Abstract
Emerging and re-emerging infections and possible bioterrorism acts will continue to challenge both the medical community and civilian populations worldwide, urging health authorities to respond rapidly and effectively. Established in 2005, the European Community (EC)-funded European Network of Biosafety-Level-4 laboratories (Euronet-P4), which brings together the laboratories in Porton Down, London, Hamburg, Marburg, Solna, Lyon and Rome, seeks to increase international collaboration in the areas of high containment laboratory biosafety and viral diagnostic capability, to strengthen Europe's capacity to respond to an infectious disease emergency, and to offer assistance to countries not equipped with such costly facilities. Network partners have agreed on a common strategy to fill the gaps identified in the field of risk group-4 agents’ laboratory diagnosis, namely the lack of standardization and of reference samples. The network has received a further 3-year funding, to offer assistance to external laboratories, and to start the planning of field activities.
Keywords: Biosafety, BSL-4 facilities, emerging infectious diseases, laboratory diagnosis
Introduction
Among the threats that the world population faces in the 21st Century, the emergence of newly-emerging or re-emerging viral diseases [1, 2] has the potential of affecting the public health systems worldwide, posing challenges of varying severity, from a due concern as in the case of the human monkeypox cases in the USA [3] to real international emergencies as has been the case with severe acute respiratory syndrome (SARS) [4], and as is feared might happen in the future with an influenza pandemic. The SARS experience in particular has taught the scientific community a very important lesson: that a global health crisis can be effectively countered and brought to an end through an international effort of communication and cooperation, as opposed to local initiatives or uncoordinated actions. This lesson has been widely accepted by the European infectious disease community and networking has become a major approach to tackling potential challenges, as demonstrated by the number of networks and collaborative projects funded by the European Community in the last few years: ENIVD (European Network for the diagnosis of Imported Viral Diseases) [5], EUNID (European Network of infectious disease physicians) [6], EURONHID (European Network of Highly Infectious Diseases) [7], ETHREAT (European training for health professionals on rapid responses to health threats) [8], ETIDE (European training for infectious disease emergencies) [9], RIVIGENE (Genomic inventory, forensic markers, and assessment of potential therapeutic and vaccine targets for viruses relevant in biological crime and terrorism) [10], the VHF-Variola PCR project [11], and Biosafety Europe [12], just to mention a few. In the present review, we present the main activities of the European Network of Biosafety-Level-4 (BSL-4) laboratories [13, 14] in the area of viral diagnostics and biosafety, and we discuss the challenges and critical points identified, as well as our future steps to ensure that Europe can be prepared to face an unexpected event involving a highly dangerous pathogen.
There are currently four levels of biosafety for laboratories [15, 16, 17, 18, 19]. The first level is for agents that do not pose a significant threat to human health. Level two is intended for those that present a low to moderate risk. Level three is where potentially lethal pathogens are handled. The fourth level of biosafety is restricted to the most dangerous pathogens known to date, among which are the causative agents of viral haemorrhagic fevers and smallpox, usually referred to as risk group 4 (RG-4) agents in international laboratory biosafety guidelines [15, 16, 17] and European legislation [18, 19]; some of these agents are also included in the list of agents likely to be used as bioweapons [20]. Although the classification of infectious agents into four risk groups varies slightly according to the WHO, the European Community or the US Centers for Disease Control and Prevention, the levels of biosafety are clearly defined on the basis of the hazard posed to the health care worker or the community. An agent belonging to a given risk group will be handled at a corresponding or higher biosafety level, depending on the procedures employed.
The BSL-4 laboratory is the conventional environment where pathogens can be handled under the safest conditions, a state-of-the-art technical facility, designed and built in compliance with the highest standards of safety and security, where only staff that have undergone extensive and continuous training programmes can be granted permission to work. This aims to ensure that all activities are performed according to the basic principles of safety, which are translated into a set of strict procedures to guarantee that no infectious agent will ever escape from the laboratory. The highest level of biosafety is always required for the development of new diagnostic tests and vaccines for RG-4 agents, as well as for testing the efficacy of new anti-viral drugs against them. The use of a BSL-4 laboratory is also strongly recommended when working on newly-recognized agents whose dangerousness has not yet been assessed.
There are currently seven internationally recognized BSL-4 laboratories in the European Union, in five countries (UK, Germany, Sweden, Italy, France) and additional facilities are under construction or are being planned [21]. To enhance preparedness for emergencies, high-containment laboratories need to share reagents, experience, and lessons learned, and this is the reason why collaboration and information sharing have become mandatory.
The European Network of BSL-4 Laboratories Responds to a Recognized Need for International Cooperation
The European Network of BSL-4 Laboratories (Euronet-P4) was created in 2005 in response to a call by the European Commission [13]. Although some form of scientific collaboration among the few BSL-4 laboratories was at that time already established [11], the aim of the European Commission was to increase collaboration and to organize the existing BSL-4 laboratories into a network of expertise to enable a rapid, effective and coordinated response to health threats to European populations resulting from natural infection by RG-4 agents or their deliberate release. The network involves six partner institutions from five EU countries (UK, Germany, Sweden, Italy and France), with the addition of three other laboratories which are currently not funded by the grant, but are involved in the planning or construction of new European BSL-4 facilities, and participate in network activities as observers (one additional representation from Germany, one from Austria and one from France). Partner laboratories and observers are listed in Table 1 .
TABLE 1.
Euronet‐P4 partners and invited observers
| Lead partner | National Institute for Infectious Diseases IRCCS ‘L. Spallanzani’ – Rome, Italy |
| Partner | Bernhard Nocht Institute for Tropical Medicine – Hamburg, Germany |
| Partner | Philipps Universität Marburg – Marburg, Germany |
| Partner | Health Protection Agency – Centre for Infections (CfI), London, and Centre for emergency preparedness and response, Porton Down UK |
| Partner | Swedish Institute for Infectious Disease Control – Solna, Sweden |
| Partner | Laboratoire P4 Jean Merieux, Inserm – Lyon, France |
| Invited observer | Österreichische Agentur für Gesundheit und Ernährungssicherheit – Wien, Austria |
| Invited observer | Robert Koch Institut – Berlin, Germany |
| Invited observer | Unité des Virus Emergents, Faculté de Médecine, Université de la Méditerranée – Marseille, France |
The work of the network focuses on the following objectives: to establish a coordinated and accessible BSL-4 infrastructure for the surveillance and diagnosis of RG-4 agents; to review current laboratory diagnostic capability for RG-4 agents and to disseminate best practice within the network; to facilitate the development of new hazard-free diagnostic tests suitable to be transferred to other non-BSL-4 laboratories in all Member States; to establish communication channels for information exchange among National, European and International Health Authorities; and to standardize policies and procedures of biosafety and biosecurity. All project results are presented and discussed in meetings that are held twice yearly, which bring together partners and observers, and are posted on the project's secured web site [13].
Biosafety: Different Standards, One Common Goal
Biosafety can be defined as the combination of structural characteristics of the facility, use of sophisticated equipment and adherence to stringent procedures, which all together ensure that any infectious agent handled within a laboratory will never reach and contaminate the external environment. It is currently a topic of much discussion, as demonstrated by the number of published guidelines and manuals [15, 16, 17, 18, 19] and articles on the subject [21, 22, 23, 24], and there is a well recognized need for set standards and minimal requirements to aid in the process of certifying both new and existing laboratories.
Existing BSL-4 laboratories are of two types: those based on the use of full-body protective suits (often referred to as ‘space suits’), and the so-called cabinet lines, also known as Class-III Biosafety cabinets or glove-boxes. The first basic principle adopted in both is physical separation between the health care worker and the infectious material. To this end, the former type relies on personnel wearing positive-pressure suits connected by hoses to an air supply, whereas, in the latter, the same goal is achieved through the use of special sealed cabinets with built-in thick rubber gloves through which the health care worker reaches inside (Fig. 1 , Fig. 2 ). Another important principle is containment, ensured by constantly maintaining the air inside the laboratory under negative pressure, and by the use of interlocked doors. Intake and exhaust air are filtered through high-efficiency particulate air filters: this is usually carried out once for intake air and twice for the exhaust. Among the procedures common to all BSL-4 facilities are the complete inactivation of all infectious waste leaving the laboratory, by autoclaving or chemical disinfection; and the lengthy exit procedures, which include a chemical shower to disinfect the external surface of the suit followed by a body shower, and the removal of all personal protective equipment in a precise order. Of the seven BSL-4 laboratories operating in Europe, four use protective suits and three use glove boxes. All are national reference centres funded by their respective National Health Authorities, and were built in compliance with national registration schemes and international guidelines. Their structural characteristics vary considerably as a consequence of the fact that they were built in different countries in the absence of specific regulations and over a period of two decades, rather than as a reflection of different biosafety requirements. The reaching of a consensus on a common standard for biosafety practices was discussed in project meetings and it soon proved to be a difficult objective to achieve; by contrast, there was unanimous agreement on the necessity to provide assistance to other European countries in the process of setting up high containment facilities, with the aim of making the experience of long-established facilities available to new ones from the initial planning stages.
FIG. 1.

Working with a class-III biosafety cabinet.
FIG. 2.
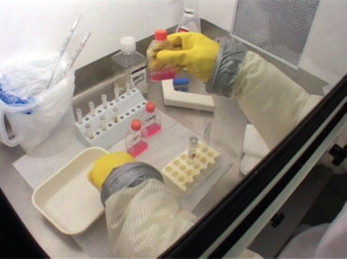
A view of the inside of a class-III biosafety cabinet.
The Laboratory Diagnosis of Highly Infectious Viral Agents: Gaps and Challenges
Public health systems and clinical laboratories worldwide are continually challenged by emerging and re-emerging viruses [25, 26, 27], owing not only to natural outbreaks (Table 2 ) [28, 29, 30, 31, 32] and potential acts of bioterrorism, but also to importation by returning travellers, as demonstrated by the recent cases of imported diseases, some of which are summarized in Table 3 [40, 41]. When one such outbreak occurs, effective infection control relies strongly on the availability of rapid and effective diagnostic tests to identify infected individuals and implement quarantine, especially for diseases for which there is no vaccine or treatment. Once again, the SARS epidemic in 2003 was an example of how the implementation of infection control measures, together with the effort of the scientific community to rapidly identify the aetiological agent, contributed to controlling the spread of the disease [42, 43, 44, 45].
TABLE 2.
Examples of outbreaks or international alerts involving RG‐4 viruses reported annually in international bulletins or in ProMed‐mail (http://www.promed‐mail.org)
| Year | Country | Disease | Number of cases | Source |
|---|---|---|---|---|
| 2000 | Uganda | Ebola | 426 (172 deaths) | WHO |
| 2003 | Congo Rep | Ebola | 35 (29 deaths) | WHO |
| 2004 | Sudan | Ebola | 17 (seven deaths) | WHO |
| 2004 | Iran | CCHF | >30 cases (five deaths) | ProMed posting |
| 2005 | Angola | Marburg | 368 (323 deaths) | WHO |
| 2005 | Pakistan | CCHF | 40 (five deaths) | ProMed posting |
| 2005 | Russia | CCHF | >100 cases | ProMed posting |
| 2006 | Iran | CCHF | 46 (three deaths) | ProMed posting |
| 2006 | Russia | CCHF | 41 (one death) | ProMed posting |
| 2006 | Turkey | CCHF | 150 (11 deaths) | Eurosurveillance weekly release 20th July 2006 |
| 2007 | Congo DR | Ebola | Up to 187 deaths | WHO |
| 2007/2008 | Uganda | Ebola | 93 (22 deaths) | WHO |
| 2007/2008 | Pakistan | CCHF | Three deaths | ProMed posting |
| 2008 | Turkey | CCHF | 37 deaths | ProMed posting |
| 2008/2009 | Congo DR | Ebola | 36 (12 deaths) | WHO |
Congo DR, Democratic Republic of the Congo; Congo Rep, Republic of the Congo; CCHF, Crimean–Congo haemorrhagic fever.
TABLE 3.
Examples of cases of suspected or proven infection with RG‐4 agents, occurring outside their natural context (importation by returning travellers, and one laboratory accident)
| Year | Country | Disease | Patient | Source |
|---|---|---|---|---|
| 1971–2003 | UK | Lassa | Ten cases in travellers returning from Sierra Leone or Nigeria (one fatal in 2000) | [33] |
| 2004 | Russia (Siberia) | Ebola | Laboratory accident involving one scientist (fatal) | [34] |
| 2006 | Germany | Lassa | Traveller returning from Sierra Leone (confirmed case) | [35] |
| 2008 | The Netherlands | Marburg | Traveller returning from Uganda (confirmed case) | [36] |
| 2009 | UK | Lassa | Traveller returning from Nigeria (confirmed case, fatal) | [37] |
| 2009 | USA | Marburg | Traveller returning from Uganda in 2008 (diagnosed retrospectively) | [38] |
| 2009 | UK | Lassa | Traveller returning from Mali (confirmed case, fatal) | [39] |
ECDC, European Centre for Disease Prevention and Control; CDC, Centers for Disease Control and Prevention.
At present, the diagnostics for emerging viruses are based essentially on molecular methods (real time or RT-PCR, sequencing, arrays), antigen detection techniques, serology, virus isolation and microscopy, although less frequently [46, 47]. In European BSL-4 laboratories, virus isolation and molecular biology assays are widely used, allowing safe identification of filoviruses, arenaviruses, orthopoxviruses and Crimean–Congo haemorrhagic fever virus; the same cannot be said for electron microscopy studies and antigen capture methods, most likely because these tests are considered to be of low added value given the reliability of molecular testing and virus isolation. Nevertheless, it should be stated that these, perhaps ‘old fashioned’ and less sophisticated, techniques could turn out to be an invaluable tool when dealing with a mutated strain that escapes the highly specific binding requirements of molecular probes, and they should be considered in the context of an epidemiological and clinical picture consistent with a specific disease and a negative real-time PCR result.
Serology diagnostics for RG-4 agents is the area that presents the greatest difficulties, essentially due to the fact that there are currently very few commercially available diagnostic tests for these pathogens, whose identification relies almost completely on the use of in-house reagents that need to be newly produced, and constantly verified and validated [48, 49, 50, 51, 52, 53]; indeed, the validation of these homemade tests is a serious challenge because of the lack of an adequate number of sera from infected patients to use as reference biological materials, as well as of materials from infected animals to be used as surrogates. It should be noted that not many commercial companies would have an interest in producing such tests, given the overall low number of cases for which diagnosis would be required in the absence of an emergency.
Importance of Networking in Preparing for Future Emergencies
The threat posed by naturally occurring infections or deliberate release of highly dangerous pathogens requires that countries act on a well established international programme of cooperation. This need has long been recognized internationally and several networks such as the WHO GOARN (Global Outbreak Alert and Response Network) and the GHSAG-LN (Global Health Security Action Group—Laboratory Network) are in operation today, whereas another network for ‘high consequence pathogens’ is being established by the WHO. In this context, it is essential that all the European BSL-4 Laboratories coordinate with one another, exchange expertise, and agree on a common strategy to improve the capacity of responding to these natural or deliberate threats to public health. Mutual recognition of laboratories and expeditious channels of communication between them for exchanging diagnostic protocols, samples, reagents when feasible, and personnel for training will be essential to secure an effective response to highly infectious disease emergencies because it can be foreseen that, in some cases, the exchange of information, expertise and materials could be made increasingly difficult by the growing strictness of both international and national (as is the case for the USA) regulations concerning bio-security [2, 54, 55].
The European Network of BSL-4 laboratories represents a good example of successful cooperation; all participants are respected as experts in the field of highly infectious diseases, and have links with all of the other European networks mentioned above [5, 6, 7, 8, 9, 10, 11, 12]. Many of the project participants are also linked with the WHO GOARN, are involved in the GHSAG-LN, and participate regularly in international exercises.
Future Perspectives
The Network is currently organizing external quality assurance exercises, to achieve standardization of the existing diagnostic tests; the preparation of a biosafety checklist is also underway, to offer new BSL-4 laboratories all the information, expertise and training needed to become a reference centre for the diagnosis of hazardous viruses in compliance with internationally recognized standards. Furthermore, a feasibility exercise regarding the development of a mobile laboratory suitable for the safe handling of highly infectious pathogens (i.e. covering technical requirements for establishment, biosafety, maintenance, deployment and operation mode in the field, and budget estimation) will represent the stepping stone towards the beginning of virological field activities. Recent publications have shown how the deployment of mobile laboratories in outbreak areas may serve the dual purpose of diagnosing infected individuals early, therefore facilitating the implementation of effective isolation of cases, and improving the available diagnostic tests through validation against human sera collected locally [56, 57, 58, 59, 60]. The purpose of a European mobile BSL-4 laboratory (which, for all technical and biosafety aspects, is also suitable for BSL-3 agents) would be to perform diagnostics within Europe or in other countries where these agents are endemic or outbreaks occur. It would not only increase European preparedness by overcoming specific geographical weaknesses represented by countries for which BSL-4 laboratories are probably too costly to build, operate and maintain, but also, and above all, it would represent an invaluable support and back-up during scaling up of investigations in outbreak-prone countries, at the same time allowing the search for more accurate and faster diagnostic tools to proceed unhindered.
All of these activities are part of a wider strategy of collaboration among European countries and international organizations such as the WHO and the GHSAG-LN, which aims to build our capacity to respond effectively to health threats.
Acknowledgements
We thank R. Iacovino for her support throughout the project. Other members of Euronet-P4 are: C. Drosten, Department of Virology, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany; K. Falk, Centre for Microbiological Preparedness, Swedish Institute for Infectious Disease Control, Solna, Sweden; M. C. Georges, Laboratoire P4 Inserm Jean Mérieux, French National Institute for Health and Medical Research, Lyon, France; H. Meyer, Bundeswehr Institute of Microbiology, Munich, Germany; S. Becker, Institute of Virology, Philipps Universitat Marburg, Marburg, Germany; M. Eickmann, Institute of Virology, Philipps Universitat Marburg, Marburg, Germany; H. Huemer, Austrian Agency for Health and Food Safety, Vienna, Austria; R. Charrel, Emerging Viruses Unit, University of Méditerranée, Marseille, France.
The authors gratefully acknowledge the advice and support of A. Werner.
Transparency Declaration
This work was supported by the EC grants Euronet-P4 (2003214) and ENP4-Lab (2006208). All authors contributed to the writing of the manuscript, and have read and approved the final version. None of them has any conflicts of interest to declare.
References
- 1.Morens DM, Fokers GK, Fauci A. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirschberg R, La Montagne J, Fauci AS. Biomedical Research – an integral component of National Security. N Engl J Med. 2004;350:21. doi: 10.1056/NEJMp048123. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Multistate outbreak of monkeypox – Illinois, Indiana, and Wisconsin. MMWR. 2003;52:537–540. [PubMed] [Google Scholar]
- 4.Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 5.ENIVD European Network for the diagnosis of Imported Viral Diseases. European Commission, DG health and consumer protection. Available from: http://ec.europa.eu/health/ph_threats/com/networks/network_enivd_en.htm and http://www.enivd.de
- 6.EUNID European Network of Infectious Disease physicians. European Commission, DG health and consumer protection. Available from: http://ec.europa.eu/health/ph_projects/2003/action2/action2_2003_04_en.htm and http://www.eunid.com
- 7.EURONHID. European Network of highly infectious diseases. European Commission, Public Health Executive Agency. Available from: http://ec.europa.eu/phea/documents/2007_5986_EN.pdf and http://www.eunid.com.
- 8.ETHREAT European training for health professionals on rapid responses to health threats. European Commission, DG health and consumer protection. Available from: http://ec.europa.eu/health/ph_projects/2004/action2/action2_2004_02_en.htm
- 9.ETIDE. European training for infectious disease emergencies. European Commission, DG health and consumer protection. Available from: http://ec.europa.eu/health/ph_projects/2005/action2/action2_2005_2_en.htm and http://www.etide.eu.
- 10.RiViGene Genomic inventory, forensic markers, and assessment of potential therapeutic and vaccine targets for viruses relevant in biological crime and terrorism. DG Research 2005. Information available from: http://www15.bni-hamburg.de/bni/others/rivigene/
- 11.VHF-Variola PCR. Development and commercial production of standardized PCR-assays for the detection of hemorrhagic fever viruses and variola virus and their implementation in the diagnostic service of EU P4 laboratories. European Commission, 6th Framework Programme on Research and technological development. Available from: http://cordis.europa.eu/fetch?CALLER=FP6_PROJ&ACTION=D&DOC=1&CAT=PROJ&QUERY=1183048901657&RCN=73904
- 12.Biosafety Europe Coordination, harmonization and exchange of Biosafety and biosecurity practices within a pan-european network. European Commission, 6th Framework Programme on Research and technological development. Available from: http://www.Ebsaweb.eu/Biosafety_Europe.html
- 13.EURONET-P4. The European Network of P4 laboratories 2005–2007. European Commission, DG Sanco and consumer protection. Available from: http://ec.europa.eu/health/ph_projects/2003/action2/action2_2003_19_en.htm and http://www.euronetp4.com.
- 14.ENP4-Lab. The European Network of P4 laboratories 2007–2010. European Commission, Public Health Executive Agency. Available from: http://ec.europa.eu/phea/documents/2007_5986_EN.pdf and http://www.euronetp4.com.
- 15.National Institutes of Health What are Biosafety Labs? Bethesda, Md: National Institute of Allergy and Infectious Diseases. Available at: http://www3.niaid.nih.gov/Biodefense/Public/Biolab.htm
- 16.World Health Organisation Laboratory Biosafety Manual, 2nd (revised) edn. Interim guidelines 2003. WHO/CDS/CSR/LYO/2003.4. Available from: http://www.who.int/csr/resources/publications/biosafety/who_cds_csr_lyo_20034/en/
- 17.US Department of health and Human Services, Public Health Service, Centers for Disease Control and Prevention and national Institutes of Health . Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th edn. US Government Printing Office; Washington, DC: 2007. [Google Scholar]
- 18.Council Directive 90/679/EEC of 26 November 1990 on the protection of workers from risks related to exposure to biological agents at work. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31990L0679:EN:HTML
- 19.Directive 2000/54/EC of the European Parliament and of the Council of 18 September2000 on the protection of workers from risks related to exposure to biological agents at work. Available from: http://europa.eu/eur-lex/pri/en/oj/dat/2000/l_262/l_26220001017en00210045.pdf
- 20.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public Health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronvall GK, Fitzgerald J, Chamberlain A, Inglesby TV, O'Toole T. High containment biodefense research laboratories: meeting report and center recommendations. Biosecur Bioterror. 2005;5:75–85. doi: 10.1089/bsp.2007.0902. [DOI] [PubMed] [Google Scholar]
- 22.Steinbrook R. Research in the Hot Zone. N Engl J Med. 2006;354:2. doi: 10.1056/NEJMp058245. [DOI] [PubMed] [Google Scholar]
- 23.Richardson L. Buying biosafety – is the price right? N Engl J Med. 2004;350:21. doi: 10.1056/NEJMp048053. [DOI] [PubMed] [Google Scholar]
- 24.Thacker PD. ‘Hot’ labs take on dangerous pathogens. JAMA. 2003;290:875–877. doi: 10.1001/jama.290.7.875. [DOI] [PubMed] [Google Scholar]
- 25.Feldmann H, Czub M, Jones S. Emerging and re-emerging infectious diseases. Med Microbiol Immunol. 2002;191:63–74. doi: 10.1007/s00430-002-0122-5. [DOI] [PubMed] [Google Scholar]
- 26.Fouchier RA, Rimmelzwaan GF, Kuiken T, Osterhaus AD. Newer respiratory virus infections: human metapneumovirus, avian influenza virus, and human coronaviruses. Curr Opin Infect Dis. 2005;18:141–146. doi: 10.1097/01.qco.0000160903.56566.84. [DOI] [PubMed] [Google Scholar]
- 27.Fauci AS. Emerging and re-emerging infectious disease: the perpetual challenge. Acad Med. 2005;80:1079–1085. doi: 10.1097/00001888-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Disease outbreaks reported: Ebola hemorrhagic fever in Uganda. Wkly Epidemiol Rec. 2000;42:337–338. [Google Scholar]
- 29.World Health Organization Outbreak(s) of Ebola haemorrhagic fever in the Republic of Congo, January–April 2003. Wkly Epidemiol Rec. 2003;78:285–289. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention Outbreak of Marburg virus hemorrhagic fever – Angola, October 1, 2004 – March 29, 2005. Morb Mortal Wkly Rep. 2005;54:308–309. [PubMed] [Google Scholar]
- 31.World Health Organization Marburg virus disease, Angola. Wkly Epidemiol Rec. 2005;80:115–117. [PubMed] [Google Scholar]
- 32.World Health Organization Ebola haemorrhagic fever in Uganda. http://www.who.int/csr/don/2007_11_30a/en/index.html
- 33.Health Protection Agency CDR Weekly, 23 September 2004; 14: 39. Available from: http://www.hpa.org.uk/cdr/archives/2004/cdr3904.pdf
- 34.Pro Med-mail Ebola, lab accident death. Archive number: 20040522. 1377, 22 May 2004. Boston, MA: International Society for Infectious Diseases. Available from: http://www.promedmail.org
- 35.Unit for Surveillance and Communcation, Unit for Preparedness and Response (Editorial team) Case of Lassa fever imported into Germany from sierra Leone (E-alert 24 July) Euro Surveill. 2006;11:3008. Avaliable from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3008 [PubMed] [Google Scholar]
- 36.Pro Med – mail. Marburg Hemorrhagic Fever – The Netherlands ex Uganda Archive number: 20080711.2 115, 11 July 2008. Boston, MA: International Society for Infectious Diseases. Available from: http://www.promedmail.org
- 37.Kitching A, Addiman S, Cathcart S. A fatal case of Lassa fever in London, January 2009. Euro Surveill. 2009;14:17–19. [PubMed] [Google Scholar]
- 38.Centers for Disease Prevention and Control (online) Outbreak Notice Update: 2008: Marburg Hemorrhagic fever, imported case – United States, 22 January 2009. Available from: http://www.cdc.gov/ncidod/dvrd/spb/outbreaks/index.htm # USA.
- 39.Atkin S, Anaraki S, Gothard P. The first case of Lassa fever imported from Mali to the United Kingdom, February 2009. Euro Surveill. 2009;14:19145. [PubMed] [Google Scholar]
- 40.Haas WH, Breuer T, Pfaff G. Imported Lassa fever in Germany: surveillance and management of contact persons. Clin Infect Dis. 2003;36:1254–1258. doi: 10.1086/374853. [DOI] [PubMed] [Google Scholar]
- 41.Gunther S, Emmerich P, Laue T. Imported Lassa fever in Germany: molecular characterization of a new lassa virus strain. Emerg Infect Dis. 2000;6:466–476. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rota PA, Oberste MS, Monroe SS. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 43.Drosten C, Gunther S, Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 44.Choi KW, Chau TN, Tsang O. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 45.Guan Y, Zheng BJ, He YQ. Isolation and characterisation of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 46.Drosten C, Kummerer BM, Schmitz H, Gunther S. Molecular diagnostics of viral hemorrhagic fevers. Antiviral Res. 2003;57:61–87. doi: 10.1016/s0166-3542(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 47.Strong JE, Grolla A, Jahrling PB, Feldmann H. Filoviruses and arenaviruses. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of molecular and clinical laboratory immunology. ASM Press; Washington, DC: 2006. pp. 774–790. [Google Scholar]
- 48.Niedrig M, Niklassson B, Lloyd G, Schmitz H, LeGuenno B. Establishing a European network for the diagnosis of ‘imported’ viral diseases (ENIVD) Euro Surveill. 1998;3:80. doi: 10.2807/esm.03.07.00108-en. Available from: http://www.eurosurveillance.org/em/v03n07/0307-223.asp [DOI] [PubMed] [Google Scholar]
- 49.Wolfel R, Paweska JT, Petersen N. Virus detection and monitoring of viral load in Crimean–Congo hemorrhagic fever virus patients. Emerg Infect Dis. 2007;13:1097–1100. doi: 10.3201/eid1307.070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhai J, Palacios G, Towner JS. Rapid molecular strategy for filovirus detection and characterization. J Clin Microbiol. 2007;45:224–226. doi: 10.1128/JCM.01893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niedrig M, Meyer H, Panning M, Drosten C. Follow-up on diagnostic proficiency of laboratories equipped to perform orthopoxvirus detection and quantification by PCR: the second international external quality assurance study. J Clin Microbiol. 2006;44:1283–1287. doi: 10.1128/JCM.44.4.1283-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niedrig M, Leitmeyer K, Lim W, Peiris M, Mackenzie JS, Zambon M. First external quality assurance of antibody diagnostic for SARS – new coronavirus. J Clin Virol. 2005;34:22–25. doi: 10.1016/j.jcv.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donoso Mantke O, Schmitz H, Zeller H, Heyman P, Papa A, Niedrig M, European Network for Diagnostics of Imported Viral Diseases (ENIVD) Quality assurance for the diagnostics of viral diseases to enhance the emergency preparedness in Europe. Euro Surveill. 2005;10:102–106. [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention, National Institutes of health . Biosafety in microbiological and biomedical laboratories (BMBL) 4th edn. Government Printing Office; Washington, DC: 1999. http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm [Google Scholar]
- 55.Committee on Research Standards to prevent the destructive application of biotechnology, National Research Council . Biotechnology research in an age of terrorism. National Academies Press; Washington, DC: 2004. [Google Scholar]
- 56.Onyango CO, Opoka ML, Ksiazek TG. Laboratory diagnosis of Ebola haermorrhagic Fever during an outbreak in Yambio, Sudan, 2004. J Infect Dis. 2007;196:S193–S198. doi: 10.1086/520609. [DOI] [PubMed] [Google Scholar]
- 57.Towner JS, Sealy TK, Ksiazek TG, Nichol ST. High-throughput molecular detection of haemorrhagic fever virus threats with applications for outbreak settings. J Infect Dis. 2007;196:S205–S212. doi: 10.1086/520601. [DOI] [PubMed] [Google Scholar]
- 58.Towner JS, Khristova ML, Sealy TK. Marburgvirus genomics and association with a large haemorrhagic fever outbreak in Angola. J Virol. 2006;80:6497–6516. doi: 10.1128/JVI.00069-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grolla A, Lucht A, Dick D, Strong JE, Feldmann H. Laboratory diagnosis of Ebola and Marburg haemorrhagic fever. Bull Soc Pathol Exot. 2005;98:205–209. [PubMed] [Google Scholar]
- 60.Towner JS, Rollin PE, Bausch DG. Rapid diagnosis of Ebola haemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


