Abstract
Specified pathogen-free cats were naturally infected with FCoV or experimentally infected with FCoV type I. Seroconversion was determined and the course of infection was monitored by measuring the FCoV loads in faeces, whole blood, plasma and/or monocytes. Tissue samples collected at necropsy were examined for viral load and histopathological changes. Experimentally infected animals started shedding virus as soon as 2 days after infection. They generally displayed the highest viral loads in colon, ileum and mesenteric lymph nodes. Seroconversion occurred 3–4 weeks post infection.
Naturally infected cats were positive for FCoV antibodies and monocyte-associated FCoV viraemia prior to death. At necropsy, most animals tested positive for viral shedding and FCoV RNA was found in spleen, mesenteric lymph nodes and bone marrow. Both experimentally and naturally infected cats remained clinically healthy. Pathological findings were restricted to generalized lymphatic hyperplasia. These findings demonstrate the presence of systemic FCoV infection with high viral loads in the absence of clinical and pathological signs.
Introduction
Feline infectious peritonitis (FIP) is a fatal disease of cats, induced by feline coronavirus (FCoV). FIP is currently the leading infectious cause of death in pet cats (Fehr et al., 1997; Pedersen, 1995). Until now, the pathogenesis of the disease was not well understood. In 1982 it was postulated that FIP was an immune-mediated disease (Jacobse-Geelset al., 1982). One manifestation of an immune-mediated disease is the antibody-dependent enhancement (ADE) frequently observed in experimental infection (Weiss and Scott, 1981). However, ADE does not appear to be widely occurring under field conditions (Addie et al., 1995; Fehr et al., 1996).
Originally, it was thought that FIP was caused by a virulent feline infectious peritonitis virus (FIPV) (Pedersen, 1976; Ward, 1970). Later, two types of FCoVs were identified, one, which is confined to the digestive tract and does not spread beyond regional lymph nodes. This is the so-called feline enteric coronavirus (FECV). The second, immunologically closely related to FECV, is capable of going systemic by infecting monocytes and macrophages and causing FIP. This latter is the so-called FIPV (Pedersen et al., 1981b; Stoddart et al., 1988; Stoddart and Scott, 1989).
Recent findings support the hypothesis that FIPVs arise from avirulent FCoVs (FECVs) due to mutations during their replication (Horzinek and Lutz, 2000; Poland et al., 1996; Vennema et al., 1998). Moreover, new techniques such as RT–PCR could demonstrate the presence of FCoV in the blood of healthy cats (Addie et al., 1996; Egberink et al., 1995; Fehr et al., 1996; Foley et al., 1997; Gunn-Moore et al., 1998; Kipar et al., 1999). These observations support the hypothesis that viraemia is not a hallmark of FIP-inducing FCoVs and that a carrier state exists where cats remain healthy despite being systemically infected. Persistently infected, healthy cats are believed to play the most important epidemiological role in FIP, because they represent a constant source of infection by shedding FCoV in their faeces (Foley et al., 1997).
Two FCoV serotypes have been identified on the basis of in vitro neutralization tests (Hohdatsu et al., 1991a, b; Pedersen et al., 1984a), both of which can cause FIP. The type I virus group represents the genuine FCoVs; they grow poorly in cell culture. Type II viruses, however, arise from recombinations between feline and canine (CCV) coronavirus(Herrewegh et al., 1998). These proliferate well in cell culture and are widely used for the study of FCoV infection. However, in the field, serotype II accounts for only 20 to 30% of all FCoV infections at least in Japan (Hohdatsu et al., 1992). In a recent study conducted in Austria, type I was found to be present in 62% of the cats studied (Posch et al., 2001).
The goal of the present study was to quantify viral loads in the intestinal tract and in various organs of cats infected with FCoV type I and to correlate these with potential clinical and pathological signs. As type I viruses are difficult to grow in cell culture, viruses originating from faeces of naturally infected animals were used for experimental studies. As natural infection occurs via the faecal–oral route, animals were inoculated perorally. Furthermore, in a prospective experiment with naturally infected animals, the presence of FCoV in faeces, blood monocytes and different organs was evaluated and correlated with clinical and pathological findings.
Materials and methods
Animals
For experimental infections, 15 specified pathogen-free (spf) kittens were provided by IFFA-Credo (Saint-Germain sur l'Arbresle, France) at either 6 or 16 weeks of age. The animals were first acclimatized by keeping them together for 4 days and were then separated into groups. After infection, the cats were clinically examined daily, weighed, and the rectal temperature was measured.
Eight spf cats were provided by HarlanWinkelmann (Borchen, Germany) at 9 weeks of age. They were acclimatized until they entered the experiment at the age of 21 weeks (Kipar et al., 1999).
Preparation of virus inoculum for infection
Viruses to be used as the source of infection were isolated from faecal samples of field cats or from the intestines of cats experimentally infected with an FCoV field strain. Based on the determination of the sequence of part of the S-gene and on the comparison with sequences deposited in the gene bank, they all belonged to type I.
Faecal samples were prepared as follows: 20 ml of ice-cold RPMI 1640 medium (Sigma, Schnelldorf, Germany) containing L-glutamine and 10% fetal calf serum (FCS, BioConcept, Allschwil, Switzerland), but no antibiotics, were added to 1.5–2 g of faeces under sterile and RNase-free conditions. The samples were incubated for 10 minutes at 4 °C with occasional shaking and finally centrifuged twice for 10 minutes at 900×g to obtain a clear supernatant. The supernatants were aliquoted into tubes of 4 ml each, frozen, and stored at −80 °C. Aliquots of these virus-containing supernatants were analysed to determine the FCoV load. In parallel, the samples were tested for the presence of parvoviruses (see below), yielding negative results. Four of these faecal extracts containing FCoV were used to infect kittens: FCoVZu1, FCoVZu2, FCoVZu3 and FCoVZu5.
The intestines (gut homogenates) were prepared as follows: the intestines including their contents were collected from three kittens that had been experimentally infected with the FCoVZu1 strain. The intestines were collected after euthanasia at peak infection and separated into duodenum, jejunum, ileum and colon. Samples were snap-frozen in liquid nitrogen and stored at −80 °C. After it became evident that the viral loads were high in intestinal samples of all three cats, the intestinal aliquots were homogenized and pooled in a total volume of 500 ml of stabilizing solution (HONDEV STAB, Intervet UK; diluted 1:4 in RPMI 1640). Homogenization was performed using an Ultra Tourax instrument (Kinematica AG, Lucerne, Switzerland) at 20,000 rpm for 15 minutes on ice at 5–6 intervals. The final volume of about 500 ml of pooled homo genate was aliquoted and stored at −80 °C. This gut homogenate was later used in a titration experiment in cats.
Infection experiments
Experimentally infected cats
Animals were infected perorally twice within 24 hours, the infectious dose was applied via an oesophageal tube. This was done in order to keep the volume of the inoculum as small as possible and to increase the chance of infection. Infection was performed while the animals were anaesthetized to avoid any unwanted reaction from the cats that could compromise any efforts to standardize the procedure. In the first infection experiment, six 6-week-old spf kittens were divided into three groups of two animals each and infected with different FCoV field strains (Table 1). In the second infection experiment, three spf kittens of 6 weeks of age were kept together as one group during the entire experiment (17 days). Each cat was inoculated perorally with two doses of the FCoVZu1 strain within 24 hours (Table 1). In the third infection experiment, six 16-week-old spf kittens were divided into three groups of two animals each and infected with ten-fold dilutions (see Table 1) of the virus strain FCoVZu1.
Table 1.
Compilation of experimental data of the three infection experiments
| Experiment | Group | Cat no. | Virus | Dose (RNA copy no.) | |
|---|---|---|---|---|---|
| Strain | Origin | ||||
| I | 1 | 3137B | FCoVZu5+FCoVZu2 | Faecal extract | 1.3×104+8.8×104 |
| 3154B | |||||
| 2 | 3132B | FCoVZu1 | Faecal extract | 0.8×106 | |
| 3141B | |||||
| 3 | 3138B | FCoVZu3 | Faecal extract | 1.5×105+1.5×105 | |
| 3139B | |||||
| II | 4 | 3312B | FCoVZu1 | Faecal extract | 8×106 |
| 3313B | |||||
| 3314B | |||||
| III | 5 | 3252B | FCoVZu1 | Gut homogenate | 3×109 |
| 3258B | |||||
| 6 | 3257B | FCoVZu1 | Gut homogenate | 0.3×109 | |
| 3265B | |||||
| 7 | 3253B | FCoVZu1 | Gut homogenate | 0.03×109 | |
| 3256B | |||||
Prior to infection, and at weekly intervals, 1 ml of blood was collected from all animals and haematological parameters as well as FCoV viral loads were determined.
FCoV shedding was determined by measuring FCoV viral load in faecal swabs. In the first two infection experiments, faecal swabs were collected on a daily basis, whereas in the third infection experiment shedding was determined during the first 5 weeks in faecal swabs collected every 2 days; in the remaining period, samples were collected weekly.
Naturally infected cats
Eight spf cats, originating from a natural FCoV contact infection experiment in which a total of 20 cats had been included, were housed together with 11 FCoV-infected, clinically healthy cats (tested positive for circulating and FCoV-specific immune complexes (Kipar et al., 1999)) originating from animal shelters, for 30 weeks from the age of21 weeks on. The eight cats survived the total observation period, whereas the remaining 12 animals of that group developed FIP (Kipar et al., 1999). The cats were clinically examined on a weekly basis throughout the experiment. Blood for serology and monocyte isolation was collected every 2 months. Viral loads and pathological changes were assessed on samples collected at necropsy.
Preparation of viral RNA from whole blood, plasma and faeces
RNA was extracted from whole EDTA blood using the QIAamp® RNA Blood Mini Kit (Qiagen, Basel, Switzerland) according to the manufacturer's protocol. For the extraction of viral RNA from plasma the QIAamp® Viral RNA Mini Kit (Qiagen) was used.
To determine the viral load in faeces, faecal swabs were inserted into the rectum and then placed in 1.5 ml RNase-free Eppendorf tubes. RNA was prepared as follows: 200 μl of phosphate buffered saline (PBS 1×) were added to each swab, the tubes were closed and briefly mixed by vortexing. The swabs were then incubated at 42 °C for 10 minutes and mixed again. After a short spin the swab was inverted in the tube and another short spin was applied. The dried swab was removed and the extract was inactivated by incubating at 95 °C for 5 minutes. Any residual faecal material was eliminated in a final centrifugation step. The faecal extracts were diluted 1:10 in PBS 1× and both the undiluted and the diluted solution were tested for the presence of FCoV RNA.
Viral RNA isolation from monocytes
As previously described (Kipar et al., 2001b), monocytes were isolated from heparin blood(4.5 ml) using Ficoll (Histopaque®-1077, Sigma). The lymphocyte-enriched fraction was resuspended in 3 ml of RPMI 1640 medium supplemented with10% FCS (BioConcept), antibiotics (Penicillin, Streptomycin) and L-glutamine and the cells were distributed in three wells of a 24-well plate. Cells were incubated for 16 hours in 5% CO2to allow monocytes to adhere to the well surface. Floating lymphocytes were washed out. The monocytes of all three wells were lysed together in 350 μl RLT Buffer (Qiagen), the lysate was homogenized over a QIAshredder® column (Qiagen) and RNA was subsequently purified using the RNeasy® Mini Kit (Qiagen) according to the manufacturer's protocol.
In naturally infected cats isolated monocytes, obtained 4 months after the beginning of the experiment and 2 months later (end of experiment), were co-cultivated with whole feline embryo cells (WFE) and RNA was isolated as described elsewhere (Gunn-Moore et al., 1998).
RNA isolation from organs
In experimentally infected animals, samples from different organs (duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver, spleen, bone marrow, kidney, thymus, lung, tonsil and brain) were collected under sterile, RNase-free conditions and directly frozen in liquid nitrogen. The tissues were then stored at −80 °C. RNA was purified by means of the ABI Prism™ 6700 Automated Nucleic Acid Workstation (Applied Biosystems (ABI), Rotkreuz, Switzerland). About 30 mg of tissue was cut out from the frozen samples and placed in a 2 ml Eppendorf tube containing 1.2 ml of ABI Lysis Buffer and a 3 mm steel bead (Schieritz & Hauenstein AG, Arlesheim, Switzerland). The samples were homogenized and lysed by mixing twice for 1 minute at 30 Hz in a Mixer Mill device (Qiagen). After mixing, the samples were incubated for 30 minutes on ice to reduce the foam that had formed during the homogenization step. After equilibration to room temperature (RT), the lysed samples were digested during 1 hour with 200 μg of proteinase K (Qiagen). The samples were then subjected to another round of homogenization with the Mixer Mill and finally stored at −20 °C for no longer than 2 days. From these lysed samples, RNA was purified using the ABI Prism™ 6700 chemistry according to the manufacturer's protocol. Negative controls containing only PBS 1× were also extracted to check eventual cross-contamination during this extraction step.
As control for the efficacy of RNA purification, the RNA was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems) by means of the ABI Prism™ 6700. The expression of the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was measured by real-time PCR (Leutenegger et al., 1999b).
In naturally infected animals, for RNA extraction, approximately 100 mg of tissue was homogenized with a tissue homogenizer (PCR Tissue Homogenizing Kit, Süd-Laborbedarf GmbH, Gauting, Germany) and lysed in each 700 μl lysis buffer. Total RNA was extracted with the RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol (Kipar et al., 2001b). RNA was dissolved in 30 μl RNase-free water.
Viral loads
FCoV viral loads were determined by one-tube real-time RT–PCR detecting a 102 bp fragment of the conserved FCoV 7b gene (Gut et al., 1999), using an automated fluorometer (TaqMan, ABI 7700, Applied Biosystems) in all samples from experimental infections and tissue samples from naturally infected cats.
The infectious dose was calculated as the RNA copy number present in the volume of the infectious inoculum given to the cats (Table 1).
Blood samples of experimentally infected animals were also tested by one-tube real-time PCR for presence of feline parvovirus (FPV), feline leukaemia virus (FeLV), feline immunodeficiency virus (FIV) and feline herpesvirus-1 (FHV-1) as described elsewhere (Hofmann-Lehmann et al., 2001;Leutenegger et al., 1999a; Vogtlin et al., 2002). In the case of FPV, a sequence in the high conserved VP-2 gene was amplified with the TaqMan® technology using the following primers and probe: PV3294f: 5′-ACTGCATCATTGATGGTTGCA-3′; PV3400r: 5′-GGTATGGTTGGTTTCCATGGA-3′ PV3375p: 5′-FAM-CCCAATGTCTCAGATCTCATAGCTGCTGG-6-TAMRA-3′.
Large intestinal content was collected from naturally infected cats at necropsy and tested by conventional RT–PCR (Herrewegh et al., 1995) and electron microscopy (negative staining technique) for FCoV (Kipar et al., 1999).
Serology
Antibody titres in serum were determined by immunofluorescence analysis (IFA) as previously described (Osterhaus et al., 1977). Circulating immune complexes were measured by competitive ELISA as described elsewhere (Schroo, 1994).
Results
Infection experiment I
To determine the infectious potential of different FCoV field strains, three groups of two cats each were infected with three different FCoV field isolates (Table 1).
Prior to infection, all cats were negative for FCoV in both blood and faeces. The faeces of the cats of group 2, infected with the FCoVZu1 strain, turned positive on day 4 post infection (p.i.) and the cats continued to shed high amounts of virus (on average 1×107FCoV RNA copies per g faeces) until euthanasia. The animals of group 1 and 3 did not shed any virus during the first 14 days after infection. The cats of group 2 were sacrificed 14 days p.i., whereas those of group 1 and group 3 were re-infected on day 14 under the same conditions as in the first inoculation. To determine whether lack of infection was a hallmark of the FCoV strain used or whether it depended of the infectious dose, group 1 was re-infected with a different FCoV strain (FCoVZu2), whereas group 3 was re-infected with the same isolate as the first time (FCoVZu3) (Table 1). All four cats then started shedding FCoV in their faeces from day 2 after re-infection on. FCoV RNA was detected in whole blood in two cats (3154B and 3139B) on days 14 and 21 p.i., in cat 3132B on day 7 p.i. and in cat 3138B on day 21 p.i. FCoV RNA was found in the plasma fraction of cat 3132B on day 14 p.i. Cat 3138B was the only animal that seroconverted (day 28 p.i.). The results are compiled in Table 2.
Table 2.
Summary of the results of the infection experiments I–III
| Experiment I | Experiment II | Experiment III | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
| Virus strain | FCoVZu5+2 | FCoVZu1 | FCoVZu3 | FCoVZu1 | FCoVZu1 | ||
| Start of shedding (days p.i.) | 2–6 a | 3–4 | 2–3 a | 2–3 | 2 | 2 | 2 |
| Intensity of shedding | +++ | ++/+++ | +++ | +++ | +++ | +++ | +++ |
| Frequency of shedding; no. of positive tests (samples tested) | 18 (58) | 22 (30) | 19 (58) | 46 (54) | 32 (48) | 37 (48) | 37 (48) |
| Rectal temperature >39 °C | 21 (58) | 6 (30) | 3 (58) | Never | 2 (36) | 10 (36) | 4 (36) |
| Clinical signs | None | None | None | None | None | None | None |
| Viraemia, cell-associated; no. of positive tests (samples tested) | 2 (10) | 1 (6) | 3 (10) | 1 (12) | 0 (22) | 0 (22) | 0 (22) |
| Viraemia, cell-free; no. of positive tests (samples tested) | 0 (4) | 1 (2) | 1 (4) | 4 (12) | 0 (22) | 0 (22) | 0 (22) |
| Seroconversion (days p.i.) | −/− | −/− | 28/− | 14/14/− | 14/22 | 14/14 | 14/22 |
| Monocyte-associated viraemia; no. of positive tests (samples tested) | 0 (2) | 1 (2) | 0 (2) | 1 (3) | 0 (22) | 0 (22) | 0 (22) |
+++=CT value from 20 to 30.
++=CT value from 30 to 40.
−=never seroconverted.
=after re-infection.
Monocytes were isolated from blood collected prior to euthanasia and cultivated overnight. From all cats tested only in one animal of group 2 (3132B) FCoV RNA could be detected in the monocytes.
Clinically, the cats showed a short episode of moderate pyrexia at days 2 and 3 p.i. The leukocyte counts were slightly elevated at the beginning of the experiment (17,000–27,000 per μl), but returned to normal values after 1 week. No further clinical signs were detected.
Blood from all animals was additionally tested for the presence of other viruses, including FPV, FeLV, FIV and FHV-1, prior to infection and during the entire experiment with negative results. The experiment was concluded at day 28 p.i.
Infection experiment II
To obtain FCoV-infected intestines for use as infection material for future experiments, three 6-week-old spf kittens were inoculated with the FCoVZu1 strain (Table 1).
All three cats were clinically healthy during the entire observation period, in spite of the fact that they all started to shed high amounts of FCoV in their faeces 2–3 days after infection and continued to shed virus each day until they were sacrificed (Table 2) on day 17 p.i.
Prior to infection, all three cats were negative for FCoV in blood as well as in faeces. All three animals were positive for viral RNA in the plasma fraction on day 7, and one cat (3314B) also on day 17 p.i. In addition, cat 3313B tested positive in whole blood on day 7. Two cats (3312B and 3313B) transiently seroconverted on day 14. Results are compiled in Table 2.
Blood from all animals was additionally tested for the presence of other viruses, namely FPV, FeLV, FIV and FHV-1, prior to infection and during the entire experiment with negative results.
Infection experiment III
To determine the optimal infectious dose as well as possible dose-dependent effects, three groups of two animals each were infected with ten-fold dilutions of FCoVZu1-infected gut homogenate (Table 1) derived from the intestines of FCoVZu1-infected cats (infection experiment II).
Regardless of the infectious dose, all animals started shedding 2 days after infection. Intensity and frequency of shedding were comparable in all three groups.
In none of the samples tested FCoV RNA was detected in blood or isolated monocytes. All cats seroconverted 2 to 3 weeks p.i. Results are compiled in Table 2.
With the exception of several one day episodes, when the cats exhibited elevated rectal temperatures (≥39 °C), all animals remained clinically healthy during the entire observation period of 80 days.
Naturally infected cats
In order to compare experimental with natural infection, we analysed data from animals thatoriginated from an experiment in which FCoV infection was established under long-term natural exposition by housing of spf cats with naturally infected cats for 5 months (Kipar et al., 1999).
All eight spf cats that survived healthy showed seroconversion with titres of 1:100 or higher when tested either 2 months or 4 months after first being housed with FCoV-infected cats. At the end of the experiment, three animals were still seropositive (titre ≥1:100; Table 3). In six cats (cat Nos. 2–5, 7, 8), circulating FCoV-specific immune complexes were detected at least at one time point, whereas at the end of the experiment all cats were negative in the competitive ELISA (Kipar et al., 1999).
Table 3.
Summary of the results obtained in the blood (antibody titres, monocyte-associated viraemia), in organs (FCoV-RT–PCR) and in large intestinal content (electron microscopy, FCoV-RT–PCR) of naturally FCoV-infected cats
| Animal | TP1 | TP2 | TP3 | TP4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFA | IFA | IFA | Monocytes | IFA | Monocytes | Spleen | Mes. lnn. | Bone marrow | Shedding | ||
| EM | RT–PCR | ||||||||||
| 1 | ⩽25 | 800 | 200 | + | 50 | + | + | − | + | − | + |
| 2 | ⩽25 | ⩽25 | 200 | + | ⩽25 | (+) | n.p. | n.p. | n.p. | (+) | + |
| 3 | ⩽25 | 100 | 800 | ++ | 400 | ++ | − | − | n.p. | + | n.p. |
| 4 | ⩽25 | 100 | 100 | + | 50 | +++ | + | − | − | − | + |
| 5 | ⩽25 | 400 | 400 | ++ | 400 | − | − | + | + | − | + |
| 6 | ⩽25 | >3200 | ⩽25 | ++ | 50 | ++ | + | − | − | − | − |
| 7 | ⩽25 | >3200 | 400 | + | 400 | − | + | + | − | + | + |
| 8 | ⩽25 | ⩽25 | 100 | + | ⩽25 | (+) | − | − | − | +++ | + |
IFA: immune fluorescence analysis (titre); EM: electron microscopy (negative staining technique); mes.lnn.: mesenteric lymph nodes; n.p.: not performed.−: negative; (+): weak positive; +: moderate positive; ++: strong positive; +++: very strong positive. TP: time point. 1: directly prior to infection; 2: 2 months p.i.; 3: 5 months p.i.; 4: 7 months p.i. (euthanasia).
Four months after first being housed with FCoV-infected cats, monocyte-associated FCoV viraemia was detected in all cats. At the end of theexperiment, six cats still exhibited a monocyte-associated viraemia. Examination of the large intestinal content, collected at necropsy, demonstrated shedding of FCoV in seven cats, either by electron microscopy and/or by RT–PCR. Results are compiled in Table 3.
All animals had remained clinically healthy during the entire experiment.
Post-mortem analysis: FCoV viral load in different tissues
To determine to what extent FCoV was present in the organs of the cats, different tissues were analysed for presence of FCoV RNA.
In infection experiment I, the cats of group 2 were sacrificed at day 14 p.i., whereas those of groups 1 and 3 were sacrificed at day 28 p.i. (14 days after re-infection), respectively. In all cats, macroscopical findings were restricted to enlargement of mesenteric lymph nodes. Samples of different organs (duodenum, jejunum, ileum, colon, mesenteric lymph nodes, liver, spleen, bone marrow, kidney, thymus, lung, tonsil and brain) were collected and examined for FCoV load. Ileum, colon and mesenteric lymph nodes displayed the highest viral loads (Fig. 1). In animals of group 2 (Fig. 1B), high viral loads were detected in duodenum, jejunum, liver, spleen and kidney. In all three groups, FCoV was detected in the entire intestine and in all organs tested, with the exception of the bone marrow.
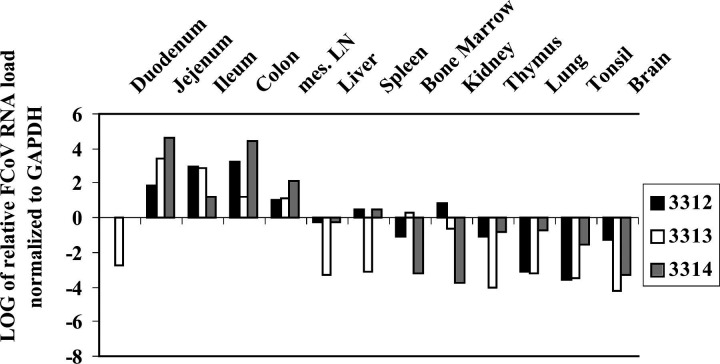
Figure 1.
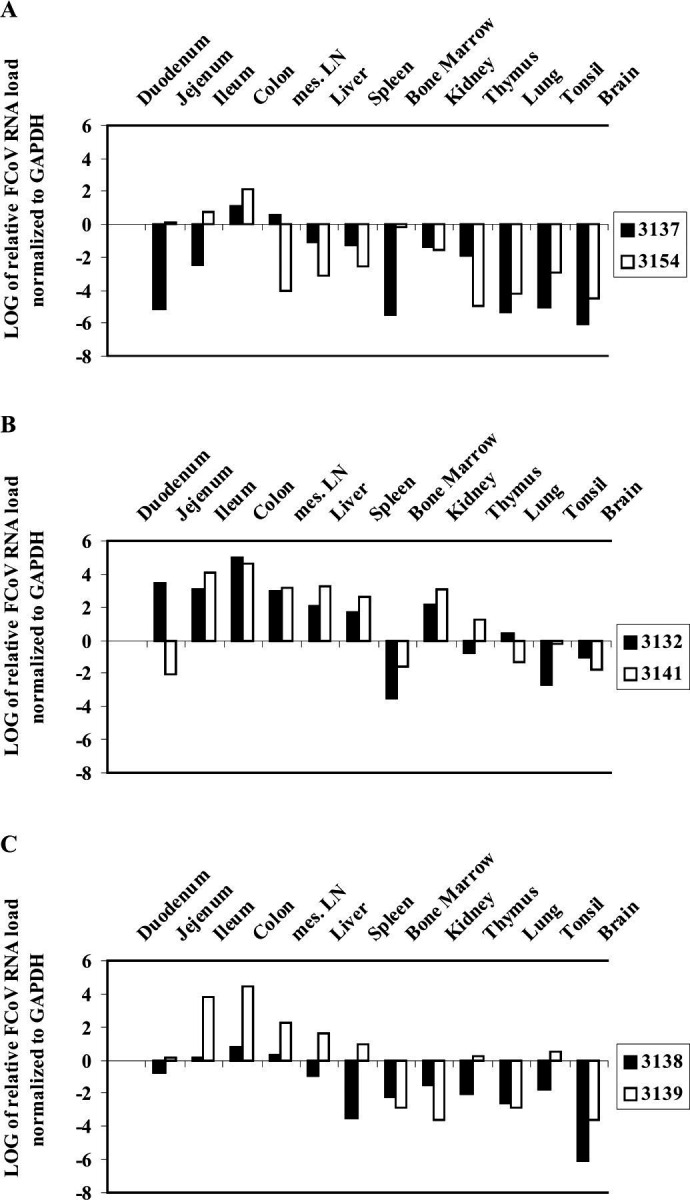
FCoV viral loads in organs of experimentally infected cats: Experiment I. Data are calculated using the comparative CT method for relative quantitation. Expression in duodenum served as calibrator. A: group 1; B: group 2; C: group 3.
In infection experiment II, the cats were sacrificed on day 17 p.i. Like in the first infection experiment, macroscopical findings were restricted to enlargement of mesenteric lymph nodes. The patterns of viral loads in the organs of these three animals were similar to those in the cats of group 2 of infection experiment I, infected with the same FCoV strain: duodenum, bone marrow and brain showed comparatively low viral loads, while jejunum, ileum, colon and mesenteric lymph nodes showed high viral loads (Fig. 2).
Figure 2.
FCoV viral loads in organs of experimentally infected cats of group 4: Experiment II. Data are calculated using the comparative CT method for relative quantitation. Expression in duodenum served as calibrator.
In infection experiment III, the animals were euthanased 80 days after infection. Macroscopical findings were again restricted to enlargement of mesenteric lymph nodes. In addition to the organs examined in the previous experiments, skin and skeletal muscle samples were analysed. In the animals, which received the lowest dose of virus (Fig. 3A), the virus was present at low levels in almost all organs. With increasing infection doses, higher viral loads were detected in the colon (Fig. 3B and C). However, due to the small number of cats (n=2 per group), the differences are not significant.
Figure 3.
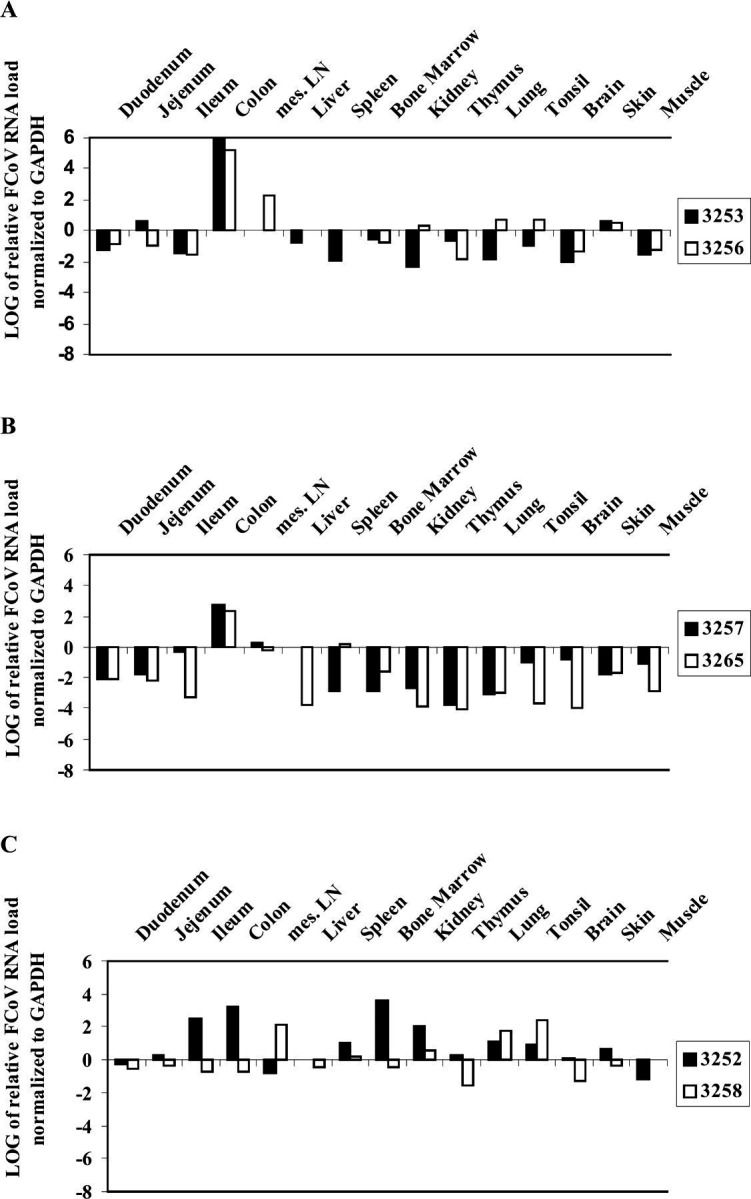
FCoV viral loads in organs of experimentally infected cats: Experiment III. Data are calculated using the comparative CT method for relative quantitation. Expression in duodenum served as calibrator. A: group 5; B: group 6; C: group 7.
In naturally infected cats, like in experimentally infected animals, macroscopical findings were restricted to enlargement of mesenteric lymph nodes. Cats tested positive for FCoV in spleen, bone marrow and/or mesenteric lymph nodes. Five of the seven cats examined were FCoV-positive in at least one of the organs. In four animals, FCoV-RNA was detected in spleen and/or bone marrow, two cats were positive in the lymph nodes. Three animals were positive in two of the three organs tested. None tested positive in all organs analysed (Table 3).
Histopathological findings
In addition, organs were subjected to histological examination for evaluation of pathological findings.
In all cats, both experimentally and naturally infected, histopathological findings were restricted to a generalized B and T cell hyperplasia in spleen and mesenterial lymph nodes. The thymus exhibited no or minimal involution. The bone marrow showed moderate to high activity. All other organs were unaltered.
Discussion
In the present study we investigated both FCoV distribution and loads in faeces, blood and organs of cats experimentally infected with different FCoV type I field strains as well as of naturally FCoV-infected cats.
So far, in the majority of experimental infection trials, the investigators used a type II FCoV (Chalmers et al., 1993; Fiscus et al., 1987;Glansbeek et al., 2002; Pedersen et al., 1981a, 1984a; Pedersen and Floyd, 1985; Vennema et al., 1990; Wasmoen et al., 1995), mainly the strain FIPV-1146 (Pedersen et al., 1984b). Infection with type II FCoVs provides reproducible results, but this serotype is not representative of FCoVs present in the field (Hohdatsu et al., 1992; Posch et al., 2001). Our study aimed to prove if cats can be reproducibly infected with an FCoV type I strain and to gain additional insight into the pathogenesis of FCoV type I infection by measuring viral loads.
Experimental infections showed that, regardless of the strain used, kittens can be infected and that faecal shedding is detectable as early as 2 days p.i. However, shedding at day 2 p.i. was observed mainly in those kittens that had received a high infectious dose (⩾106FCoV RNA copies; cats in group 2 [infection experiment I] and cats of infection experiment II and III). In kittens of groups 1 and 3 (infection experiment I) that had received a lower FCoV dose (⩽105FCoV RNA copies), shedding did not start until 2 to 3 days after re-infection witha second low-dose inoculum. From these observations it is concluded that shedding is somehow dependent on the force of infection, i.e. there seems to be a threshold dose of virus that allows the infection to manifest. The time span until seroconversion was not affected by early shedding.
Although shedding occurred at constantly high levels during the entire observation periods (between 2 and 11 weeks), clinical signs were restricted to transient episodes of moderate pyrexia in all 15 animals of this part of the study.
Our FCoV PCR results on several sections of the intestine extended the findings of Herrewegh et al. (1997), who were able to detect FCoV in colon and rectum but not in other sections of the intestine. However, we demonstrated that all field strains used for infection not only lead to infection of the colon and rectum, but of the entire intestine. Additionally, almost every parenchymal organ was shown to contain FCoV. Considering all animals together, the most frequently affected organs were: colon, mesenteric lymph nodes, liver, ileum, jejunum, kidney and spleen. Four kittens tested positive in the brain, although no clinical signs of CNS involvement were observed. This can be explained by the presence of FCoV-infected monocytes in the tissue vasculature althoughinflammatory processes were absent (Kipar et al., 2003).
High viral loads were not associated with clinical signs or pathological findings. The generalized lymphatic hyperplasia observed in all the cats has previously been shown to reflect the systemic FCoV-specific immune response cats develop after FCoV infection (Kipar et al., 1999, 2001a).
Similarly, the naturally infected animals included in this study had remained clinically healthy during the 7 months they were housed with animals dying from FIP. All these animals seroconverted and most developed circulating FCoV-specific immune complexes, supporting the fact that the occurrence of FCoV immune complexes is not restricted to the development of FIP (Kipar et al., 1999). In seven out of the eight cats examined, FCoV shedding was demonstrated at the time of necropsy. This confirms previous findings that healthy infected cats are frequently shedding virus (Foley et al., 1997; Harpold et al., 1999). Furthermore, there is a strong correlation between shedding frequency and FCoV antibody titre (Gut, 1999). Accordingly, we concluded that the high FCoV antibody titres observed in all eight animals represent a high FCoV load in all these cats (Gut, 1999).
In these naturally infected cats, 5 months after exposure to healthy FCoV-infected animals, blood monocytes were found to be positive for FCoV-RNA. Furthermore, at necropsy, monocytes were still positive in 75% of the cats. These findings are in accordance with other studies, which showed that FCoV-infected, healthy cats can maintain monocyte-associated viraemia for at least 12 months (Gunn-Moore et al., 1998). They differ from the results obtained from our experimentally infected cats where FCoV-RNA was only detected in the monocytes of one animal. However, this is likely due to the lower sensitivity of the FCoV RT-PCR, performed on RNA directly isolated from peripheral blood monocytes. In comparison, monocytes of naturally infected cats in this study as well of naturally infected cats in a previous study had been cultivated prior to FCoV RT-PCR (Gunn-Moore et al., 1998).
In addition, as in experimentally infected cats of this study, FCoV was found in the spleen, lymph nodes and/or bone marrow of most of the naturally infected cats at the time of necropsy. From these observations we can conclude that in cats infected with FCoV by natural contact with infected animals FCoV spreads systemically and into internal organs (probably via infected monocytes within the vasculature) (Kipar et al., 2003).
Several of the infected cats were found to be seronegative although they were shedding virus. This is not surprising and may be explained by the fact that in many cats, replication initially may be confined to the intestine and only later spread to the blood circulation and to the lymph nodes. As long as infection is confined to the intestine or the viral load is low, this may not induce seroconversion. Similar observations were also made in FIV infection in cats experimentally infected with low challenge doses (Boretti et al., 2000).
As FCoV could be detected in almost all organs tested from both experimental and naturally infected cats in the absence of any clinical signs and pathological findings, we concluded that development of FIP is not an imperative sequel of systemic spreading of FCoV. Originally, Pedersen and co-workers postulated that the apathogenic FCoV (designated FECV) remained restricted to the intestinal tract and that systemic infection would coincide with the development of FIP (Pedersen et al., 1981b). However, our observations support the hypothesis that the key event triggering the development of FIP is not associated with the capability of an FCoV to cause viraemia and systemic infection. Although the observation period was not sufficiently long to see animals developing FIP, and we did not sequence the virus to detect mutations, these observations support the concept that FCoV mutants with increased virulence arise during replication (Kennedy et al., 2001; Vennema et al., 1998). To further substantiate this hypothesis it would be important to determine if FCoV is replicating in the blood. A recently developed method to determine FCoV messenger RNA in peripheral blood mononuclear cells could address this question (Simons et al., 2002).
In conclusion, we have demonstrated, that SPF cats experimentally infected by different FCoV type I field strains or naturally FCoV-infected, can remain clinically healthy despite overall high viral loads in faeces and different organs. It is suggested that identical mechanisms of FCoV kinetics occur under field conditions and that high viral loads are the basis for the generation of virulent FIPV strains.
Acknowledgments
The authors would like to acknowledge ArminRüdimann for expert technical help with the experimentally infected cats as well as Edith Rhiner, Elizabeth Rogg and Beatrice Weibel for excellent technical support. Thanks are due to Dr W. Leukert for clinical supervision of the naturally infected cats. We are grateful to Mr A. Barth for perfect technical assistance.
References
- Addie D.D., Toth S., Murray G.D., Jarrett O. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus, American Journal of Veterinary Research, 56, 1995, 429–434. [PubMed] [Google Scholar]
- Addie D.D., Toth S., Herrewegh A.A., Jarrett O. Feline coronavirus in the intestinal contents of cats with feline infectious peritonitis, Veterinary Record, 139, 1996, 522–523. [DOI] [PubMed] [Google Scholar]
- Boretti F.S., Leutenegger C.M., Mislin C., Hofmann-Lehmann R., Konig S., Schroff M., Junghans C., Fehr D., Huettner S.W., Habel A., Flynn J.N., Aubert A., Pedersen N.C., Wittig B., Lutz H. Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV env gene and feline IL-12 expression, Aids, 14, 2000, 1749–1757. [DOI] [PubMed] [Google Scholar]
- Chalmers W.S., Horsburgh B.C., Baxendale W., Brown T.D. Enhancement of FIP in cats immunised with vaccinia virus recombinants expressing CCV and TGEV spike glycoproteins, Advances in Experimental Medicine & Biology, 342, 1993, 359–364. [DOI] [PubMed] [Google Scholar]
- Egberink H.F., Herrewegh A.P., Schuurman N.M., van der Linde-Sipman J.S., Horzinek M.C., de Groot R.J. FIP, easy to diagnose?, Veterinary Quarterly, 17 (Suppl 1, 1995, S24–25. [PubMed] [Google Scholar]
- Fehr D., Bolla S., Herrewegh A.A., Horzinek M.C., Lutz H. [Detection of feline coronavirus using RT–PCR: basis for the study of the pathogenesis of feline infectious peritonitis (FIP)], Schweizer Archiv für Tierheilkunde, 138, 1996, 74–79. [PubMed] [Google Scholar]
- Fehr D., Holznagel E., Bolla S., Hauser B., Herrewegh A.A., Horzinek M.C., Lutz H. Placebo-controlled evaluation of a modified life virus vaccine against feline infectious peritonitis: safety and efficacy under field conditions, Vaccine, 15, 1997, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus S.A., Rivoire B.L., Teramoto Y.A. Epitope-specific antibody responses to virulent and avirulent feline infectious peritonitis virus isolates, Journal of Clinical Microbiology, 25, 1987, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J.E., Poland A., Carlson J., Pedersen N.C. Patterns of feline coronavirus infection and fecal shedding from cats in multiple-cat environments, Journal of the American Veterinary Medical Association, 210, 1997, 1307–1312. [PubMed] [Google Scholar]
- Glansbeek H.L., Haagmans B.L., te Lintelo E.G., Egberink H.F., Duquesne V., Aubert A., Horzinek M.C., Rottier P.J. Adverse effects of feline IL-12 during DNA vaccination against feline infectious peritonitis virus, Journal of General Virology, 83, 2002, 1–10. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Gruffydd-Jones T.J., Harbour D.A. Detection of feline coronaviruses by culture and reverse transcriptase-polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis, Veterinary Microbiology, 62, 1998, 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut M., 1999. Kombination von Frühabsetzen und Vakzinierung mit Primucell FIP: Evaluation der Wirksamkeit zur Verhinderung der Felinen Infektiösen Peritonitis der Katze in einer Feldstudie, in: Diss Uni Zurich, University of Zurich, Zurich.
- Gut M., Leutenegger C.M., Huder J.B., Pedersen N.C., Lutz H. One-tube fluorogenic reverse transcription-polymerase chain reaction for the quantitation of feline coronaviruses, Journal of Virological Methods, 77, 1999, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold L.M., Legendre A.M., Kennedy M.A., Plummer P.J., Millsaps K., Rohrbach B. Fecal shedding of feline coronavirus in adult cats and kittens in an Abyssinian cattery, Journal of the American Veterinary Medical Association, 215, 1999, 948–951. [PubMed] [Google Scholar]
- Herrewegh A.A., de Groot R.J., Cepica A., Egberink H.F., Horzinek M.C., Rottier P.J. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR, Journal of Clinical Microbiology, 33, 1995, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Mahler M., Hedrich H.J., Haagmans B.L., Egberink H.F., Horzinek M.C., Rottier P.J., de Groot R.J. Persistence and evolution of feline coronavirus in a closed cat-breeding colony, Virology, 234, 1997, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus, Journal of Virology, 72, 1998, 4508–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Huder J.B., Gruber S., Boretti F., Sigrist B., Lutz H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats, Journal of General Virology, 82, 2001, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Koyama H. Characterization of monoclonal antibodies against feline infectious peritonitis virus type II and antigenic relationship between feline, porcine, and canine coronaviruses, Archives of Virology, 117, 1991a, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Sasamoto T., Okada S., Koyama H. Antigenic analysis of feline coronaviruses with monoclonal antibodies (MAbs): preparation of MAbs which discriminate between FIPV strain 79-1146 and FECV strain 79-1683, Veterinary Microbiology, 28, 1991b, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats, Journal of Veterinary Medical Science, 54, 1992, 557–562. [DOI] [PubMed] [Google Scholar]
- Horzinek M.C., Lutz H. An update on feline infectious peritonitis, Veterinary Sciences Tomorrow, 1, 2000, 1–10. [Google Scholar]
- Jacobse-Geels H.E., Daha M.R., Horzinek M.C. Antibody, immune complexes, and complement activity fluctuations in kittens with experimentally induced feline infectious peritonitis, American Journal of Veterinary Research, 43, 1982, 666–670. [PubMed] [Google Scholar]
- Kennedy M., Boedeker N., Gibbs P., Kania S. Deletions in the 7a ORF of feline coronavirus associated with an epidemic of feline infectious peritonitis, Veterinary Microbiology, 81, 2001, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., Bellmann S., Gunn-Moore D.A., Leukert W., Kohler K., Menger S., Reinacher M. Histopathological alterations of lymphatic tissues in cats without feline infectious peritonitis after long-term exposure to FIP virus, Veterinary Microbiology, 69, 1999, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar A., Kohler K., Leukert W., Reinacher M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection, Journal of Comparative Pathology, 125, 2001a, 182–191. [DOI] [PubMed] [Google Scholar]
- Kipar A., Leutenegger C.M., Hetzel U., Akens M.K., Mislin C.N., Reinacher M., Lutz H. Cytokine mRNA levels in isolated feline monocytes, Veterinary Immunology & Immunopathology, 78, 2001b, 305–315. [DOI] [PubMed] [Google Scholar]
- Kipar A., May H., Menger S., Weber M., Leukert W., Reinacher M. Granulomatous vasculitis in feline infectious peritonitis: crosstalk between activated monocytes and endothelial cells, Veterinary Pathology, 2003, submitted for publication. [DOI] [PubMed]
- Leutenegger C.M., Klein D., Hofmann-Lehmann R., Mislin C., Hummel U., Boni J., Boretti F., Guenzburg W.H., Lutz H. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system, Journal of Virological Methods, 78, 1999a, 105–116. [DOI] [PubMed] [Google Scholar]
- Leutenegger C.M., Mislin C.N., Sigrist B., Ehrengruber M.U., Hofmann-Lehmann R., Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA, Veterinary Immunology & Immunopathology, 71, 1999b, 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhaus A.D., Horzinek M.C., Reynolds D.J. Seroepidemiology of feline infectious peritonitis virus infections using transmissible gastroenteritis virus as antigen, Zentralblatt für Veterinärmedizin [B], 24, 1977, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. Serologic studies of naturally occurring feline infectious peritonitis, American Journal of Veterinary Research, 37, 1976, 1449–1453. [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture, American Journal of Veterinary Research, 42, 1981a, 363–367. [PubMed] [Google Scholar]
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis, American Journal of Veterinary Research, 42, 1981b, 368–377. [PubMed] [Google Scholar]
- Pedersen N.C., Black J.W., Boyle J.F., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenic differences between various feline coronavirus isolates, Advances in Experimental Medicine & Biology, 173, 1984a, 365–380. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Evermann J.F., McKeirnan A.J., Ott R.L. Pathogenicity studies of feline coronavirus isolates 79-1146 and 79-1683, American Journal of Veterinary Research, 45, 1984b, 2580–2585. [PubMed] [Google Scholar]
- Pedersen N.C., Floyd K. Experimental studies with three new strains of feline infectious peritonitis virus: FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4, Compendium on Continuing Education, 7, 1985, 1001–1011. [Google Scholar]
- Pedersen N.C. An overview of feline enteric coronavirus and infectious peritonitis virus infections, Feline Practice, 23, 1995, 7–21. [Google Scholar]
- Poland A.M., Vennema H., Foley J.E., Pedersen N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus, Journal of Clinical Microbiology, 34, 1996, 3180–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch A., Posch U., Kuebber-Heiss A., Stur I., Seiser M., Moestl K. Feline coronaviruses: differentiation of the types I and II by RT–PCR and their ocurrence in Austrian cat populations, Wiener Tierärzliche Monatschrift, 88, 2001, 1–9. [Google Scholar]
- Schroo S., 1994. Kompetitiver ELISA zum Nachweis von löslichen Immunkomplexen in Serum und Exsudaten FIP-verdächtiger Katzen, in: Vet. Med. Diss, Justus Liebig University Giessen, Giessen.
- Simons F., Rofina J., Rottier P.J., Vennema H., Pol J.M.A., Egberink H.F., 2002. Detection of replicating feline coronavirus in peripheral blood mononuclear cells as a potential diagnostic assay for feline infectious peritonitis, in: Second International FCoV/FIP Symposium, Glasgow.
- Stoddart C.A., Scott F.W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence, Journal of Virology, 63, 1989, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart M.E., Gaskell R.M., Harbour D.A., Pearson G.R. The sites of early viral replication in feline infectious peritonitis, Veterinary Microbiology, 18, 1988, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., de Groot R.J., Harbour D.A., Dalderup M., Gruffydd-Jones T., Horzinek M.C., Spaan W.J. Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization, Journal of Virology, 64, 1990, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses, Virology, 243, 1998, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtlin A., Fraefel C., Albini S., Leutenegger C.M., Schraner E., Spiess B., Lutz H., Ackermann M. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR, Journal of Clinical Microbiology, 40, 2002, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M. Morphogenesis of a virus in cats with experimental feline infectious peritonitis, Virology, 41, 1970, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmoen T.L., Kadakia N.P., Unfer R.C., Fickbohm B.L., Cook C.P., Chu H.J., Acree W.M. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus, Advances in Experimental Medicine & Biology, 380, 1995, 221–228. [DOI] [PubMed] [Google Scholar]
- Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever, Comparative Immunology, Microbiology & Infectious Diseases, 4, 1981, 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]