1. Introduction
The Japanese Association for Infectious Diseases (JAID) and Japanese Society of Chemotherapy (JSC) announced the “Guide for the Use of Antimicrobial Drugs” in 2001 and the “Guidelines for the Use of Antimicrobial Drugs” in 2005. Subsequently, the “The JAID/JSC guide to clinical management of infectious diseases 2011” was published. With its revision, guidelines were newly prepared.
Concerning respiratory infectious diseases, in Japan, the Japanese Respiratory Society published guidelines for the management of community-acquired pneumonia, hospital-acquired pneumonia, respiratory tract infection, and -/nursing and healthcare-associated pneumonia. Furthermore, the Japanese Society of Pediatric Pulmonology and Japanese Society for Pediatric Infectious Diseases announced the “Guidelines for the Management of Respiratory Infectious Diseases in Children in Japan”. Internationally, many guidelines, including those established by the American Thoracic Society and Infectious Diseases Society of America, have been published from various countries. Thereafter, clinical research on respiratory infectious diseases has advanced, leading to the accumulation of many outcomes regarding epidemiology, clinical diagnosis, and treatment. However, the types of microorganisms that cause respiratory infectious diseases have increased with the number of resistant bacteria. In addition, conditions have also varied with causative microorganisms through the recent compromised host's severe status. The place of treatment varies: from the outpatient clinic to the ICU. Physicians responsible for treatment also vary: practitioners, hospital doctors, pulmonologists, emergency physicians, board certified member of JAID, Japanese antimicrobial chemotherapy physician. There are a large number of options of antimicrobial drugs that are available, including new drugs; therapeutic strategies are confused. On the other hand, recently, the entity of PK-PD has been commonly recognized, and the importance of scientifically using antimicrobial drugs has been emphasized. In addition, the Japanese Society of Chemotherapy established a system for antimicrobial chemotherapy-certified physicians, and promoted the widespread, adequate use of antimicrobial drugs. Based on these, the two societies prepared the JAID/JSC Guidelines for the Treatment of Respiratory Infectious Diseases. If specific treatment guidelines can be presented, this may contribute to an improvement in the treatment responses of respiratory infectious diseases, a reduction in health expenditure, and the prevention of resistant bacteria.
The guidelines were prepared based on the EBM so that they reflected the management of respiratory infectious diseases in Japan and covered all such diseases in adults and children. To prepare the guidelines, a committee was established in 2012, and a draft was published on homepage based on an approval from the boards of directors at the two societies through a review-based consensus. Opinions were collected from the two societies' members. In Japan, there have been no such guidelines covering respiratory infectious diseases. In the future, with further advances in research, the contents of the guidelines must be revised. However, we successfully provided treatment guidelines that are the most advanced at present.
The guidelines were prepared for all clinicians to understand the Treatment of Respiratory Infectious Diseases and manage them with antimicrobial drugs adequately. They do not limit treatment by individual physicians or affect their rights to select it. The guidelines may be commonly applied for respiratory infectious disease management/research/education in Japan, improving the quality of respiratory infectious disease management, preventing an increase in the number of resistant bacteria, and contributing to national health. We hope that the guidelines will be utilized by a large number of clinicians in respiratory infectious disease management. Lastly, we thank the committee members and secretariat staff for their cooperation.
-
1.
Descriptions on the recommendation grade and evidence level
| Recommendation grade | Evidence level | ||
|---|---|---|---|
| A | Strongly recommended, | I | Randomized comparative study |
| B | General recommendation | II | Non-randomized comparative study |
| C | Comprehensive evaluation by the attending physician | III | Case report |
| IV | Specialist's opinion | ||
-
2.
Definition of first- and second-choice drugs
| First-choice drugs | Drugs to be recommended for initial treatment |
| Second-choice drugs | Alternative drugs when first-choice drugs cannot be used due to allergy, organ disorder, or local factors |
-
3.Precautions
-
-In this article, with respect to the administration method (especially doses) of antimicrobial drugs, they are recommended based on sufficient doses. Considering the products adopted at each medical institution, antibiograms, severity, underlying disease, age, and presence or absence of organ disorder, the dose should be increased or decreased if necessary.
-
-The spectra of third-generation cephems for intravenous injection, CTX and CTRX, are similar, but CTX, which is excreted in the kidney, should be primarily used when liver dysfunction is present, and CTRX, which is excreted in bile, should be primarily used when renal dysfunction is present.
-
-As quinolones exhibit antitubercular actions, patients with pulmonary tuberculosis should be excluded for use.
-
-
-
4.
A list of antimicrobial drug abbreviations and doses for neonates are presented at the end of this volume.
2. Pneumonia (Adults)
2.1. Community-acquired pneumonia
2.1.1. Empiric therapy
- - - Executive summary- - -
-
•
Patient with bacterial pneumonia should be treated primarily with high-dose penicillin (AII). In elderly patients and those with underlying lung diseases, the use of respiratory quinolones may be considered positively (BII).
-
•
In case of atypical pneumonia, a macrolide or tetracycline is the first choice. Respiratory quinolones should be reserved as alternative drugs (BII), but may be used depending on local circumstances about drug resistance (CIII).
-
•
In case of whether pneumonia or atypical pneumonia dose not diagnose, comobination with high-dose penicillin and a macrolide or tetracycline should be attempted first (BII). Respiratory quinolones should be reserved as alternative drugs (BII).
-
•
In severer cases requiring treatment in the ICU, a macrolide or new quinolone should be used aggressively in combination with a broad spectrum β-lactam such as high-dose penicillin from the beginning of treatment (AII).
- - - Explanation- - -
Community-acquired pneumonia refers to hospital-acquired pneumonia that develops 48 h or more after admission or pneumonia that develops in healthy adults on social activities other than medical practice-/nursing-associated pneumonia [1], [2], [3]. As signs and symptoms, cough, sputum, thoracic pain, and dyspnea appear, and this disease acutely occurs with systemic symptoms such as fever and general malaise [1], [2], [3]. However, these symptoms are not marked in some elderly patients. Furthermore, atypical pneumonia including Mycoplasma is characterized by a small amount of sputum, and can be differentiated (Table 1, Table 2 ) [4], [5].
Table 1.
Items used to differentiate between bacterial and atypical pneumonia [3].
|
Table 2.
Criteria for differentiation [3].
| In cases using the 6 items in Table 1: | |
| In cases where at least 4 of 6 items are satisfied | Atypical pneumonia suspected |
| In cases where 3 or less of 6 items are satisfied | Bacterial pneumonia suspected |
| The sensitivity and specificity for detecting atypical pneumonia is 77.9% and 93.0%, respectively. | |
Concerning examination, Gram staining and culture of sputum are used to identify causative microorganisms and select subsequent treatment strategies [6], [7] (AII). Kits for rapid diagnosis with urine or nasal swab are also used for auxiliary diagnosis [8], [9] (AII). A blood test shows inflammatory findings such as leukocytosis and an increase in the CRP level, facilitating a certain assessment of the disease [5], [10]. On thoracic imaging, consolidation or a ground glass-like shadow is observed [1], [2], [3], [4], [5] (II).
When patients are in an immunosuppressive state related to an underlying disease, a causative microorganism test should be performed, considering the possibility of opportunistic infection [1], [2], [3], [11], [12] (A). In elderly patients, aspiration pneumonia is frequently observed, and the management of this disorder is necessary (Refer to the section “2.4 Aspiration pneumonia” on Page. 19). In the presence of renal dysfunction, the type and dose of an antimicrobial drug must be carefully selected [11], [12] (AII).
Bacterial pneumonia should be differentiated from atypical pneumonia in accordance with “The JRS Guidelines for the Management of Community-acquired pneumonia in Adults in 2007” (edited by the Committee to Prepare Guidelines regarding Respiratory Infectious Diseases, Japanese Respiratory Society) (Table 1, Table 2) [3]. Although Legionella pneumonia is routinely classified as atypical pneumonia, various types of atypical pneumonia do not include Legionella pneumonia in this differentiation method.
-
a.Bacterial pneumonia
-
(1)Outpatient treatmentBacterial pneumonia is primarily caused by Sterptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis [1], [2], [3], [4], [5], [13], [14] (II). Basically, these types of pneumonia should be treated by orally administering high-dose penicillin [1], [2], [3], [4] (AII). In Japan, macrolide-resistant S. pneumoniae is detected in most cases; therefore, macrolides are not recommended as the first choice, differing form those in Europe and the United States [4], [5], [10], [13], [14] (AII).For outpatient treatment, β-lactamase inhibitor-containing penicillin is commonly used. Therapy with CVA/AMPC or SBTPC (2 tablets/3–4 times a day) is recommended with respect to the efficacy and suppression of resistant bacteria [1], [4], [11] (AII). However, such high-dose prescriptions are not always accepted by health insurance system in Japan, and the following prescriptions (examples) should also be considered.In elderly patients or those with underlying lung diseases such as COPD/old pulmonary tuberculosis, the use of respiratory quinolones should be considered positively from the perspective of the effects on penicillin-resistant Pneumococcus and tissue transfer [11], [14], [15] (BII). However, many new quinolones also have antimicrobial activities against Mycobacterium tuberculosis; therefore, the presence or absence of active tuberculosis must be strictly checked before administration [16] (AII).
-
(2)Hospital treatmentFor hospital treatment, injection is primarily used. However, basic concepts for drug selection are similar to those at the outpatient clinic. Considering S. pneumoniae, H. influenzae, and M. catarrhalis, high-dose penicillin or cephems, which are effective for these microorganisms, should be selected [1], [2], [3], [4] (AII). If more potent treatment is required, respiratory quinolone injection should be used [15], [17] (BII).
-
(1)
- - - Drugs to be recommended- - -
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
∗Concerning CVA/AMPC and SBTPC, up to 1000 mg of AMPC or up to 750 mg of ABPC are approved dosage in Japan.
- Combination therapy with AMPC (oral preparation) should also be considered.
- [Example] CVA/AMPC, oral (125/250 mg), 1 tablet/3 times a day + AMPC, oral (250 mg), 1 tablet/3 times a day
-
-
-
<>Second choices
-
-LVFX, oral, 500 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-MFLX, oral, 400 mg/once a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
<>Second choice
-
-LVFX, intravenous drip, 500 mg/once a day
-
-
-
♦
-
b.Atypical pneumonia
-
(1)Outpatient treatmentAtypical pneumonia is primarily caused by Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila [1], [2], [3], [4], [5], [10], [11], [13], [14] (II). The oral administration of a macrolide or tetracycline is the first choice [1], [4], [5], [7] (AII). To suppress resistant bacteria, respiratory quinolones should be reserved as alternative drugs [1], [4], [11], [12], [18] (BII).However, recently, the appearance of macrolide-resistant M. pneumoniae in adults has raised an issue in Japan. Respiratory quinolones must be used as the first choice depending on local circumstances about drug resistance [18] (CIII).
-
(2)Hospital treatment
-
(1)
- - - Drugs to be recommended- - -
-
(1)Outpatient treatment
-
♦First choices
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-MINO, oral, 100 mg twice a day
-
-
-
<>Second choices
-
-LVFX, oral, 500 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-MFLX, oral, 400 mg/once a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
-AZM, intravenous drip, 500 mg/once a day
-
-MINO, intravenous drip, 100 mg/twice a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day.
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
c.Cases in which whether the disease is bacterial pneumonia or atypical pneumonia is unclear
-
(1)Outpatient treatmentIn this case, combination therapy with high-dose penicillin and a macrolide or tetracycline should be selected as the first choice to cover both bacterial and atypical pneumonia [1], [2], [3], [4], [11], [13], [14], [17], [18] (BII).As respiratory quinolones cover both bacterial and atypical pneumonia, they are convenient, but should be reserved as alternative drugs from the perspective of suppression of resistant bacteria [1], [2], [3], [4], [11], [15], [17], [18] (BII).However, in elderly patients or those with underlying lung diseases such as COPD/old pulmonary tuberculosis, the use of respiratory quinolones should be considered positively from the perspective of the effects on penicillin-resistant Pneumococcus and tissue transfer [11], [14], [15] (BII). Recently, the appearance of macrolide-resistant M. pneumoniae in adults has raised an issue. Respiratory quinolones may be used as the first choice depending on local circumstances about drug resistance [18] (CIII).
-
(2)Hospital treatment
-
(3)Severer cases requiring treatment in the ICUIn severer cases requiring treatment in the ICU, S. pneumoniae should be initially considered, and a macrolide or new quinolone should be used aggressively in combination with a broad spectrum β-lactam such as high-dose penicillin from the beginning of treatment primarily to cover latent atypical bacteria (in particular, when L. pneumophila is not covered, the condition may become fatal) [1], [2], [3], [4], [11], [17], [18] (AII). In particular, combination therapy with a macrolide is recommended from immunological aspects to suppress excessive inflammation related to cytokines [19] (CII).
-
(1)
- - - Drugs to be recommended- - -
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
∗Concerning CVA/AMPC and SBTPC, up to 1000 mg of AMPC or up to 750 mg of ABPC are approved dosage in Japan. Combination therapy with AMPC (oral preparation) should also be considered.
- [Example] CVA/AMPC, oral (125/250 mg), 1 tablet/3 times a day + AMPC, oral (250 mg), 1 tablet/3 times a day
-
-
-
+one of the followings:
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-MINO, oral, 100 mg/twice a day
-
-
-
<>Second choices
-
-LVFX, oral, 500 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-MFLX, oral, 400 mg/once a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
+one of the followings:
-
-AZM, intravenous drip, 500 mg/once a day
-
-MINO, intravenous drip, 100 mg/twice a day
-
-CAM, oral, 200 mg/twice a day
-
-
-
<>Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(3)Severer cases requiring treatment in the ICU
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
+one of the followings:
-
-AZM, intravenous drip, 500 mg/once a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-MINO, intravenous drip, 100 mg/twice a day
-
-
-
-
2.1.2. Definitive therapy
- - - Executive summary- - -
-
-
When causative microorganisms are identified based on the results of microbial examination of good-quality sputum, blood culture, and urinary antigen (S. pneumoniae, L. pneumophila) tests and drug susceptibility testing of the causative agents, definitive therapy should be performed if possible [2], [3] (BIII).
-
-
The place of treatment and drugs should be selected in accordance with the severity of the disease [2], [3] (AII).
-
-
Antimicrobial drugs should be selected in reference to the susceptibility of isolated bacteria to antimicrobial drugs or a drug-susceptibility tendency in the area [2], [3], [13], [22] when these data are available (AII).
-
-
The administration period of an antimicrobial drug is determined in accordance with the improvement of symptoms and laboratory data, with a target of 5–7 days [2], [21] (BIII).
-
-
When the patient is infected with L. pneumophila or C. pneumoniae, the optimal administration period is about 14 days [21] (BIV).
- - - Explanation- - -
-
a.Streptococcus pneumoniae
-
-The Clinical and Laboratory Standards Institute (CLSI) has established higher criteria for breakpoints for penicillin susceptibility on the administration of parenteral antimicrobial drugs for S. pneumoniae infections other than meningitis [23], based on the following findings: patients with severe pneumonia due to S. pneumoniae with a low PCG susceptibility (MIC: 0.12–4 μg/mL) showed no difference in responses to PCG and outcome [24], [25] (II). For the treatment of pneumococcal pneumonia, the dose of penicillin should be increased [23], [26] (A).
- -
-
-Respiratory quinolones have potent anti-pneumococcal activities (III). The clinical effects of such quinolones are similar to those of high-dose AMPC [27] (II).
- -
-
-
-
b.Haemophilus influenzae
-
-The ABPC-resistant mechanism of H. influenzae involves β-lactamase production and/or PBP mutation. Previously, β-lactamase production was primarily involved, but, recently, PBP mutation-mediated β-lactamase-negative ABPC-resistant (BLNAR) strains have been increasingly detected. ABPC-resistant strains with both β-lactamase production and PBP mutation are classified as β-lactamase-positive CVA/ABPC-resistant (BLPACR) strains.
-
-According to a national survey in Japan, 49 (39.8%) and 7 (5.7%) of 123 H. influenzae strains were BLNAR and β-lactamase-producing strains, respectively [13].
-
-BLNAR strains are also resistant to first- and second-generation cephems.
-
-PIPC exhibits an antimicrobial activity against BLNAR strains. However, it is ineffective for BLPACR strains.
-
-
-
c.Klebsiella spp., Escherichia coli, Proteus spp.
-
-The proportion of extended spectrum β-lactamase (ESBL)-producing bacteria has slightly increased among isolates from respiratory samples.
- -
-
-Most ESBL-producing strains are simultaneously resistant to quinolones [30]. Antimicrobials should be selected according to the drug susceptibility of isolated bacteria.
-
-In Japan, carbapenemase-producing strains are extremely rare.
-
-
-
d.Mycoplasma pneumoniae
- -
-
-Tetracyclines exhibit potent clinical effects on macrolide-resistant M. pneumoniae [33].
- -
-
e.Legionella spp.
-
-It should be noted that pneumonia related to Legionella spp. other than L. pneumophila SG1 cannot be diagnosed using Legionella urinary antigen testing.
-
-As neither β-lactams nor aminoglycosides have antimicrobial activities against Legionella spp., which proliferates within host cells, they are clinically ineffective.
- -
-
-RFP is effective when combined with EM. The combination of EM and RFP is useful. A study suggested the effects of combination therapy with LVFX and a macrolide [38] (CIII).
-
-Although there are no marked differences in antimicrobial drug susceptibility among Legionella spp., clinical reviews to verify this are limited [39].
-
-
- f.
-
g.Staphylococcus aureus
-
-With respect to Staphylococcus aureus in Japan, there has been an increase in the number of methicillin-resistant strains even in patients with community-acquired pneumonia. In particular, recently, municipal-onset-type MRSA (CA-MRSA) with Panton-Valentine-Leucocidine (PVL) has been detected in Japan, raising an issue [41].
-
-In cases of MSSA infection (bacteremia), the clinical effects of CEZ are superior to those of VCM [42].
-
-As the susceptibility of MRSA to oral antimicrobial drugs differs among isolates, drugs should be selected according to its drug susceptibility Results.
-
-
-
h.Streptococcus spp.
- -
-
-There is no penicillin resistance, but macrolide resistance is observed at a low frequency [45].
- -
- i.
-
j.Anaerobes
-
-Most anaerobes that cause pneumonia exist in the oral cavity. Peptostreptococcus spp., Prevotella spp., and Fusobacterium spp. are involved. Mixed infection with microaerophilic Streptococci is often observed.
-
-In many cases, infection with anaerobes may be associated with aspiration.
-
-Most oral anaerobes (Prevotella spp., Fusobacterium spp., and Porphyromonas spp.) are susceptible to combination drugs consisting of penicillin and a β-lactamase inhibitor, CLDM and MNZ [48].
-
-
-
k.Pseudomonas aeruginosa
-
-In patients with chronic respiratory tract infection, Pseudomonas aeruginosa colonizes in the airway, and may cause community-acquired pneumonia [49].
-
-As the susceptibility of P. aeruginosa to antimicrobial drugs differs among clinical isolates, drugs should be selected according to its drug susceptibility results.
-
-
- - - Drugs to be recommended- - -
-
-
The drug susceptibility of each clinical isolate should be classified in accordance with the CLSI criteria [23].
-
-Establishment of prescriptions recommended in this article
-
∗Antimicrobials have been approved for specific diseases and specific causative agents by Japanese Ministry of Health and Welfare. The approvals are based on the results of clinical studies with Good Clinical Practice. As a general rule, the recommendations in this section refer to this (AII). However, recent trends in drug susceptibility are also considered.
- ∗
-
∗The recommendations without the approvals by Japanese Ministry are graded by evidence levels.
-
∗
-
[1]S. pneumoniae (PC-susceptible)
-
(1)Outpatient treatment
-
♦First choice
-
-AMPC, oral (250 mg), 2 tablets/3–4 times a day
-
-
-
<>Second choices
-
-GRNX, oral, 400 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-LVFX, oral, 500 mg/once a day
-
-TFLX, oral, 300 mg/twice a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-PCG, intravenous drip, 2,000,000 to 3,000,000 units/4 times a day
-
-ABPC, intravenous drip, 1–2 g/3–4 times a day
-
-
-
<> Second choices
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-
-
♦
-
(1)
-
[2]S. pneumoniae (PC-resistant)
-
(1)Outpatient treatment
-
♦First choices
-
-GRNX, oral, 400 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-LVFX, oral, 500 mg/once a day
-
-TFLX, oral, 300 mg/twice a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-PAPM/BP, intravenous drip, 0.5–1 g/2–4 times a day
-
-
-
♦
-
(1)
-
[3]H. influenzae (ABPC-susceptible)
-
(1)Outpatient treatment
-
♦First choice
-
-AMPC, oral (250 mg), 2 tablets/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-ABPC, intravenous drip, 1–2 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[4]H. influenzae (β-lactamase-producing)
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[5]H. influenzae [β-lactamase-negative ampicillin-resistant (BLNAR)]
-
(1)Outpatient treatment
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
(2)Hospital treatment
-
♦First choices
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-PIPC, intravenous drip, 2 g/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[6]H. influenzae [β-lactamase-positive amoxicillin clavulanate-resistant (BLPACR)]
-
(1)Outpatient treatment
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
(2)Hospital treatment
-
♦First choices
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[7]
Klebsiella spp. [non-extended-spectrum β-lactamase (ESBL)-producing bacteria]
The results of drug susceptibility testing must be confirmed.-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-CTM, intravenous drip, 1–2 g/2–3 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[8]
Klebsiella spp. (ESBL-producing bacteria)
The results of drug susceptibility testing must be confirmed.-
(1)Outpatient treatment
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
(2)Hospital treatment
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-PAPM/BP, intravenous drip, 0.5–1 g/2–4 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
(1)
-
[9]M. pneumoniae
-
(1)Outpatient treatment
-
♦First choices
-
-CAM, oral, 200 mg/twice a day
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-MINO, oral, 100 mg/twice a day
-
-
-
<> Second choices
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-LVFX, oral, 500 mg/once a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-MINO, intravenous drip, 100 mg/twice a day
-
-AZM, intravenous drip, 500 mg/once a day
-
-
-
<> Second choice
-
-LVFX, intravenous drip, 500 mg/once a day
-
-
-
♦
-
(1)
-
[10]
Legionella spp.
As a rule, hospital treatment should be performed.-
♦First choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/2–3 times a day
-
-PZFX, intravenous drip, 500 to 1,000 mg/twice a day
-
-AZM, intravenous drip, 500 mg/once a day
-
-
-
<>Second choice
-
-EM, intravenous drip, 500 mg/3 times a day + RFP, oral, 450–600 mg/once a day.
-
-
-
♦
-
[11]C. pneumoniae
-
(1)Outpatient treatment
-
♦First choices
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-MINO, oral, 100 mg/twice a day
-
-
-
<> Second choices
-
-GRNX, oral, 400 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choice
-
-MINO, intravenous drip, 100 mg/twice a day
-
-
-
<> Second choice
-
-AZM, intravenous drip, 500 mg/once a day
-
-
-
♦
-
(1)
-
[12]MSSA
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-
-
<> Second choices(The results of drug susceptibility testing must be confirmed.)
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-MINO, oral, 100 mg/twice a day
-
-CLDM, oral, 300 mg/3–4 times a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-CEZ, intravenous drip, 1–2 g/2–3 times a day
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-
-
<> Second choices
-
-MINO, intravenous drip, 100 mg/twice a day
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-
-
♦
-
(1)
-
[13]MRSA
-
(1)Outpatient treatment
- The results of drug susceptibility testing must be confirmed.
-
-ST combination drug (SMX at 400 mg/TMP at 80 mg), oral, 2 tablets/twice a day
-
-LZD, oral, 600 mg/twice a day
-
∗CA-MRSA: When MRSA is susceptible to macrolides, quinolones, tetracyclines, and CLDM, these drugs can be used.
-
∗
-
(2)Hospital treatmentRefer to the section “2.2 Hospital-acquired pneumonia- - - 2.2.3 Definitive therapy- - - (1) MRSA” (p.13).
-
(1)
-
[14]M. catarrhalis
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-
-
<> Second choices
-
-LVFX, oral, 500 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-
-
<> Second choices
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
♦
-
(1)
-
[15]Streptococcus spp.
-
(1)Outpatient treatment
-
♦First choice
-
-AMPC, oral (250 mg), 2 tablets/3–4 times a day
-
-
-
<> Second choices
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-PCG, intravenous drip, 1,000,000 to 2,000,000 units/3–4 times a day
-
-ABPC, intravenous drip, 2 g/3–4 times a day
-
-
-
<> Second choices
-
-AZM, intravenous drip, 500 mg/once a day
-
-VCM, intravenous drip, 1 g/twice a day
-
-
-
♦
-
(1)
-
[16]Anaerobes
-
(1)Outpatient treatment
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-CLDM, oral, 300 mg/3–4 times a day
-
-MNZ, oral, 500 mg/3–4 times a day
-
-
-
<> Second choices
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-
-
♦
-
(2)Hospital treatment
-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/3–4 times a day
-
-
-
<> Second choices
-
-IPM/CS, intravenous drip, 0.5–1 g/3–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-PAPM/BP, intravenous drip, 0.5–1 g/3–4 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-
-
♦
-
(1)
-
[17]
P. aeruginosa
The results of drug susceptibility testing must be confirmed.-
(1)Outpatient treatment
-
-CPFX, oral, 200 mg/3 times a day
-
-LVFX, oral, 500 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-TFLX, oral, 300 mg/twice a day
-
-
-
(2)Hospital treatmentRefer to the section “2.2 Hospital-acquired pneumonia- - - 2.2.3 Definitive therapy- - - (4) P. aeruginosa” (p. 14).
-
(1)
2.2. Hospital-acquired pneumonia
2.2.1. Empiric therapy: cases in which gram staining is not available
- - - Executive Summary- - -
-
-
As a rule, an appropriate antimicrobial drug should be administered in the early stage. If hospital-acquired pneumonia is suspected, the administration of an antimicrobial drug at a sufficient dose should be promptly started [50], [51], [52], [53], [54] (AII).
-
-
Before the administration of an antimicrobial drug, a good-quality airway sample should be collected. However, the start of treatment should not be delayed for this purpose [50], [51], [52], [53] (BII).
-
-
When selecting an antimicrobial drug, the presence or absence of risk factors for resistant bacteria should be evaluated [50], [51], [52], [53] (AII).
-
-
When the susceptibility of identified causative microorganisms is clarified, or after the treatment responsiveness is evaluated, whether or not de-escalation is possible should be reviewed [50], [51], [52], [53] (AII).
- - - Explanation- - -
Definition: Hospital-acquired pneumonia is defined as “pneumonia that newly develops 48 h or more after admission”. In many cases, treatment is difficult due to unfavorable patient conditions such as the presence of an underlying disease, immune capacity, and general condition [50], [51], [52].
Laboratory findings: Patients meeting 2 of 3 items, fever, an abnormal leukocyte count, and purulent secretes, in addition to the appearance of an abnormal shadow of the chest should be diagnosed with hospital-acquired pneumonia [50], [51], [52].
-
1)
Ventilator-associated pneumonia (VAP): VAP refers to pneumonia that newly develops 48 h or more after endotracheal intubation/ventilator initiation. Its onset within 4–5 days after endotracheal intubation is classified as early-type, and its subsequent onset as late-type [50], [51], [54], [55].
-
2)
Hospital-acquired pneumonia other than VAP: Several types of hospital-acquired pneumonia other than VAP include (1) immunodeficiency (for example, neutropenia during anticancer therapy, cell-mediated immunodeficiency related to the administration of steroids or immunosuppressive drugs) and (2) aspiration pneumonia including latent aspiration (Refer to the section “2.4 Aspiration pneumonia” on Page 19). Appropriate management and selection of antimicrobial drugs in accordance with individual conditions are necessary [50].
With respect to microorganisms that are expected, refer to the section “2.2 Hospital-acquired pneumonia- - - 2.2.2 Empiric therapy: Cases in which Gram staining is available” (p. 10).
- - - Drugs to be recommended- - -
-
a.
Cases in which there is no risk of resistant bacteria
Antimicrobial drugs should be selected, targeting Streptococcus pneumoniae, H. influenzae, and Klebsiella spp. as causative microorganisms [50], [51], [52] (BIII). Although it is difficult to estimate/identify causative microorganisms using sputum samples, bacteria that are not isolated/cultured from good-quality sputum may not be causative microorganisms. If resistant bacteria such as MRSA and P. aeruginosa are not detected on sputum culture and there is no deterioration of clinical symptoms, an initial drug should be continued [50] (BIII). In patients in whom aspiration episodes are clear, those in whom oral hygiene is not maintained, or those with consciousness disorder, drugs with anti-anaerobe activities should be selected, considering the involvement of anaerobes [50] (BIII). If an adequate antimicrobial drug is administered, the treatment period may be 7–10 days, excluding MRSA and P. aeruginosa [50], [53] (BII).-
♦First choices
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTX, intravenous drip, 1–2 g/3 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
∗If the involvement of anaerobes is suspected, SBT/ABPC should be selected.
-
∗
-
-
-
<>Second choice
-
-LVFX, intravenous drip, 500 mg/once a day (As its antimicrobial activity against anaerobes is weak, monotherapy with this drug should be avoided in patients with aspiration pneumonia.).
-
-
-
♦
-
b.
Cases in which there is a risk of multi-drug-resistant bacteria (Table 3) [51]
Table 3.
Risk factors for multi-drug-resistant bacteria.-
1.Previous use of antimicrobial drugs within 90 days
-
2.Interval of 5 days or more from admission
-
3.Admission from an area/hospital in which resistant bacteria are frequent
-
4.Immunosuppressive state or treatment
To cover multi-drug-resistant bacteria including P. aeruginosa, broad-spectrum antimicrobial drugs with anti-P. aeruginosa activities should be selected [50], [51], [52] (AIII). Considering the frequency of ESBL in each institution, carbapenems should be considered even when enteric bacteria, including Klebsiella spp. and Escherichia coli, are suspected (BIV). If P. aeruginosa is not isolated on good-quality sputum culture, a treatment option should be de-escalated to drugs for cases in which there is no risk of resistant bacteria [50], [51], [52] (AII). If aspiration is suspected, or if the involvement of gram-positive bacteria is suggested, combination therapy with CLDM must be considered (BIV). If there is a risk of MRSA carrier (Table 4 ), combination therapy with anti-MRSA drugs should also be considered.
The mean administration period of antimicrobial drugs with respect to causative bacteria in patients with an improvement was approximately 10 days. However, that for resistant bacteria such as P. aeruginosa and MRSA was approximately 12 days [53] (BII). If appropriate antimicrobial drugs can be administered after clarifying causative bacteria, a treatment period of approximately 10 days is recommended [53], [56], [57] (BII).-
♦First choices
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5 g/4 times a day or 1 g/3 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-
-
<>Second choices
-
-CFPM, intravenous drip, 1–2 g/2–4 times a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
- If the involvement of anaerobes is suspected, one of the following options should be combined with one of the above regimens:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-
-
1.
-
c.
Severe cases
One of the following options must be combined with one of the regimens for cases in which there is a risk of multi-drug-resistant bacteria. When comparing the results between patients undergoing appropriate and inappropriate treatments, the prognosis of the latter was significantly poorer [58], [59] (BII). However, a study reported that the prognosis in a group with compliance with recommended drug selection was significantly poorer than in a non-compliance group in patients in whom infection with drug-resistant bacteria in the ICU was suspected even among those in whom the etiology was bacteriologically investigated [60] (BII). Therefore, it must be considered that, even when resistant bacteria are etiologically involved, the administration of an appropriate antimicrobial drug that covers them does not always improve the prognosis.-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5 g/4 times a day or 1 g/3 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a dayOne of the following options should be combined with one of the above regimens:
-
♦First choices
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
<>Second choices
-
-AMK, intravenous drip, 15 mg/kg/once a day
-
-GM, intravenous drip, 5 mg/kg/once a day
-
-TOB, intravenous drip, 5 mg/kg/once a day
-
-
-
♦
-
-
Table 4.
Risk factors for carrying MRSA [50].
| Conditions under which anti-MRSA drug therapy should be considered (including gram stain) |
|---|
|
- - - Precautions- - -
-
-
In cases of HCAP/VAP, several types of bacteria are often isolated on sputum culture, but whether or not detected bacteria are causative microorganisms is unclear. Caution is needed when selecting an antimicrobial drug.
-
-
Drugs should be selected, considering bacteria that are problematic in each institution and their susceptibility pattern.
-
-
It is necessary to examine whether or not de-escalation is possible when causative microorganisms are identified and their susceptibility is clarified.
2.2.2. Empiric therapy: cases in which gram staining is available
-
a.
Usefulness of Gram staining and interpretation of staining findings
- - - Executive summary- - -- -
- -
- -
-
-Microorganisms that cause hospital-acquired pneumonia should be estimated based on the results of the clinical microbiological culture (CMC: Gram staining and culture) of a lower airway sample immediately before the start of treatment, and not based on bacteria isolated on active surveillance culture (ASC), which was conducted as a strategy to prevent/control infection prior to onset [66].
-
-Microorganisms that cause pneumonia or (colonization of) the lower airway should be estimated based on the presence or absence of neutrophils or phagocytosis (excluding those patients with neutropenia or functional impairment of neutrophil) [50] (BII).
- - - Explanation- - -
[Gram staining]
Diagnostic accuracy of hospital-acquired pneumonia is improved by confirming neutrophils and bacterial cells using Gram staining of airway samples. This observation has also been confirmed through an increase in the likelihood ratio of hospital-acquired pneumonia in patients with a clinical pulmonary infection score (CPIS) of 6 points or higher [61]. As bacteria isolated from the lower airways of inpatients are common colonizers in many cases, Gram staining is also useful for discerning colonization from infection by evaluating the presence or absence of neutrophil and phagocytosis. Therefore, it is desirable to combine bacterial culture with Gram staining [51], [61], [62], [63], [64], [65].
Antimicrobial-drug selection based on Gram staining findings leads to appropriate empiric therapy in two-thirds of patients with hospital-acquired pneumonia, and it can be continued as definitive therapy in many cases [62].
If there are no bacterial cells on Gram staining of lower airway sample in whom an antimicrobial regimen was not changed within the past 72 h, it is unlikely that the focus of infection/inflammation is within the lungs (lower airway) [51]. In this case, the possibility of pneumonia mimic, such as pleural effusion, atelectasis, and pulmonary edema, is suggested if the lung field opacity still remains in chest X-ray. If there is no other infectious focus, the discontinuation of an antimicrobial drug may be warranted [50], [66], [67].
A study has reported that the culture results of ASC performed as a strategy of routine infection control measure prior to the development of nosocomial pneumonia accurately predicted the causative pathogen in only 35% of cases [66]. Therefore, it is necessary to submit airway samples for clinical microbiological culture (CMC) immediately before the start of presumptive treatment.
[Causative microorganisms and their origin]
Microorganisms that cause hospital-acquired pneumonia are derived from the oropharynx, airway (including the nasal cavity and nasal sinus), digestive tract, and environment. Gastrointestinal tract-derived causative microorganisms are enteric bacteria (primarily, Klebsiella spp., E. coli and others such as Proteus spp., Enterobacter spp., Serratia spp., Morganella spp., and Citrobacter spp.). Those derived from the upper airway include S. pneumoniae, H. influenzae, Moraxella catarrhalis, S. aureus (namely methicillin sensitive strain), and oral anaerobes. Those derived from the environment include methicillin-sensitive S. aureus, Pseudomonas spp., Acinetobacter spp., and Stenotrophomonas spp. [50], [51], [65], [68].
As the above bacteria derived from the airway and gastrointestinal tract basically exert strong virulence to the airway, they can be considered a core pathogen group of hospital-acquired pneumonia. Their potential for developing airway inflammatory response is generally believed stronger than those caused by environmental pathogen [67], [68].
-
b.
Gram-positive bacteria
- - - Executive summary- - --
-As gram-positive bacteria, S. aureus and Streptococcus spp. are frequently detected. It is relatively easy to differentiate the two types of bacteria based on gram staining findings.
-
-Among various types of Streptococcus sp., S. pneumoniae, Streptococcus anginosus group, and β-Streptococcus spp. are supposed as causative microorganisms.
-
-If Streptococcus spp. is suspected as causative microorganisms, empiric therapy with penicillin is encouraged.
-
-
- - - Drugs to be recommended- - -
-
(1)Gram-positive coccus in cluster (grape cluster-like apperace)
-
♦In cases of early onset hospital-acquired pneumonia (development within the first 48 h after hospitalization) without previous administration of antimicrobial drugs, or in the absence of conditions under which environmental bacteria directly invade into the airway, such as airway aspiration or tracheotomy, MSSA may be supposed.
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CEZ, intravenous drip, 1–2 g/2–3 times a day
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MINO, intravenous drip, 100 mg/twice a day
-
-
-
<>In cases of late onset hospital-acquired pneumonia (development of 48H to 72H after hospitalization), those who have had previous antimicrobial treatment, or under tracheotomy or ventilator management, an antimicrobial drug that covers MRSA should be administered until proven, based on the susceptibility testing, otherwise.
-
-Refer to the section “2.2.3 Definitive therapy- - - (1) MRSA- - -” (p. 13).
-
-
-
♦
-
(2)
Diplococcus consisting of a pair of two cocci (GPDC: Gram-positive diplococci)
- S. pneumoniae should initially be suspected. Enterococcus is also a GPDC in microscopic appearance, but is basically considered a non-pulmonary pathogen [67].
- <> Cases in which there have been no previous treatment with antimicrobial drugs or risks of penicillin-resistant Pneumococcus
-
-PCG, intravenous drip, 2,000,000 to 3,000,000 units/4–6 times a day
-
-ABPC, intravenous drip, 2 g/4–6 times a day
-
-
-
<> Cases in which previous treatment with antimicrobial drugs or a risk of PRSP is present
-
-CTRX, intravenous drip, 1 g/twice a day or 2 g/once a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-VCM, intravenous drip, 1 g/twice a day
- (TDM should be conducted so that a trough is 15–20 μg/mL [69].)
-
-
-
(3)Gram-positive coccus in either short or long chain (GPC in chain)
- α- or β-hemolytic streptococci is indicated.
-
-PCG, intravenous drip, 2,000,000 to 3,000,000 units/4–6 times a day
-
-ABPC, intravenous drip, 2 g/4–6 times a day
-
-
-
(4)Gram-positive bacillus with a rod-like morphology (GPR: Gram-positive rod)
- Corynebacterium spp. may be indicated.
-
-VCM, intravenous drip, 1 g/twice a day
- (TDM should be conducted so that a trough is 15–20 μg/mL [69].)
-
-
-
c.
Gram-negative bacteria
- - - Executive summary- - -- -
-
-It is difficult to estimate the type of bacteria based on the morphology on Gram staining in comparison with gram-positive bacteria.
-
-Gram-negative bacteria frequently detected as causative microorganisms include enteric bacteria and P. aeruginosa.
-
-It is encouraged important to recognize the basic antimicrobial drug susceptibility pattern of each type (group) of bacteria to make sure that the empiric antimicrobial therapy is appropriate (Table 5).
Table 5.
Basic susceptibility of various pathogen groups to antimicrobial drugs.GNRa GNRb ESBL-GNRc P. aeruginosa Acinetobacter Gram(+)d ABPC +e/− +/− PIPC ++ + ++ +/− +/− SBT/ABPC ++ +f +g +h ++ TAZ/PIP ++ +f +g ++ +/− ++ CTX,CTRX ++ +i ++ CPZ ++ +i ++ + CAZ ++ +i ++ ++ + CFPM ++ ++j ++ ++ ++ Carbapenem ++ ++ ++ ++i ++ ++ Monobactam ++ + +/− +/− CPFX ++ ++i ++i ++ ++k aE.coli, K. pneumoniae, P. mirabilis, H. influenzae, and M. catarrhalis.bEnterobacter, Citrobacter, Serratia, P. vulgaris, and M. morganii.cExtended-spectrum β-lactamase(+)GNR.dExcluding MRSA and enterococcus. It must be considered that there are many penicillinas-producing strains of MSSA.eThis is limited to Susceptible E. coli, Proteus, and H. influenzae.fβ-lactamase inhibitors do not inhibit cephalosporinase activity.gClinical experience is limited.hSBT has a time-dependent antimicrobial activity against Acinetobacter (BL: BLI → 2:1, A susceptibility test with liquid medium is recommended).iBoth intrinsic resistance and resistance induced by antimicrobial drugs are probable.jThe drug may also show an antimicrobial activity against cephalosporinase (AmpC)-producing strains.kExcluding MRSA, enterococcus, and S. pneumoniae.Reference 74 was quoted/modified.- - - Drugs to be recommended- - - -
(1)Cases of early-onset hospital-acquired pneumonia in which there have been no previous administration of antimicrobial drugs or risk of resistant bacteriaAero-respiratory pathogen, such as H. influenzae and M. catarrhalis, and enteric bacteria, such as Klebsiella spp., are indicated.
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTRX, intravenous drip, 1 g/twice a day or 2 g/once a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-
-
(2)Cases of late-onset hospital-acquired pneumonia or ventilator-associated pneumonia in which the risk of resistant bacteria is high
- An antimicrobial drug with anti-pseudomonal activity that targets non-glucose-fermentative gram-negative rod should be administered [50], [51], [68] (BII).
-
-CAZ, intravenous drip, 1–2 g/4 times a day
-
-CFPM, intravenous drip, 1–2 g/4 times a day
-
-CZOP, intravenous drip, 1–2 g/2–4 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-
-
(3)In critically ill patients, carbapenem may be the first line drug, considering the involvement of multi-drug-resistant bacteria such as ESBL.
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-
-
d.
Polymicrobial infection
- - - Executive summary- - --
-If several bacterial cells differing one another in Gram staining and/or morphology are observed (polymicrobial infection), anaerobes may be involved.
-
-Polymicrobial infection commonly reflects microaspiration of oropharyngeal secretions into the lower airway.
- -
-
-In non-severe cases, the administration of antimicrobial agents with anti-MRSA activity may be withheld in the initial phase even when Staphylococcus-like bacterial cells are observed [70].
-
-
- - - Explanation- - -
When several types of bacteria differing in Gram staining and morphology are observed, the condition is commonly interpreted as aspiration pneumonia, suggesting the involvement of anaerobes. However, the number of hospital-acquired pneumonia (including VAP) caused by anaerobes have been reported relatively smaller than generally anticipated. [71], Polymicrobial infection does not always require the prompt antimicrobial therapy that covers anaerobes. Even though when aspiration pneumonia is suspected, SBT/ABPC is frequently prescribed assuming anaerobic infection, which actually works good on many occasions, it has to be acknowledged that SBT/ABPC exert good antimicrobial activity not solely against anaerobes, but also aero-enteric pathogen of pneumonia such as Streptococcus pneumonia, oral streptococci, H. influenzae, M. catarrhalis, and Klebsiella pneumonia.
Inpatients are often exposed to gram-negative bacteria residing in the hospital environment. Furthermore, there are many opportunities to undergo antimicrobial drug therapy that affects the indigenous microflora. For such reasons, gram-negative bacillus (enteric bacteria or P. aeruginosa) frequently colonize within the oropharyngeal region of the elderly patients or long-term bed-bound patients, many of whom need airway suctioning or have tracheostomy that may serve as portal of entry of environmental pathogen. Oropharyngeal microflora primarily consisting of these gram-negative bacteria can be aspirated into the airway after surgery requiring sedation or anesthesia, or during or after endoscopic examination [57], [72], [73]. Briefly, anaerobes may be an occasional pathoen in polymicrobial infection as seen on Gram staining of patients with suspected aspiration pneumonia, but S. pneumoniae, H. influenzae, S. aureus, Klebsiella spp., P. aeruginosa, and Acinetobacter spp. are more commonly involved in many cases, being similar to the microorganisms that are thought to be the major pathogen of hospital-acquired pneumonia. This is in contrast with the community-onset aspiration pneumonia, represented by lung abscess, in that anaerobes are primarily involved [67], [71].
Anaerobes involved in hospital-acquired pneumonia include facultative anaerobic α-hemolytic streptococci in the oral cavity and obligate anaerobes. Oral obligate anaerobes include gram-positive coccus (Peptostreptococcus sp.), gram-negative coccus (Veillonella sp.), and gram-negative bacillus “oral pigmented” Bacteroides (Bacteroides melaninogenicus), Prevotella sp., Porphyromonas sp., and Fusobacterium sp.). Many of these types of bacteria are susceptible to β-lactams that do not contain a β-lactamase inhibitor, new quinolones, macrolides, and tetracyclines.
Therefore, patients with hospital-acquired pneumonia may be basically treated by standard empiric therapy for hospital-acquired pneumonia even when aspiration pneumonia related to several types of bacteria is suspected [67].
- - - Drugs to be recommended- - -
-
(1)
Cases in which it is not necessary to consider the involvement of multi-drug-resistant bacteria, or early hospital-acquired pneumonia
The involvement of oral Streptococcus, oral anaerobes, S. pneumoniae, H. influenzae, and enteric bacteria should be considered.-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-
-
(2)
Late-onset hospital-acquired pneumonia or cases in which there is a risk of multi-drug-resistant bacteria
In addition to the above pathogens, the involvement of non-glucose-fermentative gram negative bacteria or ESBL-producing enteric bacteria must be considered.-
-CFPM, intravenous drip, 1–2 g/2–4 times a day
-
-CZOP, intravenous drip, 1–2 g/2–4 times a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-
2.2.3. Definitive therapy
-
a.
Rule of antimicrobial chemotherapy
- - - Executive summary- - -- -
- -
- - - Explanation- - -
If drug susceptibility test is not conducted for some reasons after the identification of causative microorganisms, an antimicrobial drug should be selected with reference to the susceptibility pattern (local sensitivity) of the identified bacteria at each institution. If the local sensitivity is not obtained, a drug should be selected based on the basic susceptibility of various pathogens to antimicrobial drugs (Table 5) [74].
In the treatment of hospital-acquired pneumonia, the duration of antimicrobial therapy generally tends to be longer than required for the following reasons: opacity on chest X-ray often remains for reasons other than pneumonia even after the start of antimicrobial drug treatment; and there may be a large number of latent non-pneumonia (or non-infectious-disease) factors that may cause increase in body temperature or CRP level in inpatients [75]. However, if appropriate antimicrobial drug treatment is performed, it is possible to complete treatment in 1 week [57]. In strains such as Enterobacter spp., Serratia spp., Citrobacter spp., and Morganella spp. (Table 5 GNRb), the expression of intrinsic antimicrobial-drug-resistance genes encoded in chromosome genes is induced during antimicrobial drug treatment, a phenomenon which is basically rarely seen in E. coli, Klebsiella spp., H. influenzae, and M. catarrhalis (Table 5 GNRa) (Table 5) [74], [76], [77]. Therefore, if adequately chosen treatment parameters are improved, antimicrobial treatment could be completed with careful follow up of patients' condition. Although it is useful to recognize these pathogens, abbreviated as SPACE (Serratia, Pseudomonas, Acinetobacter, Citrobacter, and Enterobacter), as a representative microorganism group that causes hospital-acquired pneumonia, the SPACE group is essentially a common colonizer. Therefore it is important to bear in mind that antimicrobial drug is not always indicated upon the isolation of SPACE to avoid selection of antimicrobial resistant bacteria related to unnecessary or long-term antimicrobial therapy [65], [67].
-
b.
Gram-positive bacteria
- - - Executive summary- - --
-In MRSA-infected patients, glycopeptides (VCM, TEIC) or LZD should be selected [78], [79] (AI).
- The penetration of LZD into the alveolar epithelium-lining fluid and intra-alveolar sputum is more favorable. Therefore, use of LZD should be encouraged in cases of restricted sputum expectoration, such as VAP [51] (BII).
- As DAP is inactivated by pulmonary surfactants, its use should be avoided for MRSA pneumonia.
-
-Glycopeptides should be selected as first-line drug for pneumonia caused by Corynebacterium sp [84] (AII).- - - Explanation- - -There is no significant difference in the therapeutic efficacy for MRSA pneumonia between glycopeptides and LZD. Several studies reported that the overall clinical efficacy of LZD, including the incidence of side effects, was superior to VCM in patients with hospital-acquired pneumonia caused by MRSA [85], [86]. However, since the dosing of VCM in these studies have been considered suboptimal, further study is needed [51], [87]. Some investigators have recommended that, when MRSA is susceptible to CLDM or MINO on a susceptibility test, LZD, a protein synthesis inhibitor should be administered given the possible involvement of the Panton-Valentine leukocidin [78], [88]. If a prompt improvement is achieved by the intravenous drip of LZD 600 mg q12h, or if the patient's condition is not critical, switch from the intravenous administration to an oral preparation of LZD, which shows high bioavailability [89], is encouraged. As DAP is inactivated by pulmonary surfactants, it should not be used to treat MRSA pneumonia. This may not apply to the treatment of septic pulmonary embolism [90].- - - Drugs to be recommended- - -
-
(1)MRSA
-
♦First choices
-
-VCM, intravenous drip, 1 g/twice a day
-
-TEIC, intravenous drip, 400 mg for the first 2 days/twice a day for loading, 400 mg/once a day from Day 3
-
∗TDM should be conducted so that the trough levels of VCM and TEIC range from 15 to 20 μg/mL [11].
-
-LZD, intravenous drip or oral administration, 600 mg/twice a day
-
-
-
<>Second choices
-
-ABK, intravenous drip, 300 mg/once a day (A trough level was established as ≤2 μg/mL using TDM.)
-
-ST combination drug (SMX at 400 mg/TMP at 80 mg), oral administration, 2 tablets/twice a day or intravenous drip, 960 mg/twice a day
-
-CLDM, intravenous drip, 600 mg/2–4 times a day (The results of drug susceptibility testing must be confirmed).
-
-
-
♦
-
(2)MSSARefer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [12] MSSA (2) Hospital treatment” (p. 8).
-
(3)S. pneumoniaeRefer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [1] S. pneumoniae (PC-susceptible) and [2] S. pneumoniae (PC-resistant)” (p.6).
-
(4)Corynebacterium sp.VCM and TEIC should be administered, as described for MRSA.
-
-
-
c.
Gram-negative bacteria
- - - Drugs to be recommended- - --
(1)E. coli, Klebsiella spp., Proteus spp. (non-ESBL-producing bacteria)Refer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [7] Klebsiella spp. [non-extended-spectrum β-lactamase (ESBL)-producing bacteria] (2) Hospital treatment ” (p.7).
-
(2)E. coli, Klebsiella spp., Proteus mirabilis (ESBL-producing bacteria)Refer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [8] Klebsiella spp. [ESBL-producing bacteria] (2) Hospital treatment” (p. 7).
-
(3)Enterobacter spp., Serratia spp., Citrobacter spp., Morganella spp., Proteus vulgaris
-
♦Third-generation cephems or quinolones should be administered [50], [51], [68] (AII).
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-CTX, intravenous drip, 1–2 g/2–3 times a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 1000 mg/twice a day
-
-
-
<>If a strain is estimated to constantly express cephalosporinase (highly resistant to β-lactamase inhibitor-containing β-lactams, oxyimino [=3rd generation] cephalosporin and cephamycin, through plasmid genes) on an antimicrobial drug susceptibility test, fourth-generation cephems or carbapenems should be administered.
-
-CFPM, intravenous drip, 1–2 g/4 times a day
-
-CZOP, intravenous drip, 1–2 g/4 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-
-
♦
-
(4)P. aeruginosa
- •
-
•No marked enhancement of therapeutic effects related to combination therapy with a β-lactam and aminoglycoside has been confirmed.
-
•Combination therapy with a β-lactam and new quinolone (CPFX, LVFX) may be effective, but its effects have not been investigated.
- •
-
•When performing combination therapy, the combination effects of the drugs should be measured in vitro [50] (BIII).
-
-PIPC, intravenous drip, 2–4 g/4 times a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/4 times a day
-
-CAZ, intravenous drip, 1–2 g/4 times a day
-
-CFPM, intravenous drip, 1–2 g/4 times a day
-
-CZOP, intravenous drip, 1–2 g/4 times a day
-
-AZT, intravenous drip, 1–2 g/4 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-TOB, intravenous drip, 5 mg/kg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 1000 mg/twice a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
∗Combination therapy
- the above β-lactam + TOB (intravenous drip, 5 mg/kg/once a day)
- or + CPFX (intravenous drip, 300 mg/twice a day)
- or + PZFX (intravenous drip, 1000 mg/twice a day)
-
∗Multi-drug-resistant bacteria
- CL (colistin): An initial dose (loading, 5 mg/kg) of CL should be administered as a single dose. After 24 h, administration at the following maintenance dose should be started, and continued at 12-h or 8-h intervals: 2.5 × [(1.5 × CLcre) + 30] mg
-
-
-
(5)Stenotrophomonas maltophiliaWhen this type of bacteria are isolated from airway samples, they commonly represent colonization [51].
-
-MINO, intravenous drip or oral administration (during or immediately after meals), 100 mg/twice a day
-
-ST combination drug (SMX at 400 mg/TMP at 80 mg), oral administration, 3 to 4 tablets/3 times a day or intravenous drip as TMP dose 240–320 mg/3 times a day
-
-
-
(6)M. catarrhalisRefer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [14] M. catarrhalis (2) Hospital treatment” (p. 8).
-
(7)Acinetobacter baumannii
- •
-
•It has not been sufficiently investigated whether the effects of CVA/AMPC or TAZ/PIPC are similarly effective to those of SBT/ABPC [92].
-
•Carbapenems may be effective.
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CAZ, intravenous drip, 1–2 g/4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-TOB, intravenous drip, 5 mg/kg/once a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-
-
(8)H. influenzaeRefer to the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy- - - [3] H. influenza (ABPC-susceptible), [4] H. influenza (β-lactamase-producing), [5] H. influenza (β-lactamase-negative ampicillin-resistant (BLNAR), and [6] H. influenza (β-lactamase-positive amoxicillin clavulanate-resistant (BLPACR)” (p. 6–7).- - - Explanation- - -According to some investigators, enteric bacteria are classified into 2 types: sensitive, Gram-negative rods, such as E. coli, Klebsiella pneumoniae, and P. mirabilis, which are susceptible to first-generation cephalosporin, and resistant, Gram-negative rods, such as Enterobacter spp., Serratia spp., and Citrobacter spp., which show an intrinsic or inducible resistance to third-generation cephalosporin through chromosomal AmpC genes [74], [76], [77]. In addition, the number of extended spectrum of β-lactamase (ESBL)-producing strains of E. coli, Klebsiella, and Proteus sp. that are resistant to all cephalosporin has increased. Among resistant GNRs, such as Enterobacter spp., strains that constantly produce AmpC-type β-lactamase (cephalosporinase) (plasmid type) must also be considered [76], [77].Concerning non-fermentative bacteria, their intrinsic susceptibility to antimicrobial agents differs among P. aeruginosa, Stenotrophomonas spp., and Acinetobacter spp. A study indicated that, in patients with P. aeruginosa pneumonia, monotherapy with a new quinolone might show unfavorable bacteria-eradicating effects or lead to recrudescence [90]. In some patients, combination therapy with a β-lactam (PIPC, CAZ, CFPM, or carbapenems), which has an anti-pseudomonal activity, and aminoglycoside or new quinolone may be considered [51], [90], [94]. Most strains of Stenotrophomonas spp. are susceptible to MINO or an ST combination drug.M. catarrhalis and Acinetobacter spp. are the frequent types of Gram-negative coccus detected in patients with early and late hospital-acquired pneumonia, respectively. Many strains of the former produce β-lactamase. The latter is a GNR existing in the hospital environment, and may be resistant to many antimicrobial drugs. However, in Japan, the multi-drug resistance of this type of bacteria has not widely distributed. Carbapenems and new quinolones should be selected. However, the vast majority of Acinetobaccter strains are susceptible to SBT/ABPC. In particular, SBT has an antimicrobial activity against this type of bacteria, and their susceptibility to SBT/ABPC should routinely be confirmed. Primary test drugs for an antimicrobial susceptibility of this type of bacteria (drugs appropriate for a routine examination panel) are SBT/ABPC, CAZ, IPM/CS, MEPM, GM, TOB, LVFX, and CPFX [95].Pan-sensitive strains of H. influenzae are β-lactamase (BL)-negative, ABPC-sensitive (BLNAS) strains. However, there are various resistance patterns: BL-producing, ABPC-resistant (BLPAR), BL-negative, ABPC-resistant (BLNAR), and BL-producing, AMPC/CVA-resistant (BLPACR) strains. BLNAS strains can be treated with ABPC, but SBT/ABPC therapy is required to control BLPAR strains. The administration of CTRX or new quinolones is necessary for BLNAR or BLPACR.A randomized-controlled trial with multivariate analysis has shown that factors for favorable bacteriological effects included the absence of P. aeruginosa-related pneumonia (<0.01), a higher body weight (<0.01), a low APACHE II score (severity) (0.03), and CPFX therapy (0.04) [90]. Conditions suggesting the use of new quinolones include allergy to β-lactams, the presence of or concern for nephropathy (an aminoglycoside cannot be combined with a β-lactam), necessity of covering obligate intracellular pathogen, or situations in which switching to an oral preparation is indicated [94]. An in vitro study indicated that the alveolar epithelial lining fluid (ELF) concentration of LVFX reached as high as its serum concentration. Furthermore, a prospective open-label study reported that switching of intravenous drip to oral administration decreased the ELF concentration, but it was within the range at which many pathogens are deemed sensitive based on the cumulative data of minimum inhibitory concentrations for causative microorganisms [94].
-
(1)
2.3. Nursing and healthcare-associated pneumonia
- - - Executive summary- - -
-
-
Nursing and healthcare-associated pneumonia (NHCAP) is a category independently defined in Japan based on medical circumstances.
-
-
The attending physician proposes a treatment category (Groups A to D) by evaluating what treatment is necessary as the most important item based on the patient's and his/her family's will (Fig. 1) [96].
-
-
Risk factors for resistant bacteria are categorized into two items, and initial treatment options are recommended, assuming target causative microorganisms (CIV).
-
-
In patients in whom the general condition is unfavorable due to complications or in terminal-stage patients, initial treatment options are recommended considering side effects from the perspective of innocent properties (CIV).
-
-
In Group D, in which intensive care is required, combination therapy with broad-spectrum (involving resistant bacteria and Legionella) and potent antimicrobial drugs is recommended (BI).
Fig. 1.
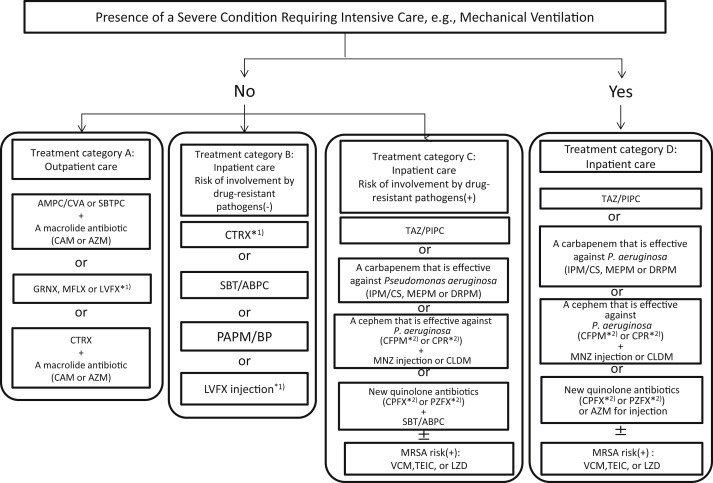
Recommendations of initial empiric antibiotic therapy [96].
Risk factors for involvement by drug-resistant pathogens.
-If no antibiotic therapy in the preceding 90 days or current tube feeding, the patient can be assumed to have no risk of involvement by drug-resistant pathogens.
-However, if past medical history indicates isolation of MRSA, the patient should be assumed to have risk of involvement by MRSA.
*1) Inappropriate when aspiration pneumonia is suspected, because it has insufficient activity against anaerobic bacteria.
*2) Because of insufficient activity against anaerobic bacteria, when used to treat suspected aspiration pneumonia, it should be used in combination with an antibiotic that has activity against anaerobic bacteria (e.g., MNZ, CLDM, SBT/ABPC).
- - - Explanation- - -
[Characteristics and classification of diseases]
In 2011, the Japanese Respiratory Society issued the “Guidelines for the Management of Nursing and Healthcare-associated Pneumonia (NHCAP)” [96], considering medical circumstances in Japan with reference to the entity of healthcare-associated pneumonia (HCAP) proposed in the Untied States [51]. The definition of NHCAP is shown in Table 6 . As this committee has no objection to the entity itself, the selection of drugs will be explained based on evidence to avoid duplications with the above guidelines.
Table 6.
Definition of NHCAP [96].
|
|
The mortality rate and frequency of resistant bacteria in patients with NHCAP are intermediate between community-acquired pneumonia (CAP) and hospital-acquired pneumonia (HAP). However, NHCAP may be primarily regarded as being similar to geriatric pneumonia [97], [98]. There is no fact that the rate at which resistant bacteria are isolated increases with severity [97]. Even when pneumonia is not severe, the host's activities of daily living (ADL) and an underlying disease/immunodeficiency reduce the prognosis in many cases [98]. As this type of pneumonia develops in a variety of uneven populations, it is difficult to simply determine severity classification. Therefore, considering various conditions, the entity of “treatment category”, involving the ethical aspects of geriatric care, was introduced based on evaluation by the attending physician who knows the patient well (Fig. 1). Frequent basic conditions or concomitant diseases for NHCAP in Japan include an advanced age, central nervous diseases, aspiration, a reduction in ADL, and tubal feeding. Their factors are aspiration pneumonia itself or risk factors, and HCAP in Japan may overlap with aspiration pneumonia [99]. On the other hand, in NHCAP patients, MRSA, P. aeruginosa, and anaerobes are more frequently isolated in comparison with CAP patients. It is necessary to switch therapeutic strategies, considering these causative microorganisms. Refer to the next section “2.4 Aspiration pneumonia” (p.19).
[Type and frequency of causative microorganisms]
Concerning causative microorganisms in NHCAP patients, resistant bacteria are frequently detected, differing from CAP patients. However, with respect to microorganisms that cause HCAP, the distribution and frequency of Streptococcus pneumoniae and H. influenzae, which are frequently isolated in CAP patients, as well as MRSA, P. aeruginosa, and Gram-negative bacillus, which are frequently detected in HAP patients, differ among countries, areas, and institutions due to their variety (III). Concerning causative microorganisms, a study reported that there was no marked difference between NHCAP and CAP [100]. On the other hand, a study in the United States indicated that S. aureus was frequently detected [101], and another study in Italy, where the rapid aging of society is advanced, as described for Japan, reported that aspiration pneumonia, H. influenzae, S. aureus, and Gram-negative bacillus were more frequent than in patients with CAP [59]. As a result, the rate of resistant bacteria increased, and inappropriate antimicrobial drugs were selected in a high proportion of patients. In addition, the mortality rate was higher than in CAP patients, suggesting the association between the two factors.
Representative causative microorganisms with respect to the presence or absence of risk factors for resistant bacteria are presented in Table 7 [96]. Of these, resistant bacteria, which are not targeted in CAP patients, were isolated in approximately 20%. However, the value was lower than in HAP patients. This is a current status of Japan (III). However, we must consider that patients in whom isolated bacteria are unclear account for approximately 50%, with the involvement of aspiration as a background factor [99]. In addition to bacteria commonly isolated in CAP patients, the frequency of enteric bacteria and anaerobes has increased [102].
Table 7.
Possible pathogens isolated from NHCAP patients [96].
| When an NHCAP patient has no risk factors for involvement by drug- resistant pathogens |
|
| When an NHCAP patient has a risk factor for involvement by drug-resistant pathogens (the following will be considered in addition to the above-mentioned pathogens) |
|
[Rules of antimicrobial drug therapy]
Risk factors for resistant bacteria in NHCAP patients include “the previous use of antimicrobial drugs for 2 days or more within 90 days” and “tubal feeding” (Table 8 ) [96] (II). A study reported that, even among severe NHCAP patients who were managed with a ventilator or in the ICU, resistant bacteria were not isolated in those who had made favorable daily life activities without a history of antimicrobial drug therapy [103]. Another study indicated that tubal feeding was an independent risk factor for infection with P. aeruginosa (odds ratio: 13.9) [104] (II). This is the reason why Treatment Category C was established in the Guidelines. Briefly, patients who do not meet the above two items are regarded as having no risk factor for resistant bacteria, and assigned to Group B. Patients meeting 1 or 2 items or those in whom MRSA was previously isolated are assigned to Group C. Respective drugs to be recommended were separately established. Patients in whom outpatient treatment is considered to be appropriate are assigned to Group A, and those in whom the attending physician considers ventilator or ICU management necessary to Group D. Drugs to be recommended were added, and a treatment category algorithm (Fig. 1) [96] was prepared. Concerning HCAP treatment in Europe and the United States, there is a gap between drugs used in clinical practice and those recommended in guidelines [105] (II). Therefore, treatment category-based empiric therapy in Japan may be acceptable in clinical practice; future investigation is necessary.
Table 8.
|
The risk of MRSA should be taken into account whenever there is past history of MRSA isolation.
When attempting to predict the isolation of drug-resistant pathogens based on the presence of these risk factors, it should be borne in mind that their sensitivity and negative predictive value are high, but their specificity and positive predictive value are low.
Drug-resistant pathogens include Pseudomonas aeruginosa, MRSA, Acinetobacter, ESBL-producing enteric bacteria, and Stenotrophomonas maltophilia.
[Administration period of antimicrobial drugs]
There is no evidence regarding the administration period of antimicrobial drugs. An administration period of 7–10 days, which is routinely adopted in the highest percentage of patients, is appropriate (BIV). When administering antimicrobial drugs for a longer period, equivalent-spectrum antimicrobial drugs should be selected, or de-escalation of antimicrobial drugs should be performed. In this case, fever, CRP, and leukocyte counts are often used as indices of the treatment response. In cases of aspiration pneumonia in which aspiration recurs during treatment despite the efficacy of antimicrobial drugs, it is necessary to evaluate whether the effects of antimicrobial drugs are not obtained or recurrence occurs.
- - - Drugs to be recommended- - -
-
a.Empiric therapy (Fig. 1) [96]
-
(1)Cases in which there is no risk of resistant bacteria and outpatient treatment is performed (Group A)According to a study, Chlamydophila spp. and M. pneumoniae accounted for 34.7 and 9.3% of patients in whom the type of microorganisms that cause NHCAP was clarified in Japan, respectively [98]. The results suggested that Chlamydophila spp. is a target of treatment, as described for CAP. Therefore, in Group A, combination therapy with a β-lactam and macrolide or monotherapy with a respiratory quinolone should be performed (BII). In Group D, an anti-P. aeruginosa drug should be combined with CPFX, PZFX, or AZM for injection, considering Legionella or Chlamydophila spp. pneumonia. However, concerning combination therapy with a macrolide (CII) in patients without “severe pneumonia requiring intensive care”, as described below, the evidence level is not always high from the perspectives of medical economics, side effects, and resistant bacteria [106]. Some studies examined the mortality rate with respect to the presence or absence of treatment covering atypical pathogens, and reported that the mortality rate was significantly lower in the presence of such treatment [17]. A recent meta-analysis also showed a difference [107].Respiratory quinolones were established as an option (BII) based on many references describing that their effects are similar to or more potent than those of combination therapy with a β-lactam and macrolide. However, this must be further examined, considering factors such as severity and the presence or absence of concomitant sepsis [108]. Furthermore, the prevalence of penicillin-resistant Pneumococcus, which has been internationally emphasized as an issue, and macrolide-resistant Pneumococcus, which has been markedly observed in Japan, was also a background factor for establishing respiratory quinolones as an option [109]. The previous use of antimicrobial drugs, which is often observed in patients with NHCAP, is considered to be a risk factor for resistant Pneumococcus [110]. A study reported that penicillin or EM resistance in HCAP patients was more advanced than in CAP patients [100]. A study indicated that the efficacy of oral therapy with LVFX was similar to that of CTRX injection therapy in 619 patients with CAP [111]. Another study reported that, among 680 patients with non-severe HCAP, oral LVFX was useful in those with no description of causative microorganisms [112]. However, when aspiration pneumonia is suspected, GRNX or MFLX should be selected, because the effects of LVFX on anaerobes are weak. Furthermore, several studies suggested the usefulness of MFLX, which is not influenced by the kidney function and does not require dose regulation, in elderly patients with NHCAP [113], [114].As treatment is completed with a single, high dose, compliance is favorable. AZM sustained-release preparations [115], [116], [117], which simultaneously cover bacteria and atypical pathogens, and STFX, which shows a favorable MIC for anaerobes, may also be recommended [118].
-
♦First choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
-
-
+one of the followings:
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-
-
<>Second choices
-
-MFLX, oral, 400 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/L to 2 times a dayor
-
-CTRX∗1, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-CTX∗1, intravenous drip, 1–2 g/2–3 times a day
-
-
-
+one of the followings:
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-CAM, oral, 200 mg/twice a day
-
-
-
♦
- *1) As the antimicrobial activity of the drug against anaerobes is insufficient, it is inappropriate under a tentative diagnosis of aspiration pneumonia.
-
(2)Cases in which there is no risk of resistant bacteria and hospital treatment is performed (Group B)In this category, we recommend monotherapy from the perspectives of causative microorganisms resembling those for CAP and side effect-based “innocent properties”. A study reported that initial treatment with narrow-spectrum antimicrobial drugs did not always lead to a poor prognosis [119]. In particular, according to two articles [120], [121], it is not necessary to consider resistant bacteria in HCAP patients in whom causative microorganisms are unclear; treatment in accordance with CAP treatment is sufficient (BII). However, an advanced age, central nervous diseases, aspiration, and a reduction in ADL are the clinical characteristics of aspiration pneumonia. The condition of NHCAP in Japan overlaps with aspiration pneumonia [122]. Therefore, in Group B, to which patients admitted for the first time, with no recent use of antimicrobial drugs, correspond, antimicrobial therapy with β-lactamase inhibitor-containing penicillins is appropriate, as described for CAP. However, when aspiration pneumonia is suspected, CTRX and LVFX should be avoided (BIV). For the management of enteric bacteria, candidate drugs for Group B, PAPM/BP, may be selected. Actually, it is not necessary to consider P. aeruginosa in patients with CAP or non-ICU HAP. A study indicated that ertapenem, which is not effective for P. aeruginosa, was useful [123], as demonstrated for PAPM/BP. However, another study reported that the widespread use of ertapenem induced the cross resistance of P. aeruginosa to other carbapenems; the use of PAPM/BP alone should be avoided [124] (BIV). In elderly persons, with a high risk of aspiration, who are repeatedly admitted and discharged, Klebsiella is often involved. TAZ/PIPC is more useful according to a study [125] (BII). In patients in whom Gram-negative bacillus is detected on Gram staining of sputum or those in whom the involvement of enteric bacteria is suspected, PAPM/BP or TAZ/PIPC should be selected (BIV).
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-CTRX∗1, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-CTX∗1, intravenous drip, 1–2 g/2–3 times a day
-
-LVFX∗1, intravenous drip, 500 mg/once a day
-
-PAPM/BP, intravenous drip, 0.5–1 g/2–4 times a day
-
-
- *1) As the antimicrobial activity of the drug against anaerobes is insufficient, it is inappropriate under a tentative diagnosis of aspiration pneumonia.
-
(3)Cases in which there is a risk of resistant bacteria and hospital treatment is performed (Group C)The target microorganisms include P. aeruginosa, MRSA, and Acinetobacter spp. in addition to frequent microorganisms that cause respiratory infection [97], [99], [103], [126]. As antimicrobial drugs, TAZ/PIPC, with an antimicrobial activity against P. aeruginosa, fourth-generation cephems, carbapenems, and quinolones (CPFX, PZFX) are recommended. TAZ/PIPC exhibits effects similar to those of IPM/CS and MEPM in patients with nursing and healthcare-associated pneumonia [127] (BII). PZFX also has an antimicrobial activity against S. pneumoniae when used at a high dose (2 g/day). When pneumonia related to atypical pathogens such as Chlamydophila spp. is suggested, quinolones should be selected. As the antimicrobial activities of fourth-generation cephems and quinolones against anaerobes are weak, these drugs should be combined with MNZ, CLDM, or SBT/ABPC. Recently, the resistance of the Bacteroides fragilis group to CLDM has advanced [128]. Therefore, in Europe and the United States, MNZ is selected as a first-choice antimicrobial drug against anaerobes. However, the rate at which the B. fragilis group is involved in oral anaerobes is low, and combination therapy with CLDM may be selected [129], [130]. Therefore, for combination therapy with fourth-generation cephems, we recommend the two drugs. When there is a risk of MRSA, such as previous admission, they should be combined with VCM, TEIC, or LZD. If there is no abscess formation, ABK is also effective.
-
♦First choices
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-
-
<>Second choices
-
-CFPM*2, intravenous drip, 1–2 g/2–4 times a day
-
-CPR∗2, intravenous drip, 1–2 g/2–4 times a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a dayor
-
-CPFX∗2, intravenous drip, 300 mg/twice a day
-
-PZFX∗2, intravenous drip, 1000 mg/twice a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-
-
♦
- *In addition to the above drugs, if MRSA infection is suspected, antimicrobial drugs should be added in accordance with the section “MRSA pneumonia”.
- *2) As the antimicrobial activity of the drug against anaerobes is insufficient, it should be combined with a drug with an antimicrobial activity against anaerobes (MNZ, CLDM, or SBT/ABPC) under a tentative diagnosis of aspiration pneumonia.
-
(4)Severe cases requiring intensive care (Group D)To cover L. pneumophila and atypical pathogens, which are rare as causative microorganisms but may cause severe conditions, Group-C antimicrobial drugs should be combined with CPFX, PZFX, or AZM injection (BI). Concerning the usefulness of combination therapy with a β-lactam and macrolide injection for severe pneumonia, evidence has been accumulated [131]. A study indicated that, in severe community-acquired pneumonia patients with sepsis or requiring ICU management, combination therapy with a β-lactam and macrolide led to a more favorable prognosis compared to that with a quinolone (I), suggesting that anti-inflammatory actions are involved in the mechanism [132]. In addition, another study reported that, among pneumonia patients with acute pulmonary disorder, both the ventilator withdrawal and survival rates in a macrolide-treated group were higher than in a non-macrolide-treated group [19] (I). Several meta-analyses also support them [107], [133].
-
♦First choices
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-
-
+one of the followings:
-
-CPFX∗2, intravenous drip, 300 mg/twice a day
-
-PZFX∗2, intravenous drip, 1000 mg/twice a day
-
-AZM, intravenous drip, 500 mg/once a day
-
-
-
<>Second choices
-
-CFPM∗2, intravenous drip, 1–2 g/2–4 times a day
-
-CPR∗2, intravenous drip, 1–2 g/2–4 times a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a day
-
-
-
+one of the followings:
-
-CPFX∗2, intravenous drip, 300 mg/twice a day
-
-PZFX∗2, intravenous drip, 1000 mg/twice a day
-
-AZM, intravenous drip, 500 mg/once a day
-
-
-
♦
- In addition to the above drugs,
- *If MRSA infection is suspected, antimicrobial drugs should be added in accordance with the section “MRSA pneumonia”.
- *2) As the antimicrobial activity of the drug against anaerobes is insufficient, it should be combined with a drug with an antimicrobial activity against anaerobes (MNZ, CLDM, or SBT/ABPC) under a tentative diagnosis of aspiration pneumonia.
-
b.Definitive therapyAntimicrobial drugs against identified causative microorganisms should be selected in accordance with the section “2.2 Hospital-acquired pneumonia” (p. 8).
-
(1)
2.4. Aspiration pneumonia
- - - Executive summary- - -
-
-
As oral indigenous bacteria, including anaerobes, cause aspiration pneumonia, β-lactamase inhibitor-containing penicillins are appropriate (BII).
-
-
In cases of nosocomial onset, it is necessary to cover Gram-negative bacillus, including P. aeruginosa.
-
-
In cases of severe ventilator-associated pneumonia (VAP), the selection of broad-spectrum antimicrobial drugs or combination therapy with them should not be hesitated (AI).
-
-
The detection rate of ESBL-producing Gram-negative bacillus has increased, and antimicrobial drugs should be carefully selected.
-
-
It is important to prevent subclinical aspiration through oral care and the prevention of gastroesophageal reflux, such as head lifting (BII).
-
-
To prevent aspiration pneumonia, it is also important to improve the nutritional status and avoid the overuse of sleeping pills/sedatives (BII).
- - - Explanation- - -
[Characteristic and classification of diseases]
Aspiration pneumonia occurs with a background factor, dysphagia, which is frequently observed in the presence of a reduction in ADL or systemic functions, especially cerebrovascular disorder. Its onset is associated with dietary ingestion in elderly persons [134]. Currently, aspiration pneumonia is accurately defined only in the Guidelines for the Management of Hospital-acquired Pneumonia (HAP) in Adults, which were prepared by the Japanese Respiratory Society [135]. The guidelines present conditions that may cause dysphagia, which were proposed by the Japanese Study Group on Aspiration Pulmonary Disease (Table 9 , modified) [136]. In our guidelines, we primarily explain antimicrobial drugs to be selected for patients with such conditions.
Table 9.
Conditions that may cause dysphagia [136] modified.
| Old/acute cerebrovascular disorder |
| Degenerative nervous and neuromuscular diseases, Parkinson's disease |
| Consciousness disorder, dementia |
| Gastroesophageal reflux, after gastrectomy (especially total gastrectomy), achalasia, scleroderma |
| Being bedridden |
| Laryngeal/pharyngeal tumors |
| Oral abnormalities (tooth occlusion disorder, inadaptation of dentures, dry mouth) |
| Tracheotomy, nasogastric tube (tubal feeding) |
| Drugs that induce dry mouth, such as sedatives, sleeping pills, and anticholinergic drugs |
The above definition is also adopted in the Guidelines for the Management of Nursing and Healthcare-associated Pneumonia (NHCAP) [96]. In elderly persons admitted to long-term care beds or nursing homes, risk factors include dysphagia and tubal feeding according to international data on pneumonia that develops in nursing homes [137], [138], [139]. In Japan, frequent underlying diseases in patients with NHCAP also include central nervous diseases and dementia, which are closely associated with aspiration. The proportion of patients after percutaneous endoscopic gastrostomy (PEG) is high [140]. However, among various types of community-acquired pneumonia, a diagnosis of aspiration pneumonia is made based on onset factor-based classification, and is not equal to NHCAP diagnosed primarily based on the place of onset or grade of nursing. According to data in Spain, aspiration pneumonia accounts for 20.6% of patients with healthcare-associated pneumonia (HCAP) requiring admission. This percentage was markedly higher than in those with community-acquired pneumonia (CAP) requiring admission (3.6%), but corresponded to no more than 1/5 [100]. On the other hand, a multicenter cooperative study involving inpatients with pneumonia in Japan, where the rapid aging of society is advanced, reported that 60.1% of patients who were admitted with CAP had aspiration pneumonia. Even in patients with CAP, which is not classified as NHCAP, the involvement of aspiration cannot be ignored [141]. Furthermore, the study indicated the involvement of aspiration in 86.7% of patients, aged over 70 years, with CAP/HAP [141]. In the future, the significance of distinguishing aspiration pneumonia among patients with NHCAP or HAP and changing therapeutic strategies should be examined. However, NHCAP more markedly affects ADL compared to CAP, and the aspect of elderly pneumonia is emphasized; it may be significant to positively diagnose aspiration pneumonia and establish therapeutic strategies different from those for CAP [142].
Concerning HAP, a reduction in the immune function is a background factor. HAP has two aspects: pneumonia with a high risk of resistant bacteria and that in which central nervous disease-related aspiration is involved. In the Guidelines for the Management of Hospital-acquired Pneumonia in Adults, which were prepared by the Japanese Respiratory Society, Mendelson syndrome and VAP are categorized as a group, and 3 classifications, involving diffuse deglutition-related bronchiolitis, in which there are no findings of pneumonia, are proposed. In addition, a flow chart for diagnosis is presented [135] (Fig. 2 ). With respect to the condition and treatment of VAP, refer to a review described by Chastre et al. [55] Management other than antimicrobial drug therapy should also be considered, and bundle (Table 10 )-based prevention should be performed [143] (AII).
Fig. 2.
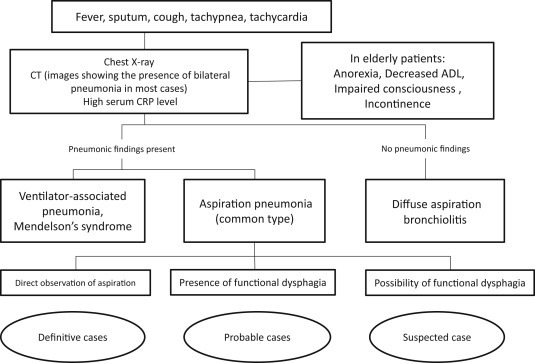
Diagnostic flow chart for aspiration pulmonary disorders [96].
Table 10.
Bundles for the prevention of ventilator-associated pneumonia.
|
In addition to the above items, methods to prevent aspiration pneumonia include oral care, the administration of drugs that improve the deglutition function, such as ACE inhibitors and cilostazol, improvement in the nutritional status, eating/swallowing rehabilitation, and anti-Pneumococcus vaccination.
[Type and frequency of causative microorganisms]
Streptococcus pneumoniae, S. aureus, and Enterobacteriacae have been reported. A study indicated that K. pneumoniae was frequent [144]. The involvement of oral indigenous bacteria, such as Streptococcus anginosus group. and anaerobes, has been suggested [145], [146]. In cases of nosocomial onset, Gram-negative bacillus, including P. aeruginosa, must also be considered. Concerning E. coli, Klebsiella spp., and Proteus spp., the number of ESBL-producing strains may increase in the future.
[Rules of antimicrobial drug therapy]
If appropriate antimicrobial drug therapy is not selected under a diagnosis of aspiration-related pneumonia, insufficient treatment may lead to a fatal condition, or excessive treatment may increase the number of resistant bacteria, showing negative effects. There may be differences in options for empiric therapy between patients with VAP (most patients show severe conditions) and those with diffuse deglutition-related bronchiolitis, in whom the start of treatment is not accelerated. On the other hand, approaches to prevent pneumonia after aspiration or avoid aspiration are important. Oral care, head lifting, and improvement in the nutritional status must be considered, and the overuse of sleeping pills/sedatives should be avoided (BII).
The best option for standard-type aspiration pneumonia is an antimicrobial drug that exists an antimicrobial activity against both aerobes and anaerobes. SBT/ABPC and TAZ/PIPC are effective for anaerobes frequently isolated in the respiratory system, such as Fusobacterium spp., Prevotella spp., and Peptostreptococcus spp. [147], [148]. As the resistance rates of these types of bacteria to the two regimens are low, these regimens are also recommended as first choices in the guidelines established by the Japanese Association for Anaerobic Infection Research [126].
However, a study reported that the previous administration of antimicrobial drugs and ADL were correlated with the frequency of Enterobacteriacae- or P. aeruginosa-related pneumonia [149]. A retrospective study involving 90 patients with aspiration pneumonia showed that the frequency of K. pneumoniae-related pneumonia was 25% [150]. Based on these studies, drugs to be selected should be changed in accordance with the previous administration of antimicrobial chemotherapeutic drugs in patients admitted to general or medical wards. Among patients with hospital-acquired pneumonia, broad-spectrum drugs should be selected in those with severe aspiration pneumonia or VAP (BII). When causative microorganisms are identified and an improvement in the condition is achieved, de-escalation should be performed.
- - - Drugs to be recommended- - -
-
a.Empiric therapy
-
➀No risk of resistant bacteriaDrugs with potent antimicrobial activities against oral anaerobes are presented. However, no article has provided high-level evidence regarding aspiration pneumonia. As the following drugs affect the intestinal flora, antimicrobial drug-associated diarrhea may occur. If symptom improvement is delayed, patients must be promptly admitted, and drip infusion therapy should be performed (outpatient treatment should not be prolonged).
-
(1)Outpatient treatment
-
♦First choices
- - CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
- - SBTPC, oral (375 mg), 2 tablets/3–4 times a day
-
<>Second choices
- - MFLX, oral, 400 mg/once a day
- - STFX, oral, 100 mg/1–2 times a day
- - GRNX, oral, 400 mg/once a day
-
♦
-
(2)Hospital treatmentWhen a diagnosis of aspiration pneumonia is made, SBT/ABPC is most frequently used in Japan [151]. Kaneko et al. reported that the sensitivity of oral anaerobes that may cause aspiration pneumonia, such as Peptostreptococcus spp., Prevotella spp., and Fusobacterium spp., to SBT/ABPC was 100%, similar to that to TAZ/PIPC [152].The effects of CLDM on aspiration pneumonia or a pulmonary abscess are similar to those of SBT/ABPC (BI) [145]. SBT/ABPC and CLDM showed similar effects and tolerance on aspiration pneumonia (67.5 and 63.5%, respectively) [147]. Oral anaerobes, excluding Bacteroides spp., are still susceptible to CLDM. A randomized clinical trial (RCT) indicated that CLDM was more potent than cephems [153].
-
♦First choice
- - SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
<>Second choice
- - CLDM, intravenous drip, 600 mg/2–4 times a day
-
♦
-
(1)
-
➁Cases in which there is a risk of resistant bacteria or severe casesIn cases in which there is a risk of resistant bacteria or severe cases, drugs should be selected in accordance with options for Group C for NHCAP. Tubal feeding is a risk factor for aspiration, and is also a risk factor for resistant bacteria [97]. When the involvement of Enterobacteriacae, such as K. pneumoniae and E. coli, is suggested, empiric therapy should be selected in accordance with cases in which there is a risk of resistant bacteria [55]. In Japan, the proportion of ESBL-producing bacteria on sputum culture in patients with respiratory infectious diseases is 5% or less [13], but the number of ESBL-producing bacteria has slightly increased; this must be considered in the future [154], [155]. A study reported that the clinical effects of TAZ/PIPC on non-ESBL-producing K. pneumoniae were more potent than those of SBT/ABPC; caution is needed [125]. It must be considered that, in cases of aspiration pneumonia classified as HCAP, Enterobacteriacae is isolated at a frequency that cannot be ignored. Concerning aspiration pneumonia that occurs in hospitals, some reviews proposed that antimicrobial drugs should be selected, regarding the condition as hospital-acquired pneumonia; empiric therapy may be selected in accordance with the Guidelines for the Management of Hospital-acquired Pneumonia, which were published by the Japanese Respiratory Society [156], [157]. According to a study, BIPM, which does not cause kidney dysfunction in elderly patients, is also effective (CIV); therefore, it is presented as an option for cases in which there is a risk of resistant bacteria [158].The mortality rate in patients with VAP is high. If causative microorganisms cannot be initially covered, the mortality rate may increase [51]. Therefore, drugs should be selected, regarding the condition as severe aspiration pneumonia. Three studies reported that, in a group in which TAZ/PIPC was selected as a drug to be combined with aminoglycosides for VAP treatment, the mortality rate was lower than in a group in which CAZ was selected [159], [160], [161]. In particular, when pneumonia was caused by P. aeruginosa, the clinical effects of TAZ/PIPC were more potent than those of IPM/CS (BII). For monotherapy, information on the culture of protected specimen brush (PSB) samples or broncho-alveolar lavage (BAL) fluid is strongly recommended [70], [162]. On the other hand, an observational study indicated that three-drug therapy (two anti-P. aeruginosa drugs + an anti-MRSA drug) deteriorated the prognosis; an RCT should be conducted in the future [60]. Therefore, if there is a risk of resistant bacteria, at least broad-spectrum antimicrobial drugs must be used for empiric therapy. However, assuming causative microorganisms with reference to Gram staining reactions, minimum necessary antimicrobial drugs should be selected based on local factors (antimicrobial drug susceptibility pattern of each type of bacteria in each hospital). Recently, the entity of ventilator-associated tracheobronchitis (VAT) was proposed, and the disadvantages of aggressive treatment have been discussed [163]. In a multicenter cooperative study, patients with VAT, which occurred in the ICU, were divided into two groups with and without antimicrobial drug therapy, and the results were compared. In the former, the incidence of VAP was significantly lower, and the mechanical ventilation-free period was significantly longer. In addition, the ICU mortality rate was significantly lower. On the other hand, there was no significant difference in the appearance of resistant bacteria between the two groups [164].
-
♦First choices
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-
-
<>Second choices
-
-CFPM, intravenous drip, 1–2 g/2–4 times a day
-
-CPR, intravenous drip, 1–2 g/2–4 times a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a dayor
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a day
-
-SBT/ABPC, intravenous drip, 1.5–3 g/3–4 times a day
-
-
-
♦
-
➀
-
*If MRSA infection is suspected, antimicrobial drugs should be administered in accordance with the section “MRSA pneumonia” in addition to the above drugs.
[Administration period of antimicrobial drugs]
It is recommended that the treatment period of hospital-acquired pneumonia should be 7–10 days. However, the treatment period should be 14 days in patients with pneumonia related to non-glucose-fermenting bacteria such as P. aeruginosa [2] (BII). Concerning VAP, a study reported that there was no difference in the clinical effects between 8 and 15 days [57].
-
b.
Definitive therapy
To control identified causative microorganisms, antimicrobial drugs should be selected in accordance with the section “Hospital-acquired pneumonia”. If MRSA infection is suspected, antimicrobial drugs should be selected in accordance with the section “MRSA pneumonia”.
2.5. Fungal/viral pneumonia
-
a.
Invasive pulmonary aspergillosis (IPA)
- - - Executive summary- - --
-In the management of IPA, effective treatment should be started as early as possible [165] (AII).
- -
- -
- -
-
-As the effects of combination therapy with an azole and AMPH-B preparation antagonize in some strains, a combination of these drugs should be avoided [174] (AIII).
-
-For the target treatment of this disease, an antifungal drug of which the class is different from that of a drug used for preventive administration should be used (BIII).- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease develops in severe immunocompromised hosts such as patients received chemotherapy for leukemia or hematopoietic stem cell/organ transplantation. Symptoms such as fever that does not respond to broad-spectrum antimicrobial drugs, cough, dyspnea, sputum, and bloody sputum/hemoptysis are observed.
-
-Laboratory findings: Chest X-ray shows an infiltrative shadow (typically, a wedge shadow involving the pleura as the base). On thoracic CT, infiltrative and nodular shadows (with a halo sign in some cases) are observed. In the recovery phase of neutrophils, an air crescent sign is noted. An increase in the inflammatory marker such as the CRP level, Aspergillus galactomannan antigen-positive reactions, and an increase in the (1 → 3)-β-D-glucan level are useful for diagnosis. However, neither the sensitivity nor specificity is sufficient. The results should be carefully evaluated.
-
-Causative microorganisms: Aspergillus fumigatus is frequently detected, but, recently, an increase in the number of patients with non-fumigatus Aspergillus-related IPA has been indicated.
-
-Specific condition: Lesions are sometimes formed in the nasal sinus and brain; caution is needed.
-
-Early diagnosis: Early treatment is important for successful treatment for this disease.- - - Drugs to be recommended- - -A study reported that, in a group in which VRCZ was used for the initial treatment of IPA, the results of treatment were more favorable than in a group in which d-AMPH was used [166]. Furthermore, another study indicated that therapy with L-AMB at 3 mg/kg/day was safer than that at 10 mg/kg/day, although there was no significant difference in the clinical efficacy [167]. CPFG, MCFG, and ITCZ also have anti-Aspergillus activities, and can be used. It is important to consider different strategies in accordance with the appearance of the host's allergy or adverse events and interactions with drugs used to treat an underlying disease.
-
♦First choices
-
-VRCZ, intravenous drip, 6.0 mg/kg/twice a day on Day 1 (loading dose), 3.0–4.0 mg/kg/twice a day on Day 2 or later
-
-L-AMB, intravenous drip, 2.5–5.0 mg/kg/once a day
-
-
-
<>Second choices
-
-CPFG, intravenous drip, 70 mg/once a day on Day 1 (loading dose), 50 mg/once a day on Day 2 or later
-
-MCFG, intravenous drip, 150–300 mg/once a day
-
-ITCZ, intravenous drip, 200 mg/twice a day on Days 1 and 2 (loading dose), 200 mg/once a day from Day 3 until Day 14. If treatment is further continued, ITCZ capsules (200 mg) should be administered immediately after meals twice a day, or ITCZ oral solution (20 mL) (200 mg as ITCZ) should be administered after fasting once a day.
-
-
-
-
-
b.
Chronic progressive pulmonary aspergillosis (CPPA)
- - - Executive summary- - --
-In Japan, various disease types such as aspergilloma with infiltration and enlargement of an existing cavity are included. CPPA includes various diseases such as chronic necrotizing pulmonary aspergillosis (CNPA), chronic cavitary pulmonary aspergillosis (CCPA), and chronic fibrosing pulmonary aspergillosis (CFPA). It refers to a series of syndrome for which the administration of antifungal drugs is essential.
-
-Treatment should be started with injection. If symptoms and findings are stabilized, injection should be switched to oral drugs.
- -
-
-Initial treatment with ITCZ, VRCZ, or L-AMB can also be selected in accordance with the host's underlying disease or drugs used to treat the underlying disease.
-
-For maintenance therapy, ITCZ and VRCZ oral preparations are recommended (AIII).- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease develops in hosts with organic diseases such as a cavity or cystic disease of the lung or bronchus. Symptoms such as fever, sputum, bloody sputum/hemoptysis, and dyspnea are observed.
-
-Laboratory findings: Chest X-ray and CT show an infiltrative shadow, enlargement of a cavity, thickening of the cavity wall/pleura, and a niveau in the cavity. There is an increase in the CRP level in many patients. Most patients are positive for anti-Aspergillus precipitating antibody. Neither Aspergillus galactomannan antigen nor β-D-glucan is a clue to diagnosis.
-
-Causative microorganisms: A. fumigatus is frequently detected. Non-fumigatus Aspergillus-related CPPA is also often observed.- - - Drugs to be recommended- - -A clinical study in Japan indicated that there was no marked difference in the efficacy of treatment between MCFG- and VRCZ-treated groups, whereas MCFG was safer [175]. Another study reported that there was no difference in treatment results between MCFG and CPFG [176]. In the phase of severe symptoms such as fever and bloody sputum, treatment should be started using these injections. Subsequently, if the condition becomes stable, switching to oral preparations can be considered. Currently, there are no criteria for the completion of treatment.
-
(1)Initial treatment
-
♦First choices
-
-MCFG, intravenous drip, 150–300 mg/once a day
-
-CPFG, intravenous drip, 70 mg/once a day on Day 1 (loading dose), 50 mg/once a day on Day 2 or later
-
-VRCZ, intravenous drip, 6.0 mg/kg/twice a day on Day 1 (loading dose), 3.0–4.0 mg/kg/twice a day on Day 2 or later
-
-
-
<>Second choices
-
-ITCZ, intravenous drip, 200 mg/twice a day on Days 1 and 2 (loading dose), 200 mg/once a day from Day 3 until Day 14. If treatment is further continued, refer to the section “(2) Maintenance therapy”.
-
-L-AMB, intravenous drip, 2.5–5.0 mg/kg/once a day
-
-
-
♦
-
(2)Maintenance therapy
-
♦First choices
-
-ITCZ oral solution, oral, 20 mL (200 mg as ITCZ)/once a day (administered after fasting)
-
-(Switching from ITCZ injection) ITCZ capsules, oral, 200 mg/twice a day (administered immediately after meals)
-
-(Switching from drugs other than ITCZ injection or favorable conditions) ITCZ capsules, oral, 200 mg/once a day (administered immediately after meals)
-
-VRCZ tablets
- • (Body weight: 40 kg or more) oral, 300 mg/twice a day on Day 1 (loading dose), 150 or 200 mg/twice a day (administered between meals) on Day 2 or later
- • (Body weight: less than 40 kg) oral, 150 mg/twice a day on Day 1 (loading dose), 100 mg/twice a day (administered between meals) on Day 2 or later
-
-
-
♦
-
-
-
c.
Pulmonary aspergilloma
- - - Executive summary- - --
-The purpose of treatment is the prevention or treatment of hemoptysis. When there are no symptoms, follow-up is continued without treatment in some cases.
-
-As a rule, resection should be performed for radical treatment [177].
-
-When treatment is necessary, resection should be considered, if possible, by comprehensively evaluating the age, pulmonary function, and degree of pulmonary destruction/pleural adhesion.
-
-When resection is considered to be impossible, antifungal therapy should be performed if necessary.
-
-Oral treatment with ITCZ or VRCZ is recommended (BIII).- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease develops in hosts with preexisting cavities such as old pulmonary tuberculosis, pulmonary cysts, and bronchiectasis. The condition is asymptomatic in some patients, whereas symptoms such as sputum and bloody sputum/hemoptysis are observed in others.
-
-Laboratory findings: Chest X-ray and CT show a cavity, intracavitary fungas balls, and thickening of the cavity wall/pleura. Anti-Aspergillus precipitating antibody–positive reactions are obtained. The enhancement of the inflammatory marker is noted in some cases.
-
-Causative microorganisms: A. fumigatus is frequently detected.
-
-Pulmonary aspergilloma is classified into two types: simple and complex aspergilloma based on the grade of difficulty in resection. The former refers to aspergilloma formation in a focus with a thin wall, such as a cyst, without accessory lesions at the periphery. The latter refers to aspergilloma formation in a cavity derived from a strongly destructed existing structure of the lung, such as old pulmonary tuberculosis and bronchiectasis, with marked destructive lesions or pleural adhesion at the periphery of the cavity.- - - Drugs to be recommended- - -Resection should be selected as a first choice. When resection is impossible, medical treatment can be considered.In the treatment of aspergilloma, oral drugs are usually selected. Although there is no evidence, ITCZ capsules/oral solution and VRCZ tablets should be used.
-
-ITCZ capsules, oral, 200 mg/once a day (administered immediately after meals)
-
-ITCZ oral solution, oral, 20 mL (200 mg as ITCZ)/once a day (administered after fasting)
-
-VRCZ tablets
-
•(Body weight: 40 kg or more) oral, 300 mg/twice a day on Day 1 (loading dose), 150 or 200 mg/twice a day (administered between meals) on Day 2 or later
-
•(Body weight: less than 40 kg) oral, 150 mg/twice a day on Day 1 (loading dose), 100 mg/twice a day (administered between meals) on Day 2 or later
-
•
-
-
-
d.
Primary pulmonary cryptococcosis
- - - Executive summary- - --
-No study has prospectively examined treatment for pulmonary cryptococcosis in patients without underlying diseases.
-
-FLCZ oral preparations should be selected [178] (AII).
-
-ITCZ capsules/oral solution and VRCZ tablets can also be used (BIII).
-
-In severe cases, drugs should be selected in accordance with cases in which underlying diseases are present (BIV).
-
-Cryptococcus glucuronoxylomannan antigen is useful for the diagnosis of this disease, but cannot be used to evaluate the treatment response or as an index for the completion of treatment.
-
-To confirm the presence or absence of inflammation involving the central nervous system, glucuronoxylomannan antigen or Cryptococcus cells in cerebrospinal fluid should be investigated, even when there is no marked meningeal irritation sign (BIII).
-
-If meningitis is present, initial treatment with an AMPH-B preparation, such as L-AMB, and 5-FC should be performed for 2 weeks or more. Subsequently, treatment should be continued using FLCZ or F-FLCZ.
-
-In Japan, Cryptococcus gattii infection has also been reported. If possible, causative fungus must be isolated/identified.- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease is often asymptomatic, and is detected on a health checkup in many cases.
-
-Laboratory findings: Chest X-ray and CT show solitary or multiple nodular and infiltrative shadows.
-
-Some cavities are observed in a lot of cases. There is no enhancement of the inflammatory marker in many cases, but glucuronoxylomannan antigen-positive reactions are detected.
-
-Causative microorganisms: This disease is caused by Cryptococcus neoformans. Recently, infection with C. gattii has been reported in Vancouver, Canada and the North area of the West Coast of the United States of America; caution is needed.
-
-C. gattii is primarily distributed in the tropical and subtropical zones. Infection in humans has been considered to be rare. However, since 1999, patients infected with C. gattii have been reported in the Pacific Coast of North America. Even healthy adults are infected with C. gattii, and the mortality rate is high.- - - Drugs to be recommended- - -Although there is no evidence regarding pulmonary cryptococcosis, FLCZ tablets, which have a potent activity against Cryptococcus, are frequently selected when the patient's condition is stable in the absence of an underlying disease. Azoles other than this drug can also be selected.
-
♦First choice
-
-FLCZ, oral, 400–800 mg/once a day on Days 1 and 2 (loading dose), 200–400 mg/once a day on Day 3 or later
-
-
-
<>Second choices
-
-ITCZ capsules, oral, 200 mg/once a day (administered immediately after meals)
-
-ITCZ oral solution, oral, 20 mL (200 mg as ITCZ)/once a day (administered after fasting)
-
-VRCZ tablets
-
•(Body weight: 40 kg or more) oral, 300 mg/twice a day on Day 1 (loading dose), 150 or 200 mg/twice a day (administered between meals) on Day 2 or later
-
•(Body weight: less than 40 kg) oral, 150 mg/twice a day on Day 1 (loading dose), 100 mg/twice a day (administered between meals) on Day 2 or later
-
•
-
-
-
-
-
e.
Pulmonary cryptococcosis- - - in the presence of an underlying disease (non-HIV infection)- - -
- - - Executive summary- - --
-Initial treatment with F-FLCZ injection should be performed [179] (AIII).
-
-ITCZ and VRCZ injections can also be used (BIII).
-
-In severe cases, treatment with L-AMB + 5-FC should be selected [179] (AIII).
-
-Cryptococcus antigen is useful for diagnosis, but cannot be used to evaluate the treatment response or as an index for the completion of treatment.
-
-To confirm the presence or absence of inflammation involving the central nervous system, glucuronoxylomannan antigen or Cryptococcus cells in cerebrospinal fluid should be investigated, even when there is no marked meningeal irritation sign (BIII).
-
-If meningitis is present, initial treatment with an AMPH-B preparation, such as L-AMB, and 5-FC should be performed for 2 weeks or more. Subsequently, treatment should be continued using FLCZ or F-FLCZ.- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease develops as opportunistic infection in hosts with malignant tumors or renal failure, those receiving steroids or immunosuppressive drugs, and patients with AIDS. The symptoms of this disease are more marked than those of primary pulmonary cryptococcosis: fever, general malaise, cough, sputum, bloody sputum, dyspnea, and thoracic pain.
-
-Laboratory findings: Chest X-ray and CT show solitary or multiple nodular and infiltrative shadows. Cavitary lesions are observed in a lot of cases. Cryptococcus glucuronoxylomannan antigen-positive reactions are detected.
-
-Causative microorganisms: This disease is caused by C. neoformans.
-
-Specific condition: If this disease develops in AIDS patients, it may deteriorate to a systemic infectious disease in the early stage, particularly causing meningoencephalitis. Therefore, treatment in accordance with Cryptococcus encephalomeningitis should be performed.- - - Drugs to be recommended- - -Pulmonary cryptococcosis in the host with an underlying disease more frequently leads to a severe condition compared to primary pulmonary cryptococcosis. Although there is no evidence, azoles with anti-Cryptococcus activities should be used for initial treatment. When azoles cannot be used, or when clinical effects are not satisfactory, combination therapy with L-AMB and 5-FC should be considered.
-
♦First choices
-
-F-FLCZ, intravenous drip, 800 mg/once a day on Days 1 and 2 (loading dose), 400 mg/once a day on Day 3 or later
-
-ITCZ, intravenous drip, 200 mg/twice a day on Days 1 and 2 (loading dose), 200 mg/once a day from Day 3 until Day 14. If treatment is further continued, ITCZ capsules (200 mg) should be administered immediately after meals twice a day, or ITCZ oral solution (20 mL) (200 mg as ITCZ) should be administered after fasting once a day.
-
-VRCZ, intravenous drip, 6.0 mg/kg/twice a day on Day 1 (loading dose), 3.0–4.0 mg/kg/twice a day on Day 2 or later
-
-
-
<>Second choice
-
-L-AMB, intravenous drip, 2.5–6.0 mg/kg/once a day + 5-FC tablets, oral, 25 mg/kg/4 times a day
-
-
-
-
-
f.
Pulmonary zygomycosis
- - - Executive summary- - --
-When this disease is suspected, an effective antifungal drug should be administered as early as possible (A).
-
-This disease may develop as breakthrough infection during azole therapy (BII).
-
-Treatment with high-dose L-AMB should be performed [180] (AII).
-
-If the lesion is localized, resection should be considered.
-
-Combination therapy with an iron chelating agent and L-AMB should be avoided [181] (AI).- - - Explanation- - -[Characteristics of the disease]
-
-Symptoms: This disease develops as opportunistic infection in patients with severe diabetes, those after organ/hematopoietic stem cell transplantation, those with neutropenia, and those with malignant tumors. It rapidly exacerbates, showing an unfavorable prognosis. In some patients, autopsy leads to a definitive diagnosis. Fever, dyspnea, and bloody sputum/hemoptysis are often observed.
- -Laboratory findings: Thoracic CT shows infiltrative/nodular (±halo sign) shadows and air crescent signs. In some patients, reversed halo signs are observed. Serological diagnosis cannot be applied.
-
-Causative microorganisms: In many cases, this disease is caused by 4 classes of Mucoraceae: Rhizopus, Rhizomucor, Mucor, and Absidia. The most frequent type is Rhizopus oryzae. Recently, an increase in the incidence of infection with Cunninghamella has also been indicated.
-
-Specific condition: Nasal/brain-type, dermal, and disseminated zygomycosis is observed.- - - Drugs to be recommended- - -Currently, only AMPH preparations may be clinically useful for treating zygomycosis among antifungal drugs that are available in clinical practice in Japan. As high-dose therapy must be started as early as possible, not d-AMPH but L-AMB should be selected.
-
-L-AMB, intravenous drip, 5 mg/kg/once a dayPrecautions for the use of antifungal drugs (Confirm the package inserts.)
-
➀VRCZVision disorder, liver dysfunction, and neurological/mental adverse events may occur.Combination therapy with RFP, RBT, efavirenz, ritonavir, carbamazepine, long-acting barbiturate, pimozide, quinidine sulfate, ergot alkaloid, or triazolam is contraindicated. This drug is also contraindicated for pregnant women. As a rule, it is contraindicated for patients with a Ccr of <30 mL/min (injection only).As the blood concentration of VRCZ may vary, TDM should be conducted. In patients with mild to moderate liver dysfunction, the dose should be regulated.
-
➁ITCZHepatopathy and congestive heart failure may occur.Combination therapy with pimozide, quinidine, bepridil, simvastatin, triazolam, azelnidipine, ergotamine, nisoldipine, dihydroergotamine, vardenafil, eplerenone, blonanserin, sildenafil, tadalafil, aliskiren, dabigatran(ITCZ oral only), rivaroxaban, ergometrine, or methylergometrine is contraindicated. This drug is contraindicated for patients with severe liver diseases, pregnant women. Patients with a Ccr of <30 mL/min are also contraindicated (injection only).
-
➂FLCZHepatopathy and a prolongation of QT may occur.Combination therapy with triazolam, ergotamine, dihydroergotamine, quinidine, or pimozide is contraindicated. This drug is also contraindicated for pregnant women.
-
➃F-FLCZCombination therapy with triazolam, ergotamine, dihydroergotamine, quinidine, or pimozide is contraindicated. This drug is also contraindicated for pregnant women.
-
➄L-AMBAdverse events such as nephropathy, hypopotassiumemia, and fever may occur.This drug is contraindicated during leukocyte transfusion.
-
➅CPFGThis drug is safe, but hepatopathy may occur.Caution is needed for combination therapy with cyclosporin, tacrolimus, RFP, efavirenz, nevirapine, phenytoin, dexamethasone, or carbamazepine.
-
➆MCFGThis drug is safe, but hepatopathy may occur.
-
➇5-FCAnorexia and myelopathy may occur.Combination therapy with tegafur-/gimeracil-/oteracil potassium-containing drugs is contraindicated. Even after the discontinuation of tegafur-/gimeracil-/oteracil potassium-containing drugs, combination therapy should be avoided within 7 days. This drug is also contraindicated for pregnant women.
-
➀
-
-
-
g.
Pneumocystis jirovecii pneumonia (PCP)
- - - Executive summary- - --
-Initial treatment with an ST combination drug should be performed [182] (AI).
-
-When an ST combination drug cannot be used, treatment with pentamidine or atovaquone should be performed (AI).
-
-To HIV-infected patients with respiratory failure (PaO2: <70 mmHg or A-aDO2: >35 mmHg in room air), corticosteroids should be administered for adjuvant therapy [183] (AI).
-
-Even in the absence of HIV infection, corticosteroids is recommended to patients with respiratory failure (PaO2: <70 mmHg or A-aDO2: >35 mmHg in room air) for adjuvant therapy (AIII).- - - Explanation- - -[Characteristic of the disease]
-
-Symptoms: This disease develops as opportunistic infection in patients taking steroids or immunosuppressive drugs over a long period and those infected with HIV. Three major signs consist of fever, dry cough, and dyspnea. The mode of PCP onset in HIV-infected patients is slower than in non-HIV-infected patients. In the former, fever and hypoxemia are relatively mild, and the mortality rate is low.
-
-Laboratory findings: Typical chest X-ray and CT findings include a diffuse, ground glass opacity extending from the pulmonary hilum to the bilateral sides. On CT, a ground glass opacity expanding like a map is observed in some cases. In addition, various shadows such as multiple nodes/cysts are detected. The levels of CRP, LDH, KL-6, and (1 → 3)-β-D-glucan increase. A definitive diagnosis is made by confirming Pneumocystis jiroveciil cells using Diff-Quick or Grocott staining of sputum/BALF.- - - Drugs to be recommended- - -ST combination drugs are used as a gold standard of PCP treatment. However, there have been a large number of patients in whom treatment was discontinued due to their side effects. Recently, atovaquone also became available in Japan as a second-choice drug. In patients with mild PCP, atovaquone tablets were as effective as ST combination drugs. In those with moderate PCP, ST combination drugs were more effective, but there was no significant difference due to a small number of subjects. In atovaquone-treated patients, the incidence of adverse events for which administration was discontinued was lower than in ST combination drug-treated patients, suggesting that the tolerance is high [184].
-
♦First choicesThe administration period should be 21 days in HIV-infected patients and 14 days in non-HIV-infected patients. A target daily dose of trimethoprim should be 15–20 mg/kg.
-
-ST combination drug, oral, 3 to 4 tablets/3 times a day
-
-ST combination drug, intravenous drip, 240–320 mg as trimethoprim/3 times a day (infused over 1–2 h)
-
-
-
<>Second choices
-
-Pentamidine, intravenous drip, 4 mg/kg/once a day (infused over 1–2 h)
-
-Atovaquone oral suspension, 5 mL (750 mg as atovaquone)/twice a day for 21 days (orally administered after meals)
-
∗Adjuvant therapy
-
∗
- In patients with a PaO2 of <70 mmHg or A-aDO2 of >35 mmHg in room air, one of the above drugs should be combined with a corticosteroid from the start of treatment. However, the dose may be reduced or administration may be discontinued in the early phase in accordance with symptoms. When the respiratory state is extremely unfavorable, pulse therapy should also be considered.
- Prednisolone Days 1–5: Oral, 30–40 mg/twice a day
- Days 6–10: Oral, 15–20 mg/twice a day
-
Days 11–21: Oral, 7.5–10 mg/twice a day- - - Precautions for each drug- - - (Confirm the package inserts.)
-
➀ST combination drug (Baktar tablets)Fever, exanthema, digestive symptoms, hepatopathy, nephropathy, and blood disorder may occur.This drug may interact with methotrexate, sulfadoxine, pyrimethamine, diaphenylsulfone, sulfonyl amide/sulfonylurea oral drugs for diabetes, warfarin, phenytoin, cyclosporin, zidovudine, digoxin, tricyclic antidepressants, and lamivudine.This drug is contraindicated for neonates, low-birth-weight infants, pregnant women, and patients with G-6-PD deficiency. In patients with renal dysfunction, dose reduction must be considered.
-
➁PentamidineSide effects such as hypoglycemia, hypotension, nephropathy, taste disorder, numbness of the tongue/lips, ventricular arrhythmia, exanthema, and fever may occur. Combination therapy with zalcitabine, PFA, or amiodarone is contraindicated. This drug is contraindicated for patients with severe ventilatory disturbance.
-
➂AtovaquoneNausea/vomiting, exanthema, and diarrhea may occur. This drug should be carefully administered to patients with severe kidney or liver dysfunction. This drug may interact with RFP, RBT, tetracycline, metoclopramide, zidovudine, acetaminophen, benzodiazepines, aciclovir, opioid analgesic drugs, cephalosporin antibiotics, antidiarrheal drugs/laxatives, and indinavir.
-
-
-
-
-
h.
Cytomegalovirus (CMV) pneumonia
- - - Executive summary- - --
-In the field of transplantation, preemptive treatment with GCV should be conducted through CMV antigenemia test monitoring.
-
-The efficacy of preemptive treatment with vGCV or PFA is similar to that of GCV.
-
-If a diagnosis of CMV pneumonia is made, treatment with GCV should be promptly started [185] (AII).
- -
-
-Combination therapy with an antiviral drug and high-dose immunoglobulin should be performed [188] (AIII).- - - Explanation- - -[Characteristic of the disease]
-
-Symptoms: In most healthy adults, latent infection persists after the initial infection with CMV during childhood. However, when cellular immunodeficiency occurs, this disease develops, leading to a severe condition. It frequently occurs after hematopoietic stem cell/organ transplantation or in patients with AIDS. Symptoms such as fever, general malaise, dry cough, dyspnea, and tachypnea are observed.
-
-Laboratory findings: Chest X-ray and CT show ground glass opacity extending from the pulmonary hilum to the bilateral sides. On CT, a microgranular shadow and thickening of the interlobular septa are sometimes observed. In the initial phase, there is no abnormal shadow on chest X-ray in about one-third of patients; caution is needed. Leukopenia, thrombopenia, atypical lymphocytes, and hypoxemia are noted. A definitive diagnosis is made by verifying inclusion cells, which are called owl's eyes, on histopathological examination. For clinical diagnosis, the antigenemia method is commonly used.
-
-In severely immunosuppressed patients, such as those after transplantation, empiric therapy is sometimes necessary.
-
-Specific condition: This disease causes retinitis, gastroenteritis, hepatitis, or encephalitis in some cases. Other types of opportunistic infection, such as Pneumocystis pneumonia, may concomitantly occur.- - - Antimicrobial drugs to be recommended- - -A first-choice drug for CMV pneumonia treatment is GCV, which has been frequently used. PFA has been used to treat CMV infection in AIDS patients, but experience on its use is limited in patients after hematopoietic stem cell transplantation.
-
(1)Initial administration
-
♦First choice
-
-GCV, intravenous drip, 5 mg/kg (over 1 h or more)/every 12 h for 2–3 weeks + anti-CMV high-titer gamma globulin, intravenous drip, 2.5–5 g/once a day for the first 3 days
-
-
-
<>Second choices
-
-PFA, intravenous drip, 60 mg/kg (over 1 h or more)/3 times a day, every 8 h for 2–3 weeks or more + anti-CMV high-titer gamma globulin, intravenous drip, 2.5–5 g/once a day for the first 3 daysor
-
-PFA, intravenous drip, 90 mg/kg (over 2 h or more)/twice a day, every 12 h for 2–3 weeks or more + anti-CMV high-titer gamma globulin, intravenous drip, 2.5–5 g/once a day for the first 3 days
-
-
-
♦
-
(2)Maintenance administration
-
♦First choices
-
-GCV, intravenous drip, 5 mg/kg (over 1 h or more)/once a day, 7 days a weekor
-
-GCV, intravenous drip, 6 mg/kg (over 1 h or more)/once a day, 5 days a week
- * This regimen should be completed after confirming the disappearance of clinical symptoms and negative reactions on two consecutive CMV antigenemia tests.
-
-
-
<>Second choice
-
-PFA, intravenous drip, 90–120 mg/kg (over 2 h or more)/once a day(In clinical practice in Japan, there have been few case reports on once-a-day administration at 120 mg/kg as maintenance therapy. A dose exceeding 120 mg/kg should be avoided. For administration at 120 mg/kg, twice-a-day administration at 60 mg/kg is commonly selected.)
-
* This regimen should be completed after confirming the disappearance of clinical symptoms and negative reactions on two consecutive CMV antigenemia tests.- - - Precautions for each drug- - - (Confirm the package inserts.)
-
-
-
➀GCVSevere leukopenia, neutropenia, anemia, thrombopenia, pancytopenia, aplastic anemia, and bone marrow suppression may occur. An animal experiment showed that this drug induced transient or irreversible spermatogenic dysfunction and reduced fertility. In humans, this drug may cause spermatogenic dysfunction. An animal experiment demonstrated the teratogenicity, mutagenicity, and carcinogenicity of this drug. In the presence of renal hypofunction, it is necessary to regulate the dose.This drug is contraindicated for patients with marked bone marrow suppression (neutrophil count: <500/mm3 or platelet count: <25,000/mm3) and pregnant women.It may interact with didanosine, zidovudine, IPM/CS, bone marrow-suppressing and kidney function-affecting drugs, zalcitabine, ST combination drugs, cyclosporin, probenecid, and mycophenolate mofetil.
-
➁VGCVThis is a prodrug of GCV.
-
➂PFAAcute renal failure, shock, heart failure, thrombophlebitis, and convulsion may occur.It is necessary to regulate the dose in accordance with the kidney function. Combination therapy with pentamidine is contraindicated. This drug is contraindicated for patients with a Ccr of <0.4 mL/min/kg.
-
♦
-
-
3. Pneumonia (Children)
3.1. Community-acquired pneumonia
- - - Executive summary- - -
For the treatment of community-acquired pneumonia in children, antimicrobial drugs should be selected, considering age and severity.
- - - Explanation- - -
[Characteristics and classification of the disease]
Patients with acute respiratory infectious disease symptoms, such as fever, nasal discharge, pharyngeal pain, and cough, and the appearance of a new infiltrative shadow in the lung on imaging examinations such as chest X-ray and CT are regarded as having pneumonia [189]. In patients with pneumonia, thoracic auscultation findings often include accessory murmurs and the attenuation of respiratory sounds. Most patients with respiratory infectious diseases consult hospitals with fever and cough. The lesion site of the airway is estimated based on symptoms and physical findings (Fig. 3 ) [189]. In addition to thoracic findings, it is necessary to check the presence or absence of dyspnea signs, such as tachypnea, nasal alar breathing, retractive breathing, shoulder breathing, orthopnea, groaning, and cyanosis. To consider the need of antimicrobial-drug administration and options of antimicrobial drugs, pneumonia is classified into three types: bacterial, viral, and atypical pneumonia based on causative microorganisms [189].
Fig. 3.
Differentiation of major childhood respiratory infectious diseases [189].
[Type and frequency of causative microorganisms]
Microorganisms that cause childhood community-acquired pneumonia differ among ages. According to the data on investigation of causative microorganisms based on lavage sputum culture in Japan, bacterial and viral pneumonia is frequent in infants/children aged 1 year or younger. In those aged 2–6 years, the incidences of bacterial, viral, and atypical pneumonia are similar. In those aged over 6 years, the incidence of atypical pneumonia is the highest [189] (Fig. 4 ). In a similar proportion of children with bacterial pneumonia, non-capsule H. influenzae and Streptococcus pneumoniae are involved, respectively. Mixed infection with viruses is often observed. In Europe and the United States, lavage sputum culture is not conducted, and data on causative microorganisms are insufficient. However, a review reported similar findings [191] (Table 11 ) [189]. In children, it is not easy to investigate causative microorganisms. In institutions in which it is impossible to investigate causative microorganisms, treatment must be performed based on the statistical frequency of causative microorganisms described below. However, in those in which investigation is possible, the etiology should be investigated if possible.
Fig. 4.
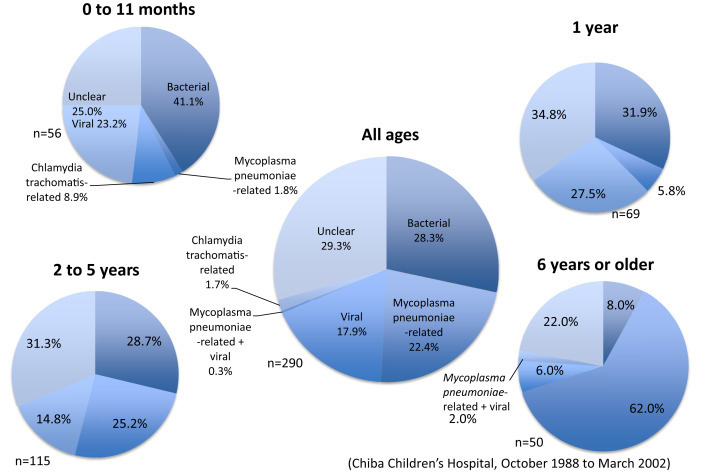
Causative microorganisms with respect to age in children with community-acquired pneumonia [190].
Table 11.
Age-related distribution of microorganisms that cause pneumonia in children [189].
| Immediately after birth to 20 days after birth | Streptococcus group B |
| Gram-negative enteric bacteria | |
| Cytomegalovirus | |
| Listeria monocytogenes | |
| 3 weeks–3 months | Chlamydia trachomatis |
| RS virus | |
| Parainfluenza virus 3 | |
| Pneumococcus | |
| Bordetella pertussis | |
| Staphylococcus aureus | |
| 4 months–4 years | RS virus |
| Parainfluenza virus | |
| Influenza virus | |
| Adenovirus | |
| Rhinovirus | |
| Pneumococcus | |
| Haemophilus influenzae | |
| Mycoplasma pneumoniae | |
| Mycobacterium tuberculosis | |
| 5–15 years | Mycoplasma pneumoniae |
| Chlamydia pneumoniae | |
| Pneumococcus | |
| Mycobacterium tuberculosis |
[Rules of antimicrobial drug therapy]
It is important to improve the efficacy of treatment by accurately predicting causative microorganisms and selecting an appropriate antimicrobial drug and its administration method. Whether or not an antimicrobial drug should be indicated must be comprehensively evaluated by differentiating bacterial, viral, and atypical pneumonia with reference to age, severity, clinical symptoms, physical findings, laboratory data, and X-ray findings [189]. As a rule, a single antimicrobial drug should be selected. When the type of microorganisms that caused pneumonia is identified, an antimicrobial drug should be selected through de-escalation, considering drug susceptibility and pharmacokinetics.
[Clinical symptoms, physical findings]
Wet cough and tachypnea are frequently observed in children with bacterial pneumonia. The proportion of labored breathing-free patients is high in children with Mycoplasma pneumonia [191], [192]. Auscultation findings include intermittent accessory murmurs (rales) regardless of the type of pneumonia. In children with Mycoplasma pneumonia, the proportion of those in whom auscultation findings are not marked is significantly higher than in other groups. In children with Chlamydia pneumonia, fever is mild, and cough is protracted. Thus, clinical symptoms and physical findings show characteristics related to causative microorganisms, but it is difficult to identify causative microorganisms based on clinical symptoms and physical findings alone in individual patients [193], [194], [195].
[Laboratory findings]
Concerning laboratory findings on admission in children with bacterial and viral pneumonia, there are significant differences in the leukocyte count, CRP level, and erythrocyte sedimentation rate between the two groups (p < 0.01). However, measurements overlapping in about one-third of patients are presented [195] (Fig. 5 ). Briefly, it is impossible to accurately differentiate bacterial from viral pneumonia based on inflammatory responses reflected by the leukocyte count, CRP level, and erythrocyte sedimentation rate. Mycoplasma pneumonia is characterized by increases in the CRP level and erythrocyte sedimentation rate, but many patients show normal leukocyte counts or a slight decrease in this parameter. Furthermore, it is difficult to differentiate Mycoplasma from viral pneumonia based on laboratory data [196].
Fig. 5.
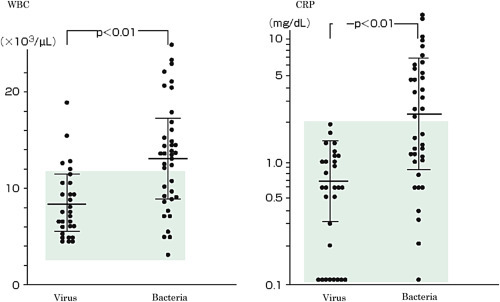
Inflammatory response on admission in children with pneumonia [196].
[Chest X-ray]
Chest X-ray findings show characteristics related to causative microorganisms to some degree, but it is difficult to identify causative microorganisms in individual patients [197].
[Classification of severity]
It is important to evaluate the severity of pneumonia, for selecting outpatient or hospital treatment and reviewing the necessity of antimicrobial drugs and route of administration (oral or intravenous). The classification of severity in the Guidelines for the Management of Respiratory Infectious Diseases in Children in Japan 2011 is presented [189] (Table 12 ). However, a consensus regarding the severity classification of childhood pneumonia has not been reached in Japan or internationally. This should be examined in the future.
Table 12.
Classification of the severity of community-acquired pneumonia in children [189].
| Mild | Moderate | Severe | |
|---|---|---|---|
| General condition | Favorable | Unfavorable | |
| Cyanosis | Absent | Present | |
| Respiratory rate | Normal | Polypnea | |
| Labored breathing∗1 (groaning, nasal alar breathing, retractive breathing) | Absent | Present | |
| Shadow on chest X-ray | 1/3 or smaller of the unilateral lung | 2/3 or larger of the unilateral lung | |
| Pleural effusion | Absent | Present | |
| SpO2 | >96% | <90%∗2 | |
| Circulatory failure | Absent | Present∗2 | |
| Artificial respiration management | Unnecessary | Necessary∗2 | |
| Criteria | Meeting all of the above criteria | Neither mild nor severe cases | ∗2: Meeting one of the above criteria |
Respiratory rate with respect to age (times/min): Neonates <60, Infants <50, Children <40, School children <30.
[Standards for outpatient/hospital treatment]
As a rule, outpatient treatment should be performed in mild-status patients evaluated according to the severity classification, and hospital treatment in mild-status patients with dehydration. In addition, it is necessary to determine admission when outpatient treatment does not lead to an improvement in symptoms or considering social adaptation [189] (Table 13 ).
Table 13.
Standards for admission due to community-acquired pneumonia in children [189].
|
[Initial antimicrobial drug therapy]
When examining children with pneumonia, treatment must be started without any precise information about causative microorganism in many cases. Basically, empiric therapy should be performed, considering the severity of pneumonia and causative microorganisms.
-
-
The type of causative microorganisms depends on age and severity. Therefore, the necessity of antimicrobial drugs should be examined, and selected, considering age and severity. In addition, bacterial, viral, or atypical pneumonia should be differentiated, and comprehensively evaluated in reference to clinical symptoms, physical findings, laboratory findings, and X-ray findings [189].
-
-
Recently, the number of drug-resistant strains of S. pneumoniae, H. influenzae, M. catarrhalis, and M. pneumoniae, which cause pneumonia, has increased [198], [199], [200], [201] (Fig. 6, Fig. 7) [189], [199]. For pneumonia treatment, it is important to perform antimicrobial drug therapy, considering resistant microorganisms.
-
-
When bacterial pneumonia is suspected, antimicrobial drug therapy that covers S. pneumoniae must be considered, as S. pneumoniae shows the strongest pathogenicity. Concerning treatment for S. pneumoniae pneumonia, resistance criteria have been established, assuming meningitis treatment. However, in January 2008, the Criteria for the Drug Susceptibility of S. pneumoniae, which were prepared by the Clinical and Laboratory Standards Institute (CLSI) (U.S.A.), were revised, and it was recommended that strains, isolated from S. pneumoniae-infected patients other than those with meningitis, with a PCG-MIC or AMPC-MIC of 2 μg/mL or less should be regarded as susceptible (Fig. 8) [203]. Currently, the susceptibility of S. pneumoniae isolated as causative microorganisms, that is, PCG-MIC, is 2 μg/mL or less in most strains. Briefly, the drug resistance of S. pneumoniae is not problematic in patients with respiratory infectious diseases. S. pneumoniae infection can be managed with standard-dose synthetic penicillins (AMPC, ABPC).
-
-
Concerning treatment for H. influenzae pneumonia, strains for which the MIC is evaluated as 1 μg/mL or less using the microliquid dilution method should be regarded as susceptible, those for which the MIC is 2 μg/mL as intermediate, and those for which the MIC is 4 μg/mL or more as resistant according to the Criteria for the ABPC Resistance of H. influenzae, which were prepared by the CLSI.
Fig. 6.
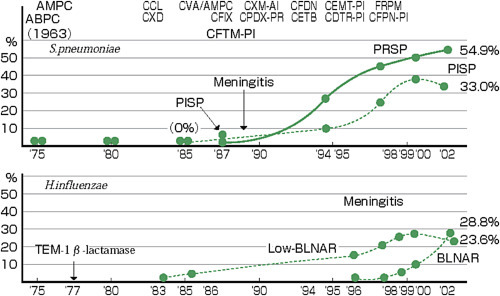
Increases in the number of resistant strains of S. pneumoniae and H. influenzae derived from respiratory infectious diseases [199].
Fig. 7.
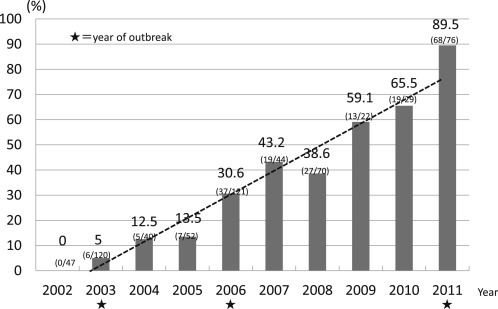
Increase in the number of macrolide-resistant M. pneumoniae strains [189].
Fig. 8.
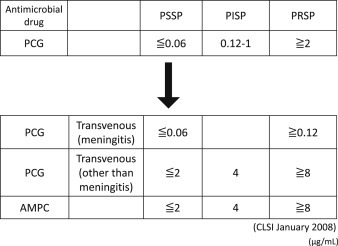
Changes in the criteria for the drug susceptibility of Pneumococcus established by the Clinical and Laboratory Standards Institute (CLSI) [203].
In particular, acute bronchitis/pneumonia related to intermediately susceptible H. influenzae (MIC: 2 μg/mL) can be managed with oral AMPC or ABPC intravenous injection therapies [204]. Recently, the number of ABPC-susceptible strains has annually decreased [198], [200]. The proportion of BLNAR strains (MIC: 4 μg/mL or more) has increased, raising an issue with respect to drug selection. When the involvement of BLNAR strains is suspected, high-dose AMPC or new oral cephems may be necessary at outpatient clinics [208].
The efficacy of outpatient antimicrobial drugs for BLNAR strains, which will increase in the future, should be carefully monitored. Concerning hospital treatment, the clinical effects of ABPC intravenous injection for 3–4 days until the results of a susceptibility test were clarified were investigated, and approximately 80% of patients responded to this therapy. There was no exacerbation in any patient [189]. In non-responders or patients in whom clinical effects are insufficient, it is necessary to switch the antimicrobial drug. PIPC, CTX, and CTRX have stable antimicrobial activities. When reviewing the clinical effects of PIPC on childhood bronchopulmonary infection, the response rate was 95%; the results were satisfactory [205].
-
-
Concerning treatment for M. catarrhalis pneumonia, M. catarrhalis produces β-lactamase. However, when examining the clinical course, AMPC is effective [205], [206]. This is because the enzymatic activity of β-lactamase produced by M. catarrhalis is low [207].
-
-
Concerning treatment for Mycoplasma pneumonia, a recent increase in the number of macrolide-resistant M. pneumoniae strains must be considered [202]. In Japan, an outbreak of M. pneumoniae infection occurred in mid-2011, and persisted until 2012. The outbreak involved a large number of patients with macrolide-resistant M. pneumoniae infection. Diagnosis and treatment were confused [209], [210]. Concepts regarding the diagnosis and treatment of childhood Mycoplasma pneumonia proposed by the Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children and Vaccination/Infection Control Committee, Japan Pediatric Society as of February 19, 2013 are presented [211] (Table 14).
Table 14.
Concepts regarding the diagnosis and treatment of childhood Mycoplasma pneumonia [211].
|
[Evaluation of the treatment response and administration period]
The administration period of antimicrobial drugs is shown in Table 15 [211].
Table 15.
Administration period of major antimicrobial drugs to be used for the treatment of Mycoplasma/Chlamydia pneumonia211).
| Antimicrobial drug | Administration period |
|---|---|
| Erythromycin ethylsuccinate | 14 days |
| Clarithromycin | 10 days |
| Azithromycin | 3 days |
| Tosufloxacin tosilate hydratea | 7–14 days |
| Minocycline | 7–14 days |
Indications for tosufloxacin tosilate granules for children include pneumonia. However, Mycoplasma pneumoniae is not included in bacterial types for which this preparation may be indicated.
To treat community-acquired pneumonia, antimicrobial drugs should be administered for 3–7 days. The treatment response should be evaluated after 2–3 days. In children, disease progression is often prompt, and the first evaluation should be performed after 2 days in younger and severe-status children, and not after 3 days [189]. If an improvement in clinical symptoms or laboratory data is achieved, the same antimicrobial drug should be continued until an appropriate antimicrobial drug and drug susceptibility are clarified. With respect to the administration period of antimicrobial drugs, factors such as the type of causative microorganisms and patient background differ among individual patients; therefore, it is difficult to establish standardized criteria. As M. pneumoniae and C. pneumoniae slowly proliferate, the treatment period is prolonged (Table 15). In patients infected with general bacteria, the administration of antimicrobial drugs can be discontinued 3 days after pyretolysis [189]. However, further antimicrobial drug therapy is necessary to treat S. aureus pneumonia.
[Management for non-responders to antimicrobial drugs]
When there are no therapeutic effects of antimicrobial drugs on pneumonia, whether or not a diagnosis of pneumonia is correct should be initially investigated [189]. The possibility of diseases other than pneumonia, with a pneumonia-like shadow, must be reviewed (Table 16 ). If it can be ruled out, whether or not the expected type of pathogenic microorganisms is correct should be examined. If the type of causative microorganisms is the same as expected, the possibility of resistant microorganisms should be considered. New therapeutic strategies should be devised carefully and promptly. When the condition further exacerbates despite treatment switching, additional examination should be conducted.
Table 16.
Conditions with pneumonia-like shadows related to factors other than pneumonia [189].
|
- - - Drugs to be recommended- - -
-
1.Empiric therapy
-
➀Two months after birth to 5 years
-
(1)Outpatient clinic (Mild)
-
♦First choices
- 1) Cases in which there is no risk of resistant bacteria
- - AMPC, oral, 10–15 mg/kg/3 times a day
- - SBTPC, oral, 10 mg/kg/3 times a day
- - CDTR-PI, oral, 3 mg/kg/3 times a day
- - CFPN-PI, oral, 3 mg/kg/3 times a day
- - CFTM-PI, oral, 3 mg/kg/3 times a day
- 2) Cases in which infection with resistant bacteria is suspected
- i) Two years or younger, ii) Pretreatment with an antimicrobial drug (within 2 weeks), iii) Concomitant development of otitis media, iv) History of pneumonia/repeated otitis media
- - AMPC, oral, 20–30 mg/kg/3 times a day
- - CVA/AMPC (1:14 preparation), oral, 48.2 mg/kg/twice a day
- - CDTR-PI, oral, 6 mg/kg/3 times a day
- - CFPN-PI, oral, 6 mg/kg/3 times a day
- - CFTM-PI, oral, 6 mg/kg/3 times a day
- 3) Cases in which onset/recurrence/recrudescence is observed despite previous treatment 2)
- - TBPM-PI, oral, 4–6 mg/kg/twice a day
- - TFLX, oral, 6 mg/kg/twice a day
-
<>Second choices
- - AZM, oral, 10 mg/kg/once a day for 3 days
- - CAM, oral, 7.5 mg/kg/twice a day
-
♦
-
(2)Admission (Moderate, general ward)
-
♦First choices
- - ABPC, intravenous injection or drip, 50 mg/kg/3 times a day
- - PIPC, intravenous injection or drip, 50 mg/kg/3 times a day
- - SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
- * When M. pneumoniae, Chlamydia trachomatis, or C. pneumoniae infection is strongly suspected, one of the above regimens should be combined with a macrolide [With respect to the administration method/dose, refer to the section “➁ Six years or older- - - (1) Outpatient clinic (Mild)”.].
-
<>Second choices
- - CTX, intravenous injection or drip, 40 mg/kg/3 times a day
- - CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
-
♦
-
(3)Admission (Severe, ICU)
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
- * When legionellosis cannot be ruled out, one of the above regimens should be combined with a macrolide [With respect to the administration method/dose, refer to the section “➁ Six years or older- - - (2) Admission (Moderate, general ward)”.].
-
-
-
(1)
-
➁Six years or older
-
(1)Outpatient clinic (Mild)
-
♦First choices
- - AZM, oral, 10 mg/kg/once a day for 3 days
- - CAM, oral, 7.5 mg/kg/twice a day
-
<>Second choices
- - AMPC, oral, 10–15 mg/kg/3 times a day
- - SBTPC, oral, 10 mg/kg/3 times a day
- - CDTR-PI, oral, 3 mg/kg/3 times a day
- - CFPN-PI, oral, 3 mg/kg/3 times a day
- - CFTM-PI, oral, 3 mg/kg/3 times a day
- - MINO, oral, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
-
♦
-
(2)Admission (Moderate, general ward)
-
♦First choices
- 1) Cases in which bacterial pneumonia is suspected
- - ABPC, intravenous injection or drip, 50 mg/kg/3 times a day
- - PIPC, intravenous injection or drip, 50 mg/kg/3 times a day
- - SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
- - CTX, intravenous injection or drip, 40 mg/kg/3 times a day
- - CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
- 2) Cases in which atypical pneumonia is suspected
- - AZM, oral, 10 mg/kg/once a day for 3 days
- - CAM, oral, 7.5 mg/kg/twice a day
- - EM, intravenous drip, 10 mg/kg/3–4 times a day
- - MINO, oral or intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
- 3) Cases in which it is impossible to differentiate bacterial from atypical pneumonia
-
♦
- One drug each should be selected from choices 1) and 2), and combined.
-
(3)Admission (Severe, ICU)
-
♦First choices
- - PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
- - MEPM, intravenous drip, 20 mg/kg/3 times a day
- - TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
- * When legionellosis cannot be ruled out, one of the above regimens should be combined with a macrolide [With respect to the administration method/dose, refer to the section “➁ Six years or older- - - (2) Admission (Moderate, general ward)”.].
-
♦
-
(1)
-
➀
-
2.Definitive therapy
-
➀S. pneumoniae
-
▪ PCG MIC ≤ 2 μg/mL
-
-AMPC, oral, 10–15 mg/kg/3 times a day
-
-ABPC, intravenous injection or drip, 30–50 mg/kg/3–4 times a day
-
-
-
▪ PCG MIC ≥ 4 μg/mL
-
-FRPM, oral, 10 mg/kg/3 times a day
-
-TBPM-PI, oral, 4–6 mg/kg/twice a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3 times a day
-
-CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
▪ PCG MIC ≤ 2 μg/mL
-
➁H. influenzae
-
▪ BLNAS
-
-AMPC, oral, 10–15 mg/kg/3 times a day
-
-ABPC, intravenous injection or drip, 50 mg/kg/3–4 times a day
-
-
-
▪ BLPAR
-
-CVA/AMPC (1:14 preparation), oral, 48.2 mg/kg/twice a day
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3 times a day
-
-CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
-
-
-
▪ BLNAR
-
-CDTR-PI, oral, 6 mg/kg/3 times a day
-
-TFLX, oral, 6 mg/kg/twice a day
-
-PIPC, intravenous injection or drip, 50 mg/kg/3 times a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3 times a day
-
-CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
-
-
-
▪ BLPACR
-
-CDTR-PI, oral, 6 mg/kg/3 times a day
-
-TFLX, oral, 6 mg/kg/twice a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3 times a day
-
-CTRX, intravenous injection or drip, 25–60 mg/kg/once to twice a day (50–60 mg/kg/day)
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-
-
▪ BLNAS
-
➂M. catarrhalis
-
-CVA/AMPC (1:14 preparation), oral, 48.2 mg/kg/twice a day
-
-AZM, oral, 10 mg/kg/once a day for 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
-
-
-
➃Streptococcus pyogenes (Group A β-Streptococcus)
-
-PCG, intravenous drip, 50,000 units/kg/4 times a day
-
-ABPC, intravenous injection or drip, 30–50 mg/kg/3–4 times a day
-
-
-
➄Staphylococcus aureus
-
▪ MSSA
-
-ABPC/MCIPC, intravenous injection or drip, 40 mg/kg/3 times a day
-
-CEZ, intravenous injection or drip, 30 mg/kg/3 times a day
-
-
-
▪ MRSA
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-LZD, intravenous drip or oral, 10 mg/kg/every 8 h, 3 times a day
-
-
-
▪ MSSA
-
➅Bordetella pertussis
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day for 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-PIPC, intravenous injection or drip, 50 mg/kg/3 times a day
-
-
-
➆Legionella
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day for 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-EM, intravenous drip, 10 mg/kg/3–4 times a day
-
-
-
➇M. pneumoniae
-
▪Macrolide-susceptible
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day for 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-EM, intravenous drip, 10 mg/kg/3–4 times a day
-
-
-
▪Macrolide-resistant
-
-MINO, oral or intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
-
-TFLX, oral, 6 mg/kg/twice a day
-
-
-
▪
-
➈Chlamydia (C. trachomatis, C. pneumoniae, Chlamydia psittaci)
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day for 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-EM, intravenous drip, 10 mg/kg/3–4 times a day
-
-MINO, oral or intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
-
-
-
➀
3.2. Hospital-acquired pneumonia (including ventilator-associated pneumonia: VAP)
- - - Executive summary- - -
For the treatment of hospital-acquired pneumonia in children, antimicrobial drugs should be selected, considering severity and the involvement of resistant bacteria. Empiric therapy should be started by combining two drugs if necessary, considering various resistant microorganisms, differing from that for community-acquired pneumonia (BIII).
[Characteristics and classification of the disease]
Hospital-acquired pneumonia is defined as pneumonia that newly develops 48 h or more after admission. Ventilator-associated pneumonia is defined as pneumonia that develops 48 h or more after endotracheal intubation [211]. These conditions may become severe due to an underlying disease, reduced immune capacity, or the deterioration of the general condition, and are caused by drug-resistant microorganisms in many cases; treatment is difficult in most cases.
[Type and frequency of causative microorganisms]
Not only microorganisms acquired in the community but also those existing in the hospital environment cause hospital-acquired pneumonia in children, as reported in the adult field. Bacteria that cause community-acquired pneumonia (S. pneumoniae, H. influenzae), enteric bacteria (E. coli, K. pneumoniae), S. aureus, non-glucose-fermenting bacteria, such as P. aeruginosa, and Acinetobacter spp., and anaerobes cause hospital-acquired pneumonia [212]. In addition, not only general bacteria but also fungus and viruses sometimes cause hospital-acquired pneumonia in patients with immunodeficiency. Among patients with nosocomial infection, causative microorganisms differ, and drug-resistant microorganisms are involved in many cases. In children, it is not easy to investigate causative microorganisms, but, if drug susceptibility is clarified, it contributes to successful treatment. Therefore, lavage or aspiration sputum culture should be conducted to investigate the etiology, if possible [189], [211].
[Rules of antimicrobial drug therapy]
Basically, empiric therapy should be performed, considering the severity of pneumonia, an underlying disease, and causative microorganisms. In particular, the involvement of drug-resistant microorganisms, such as MRSA, extended-spectrum β-lactamase (ESBL)-producing bacteria, and multi-drug-resistant P. aeruginosa (MDRP), must always be considered for treatment. Empiric therapy should be started by combining two drugs if necessary, considering various resistant microorganisms, differing from that for community-acquired pneumonia [211]. As the state of resistant bacteria differs among institutions, antimicrobial drug options should be customized based on records on antimicrobial drug susceptibility (antibiograms) at each institution.
A consensus regarding the administration period of antimicrobial drugs has not been reached. With respect to the administration period of antimicrobial drugs, factors such as the type of causative microorganisms and patient background differ among individual patients with nosocomial infection; therefore, it is difficult to establish standardized criteria. However, when complications such as severe immunodeficiency, pulmonary suppuration, lung abscess, and pleuritis are absent, antimicrobial drugs should be administered for 5 days after pyretolysis (7–10 days) [50]. Considering an underlying disease or the immune state, flexible management must be performed. In children, disease progression is often prompt, and the first evaluation should be performed after 2 days in younger and severe-status children, and not after 3 days [189]. If an improvement in clinical symptoms or laboratory data is achieved, the same antimicrobial drug should be continued until an appropriate antimicrobial drug and drug susceptibility are clarified. When the type of microorganisms that caused pneumonia is identified, a target-focused antimicrobial drug should be selected through de-escalation, considering drug susceptibility and pharmacokinetics [211].
Concerning multi-drug-resistant microorganisms, it is important to promote standard preventive strategies and those to control nosocomial infection, such as the prevention of droplet/contact infection, thoroughly. Furthermore, oral care and devised postures (if there is no medical contraindication, the head should be lifted at 30–45°.) are necessary to prevent VAP [211].
In this article, the classification of severity (Table 12, p. 30) in the Guidelines for the Management of Respiratory Infectious Diseases in Children in Japan 2011 was used [189] (Refer to the section “3. Pneumonia (Children), 3.1 Community-acquired pneumonia”.).
- - - Drugs to be recommended- - -
-
1.Empiric therapy
-
(1)Mild (General ward)
-
♦First choices
-
-CAZ, intravenous injection or drip, 50 mg/kg/3 times a day
-
-CZOP, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CPR, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-
- If necessary, one of the above regimens should be combined with one of the following drugs:
-
1)Cases in which anaerobe infection is suspected (such as aspiration pneumonia)
-
-CLDM, intravenous drip, 10 mg/kg/3 times a day
-
-
-
2)Cases in which MRSA infection is suspected
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-
-
♦
-
(2)Moderate (General ward, including VAP)
-
♦First choices
-
-CAZ, intravenous injection or drip, 50 mg/kg/3 times a day
-
-CZOP, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CPR, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-
-
+one of the followings:
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-
-
♦
-
(3)Severe (ICU, including VAP)
-
♦First choices
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-
-
+one of the followings:
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-
-
♦
-
(1)
[Note] When legionellosis cannot be ruled out in the severest cases, one of the above regimens should be combined with a macrolide (With respect to the administration method/dose, refer to the section “3.1 Community-acquired pneumonia- - - ➁ Six years or older- - - (2) Admission (Moderate, general ward)” (p. 33).
-
2.Definitive therapy
-
➀Enteric bacteria (E. coli, K. pneumoniae, Proteus spp.)
-
♦Non-ESBL-producing bacteria
-
-CTX, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CTRX, intravenous injection or drip, 50 mg/kg/twice a day
-
-
-
▪ESBL-producing bacteria
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
♦
-
➁Enterobacter spp., Serratia spp., Citrobacter spp.
-
-CPR, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CFPM, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
➂P. aeruginosa
-
-CAZ, intravenous injection or drip, 50 mg/kg/4 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a dayIf necessary, one of the above regimens should be combined with one of the following drugs:
-
-AMK, intravenous drip, 5–7.5 mg/kg/twice a day
-
-TOB, intravenous drip, 2–3 mg/kg/twice a day
-
-
-
➃Stenotrophomonas maltophilia
-
-ST combination drug, intravenous drip or oral, SMX 25 mg/TMP 5 mg/kg/3–4 times a day
-
-MINO, intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
-
-
-
➄Acinetobacter baumannii
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3–4 times a day
-
-
- [Note] If there is no susceptibility, combination therapy with aminoglycosides should be reviewed using the checker-board method. CL administration should also be considered.
-
➅S. aureus
-
▪MSSA
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3–4 times a day
-
-CEZ, intravenous injection or drip, 50 mg/kg/3 times a day
-
-
-
▪MRSA
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-LZD, intravenous drip or oral, 10 mg/kg/every 8 h, 3 times a day
-
-
-
▪
-
➆Anaerobes
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-CLDM, intravenous drip, 10 mg/kg/3 times a day
-
-
-
➀
3.3. Pneumonia in the presence of immunodeficiency/blood diseases
- - - Executive summary- - -
In children with immunodeficiency-/blood disease-related pneumonia, antimicrobial drugs should be selected, considering an underlying disease, the grade of immunodeficiency, and involvement of various causative microorganisms. Initial antimicrobial drug therapy should be started by combining two drugs if necessary, considering various causative microorganisms, differing from that for community-acquired pneumonia (BIII).
[Characteristics and classification of the disease]
As immunodeficiency-/blood disease-related pneumonia in children often develops in hospitals, it has the characteristics of hospital-acquired pneumonia in many cases. It may become severe due to the patient's unfavorable conditions, such as an underlying disease, reduced immune capacity, and the deterioration of the general condition. Even non-pathogenic microorganisms may cause pneumonia in many cases. Furthermore, drug-resistant microorganisms often cause pneumonia; treatment is difficult in many cases [213], [214]. To achieve multidisciplinary, comprehensive treatment to save children, it is necessary to cooperate with other special fields.
[Type and frequency of causative microorganisms]
Not only microorganisms acquired in the community in the presence of various immunodeficiency states but also non-pathogenic microorganisms existing in the hospital environment cause immunodeficiency-/blood disease-related pneumonia in children. Bacteria that cause community-acquired pneumonia (S. pneumoniae, H. influenzae), enteric bacteria (E. coli, K. pneumoniae), S. aureus, non-glucose-fermenting bacteria, such as P. aeruginosa, and Acinetobacter spp., and anaerobes cause this type of pneumonia. In addition, not only general bacteria but also fungus and viruses often cause this type of pneumonia. Furthermore, drug-resistant microorganisms are involved in many cases, as indicated for hospital-acquired pneumonia [213], [214].
[Type of immunodeficiency, causative microorganisms to be monitored, and precautions for diagnosis]
-
➀
Humoral immunodeficiency: As bacterial opsonization and complement activation are affected, patients with humoral immunodeficiency are prone to be infected with general bacteria. Among immunodeficiency patients with hyper-IgM-emia, Pneumocystis pneumonia should be considered in those with conditions related to CD40 ligand abnormalities.
-
➁
Cellular immunodeficiency: In addition to infection with general bacteria, infection with intracellular parasitic bacteria, fungus, or Protozoa may become severe, and be protracted. As the differentiation and induction of B and killer T cells by CD4-positive lymphocytes are affected, the eradication of virus-infected cells is inhibited (Table 17) [215].
Table 17.
Rate of decrease in the number of CD4-positive lymphocytes and risk of infection with causative microorganisms [214].CD4-positive Causative microorganisms 200–500/μL General bacteria, Mycobacterium tuberculosis 50–199/μL Cryptococcus, Toxoplasma ≤49/μL Cytomegalovirus, nontuberculous Mycobacteria -
➂
Neutrophil abnormalities: Neutrophil abnormalities are classified into two types: neutropenia and neutrophil functional disorders. Patients with a peripheral blood absolute neutrophil count (ANC) of <500/μL or those in whom the ANC is estimated to reach <500/μL within 48 h are regarded as having neutropenia [215]. Of these, the risk is higher in patients with an ANC of 100/μL or less in whom the period is estimated to exceed 7 days. Many patients with neutropenia do not show purulent sputum or abnormal findings on chest X-ray even in the presence of pneumonia. Therefore, when fever persists, thoracic CT should be performed in the early stage. All microorganisms including general bacteria (gram-positive bacteria, gram-negative bacteria), fungus, and viruses may cause pneumonia. In particular, in addition to neutropenia early after homologous hematopoietic stem cell transplantation, infection-prone features associated with humoral/cellular immunodeficiency related to the administration of immunosuppressives persist over a long period. Furthermore, the concomitant development of acute/chronic graft-versus-host disease (GVHD) is a risk factor for the onset of pneumonia. In addition, non-infectious pulmonary disorder related to drugs/radiation for pretreatment may occur, and it is important to differentiate it from infectious diseases (Fig. 9) [214], [216].
A representative neutrophil functional disorder, chronic granulomatosis, induces active oxygen production disorder of neutrophils, affecting bactericidal actions. Therefore, patients with this disorder are prone to be infected with non-H2O2-producing catalase-positive bacteria (S. aureus, K. pneumoniae, E. coli, Candida spp., Aspergillus spp.).
-
➃
Complement deficiency: Patients with complement deficiency are prone to be infected with bacteria with capsules, such as S. pneumoniae, H. influenzae (capsule strains), and Neisseria meningitidis [214].
Fig. 9.
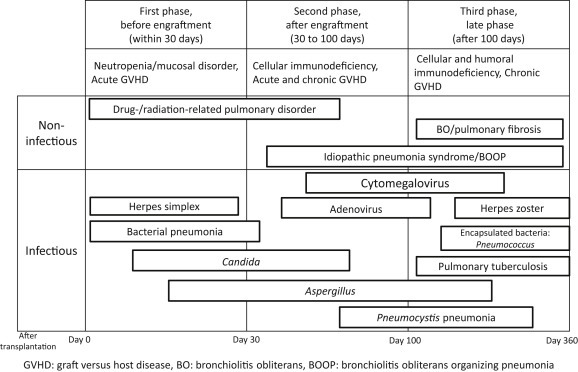
Pulmonary complications after homologous hematopoietic stem cell transplantation [213].
In children, it is not easy to investigate causative microorganisms, but, if drug susceptibility is clarified, it contributes to successful treatment. Therefore, various cultures should be conducted to investigate the etiology, if possible. In addition, testing of various antigens, such as urinary S. pneumoniae/Legionella antigens, β-D-glucan, Aspergillus antigen, Cryptococcus antigen, Candida antigen, and Cytomegalovirus antigen, and tests using nucleic acid amplification methods, such as the PCR method, should be utilized, if possible.
[Rules of antimicrobial drug therapy]
Initial antimicrobial drug therapy should be performed, considering the severity of pneumonia and an underlying disease. For the treatment of immunodeficiency-/blood disease-related pneumonia in children, antimicrobial drug therapy should also be basically selected, considering causative microorganisms. As described for nosocomial infection, the involvement of drug-resistant microorganisms, such as MRSA, extended-spectrum β-lactamase (ESBL)-producing bacteria, and multi-drug-resistant P. aeruginosa (MDRP), must always be considered for treatment. Initial antimicrobial drug therapy should be started by combining two drugs, if necessary, considering various resistant microorganisms, differing from that for community-acquired pneumonia [213], [214]. As the state of resistant bacteria differs among institutions, antimicrobial drug options should be customized based on records on antimicrobial drug susceptibility (antibiograms) at each institution.
A consensus regarding the administration period of antimicrobial drugs has not been reached. With respect to the administration period of antimicrobial drugs, factors such as the type of causative microorganisms and patient background differ among individual patients with nosocomial infection; therefore, it is difficult to establish standardized criteria. In children, disease progression is often prompt, and the first evaluation should be performed after 2 days in younger and severe-status children, and not after 3 days. If an improvement in clinical symptoms or laboratory data is achieved, the same antimicrobial drug should be continued until an appropriate antimicrobial drug and drug susceptibility are clarified. When the type of microorganisms that caused pneumonia is identified, a target-focused antimicrobial drug should be selected through de-escalation, considering drug susceptibility and pharmacokinetics [189].
Monitoring culture of the airway is useful for treating immunodeficiency-/blood disease-related pneumonia in children [214].
General preventive methods are presented in Table 18 [213]. In addition, long-term low-dose macrolide therapy or the intermittent administration of β-lactams to prevent P. aeruginosa fixation is effective in some patients with chronic bronchitis [217], [218]. In those with chronic granulomatosis, the oral administration of ITCZ (4–6 mg/kg/day, maximum: 100 mg/day) or subcutaneous injection of IFN-γ (250,000 domestic standard units/m2, 1–3 times a week) is useful for preventing infection [219].
Table 18.
Prevention of immunodeficiency-/blood disease-related pneumonia in children [213].
| Pathogen | Prevention |
|---|---|
| Bacteria | Sterilization of the intestinal tract with polymyxin B In children, there is no evidence regarding the usefulness of prevention with new quinolones. |
| Fungus | In the presence of severe neutropenia (100/μL or less), the preventive administration of fluconazole should be performed. Use of HEPA filters ST combination drugs for Pneumocystis pneumonia |
| Virus | Compliance with standard preventive strategies The administration of gamma-globulin preparations should be considered using a serum IgG level of 400 mg/dL or less as an index. |
In this article, the classification of severity (Table 12, p. 30) in the Guidelines for the Management of Respiratory Infectious Diseases in Children in Japan 2011 was used (Refer to the section “3. Pneumonia (Children), 3.1 Community-acquired pneumonia”.).
- - - Drugs to be recommended- - -
-
1.Empiric therapy
-
(1)Pneumonia related to mild immunodeficiency in the initial phase after admission
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3–4 times a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CTRX, intravenous injection or drip, 50 mg/kg/twice a day
-
-
-
(2)Pneumonia related to moderate/severe immunodeficiency
-
-CAZ, intravenous injection or drip, 50 mg/kg/3–4 times a day
-
-CZOP, intravenous injection or drip, 50 mg/kg/3–4 times a day
-
-CPR, intravenous injection or drip, 50 mg/kg/3–4 times a dayIn severe cases,
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a dayWhen there is no response,
-
-AMK, intravenous drip, 5–7.5 mg/kg/twice a day
-
-TOB, intravenous drip, 3.3 mg/kg/3 times a day
-
-
-
(1)
If necessary, an antifungal drug (MCFG) and ST combination drug should be additionally administered.
When Aspergillus infection is suspected, treatment should be started with VRCZ or L-AMB instead of MCFG.
In patients with cellular immunodeficiency, one of the above drugs should be combined with MCFG and an ST combination drug in the early stage.
In those with neutrophil abnormalities, one of the above drugs should be combined with MCFG in the early stage.
When legionellosis cannot be ruled out in the severest cases, one of the above regimens should be combined with a macrolide (With respect to the administration method/dose, refer to the section “3.1 Community-acquired pneumonia- - - ➁ Six years or older- - - (2) Admission (Moderate, general ward)” (p. 32).).
-
2.Definitive therapy
-
➀Enteric bacteria (E. coli, K. pneumoniae, Proteus spp.)
-
▪Non-ESBL-producing bacteria
-
-CTX, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CTRX, intravenous injection or drip, 50 mg/kg/twice a day
-
-
-
▪ESBL-producing bacteria
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
▪
-
➁Enterobacter spp., Serratia spp., Citrobacter spp.
-
-CPR, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CFPM, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
➂S. aureus
-
▪MSSA
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3–4 times a day
-
-CEZ, intravenous injection or drip, 50 mg/kg/3 times a day
-
-
-
▪MRSA
-
-VCM, intravenous drip, 15 mg/kg/3 times a day
-
-TEIC, intravenous drip, 10 mg/kg/every 12 h, 3 times, subsequently: 6–10 mg/kg/once a day
-
-ABK, intravenous drip, 4–6 mg/kg/once a day
-
-LZD, intravenous drip or oral, 10 mg/kg/every 8 h, 3 times a day
-
-
-
▪
-
➃P. aeruginosa
-
-CAZ, intravenous injection or drip, 50 mg/kg/4 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-AMK, intravenous drip, 5–7.5 mg/kg/twice a day
-
-TOB, intravenous drip, 2–3 mg/kg/twice a day
-
-
-
➄S. maltophilia
-
-ST combination drug, intravenous drip or oral, SMX 25 mg/TMP 5 mg/kg/3–4 times a day
-
-MINO, intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders to other drugs.)
-
-
-
➅A. baumannii
-
-IPM/CS, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3–4 times a day
-
-
- [Note] If there is no susceptibility, combination therapy with aminoglycosides should be reviewed using the checker-board method. CL administration should also be considered.
-
➆Anaerobes
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-CLDM, intravenous drip, 10 mg/kg/3 times a day
-
-
-
➇Pneumocystis jirovecii
-
-ST combination drug, intravenous drip or oral, SMX 25 mg/TMP 5 mg/kg/3–4 times a day
-
-Pentamidine, intravenous drip, 4 mg/kg/once a day
-
-
-
➈C. neoformans, FLCZ-susceptible Candida (primarily C. albicans)
-
-FLCZ, intravenous drip, 10–12 mg/kg/once a day
-
-
-
➉Aspergillus, FLCZ-resistant Candida
-
-MCFG, intravenous drip, 3–6 mg/kg/once a day
-
-VRCZ, intravenous drip, 9 mg/kg/twice a day on Day 1, 8 mg/kg/twice a day on Day 2 or later. During the administration period, the blood concentration of this drug should be monitored.
-
-L-AMB, intravenous drip, 2.5–5 mg/kg/once a day
-
-
-
⑪Cytomegalovirus
-
-GCV, intravenous drip, 10 mg/kg/twice a day
-
-
-
➀
[Note] In the presence of GCV resistance, the intravenous drip of PFA (foscarnet), 60 mg/kg/3 times a day should be performed.
3.4. Neonatal pneumonia
- - - Executive summary- - -
In the treatment of neonatal pneumonia, antimicrobial drugs should be selected after differentiating congenital from acquired pneumonia.
Initial antimicrobial drug therapy should be started by combining two drugs, regarding the condition as severe systemic infection and considering various causative microorganisms. The administration method and dose should be selected based on the age (days) and birth weight (BIII).
[Characteristics and classification of the disease]
The incidence of neonatal pneumonia is not high. According to Sakata, pneumonia occurred in only 40 (0.25%) of approximately 16,000 neonates who were admitted during a 10-year period [220]. Neonatal pneumonia is frequently observed as a portion of systemic infection represented by sepsis, and not as a single condition. It is classified into two types: congenital and acquired pneumonia [221].
In most cases, neonatal pneumonia does not cause any typical symptoms such as fever or cough in comparison with infantile/childhood pneumonia. The neonatal protective capacity against infection is physiologically immature, often leading to a severe condition. In the neonatal phase, artificial respiration management is performed in many cases, and ventilator-associated pneumonia (VAP) is frequently observed [222], [223], [224]. In addition, many neonates are admitted to the neonatal intensive care unit (NICU) for a long period; therefore, the incidence of nosocomial infection is high.
[Type and frequency of causative microorganisms]
Several types of congenital pneumonia include transplacental infection, intrauterine infection related to aspiration of infected amniotic fluid on premature rupture, and birth canal infection with vaginal discharge on delivery. These types of congenital pneumonia are primarily caused by various viruses (Cytomegalovirus, herpes simplex virus), bacteria (Streptococcus agalactiae (GBS), E. coli, Listeria monocytogenes), Chlamydia, and fungus.
The clinical onset of perinatal pneumonia is frequently observed immediately to 7 days after birth [221].
Postnatal pneumonia usually develops 2 weeks or more after birth. It is caused by viruses and bacteria. Viral infection may occur when RS virus, parainfluenza virus, or adenovirus prevails in the ward. However, it rarely leads to the onset of pneumonia. Frequent causative bacteria include S. aureus, E. coli, P. aeruginosa, Acinetobacter spp., Enterobacter spp., and Legionella spp., which exist in the environment. These often cause nosocomial infection [221]. In premature babies, Gram staining of tracheal aspirate samples is useful for selecting antimicrobial drugs to be administered in the initial phase [225].
In children, it is not easy to investigate causative microorganisms, but, if drug susceptibility is clarified, it contributes to successful treatment. Therefore, blood or bronchial lavage fluid culture should be conducted to investigate the etiology, if possible.
[Rules of antimicrobial drug therapy]
As many neonates have systemic infection in an immunodeficiency state, the same administration method and dose as selected for the treatment of sepsis should be used [221]. Therefore, the severity of pneumonia is not considered. When bacterial pneumonia is suspected, combination therapy with ABPC and CTX is commonly selected as initial treatment. Considering L. monocytogenes infection, combination therapy with ABPC is recommended. After causative microorganisms are identified, antimicrobial drugs should be selected with reference to their drug susceptibility. In cases of VAP, antimicrobial drug options should be customized based on records on the drug susceptibility (antibiograms) of microorganisms that cause nosocomial infection at each institution.
In neonates, there are marked age-related differences in the pharmacokinetics of antimicrobial drugs; therefore, these drugs must be carefully administered in the neonatal phase. Concretely, the same dose as established in the field of pediatrics should be used, and the administration interval should be prolonged in accordance with age [221]. In cases of severe asphyxia or acute renal failure, the administration interval must be further prolonged. It should be shortened with an improvement in the kidney function [226], [227], [228], [229].
A consensus regarding the administration period of antimicrobial drugs has not been reached. However, the standard administration period is 14 days. With respect to the administration method/dose, refer to the section “XI. Doses for neonates”.
In neonates, disease progression is often prompt, and the treatment response should be evaluated after 2 days, and not after 3 days. If an improvement in clinical symptoms or laboratory data is achieved, the same antimicrobial drug should be continued until an appropriate antimicrobial drug and drug susceptibility are clarified. When the type of microorganisms that caused pneumonia is identified, a target-focused antimicrobial drug should be selected through de-escalation, considering drug susceptibility and pharmacokinetics [189].
- - - Drugs to be recommended- - -
-
1.
Empiric therapy
[0 days–4 weeks after birth]-
(1)Non-hospital-acquired pneumonia
-
♦First choice
-
-ABPC, intravenous injection or drip
- +
-
-CTX, intravenous injection or drip
-
-
-
<>Second choices
-
-ABPC, intravenous injection or drip
-
-
-
+one of the followings:
-
-GM, intravenous drip
-
-AMK, intravenous drip
-
-
-
♦
-
(2)Hospital-acquired pneumonia (including VAP)
-
♦First choices
-
-CAZ, intravenous injection or drip
-
-CZOP, intravenous injection or drip
-
-
-
+one of the followings:
-
-GM, intravenous drip
-
-AMK, intravenous drip
-
-TOB, intravenous drip
-
-ABK, intravenous drip (cases in which the involvement of MRSA is suspected)
-
-
-
<>Second choices
-
-MEPM, intravenous drip
-
-
-
+one of the followings:
-
-VCM, intravenous drip
-
-TEIC, intravenous drip
-
-ABK, intravenous drip (cases in which the involvement of MRSA is suspected)
-
-
-
♦
-
(1)
-
2.Definitive therapy
-
➀S. agalactiae
-
▪ABPC-susceptible bacteria
-
-ABPC, intravenous injection or drip
-
-
-
▪Moderately ABPC-resistant bacteria
-
-ABPC, intravenous injection or drip + GM or AMK, intravenous drip
-
-MEPM, intravenous drip
-
-
-
▪
-
➁Enteric bacteria (E. coli, K. pneumoniae)
-
-CTX, intravenous injection or drip
-
-MEPM, intravenous drip (ESBL-producing bacteria)
-
-
-
➂MSSA
-
-CEZ, intravenous injection or drip
-
-
-
➃MRSA
-
-VCM, intravenous drip
-
-TEIC, intravenous drip
-
-ABK, intravenous drip
-
-
-
➄Listeria spp.
-
-ABPC, intravenous injection or drip
-
-MEPM, intravenous drip
-
-
-
➅Enterococcus
-
-ABPC, intravenous injection or drip ± GM or AMK, intravenous drip
-
-VCM, intravenous drip
-
-
-
➆P. aeruginosa
-
-CAZ, intravenous injection or drip
-
-GM, intravenous drip
-
-AMK, intravenous drip
-
-TOB, intravenous drip
-
-MEPM, intravenous drip
-
-
-
➇C. trachomatis
-
-EM, oral
-
-
-
➀
4. Pyothorax
4.1. Adults
- - - Executive summary- - -
-
-
Pyothorax refers to a condition in which pus accumulates in the thoracic cavity. Usually, pleural effusion puncture is performed, and a diagnosis of pyothorax is made based on the results of various examinations, such as (macroscopic) purulent pleural effusion, microorganisms detected on Gram staining or culture of pleural effusion, or pleural effusion pH: <7.2 (BIII).
-
-
In patients with acute pyothorax related to community-acquired pneumonia, treatment should be performed in accordance with that for community-acquired pneumonia, considering microorganisms that cause community-acquired pneumonia, such as Streptococcus pneumoniae (BIII).
-
-
In patients with slowly progressing pyothorax, mixed infection with oral aerobes/anaerobes is frequently observed. Combination therapy with PCG or ABPC and CLDM or MNZ, which show anti-anaerobe activities, or therapy with a single antimicrobial drug with an anti-anaerobe activity, such as SBT/ABPC, should be selected (BIII).
-
-
When there is a risk of multi-drug-resistant bacteria, monotherapy with a carbapenem, combination therapy with a fourth-generation cephalosporin and CLDM or MNZ, and combination therapy with a quinolone and CLDM or MNZ should be considered, assuming ESBL-producing enteric bacteria, resistant P. aeruginosa, anaerobes, and Acinetobacter (BIII).
-
-
If the results of culture/susceptibility tests are clarified, antimicrobial drugs should be changed in accordance with them (AIII).
-
-
The penetration of aminoglycosides to the thoracic cavity is poor, and their activities reduce when the pH is low. Therefore, the use of aminoglycosides should be avoided as a rule (BIII).
-
-
If a diagnosis of pyothorax is made, the administration of an appropriate antimicrobial drug should be started, and drainage must be performed (AII). If possible, the attending physician should consult a surgeon in the early stage (AIII).
-
-
In some patients with marked pleural thickening or multilocular pleural effusion, thoracoscopic debridement is necessary (BIII). In addition, a fibrinolytic drug, such as streptokinase, is administered through a thoracic drain, or surgical interventions such as thoracotomy or decortication is performed in some cases (BIII).
- - - Explanation- - -
[Diagnosis]
-
-
Pyothorax is defined as a condition in which pus accumulates in the thoracic cavity, but this definition has no diagnostic objectivity. For this reason, usually, pleural effusion puncture is performed, and a diagnosis of pyothorax is made based on the results of various examinations, such as (macroscopic) purulent pleural effusion, microorganisms detected on Gram staining or culture of pleural effusion, or pleural effusion pH: <7.2 [230], [231].
[Causative microorganisms]
-
-
Pleural effusion appears in 30–40% of patients with bacterial pneumonia, but leads to pyothorax in 0.5–2%. Other etiological factors for pyothorax include surgery, trauma, and esophageal perforation.
-
-
Microorganisms that cause pyothorax depend on its etiology and course. In the presence of bacterial pneumonia, pyothorax is caused by the same microorganisms as caused bacterial pneumonia. Furthermore, acute pyothorax is frequently caused by S. pneumoniae and S. aureus. However, in many patients with chronic pyothorax, mixed infection primarily with anaerobes is involved. Among anaerobes, Fusobacterium spp. (especially Fusobacterium nucleatum), Prevotella spp., Peptostreptococcus spp., and Bacteroides spp. are frequently detected [232], [233], [234]. According to recent studies, the detection rate of the Streptococcus anginosus group is high [235], [236].
-
-
In many cases, pyothorax with a relatively slow course is associated with M. tuberculosis. It must be considered that pulmonary lesions do not always concurrently exist with tuberculous pleuritis.
[Treatment]
-
-
No randomized comparative study regarding antimicrobial drug treatment for pyothorax has been conducted. An antimicrobial drug with an activity against microorganisms expected or obtained on culture should be selected and administered. In acute-onset patients in whom there is no risk of resistant bacteria, for example, those with pyothorax accompanying community-acquired pneumonia, antimicrobial drugs such as PCG and ABPC should be initially selected, considering S. pneumoniae. These drugs simultaneously cover Fusobacterium, Peptostreptococcus, and the viridans Streptococcus group. However, Prevotella and Bacteroides produce β-lactamase; therefore, when the results of culture are not obtained, combination therapy with antimicrobial drugs with activities against anaerobes, such as CLDM and MNZ, or therapy with such a single drug, such as SBT/ABPC, should be selected.
-
-
When there is a risk of multi-drug-resistant bacteria, monotherapy with a carbapenem should be selected as a first choice, assuming ESBL-producing enteric bacteria, resistant P. aeruginosa, and Acinetobacter. A fourth-generation cephalosporin should be combined with CLDM, or a quinolone should be combined with CLDM or SBT/ABPC.
-
-
If the results of culture/susceptibility tests are clarified, antimicrobial drugs should be changed in accordance with them. However, the culture of anaerobes is difficult, or is not conducted in some cases. Therefore, this should be confirmed to the laboratory. When only one type of bacteria are detected on a culture test despite several types of bacteria detected on Gram staining of pleural effusion, anaerobes must also be considered.
-
-
The penetration of aminoglycosides to the thoracic cavity is poor, and their activities reduce when the pH is low. Therefore, for pyothorax treatment, the use of aminoglycosides should be generally avoided [237], [238], [239], [240].
[Treatment period]
-
-
The pyothorax treatment period has not been established. However, when pneumonia promptly responds to treatment and thoracic drainage is successfully achieved in patients with pneumonia-related pyothorax, a treatment period of 10–14 days is required. In patients in whom drainage is unsuccessful, those with marked pleural thickening, or those with encapsulated/septum-like pyothorax, a treatment period of about 4 weeks is often required.
[Treatments other than antimicrobial drug therapy]
-
-
After a diagnosis of pyothorax is made, the administration of an appropriate antimicrobial drug should be promptly started, and drainage is necessary. When the pleural fluid is purulent and viscous in the absence of a multilocular pattern, chest-tube insertion is routinely performed. The position of insertion should be confirmed using thoracic CT within 24 h after insertion. Wozniak et al. performed multivariate analysis involving 104 patients with pyothorax and indicated that failure in the first drainage was strongly correlated with mortality, suggesting the necessity of early consultation with surgeons [241].
-
-
In some patients with marked pleural thickening or multilocular pleural effusion, thoracoscopic debridement is necessary. In addition, a fibrinolytic drug, such as streptokinase, is administered through a thoracic drain, or surgerical interventions such as thoracotomy or decortication is performed in some cases [242].
- - - Drugs to be recommended- - -
-
1.Empiric therapy
-
➀Cases in which there is no risk of multi-drug-resistant bacteria
-
♦First choice
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-
-
<>Second choices
-
-PCG, intravenous drip, 2,000,000 to 3,000,000 units/4 times a day
-
-ABPC, intravenous drip, 2 g/3–4 times a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a day
-
-
-
♦
-
➁Cases in which there is a risk of multi-drug-resistant bacteria
-
♦First choices
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-
-
<>Second choices (I)
-
-CFPM, intravenous drip, 1–2 g/2–4 times a day
-
-CZOP, intravenous drip, 1–2 g/2–4 times a day
-
-CPR, intravenous drip, 1–2 g/2–4 times a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a day
-
-
-
<>Second choices (II)
-
-LVFX, intravenous drip, 500 mg/once a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-
-
+one of the followings:
-
-CLDM, intravenous drip, 600 mg/2–4 times a day
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-MNZ, intravenous drip, 500 mg/4 times a day
-
-
-
∗With respect to the risk of multi-drug-resistant bacteria, refer to the section “2.2 Hospital-acquired pneumonia” Table 3 (p. 10).
-
∗In particular, MRSA infection must be considered in patients with nosocomial onset or the previous administration of antimicrobial drugs. When Staphylococcus infection is suspected on Gram staining of pleural effusion, an anti-MRSA drug should be used as an empiric therapy. If MSSA is identified as causative bacteria, de-escalation should be performed.
-
♦
-
➀
-
2.Definitive therapy
-
-Antimicrobial drugs against causative microorganisms identified should be selected in accordance with the section “2.1 Community-acquired pneumonia- - - 2.1.2 Definitive therapy” (p. 4) or “2.2 Hospital-acquired pneumonia- - - 2.2.3 Definitive therapy” (p. 13).
-
-If MRSA infection is suspected, antimicrobial drugs should be selected in accordance with the section “2.2 Hospital-acquired pneumonia- - - 2.2.3 Definitive therapy- - - (1) MRSA” (p. 13).
-
-Tuberculous pleuritis should be treated in accordance with the treatment of pulmonary tuberculosis (p. 42).
-
-
4.2. Children
- - - Executive summary- - -
For the treatment of pyothorax in children, antimicrobial drugs should be administered after investigating the etiology using thoracentesis or blood culture, if possible. In addition to antimicrobial drug therapy, treatment for retention fluid (pleural effusion drainage, continuous drainage) must also be considered (BIII).
[Characteristics and classification of the disease]
Pleuritis refers to inflammation of the pleura. Fluid (pleural effusion) is retained in the pleural cavity. Pleuritis is classified into 3 types: fibrinous (dry) pleuritis, exudative (wet) pleuritis, and purulent pleuritis (pyothorax) based on conditions [243]. On auscultation, the attenuation of respiratory sounds, as well as pleural friction rubs, are heard. Percussion dullness is noted. In the chronic stage, localized pleural thickening in various shapes is observed. When pus is obtained on thoracentesis, a definitive diagnosis of pyothorax is made. When the protein level in non-purulent pleural effusion is high, tuberculous pleuritis should be differentiated [244].
[Type and frequency of causative microorganisms]
Previously, pyothorax was associated with S. aureus in many cases. However, recently, such cases have been rare. According to data form a national survey in the former half of the 1990's, there were only a few patients with S. pneumoniae- or anaerobe-related pyothorax [243]. In many cases, pleuritis follows M. pneumoniae- or virus-related pneumonia. Decubitus-view imaging shows the retention of pleural effusion in approximately 20% of patients with Mycoplasma pneumonia [245]. If the drug susceptibility of causative microorganisms is clarified, it contributes to successful treatment. Therefore, pleural effusion obtained by thoracentesis should be cultured to investigate the etiology, if possible.
[Rules of antimicrobial drug therapy]
When bacterial infection-related pyothorax is suspected, the intravenous injection or drip of SBT/ABPC, CTX, or CTRX should be selected in community-onset patients without an underlying disease. On the other hand, combination therapy with SBT/ABPC (intravenous injection or drip) and CLDM (intravenous drip) or carbapenem therapy (intravenous drip) should be started in those with an underlying disease or nosocomial onset, considering S. pneumoniae, anaerobes, H. influenzae, and S. aureus [243]. Based on the Gram staining reactions of pleural effusion, causative microorganisms should be estimated, and antimicrobial drugs must be reviewed. If necessary, tuberculosis should also be investigated.
The administration period of antimicrobial drugs must be longer than that for pneumonia. As the type of causative microorganisms, patient background, and state of retention-fluid drainage differ among individual patients, it is difficult to establish standardized criteria. However, target administration periods for S. pyogenes (GAS)-, S. pneumoniae-, and S. aureus-related pyothorax are 10, 14, and 21 days or more, respectively.
The treatment response should be evaluated 3–4 days after the start of administration. If an improvement in clinical symptoms or laboratory data is achieved, the same antimicrobial drug should be continued until causative microorganisms and their drug susceptibility are clarified. When the type of microorganisms that caused pneumonia is identified, a target-focused antimicrobial drug should be selected through de-escalation, considering drug susceptibility and pharmacokinetics [189].
For the treatment of pyothorax, treatment for retention fluid (pleural effusion drainage) is also a basic procedure in addition to antimicrobial drug therapy. If necessary, continuous drainage should be performed (Table 19 ) [243]. If pleural thickening leads to underexpanded lung, decortication should be indicated. The widespread application of thoracoscopic surgery has facilitated minimally invasive surgery [246].
Table 19.
Indications for continuous drainage [243].
|
- - - Drugs to be recommended- - -
-
1.Empiric therapy
-
(1)Community onset (without an underlying disease)
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
-
-CTX, intravenous injection or drip, 40 mg/kg/3–4 times a day
-
-CTRX, intravenous injection or drip, 50 mg/kg/twice a day
-
-
-
(2)Community-acquired infection (with an underlying disease), nosocomial onset
-
-CLDM, intravenous drip, 10 mg/kg/3 times a day
- +
-
-SBT/ABPC, intravenous injection or drip, 75 mg/kg/3 times a day
- or one of the following drugs alone should be administered:
-
-TAZ/PIPC, intravenous drip, 112.5 mg/kg/3 times a day
-
-PAPM/BP, intravenous drip, 20 mg/kg/3 times a day
-
-MEPM, intravenous drip, 20 mg/kg/3 times a day
-
-DRPM, intravenous drip, 20 mg/kg/3 times a day
-
-
-
(1)
-
2.
Definitive therapy
Refer to the section “3.1 Community-acquired pneumonia- - - 3.1.2 Definitive therapy” (p. 33) or “3.2 Hospital-acquired pneumonia- - -3.2.2 Definitive therapy” (p. 34).
5. Mycobacterium infection
5.1. Adults
-
1.
Pulmonary tuberculosis
- - - Executive summary- - --
-As initial treatment, four drugs (INH, RFP, and PZA + EB or SM) should be administered for 2 months. Subsequently, as a rule, standard treatment (A), in which two drugs, INH and RFP, are administered for 4 months, should be performed as maintenance treatment (AI).
-
-When PZA cannot be used for initial treatment for some reason, INH, RFP, and EB or SM, should be administered for 2 months as initial treatment. Subsequently, standard treatment (B), in which two drugs, INH and RFP, are administered for 7 months, should be performed as maintenance treatment (AII).
-
-In patients in whom chest X-ray shows a cavity on initial consultation to during initial treatment and the septum culture is still positive at the completion of initial treatment, maintenance treatment should be prolonged over 3 months. In addition, the prolongation of maintenance treatment should also be considered in patients with severe tuberculosis, such as military tuberculosis and tuberculosis of the central nervous system, those with immune depression, and those with relapse of tuberculosis (AII).
-
-For the treatment of latent tuberculosis infection, INH should be administered for 6 or 9 months (AI). When M. tuberculosis, as the source of infection, is resistant to INH, or when the oral administration of INH is difficult due to side effects, RFP should be used as a second-choice drug for 4 or 6 months (AI).- - - Explanation- - -
-
-Standard treatment for tuberculosis in the 1950's was combination therapy with INH, SM, and PAS for 18 months. In 1968, RFP became commercially available. In the 1970's, controlled studies in England indicated the usefulness of short-term combination chemotherapy with RFP for 6 months [248], [249]. Thereafter, the efficacy of short-term combination chemotherapy with PZA for 2 months after the start of treatment was demonstrated [250], [251], [252]. In 1998, the British Thoracic Society recommended that conventional drugs, INH, RFP, and PZA, should be combined with SM or EB for the first 2 months, assuming that the rate of INH-resistant M. tuberculosis will increase. It was also recommended that, if a smear test becomes negative within 2 months and there is no drug resistance, INH and RFP, should be used for the subsequent 4 months (total: 6-month treatment) [253].
-
-Both the Guidelines for the Management of Tuberculosis in Japan, Revision No. 2, which was published in 2012, and Document regarding Tuberculosis Treatment, which was agreed by the ATS/CDC/IDSA in the United States and announced in 2003, recommended that INH, RFP, PZA, and EB or SM, should be administered for the first 2 months, and that INH and RFP, should be administered for the subsequent 4 months, as the most standard, evidence-based treatment method [254], [255]. In particular, this method is named “standard treatment (A)” in the Guidelines for the Management of Tuberculosis in 2012 in Japan. In this article, this name is also used.
-
-The secondary assessment of a study examining the efficacy of rifapentine and INH showed that factors for unsuccessful treatment/recurrence included a cavity on chest X-ray at the start of treatment and positive findings on culture at the completion of initial treatment for 2 months [256]. Similarly, when the treatment period was extended from 6 to 8 months in patients with silicotuberculosis, in whom the unsuccessful treatment/recurrence rates are high, the recurrence rate decreased from 22 to 7%. Therefore, various guidelines recommend that maintenance treatment should be prolonged over 3 months in patients with a cavity and those showing positive findings on septum culture at the completion of initial treatment [254], [255].
-
-In 1990, Combs et al. compared the results of treatment between a group treated with INH, RFP, and PZA for 2 months and, then, with INH and RFP for 4 months and that treated with INH and RFP for 9 months, and reported that the efficacy and incidence of side effects were similar [257]. Based on such a study, the following regimen is recommended as standard treatment (B) in the Guidelines for the Management of Tuberculosis in 2012 in Japan: when PZA cannot be used for some reason, INH, RFP, and EB or SM, are administered for the first 2 months, and, subsequently, two drugs, INH and RFP, are administered for 7 months.
-
-Patients who are infected with M. tuberculosis, but do not develop tuberculosis are regarded as having latent tuberculosis infection (LTBI). INH administration for LTBI decreases the incidence of tuberculosis by 25–92% [258]. Previously, this was called preventive therapy, but is currently termed LTBI treatment. The decrease in the incidence of tuberculosis is correlated with compliance with INH, and more marked preventive effects may be achieved when compliance is higher [258], [259], [260]. In a study involving INH administration to LTBI patients with an old shadow on chest X-ray, this therapy inhibited the onset of tuberculosis in 65 and 75% of patients treated for 6 and 12 months, respectively (there was no significant difference between the two groups) [261]. Based on the data, some studies recommended that the period of standard INH administration for LTBI should be 9 months [262], [263], [264]. However, a consensus regarding an effective administration period (6 or 9 months) has not been reached from various aspects including the efficacy, compliance, expenses, and incidence of side effects. Actually, the administration period should be determined based on compliance and the incidence of side effects.
-
-In LTBI treatment, RFP should be used for 4 or 6 months as an alternative drug when the oral administration of INH is impossible for some reason. The preventive effects of RFP on the onset of tuberculosis in LTBI patients may be similar to those of INH. Furthermore, the incidence of liver dysfunction is lower than that related to INH [265], [266], [267]. However, for the use of RFP, drug interactions must be considered.- - - Drugs to be recommended- - -
-
♦First choice
-
-INH, oral, 5 mg/kg/once a day (maximum: 300 mg/day) + RFP, oral, 10 mg/kg/once a day (maximum: 600 mg/day, orally administered before meals as a rule) + PZA, oral, 25 mg/kg/once a day (maximum: 1500 mg/day) + EB, oral, 15 mg/kg/once a day (maximum: 750 mg/day) or SM, intramuscular injection, 15 mg/kg/once a day (maximum: 750 mg/day)/2–3 times a week.
-
∗The above 4 drugs should be administered for 2 months, and, subsequently, two drugs, INH and RFP, should be administered for 4 months.
-
∗
-
-
-
<>Second choice
-
-INH, oral, 5 mg/kg/once a day (maximum: 300 mg/day) + RFP, oral, 10 mg/kg/once a day (maximum: 600 mg/day, orally administered before meals as a rule) + EB, oral, 15 mg/kg/once a day (maximum: 750 mg/day) or SM, intramuscular injection, 15 mg/kg/once a day (maximum: 750 mg/day)/2–3 times a week.
-
∗The above 3 drugs should be administered for 2 months, and, subsequently, two drugs, INH and RFP, should be administered for 7 months.
-
∗
-
-
-
-
-
2.Non-tuberculous Mycobacterium infection
-
a.Mycobacterium avium complex (MAC)- - - Executive summary- - -
-
-To treat pulmonary infection with M. avium complex in HIV-negative patients, CAM, RFP, and EB should be administered to those with nodular/bronchodilatation type infection (AI). In severe-status patients and those with cavity-type lesions, the intramuscular injection of SM or KM should be added (BIV).
-
-The treatment period should be at least 12 months after culture becomes negative, but there are no criteria regarding an optimal treatment period (BIV).- - - Explanation- - -
-
-In the 1990's, CAM was introduced for the treatment of pulmonary infection with M. avium complex. Before then, combination therapy with antituberculous drugs, such as INH, RFP, and EB, had been used. According to a report from the Research Committee of the British Respiratory Society in 2002, 27 of 75 HIV-negative patients with pulmonary MAC infection died within 5 years, treatment was unsuccessful in 11, and recurrence was noted in 10. CAM was not used in any patient, and there was no correlation between the susceptibility to various antituberculous drugs, such as INH, RFP, and EB, and prognosis [268].
-
-The introduction of CAM for the treatment of pulmonary MAC infection in the 1990's has markedly improved the treatment response and prognosis. Wallace et al. performed combination therapy with CAM (1000 mg/day), EB, RFP (or RBT), and SM in 39 patients with pulmonary MAC infection, and reported that 91% of the patients became negative for MAC [269]. In Japan, Tanaka et al. conducted combination therapy with CAM (10 mg/kg) and EB/RFP/KM in 39 patients, and indicated that 89.5% of those who underwent initial treatment became negative for MAC [270]. Concerning CAM, many studies have reported a correlation between the in vitro drug susceptibility and treatment response [271], [272], [273]. Furthermore, a study indicated that AZM was as effective as CAM [274].
-
-RBT, which was published in the drug price in NHI scheme in Japan in 2008, was also approved for tuberculosis and non-tuberculous Mycobacterium infection, including pulmonary MAC infection. The drug interactions of RBT are less marked than those of RFP, and RBT is used as a first-choice drug for disseminated MAC infection in HIV-infected patients [275]. On the other hand, RBT induces side effects such as uveitis. In elderly patients, in whom pulmonary MAC infection frequently develops, various side effects, such as gastrointestinal disorder, make long-term therapy difficult [276]. In addition, no study has indicated that RBT is more effective than RFP for pulmonary MAC infection in non-HIV-infected patients. Therefore, RFP should be selected as a first-choice drug for pulmonary MAC infection in non-HIV-infected patients.
-
-Kobashi et al. divided 146 patients with pulmonary MAC infection into two groups: a group treated with CAM, RFP, EB, and SM (intramuscularly injected at 15 mg/kg 3 times a week for 3 months) and a group treated with saline, and conducted a randomized, double-blind, comparative study [277]. In the SM-treated group, the rate at which the culture of sputum became negative was higher than in the saline-treated group (71.2 vs. 50.7%, respectively). There has been no high-quality study demonstrating the usefulness of combination therapy with aminoglycosides other than their study. However, various guidelines recommend that combination therapy with SM, AMK, or KM should be performed for 2–3 months in the initial phase of treatment in patients with a cavity or severe nodular/bronchodilatation type infection based on experience [276], [278], [279]. Guidelines in the United States recommend SM or AMK, and comment that no study has showed which of two drugs, SM and AMK, is more effective, although SM has been more frequently used [276]. In Japan, SM or KM is recommended [279].
-
-Based on the results of these studies, the Non-tuberculous Mycobacterium Infection Control Committee, Japanese Society for Tuberculosis recommends the following doses and administration methods for chemotherapy for pulmonary MAC infection in the “Opinions regarding Chemotherapy for Pulmonary Non-tuberculous Mycobacterium Infection- - - Revision in 2012”: RFP: 10 mg/kg (up to 600 mg)/day, once a day, EB: 15 mg/kg (up to 750 mg)/day, once a day, CAM: 600–800 mg/day (15–20 mg/kg), once a day or two divided doses (800 mg: two divided doses), and SM or KM: 15 mg/kg or less (up to 1000 mg), intramuscularly injected 2 or 3 times a week [279].
-
-A cooperative statement on non-tuberculous Mycobacterium infection by the American Thoracic Society/Infectious Diseases Society of America recommends therapy with CAM at 500 to 1000 mg/day or AZM at 250 mg/day, EB at 15 mg/kg/day, and RFP at 10 mg/kg/day (up to 600 mg) for patients with a cavity or severe nodular/bronchodilatation type infection. In addition, it is recommended that combination therapy with SM or AMK (8–10 mg/kg, 2–3 times a week, patients aged over 50 years: 500 mg or less) for 2–3 months in the initial phase should be considered [276], [280].
-
-The treatment period is established as about 1 year after the culture becomes negative in the above guidelines, but this is not based on evidence. In the Guidelines for the Management of Non-tuberculous Mycobacterium Infection, which were published by the British Thoracic Society, the treatment period of pulmonary MAC infection is established as 2 years. In the future, an optimal treatment period should be investigated.- - - Drugs to be recommended- - -
-
-CAM, oral, 200 mg/3 times a day or 400 mg/twice a day + RFP, oral, 10 mg/kg/once a day (maximum: 600 mg/day, orally administered before meals as a rule) + EB, oral, 15 mg/kg/once a day (maximum: 750 mg/day)
-
∗Severe-status patients and those with cavity-type lesionsIn addition to the above regimen, the intramuscular injection of SM or KM should be added.
-
∗The treatment period should be at least 12 months after culture becomes negative.
-
∗
-
-
-
b.Mycobacterium kansasii- - - Executive summary- - -
-
-To treat pulmonary infection with M. kansasii in HIV-negative patients, INH, RFP, and EB should be administered (AII).
-
-The treatment period should be at least 12 months after culture becomes negative (AI).
- - - - Explanation- - -
-
-Pulmonary infection with M. kansasii is a type of pulmonary non-tuberculous Mycobacterium infection on which drug effects are the most potent. The efficacy of INH, RFP, EB, CAM, and MFLX in vitro has been confirmed. In particular, RFP is an important drug in the treatment of pulmonary infection with M. kansasii. In a retrospective study involving 244 patients with pulmonary infection with M. kansasii, all 32 patients who underwent treatment with RFP became negative for M. kansasii in sputum within 6 months, whereas 80% of 130 patients who underwent RFP-free treatment became negative for M. kansasii in sputum within 6 months [281]. According to a study in Japan, 3 (approximately 1%) of 314 M. kansasii strains were resistant to RFP [282].
-
-Concerning the treatment period, in a prospective study involving 28 non-HIV-infected patients with pulmonary infection with M. kansasii, 14 were treated with 3 drugs, INH, RFP, and EB, for 12 months (EB: for the first 6 months only), and the other 14 were treated with a similar regimen for 18 months. Recurrence was noted in 1 in the former [283]. In both a cooperative statement on non-tuberculous Mycobacterium infection by the American Thoracic Society/Infectious Diseases Society of America and the “Opinions regarding Chemotherapy for Pulmonary Non-tuberculous Mycobacterium Infection- - - Revision in 2012” published by the Non-tuberculous Mycobacterium Infection Control Committee, Japanese Society for Tuberculosis, the treatment period is established as at least 12 months after culture results become negative [276], [279].
- - - - Drugs to be recommended- - -
-
-INH, oral, 5 mg/kg/once a day (maximum: 300 mg/day) + RFP, oral, 10 mg/kg/once a day (maximum: 600 mg/day) + EB, oral, 15 mg/kg/once a day (maximum: 750 mg/day)
-
∗The treatment period should be at least 12 months after culture becomes negative.
-
∗
-
-
-
c.Mycobacterium abscessus- - - Executive summary- - -
-
-There is no drug (combination) that can be recommended to treat pulmonary infection with Mycobacterium abscessus.
-
-The drug susceptibility of M. abscessus varies, and a drug susceptibility test should be generally performed. Usually, M. abscessus is susceptible to CAM, AMK, CFX, and IPM/CS. However, it is unclear whether there is a correlation between the drug susceptibility and clinical effects.
-
-Combination therapy with CAM and several intravenous antimicrobial drugs (AMK, CFX, and IPM/CS) may control the symptoms and progression of pulmonary infection with M. abscessus (BIII).
-
-In patients with pulmonary infection with M. abscessus in whom the lesion is localized, cure can be targeted by combining the above drugs with surgical resection (BIII).
-
-
-
a.
- - - Explanation- - -
-
-
M. abscessus is a type of environmental bacteria that proliferate in soil and tap water. It belongs to rapidly growing mycobacteria (RGM). In the United States, Korea, and Taiwan, it is third most commonly detected as a type of non-tuberculous Mycobacterium, following MAC and M. kansasii. Previously, there were many case reports on skin/soft tissue or bone infection. However, recently, respiratory infection has been increasingly reported, accounting for 80% of patients with respiratory infection with RGM [284].
-
-
M. abscessus is one of the most resistant types of Mycobacterium. In particular, pulmonary infection with M. abscessus is refractory [285]. M. abscessus is resistant to representative oral drugs for Mycobacterium infection, such as INH, RFP, and EB. It may be susceptible to CAM among oral antimicrobial drugs. Its susceptibility to other antimicrobial drugs varies. As a rule, a drug susceptibility test should be performed. Although M. abscessus is sometimes susceptible to AMK, CFX, and IPM/CS, it is unclear whether there is a correlation between the drug susceptibility and clinical effects. In vitro, M. abscessus is sometimes susceptible to LZD, TGC (tigecycline), and ketolides, but it is unclear whether there is a correlation between the drug susceptibility and clinical effects [286], [287].
-
-
Combination therapy with CAM and several intravenous antimicrobial drugs (AMK, CFX, and IPM/CS) may control the symptoms and progression of pulmonary infection with M. abscessus [288], [289]. However, actually, hospitalization is required to administer these intravenous antimicrobials, and the administration period is limited to 2–3 months. Subsequently, treatment with oral drugs is performed, but CAM is the only reliable oral drug, as described above. On the other hand, monotherapy with CAM should be avoided from the perspective of resistance induction. Although some studies reported combination therapy with LZD or quinolones, its efficacy has not been established.
-
-
Based on such a background, a combination of surgical resection of the lesion and combination chemotherapy is the only treatment that is expected to achieve the complete cure of pulmonary infection with M. abscessus in which the lesion is localized [284], [288], [289].
- - - Drugs to be recommended- - -
-
♦
First choice
- Based on the results of a drug susceptibility test, the following antimicrobial drugs should be combined:
-
-CAM, oral, 200 mg/3 times a day or 400 mg/twice a day
- + AMK, intravenous drip, 15 mg/kg/once a day
- + IPM/CS, intravenous drip, 0.5 g/4 times a day or 1 g/3 times a day
- * Surgery must be considered. The treatment period should be at least 12 months after culture becomes negative.
-
-
5.2. Children
- - - Executive summary- - -
-
-
For the treatment of childhood tuberculosis, several drugs should be combined, and administered for a specific period (AII).
-
-
For the treatment of non-tuberculous Mycobacterium infection, several drugs should be combined, and administered for a specific period. However, Mycobacterium often resists treatment. If there is no treatment response, surgery must be considered (CIII).
- - - Explanation- - -
[Characteristics and classification of the disease]
In Japan, tuberculosis is still an important infectious disease. When encountering patients with chronic infection who do not respond to general antimicrobial drugs, tuberculosis should be considered for differential diagnosis. Mycobacteria that can be cultured are classified into two types: M. tuberculosis complex and non-tuberculous Mycobacteria (NTM) [247]. M. tuberculosis is a major type of M. tuberculosis complex, and has a strong infectivity from humans to humans. Childhood tuberculosis is classified into two types based on age [247]. Briefly, primary tuberculosis represented by hilar lymph node tuberculosis and meningitis, which develop following primary infection, is characteristic of infants and children. The interval from infection until onset is short, and the morbidity rate is high. In addition, this disease may lead to a severe condition. Pulmonary/hilar lymph node tuberculosis in infants and children is asymptomatic, or the general condition is favorable even in the presence of fever or cough in many cases. When primary tuberculosis is detected based on dyspnea or an unfavorable general condition in addition to fever or cough, many infants/children have military tuberculosis or meningitis. On the other hand, secondary tuberculosis with a cavity lesion or nodular shadow in the lung field is frequent in junior high school students or older. Symptoms such as cough, sputum, fever, and thoracic pain are often observed. In most children, the source of infection can be clarified through detailed peripheral contact screening at the time of onset. Usually, the source of infection is clarified in 2/3 to 3/4 of children. It is often their fathers/mothers or grandfathers/grandmothers [290], [291], [292].
As childhood tuberculosis does not form a cavity in the lung field, the M. tuberculosis level in the focus is lower than in adults. In many cases, it is difficult to bacteriologically or histologically make a definitive diagnosis in comparison with adult tuberculosis. Usually, it is possible to make a definitive diagnosis by comprehensively evaluating epidemiological/clinical information such as opportunities for the source of infection to contact with tuberculosis patients, tuberculin reaction- or QuantiFERON TB (QFT)-based verification of infection, imaging findings suggestive of tuberculosis, such as chest X-ray findings, verification of M. tuberculosis from sputum or gastric juice, and individuals' resistance including the grade of BCG vaccination-acquired immunity and age, as well as by considering treatment responses in some cases. QFT is a very useful testing method to quantitatively measure IFN-γ and diagnose tuberculosis infection without being influenced by BCG. However, assessment in infants/children should be further examined in the future [293]. When a definitive diagnosis of pulmonary tuberculosis cannot be made based on chest X-ray findings alone, thoracic CT, which facilitates the detailed evaluation of the presence or absence and extent of tuberculous lesions, is useful for diagnosis. Furthermore, imaging findings of tuberculosis do not change in a short period in many cases.
Non-tuberculous Mycobacterium belongs to Mycobacterium, the same category as reported for M. tuberculosis. Therefore, it is often detected as a Mycobacterium-positive smear of sputum, that is, Gaffky's positive reaction. Initially, some patients are regarded as having infectious tuberculosis, and admitted to a tuberculosis ward. Symptoms and imaging findings are also similar between non-tuberculous Mycobacterium- and M. tuberculosis-infected patients. Unless detected bacteria are identified, or unless either gene is detected using the nucleic acid amplification method, it is difficult to differentiate the two types of bacteria. However, it is important to recognize that tuberculosis and non-tuberculous Mycobacterium infection are different diseases [276], [294]. The most important point is that non-tuberculous Mycobacterium infection does not transmit from humans to humans, differing from tuberculosis, an infectious disease in humans. Therefore, it is not necessary to isolate the patient, and, as a rule, patients requiring admission should be managed in a general ward. As there are no public hygiene-associated problems, it is not necessary to submit a report to a health center.
[Type and frequency of causative microorganisms]
In Japan, the number of patients with childhood tuberculosis has markedly decreased. The number of newly registered patients with tuberculosis decreased from 53,229 (1963) to 95 (2008) in children aged 0–14 years [290], [291], [292]. However, a decrease in the incidence of smear-positive pulmonary tuberculosis, which is important as the source of infection, is not marked in great urban areas. We cannot conclude that the opportunity of infection in children is favorably decreasing; caution is needed.
The number of patients who newly develop non-tuberculous Mycobacterium infection in Japan is estimated to be approximately 8000. In the adult field, it accounts for about 1/3 of that of patients who newly develop tuberculosis. However, it is relatively low in children. Approximately 80% of patients with non-tuberculous Mycobacterium infection are infected with M. avium complex (M. avium and Mycoboterium intracellulare, pulmonary MAC infection), and approximately 15% are infected with M. cansasii.
[Rules of antimicrobial drug therapy]
-
-
The characteristics of antitubercular chemotherapy in children are that children are tolerable to a relatively high dose per body weight in comparison with adults with respect to pharmacokinetics, and that the incidence of side effects is low [247].
In the pediatric field, 6-month treatment with INH, RFP, and PZA for childhood pulmonary tuberculosis is internationally selected as standard chemotherapy: three drugs, INH, RFP, and PZA, are administered every day for the first 2 months, and INH and RFP every day for the subsequent 4 months. When drug resistance is suspected, these drugs should be combined with SM or EB in the initial phase until the results of a resistance test are clarified. In patients with secondary tuberculosis, 4-drug combination therapy with INH, RFP, PZA, and SM (or EB) should be initially performed. In addition, as a rule, follow-up must be continued for 2 years after the completion of treatment [290], [291], [292].
On the other hand, drug resistance, referral to another hospital, and discontinued treatment are present among patients who drop out of treatment, although the number of such patients is small. It is necessary to support the resistance and continuation of treatment. In particular, recently, the number of patients in whom it is difficult to continue treatment has increased. Potent compliance support must be considered in connection with direct observed therapy (DOT) by health centers and welfare activities [247]. Side effects during treatment include liver dysfunction. However, if the maximum AST or ALT levels are approximately 100, administration should be carefully continued without discontinuing treatment. If these levels exceed 100, treatment should be transiently discontinued, and additional administration at a low dose should be conducted after confirming the normalization of the liver function. The dose should be gradually increased. Liver dysfunction requiring a change of treatment is not frequent. Furthermore, there is an increase in the serum uric acid level, but continuous treatment leads to normalization. There have been few patients with arthralgia.
-
-
Prevention of tuberculosis: To prevent the onset of tuberculosis in uninfected persons, BCG vaccination should be performed. Concerning its efficacy, a consensus regarding its potent preventive effects on severe disseminated tuberculosis, such as tuberculous meningitis and military tuberculosis, has been reached. Considering the importance of tuberculous meningitis prevention, BCG vaccination in the early phase of infancy (5–8 months after birth, or earlier in accordance with the state of peripheral tuberculosis prevalence) is still necessary in Japan [290].
-
-
Treatment for latent tuberculosis: To prevent the onset of tuberculosis in persons with a history of tuberculosis, treatment for latent tuberculosis (conventional chemoprevention) should be conducted. A large-scale controlled study reported that INH therapy decreased the incidence of tuberculosis by approximately 50–60%. For drug administration, the risk of tuberculosis onset should be concretely and flexibly evaluated based on the tuberculin reaction, opportunity of infection, age, and state of BCG vaccination in individual patients [247].
-
-
Treatment for non-tuberculous Mycobacterium infection: Non-tuberculous Mycobacterium infection is refractory despite combination therapy with antitubercular drugs. In particular, there is no evidence regarding treatment in children [276]. The effects of monotherapy are weak, and monotherapy with CAM may lead to the appearance of CAM-resistant bacteria within a few months [279]; therefore, this therapy should be avoided. The responses of M. kansasii to antitubercular drugs are relatively favorable, and cure may be achieved. However, pulmonary MAC infection is often resistant to treatment. If there is no response, surgery must be considered. Recurrence after the completion of treatment is also often observed.
- - - Drugs to be recommended- - -
-
➀M. tuberculosis
-
♦First choice
-
-RFP + INH + PZA/2 months
- subsequently: RFP + INH/4 months
-
-
-
<>Second choices (secondary tuberculosis)
-
-RFP + INH + PZA + SM or EB/2 months
- subsequently: RFP + INH or RFP + INH + EB/4 months
-
∗Cases in which PZA administration is impossible
-
∗
-
-RFP + INH + SM or EB/6 months
- subsequently: RFP + INH or RFP + INH + EB/3 months
-
∗Administration method/doses of antitubercular drugs
-
∗
-
-
-
♦
INH: oral, 10–15 mg/kg/once a day (maximum: 400 mg/day)
RFP: oral, 10–20 mg/kg/once a day (maximum: 450 mg/day)
PZA: oral, 20–30 mg/kg/once a day (maximum: 1.2 g/day)
SM: intramuscular injection, 20–40 mg/kg/once a day (maximum: 750 mg/day)
EB: oral, 15–25 mg/kg/once a day (maximum: 750 mg/day)
-
➁M. avium complex
-
-RFP, oral, 10 mg/kg (maximum: 600 mg)/once a day + EB, oral, 15 mg/kg (maximum: 750 mg)/once a day + CAM, oral, 7.5–10 mg/kg (maximum: 400 mg, 800 mg/day)/twice a day
-
∗In severe cases, the above drugs should be combined with the intramuscular injection of SM or KM at 15 mg/kg (maximum: 1000 mg)/once a day, 2 or 3 times a week.
-
∗If there is no response, surgery must be considered.
-
∗
-
-
-
➂M. kansasii
-
-Combination therapy with INH, oral, 5 mg/kg (maximum: 300 mg)/once a day + RFP, oral, 10 mg/kg (maximum: 600 mg)/once a day + EB, oral, 15 mg/kg (maximum: 750 mg)/once a day should be performed for 1 year after culture test reactions become negative.
-
∗As the administration period is longer than that for tuberculosis patients, the development of vision disorder should be considered even at these doses.
-
∗If there is no response, surgery must be considered.
-
∗
-
-
6. Lower respiratory infectious disease (Adults)
6.1. Acute bronchitis
- - - Executive summary- - -
-
-
Viruses comprise the greater portion of causative microorganisms.
-
-
When there are no complications such as chronic respiratory diseases, the administration of antimicrobial drugs for acute bronchitis is not recommended as a rule (AI).
-
-
Treatment with macrolides is indicated for patients with pertussis (AI). Treatment after the catarrhal period does not reduce the degree or duration of cough, but antimicrobial drugs are necessary to prevent infection to peripheral persons.
-
-
As first-choice antimicrobial drugs for acute bronchitis caused by M. pneumoniae or C. pneumoniae, macrolides should be selected (CIII).
- - - Explanation- - -
Acute bronchitis is characterized by cough that persists for 5 days or more. In most cases, cough persists for 1–3 weeks, but spontaneously subsides [295], [296]. Sputum is present in some cases, but is absent in others. Sputum may be purulent even when viral infection is etiologically involved. Neither chest X-ray nor CT shows the appearance of a new abnormal shadow, differing from pneumonia.
Viruses such as influenza virus A/B, rhinovirus, coronavirus, adenovirus, RS virus, human metapneumovirus, and parainfluenza virus account for approximately 90% of causative microorganisms [295], [296], [297], [298]. In addition, B. pertussis, M. pneumoniae, and C. pneumoniae account for approximately 10% [295], [296], [297], [298]. There is no evidence that infection with other bacteria directly causes acute bronchitis in adults without an underlying disease [296]. However, in a study using the transtracheal aspiration method in Japan, H. influenzae, S. pneumoniae, and M. catarrhalis were primarily isolated in patients diagnosed with bacterial acute bronchitis in the absence of a chronic lower respiratory infectious disease as an underlying disease [299].
In cases of pertussis, cough persists particularly over a long period, and paroxysmal coughing, inspiratory whooping, and vomiting after coughing may occur [300]. Acute bronchitis caused by M. pneumoniae also induces severe, persistent cough. In cases of influenza, fever, headache, general malaise, and arthralgia are observed. Furthermore, acute viral bronchitis may lead to acute bacterial exacerbation in patients with chronic respiratory lesions as underlying diseases; fever and an increase in the amount of purulent sputum are observed.
As a rule, when an underlying disease or complication is absent, the routine administration of antimicrobial drugs for acute bronchitis is not recommended [297], [298]. To control symptoms such as cough, symptomatic therapy should be performed if necessary. On the other hand, antimicrobial drug treatment with macrolides is indicated for patients with pertussis. Treatment after the catarrhal period does not reduce the degree or duration of cough, but antimicrobial drugs are necessary to prevent infection to peripheral persons [301], [302]. When performing antimicrobial drug treatment for acute bronchitis caused by M. pneumoniae or C. pneumoniae, macrolides should be selected as first-choice drugs. However, an increase in macrolide-resistant M. pneumoniae must be considered [303]. In cases of influenza, anti-influenza therapy should be conducted within 48 h after onset [304].
In patients with underlying diseases or elderly persons with complications, bacterial (e.g., S. pneumoniae) infection may occur following viral infection, although this is not frequent in healthy adults. When acute bronchitis related to bacterial infection, including secondary infection, is strongly suspected based on cough/sputum, fever, leukocytosis, or findings suggestive of the presence of causative microorganisms on Gram staining of sputum despite the absence of a new infiltrative shadow on chest X-ray, antimicrobial drug treatment is considered in accordance with treatment for community-acquired bacterial pneumonia [305], [306].
- - - Drugs to be recommended- - -
-
➀
Acute bronchitis caused by viruses
When there are no complications such as chronic respiratory diseases, the administration of antimicrobial drugs for acute bronchitis are not recommended as a rule (With respect to the selection of antimicrobial drugs for acute bronchitis complicated by chronic respiratory diseases with secondary bacterial infection, refer to the section “6.2 Respiratory tract infection in the presence of chronic respiratory disease(COPD, bronchiectasis, old pulmonary tuberculosis.)” (p. 47)
-
➁Pertussis
-
-EM, oral, 400 mg/3 times a day, 14 days
-
-CAM, oral, 200 mg/twice a day, 7 days
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-
-
➂Acute bronchitis caused by M. pneumoniae or C. pneumoniae
-
→Refer to the section “2. Pneumonia (Adults) 2.1 Community-acquired pneumonia 2.1.1 Empiric therapy b. Atypical pneumonia” (p. 3).
-
→
-
➃Influenza
-
→Refer to the section “8. Influenza” (p. 52).
-
→
-
➄Cases in which bacterial infection is strongly suspected as an etiological factor
-
a.Empiric therapy
-
→Refer to the section “2. Pneumonia (Adults) 2.1 Community-acquired pneumonia 2.1.1 Empiric therapy a. Bacterial pneumonia” (p. 3).
-
→
-
b.Definitive therapy
-
→Refer to the section “2.1 Community-acquired pneumonia 2.1.2 Definitive therapy” (p. 4).
-
→
-
a.
6.2. Respiratory tract infection in the presence of chronic respiratory disease (COPD, bronchiectasis, old pulmonary tuberculosis)
- - - Executive summary- - -
-
-
Infectious exacerbation refers to the bacterial infection-related exacerbation of symptoms, such as cough, purulent sputum, fever, and shortness of breath, from a chronic, stable state [305], [307], [308].
-
-
Inflammatory responses involving the leukocyte count and CRP level are enhanced, and PaO2 is often reduced on blood gas analysis.
-
-
Chest X-ray and CT are useful for evaluating an underlying disease and differentiating chronic respiratory diseases from other diseases with a shadow.
-
-
At the time of persistent infection, cough and sputum are continuously eliminated, and a slight inflammatory response is sometimes observed on a blood test. In such cases, antimicrobial drugs should not be administered as a rule. Gram staining of sputum is useful for evaluating infection [309] (AIII).
-
-
Frequent causative microorganisms include H. influenzae, P. aeruginosa, M. catarrhalis, and S. pneumoniae. Although persistent infection with P. aeruginosa is noted in many cases, it must be differentiated from acute exacerbation based on clinical symptoms and laboratory data. In addition, S. aureus and K. pneumoniae should be considered (Table 20 ) [310], [311].
-
-
Selection of antimicrobial drugs: Respiratory quinolones have potent antimicrobial activities against all types of causative microorganisms, and their clinical effects are marked; therefore, they are first-choice drugs [15], [312], [313], [314] (AI). The use of β-lactams and macrolides must be considered in individual patients.
-
-
The administration period of antimicrobial drugs should be 5–7 days [315], [316], [317], [318] (BII).
Table 20.
Primary causative microorganisms.
| Bacteria | Virus | Atypical pathogen |
|---|---|---|
| Haemophilus influenzae | Rhinovirus | Chlamidophila pneumonie |
| Streptcoccus pneumoniae | Parainfluenza | Mycoplasma pneumoniae |
| Moraxella catarrhalis | Influenza | |
| Pseudomonas aeruginosa | Respiratory syncytial virus | |
| Enterobacteriaceae | Coronavirus | |
| Haemophilus haemolyticus | Adenovirus | |
| Haemophilus parainfluenzae | Human metapneumovirus | |
| Staphylococcus aureus |
- - - Explanation- - -
[Clinical symptoms]
Respiratory tract infection in the presence of chronic respiratory disease refers to the new appearance of infectious symptoms, such as fever and shortness of breath, in addition to bacterial infection-related respiratory symptoms, such as increases in the frequency of cough, volume of purulent sputum, and degree of purulence, from the chronic, stable conditions of underlying diseases, such as COPD, bronchiectasis, and old pulmonary tuberculosis. Concerning laboratory data, inflammatory responses involving the leukocyte count and CRP level are enhanced, and PaO2 is often reduced on blood gas analysis.
[Imaging findings]
Imaging findings are necessary to differentiate chronic respiratory disease-related airway infection from pneumonia. The absence of a shadow must be confirmed. CT should also be performed to evaluate underlying diseases such as pulmonary emphysema and bronchiectasis.
[Estimation of causative microorganisms and Gram staining]
It is possible to collect sputum in many patients. Gram staining is useful for predicting causative microorganisms or differentiating respiratory tract infection in the presence of chronic respiratory disease from persistent infection. According to a study, the tone of sputum suggests the presence of pathogenic microorganisms rather than the degree of purulence; macroscopic examination is also necessary [319]. Sputum involves much information, and is the most important sample. Samples should be collected before the administration of antimicrobial drugs. Those collected on waking-up early in the morning are ideal. To evaluate the degree of sputum purulence, the Miller & Jones classification [305] (Table 21 ) is used, but, if samples are evaluated as P2 or higher, causative microorganisms may be predicted using Gram staining. On Gram staining, an area where the number of inflammatory cells is large should be initially searched at a low magnification, and detailed observation should be conducted at a high magnification. Before the administration of antimicrobial drugs, sputum should always be submitted for a susceptibility test.
Table 21.
Miller & Jones classification of purulent sputum.
| M1: Saliva, complete mucous sputum |
| M2: Mucous sputum containing a small volume of purulent sputum |
| P1: Sputum in which the purulent area comprises 1/3 or smaller |
| P2: Sputum in which the purulent area comprises 1/3 to 2/3 |
| P3: Sputum in which the purulent area comprises 2/3 or greater |
[Causative microorganisms]
As causative microorganisms, H. influenzae, P. aeruginosa, M. catarrhalis, and S. pneumoniae are frequently detected. Persistent infection with P. aeruginosa is often observed, but it must be differentiated from acute exacerbation based on clinical symptoms and laboratory data. In addition, S. aureus and K. pneumoniae should be considered [310]. The involvement of atypical pathogens such as C. pneumoniae or mixed infection with viruses and bacteria must also be considered.
[Treatment]
The purpose of treatment is to reduce clinical symptoms, prevent recurrence, prolong the interval until subsequent exacerbation, and inhibit lung tissue damage. The administration of appropriate antimicrobial drugs relieves clinical symptoms, and maintains the respiratory function [320]. On the other hand, inappropriate antimicrobial drugs may deteriorate the prognosis, inducing recurrence.
In Japan, the resistance of S. pneumoniae and H. influenzae to macrolides and β-lactams is advanced [13], [29]. Several studies have reported that new quinolones are more useful than β-lactams [15], [312], [313], [314], [315]. Respiratory quinolones have potent antimicrobial activities against all types of causative microorganisms, and against resistant bacteria [321], [322], [323]. Concerning the administration period, a study indicated that the efficacy of administration for 5 days was similar to that for 7 days, and that the former was safer than the latter. The administration period should be shortened [324].
- - - Drugs to be recommended- - -
-
a.
Empiric therapy
Internationally, some studies have supported the usefulness of β-lactams [325], [326]. However, in Japan, the resistance of S. pneumoniae and H. influenzae to macrolides and β-lactams is advanced, and P. aeruginosa is also sometimes isolated. Therefore, the use of β-lactams and macrolides is limited to patients without risk factors. An international comparative study reported that the efficacy of AZM sustained-release preparations was similar to that of new quinolones [327]. However, in Japan, the long-term administration of macrolides is performed in many patients; therefore, circumstances differ.-
(1)Outpatient treatment
-
♦First choices
-
-LVFX, oral, 500 mg/once a day
-
-GRNX, oral, 400 mg/once a day
-
-MFLX, oral, 400 mg/once a day
-
-STFX, oral, 100 mg/1–2 times a day
-
-
- These 4 drugs have potent antimicrobial activities against all types of causative microorganisms predicted, and are recommended as first-choice drugs (AII).
-
<>Second choices
-
-CVA/AMPC, oral (125/250 mg), 2 tablets/3–4 times a day
-
-SBTPC, oral (375 mg), 1 tablet/3 times a day
-
-AZM sustained-release preparation, oral, 2 g/single dose
-
-
-
♦
-
(2)Hospital treatment
-
<>Mild cases
-
-CTRX, intravenous drip, 2 g/once a day or 1 g/twice a day
-
-LVFX, intravenous drip, 500 mg/once a day
-
-SBT/ABPC, intravenous drip, 3 g/3–4 times a day
-
-
-
<>Severe cases (P. aeruginosa must be considered.)
-
-MEPM, intravenous drip, 1 g/2–3 times a day
-
-DRPM, intravenous drip, 0.5–1 g/3 times a day
-
-BIPM, intravenous drip, 0.3–0.6 g/3–4 times a day
-
-IPM/CS, intravenous drip, 0.5–1 g/2–4 times a day
-
-TAZ/PIPC, intravenous drip, 4.5 g/3–4 times a day
-
-PZFX, intravenous drip, 500 to 1000 mg/twice a day
-
-CPFX, intravenous drip, 300 mg/twice a day
-
-CAZ, intravenous drip, 1–2 g/2–4 times a day
-
-CFPM, intravenous drip, 1–2 g/2–4 times a day
-
-CZOP, intravenous drip, 1–2 g/2–4 times a day
-
-CPR, intravenous drip, 1–2 g/2–4 times a day
-
-
-
∗Combination therapy with aminoglycosides should be considered in accordance with individual patients.
-
-AMK, intravenous drip, 200 mg/twice a day
-
-GM, intravenous drip, 60 mg/twice a day
-
-TOB, intravenous drip, 90 mg/twice a day
-
-
-
<>
-
(1)
-
b.
Definitive therapy
Refer to the section “2.1 Community-acquired pneumonia 2.1.2 Definitive therapy” (p. 4).-
➀H. influenzaeBeta-lactamase-producing strains are detected in approximately 10–20% of H. influenzae strains. Beta-lactamase-negative, ampicillin-resistant (BLNAR) strains account for approximately 20%. Therefore, when drug susceptibility is unclear, new quinolones should be selected as first-choice oral antimicrobial drugs. If drug susceptibility is clarified, they should be switched to effective and narrow-spectrum drugs. As injection, penicillins should be initially selected, followed by β-lactamase inhibitor-containing penicillins, carbapenems, and new quinolones.
-
➁M. catarrhalisBeta-lactamase-producing strains account for 100% of M. catarrhalis strains. As oral antimicrobial drugs, macrolides should be initially selected, followed by β-lactamase inhibitor-containing penicillins, second-/third-generation cephems, and new quinolones. As injection, β-lactamase inhibitor-containing penicillins, second-/third-generation cephems, new quinolones, or carbapenems should be selected.
-
➂P. aeruginosaAs oral drugs, new quinolones should be selected. As injection, anti-P. aeruginosa penicillins, cephems, monobactums, carbapenems, or new quinolones should be selected. As the drug susceptibility of this type of bacteria markedly differs among strains, drugs should be selected based on the results of culture tests.
-
➃S. pneumoniaeAs oral drugs, penicillins should be initially selected, followed by new quinolones. In patients with a risk of resistant bacteria, respiratory quinolones such as LVFX and GRNX should be selected. As injection, penicillins or CTRX should be selected, but carbapenems must be considered in severe-status patients.
-
➄S. aureusConsidering Methicillin-sensitive S. aureus (MSSA), drugs such as β-lactamase inhibitor-containing penicillins, first-/second-generation cephems, and carbapenems should be selected. When Methicillin-resistant S. aureus (MRSA) is identified, anti-MRSA drugs should be selected.
-
➅K. pneumoniaeSecond-generation cephems should be selected as first-choice drugs. Second-choice drugs include β-lactamase inhibitor-containing penicillins, second-/third-generation cephems, carbapenems, and new quinolones.
-
➀
6.3. Diffuse panbronchiolitis
- - - Executive summary- - -
-
-
First-choice treatment for diffuse panbronchiolitis (DPB) consists of EM to be administered at 200 mg 2–3 times a day (AII).
-
-
As a second-choice drug for patients who do not respond to EM or those in whom continuous administration is difficult, EM should be switched to CAM to be administered at 200 mg once to twice a day or RXM to be administered at 150 mg once to twice a day (AIII).
-
-
In patients in whom treatment with 14-membered ring macrolides is difficult, a 15-membered ring macrolide, AZM, to be administered at 250 mg 2–3 times a week, must be considered as an alternative treatment (BIII).
-
-
Initially, the oral administration of EM should be continued for 6 months to be evaluated its clinical effects. If symptoms or laboratory findings become stable through improvements, treatment should be continued for a total of 2 years (BIII).
-
-
At the time of acute exacerbation, an antimicrobial drug that covers H. influenzae, S. pneumoniae, M. catarrhalis, and P. aeruginosa should be additionally administered (BIII).
- - - Explanation- - -
[Characteristics/classification of the disease]
DPB is a chronic inflammatory disease of the respiratory tract, which is frequently observed in East Asians including Japanese. There is no gender difference, and this disease frequently develops in persons aged 40–50 years. It is often detected in patients with a history of chronic sinusitis or in those with the concomitant development of chronic sinusitis. This disease is classified as the category of sinobronchial syndrome.
[Symptoms]
The most typical symptoms of DPB are persistent cough and purulent sputum. Symptoms such as exertional shortness of breath and dyspnea appear in accordance with disease progression. In patients with a complication of chronic sinusitis, purulent nasal discharge and nasal obstruction are observed.
[Laboratory findings]
Chest X-ray shows pulmonary overexpansion or a diffuse scattered nodular shadow. Thoracic HRCT reveals a diffuse centrilobular nodular shadow. Furthermore, obstructive respiratory dysfunction, hypoxemia, and an increase in the cold agglutinin value (64-fold or more on the hemagglutination method) are observed.
[Type and frequency of causative microorganisms]
In patients with DPB, persistent respiratory tract infection with H. influenzae, S. pneumoniae, or M. catarrhalis is often observed. However, the incidence of persistent infection with P. aeruginosa increases with progression.
[Long-term macrolide therapy]
Previously, the prognosis of DPB was unfavorable; respiratory failure gradually progressed through repeated acute exacerbation related to respiratory tract infection, leading to a fatal outcome; however, the prognosis of DPB has been markedly improved since long-term macrolide therapy with low-dose administration of EM or other 14-membered ring macrolides was established [328], [329], [330], [331], [332], [333], [334], [335], [336], [337], [338], [339], [340]. Early diagnosis/treatment have facilitated the complete cure of DPB. Therefore, if once a diagnosis of DPB is made, long-term macrolide therapy should be started promptly.
- - - Drugs to be recommended- - -
-
➀Persistent infection
-
-The oral administration of EM at 400–600 mg/day should be continued for 6 months to be evaluated its clinical effects [328].
-
-In many cases, an improvement in symptoms (such as a decrease in the volume of sputum) will be achieved within 1–3 months after the start of administration.
-
-In addition, an improvement in imaging findings or the respiratory function will be achieved after 3–6 months of treatment.
- -
-
-When symptoms persist even after 2-year treatment, treatment can be further continued.
-
-When there is no response to EM at 600 mg/day, or when the oral administration of EM is not able to be continued due to gastrointestinal complaints, consider to switch from EM to other 14-membered ring macrolides, such as CAM at 200–400 mg/day and RXM at 150–300 mg/day, as second-choice drugs [328], [342], [343], [344].
-
-When there is no response to any 14-membered ring macrolides, AZM at 250 mg (to be administered 2–3 times a week) should be considered as an alternative treatment [345].
-
-Sixteen-membered ring macrolides are not to be effective.
-
-It does not matter to the clinical effects of long-term macrolide therapy whether the drug susceptibility of bacteria that persistently infect the host (such as P. aeruginosa) is present or not. For example, even when there is no disappearance of P. aeruginosa on sputum culture, clinical symptoms of DPB will be improved by long-term macrolide therapy. Therefore, the effects of long-term macrolide therapy should be comprehensively evaluated based on changes of symptoms or other laboratory findings as well as the results of sputum culture.
-
-In patients with bronchiectasis or chronic bronchitis, persistent bacterial infection of the lower respiratory tract is also often observed. Several studies have reported the usefulness of long-term macrolide therapy for the management of such a condition, as indicated for DPB treatment [346], [347], [348].
-
-Concerning chronic obstructive pulmonary disease (COPD), long-term macrolide therapy with EM or AZM decreases the risk of acutely exacerbating COPD [349], [350].
-
♦First choice
-
-EM, oral, 200 mg/2–3 times a day
-
-
-
<>Second choices
-
-CAM, oral, 200 mg/once to twice a day
-
-RXM, oral, 150 mg/once to twice a day
-
-
-
♦
-
-
-
➁Acute exacerbation
-
-During the clinical course of DPB, acute exacerbations may develop in some cases, as reported for other chronic respiratory diseases. Symptoms such as increases in the frequency of cough/volume of purulent sputum, fever, or respiratory failure will rapidly progress.
-
-As causative microorganisms at the time of acute exacerbation, H. influenzae, S. pneumoniae, M. catarrhalis, and P. aeruginosa are frequently detected, as indicated for persistent infection.
-
-During acute exacerbations, an antimicrobial drug should be additionally administered to cover these causative microorganisms [351].
-
a.Empiric therapyAntimicrobial drugs should be selected in accordance with the section “6.2 Respiratory tract infection in the presence of chronic respiratory disease (COPD, bronchiectasis, old pulmonary tuberculosis, etc.)- - - a. Empiric therapy- - -” (p. 48).
-
b.Definitive therapyAntimicrobial drugs should be selected in accordance with the section “6.2 Respiratory tract infection in the presence of chronic respiratory disease (COPD, bronchiectasis, old pulmonary tuberculosis, etc.)- - - b. Definitive therapy- - -” (p. 48).
-
-
7. Lower respiratory infectious disease (Children)
7.1. Croup syndrome
- - - Executive summary- - -
Croup syndrome is caused by viruses, and antimicrobial drugs are not necessary (AI).
- - - Explanation- - -
[Characteristics and classification of the disease]
Croup syndrome is characterized by acute laryngeal stenosis-associated respiratory disturbance such as barking cough, hoarseness, and inspiratory stridor. Most lesions involve not only the larynx but also the trachea/bronchus. This disease is sometimes called laryngotracheobronchitis [352]. Etiological factors are classified into two types: infectious and non-infectious (allergy-/foreign body-related) factors [352], [353]. The incidence of infectious croup syndrome is high in infants/children aged 7 months–3 years [354], [355].
[Type and frequency of causative microorganisms]
Croup syndrome is primarily caused by viruses. Parainfluenza virus type 1 is the most common virus [356]. In addition, parainfluenza virus type 2/3, influenza A/B virus, RS virus, human metapneumovirus, coronavirus, adenovirus, and measles virus are relatively frequently isolated [353], [357].
[Rules of antimicrobial drug therapy]
In most cases, croup syndrome is caused by viruses, and antimicrobial drugs are not necessary. Therefore, no study has evaluated the efficacy of antimicrobial drugs in patients with croup syndrome. There are no treatment guidelines regarding croup syndrome in which antimicrobial drugs are recommended [358], [359].
7.2. Bronchiolitis
- - - Executive summary- - -
Bronchiolitis is caused by viruses, and the administration of antimicrobial drugs is not necessary (AI).
- - - Explanation- - -
[Characteristics and classification of the disease]
Bronchiolitis is an acute, inflammatory, obstructive disease involving the bronchiole. Narrowing of the bronchiolar lumen related to mucosal epithelial injury, inflammatory-cell infiltration, interstitial edema, or an increase in mucus secretion causes air trapping in the peripheral respiratory tract, leading to obstructive respiratory disorder. This disease frequently develops in children aged 2 years or younger. However, infants aged 11 months or younger account for 80% or more [360].
[Type and frequency of causative microorganisms]
Bronchiolitis is primarily caused by viruses. RS virus accounts for 60–80%. In addition, parainfluenza virus, human metapneumovirus, adenovirus, and influenza virus are relatively frequently isolated [361], [362], [363].
[Rules of antimicrobial drug therapy]
In most cases, bronchiolitis is caused by viruses, and antimicrobial drugs are not necessary. Basic treatment is symptomatic therapy. In double-blind comparative studies involving ABPC and non-treated groups [364], AZM and non-treated groups [365], and ABPC intravenous injection/oral EM and non-treated groups [366], respectively, there were no significant differences in the admission period or symptom improvement. However, a small-scale double-blind comparative study reported that the interval until recovery in the CAM-treated group was shorter than in the non-treated group [367]. According to another study, the incidence of secondary bacterial infection during the course of RS virus-related bronchiolitis was 1.2%, and there was no difference between antimicrobial drug-treated and non-treated groups [368]. Therefore, it is not necessary to administer antimicrobial drugs to children with bronchiolitis for routine treatment or the prevention of secondary bacterial infection. However, follow-up must be carefully continued during the course of bronchiolitis. When a diagnosis of secondary bacterial infection-related pneumonia or otitis media is made, antimicrobial drug therapy should be started.
7.3. Bacterial tracheitis
- - - Executive summary- - -
Bacterial tracheitis is a bacterial disease with the rapid progression of dyspnea. If symptoms are progressive, antimicrobial drugs should be used even when a definitive diagnosis is not made (AIII).
- - - Explanation- - -
[Characteristics and classification of the disease]
Fever and croup syndrome-like cough/stridor initially appear, and respiratory disorder rapidly progresses, but there is no specific posture, salivation, or dysphagia, which are characteristic of acute epiglottitis. A definitive diagnosis can be made based on characteristic clinical features and purulent secretion in the respiratory tract. In some cases, a lateral view of the larynx on X-ray shows stenosis below the larynx [352]. This disease frequently develops in children aged 3–8 years [369].
[Type and frequency of causative microorganisms]
S. aureus-related tracheitis accounts for approximately 60%, followed by that related to M. catarrhalis, H. influenzae, Streptococcus pneumoniae, and Streptococcus pyogenes[370], [371], [372], [373]. Mixed infection with viruses and bacteria is frequent, and parainfluenza virus type I [373] and influenza A virus [374] are often detected.
[Rules of antimicrobial drug therapy]
Antimicrobial drugs should be intravenously administered for the following reasons: this disease rapidly progresses, and oral administration is difficult in many cases. As empiric therapy, combination therapy with VCM, which may be effective for infection with S. aureus (including MRSA), and third-generation cephems (CTRX, CTX), which have potent antimicrobial activities against M. catarrhalis, H. influenzae, S. pneumoniae, and S. pyogenes, should be performed. The administration period is 10–14 days [369].
- - - Drugs to be recommended- - -
Refer to the section “3. Pneumonia (Children)- - - Drugs to be recommended 2. Definitive therapy- - -” (p. 33).
7.4. Acute bronchitis
- - - Executive summary- - -
Acute bronchitis is primarily caused by viruses, and the necessity of antimicrobial drug administration is low (AI).
When acute bronchitis is caused by M. pneumoniae, C. pneumoniae, or B. pertussis, antimicrobial drugs should be administered if necessary (AIII).
Secondary infection with S. pneumoniae or H. influenzae may occur, although its incidence is unclear. Therefore, when there is no improvement, the administration of antimicrobial drugs should be considered (AIII).
- - - Explanation- - -
[Characteristics and classification of the disease]
Bronchitis causes symptoms such as cough, fever, and general malaise. Various causative microorganisms induce inflammation of the epithelial tracheobronchial tissue, leading to the onset of bronchitis. Clinically, there are no special findings on auscultation, or only rough respiratory sounds (intermittent accessory murmurs) are heard. Chest X-ray does not also show any marked infiltrative shadow. Usually, patients in whom the interval after onset is less than 3 weeks are regarded as having acute bronchitis [360].
However, bronchitis diagnosed in Japan slightly differs from that in Europe and the United States. The latter primarily causes persistent cough. In Japan, patients in whom there are no findings on chest X-ray despite clinical signs of pneumonia or those in whom chest X-ray is not performed are often diagnosed with bronchitis; the disease entity must be arranged.
[Type and frequency of causative microorganisms]
Viruses, such as rhinovirus, influenza virus, RS virus, adenovirus, parainfluenza virus, human metapneumovirus, and human bocavirus, account for 90% of causative microorganisms. M. pneumoniae, C. pneumoniae, and B. pertussis also cause bronchitis, although such cases are relatively rare [360], [375].
[Rules of antimicrobial drug therapy]
Acute bronchitis is primarily caused by viruses, and the administration of antimicrobial drugs is not necessary. A meta-analysis compared adults to whom antimicrobial drugs were administered for bronchitis treatment with non-treated adults, and indicated that there was no difference in the efficacy [376]. Few reports on clinical studies involving children have been published, and the scale is small; objective data are insufficient, but no study has reported that antimicrobial drugs are effective [377], [378], [379]. However, if secondary bacterial infection following viral infection causes fever, purulent sputum, leukocytosis, or an increase in the CRP level, antimicrobial drugs should be administered, considering S. pneumoniae and H. influenzae.
Other indications for antimicrobial drug administration include M. pneumoniae-, C. pneumoniae-, or B. pertussis-related bronchitis with protracted cough. As M. pneumoniae- or C. pneumoniae-related bronchitis tends to show spontaneous cure, the administration of antimicrobial drugs is not always necessary, but the necessity of administration should be evaluated, considering the severity of symptoms and course (With respect to indications and administration methods, refer to the section “3. Pneumonia (Children)”.). First-choice drugs for M. pneumoniae-, C. pneumoniae-, or B. pertussis-related bronchitis are macrolides. In patients with B. pertussis-related bronchitis, antimicrobial drugs relieve symptoms only during the catarrhal period, but, if B. pertussis-related bronchitis is suspected based on clinical symptoms, previous vaccination, lymphocyte-predominant leukocytosis, an anti-PT antibody titer, and LAMP findings, antimicrobial drugs should be used. However, 16-membered ring macrolides are ineffective for B. pertussis-related bronchitis.
Symptoms are similar to those of pneumonia, but, when chest X-ray does not show any abnormalities, or, when it is impossible to strictly differentiate bronchitis from pneumonia due to difficulty in chest X-ray, treatment should be performed in accordance with pneumonia.
- - - Drugs to be recommended- - -
-
1.
Empiric therapy
Secondary bacterial infection after viral infection (Cases in which fever, purulent sputum, leukocytosis, or an increase in the CRP level is observed)-
♦First choices
-
-AMPC, oral, 10–15 mg/kg/3 times a day
-
-SBTPC, oral, 10 mg/kg/3 times a day
-
-CDTR-PI, oral, 3 mg/kg/3 times a day
-
-CFPN-PI, oral, 3 mg/kg/3 times a day
-
-CFTM-PI, oral, 3 mg/kg/3 times a day
-
-
-
<>Second choices
-
-AZM, oral, 10 mg/kg/once a day, 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-
-
♦
-
2.Definitive therapy
-
➀B. pertussis
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-AZM, oral, 10 mg/kg/once a day, 3 days
-
-
-
➁M. pneumoniae
-
▪Macrolide-sensitive strains
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day, 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-
-
▪Macrolide-resistant strains
-
-MINO, oral or intravenous drip, 1–2 mg/kg/twice a day (In children aged 7 years or younger, the use of this drug is limited to those in whom other drugs cannot be used or non-responders.)
-
-TFLX, oral, 6 mg/kg/twice a day (Administration is limited to children aged 7 years or younger in whom MINO cannot be used.)
-
-
-
▪
-
➂Chlamydia (C. pneumoniae, C. psittaci, C. trachomatis)
-
-EM, oral, 10–15 mg/kg/3 times a day
-
-AZM, oral, 10 mg/kg/once a day, 3 days
-
-CAM, oral, 7.5 mg/kg/twice a day
-
-
-
➀
8. Influenza
8.1. Adults
- - - Executive summary- - -
-
-
Both M2 protein inhibitors and neuraminidase inhibitors (NAIs) are commercially available as anti-influenza drugs as of July 2013.
-
-
Influenza viruses A (H3N2) and A (H1N1) pdm09 (seasonal influenza) have been reported to be resistant to amantadine, an M2 protein inhibitor which can be used in Japan. The use of this drug as an anti-influenza drug should be avoided for a while [380], [381].
-
-
During the influenza outbreak period, anti-influenza therapy should be promptly started based on a clinical diagnosis even when patients with influenza-like symptoms show negative results on a rapid diagnosis kits (because influenza cannot be completely ruled out) [382] (AI).
-
-
NAIs significantly improve influenza survival, and NAI administration within 2 days after onset significantly reduces the rate at which the condition becomes severe [383], [384] (AI).
-
-Currently, the following NAIs can be selected in Japan. During the outbreak period, an appropriate drug should be selected based on the patient background and latest information on a prevalent influenza strain:
-
-Oseltamivir (oral), Efficacy: A (H1N1) pdm09, A (H3N2), B, Resistance: H275Y mutant
-
-Zanamivir (inhalation), Efficacy: type A/B
-
-Laninamivir (inhalation), Efficacy: type A/B
-
-Peramivir (intravenous drip), Efficacy: type A/B
-
-
- - - Drugs to be recommended- - -
There is no meta-analysis of anti-influenza drugs other than oseltamivir and zanamivir as of July 2013. However, it has been shown that the early introduction of anti-influenza therapy for influenza significantly inhibits not only the mortality and admission rates but also the incidences of influenza-associated pneumonia, otitis media, and ischemic heart disease in comparison with symptomatic therapy. NAIs such as laninamivir and peramivir have also been confirmed to be as effective as oseltamivir at the time of development [385] (AI).
-
<>Outpatient treatment
-
-Oseltamivir, oral, 75 mg/twice a day, 5 days (As a rule, administration to children/adolescents aged 10–19 years should be avoided.)
-
-Zanamivir, inhalation, 10 mg/twice a day, 5 days
-
-Laninamivir, inhalation, 40 mg/single dose
-
-Peramivir, intravenous drip, 300 mg/single dose
-
-
-
<>Hospital treatment
-
➀Patients with severe, life-threatening influenzaIn patients with severe, life-threatening influenza requiring admission, respiratory failure or encephalopathy is present. In either case, the complication must be treated, but, as a rule, NAIs should be introduced within 48 h after the onset of influenza to obtain their effects.
-
-Oseltamivir, oral, 75 mg/twice a day, 5 days (As a rule, administration to children/adolescents aged 10–19 years should be avoided.)
-
-Peramivir, intravenous drip, 600 mg/single dose (This drug can be repeatedly administered every day in accordance with symptoms.)
-
-
-
➁Non-life-threatening influenza patients with pneumonia
-
-Oseltamivir, oral, 75 mg/twice a day, 5 days (As a rule, administration to children/adolescents aged 10–19 years should be avoided.)
-
-Peramivir, intravenous drip, 300 mg (600 mg for patients in whom the condition may become severe)/single dose (This drug can be repeatedly administered every day in accordance with symptoms.)
-
-
-
➂Non-life-threatening influenza patients without pneumonia
-
-Oseltamivir, oral, 75 mg/twice a day, 5 days (As a rule, administration to children/adolescents aged 10–19 years should be avoided.)
-
-Zanamivir, inhalation, 10 mg/twice a day, 5 days
-
-Laninamivir, inhalation, 40 mg/single dose
-
-Peramivir, intravenous drip, 300 mg/single dose (This drug can be repeatedly administered every day in accordance with symptoms.)
-
∗Patients with A (H7N9)
-
∗
-
-Basic treatment is the early administration of anti-influenza drugs [386].
-
-According to an article, there were no significant differences in the viral level or mortality rate 5 days after the start of administration between double- and standard-dose oseltamivir therapies [387]. However, treatment was not started within 48 h after onset in all patients in the article.
-
-
-
➀
8.2. Children
- - - Executive summary- - -
Influenza is caused by influenza virus. Antimicrobial drugs are not necessary. It is recommended that neuraminidase inhibitors should be administered within 48 h after onset (AI).
If pneumonia or otitis media occurs through secondary bacterial infection, the administration of antimicrobial drugs must be considered (BIII).
- - - Explanation- - -
[Characteristics and classification of the disease]
Influenza often appears with sudden fever and shivering/headache/general malaise/muscular pain/dry cough, followed by marked respiratory or digestive symptoms. In underlying disease-free children, recovery is achieved after 3–7 days [388], [389]. However, during the outbreak period, even underlying disease-free children are often admitted with serious symptoms such as encephalopathy, myocarditis, and pneumonia requiring artificial respiration. Although the outbreak period is from December until March every year, outbreaks are observed in the summer in some areas [388], [389].
[Type and frequency of causative microorganisms]
Since influenza A (H1N1) pdm09 prevailed in 2009, the outbreaks of 3 types of influenza, A (H1N1) pdm09, A (H3N2), and B, have been repeated. Their incidences differ among years.
[Rules of antimicrobial drug therapy]
No antimicrobial drug is indicated for influenza. It is recommended that neuraminidase inhibitors should be administered within 48 h after onset [390]. The doses of neuraminidase inhibitors approved in Japan are presented in Table 22 . During the course of influenza, bacterial infection such as pneumonia and otitis media may occur. Previously, the incidence of secondary bacterial infection in children exceeded 10% [391], but it has been 3% or less since neuraminidase inhibitors were developed [392], [393]. However, bacterial infection is observed in 25–33% of severe-status patients requiring intensive care [394], [395]. Streptococcus pneumoniae, S. aureus, and H. influenzae are frequently detected as causative microorganisms [388], [389], [394], [395].
Table 22.
Standard doses of neuraminidase inhibitors in children.
| Drug name | Administration method | Dose |
|---|---|---|
| Oseltamivira | Oral | 2 mg/kg, twice a day, 5 days (The use of this drug should be avoided in children/adolescents aged 10–19 years.) |
| Zanamivirb | Inhalation | 10 mg, twice a day, 5 days |
| Laninamivir | Inhalation | 10 years or older: 40 mg 9 years or younger: 20 mg, single dose |
| Peramivir | Intravenous drip | 10 mg/kg, once a day |
The preventive administration of this drug at 2 mg/kg (once a day) for 10 days was approved, but is not covered by health insurance.
The preventive administration of this drug at 10 mg (once a day) for 10 days was approved, but is not covered by health insurance.
No study has demonstrated that the prophylactic administration of antimicrobial drugs at the onset of influenza prevents secondary bacterial infection. Among children with influenza, antimicrobial drug therapy should be considered in those in whom signs of pneumonia or otitis media do not subside despite the administration of a neuraminidase inhibitor.
9. Parasitic diseases of the respiratory system
- - - Executive summary- - -
-
-
Parasitic diseases are widely distributed throughout the world. In addition to domestic infection, Japanese travelers may be infected in overseas endemic areas. Furthermore, parasitic diseases always considered in foreign patients from endemic areas [396] (BIV).
-
-
When peripheral blood eosinophilia is observed in addition to respiratory symptoms and abnormal findings of the chest imaging, examinations should be performed to differentiate parasitic diseases [396] (BIV).
-
-
Parasitic diseases of the respiratory system are caused by Paragonimus spp., Ascaris lumbricoides, Ancylostoma duodenale, Necator americanus, Strongyloides stercoralis, Toxocara canis, Toxocara cati, Wuchereria bancrofti, Brugia malayi, or Dirofilaria immitis.
-
-
There are two major diagnostic tests for parasitic diseases: (1) detection of parasite eggs or larvae in sputum or stools, and (2) detection of parasite-specific antibodies using serum or pleural effusion samples (immunodiagnosis).
-
-
An anti-parasite antibody screening test against 12 species of parasite is commercially available; Paragonimus spp., Strongyloides sp., T. canis, D. immitis, Ascaris suum, Anisakis simplex, Gnathostoma spp., Fasciola hepatica, Clonorchis sinensis, Spirometra erinaceieuropaei, and Cysticercus cellulosae.
-
-
Diagnosis/treatment consultations regarding parasitic diseases by the Japanese Society of Parasitology are available (as of 2013). Refer to the homepage (http://jsp.tm.nagasaki-u.ac.jp).
- - - Explanation- - -
-
■
Paragonimus spp.
Several Paragonimus species are known to cause human infection. Paragonimus westermani and P. miyazakii are distributed in Japan. Infection occurs through the consumption of freshwater crabs (intermediate host: Eriocheir japonica, E. sinensis, Geothelphusa dehaani) or wild boars (paratenic host:Sus scrofa) contaminated with metacercaria (infective larvae) as a raw or insufficiently cooked food [397].
Typical symptoms are cough, sputum, thoracic pain, and exertional dyspnea. In patients with such symptoms, the presence of peripheral blood eosinophilia suggests this disease. In many cases, a diagnosis is made based on the peripheral blood eosinophilia, a history taking of food and immunodiagnosis. Some of paragonimiasis patients are asymptomatic, and the presence of lung lesions is detected on a health checkup. Extrapulmonary paragonimiasis such as cutaneous and cerebral paragonimiasis are classically known form of the ectopic infection with Paragonimus spp. In cases of cutaneous paragonimiasis, a slowly moving nodular lesion in the subcutaneous tissue is a characteristic symptom. The worms may migrate through mediastinal soft tissues to the brain, causing eosinophilic meningitis or cerebral paragonimiasis in some cases [397]. Since Paragonimus spp. is widely distributed around the world, Japanese travel to endemic area such as China, Korea, Thailand, and Philippines possible to infect with Paragonimus spp. As well as foreign patients from endemic areas, this disease always is considered when a patient has respiratory symptoms and/or lung lesions with eosinophilia [398].
Chest X-ray findings vary: not only pulmonary parenchymal lesions, such as nodular (±cavity formation) and infiltrative shadows, but also pleural lesions, such as the retention of pleural effusion and pneumothorax, are sometimes observed [399]. Many patients show peripheral blood eosinophilia and/or elevated serum total IgE. Immunodiagnosis to detect parasite-specific antibody has been proven as the most useful and reliable tool. Not only patient's serum but also pleural effusion could examine by immunological test. Commercially available anti-parasite antibody screening test is including Paragomimus spp. In Japan, egg-detection rate among paragonimiasis patients nowadays is low; for example 51.2% in sputum and 53.8% in BALF [66], 66.7% in bronchoscopic aspirate [397], [399].
After a definitive diagnosis is made, oral treatment should be started. The type of treatment to be selected, outpatient or hospital treatment, depends on the patient's general condition. However, usually, outpatient treatment is possible. In patients having pleural effusion, pleural fluid must be extensively drained off before starting chemotherapy [400] (BIII). In patients with chronically encapsulated pleural effusion, surgery is required [401] (CIII).
- - - Drugs to be recommended- - --
♦First choice (same dose for adults and children)
-
-Praziquantel, oral, 25 mg/kg/3 times a day, 2–3 days [402] (AIII)
-
-
-
♦
-
■
A. lumbricoides, A. duodenale, N. americanus
The larvae of these parasites pass through the lung in the human body, causing asthma- or pneumonia-like symptoms such as transient fever, cough, and dyspnea. On chest X-ray, transient nodular/infiltrative shadows are observed. Many patients show peripheral blood eosinophilia and/or elevated serum total IgE. Previously, the condition was called Loffler syndrome. Currently, it is classified as simple pulmonary eosinophilia in the category of PIE syndrome [402].
Symptoms appear 1–2 weeks after the oral ingestion of A. lumbricoides embryonated eggs. The latency period of percutaneous infection with N. americanus larvae or percutaneous/oral infection with A. duodenale larvae is approximately 10 days. Larvae appeared in the small intestine penetrate the intestinal wall, enter the portal vein, migrate through liver to heart/lung. Larvae penetrate the human skin transfer to the lung with blood flow. Then these larvae migrate up the bronchi and trachea, over the epiglottis, down the esophagus/stomach, and reach the small intestine. In the intestine the larvae develop into mature adults [403].
Simple pulmonary eosinophilia related to parasites spontaneously resolved in a few weeks. In many cases, a definitive diagnosis is made based on parasite eggs detected on a stool examination, although larvae are sometimes detected in sputum [403].
- - - Drugs to be recommended- - --
♦First choice (same dose for adults and children)
-
-Pyrantel pamoate, oral, 10 mg/kg/single-dose administration
-
∗Dry syrup for children is available.
-
∗This drug is effective for adult worms in the intestinal tract, but not for larvae migrating in the human body. Therefore, a stool examination should be performed 2 weeks after oral administration. If parasite eggs are detected, additional administration should be conducted.
-
∗
-
-
-
<>Second choices (same dose for adults and children)
-
-Albendazol, oral, 400 mg/single-dose administration
-
-Mebendazole, oral, 100 mg/twice a day, 3 days, or 500 mg/single-dose administration
-
-Ivermectin, oral, 150–200 μg/kg/single-dose administration (after fasting)
-
-
-
♦
-
■
S. stercoralis
In Japan, S. stercoralis is distributed in Nansei Islands, chain of islands extending from southwestern Kyushu to northern Taiwan. There are few young persons newly infected with S. stercoralis, but the incidence of infection is high in elderly persons [404]. Internationally, S. stercoralis is widely distributed in tropical/subtropical areas. Not only domestic infection but also Japanese travel to endemic area possible to infect with this parasite. Foreign patients from endemic areas, strongyloidiasis always considered when a patient has respiratory symptoms and/or lung lesions with eosinophilia.
S. stercoralis percutaneously infects humans. Larvae penetrate the human skin transfer to the lung with blood flow. Then these larvae migrate up the bronchi and trachea, over the epiglottis, down the esophagus/stomach, and reach the small intestine. In the intestine the larvae develop into mature adults. Parasite eggs delivered by adults are hatched while descending the intestinal tract, and larvae excreted in stools. Some larvae developed to infective form again invade/transfer through the mucosa around the anus, maintaining a life cycle in the human body. Such a mode of infection is termed autoinfection.
With the transfer of larvae to the lung, asthma- or pneumonia-like symptoms, such as transient fever, cough, and dyspnea, appear, as indicated for simple pulmonary eosinophilia related to A. lumbricoides, A. duodenale, or N. americanus. On chest X-ray, transient nodular/infiltrative shadows are observed. Many patients show peripheral blood eosinophilia and/or an increase in the total IgE level.
In immunocompromised patients, suppressing the cell-mediated immunity such as ATL patients, HIV/AIDS patients, those receiving immunosuppressive drugs, the number of S. stercoralis increases through acceleration of autoinfection, and larvae are disseminated in various organs, leading to a severe condition (disseminated strongyloidiasis). In such cases, stridor, bloody sputum, tachypnea, protein-losing gastroenteritis, ileus, and mobile exanthema are observed. Furthermore, severe pneumonia related to enteric bacteria disseminated with larvae, lung abscess, or bacterial meningitis concomitantly occurs [403]. A diagnosis is made based on S. stercoralis larvae detected in stools/sputum. Immunological diagnosis is also useful [403].
- - - Drugs to be recommended- - --
♦First choice (same dose for adults and children)
-
-Ivermectin, oral, 200 μg/kg/single-dose administration (after fasting), additional administration at the same dose after 2 weeks (AI)
-
-
-
<>Second choice (same dose for adults and children)
-
-Albendazol, oral, 400 mg/twice a day, 7 days (BI)
-
-
-
♦
-
■
T. canis, T. cati
Infection to humans occurs through the oral ingestion of the embryonated eggs of T. canis or T. cati [407]. It also occurs through the consumption of beef/chicken liver or meat contaminated with larvae as a raw or insufficiently heated food. This is considered to be a dominant route of infection in Japan [407]. As humans are not definitive host for T. canis and T. cati, they do not become adults in the human body [408]. Larvae invading the human body transfer to various organs with blood flow through the intestinal mucosa. Target organs are the lung, liver, eyes, and central nervous system including spinal cord. Toxocariasis is a typical larva migrans.
Lesions are often observed in the lung/liver (visceral larva migrans). There are few symptoms, or nonspecific symptoms are present. In many cases, a diagnosis is made based on peripheral blood eosinophilia and multiple nodular shadows of the lung or liver on CT. Ocular or central nervous symptoms appear in some patients. It is impossible to make a diagnosis on a stool examination due to larva migrans. Immunological diagnosis is useful. After a definitive diagnosis is made, treatment should be started. When visceral larva migrans is asymptomatic, follow-up may be continued.
- - - Drugs to be recommended- - --
♦First choice (same dose for adults and children)
-
-Albendazol, oral, 5 mg/kg/2 or 3 times a day, 4–8 weeks [409] (BIII)
-
∗This drug should be taken with meals for 28 days. A 14-day period of discontinuation should be established and then restarted drug if required.
-
∗
-
-
- <>
-
♦
-
■
W. bancrofti, B. malayi
W. bancrofti and B. malayi are called lymphatic filaria. Infection occurs when these parasites are transmitted to humans through mosquitoes. The pathogenesis of lymphatic filariasis is relate to structural and functional abnormality of lymphatic channels induced by parasitized adult worms. Infection with W. bancrofti is characterized by febrile attacks, lymphedema/elephantiasis, hydrocele, and chyluria. In patients infected with B. malayi, neither hydrocele nor chyluria is observed, and lymphedema of the lower limbs/elephantiasis are localized in the lower thighs. Microfilaria produced by adult worms is not pathogenic, but rarely induces allergic reactions in the lung, contributing to tropical pulmonary eosinophilia (TPE) [403]. This condition shows a chronic course, differing from simple pulmonary eosinophilia. Cough, dyspnea, and stridor with exacerbation at night are observed. Fever, malaise, and weight loss are noted in some patients. On chest X-ray, bilateral reticular nodular lesions are detected. Peripheral blood eosinophilia and an increase in the anti-filaria antibody titer are observed. In case of TPE, no microfilaria is detected [403]. If treatment is not performed, the condition may gradually exacerbate. It is important to adequately make a diagnosis for the differentiation of this disease. In the world, 130,000,000 persons are infected with lymphatic filaria, but TPE occurs in less than 0.5% of these. The risk of this disease in travelers is unclear, and most patients consist of foreign persons from endemic areas [412].
- - - Drugs to be recommended- - --
♦First choice (same dose for adults and children)
-
-Diethylcarbamazine, 2 mg/kg/3 times a day, 12 days
-
∗In cases of malayan filariasis, marked side effects, such as digestive symptoms, fever, lymphangitis/lymphadenitis, and orchitis/epididymitis, may appear. Therefore, a half dose (1 mg/kg) should be administered 3 times a day for 12 days [413].
-
∗
-
-
-
♦
-
■
D. immitis
D. immitis parasitize in the dog right ventricle and pulmonary artery, where mature female worms produce microfilaria that circulate in peripheral blood. Infection to humans is mediated by microfilaria-ingesting mosquitoes. D. immitis larvae in human subcutaneous tissue inserted by mosquito bite, some larvae migrate to the heart and die. Dead worms produce infarcts then they lodge in pulmonary vessels. There are few symptoms [402]. In patients with symptoms, thoracic pain, cough, bloody sputum, stridor, and fever have been reported. Typical chest X-ray findings include solitary coin lesions. In many cases, an abnormal shadow of the chest X-ray is indicated on a health checkup, and D. immitis infection is pathologically diagnosed from a tissue specimen extirpated under a tentative diagnosis of lung cancer. At this point, peripheral blood eosinophilia is rarely observed [403].
- - - Drugs to be recommended (same dose for adults and children)- - --
-As a diagnosis is made using pathological specimens in many patients, oral treatment is not necessary.
-
-If necessary, diethylcarbamazine at 2 mg/kg should be orally administered 3 times a day for 12 days.
- - - - Precautions for each drug- - -
-
➀PraziquantelIn the Japanese package inserts, it is described that this drug at 40 mg/kg/day should be orally administered twice a day for 2 days for the treatment of paragominiasis. However, we recommend administration at 75 mg/kg/day (3 times a day) for 2–3 days based on Reference No. 400.The incidence of side effects is low, but fever, abdominal discomfort, nausea, diarrhea, and headache are sometimes observed.Drug to be contraindicated for combination therapy: RFPPrecautions for combination therapy: Decrease in the blood concentration: dexamethasone, phenytoin, carbamazepine, chloroquineIncrease in the blood concentration: cimetidine
-
➁Pyrantel pamoatePatients can take this drug regardless of meals. There are few side effects.
-
➂AlbendazolThis drug should be taken with meals for 28 days. A 14-day period of discontinuation should be established and then restarted drug if required.As liver dysfunction is frequently observed, caution is needed during the administration period. Bone marrow suppression and Stevens-Johnson syndrome must also be considered.Precautions for combination therapy: Decrease in the blood concentration: ritonavir, phenytoin, carbamazepine, phenobarbitalIncrease in the blood concentration: praziquantel
-
➃MebendazoleAlthough this drug has been used in few children, the same dose as established for adults is used. In children weighing 20 kg or less, a half dose should be used. This drug is contraindicated for pregnant women or those who may be pregnant. Combination therapy with cimetidine may increase the blood concentration of mebendazole. Combination therapy with metronidazole may cause toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome.
-
➄IvermectinAfter fasting, this drug should be taken with water. The incidence of side effects is low, but nausea/vomiting and mild hepatic disorder are sometimes observed. The safety of this drug in pregnant women or children weighing less than 15 kg has not been established. To these patients, this drug should be administered only when its therapeutic advantage is considered to exceed its risk.
-
➅DiethylcarbamazineAs side effects, fever, lymphangitis/lymphadenitis, and orchitis/epididymitis, which result from anti-parasitic actions, are observed in addition to abdominal discomfort, nausea, and abdominal pain. The safety of this drug in pregnant women has not been established.
-
-
Conflict of interest
Nobuki Aoki:
Speaker's honorarium from Daiichi Sankyo Co., Ltd.
Yosuke Aoki:
Speaker's honorarium from MSD K.K. and Taisho Toyama Pharmaceutical Co., Ltd.
Satoshi Iwata:
Speaker's honorarium from Taisho Toyama Pharmaceutical Co., Ltd., MSD K.K., Glaxo SmithKline K.K.
Grant from Nikon Corporation.
Kazunobu Ouchi:
Speaker's honorarium from Taisho Toyama Pharmaceutical Co., Ltd., Glaxo SmithKline K.K., Pfizer Japan Inc., and Meiji Seika Pharma CO., Ltd.
Junichi Kadota:
Speaker's honorarium from Pfizer Japan Inc., Taisho Toyama Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., MSD K.K., and Daiichi Sankyo Co., Ltd.
Manuscript honorarium from Nankodo Co., Ltd.
Donation from Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc.
Osamu Kobayashi:
Leadership position at Toyama Chemical Co., Ltd.
Hiroshi Sakata:
Speaker's honorarium from Meiji Seika Pharma Co., Ltd.
Masahumi Seki:
Speaker's honorarium from Shionogi & Co., Ltd., and Taisho Toyama Pharmaceutical Co., Ltd.
Katsunori Yanagihara:
Speaker's honorarium from Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Taisho Toyama Pharmaceutical Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd.
Fund from Meiji Seika Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., and Pfizer Japan Inc.
Koichiro Yoshida:
Speaker's honorarium from Sumitomo Dainippon Pharma Co., Ltd.
Donation from Sumitomo Dainippon Pharma Co., Ltd.
Doses for neonates
Upper row: Dose (mg/kg), Lower row: Frequency of administration per day
| Body weight | < 1.2 kg | 1.2∼2 kg | ≧2 kg | ||
| After birth (days) | ∼28 | ∼7 | 7∼28 | ∼7 | 7∼28 |
| ABPC | |||||
| Meningitis (Cases in which meningitis cannot be ruled out) | 50 2 |
50 2 |
50 3 |
50 3 |
50 4 |
| Other infectious diseases | 25 2 |
25 2 |
25 3 |
25 3 |
25 4 |
| SBT/ABPC | - - |
50 2 |
50 2 |
50 2 |
50 2 |
| TAZ/PIPC | - - |
100 2 |
100 3 |
100 2 |
100 2 |
| CEZ | 20 2 |
20 2 |
20 2 |
20 3 |
|
| CTX | 50 2 |
50 2 |
50 3 |
50 2 |
50 4 |
| CTRX | 50 1 |
50 1 |
50 1 |
75 1 |
|
| CAZ | 50 2 |
50 2 |
50 3 |
50 3 |
50 3 |
| CZOP | |||||
| Severe/refractory infectious disease | 40 3∼4 |
40 3∼4 |
40 3∼4 |
40 3∼4 |
|
| Other infectious diseases | 20 2∼3 |
20 3∼4 |
20 2∼3 |
20 3∼4 |
|
| MEPM | |||||
| Meningitis | 40 3 |
40 3 |
40 3 |
40 3 |
40 3 |
| Other infectious diseases | 20 2 |
20 3 |
20 3 |
30 3 |
30 3 |
| IPM/CS | - - |
20 2 |
20 2 |
20 2 |
20 3 |
| GM | 2.5 Every 18 h |
2.5 2 |
2.5 3 |
2.5 2 |
2.5 3 |
| (Peak 5∼15 μg/mL, Trough value< 2 μg/mL) | |||||
| ABK | 2∼6 1 |
2∼6 1 |
2∼6 1 |
2∼6 1 |
|
| (Peak 9∼12 μg/mL, Trough value< 2 μg/mL) | |||||
| AMK | 7.5 2 |
7.5 2 |
7.5 3 |
10 2 |
10 3 |
| (Peak 20∼30 μg/mL, Trough value< 10 μg/mL) | |||||
| TOB | 2.5 Every 1 h or 18 h |
2 Every 2 h or 18 h |
2 2∼3 |
2 2 |
2 3 |
| (Peak 5∼12 μg/mL, Trough value< 2 μg/mL) | |||||
| VCM | 15 1 |
10 2 |
10 2 |
10 3 |
10 3 |
| (Trough value< 10∼20 μg/mL) | |||||
| TEIC | First dose: 16 mg/kg, Subsequently, the drug at 8 mg/kg should be administered over 30 minutes or more at 24-h intervals. | ||||
| CLDM | 5 2 |
5 3 |
5 3 |
5 4 |
|
| AZT | 30 2 |
30 3 |
30 2 |
40 3 |
|
| ACV | 20 2 |
20 3 |
20 3 |
20 3 |
|
| AMPH-B (Daily dose) | 1 | 1 | 1 | 1 | |
| L-AMB (Daily dose) | 5 | 5 | 5 | 5 | |
| FLCZ | 12 Every 48 h |
12 1 |
12 Every 48 h |
12 1 |
|
| EM (Oral) | - - |
10 2 |
10 3 |
10 2 |
10 3 |
References
- 1.Donowitz G.R. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Mandell G.L., Bennett J.E., Dolin R., editors. Churchill Livingstone Elsevier; Philadelphia: 2010. Acute pneumonia; pp. 891–916. [Google Scholar]
- 2.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C., et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The committee for the Japanese Respiratory Society guidelines for the management of respiratory infections . The Japanese Respiratory Society; Tokyo: 2006. The Japanese respiratory society guidelines for the management of community-acquired pneumonia in adults. [Google Scholar]
- 4.Watanabe A., Goto H., Kohno S., Matsushima T., Abe S., Aoki N., et al. Nationwide survey on the 2005 guide-lines for the management of community-acquired adult pneumonia : validation of differentiation between bacterial pneumonia and a typical pneumonia. Respir Investig. 2012;50:23–32. doi: 10.1016/j.resinv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Kohno S., Seki M., Watanabe A., CAP Study Group Evaluation of an assessment system for the JRS 2005: a-DROP for the management of CAP in adults. Intern Med. 2011;50:1183–1189. doi: 10.2169/internalmedicine.50.4651. [DOI] [PubMed] [Google Scholar]
- 6.Gleckman R., DeVita J., Hibert D., Pelletier C., Martin R. Sputum gram stain assessment in community-acquired bacteremic pneumonia. J Clin Microbiol. 1988;26:846–849. doi: 10.1128/jcm.26.5.846-849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Vázquez E., Marcos M.A., Mensa J., de Roux A., Puig J., Font C., et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164:1807–1811. doi: 10.1001/archinte.164.16.1807. [DOI] [PubMed] [Google Scholar]
- 8.Helbig J.H., Uldum S.A., Lück P.C., Harrison T.G. Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urin Antigen EIA. J Med Microbiol. 2001;50:509–516. doi: 10.1099/0022-1317-50-6-509. [DOI] [PubMed] [Google Scholar]
- 9.Marcos M.A., Jiménez de Anta M.T., de la Bellacasa J.P., González J., Martínez E., Grancia E., et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21:209–214. doi: 10.1183/09031936.03.00058802. [DOI] [PubMed] [Google Scholar]
- 10.Seki M., Watanabe A., Mikasa K., Kadota J., Kohno S. Revision of the severity rating and classification of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology. 2008;13:880–885. doi: 10.1111/j.1440-1843.2008.01348.x. [DOI] [PubMed] [Google Scholar]
- 11.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . The Japanese Respiratory Society; 2011. The guidelines for the management of nursing and healthcare-associated pneumonia. [Google Scholar]
- 12.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . The Japanese Respiratory Society; 2007. The guidelines for the management of hospital-acquired pneumonia in adults. [Google Scholar]
- 13.Watanabe A., Yanagihara K., Matsumoto T., Kohno S., Aoki N., Oguri T., et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Surveillance Committee of Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Clinical Microbiology in 2009: general view of the pathogens' antibacterial susceptibility. J Infect Chemother. 2012;18:609–620. doi: 10.1007/s10156-012-0434-3. [DOI] [PubMed] [Google Scholar]
- 14.Saito A., Kohno S., Matsushima T., Watanabe A., Oizumi K., Yamaguchi K., et al. Prospective multi center study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother. 2006;12:63–69. doi: 10.1007/s10156-005-0425-8. [DOI] [PubMed] [Google Scholar]
- 15.Higashiyama Y., Watanabe A., Aoki N., Niki Y., Kohno S. Clinical evaluation of levofloxacin versus oral β-lactams for acute exacerbation of COPD. Jpn J Chemother. 2009;56:33–48. [Google Scholar]
- 16.van der Heijden Y.F., Maruri F., Blackman A., Holt E., Warkentin J.V., Shepherd B.E., et al. Fluoroquinolone exposure prior to tuberculosis diagnosis is associated with an increased risk of death. Int J Tuberc Lung Dis. 2012;16:1162–1167. doi: 10.5588/ijtld.12.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold F.W., Summersgill J.T., Lajoie A.S., Peyrani P., Marrie T.J., Rossi P., et al. A Worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;175:1086–1093. doi: 10.1164/rccm.200603-350OC. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Infectious Diseases, Infectious Disease Surveillance Center Infectious diseases to be focused on Mycoplasma pneumoniae. IDWR. 2012;39:7–9. [Google Scholar]
- 19.Martin-Loeches I., Lisboa T., Rodriguez A., Putensen C., Annane D., Garnacho-Montero J., et al. Combination anti-biotic therapy with macrorides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med. 2010;36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 20.Paterson D.L., Ko W.C., Von Gottberg A., Mohapatra S., Casellas J.M., Goossens H., et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis. 2004;30:31–37. doi: 10.1086/420816. [DOI] [PubMed] [Google Scholar]
- 21.Mandell G.L., Bennett J.E., Dolin R., editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Churchill Livingstone Elsevier; Philadelphia: 2010. [Google Scholar]
- 22.Goto H., Shimada K., Ikemoto H., Oguri T., the Study group on antimicrobial susceptibility of pathogens isolated from respiratory infections Antimicrobial susceptibility of pathogens isolated from more than 10000 patients with infectious respiratory diseases: a 25-year longitudinal study. J Infect Chemother. 2009;15:347–360. doi: 10.1007/s10156-009-0719-3. [DOI] [PubMed] [Google Scholar]
- 23.Clinical Laboratory Standard Institute . CLSI; 2013. Performance standards for antimicrobial susceptibility testing. Twenty-third informational supplement. M100–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallares R., Liñares J., Vadillo M., Cabellos C., Manresa F., Viladrich P.F., et al. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein M.P., Klugman K.P., Jones R.N. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with anti microbial susceptibility in an era of resistance. Clin Infect Dis. 2009;48:1596–1600. doi: 10.1086/598975. [DOI] [PubMed] [Google Scholar]
- 26.Lim W.S., Baudouin S.V., George R.C., Hill A.T., Jamieson C., Le Jeune I. BTS guidelines for the management of community acquired pneumonia in adults: 2009 update. Thorax. 2009;64:1–55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 27.Petitpretz P., Arvis P., Marel M., Moita J., Urueta J., CAP 5 Moxifloxacin Study Group Oral moxifloxacin vs high-dosage amoxicillin in the treatment of mild-to-moderate, community-acquired, suspected pneumococcal pneumonia in adults. Chest. 2001;119:185–195. doi: 10.1378/chest.119.1.185. [DOI] [PubMed] [Google Scholar]
- 28.Taba H., Kusano N. Spafloxacin resistance in clinical isolates of Streptococcus pneumoniae: involvement of multiple mutations in gyrA and parC genes. Antimicrob Agents Chemother. 1998;42:2193–2196. doi: 10.1128/aac.42.9.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niki Y., Hanaki H., Matsumoto T., Yagisawa M., Kohno S., Aoki N., et al. Nationwide surveillance of bacterial respiratory pathogens condected by Japanese Society of Chemotherapy in 2008: general view of the pathogens' antibacterial susceptibility. J Infect Chemother. 2011;17:510–523. doi: 10.1007/s10156-011-0214-5. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda H., Yano H., Hirakata Y., Arai K., Endo S., Kanamori H., et al. Molecular characteristics of extended-spectrum β-lactamase-producing Escherichia coli in Japan: emergence of CTX-M-15-producing E. coli ST131. Diagn Microbiol Infect Dis. 2012;74:201–203. doi: 10.1016/j.diagmicrobio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Morozumi M., Takahasi T., Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother. 2010;16:78–86. doi: 10.1007/s10156-009-0021-4. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita N., Kawai Y., Akaike H., Ouchi K., Hayashi T., Kurihara T., et al. Macrolide-resistant Mycoplasma pneumoniae in adolescents with community-acquired pneumonia. BMC Infect Dis. 2012;12:126. doi: 10.1186/1471-2334-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada T., Morozumi M., Tajima T., Hasegawa M., Sakata H., Ohnari S., et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–1649. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino K., Inoue K., Murakami Y., Kurosaka Y., Namba K., Kashimoto Y., et al. In vitro and in vivo antibacterial activities of DC-159a, a new fluoroquinolone. Antimicrob Agents Chemother. 2008;52:65–76. doi: 10.1128/AAC.00853-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akaike H., Miyashita N., Kubo M., Kawai Y., Tanaka T., Ogita S., et al. In vitro activities of 11 antimicrobial agents against macrolide-resistant Mycoplasma pneumoniae isolates from pediatric patients: results from a multicenter surveillance study. Jpn J Infect Dis. 2012;65:535–538. doi: 10.7883/yoken.65.535. [DOI] [PubMed] [Google Scholar]
- 36.Blázquez Garrido R.M., Espinosa Parra F.J., Alemany Francés L., Ramos Guevara R.M., Sánchez-Nieto J.M., Segovia Hernández M., et al. Antimicrobial chemotherapy for Legionnaires disease: levofloxacin versus macrolides. Clin Infect Dis. 2005;40:800–806. doi: 10.1086/428049. [DOI] [PubMed] [Google Scholar]
- 37.Griffin A.T., Payrani P., Wiemken T., Arnold F. Macrolides versus quinolones in Legionella pneumonia: results from the community-acquired pneumonia organization international study. Int J Tuberc Lung Dis. 2010;14:495–499. [PubMed] [Google Scholar]
- 38.Rello J., Gattarello S., Souto J., Sole Violan J., Valles J., Peredo R., et al. Community-acquired Legionella pneumonia in the intensive care unit: impact on survival of combined anti biotic therapy. Med Intensiva. 2013;37:320–326. doi: 10.1016/j.medin.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Muder R.R., Yu V.L. Infection due to Legionella species other than L. pneumophila. Clin Infect Dis. 2002;35:990–998. doi: 10.1086/342884. [DOI] [PubMed] [Google Scholar]
- 40.Kuo C.C., Jackson L.A., Lee A., Grayston J.T. In vitro activities of azithromycin, clarithromycin, and other antibiotics against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1996;40:2669–2670. doi: 10.1128/aac.40.11.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mine Y., Nakasone I., Yamamoto Y., Utani A., Yamane N., Uezato H., et al. Dissemination of Panton-Valentine leukocidine positive metchicillin-resistant Staphylococcus aureus in Okinawa, Japan. J Dermatol. 2013;40:34–38. doi: 10.1111/j.1346-8138.2012.01569.x. [DOI] [PubMed] [Google Scholar]
- 42.Schweizer M.L., Furuno J.P., Harris A.D., Johnson J.K., Shardell M.D., McGregor J.C., et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in metchicillin susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinzato T., Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Inf Dis. 1995;21:S238–S243. doi: 10.1093/clind/21.supplement_3.s238. [DOI] [PubMed] [Google Scholar]
- 44.Barnham M., Weightman N., Anderson A., Pagan F., Chapman S. Review of 17 cases of pneumonia caused by Streptococcus pyogenes. Eur J Clin Microbiol Infect Dis. 1999;18:506–509. doi: 10.1007/s100960050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wajima T., Maruyama S.Y., Sunaoshi K., Nakayama E., Sunakawa K., Ubukata K. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and non invasive diseases. J Med Microbiol. 2008;57:1383–1388. doi: 10.1099/jmm.0.2008/002642-0. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N., Fujita J., Shinzato T., Higa F., Tateyama M., Tohyama M., et al. In vitro activities of sitafloxacin compared with several fluoroquinolones against Streptococcus anginosus and Streptococcus constellatus. Int J Antimicrob Agents. 2006;27:171–173. doi: 10.1016/j.ijantimicag.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Verduin C.M., Hol C., Fleer A., van Dijk, van Belkum A. Moraxella catarrhalis: from emerging to established pathogen. Clin Microbiol Rev. 2002;15:125–144. doi: 10.1128/CMR.15.1.125-144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuriyama T., Williams D.W., Yanagisawa M., Iwahara K., Shimizu C., Nakagawa K., et al. Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Microbiol Immunol. 2007;22:285–288. doi: 10.1111/j.1399-302X.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 49.Arancibia F., Bauer T.T., Eqig S., Mensa J., Gonzalez J., Niederman M.S., et al. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Inter Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 50.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . The Japanese Respiratory Society; Tokyo: 2008. The guidelines for the management of hospital-acquired pneumonia in adults. [Google Scholar]
- 51.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 52.Masterton R.G., Galloway A., French G., Street M., Armstrong J., Brown E., et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2008;62:5–34. doi: 10.1093/jac/dkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe A., Yanagihara K., Kohno S., Matsushima T. HAP study group: multicenter survey on hospital-acquired pneumonia and the clinical efficacy of first-line antibiotics in Japan. Intern Med. 2008;47:245–254. doi: 10.2169/internalmedicine.47.0577. [DOI] [PubMed] [Google Scholar]
- 54.Rello J., Paiva J.A., Baraibar J., Barcenilla F., Bodi M., Castander D., et al. International conference for the development of consensus on the diagnosis and treatment of ventilator-associated pneumonia. Chest. 2001;120:955–970. doi: 10.1378/chest.120.3.955. [DOI] [PubMed] [Google Scholar]
- 55.Chastre J., Fagon J.Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 56.Singh N., Rogers P., Atwood C.W., Wagener M.M., Yu V.L. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 57.Chastre J., Wolff M., Fagon J.Y., Chevret S., Thomas F., Wermert D., et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 58.Celis R., Torres A., Gatell J.M., Almela M., Rodríguez-Roisin R., Agustí-Vidal A. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93:318–324. doi: 10.1378/chest.93.2.318. [DOI] [PubMed] [Google Scholar]
- 59.Venditti M., Falcone M., Corrao S., Licata G., Serra P., Study Group of the Italian Society of Internal Medicine Outcomes of patients hospitalized with community-acquired, health care-associated and hospital-acquired pneumonia. Ann Intern Med. 2009;150:19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 60.Kett D.H., Cano E., Quartin A.A., Mangino J.E., Zervos M.J., Peyrani P., et al. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11:181–189. doi: 10.1016/S1473-3099(10)70314-5. [DOI] [PubMed] [Google Scholar]
- 61.Fartoukh M., Maître B., Honoré S., Cerf C., Zahar J.R., Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–179. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 62.Blot F., Raynard B., Chachaty E., Tancrède H., Antoun S., Nitenberg G. Value of Gram stain examination of lower respiratory tract secretions for early diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 2000;162:1731–1737. doi: 10.1164/ajrccm.162.5.9908088. [DOI] [PubMed] [Google Scholar]
- 63.Chastre J., Fagon J.Y., Bornet-Lecso M., Calvat S., Dombret M.C., al Khani R., et al. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152:231–240. doi: 10.1164/ajrccm.152.1.7599829. [DOI] [PubMed] [Google Scholar]
- 64.Fagon J.Y., Chastre J., Wolff M., Gervais C., Parer-Aubas S., Stéphan F., et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia: a randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 65.Koulent D., Rello J. Hospital-acquired pneumonia in the 21st century: a review of existing treatment options and their impact on patient care. Expert Opin Pharmacother. 2006;7:1555–1569. doi: 10.1517/14656566.7.12.1555. [DOI] [PubMed] [Google Scholar]
- 66.Hayon J., Figliolini C., Combes A., Trouillet J.L., Kassis N., Dombret M.C., et al. Role of serial routine microbio-logic culture results in the initial management of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:41–46. doi: 10.1164/ajrccm.165.1.2105077. [DOI] [PubMed] [Google Scholar]
- 67.Cunha B.A. 3rd ed. Physician's Press; Boston: 2010. Pneumonia essentials. [Google Scholar]
- 68.Rotstein C., Evans G., Born A., Grossman R., Light R.B., Magder S., et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008;19:19–53. doi: 10.1155/2008/593289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The committee for the Japanese Society of Chemotherapy guidelines for TDM of antimicrobial agents. Guidelines for TDM of antimicrobial agents. http://www.chemotherapy.or.jp/guideline/tdm_executive-summary.pdf.
- 70.Marik P.E., Careau P. The role of an aerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115:175–183. doi: 10.1378/chest.115.1.178. [DOI] [PubMed] [Google Scholar]
- 71.Craven D.E., Chroneou A. In: Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Mandell G.L., Bennett J.E., Dolin R., editors. Churchill Livingstone Elsevier; Philadelphia: 2010. Nosocomial pneumonia; pp. 3717–3724. [Google Scholar]
- 72.O'Neal P.V., Brown N., Munro C. Physiologic factors contributing to a transition in oral immunity among mechanically ventilated adults. Biol Res Nurs. 2002;3:132–139. doi: 10.1177/1099800402003003003. [DOI] [PubMed] [Google Scholar]
- 73.Hull M.W., Chow A.W. Indigenous microflora and innate immunity of the head and neck. Infect Dis Clin N Am. 2007;21:265–282. doi: 10.1016/j.idc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Chastre J. Antimicrobial treatment of hospital-acquired pneumonia. Infect Dis Clin N Am. 2003;17:727–737. doi: 10.1016/s0891-5520(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 75.Cunha B.A. In: Nosocomial fever of unknown origin. Fever of unknown origin. Cunha B.A., editor. Informa health-care; NY: 2007. pp. 101–108. [Google Scholar]
- 76.Jacoby G., Bush K. In: Frontiers in antimicrobial resistance: a tribute to Stuart B. Levy. White D.G., Alekshun M.N., McDermott P.F., editors. ASM Press; Washington DC: 2005. β-lactam resistance in the 21st century; pp. 299–313. [Google Scholar]
- 77.Bush K., Jacoby G.A. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gould F.K., Brindle R., Chadwick P.R., Fraise A.P., Hill S., Nathwani D., et al. MRSA Working Party of he British Society for Antimicrobial Chemotherapy Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 79.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J., et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:1–38. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 80.Kohno S., Yamaguchi K., Aikawa N., Sumiyama Y., Odagiri S., Aoki N., et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother. 2007;60:1361–1369. doi: 10.1093/jac/dkm369. [DOI] [PubMed] [Google Scholar]
- 81.Kainer M.A., Devasia R.A., Jones T.F., Simmons B.P., Melton K., Chow S., et al. Response to emerging infection leading to outbreak of linezolid-resistant enterococci. Emerg Infect Dis. 2007;13:1024–1030. doi: 10.3201/eid1307.070019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobbs T., Patel M., Waites K.B., Moser S.A., Stamm A.M., Hoesley C.J. Nosocomial spread of Enterococcus faecium resistant to vancomycin and linezolid in a tertiary medical center. J Clin Microbiol. 2006;44:3368–3370. doi: 10.1128/JCM.00850-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonora M.G., Solbiati M., Stepan E., Zorzi A., Luzzani A., Rosaria M., et al. Emergence of linezolid resistance in the vancomycin-resistant Enterococcus faecium multilocus sequence typing C1 epidemic lineage. J Clin Micro Biol. 2006;44:1153–1155. doi: 10.1128/JCM.44.3.1153-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Funke G., von Graevenitz A., Clarridge J.E., III, Bernard K.A. Clinical microbiology of Coryneform bacteria. Clin Microbiol Rev. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wunderink R.G., Rello J., Cammarata S.K., Croos-Dabrera R.V., Kollef M.H. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. [PubMed] [Google Scholar]
- 86.Rubinstein E., Cammarata S., Oliphant T., Wunderink R.G., the Linezolid Nosocomial Pneumonia Study Group Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multi center study. Clin Infect Dis. 2001;32:402–412. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 87.Nathwani D., Morgan M., Masterton R.G., Dryden M., Cookson B.D., French G., et al. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61:976–994. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 88.Cunha B.A. Oral antibiotic therapy of serious systemic infections. Med Clin N Am. 2006;90:1197–1222. doi: 10.1016/j.mcna.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Fowler V.G., Jr., Boucher H.W., Corey G.R., Abrutyn E., Karchmer A.W., Rupp M.E., et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. New Eng J Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 90.Fink M.P., Snydman D.R., Niederman M.S., Leeper K.V., Jr., Johnson R.H., Heard S.O., et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intra-venous ciprofloxacin with imipenem-cilastatin. Antimicrob Agents Chemother. 1994;38:547–557. doi: 10.1128/aac.38.3.547. The Severe Pneumonia Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trouillet J.L., Chastre J., Vuagnat A., Joly-Guillou M.L., Combaux D., Dombret M.C., et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 92.Munoz-Price L.S., Weinstein R.A. Acinetobacter infection. New Eng J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 93.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Álvarez-Lerma F., Grau S., Álvarez-Beltrán M. Levofloxacin in the treatment of ventilator-associated pneumonia. Clin Microbiol Infect. 2006;12:81–92. doi: 10.1111/j.1469-0691.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- 95.Clinical Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne PA: 2010. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. M100–S20. [Google Scholar]
- 96.The committee for the Japanese Respiratory Society guidelines for the management of nursing and healthcare associated pneumonia . The Japanese Respiratory Society; Tokyo: 2011. The Japanese Respiratory Society guidelines for the management of nursing and healthcare associated pneumonia. [Google Scholar]
- 97.Shindo Y., Sato S., Maruyama E., Ohashi T., Ogawa M., Hashimoto N., et al. Health-care-associated pneumonia among hospitalizes patients in a Japanese community hospital. Chest. 2009;135:633–640. doi: 10.1378/chest.08-1357. [DOI] [PubMed] [Google Scholar]
- 98.Maruyama T., Niederman M.S., Kobayashi T., Kobayashi H., Takagi T., D'Alessandro-Gabazza C.N., et al. A prospective comparison of nursing home-acquired pneumonia with health care associated pneumonia in non-intubated elderly. Resp Med. 2008;102:1287–1295. doi: 10.1016/j.rmed.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 99.Seki M., Hashiguchi K., Tanaka A., Kosai K., Kakugawa T., Awaya Y., et al. Characteristics and disease severity of healthcare-associated pneumonia among patients in a hospital in Kitakyushu. Jpn J Infect Chemother. 2010;17:363–369. doi: 10.1007/s10156-010-0127-8. [DOI] [PubMed] [Google Scholar]
- 100.Carratalà J., Mykietiuk A., Fernández-Sabé N., Suárez C., Dorca J., Verdaguer R., et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 101.Kollef M.H., Shorr A., Tabak Y.P., Gupta V., Liu L.Z., Johannes R.S. Epidemiology and outcomes of healthcare-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 102.Craven D.E. What is health-care associated pneumonia, and how should it be treated? Curr Opin Infect Dis. 2006;19:153–160. doi: 10.1097/01.qco.0000216626.05821.40. [DOI] [PubMed] [Google Scholar]
- 103.El Solh A.A., Pietrantoni C., Bhat A., Bhora M., Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home acquired pneumonia. Clin Infect Dis. 2004;39:474–480. doi: 10.1086/422317. [DOI] [PubMed] [Google Scholar]
- 104.von Baum H., Welte T., Marre R., Suttorp N., Ewig S., CAPNETZ study group Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Resp J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- 105.Seymann G.B., Di Franceso L., Sharpe B., Rohde J., Fedullo P., Schneir A., et al. The HCAP gap: differences between self-reported practice patterns and published guidelines for health care-associated pneumonia. Clin Infect Dis. 2009;49:1868–1874. doi: 10.1086/648429. [DOI] [PubMed] [Google Scholar]
- 106.Oosterheert J.J., Bonten M.J.M., Hac E., Schneider M.M., Hoepelman I.M. How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community-acquired pneumonia? A systematic review. J Antimicrob Chemother. 2003;52:555–563. doi: 10.1093/jac/dkg413. [DOI] [PubMed] [Google Scholar]
- 107.Asadi L., Sligl W.I., Eurich D.T., Colmers I.N., Tjosvold L., Marrie T.J., et al. Macroride-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2012;55:371–380. doi: 10.1093/cid/cis414. [DOI] [PubMed] [Google Scholar]
- 108.Vardakas K.Z., Siempos I.I., Grammatikos A., Athanassa Z., Korbila I.P., Falagas M.E. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:1269–1277. doi: 10.1503/cmaj.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baddour L.M., Yu V.L., Klugman K.P., Feldman C., Ortqvist A., Rello J., et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 110.Pallares R., Gudior F., Liñares J., Ariza J., Rufi G., Murgui L., et al. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N Engl J Med. 1987;317:18–22. doi: 10.1056/NEJM198707023170104. [DOI] [PubMed] [Google Scholar]
- 111.Norrby S.R., Petermann W., Willcox P.A., Vetter N., Salewski E. A comparative study of levofloxacin and ceftriaxone in the treatment of hospitalized patients with pneumonia. Scand J Infect Dis. 1998;30:397–404. doi: 10.1080/00365549850160710. [DOI] [PubMed] [Google Scholar]
- 112.Loeb M., Carusone S.C., Goeree R., Walter S.D., Brazil K., Krueger P., et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295:2503–2510. doi: 10.1001/jama.295.21.2503. [DOI] [PubMed] [Google Scholar]
- 113.Finch R., Schürmann D., Collins O., Kubin R., McGivern J., Bobbaers H., et al. Randomized controlled trial of sequential intravenous (i.v.) and oral moxifloxacin compared with sequential i.v. and oral co-amoxiclav with or without clarithromycin in patients with community-acquired pneumonia requiring initial parenteral treatment. Antimicrob Agents Chemother. 2002;46:1746–1754. doi: 10.1128/AAC.46.6.1746-1754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Torres A., Garau J., Arvis P., Carlet J., Choudhri S., Kureishi A., et al. Moxifloxacin monotherapy is effective in hospitalized patients with community-acquired pneumonia: the MOTIV study―a randomized clinical trial. Clin Infect Dis. 2008;46:1499–1509. doi: 10.1086/587519. [DOI] [PubMed] [Google Scholar]
- 115.Vergis E.N., Indorf A., File T.M., Jr., Phillips J., Bates J., Tan J., et al. Azithromycin vs cefuroxime plus erythromycin for empirical treatment of community-acquired pneumonia in hospitalized patients: a prospective, randomized, multicenter trial. Arch Intern Med. 2000;160:1294–1300. doi: 10.1001/archinte.160.9.1294. [DOI] [PubMed] [Google Scholar]
- 116.Contopoulos-Ioannidis D.G., Ioannidis J.P., Chew P., Lau J. Meta-analysis of randomized controlled trials on the comparative efficacy and safety of azithromycin against other antibiotics for lower respiratory tract infections. J Antimicrob Chemother. 2001;48:691–703. doi: 10.1093/jac/48.5.691. [DOI] [PubMed] [Google Scholar]
- 117.Yanagihara K., Izumikawa K., Higa F., Tateyama M., Tokimatsu I., Hiramatsu K., et al. Efficacy of azithromycin in the treatment of community-acquired pneumonia, including patients with macrolide-resistant Streptococcus pneumoniae infection. Intern Med. 2009;48:527–535. doi: 10.2169/internalmedicine.48.1482. [DOI] [PubMed] [Google Scholar]
- 118.Fujisawa K., Chiba M., Tanaka M., Sato K. In vitro antibacterial activity of DX-619, a novel des-fluoro (6) quinolone. Antimicrob Agents Chemother. 2005;49:3040–3045. doi: 10.1128/AAC.49.7.3040-3045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hedlund J., Ortqvist A., Ahlqvist T., Augustinsson A., Beckman H., Blanck C., et al. Management of patients with community-acquired pneumonia treated in hospital in Sweden. Scand J Infect Dis. 2002;34:887–892. doi: 10.1080/0036554021000026965. [DOI] [PubMed] [Google Scholar]
- 120.Labelle A.J., Arnold H., Reichley R.M., Micek S.T., Kollef M.H. A comparison of culture-positive and culture-negative health-care-associated pneumonia. Chest. 2010;137:1130–1137. doi: 10.1378/chest.09-1652. [DOI] [PubMed] [Google Scholar]
- 121.El-Solh A.A., Akinnusi M.E., Alfarah Z., Patel A. Effect of antibiotic guidelines on outcomes of hospitalized patients with nursing home-acquired pneumonia. J Am Geriatr Soc. 2009;57:1030–1035. doi: 10.1111/j.1532-5415.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 122.Teramoto S., Kawashima M., Komiya K., Shoji S. Health-care-associated pneumonia is primarily due to aspiration pneumonia. Chest. 2009;136:1702–1703. doi: 10.1378/chest.09-1204. [DOI] [PubMed] [Google Scholar]
- 123.Yacovlev S.V., Strachounski L.S., Woods G.L., Adeyi B., McCarroll K.A., Ginanni J.A., et al. Ertapenem versus cefepime for initial empirical treatment of pneumonia acquired in skilled-care facilities or in hospitals outside the intensive care unit. Eur J Clin Microbiol Infect Dis. 2006;25:633–641. doi: 10.1007/s10096-006-0193-0. [DOI] [PubMed] [Google Scholar]
- 124.Livemore D.M., Mushtaq S., Warner M. Selectivity of ertapnem for Psudomonas aeruginosa mutants cross-resistant to other carbapenems. J Antimicrob Chemother. 2005;55:306–311. doi: 10.1093/jac/dki009. [DOI] [PubMed] [Google Scholar]
- 125.Tsukada H., Sakai K., Cho H., Kimura Y., Tetsuka T., Nakajima H., et al. Retrospective investigation of the clinical effects of tazobactam/piperacillin and sulbactam/ampicillin on aspiration pneumonia caused by Klebsiella pneumoniae. J Infect Chemother. 2012;18:715–721. doi: 10.1007/s10156-012-0407-6. [DOI] [PubMed] [Google Scholar]
- 126.Japanese Society of Chemotherapy and Journal of Japanese Association for Anaerobic Infection Research . Kyowa Kikaku Ltd.; Tokyo: 2007. Guidelines for treatment of anaerobic infections 2007. [Google Scholar]
- 127.Yamamoto Y., Izumikawa K., Morinaga Y., Nakamura S., Kurihara S., Imamura Y., et al. Prospective randomized comparison study of piperacillin/tazobactam and meropenem for health care-associated pneumonia in Japan. J Infect Chemother. 2013;19:291–298. doi: 10.1007/s10156-013-0552-6. [DOI] [PubMed] [Google Scholar]
- 128.Fukuyama H., Ishida T., Tachibana H., Iga C., Nakagawa H., Ito A., et al. Clinical evaluation of bedridden patients with pneumonia receiving home health care. J Jpn Respir Soc. 2010;48:906–911. [PubMed] [Google Scholar]
- 129.Snydman D.R., Jacobus N.V., McDermott L.A., Ruthazer R., Goldstein E.J., Finegold S.M., et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends for 1997―2000. Clin Infect Dis. 2002;35:S126–S134. doi: 10.1086/341934. [DOI] [PubMed] [Google Scholar]
- 130.Mikasa K., Konishi M., Maeda K. Examination of anaerobes in patients with respiratory infectious diseases using the transtracheal aspiration method. Jpn Soc Anaerobe Infect. 2003;33:50–60. [Google Scholar]
- 131.Brown R.B., Iannini P., Gross P., Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest. 2003;123:1503–1511. doi: 10.1378/chest.123.5.1503. [DOI] [PubMed] [Google Scholar]
- 132.Waterer G.W., Rello J., Wunderink R.G. Management of community-acquired pneumonia. Am J Respir Crit Care Med. 2011;183:157–164. doi: 10.1164/rccm.201002-0272CI. [DOI] [PubMed] [Google Scholar]
- 133.Walkey A.J., Wiener R.S. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141:1153–1159. doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mrik P.E. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665–671. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 135.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . The Japanese Respiratory Society; Tokyo: 2008. The guidelines for the management of hospital-acquired pneumonia in adults. [Google Scholar]
- 136.Japanese Study Group on Aspiration Pulmonary Disease . Pfizer Japan Inc.; Tokyo: 2003. Diagnosis and treatment for aspiration pulmonary disease. [Google Scholar]
- 137.Marie T.J. Epidemiology of community-acquired pneumonia in the elderly. Semin Respir Infect. 1990;5:260–268. [PubMed] [Google Scholar]
- 138.Loeb M., McGeer A., McArthur M., Walter S., Simor A.E. Risk factors for pneumonia and other lower respiratory tract infection in elderly residents of long term care facilities. Arch Intern Med. 1999;159:2058–2064. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 139.Muder R.R. Pneumonia in residents of long-term care facilities: epidemiology, management, and prevention. Am J Med. 1998;105:319–330. doi: 10.1016/s0002-9343(98)00262-9. [DOI] [PubMed] [Google Scholar]
- 140.Tani S., Tomioka H., Kaneda T., Kubota T., Kaneko M., Fujii H. Hospitalized nursing home-acquired pneumonia: comparison with community-acquired pneumonia in older adults. J Jpn Respir Soc. 2009;47:355–361. [PubMed] [Google Scholar]
- 141.Teramoto S., Fukuchi Y., Sasaki H., Sato K., Sekizawa K., Matsuse T., et al. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multi-center, prospective study in Japan. J Am Geriatr Soc. 2008;56:577–579. doi: 10.1111/j.1532-5415.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 142.Ewig S., Welte T., Chastre J., Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lance Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 143.Rosenthal V.D., Rodrigues C., Álvarez-Moreno C., Madani N., Mitrev Z., Ye G., et al. Effectiveness of a multi dimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Crit Care Med. 2012;40:3121–3128. doi: 10.1097/CCM.0b013e3182657916. [DOI] [PubMed] [Google Scholar]
- 144.Gilbert D.N., Moellering R.C. Jr., Eliopoulos G.M., Chambers H.F., Saag M.S., editors. The Sanford guide to antimicrobial therapy 2011. 41st ed. Antimicrobial Therapy Inc; VA, USA: 2011. [Google Scholar]
- 145.Higa F., Saito A. Pathogenic bacteria in aspiration pneumonia. Geriatr Med. 1997;35:153–156. [Google Scholar]
- 146.El-Solh A.A., Pietrantoni C., Bhat A., Aquilina A.T., Okada M., Grover V., et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 147.Allewelt M., Schüler P., Bölcskei P.L., Mauch H., Lode H. Ampicillin + sulbactam vs clindamysin ± cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect. 2004;10:163–170. doi: 10.1111/j.1469-0691.2004.00774.x. [DOI] [PubMed] [Google Scholar]
- 148.Ito I., Kadowaki S., Tanabe N., Haruna A., Kase M., Yasutomo Y., et al. Tazobactam/piperacillin for moderate-to-severe pneumonia in patients with risk for aspiration: comparison with imipenem/cilastatin. Pulm Pharmacol Ther. 2010;23:403–410. doi: 10.1016/j.pupt.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 149.El-Solh A.A., Pietrantoni C., Bhat A., Bhora M., Berbary E. Indicators of potentially drug-resistant bacteria in severe nursing home-acquired pneumonia. Clin Infect Dis. 2004;39:474–480. doi: 10.1086/422317. [DOI] [PubMed] [Google Scholar]
- 150.Wang J.L., Chen K.Y., Fang C.T., Hsueh P.R., Yang P.C., Chang S.C. Changing bacteriology of adult community- acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis. 2005;40:915–922. doi: 10.1086/428574. [DOI] [PubMed] [Google Scholar]
- 151.Shiraishi T., Sakamoto A., Iwatake S., Nakamura Y., Yokoyama H. Survey regarding antimicrobial drug therapy for aspiration pneumonia. J Jpn Soc Hosp Pharm. 2009;45:1501–1504. [Google Scholar]
- 152.Kaneko A., Yamane N., Watanabe D., Mizusawa N., Matsuzaki K., Hasegawa M. Treatment of aspiration pneumonia based on the antimicrobial susceptibility pattern of oral bacteria pathogens. Jpn J Chemother. 2007;55:378–381. [Google Scholar]
- 153.Kadowaki M., Demura Y., Mizuno S., Uesaka D., Ameshima S., Miyamori I., et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127:1276–1282. doi: 10.1378/chest.127.4.1276. [DOI] [PubMed] [Google Scholar]
- 154.Hirakata Y., Matsuda J., Miyazaki Y., Kamihira S., Kawakami S., Miyazawa Y., et al. Regional variation in the prevalence of extended-spectrum β-lactamase-producing clinical isolates in the Asia-Pacific region (SENTRY 1998―2002) Diagn Microbiol Infect Dis. 2005;52:323–329. doi: 10.1016/j.diagmicrobio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 155.Muratani T. Special issue, current topics on urological infectious diseases- - - Refractory factors and management- - - , 2 resistance of bacteria isolated from patients with urinary tract infection to antimicrobial drugs and methods to prevent drug resistance. Antibiot Chemother. 2009;25:413–423. [Google Scholar]
- 156.Bartlett JG. Aspiration pneumonia in adults UpToDate, Topic 7024 Version 9.0. This topic last updated: Jul 06, 2015.
- 157.Bartlett J.G. Anaerobic bacterial infection of the lung. Anaelobe. 2012;18:35–39. doi: 10.1016/j.anaerobe.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 158.Karino F., Deguchi N., Kanda H., Ohe M., Kondo K., Tada M., et al. Evaluation of the efficacy and safety of biapenem against pneumonia in the elderly and a study on its pharmacokinetics. J Infect Chemother. 2013;19:98–102. doi: 10.1007/s10156-012-0463-y. [DOI] [PubMed] [Google Scholar]
- 159.Brun-Buisson C., Sollet J.P., Schweich H., Brière S., Petit C. Treatment of ventilator-associated pneumonia with piperacillin-tazobactam/amikacin versus ceftazidime/amikacin: a multicenter, randomized controlled trial. Clin Infect Dis. 1998;26:346–354. doi: 10.1086/516294. VAP Study Group. [DOI] [PubMed] [Google Scholar]
- 160.Jaccard C., Troillet N., Harbarth S., Zanetti G., Aymon D., Schneider R., et al. Prospective randomized comparison of imipenem-cilastatin and piperacillin-tazobactam in nosocomial pneumonia or peritonitis. Antimicrob Agents Chemother. 1998;42:2966–2972. doi: 10.1128/aac.42.11.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crouch B.S., Wunderink R.G., Jones C.B., Jones C.B., Leeper K.V., Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 162.Matsushima A., Tasaki O., Shimizu K., Tomono K., Ogura H., Shimazu T., et al. Preemptive antibiotic treatment based on gram staining reduced the incidence of ARDS in mechanically ventilated patients. J Trauma. 2008;65:309–315. doi: 10.1097/TA.0b013e31817c96f6. [DOI] [PubMed] [Google Scholar]
- 163.Craven D.E., Chroneou A., Zias N., Hjalmarson K.I. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135:521–528. doi: 10.1378/chest.08-1617. [DOI] [PubMed] [Google Scholar]
- 164.Nseir S., Favory R., Jozefowicz E., Decamps F., Dewavrin F., Brunin G., et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008;12:R62. doi: 10.1186/cc6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Greene R.E., Schlamm H.T., Oestmann J.W., Stark P., Durand C., Lortholary O., et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 166.Herbrecht R., Denning D.W., Patterson T.F., Bennett J.E., Greene R.E., Oestmann J.W., et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 167.Cornely O.A., Maertens J., Bresnik M., Ebrahimi R., Ullmann A.J., Bouza E., et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 168.Maertens J., Egerer G., Shin W.S., Reichert D., Stek M., Chandwani S., et al. Caspofungin use in daily clinical practice for treatment of invasive aspergillosis: results of a prospective observational registry. BMC Infect Dis. 2010;10:182. doi: 10.1186/1471-2334-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Denning D.W., Marr K.A., Lau W.M., Facklam D.P., Ratanatharathorn V., Becker C., et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337–349. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Caillot D., Bassaris H., McGeer A., Arthur C., Prentice H.G., Seifert W., et al. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis. 2001;33:e83–e90. doi: 10.1086/323020. [DOI] [PubMed] [Google Scholar]
- 171.Singh N., Limaye A.P., Forrest G., Safdar N., Muñoz P., Pursell K., et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006;81:320–326. doi: 10.1097/01.tp.0000202421.94822.f7. [DOI] [PubMed] [Google Scholar]
- 172.Marr K.A., Boeckh M., Carter R.A., Kim H.W., Corey L. Combination antifungal therapy for invasive aspergillosis. Clin Infect Dis. 2004;39:797–802. doi: 10.1086/423380. [DOI] [PubMed] [Google Scholar]
- 173.Caillot D., Thiébaut A., Herbrecht R., de Botton S., Pigneux A., Bernard F., et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies. Cancer. 2007;110:2740–2746. doi: 10.1002/cncr.23109. a randomized pilot study (Combistrat trial) [DOI] [PubMed] [Google Scholar]
- 174.Meletiadis J., Petraitis V., Petraitiene R., Lin P., Stergiopoulou T., Kelaher A.M., et al. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation. J Infect Dis. 2006;194:1008–1018. doi: 10.1086/506617. [DOI] [PubMed] [Google Scholar]
- 175.Kohno S., Izumikawa K., Ogawa K., Kurashima A., Okimoto N., Amitani R., et al. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: a multicenter trial in Japan. J Infect. 2010;61:410–418. doi: 10.1016/j.jinf.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 176.Kohno S., Izumikawa K., Yoshida M., Takesue Y., Oka S., Kamei K., et al. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur J Clin Microbiol Infect Dis. 2013;32:387–397. doi: 10.1007/s10096-012-1754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Regnard J.F., Icard P., Nicolosi M., Spagiarri L., Magdeleinat P., Jauffret B., et al. Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg. 2000;69:898–903. doi: 10.1016/s0003-4975(99)01334-x. [DOI] [PubMed] [Google Scholar]
- 178.Yamaguchi H., Ikemoto H., Watanabe K., Ito A., Hara K., Kohno S. Fluconazole monotherapy for cryptococcosis in non-AIDS patients. Eur J Clin. Microbiol Infect Dis. 1996;15:787–792. doi: 10.1007/BF01701520. [DOI] [PubMed] [Google Scholar]
- 179.Pappas P.G., Perfect J.R., Cloud G.A., Larsen R.A., Pankey G.A., Lancaster D.J., et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 180.Shoham S., Magill S.S., Merz W.G., Gonzalez C., Seibel N., Buchanan W.L., et al. Primary treatment of zygomycosis with liposomal amphotericin B: analysis of 28 cases. Med Mycol. 2010;48:511–517. doi: 10.3109/13693780903311944. [DOI] [PubMed] [Google Scholar]
- 181.Spellberg B., Ibrahim A.S., Chin-Hong P.V., Kontoyiannis D.P., Morris M.I., Perfect J.R., et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas C.F., Jr., Limper A.H. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 183.The National Institute of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323:1500–1504. doi: 10.1056/NEJM199011223232131. [DOI] [PubMed] [Google Scholar]
- 184.Hughes W., Leoung G., Kramer F., Bozzette S.A., Safrin S., Frame P., et al. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med. 1993;328:1521–1527. doi: 10.1056/NEJM199305273282103. [DOI] [PubMed] [Google Scholar]
- 185.Kotton C.N., Kumar D., Caliendo A.M., Asberg A., Chou S., Snydman D.R., et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 186.Asberg A., Humar A., Rollag H., Jardine A.G., Mouras H., Pescovitz M.D., et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transpl. 2007;7:2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 187.Moretti S., Zikos P., Van Lint M.T., Tedone E., Occhini D., Gualandi F., et al. Forscarnet vs ganciclovir for cytomegalovirus (CMV) antigenemia after allogeneic hemopoietic stem cell transplantation (HSCT): a randomised study. Bone Marrow Transpl. 1998;22:175–180. doi: 10.1038/sj.bmt.1701302. [DOI] [PubMed] [Google Scholar]
- 188.Ljungman P., Engelhard D., Link H., Biron P., Brandt L., Brunet S., et al. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: experience of European Bone Marrow Transplant Group. Clin Infect Dis. 1992;14:831–835. doi: 10.1093/clinids/14.4.831. [DOI] [PubMed] [Google Scholar]
- 189.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Pneumonia. The guidelines for the management of respiratory infectious diseases in children in Japan 2011; pp. 29–49. [Google Scholar]
- 190.Nakamura A. Limitations of the etiological diagnosis of bronchopulmonary infectious diseases- - - Appearance of EMB. J Jpn Pediatr Soc. 2003;107:1067–1073. [Google Scholar]
- 191.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–433. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 192.Kurosaki T., Ishiwada N., Fifth Pediatric Respiration Seminar Review of chest X-ray findings of pneumonia, childhood pneumonia with respect to 3 causative pathogens. J Jpn Soc Pediatr Pulmonol. 1998;9:124–134. [Google Scholar]
- 193.World Health Organization: Programme for the control of acute respiratory infections: technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. WHO/ARI/91. 20.
- 194.Turner R.B., Lande A.E., Chase P., Hilton N., Weinberg D. Pneumonia in pediatric outpatients: cause and clinical manifestations. J Pediatr. 1987;111:194–200. doi: 10.1016/S0022-3476(87)80066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ishiwada N., Kurosaki T., Toba T., Niimi H. Etiology of pediatric inpatients with pneumonia–analysis of clinical symptoms, physical examination and simple laboratory findings. J Jpn Assoc Infect Dis. 1995;69:284–290. doi: 10.11150/kansenshogakuzasshi1970.69.284. [DOI] [PubMed] [Google Scholar]
- 196.Kurosaki T. Basic examination regarding “the Guidelines for the Management of Pneumonia in Children”, selection of treatment. J Jpn Pediatr Soc. 2003;14:198–204. [Google Scholar]
- 197.Pönkä A., Sarna S. Differential diagnosis of viral, mycoplasmal and bacteraemic pneumococcal pneumonias on admission to hospital. Eur J Respir Dis. 1983;64:360–368. [PubMed] [Google Scholar]
- 198.Ishiwada N., Kurosaki T., Toba T., Ohta F., Saito N., Tamai K., et al. Current status of pneumonia in children, review of chest X-ray findings. J Jpn Pediatr Soc. 1994;98:2012–2016. [Google Scholar]
- 199.Ubukata K. Problems associated with high prevalence of multidrug-resistant bacteria in patients with community-acquired infections. J Infect Chemother. 2003;9:285–291. doi: 10.1007/s10156-003-0278-y. [DOI] [PubMed] [Google Scholar]
- 200.Sato Y., Toyonaga Y., Hanaki H., Nonoyama M., Oishi T., Sunakawa K. Nationwide survey of the development of drug-resistant pathogens in the pediatric field: drug sensitivity of Streptococcus pneumonia in Japan. J Infect Chemother. 2009;15:396–401. doi: 10.1007/s10156-009-0723-7. [DOI] [PubMed] [Google Scholar]
- 201.Sakata H., Toyonaga Y., Sato Y., Hanaki H., Nonoyama M., Oishi T., et al. Nationwide survey of the development of drug-resistance in the pediatric field: drug sensitivity of Haemophilus influenzae in Japan. J Infect Chemother. 2009;15:402–409. doi: 10.1007/s10156-009-0729-1. [DOI] [PubMed] [Google Scholar]
- 202.Morozumi M., Takahashi T., Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother. 2010;16:78–86. doi: 10.1007/s10156-009-0021-4. [DOI] [PubMed] [Google Scholar]
- 203.Clinical and Laboratory Standard Institute . vol. 28. 2008. (Performance standards for antimicrobial susceptibility testing). 18th Informational Supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kurosaki T., Ohta H., Tamai K., Hoshioka A., Takahashi Y., Omata T., et al. Annual change of ampicillin susceptibility of H. influenzae and the clinical efficacy of penicillins in bronchopulmonary infections caused by β-lactamase negative H. influenzae with an ampicillin-minimum inhibitory concentration (MIC) of 2 μg/ml. Jpn J Pediatr Pulmonol. 2001;12:18–23. [Google Scholar]
- 205.Sudo F., Ishiwada N., Hoshino T., Fukasawa C., Inami Y., Hishiki H., et al. Clinical effects of piperacillin and tazobactam/piperacillin on Haemophilus influenzae lower respiratory tract infection in pediatric patients. J Jpn Assoc Infect Dis. 2005;79:637–643. doi: 10.11150/kansenshogakuzasshi1970.79.637. [DOI] [PubMed] [Google Scholar]
- 206.Oshima H., Kurosaki T., Ohta F., Tamai K., Uehara S., Niimi H. Clinical studies on lower respiratory tract infections caused by Moraxella catarrhalis in childhood (1st report) J Jpn Pediatr Soc. 1998;102:23–28. [Google Scholar]
- 207.Oshima H., Kurosaki T., Ishikawa N., Uehara S., Niimi H. Clinical studies on lower respiratory tract infections caused by Moraxella catarrhalis in childhood (2nd report)-indirect pathogenicity of Moraxella catarrhalis. J Jpn Pediatr Soc. 1998;102:131–134. [Google Scholar]
- 208.Mogi A., Nishi J., Yoshinaga M., Harada H., Narahara S., Kawakami K., et al. Increased prevalence of penicillin-resistant viridans group streptococci in Japanese children with upper respiratory infection treated by beta-lactam agents and in those with oncohematologic diseases. Pediatr Infect Dis J. 1997;16:1140–1144. doi: 10.1097/00006454-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 209.Ubukata K., Morokado M., Iwata T. Strong macrolide resistance in children/pandemic of Mycoplasma pneumoniae infection. IASR. 2011;32:337–339. [Google Scholar]
- 210.Kurosaki T., Ouchi K. Macrolide resistance of Mycoplasma pneumoniae in primary hospitals. IASR. 2012;33:267–268. [Google Scholar]
- 211.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children, Vaccination/Infectious Disease Control Committee of the Japan Pediatric Society . 2013. Supplement revision (2013) of the guidelines for the management of respiratory infectious diseases in Children in 2011 regarding the diagnosis and treatment of pneumonia with Mycoplasma pneumoniae in children. [Google Scholar]
- 212.Foglia E., Meier M.D., Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev. 2007;20:409–425. doi: 10.1128/CMR.00041-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Pneumonia in the presence of an underlying disease 1. Blood disease. Guidelines for the management of respiratory infectious diseases in children 2011; pp. 52–55. [Google Scholar]
- 214.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Pneumonia in the presence of an underlying disease 2. Immunodeficiency. Guidelines for the management of respiratory infectious diseases in children 2011; pp. 55–58. [Google Scholar]
- 215.Freifeld A.G., Bow E.J., Sepkowitz K.A., Boeckh M.J., Ito J.I., Mullen C.A., et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 216.Misawa M., Masaoka T. Iyaku (Medicine and Drug) Journal Co., Ltd; Osaka: 2006. Indicative therapy during blood disease treatment; pp. 147–157. [Google Scholar]
- 217.Kuroki H., Ishikawa N., Hoshioka A., Shimojo N., Kawano Y., Uehara S., et al. Experience on long-term therapy with macrolides in the field of pediatrics. J Jpn Pediatr Soc. 1996;100:1194–1201. [Google Scholar]
- 218.Shishido H. Van Medical co., Ltd.; Tokyo: 1995. Refractory respiratory infectious diseases; pp. 57–60. [Google Scholar]
- 219.Gallin J.I., Alling D.W., Malech H.L., Wesley R., Koziol D., Marciano B., et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–2422. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 220.Sakata H. Special issue- - - Respiratory infectious disease- - - neonatal pneumonia. J Jpn Pediatr Med. 2004;36:104–107. [Google Scholar]
- 221.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Pneumonia in the presence of an underlying disease 2. Neonates. Guidelines for the management of respiratory infectious diseases in children 2011; pp. 58–62. [Google Scholar]
- 222.Jeong I.S., Jeong J.S., Choi E.O. Nosocomial infection in a newborn intensive care unit (NICU), South Korea. BMC Infect Dis. 2006;6:103. doi: 10.1186/1471-2334-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Couto R.C., Carvalho E.A., Pedrosa T.M., Pedroso E.R., Neto M.C., Biscione F.M. A 10-year prospective surveillance of nosocomial infections in neonatal intensive care units. Am J Infect Control. 2007;35:183–189. doi: 10.1016/j.ajic.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 224.Yuan T.M., Chen L.H., Yu H.M. Risk factors and outcomes for ventilator-associated pneumonia in neonatal intensive care unit patients. J Perinat Med. 2007;35:334–338. doi: 10.1515/JPM.2007.065. [DOI] [PubMed] [Google Scholar]
- 225.Katayama Y., Minami H., Enomoto M., Takano T., Hayashi S., Lee Y.K. Usefulness of Gram staining of tracheal aspirates in initial therapy for ventilator-associated pneumonia in extremely preterm neonates. J Perinatol. 2010;30:270–274. doi: 10.1038/jp.2009.144. [DOI] [PubMed] [Google Scholar]
- 226.Sato Y. Childhood infectious diseases--I. Review of childhood infectious diseases with respect to the type of infection, 13. Neonatal infectious diseases. Antibiot Chemother. 2009;25:985–994. [Google Scholar]
- 227.Sato Y. Chapter 2. Various infectious diseases and how to select/use drugs, neonatal period, neonatal infectious diseases. Pediatr Clin Pract Pixis. 2009;11:252–257. Nakayama Shoten Co. Ltd., Tokyo. [Google Scholar]
- 228.Sato Y. Neonatal care/basic knowledge V. Management method, use of antimicrobial drugs. J Jpn Pediatr Pract. 2010;73:1577–1584. [Google Scholar]
- 229.Sato Y. Antimicrobial/antiviral drugs for severe infectious diseases III. Clues to diagnosis and selection of drugs in patients with various infectious diseases/conditions, infectious diseases in neonates. J Jpn Pediatr Pract. 2010;73:2035–2041. [Google Scholar]
- 230.Colice G.L., Curtis A., Deslauriers J., Heffner J., Light R., Littenberg B., et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;118:1158–1171. doi: 10.1378/chest.118.4.1158. [DOI] [PubMed] [Google Scholar]
- 231.Light R.W. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 232.Brook I., Frazier E.H. Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest. 1993;103:1502–1507. doi: 10.1378/chest.103.5.1502. [DOI] [PubMed] [Google Scholar]
- 233.Civen R., Jousimies-Somer H., Marina M., Borenstein L., Shah H., Finegold S.M. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin Infect Dis. 1995;20:S224–S229. doi: 10.1093/clinids/20.supplement_2.s224. [DOI] [PubMed] [Google Scholar]
- 234.Boyanova L., Vladimir D., Gergova G., Iotov Dragomir, Petrov D., Osmanliev D., et al. Anaerobic microbiology in 198 cases of pleural empyema: a Bulgarian study. Anaerobe. 2004;10:261–267. doi: 10.1016/j.anaerobe.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 235.Ahmed R.A., Marrie T.J., Huang J.Q. Thoracic empyema in patients with community-acquired pneumonia. Am J Med. 2006;119:877–883. doi: 10.1016/j.amjmed.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 236.Maskell N.A., Davies C.W., Nunn A.J., Hedley E.L., Gleeson F.V., Miller R., et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 237.Hughes C.E., Van Scoy R.E. Antibiotic therapy of pleural empyema. Semin Respir Infect. 1991;6:94–102. [PubMed] [Google Scholar]
- 238.Shohet I., Yellin A., Meyerovitch J., Rubinstein E. Pharmacokinetics and therapeutic efficacy of gentamicin in an experimental pleural empyema rabbit model. Antimicrob Agents Chemother. 1987;31:982–985. doi: 10.1128/aac.31.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Thys J.P., Serruys-Schoutens E., Rocmans P., Herchuelz A., Vanderlinden M.P., Yourassowsky E. Amikacin concentrations in uninfected post thoracotomy pleural fluid and in serum after intravenous and intrapleural injection. Chest. 1984;85:502–505. doi: 10.1378/chest.85.4.502. [DOI] [PubMed] [Google Scholar]
- 240.Thys J.P., Vanderhoeft P., Herchuelz A., Bergmann P., Yourassowsky E. Penetration of aminoglycosides in uninfected pleural exudates and in pleural empyemas. Chest. 1988;93:530–532. doi: 10.1378/chest.93.3.530. [DOI] [PubMed] [Google Scholar]
- 241.Wozniak C.J., Paull D.E., Moezzi J.E., Scott R.P., Anstadt M.P., York V.V., et al. Choice of first intervention is related to outcomes in the management of empyema; discussion 30―1. Ann Thorac Surg. 2009;87:1525–1530. doi: 10.1016/j.athoracsur.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 242.Davies H.E., Davies R.J., Davies C.W. Management of pleural infection in adults: British thoracic society pleural disease guideline 2010. Thorax. 2010;65:ii41–ii53. doi: 10.1136/thx.2010.137000. [DOI] [PubMed] [Google Scholar]
- 243.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Pleuritis/Pyothorax. Guidelines for the management of respiratory infectious diseases in children 2011; pp. 50–51. [Google Scholar]
- 244.Chakrabarti B., Davies P.D. Pleural tuberculosis. Monaldi Arch Chest Dis. 2006;65:26–33. doi: 10.4081/monaldi.2006.582. [DOI] [PubMed] [Google Scholar]
- 245.Odagiri K. Radiological study of mycoplasma pneumonia in children. Yokohama Med J. 1979;30:113–122. [Google Scholar]
- 246.Pinkerton H.J. In: Principle and practice of pediatric surgery. Oldham K.T., Colombani P.M., Foglia R.P., Skinner M.A., editors. Lippincott Williams & Wilkins; Philadelphia: 2005. Lung; pp. 951–982. [Google Scholar]
- 247.Committee to Prepare the Guidelines for the Management of Respiratory Infectious Diseases in Children . Kyowa Kikaku Ltd.; Tokyo: 2011. Primary diseases for which vaccination should be performed 5. Tuberculosis. Guidelines for the management of respiratory infectious diseases in children 2011; pp. 84–88. [Google Scholar]
- 248.East African/British Medical Research Councils Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1973;1:1331–1338. Second report. [PubMed] [Google Scholar]
- 249.East African/British Medical Research Councils Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1974;2:237–240. Third report. [PubMed] [Google Scholar]
- 250.Hong Kong Chest Service/British Medical Research Council Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Am Rev Respir Dis. 1991;143:700–706. doi: 10.1164/ajrccm/143.4_Pt_1.700. Results at 30 months. [DOI] [PubMed] [Google Scholar]
- 251.British Thoracic Society A controlled trial of 6 months' chemotherapy in pulmonary tuberculosis. Final report: results during the 36 months after the end of chemotherapy and beyond. Br J Dis Chest. 1984;78:330–336. [PubMed] [Google Scholar]
- 252.Ormerod L.P., Horsfield N. Short-course antituberculous chemotherapy for pulmonary and pleural disease: 5 years' experience in clinical practice. Br J Dis Chest. 1987;81:268–271. doi: 10.1016/0007-0971(87)90160-4. [DOI] [PubMed] [Google Scholar]
- 253.Joint Tuberculosis Committee of the British Thoracic Society Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1998;53:536–548. [PMC free article] [PubMed] [Google Scholar]
- 254.Blumberg H.M., Burman W.J., Chaisson R.E., Daley C.L., Etkind S.C., Friedman L.N., et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 255.The Japanese Society for Tuberculosis . 2nd ed. Nankodo; Tokyo: 2012. Guidelines for the management of tuberculosis. [Google Scholar]
- 256.Benator D., Bhattacharya M., Bozeman L., Burman W., Cantazaro A., Chaisson R., et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360:528–534. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 257.Combs D.L., O'Brien R.J., Geiter L.J. USPHS tuberculosis short-course chemotherapy trial 21:effectiveness, toxicity, and acceptability. The report of final results. Ann Intern Med. 1990;112:397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 258.Ferebee S.H. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 259.Curry F.J. Prophylactic effect of isoniazid in young tuberculin reactors. N Engl J Med. 1967;277:562–567. doi: 10.1056/NEJM196709142771103. [DOI] [PubMed] [Google Scholar]
- 260.Jenkins D., Davidson F.F. Isoniazid chemoprophylaxis of tuberculosis. Calif Med. 1972;116:1–5. [PMC free article] [PubMed] [Google Scholar]
- 261.International Union Against Tuberculosis Committee on Prophylaxis Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–564. [PMC free article] [PubMed] [Google Scholar]
- 262.Jasmer R.M., Nahid P., Hopewell P.C. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–1866. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 263.Comstock G.W. How much isoniazid is needed for prevention of tuberculosis among immune competent adults? Int J Tuberc Lung Dis. 1999;3:847–850. [PubMed] [Google Scholar]
- 264.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med 2000; 161: S221―S247. [DOI] [PubMed]
- 265.Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis. 1992;145:36–41. doi: 10.1164/ajrccm/145.1.36. [DOI] [PubMed] [Google Scholar]
- 266.Villarino M.E., Ridzon R., Weismuller P.C., Elcock M., Maxwell R.M., Meador J., et al. Rifampin preventive therapy for tuberculosis infection: experience with 157 adolescents. Am J Respir Crit Care Med. 1997;155:1735–1738. doi: 10.1164/ajrccm.155.5.9154885. [DOI] [PubMed] [Google Scholar]
- 267.Menzies D., Long R., Trajman A., Dion M.J., Yang J., Al Jahdali H., et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Ann Intern Med. 2008;149:689–697. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- 268.The Research Committee of the British Thoracic Society Pulmonary disease caused by Mycobacterium avium- intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardized treatment. Int J Tuberc Lung Dis. 2002;6:628–634. [PubMed] [Google Scholar]
- 269.Wallace R.J., Jr., Brown B.A., Griffith D.E., Girard W.M., Murphy D.T. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 270.Tanaka E., Kimoto T., Tsuyuguchi K., Watanabe I., Matsumoto H., Niimi A., et al. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1999;160:866–872. doi: 10.1164/ajrccm.160.3.9811086. [DOI] [PubMed] [Google Scholar]
- 271.Dautzenberg B., Piperno D., Diot P., Truffot-Pernot C., Chauvin J.P. Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Chest. 1995;107:1035–1040. doi: 10.1378/chest.107.4.1035. Clarithromycin Study Group of France. [DOI] [PubMed] [Google Scholar]
- 272.Wallace R.J., Jr., Brown B.A., Griffith D.E., Girard W.M., Murphy D.T., Onyi G.O., et al. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994;149:1335–1341. doi: 10.1164/ajrccm.149.5.8173775. [DOI] [PubMed] [Google Scholar]
- 273.Griffith D.E., Brown-Elliott B.A., Langsjoen B., Zhang Y., Pan X., Girard W., et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928–934. doi: 10.1164/rccm.200603-450OC. [DOI] [PubMed] [Google Scholar]
- 274.Griffith D.E., Brown B.A., Girard W.M., Murphy D.T., Wallace R.J., Jr. Azithromycin activity against Mycobacterium avium complex lung disease in patients who were not infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:983–989. doi: 10.1093/clinids/23.5.983. [DOI] [PubMed] [Google Scholar]
- 275.Nightingale S.D., Cameron D.W., Gordin F.M., Sullam P.M., Cohn D.L., Chaisson R.E., et al. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 276.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 277.Kobashi Y., Matsushima T., Oka M. A double-blind randomized study of aminoglycoside infusion with combined therapy for pulmonary Mycobacterium avium complex disease. Respir Med. 2007;101:130–138. doi: 10.1016/j.rmed.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 278.Sub committee of the Joint Tuberculosis Committee of the British Thoracic Society Management of opportunist mycobacterial infections: joint tuberculosis committee guidelines 1999. Thorax. 2000;55:210–218. doi: 10.1136/thorax.55.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.The nontuberculous mycobacteriosis control committee of the Japanese Society for Tuberculosis, The scientific assembly for infection and tuberculosis of the Japanese Respiratory Society Guidelines for chemotherapy of pulmonary nontuberculous mycobacterial disease – 2012 revised version. Tuberculosis. 2012;87:83–86. [PubMed] [Google Scholar]
- 280.Medical Section of the American Lung Association Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Am J Respir Crit Care Med. 1997;156:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 281.Pezzia W., Raleigh J.W., Bailey M.C., Toth E.A., Silverblatt J. Treatment of pulmonary disease due to Mycobacterium kansasii: recent experience with rifampin. Rev Infect Dis. 1981;3:1035–1039. doi: 10.1093/clinids/3.5.1035. [DOI] [PubMed] [Google Scholar]
- 282.Yoshida S., Suzuki K., Tsuyuguchi K., Iwamoto T., Tomita M., Okada M., et al. Detection of rpoB mutations in rifampicin-resistant Mycobacterium Kansasii. Tuberculosis. 2006;81:475–479. [PubMed] [Google Scholar]
- 283.Sauret J., Hernández-Flix S., Castro E., Hernández L., Ausina V., Coll P. Treatment of pulmonary disease caused by Mycobacterium kansasii: results of 18 vs 12 months' chemotherapy. Tuber Lung Dis. 1995;76:104–108. doi: 10.1016/0962-8479(95)90550-2. [DOI] [PubMed] [Google Scholar]
- 284.Griffith D.E., Girard W.M., Wallace R.J., Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 285.Brown-Elliott B.A., Wallace R.J., Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Daley C.L., Griffith D.E. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002;23:623–632. doi: 10.1016/s0272-5231(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 287.Cremades R., Santos A., Rodríguez J.C., Garcia-Pachón E., Ruiz M., Royo G. Mycobacterium abscessus from respiratory isolates: activities of drug combinations. J Infect Chemother. 2009;15:46–48. doi: 10.1007/s10156-008-0651-y. [DOI] [PubMed] [Google Scholar]
- 288.Jeon K., Kwon O.J., Lee N.Y., Kim B.J., Kook Y.H., Lee S.H., et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009;180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 289.Jarand J., Levin A., Zhang L., Huitt G., Mitchell J.D., Daley C.L. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011;52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 290.Supervised by the Tuberculosis and Infectious Diseases Control Division, Health Service Bureau, Ministry of Health, Labour and Welfare . Japan Anti-Tuberculosis Associaiton; Tokyo: 2009. Statistics of tuberculosis in 2009. [Google Scholar]
- 291.Takamatsu I. Special issue- - - New vaccination system of BCG-for the prevention of tuberculosis based on scientific grounds- Jpn J Public Health. 2006;70:266–270. [Google Scholar]
- 292.Mori T. Usefulness of interferon-gamma release assays for diagnosing TB infection and problems with these assays. J Infect Chemother. 2009;15:143–155. doi: 10.1007/s10156-009-0686-8. [DOI] [PubMed] [Google Scholar]
- 293.Tokunaga O: Subsidy for Scientific Research by the Ministry of Health, Labour and Welfare in 2009, Research business for new/old infectious diseases such as new influenza “Evaluation of tuberculosis control strategies and study regarding the development/application of new diagnostic/treatment techniques” (Kato Group), substudy “Evaluation of tuberculosis control strategies for children/medical practice”, report.
- 294.Kurashima A. Japanese new guidelines for nontuberculous mycobacterial pulmonary disease. Tuberculosis. 2010;85:87–93. [PubMed] [Google Scholar]
- 295.Wenzel R.P., Fowler A.A., 3rd Clinical practice. Acute bronchitis. N Engl J Med. 2006;355:2125–2130. doi: 10.1056/NEJMcp061493. [DOI] [PubMed] [Google Scholar]
- 296.Gonzales R., Sande M.A. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133:981–991. doi: 10.7326/0003-4819-133-12-200012190-00014. [DOI] [PubMed] [Google Scholar]
- 297.Gonzales R., Bartlett J.G., Besser R.E., Cooper R.J., Hickner J.M., Hoffman J.R., et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134:521–529. doi: 10.7326/0003-4819-134-6-200103200-00021. [DOI] [PubMed] [Google Scholar]
- 298.Braman S.S. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:95S–103S. doi: 10.1378/chest.129.1_suppl.95S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Konishi M., Sawaki M., Mikasa K., Sakamoto M., Maeda K., Takeuchi S., et al. Clinical study of acute bacterial bronchitis. J Jpn Assoc Infect Dis. 1993;67:452–458. doi: 10.11150/kansenshogakuzasshi1970.67.452. [DOI] [PubMed] [Google Scholar]
- 300.Rothstein E. wards K.:Health burden of pertussis in adolescents and adults. Pediatr Infect Dis J. 2005;24:S44–S47. doi: 10.1097/01.inf.0000160912.58660.87. [DOI] [PubMed] [Google Scholar]
- 301.Tiwari T., Murphy T.V., Moran J. Recommended antimicrobial agents for the treatment and post-exposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54:1–16. [PubMed] [Google Scholar]
- 302.Altunaiji S., Kukuruzovic R., Curtis N., Massie J. Antibiotics for whooping cough (pertussis) Cochrane Database Syst Rev. 2005:2005. doi: 10.1002/14651858.CD004404.pub2. CD004404. [DOI] [PubMed] [Google Scholar]
- 303.Morozumi M., Iwata S., Hasegawa K., Chiba N., Takayanagi R., Matsubara K., et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2008;52:348–350. doi: 10.1128/AAC.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hsu J., Santesso N., Mustafa R., Brozek J., Chen Y.L., Hopkins J.P., et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156:512–524. doi: 10.7326/0003-4819-156-7-201204030-00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . The Japanese Respiratory Society; Tokyo: 2003. The guidelines for the management of respiratory tract infection. Basic concept of respiratory tract infection in adults. [Google Scholar]
- 306.Tan T., Little P., Stokes T. Antibiotic prescribing for self limiting respiratory tract infections in primary care: summary of NICE guidance. BMJ. 2008;337:a437. doi: 10.1136/bmj.a437. [DOI] [PubMed] [Google Scholar]
- 307.Anthonisen N.R., Manfreda J., Warren C.P., Hershfield E.S., Harding G.K., Nelson N.A. Antibiotic therapy in exacerbation of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 308.Miravitlles M., Anzuet o A., Ewig S., Legnani D., Stauch K. Characterisation of exacerbations of chronic bronchitis and COPD in Europe: the GIANT study. Ther Adv Respir Dis. 2009;3:267–277. doi: 10.1177/1753465809352791. [DOI] [PubMed] [Google Scholar]
- 309.Burley C.J., Masterton R.G., Lovell D.P. Indicators of bacterial infection in patients with acute exacerbation of chronic bronchitis for application in clinical trials of anti bacterial drugs. J Infect. 2007;55:226–232. doi: 10.1016/j.jinf.2007.05.181. [DOI] [PubMed] [Google Scholar]
- 310.Sethi S., Murphy T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 311.Hui D.S., Ip M., Ling T., Chang S.C., Liao C.H., Yoo C.G., et al. A multicentre surveillance study on the characteristics, bacterial aetiologies and in vitro antibiotic susceptibilities in patients with acute exacerbations of chronic bronchitis. Respirology. 2011;16:532–539. doi: 10.1111/j.1440-1843.2011.01943.x. [DOI] [PubMed] [Google Scholar]
- 312.Kohno S., Watanabe A., Aoki N., Niki Y., Kadota J., Fujita J., et al. Clinical response of levofloxacine 500 mg qd to respiratory tract infection. Jpn J Chemother. 2009;57:20–33. [Google Scholar]
- 313.Saito A., Tanigawara Y., Watanabe A., Aoki N., Niki Y., Kohno S., et al. Open study of sitafloxacin in patients with respiratory tract infections – PK/PD study. Jpn J Chemother. 2008;56:63–80. [Google Scholar]
- 314.Chuchalin A., Zakharova M., Doki D., Tokic M., Marschall H.P., Petri T. Efficacy and safety of moxifloxacin in acute exacerbations of chronic bronchitis: a prospective, multicenter, observational study (AVANTI) BMC Pulm Med. 2013;23:5. doi: 10.1186/1471-2466-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Starakis I., Gogos C.A., Bassaris H. Five-day moxifloxacin therapy compared with 7-day co-amoxiclav therapy for the treatment of acute exacerbation of chronic bronchitis. Int J Antimicrob Agents. 2004;23:129–137. doi: 10.1016/j.ijantimicag.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 316.Grossman R.F., Ambrusz M.E., Fisher A.C., Khashab M.M., Kahn J.B. Levofloxacin 750 mg QD for five days versus amoxicillin/clavulanate 875 mg/125 mg BID for ten days for treatment of acute bacterial exacerbation of chronic bronchitis: a post hoc analysis of data from severely ill patients. Clin Ther. 2006;28:1175–1180. doi: 10.1016/j.clinthera.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 317.El Moussaoui R., Roede B.M., Speelman P., Bresser P., Prins J.M., Bossuyt P.M. short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415–422. doi: 10.1136/thx.2007.090613. [DOI] [PubMed] [Google Scholar]
- 318.Falagas M.E., Avgeri S.G., Matthaiou D.K., Dimopoulos G., Siempos I.I. Short- versus long- duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442–450. doi: 10.1093/jac/dkn201. [DOI] [PubMed] [Google Scholar]
- 319.Miravitlles M., Kruesmann F., Haverstock D., Perroncel R., Choudhri S.H., Arvis P. Sputum colour and bacteria in chronic bronchitis exacerbations: a pooled analysis. Eur Respir J. 2012;39:1354–1360. doi: 10.1183/09031936.00042111. [DOI] [PubMed] [Google Scholar]
- 320.Adams S.G., Melo J., Luther M., Anzueto A. Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPD. Chest. 2000;117:1345–1352. doi: 10.1378/chest.117.5.1345. [DOI] [PubMed] [Google Scholar]
- 321.Takahata M., Fukuda Y., Futakuchi Y., Sugiura Y., Hisada H., Mizunaga S., et al. In vitro antibacterial activity of garenoxacin. Jpn J Chemother. 2007;55:1–20. [Google Scholar]
- 322.Araki N., Yanagihara K., Matsukawa Y., Harada Y., Migiyama Y., Nagaoka K., et al. Molecular characterization of quinolone-insensitive Streptococcus pneumonia isolates from Japanese patients. J Infect Chemother. 2013;19:356–359. doi: 10.1007/s10156-012-0461-0. [DOI] [PubMed] [Google Scholar]
- 323.Nakamura S., Yanagihara K., Morinaga Y., Izumikawa K., Seki M., Kakeya H., et al. Comparative mutant prevention concentration and mutant selection window of sitafloxacin versus other quinolones using strains of Haemophilus influenzae with decreasing susceptibility to levofloxacin. Int J Antimicrob Agents. 2009;33:489–490. doi: 10.1016/j.ijantimicag.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 324.Masterton R.G., Burley C.J. Randomized, double-blind study comparing 5- and 7-day regimens of oral levofloxacin in patients with acute exacerbation of chronic bronchitis. Int J Antimicrob Agents. 2001;18:503–512. doi: 10.1016/s0924-8579(01)00435-6. [DOI] [PubMed] [Google Scholar]
- 325.Granizo J.J., Giménez M.J., Barberán J., Coronel P., Gimeno M., Aguilar L. The efficacy of cefditoren pivoxil in the treatment of lower respiratory tract infections, with a focus on the per-pathogen bacteriologic response in infections caused by Streptococcus pneumoniae and Haemophilus influenzae: a pooled analysis of seven clinical trials. Clin Ther. 2006;28:2061–2069. doi: 10.1016/j.clinthera.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 326.Anzueto A., Bishai W.R., Pottumarthy S. Role of oral extended-spectrum cephems in the treatment of acute exacerbation of chronic bronchitis. Diagn Microbiol Infect Dis. 2007;57:31S–38S. doi: 10.1016/j.diagmicrobio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 327.Zervos M., Martinez F.J., Amsden G.W., Rothermel C.D., Treadway G. Efficacy and safety of 3-day azithromycin versus 5-day moxifloxacin for the treatment of acute bacterial exacerbations of chronic bronchitis. Int J Antimicrob Agents. 2007;29:56–61. doi: 10.1016/j.ijantimicag.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 328.Nakata K., Taguchi Y., Kudo S. 2000. Final draft report of the treatment guideline of DPB. Diffuse lung disease study group, scientific research specific disease study business by the ministry of health, labour and welfare; p. 111. Study report in 1999. [Google Scholar]
- 329.Yang M., Dong B.R., Lu J., Lin X., Wu H.M. Macrolides for diffuse panbronchiolitis. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD007716.pub2. CD007716. [DOI] [PubMed] [Google Scholar]
- 330.Kudo S., Uetake K., Hagiwara K., Hirayama M., Kyo E., Kimura H., et al. Study regarding the clinical efficacy of long-term therapy with low-dose erythromycin for diffuse panbronchiolitis. Jpn J Thorac Dis. 1987;25:632–642. [PubMed] [Google Scholar]
- 331.Yamamoto M., Kondo A., Tamura M., Izumi T., Ina Y., Noda M. Long-term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis. Jpn J Thorac Dis. 1990;28:1305–1313. [PubMed] [Google Scholar]
- 332.Nagai H., Shishido H., Yoneda R., Yamaguchi E., Tamura A., Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58:145–149. doi: 10.1159/000195915. [DOI] [PubMed] [Google Scholar]
- 333.Therapeutic effects of erythromycin on DPB- - - Double-blind study- - - . Report by the diffuse lung disease Survey/Study group of the Ministry of Health and Welfare in 1990. 1991. pp. 18–20. [Google Scholar]
- 334.Akira M., Higashihara T., Sakatani M., Hara H. Diffuse panbronchiolitis: follow-up CT examination. Radiology. 1993;189:559–562. doi: 10.1148/radiology.189.2.8210390. [DOI] [PubMed] [Google Scholar]
- 335.Ohno S., Sugiyama Y., Kitamura S. Effects of long-term erythromycin therapy on diffuse panbronchiolitis leading to chronic respiratory failure. Jpn J Thorac Dis. 1993;31:1251–1256. [PubMed] [Google Scholar]
- 336.Fujii T., Kadota J., Kawakami K., Iida K., Shirai R., Kaseda M., et al. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thotrax. 1995;50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Kudo S., Azuma A., Yamamoto M., Izumi T., Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157:1829–1832. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- 338.Keicho N., Kudo S. Diffuse panbronchiolitis: role of macrolides in therapy. Am J Respir Med. 2002;1:119–131. doi: 10.1007/BF03256601. [DOI] [PubMed] [Google Scholar]
- 339.Kadota J., Mukae H., Tomono K., Kohno S., Nasu M. Efficacy of lomg-term macrolide antibiotic therapy in patients with diffuse panbronchiolitis: comparison between HLA-B54-positive and negative cases. Int J Antimicrob Agents. 2004;24:550–554. doi: 10.1016/j.ijantimicag.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 340.Kadota J., Mukae H., Mizunoe S., Kishi K., Tokimatsu I., Nagai H., et al. Long-term macrolide antibiotic therapy in the treatment of chronic small airway disease clinically mimicking diffuse panbronchiolitis. Intern Med. 2005;44:200–206. doi: 10.2169/internalmedicine.44.200. [DOI] [PubMed] [Google Scholar]
- 341.Shirai R., Abe K., Yoshinaga M., Ishimatsu Y., Matsubara Y., Kawakami K., et al. Analysis of cases allowed to cease erythromycin therapy for diffuse panbronchiolitis–comparative study between patients with cessation of the therapy and patients continuing the therapy. J Jpn Assoc Infect Dis. 1997;71:1155–1161. doi: 10.11150/kansenshogakuzasshi1970.71.1155. [DOI] [PubMed] [Google Scholar]
- 342.Kadota J., Mukae H., Ishii H., Nagata T., Kaida H. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir Med. 2003;97:844–850. doi: 10.1016/s0954-6111(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 343.Kadota J., Sakito O., Kohno S., Abe K., Shirai R., Kawakami K., et al. Roxithromycin treatment in patients with chronic lower respiratory tract disease–its clinical efficacy and effect on cytokine. J Jpn Assoc Infect Dis. 1994;68:27–33. doi: 10.11150/kansenshogakuzasshi1970.68.27. [DOI] [PubMed] [Google Scholar]
- 344.Nakamura H., Fujishima S., Inoue T., Ohkubo Y., Soejima K., Waki Y., et al. Clinical and immune regulatory effects of roxithromycin therapy for chronic respiratory tract infection. Eur Respir J. 1999;13:1371–1379. doi: 10.1183/09031936.99.13613809. [DOI] [PubMed] [Google Scholar]
- 345.Kobayashi H., Takeda H., Sakayori S., Kawakami Y., Otsuka Y., Tamura M., et al. Study on azithromycin in treatment of diffuse panbronchiolitis. J Jpn Assoc Infect Dis. 1995;69:711–722. doi: 10.11150/kansenshogakuzasshi1970.69.711. [DOI] [PubMed] [Google Scholar]
- 346.Anwar G.A., Bourke S.C., Afolabi G., Middleton P., Ward C., Rutherford R.M. Effects of long-term low-dose azithromycin in patients with non-CF bronchiectasis. Respir Med. 2008;102:1494–1496. doi: 10.1016/j.rmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 347.Serisier D.J., Martin M.L., McGuckin M.A., Lourie R., Chen A.C., Brain B., et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309:1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- 348.Altenburg J., de Graaff C.S., Stienstra Y., Sloos J.H., van Haren E.H.J., Koppers R.J.H., et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309:1251–1259. doi: 10.1001/jama.2013.1937. [DOI] [PubMed] [Google Scholar]
- 349.Yamaya M., Azuma A., Tanaka H., Takizawa H., Chida K., Taguchi Y., et al. Inhibitory effects of macrolide antibiotics on exacerbations and hospitalization in chronic obstructive pulmonary disease in Japan: a retrospective multicenter analysis. J Am Geriatr Soc. 2009;56:1358–1360. doi: 10.1111/j.1532-5415.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 350.Albert R.K., Connett J., Bailey W.C., Casaburi R., Cooper J.A.D., Jr., Criner G.J., et al. Azithromycin for prevention of exacerbations of COPD. New Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.The committee for the Japanese Respiratory Society guidelines for the management of respiratory tract infection . 2003. Chapter IX. Acute exacerbation of diffuse panbronchiolitis and continuous infection. “The guidelines for the management of respiratory tract infection” basic concept of respiratory tract infection management in adults. Tokyo; pp. 43–45. [Google Scholar]
- 352.Roosevelt G.E. In: Nelson textbook of pediatrics. 19th ed. Kliegman R.M., Behrman R.E., Jenson H.B., Stanton B.F., editors. Saunders; Philadelphia: 2011. Acute inflammatory upper airway obstruction (Croup, epiglottitis, laryngitis, and bacterial tracheitis) pp. 1445–1450. [Google Scholar]
- 353.Cherry J.D. In: Feigin and Cherry's textbook of pediatric infectious diseases. 6th ed. Feigin R.D., Cherry J., Demmler-Harrison G.J., Kaplan S.L., editors. Saunders; Philadelphia: 2009. Croup (laryngitis, laryngotracheitis, spasmodic croup, laryngotracheobronchitis, bacterial tracheitis) pp. 254–268. [Google Scholar]
- 354.Denny F.W., Murphy T.F., Clyde W.A., Jr., Collier A.M., Henderson F.W. Croup: an 11-year study in a pediatric practice. Pediatrics. 1983;71:871–876. [PubMed] [Google Scholar]
- 355.Segal A.O., Crighton E.J., Moineddin R., Mamdani M., Upshur R.E. Croup hospitalizations in Ontario: a 14-year time-series analysis. Pediatrics. 2005;116:51–55. doi: 10.1542/peds.2004-1892. [DOI] [PubMed] [Google Scholar]
- 356.Klassen T.P. Croup. A current perspective. Pediatr Clin North Am. 1999;46:1167–1178. doi: 10.1016/s0031-3955(05)70180-2. [DOI] [PubMed] [Google Scholar]
- 357.Rihkanen H., Rönkkö E., Nieminen T., Komsi K.L., Räty R., Saxen H., et al. Respiratory viruses in laryngeal croup of young children. J Pediatr. 2008;152:661–665. doi: 10.1016/j.jpeds.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zoorob R., Sidani M., Murray J. Croup: an overview. Am Fam Physician. 2011;8:1067–1073. [PubMed] [Google Scholar]
- 359.Rajapaksa S., Starr M. Croup-assessment and management. Aust Fam Physician. 2010;39:280–282. [PubMed] [Google Scholar]
- 360.Watts K.D., Goodman D.M. In: Nelson text book of pediatrics. 19th ed. Kliegman R.M., Behrman R.E., Jenson H.B., Stanton B.F., editors. Saunders; Philadelphia: 2011. Wheezing, bronchiolitis, and bronchitis; pp. 1456–1460. [Google Scholar]
- 361.Welliver R.C. In: Feigin and Cherry's textbook of pediatric infectious diseases. 6th ed. Feigin R.D., et al., editors. Saunders; 2009. Bronchiolitis; pp. 138–146. [Google Scholar]
- 362.Shay D.K., Holman R.C., Newman R.D., Liu L.L., Stout J.W., Anderson L.J. Bronchiolitis-associated hospitalizations among US children, 1980―1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 363.Mansbach J.M., McAdam A.J., Clark S., Hain P.D., Flood R.G., Acholonu U., et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med. 2008;15:111–118. doi: 10.1111/j.1553-2712.2007.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Field C.M., Connolly J.H., Murtagh G., Slattery C.M., Turkington E.E. Antibiotic treatment of epidemic bronchiolitis–a double-blind trial. Br Med J. 1966;1:83–85. doi: 10.1136/bmj.1.5479.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kneyber M.C., van Woensel J.B., Uijtendaal E., Uiterwaal C.S., Kimpen J.L. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol. 2008;43:142–149. doi: 10.1002/ppul.20748. [DOI] [PubMed] [Google Scholar]
- 366.Kabir A.R., Mollah A.H., Anwar K.S., Rahman A.K., Amin R., Rahman M.E. Management of bronchiolitis without antibiotics: a multicentre randomized control trial in Bangladesh. Acta Paediatr. 2009;98:1593–1599. doi: 10.1111/j.1651-2227.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- 367.Tahan F., Ozcan A., Koc N. Clarithromycin in the treatment of RSV bronchiolitis: a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29:91–97. doi: 10.1183/09031936.00029206. [DOI] [PubMed] [Google Scholar]
- 368.Hall C.B., Powell K.R., Schnabel K.C., Gala C.L., Pincus P.H. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection. J Pediatr. 1988;113:266–271. [PubMed] [Google Scholar]
- 369.Al-Mutair i B., Kirk V. Bacterial tracheitis in children: approach to diagnosis and treatment. Paediatr Child Health. 2004;9:25–30. doi: 10.1093/pch/9.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Jones R., Santos J.I., Overall J.C., Jr. Bacterial tracheitis. JAMA. 1979;242:721–726. [PubMed] [Google Scholar]
- 371.Wong V.K., Mason W.H. Branhamella catarrhalis as a cause of bacterial tracheitis. Pediatr Infect Dis J. 1987;6:945–946. doi: 10.1097/00006454-198710000-00023. [DOI] [PubMed] [Google Scholar]
- 372.Donnelly B.W., McMillan J.A., Weiner L.B. Bacterial tracheitis: report of eight new cases and review. Rev Infect Dis. 1990;12:729–735. doi: 10.1093/clinids/164.5.729. [DOI] [PubMed] [Google Scholar]
- 373.Bernstein T., Brilli R., Jacobs B. Is bacterial tracheitis changing? A 14-month experience in a pediatric intensive care unit. Clin Infect Dis. 1998;27:458–462. doi: 10.1086/514681. [DOI] [PubMed] [Google Scholar]
- 374.Liston S.L., Gehrz R.C., Siegel L.G., Tilelli J. Bacterial tracheitis. Am J Dis Child. 1983;137:764–767. doi: 10.1001/archpedi.1983.02140340044012. [DOI] [PubMed] [Google Scholar]
- 375.Cherry J.D., Nieves D.J. In: Feigin and Cherry's textbook of pediatric infectious diseases. 6th ed. Feigin R.D., et al., editors. Saunders; Philadelphia: 2009. Bronchitis; pp. 269–272. [Google Scholar]
- 376.Smucny J., Fahey T., Becker L., Glazier R. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;4 doi: 10.1002/14651858.CD000245.pub2. CD000245. [DOI] [PubMed] [Google Scholar]
- 377.Gordon M., Lovell S., Dugdale A.E. The value of antibiotics in minor respiratory illness in children. A controlled trial. Med J Aust. 1974;1:304–306. [PubMed] [Google Scholar]
- 378.Taylor B., Abbott G.D., Kerr M.M., Fergusson D.M. Amoxycillin and co-trimoxazole in presumed viral respiratory infections of childhood: placebo-controlled trial. Br Med J. 1977;2:552–554. doi: 10.1136/bmj.2.6086.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.O'Brien K.L., Dowell S.F., Schwartz B., Marcy S.M., Phillips W.R., Gerber M.A. Cough illness/Bronchitis―principles of judicious use of antimicrobial agents. Pediatrics. 1998;101:178–181. [Google Scholar]
- 380.Centers of Disease Prevention (CDC) High levels of adamantane resistance among A (H3N2) viruses and interim guidelines for use of anti-viral agents–United States, 2005―06 influenza season. MMWR Morb Mortal Wkly Rep. 2006;55:44–46. [PubMed] [Google Scholar]
- 381.Centers of Disease Prevention (CDC) Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–435. [PubMed] [Google Scholar]
- 382.Chartrand C., Leeflang M., Minion J., Brewer T., pai M. Accuracy of rapid influenza diagnostic tests. A meta-analysis. Ann Intern Med. 2012;156:500–504. doi: 10.7326/0003-4819-156-7-201204030-00403. [DOI] [PubMed] [Google Scholar]
- 383.Domínguez-Cherit G., Lapinsky S.E., Macias A.E., Espinosa-perez L., dela Torre A., Poblano-Morales M., et al. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 384.Ariano R.E., Sitar D.S., Zelenitsky S.A., Zarychansk R., Pisipati A., Ahem S., et al. Enteric absorption and pharmaco-kinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ. 2010;182:357–363. doi: 10.1503/cmaj.092127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Hsu J., Santesso N., Mustafa R., Brozek J.,C., hen Y.L., Hopkins J.P. Antivials for treatment of influenza. A systemic review and meta-analysis of observation studies. Ann Intern Med. 2013;156:512–524. doi: 10.7326/0003-4819-156-7-201204030-00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Influenza Committee of the Japanese Association for Infectious Diseases, Proposal by the Japanese Association for Infectious Diseases, Strategies to control avian influenza (H7N9) [Draft]. http://www.kansensho.or.jp/influenza/pdf/1305_teigen.pdf.
- 387.South East Asia Innfectious Disease Clinical Research Network Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double -blind randomised controlled trial. BMJ. 2013;346:f3039. doi: 10.1136/bmj.f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Wright P.F. In: Nelson textbook of pediatrics. 19th ed. Kliegman R.M., Behrman R.E., Jenson H.B., Stanton B.F., editors. Saunders; Philadelphia: 2011. Influenza viruses; pp. 1121–1125. [Google Scholar]
- 389.Glezen W.P. In: Feigin and Cherry's textbook of pediatric infectious diseases. 6th ed. Cherry J., Demmler-Harrison G.J., Kaplan S.L., editors. Saunders; Philadelphia: 2009. Influenza viruses; pp. 2395–2414. [Google Scholar]
- 390.Ameican Academy of Peiatrics . Red book. 29th ed. 2012. Haemophilus influenza infections; pp. 439–453. [Google Scholar]
- 391.Jordan W.S., Jr., Denny F.W., Jr., Badger G.F., Curtiss C., Dingle J.H., Oseasohn R., et al. A study of illness in a group of clevel and families. XVII. The occurrence of Asian influenza. Am J Hyg. 1958;68:190–212. doi: 10.1093/oxfordjournals.aje.a119962. [DOI] [PubMed] [Google Scholar]
- 392.Wieching A., Benser J., Kohlhauser-Vollmuth C., Weissbrich B., Streng A., Liese J.G. Clinical characteristics of pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Northern Bavaria, Germany. BMC Res Notes. 2012;5:304. doi: 10.1186/1756-0500-5-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Gutierrez C., Nazar G.A., Torres J.P. Otolaryngological complications in patients infected with the influenza A (H1N1) virus. Otolaryngol Head Neck Surg. 2012;146:478–482. doi: 10.1177/0194599811425765. [DOI] [PubMed] [Google Scholar]
- 394.Streng A., Grote V., Liese J.G. Severe influenza cases in paediatric intensive care units in Germany during the pre-pandemic seasons 2005 to 2008. BMC Infect Dis. 2011;11:233. doi: 10.1186/1471-2334-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Randolph A.G., Vaughn F., Sullivan R., Rubinson L., Thompson B.T., Yoon G., et al. Critically ill children during the 2009―2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–e1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Nakamura-Uchiyama F., Hiromatsu K., Ishiwata K., Sakamoto Y., Nawa Y. The current status of parasitic diseases in Japan. Intern Med. 2003;42:222–236. doi: 10.2169/internalmedicine.42.222. [DOI] [PubMed] [Google Scholar]
- 397.Nakamura-Uchiyama F., Nawa Y. In: Tropical lung disease. 2nd ed. Sharma O.P., editor. Taylor and Francis; NY: 2006. Paragonimiasis; pp. 295–326. [Google Scholar]
- 398.Obara A., Nakamura-Uchiyama F., Hiromatsu K., Nawa Y. Paragonimiasis cases recently found among immigrants in Japan. Intern Med. 2004;43:388–392. doi: 10.2169/internalmedicine.43.388. [DOI] [PubMed] [Google Scholar]
- 399.Mukae H., Taniguchi H., Matsumoto N., Iiboshi H., Ashitani J., Matsukura S., et al. Clinicoradiologic features of pleuropulmonary Paragonimus westermani on Kyusyu Island, Japan. Chest. 2001;120:514–520. doi: 10.1378/chest.120.2.514. [DOI] [PubMed] [Google Scholar]
- 400.Uchiyama F., Ishiwata K., Nawa Y. The 5th Asian-Pacific Congress for parasitic Zoonosis. Chiba. 1998. Efficacy of chemotherapy of paragonimiasis in southern Kyushu, Japan. [Google Scholar]
- 401.Tomita M., Ishinari H., Matsuzaki Y., Shibata K., Koga Y., Yamaguchi R., et al. A case of chronic pleural empyema by Paragonimus westermani infection resistant to chemotherapy and cured by surgical decortication. J pn J Parasitol. 1996;45:242–246. [Google Scholar]
- 402.Rim H.J. Paragonimiasis: experimental and clinical experience with praziquantel in Korea. Arzneimittelforschung. 1984;34:1197–1203. [PubMed] [Google Scholar]
- 403.Kunst H., Mack D., Kon O.M., Banerjee A.K., Chiodini P., Grant A. Parasitic infections of the lung:a guide for the respiratory physician. Thorax. 2011;66:528–536. doi: 10.1136/thx.2009.132217. [DOI] [PubMed] [Google Scholar]
- 404.Asato R., Nakasone T., Yoshida C., Arakaki T., Ikeshiro T., Murakami H., et al. Current status of strongyloides infection in Okinawa. Jpn Jpn J Trop Med Hyg. 1992;20:169–173. [Google Scholar]
- 405.Mokhlesi B., Shulzhenko O., Garimella P.S., Kuma L., Monti C. Pulmonary strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004;11:6–13. doi: 10.1097/01.cpm.0000107609.50629.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Fusco D.N., Downs J.A., Satlin M.J., Pahuja M., Ramos L., Barie P.S., et al. Non-oral treatment with ivermectin for disseminated strongyloidiasis. Am J Trop Med Hyg. 2010;83:879–883. doi: 10.4269/ajtmh.2010.10-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Smith H., Holland C., Taylor M., Magnaval J.F., Schants P., Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 408.Akao N., Ohta N. Toxocariasis in Japan. Parasitol Int. 2007;56:87–93. doi: 10.1016/j.parint.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 409.Yoshikawa M., Koyama N., Hontsu S., Yamamoto Y., Mikasa K., Kimura H. Lessons from eight cases of adult pulmonary toxocariasis :abridged republication. Respirology. 2011;16:1014–1015. doi: 10.1111/j.1440-1843.2011.02000.x. [DOI] [PubMed] [Google Scholar]
- 410.Stürchler D., Schubarth P., Gualzata M., Gottstein B., Oettli A. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989;83:473–478. doi: 10.1080/00034983.1989.11812374. [DOI] [PubMed] [Google Scholar]
- 411.Hossack J., Ricketts P., Te H.S., Hart J. A case of adult hepatic toxocariasis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:344–348. doi: 10.1038/ncpgasthep1140. [DOI] [PubMed] [Google Scholar]
- 412.Boggild A.K., Keystone J.S., Kain K.C. Tropical pulmonary eosinophilia: a case series in a setting of nonendemicity. Clin Infect Dis. 2004;39:1123–1128. doi: 10.1086/423964. [DOI] [PubMed] [Google Scholar]
- 413.Study group regarding the establishment of medical management by appropriate treatment with rare-disease drugs for imported tropical diseases/parasitosis . 2010. Guidelines for drug therapy for parasitosis. Study group regarding drugs for tropical diseases. Tokyo. [Google Scholar]


