditis in 4 cases of suspected sudden infant death syndrome: diagnosis by immunohistochemical and molecular-pathologic investigations. Pathol Res Pract. 2002;198:689–696. doi: 10.1078/0344-0338-00322. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi R., Bajanowski T., Kahl B., Wiegand P. CMV-DNA detection in parenchymatous organs in cases of sudden infant death syndrome. Int J Legal Med. 1995;107:291–295. doi: 10.1007/BF01246875. [DOI] [PubMed] [Google Scholar]
- 24.Püschel K., Hashimoto Y., Löning T., Lignitz E. Cytomegalic inclusion disease of the salivary glands in sudden infant death syndrome. Z Rechtsmed. 1988;99:281–289. doi: 10.1007/BF00204439. [DOI] [PubMed] [Google Scholar]
- 25.Grangeot-Keros L., Broyer M., Briand E., Gut J.P., Turkoglü S., Chretien P. Enterovirus in sudden unexpected deaths in infants. Pediatr Infect Dis J. 1996;15:123–128. doi: 10.1097/00006454-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Asano Y., Yoshikawa T., Urisu A., Yazaki T., Miziquchi Y., Kurata T. Varicella-zoster virus replication site in internal organs of an otherwise healthy child with varicella and sudden death. Acta Paediatr Jpn. 1993;35:348–351. doi: 10.1111/j.1442-200x.1993.tb03068.x. [DOI] [PubMed] [Google Scholar]
- 27.Grimprel E., Levy C., De La Rocque F., Cohen R., Soubeyrand B., Caulin E. Paediatric varicella hospitalisations in France: a nationwide survey. Clin Microbiol Infect. 2007;13:546–549. doi: 10.1111/j.1469-0691.2007.01706.x. [DOI] [PubMed] [Google Scholar]
- 28.Heininger U., Sewaed J.F. Varicella. Lancet. 2006;368:1365–1376. doi: 10.1016/S0140-6736(06)69561-5. [DOI] [PubMed] [Google Scholar]
- 29.van der Zwet W.C., Vandenbroucke-Grauls C.M., van Elburg R.M., Cranendonk A., Zaaijer H.L. Neonatal antibody titers against varicella-zoster virus in relation to gestational age, birth weight, and maternal titer. Pediatrics. 2002;109:79–85. doi: 10.1542/peds.109.1.79. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg J.P., Westerbeek E.A., van der Klis F.R., Berbers G.A., van Elburg R.M. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87:67–72. doi: 10.1016/j.earlhumdev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Santos R.A., Hatfield C.C., Cole N.L., Padilla J.A., Moffat J.F., Arvin A.M. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures abd SCID-hu mice. Virology. 2000;275:306–317. doi: 10.1006/viro.2000.0507. [DOI] [PubMed] [Google Scholar]
- 32.Santos R.A., Padilla J.A., Hatfield C.C., Grose C. Antigenic variation of varicella zoster virus Fc receptor gE: loss of major B cell epitope in the ectodomain. Virology. 1998;249:21–31. doi: 10.1006/viro.1998.9313. [DOI] [PubMed] [Google Scholar]
- 33.Spencer J.V., Lockridge K.M., Barry P.A., Lin G., Tsang M., Penfold M.E. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76:1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dettmeyer R., Baasner A., Schlamann M., Padosch S.A., Haag C., Kandolf R. Role of virus-induced myocardial affections in sudden infant death syndrome: a prospective postmortem study. Pediatr Res. 2004;55:947–952. doi: 10.1203/01.pdr.0000127022.45831.54. [DOI] [PubMed] [Google Scholar]
- 35.Gazziero A., Guzzardo V., Aldighieri E., Fassina A. Morphological quality and nucleic acid preservation in cytopathology. J Clin Pathol. 2009;62:429–434. doi: 10.1136/jcp.2008.059808. [DOI] [PubMed] [Google Scholar]