Abstract
Despite tremendous resources being invested in prevention and treatment, breast cancer remains a leading cause of cancer deaths in women globally. The available treatment modalities are very costly and produces severe side effects. Drug repurposing that relate to new uses for old drugs has emerged as a novel approach for drug development. Repositioning of old, clinically approved, off patent non-cancer drugs with known targets, into newer indication is like using old weapons for new battle. The advances in genomics, proteomics and information computational biology has facilitated the process of drug repurposing. Repositioning approach not only fastens the process of drug development but also offers more effective, cheaper, safer drugs with lesser/known side effects. During the last decade, drugs such as alkylating agents, anthracyclins, antimetabolite, CDK4/6 inhibitor, aromatase inhibitor, mTOR inhibitor and mitotic inhibitors has been repositioned for breast cancer treatment. The repositioned drugs have been successfully used for the treatment of most aggressive triple negative breast cancer. The literature review suggest that serendipity plays a major role in the drug development. This article describes the comprehensive overview of the current scenario of drug repurposing for the breast cancer treatment. The strategies as well as several examples of repurposed drugs are provided. The challenges associated with drug repurposing are discussed.
Keywords: Breast cancer, Multitargeting, Non-cancer drug, OMICS, Serendipity
1. Introduction
Breast cancer, one of the most common cancer in women, is globally diagnosed by physical examination, breast scan and tissue biopsies. A total of 2.089 million new cases of breast cancer were identified in 2018, of which ∼627,000 cases died. This accounted for approximately 15% of all cancer deaths among women globally [1]. In 2018, the highest numbers of breast cancer cases were reported from Asia (9,11,014) followed by Europe (5,22,512), North America (2,62,347), Latin America and Caribbean (199,734), Africa (1,68,690) and Oceania (24,551) [1]. Although very common in women, breast cancer rarely occurs in men [2]. A vast heterogeneity in breast cancer molecular profiling has been reported [3]. Based on the ER/PR and HER2 expression, breast cancer can be of 4 types: (i) ER/PR+, HER2+; (ii) ER/PR−, HER2+; (iii) ER/PR+, HER2−; and (iv) ER/PR−, HER2− [4]. The most aggressive breast cancer is ER/PR−, HER2− (also called triple negative breast cancer, TNBC) because no receptor is present on these tumors. It is estimated that 70% of the breast cancers are hormone receptor positive (70%) and can be treated with corresponding therapies [5,6]. In addition to hormone receptors, other biomarkers used for clinical, pathological and molecular characterization of breast cancer include Ki67, p53, CA153, CEA, BRCA1/BRCA2 etc. [7]. These molecular biomarkers in breast cancer are used for diagnosis, staging and grading, therapeutic intervention, prognosis as well as clinical management of recurrent and metastatic cases [8].
2. Current breast cancer therapy
Unlike a decade ago, clinicians today have multiple choices for breast cancer treatment depending on the size, stage, grade, metastatic behavior, aggressiveness and intrinsic molecular subtyping of tumor, age, menopausal status, overall health, comorbidities, and preferences of the patient [[9], [10], [11], [12], [13]]. Chemotherapy, hormone therapy, immunotherapy, radiotherapy, and surgery are the common modalities for breast cancer [10,14]. Primary choice of treatment usually includes surgery with the aim of complete resection of the major tumor mass on the first hand. Breast conserving (lumpectomy) and breast reconstruction surgeries, mastectomy or lymph node dissections are performed initially in the breast cancer patients [15]. Surgery may be preceded with the systemic neoadjuvant therapies in order to shrink the tumor for effective surgery and to maximize breast conservation. For example, in HER2+ cases, Trastuzumab (Herceptin) and Pertuzumab (Perjeta) are given as neoadjuvant therapy and only Trastuzumab is continued post-surgery [16,17]. Surgery is mostly followed by chemotherapy or radiotherapy to further destroy the remnant micrometastatic cancer cells that have escaped the breast or lymph nodes, to decrease the chances of recurrence and to increase the overall patient survival [14,15]. The adjuvant (additional) therapies i.e. hormone or targeted therapy are provided to breast cancer patients depending on the expression of hormone receptor or target protein, respectively [9]. The ER/PR + breast cancer patients are given tamoxifen and aromatase inhibitor together or alternatively after the surgical resection [18,19]. Likewise, trastuzumab (herceptin) is mostly recommended to the HER2+ breast cancer patients [20]. TNBC patients are difficult to treat, with unfavorable prognosis and are generally administered with the standard chemotherapy along with the PARP inhibitors or the DNA-targeting platinum drug (carboplatin) [21,22]. Another strategy is based on the holistic approach of patient recovery i.e. complementary and alternative medicine. In the holistic approach, the patients are advised for several life style changes like exercise, yoga, meditation, acupuncture and/or Ayurvedic treatments in addition to the standard therapies [23].
The United States FDA has approved a number of drugs for the breast cancer therapy (Table 1 ). However, these drugs are very costly and produce numerous side effects [6,24]. The common reported side effects in patients include fatigue, headaches, pain, numbness, dental issues, lymphedema, musculoskeletal symptoms, heart problems, blood clots, menstrual and menopausal symptoms, infertility, bone loss, and memory loss. These side effects are hard to be tolerated by the patients who are already weakened by the disease. Eventually these also affect the decision for further choice of treatment and impair the quality of life. This necessitates the development of novel drugs for breast cancer therapy.
Table 1.
A list of clinically approved drugs for breast cancer therapy.
| Drug |
Type of breast cancer | Molecular mechanism | Side effects | ||
|---|---|---|---|---|---|
| Chemical name | Commercial name | Category | |||
| Abemaciclib | Verzenio | CDK4/6 inhibitor | ER+, PR+, HER2-, advanced and metastatic | Controls cell division | Abdominal pain, anemia, decreased appetite, diarrhea, fatigue, headache, infections, nausea, neutropenia, leukopenia, thrombocytopenia, vomiting |
| Ado-transtuzumab- Emstansine | Kadcyla | Targeted therapy | HER2+, early stage | Reduces cell growth | Anemia; bone, joint and muscle pain; constipation; headache; heart, lung and liver problems; low blood platelet count; low potassium levels; nausea; nerve damage; tiredness/fatigue |
| Albumin bound-paclitaxel | Abraxane | Taxanes | Advanced stage | Inhibits growth of dividing cancer cells | Neutropenia, leukopenia, anemia, edema, hair loss, nausea, vomiting, diarrhea, neuropathy, muscle or joint pain |
| Anastrazole | Arimidex | Aromatase inhibitor | ER+, PR+ | – | Depression, fatigue, headache, hot flashes, increased or decreased appetite, joint pain, lower bone density, mild diarrhea, mood swings, nausea, sweating, temporary hair thinning, vaginal dryness, weakness |
| Atezolizumab | Tecentriq | Immune checkpoint inhibitor | TNBC, PD-1+, Advanced stage | Elevates anti-tumour immuno response | Lung, liver, intestinal and hormone gland problems; infection; hair loss; neuropathy; nausea; diarrhea; anemia; constipation; cough; headache; neutropenia; low appetite; vomiting; fatigue |
| Bevacizumab | Avastin | Targeted therapy | HER2- | Anti-angiogenic | Bleeding from nose, high blood pressure, proteinuria, kidney malfunction, fatigue, neutropenia, leukopenia, diarrhea |
| Capecitabine | Xeloda | Antimetabolite | Metastatic | False incorporation in DNA | Decreased appetite, dehydration, diarrhea, hand-foot syndrome, irregular periods, mouth and throat sores, nausea, vomiting |
| Carboplatin | Paraplatin | Platinum based | Advanced stage | Damages genetic material | Fatigue, nausea, leukopenia, kidney and nerve damage, hair loss, loss of appetite, irregular periods |
| Cyclophosphamide | Cytoxan | Alkylating agent | Early and advanced stages | Damages genetic material | Appetite loss, diarrhea, hair loss, irregular periods, leukopenia, mouth sores, nausea, vomiting |
| Darbepoetin alfa | Aranesp | Erythropoiesis stimulating agent | To treat anemia caused by chemotherapy | – | Abdominal pain, blood clots, constipation, diarrhea, headache, heart problems, joint and muscle pain, nausea, stroke, vomiting |
| Daunorubicin | Cerubidine | Anthracyclins | Early and advanced stages | Damages genetic material | Nausea, vomiting, loss of appetite, stomach pain, diarrhea, difficulty swallowing, hair changes, skin sensitivity, rash, nail changes, irregular periods |
| Denosumab | Xgeva | RANKL inhibitor | Advanced stage | Limits osteoclasts activity | Bone pain, nausea, vomiting, fever, fatigue, constipation, diarrhea, loss of appetite, teary eyes, heartburn, mouth sores, depression, vaginal discharge, hand-foot syndrome, hair changes, osteonecrosis of the jaw |
| Docetaxel | Taxotere | Taxane | Early and advanced stages | Interferes with cell division | Allergic reactions; constipation; fatigue; fluid retention; hair loss; irregular periods; leukopenia; muscle, bone or joint pain; nail changes; nausea; neuropathy; sores; susceptibility to infection; taste changes; vomiting; watery eyes |
| Doxorubicin | Adriamycin | Anthracyclins | Advanced stage | Damage genes | Anemia, diarrhea, edema, hair loss, infections, leukopenia, muscle or joint pain, nausea, nerve damage, vomiting |
| Epirubicin | Ellence | Anthracyclins | Early and advanced stages | Damages genetic material | Nausea, vomiting, diarrhea, mouth sores, hair loss, leukopenia, neutropenia, irregular periods |
| Epoetin | Epogen | Erythropoiesis stimulating agent | To treat anemia caused by chemotherapy | – | Abdominal, joint and muscle pain; blood clots; constipation; diarrhea; headache; heart problems; injection site pain; nausea; stroke; vomiting |
| Epoetin alfa | Procrit | Erythropoiesis stimulating agent | Combination to treat anemia | Makes more red blood cells | Blood clots, constipation, diarrhea, headache, heart problems, itching, joint pain, muscle pain, nausea, neuropathy, rash, sleeping problems, stomach pain, stroke, vomiting |
| Eribulin | Halaven | Microtubule inhibitor | Advanced stages | Disrupt cell division | Leukopenia, neutropenia, anemia, fatigue, weakness, hair loss, neuropathy, nausea, fever, constipation, irregular periods |
| Everolimus | Afinitor | mTOR inhibitor | HER2+, HER2- | Interferes with mTOR kinase | Lung, breathing and kidney problems, mouth sores, infections, rash, fatigue, diarrhea, decreased appetite |
| Exemestane | Aromasin | Aromatase inhibitor | ER+, PR+ | Lowers ER amount | Hot flashes, mood swings, depression, nausea, fatigue, increased sweating, increased appetite, weakened bones |
| Filgrastim | Neupogen | GCSF | Combination | Increases neutrophils | Bone, joint, stomach and muscle pain; headache; nosebleeds; spleen rupture; fever; trouble breathing; rash; itching |
| Filgrastim-sndz | Zarxio | GCSF | Combination | Increases neutrophils | Bone, joint, stomach and muscle pain; headache; nosebleeds; spleen rupture; fever; trouble breathing; rash; itching |
| Fluorouracil | Adrucil | Antimetabolite | Early and advanced stages | False nucleotide incorporation | Diarrhea, loss of appetite, mouth sores, nausea, taste changes, vision problems, vomiting, leukopenia, neutropenia, hand-foot syndrome, irregular periods |
| Fluoxymesterone | Halostein | Hormone therapy | ER+, PR+, advanced stages | Lowers estrogen amount | Headache, facial hair growth, nausea, acne, anxiety, insomnia |
| Fulvestrant | Faslodex | HT-ERD | ER+, PR+, HER2-, Advanced satge | Binds to ER | Back pain, constipation, diarrhea, headache, hot flashes, injection site pain, nausea, sore throat, stomach/abdominal pain, vomiting |
| Gemcitabine | Gemzar | Antimetabolite | Advanced stages | False nucleotide incorporation | Fatigue, leukopenia, neutropenia, hair thinning, nausea, anemia, diarrhea, irregular periods |
| Goserelin | Zoladex | HT-LHRH | ER+, PR+, Early stage | Reduces amount of estrogen | Bone pain, breast tenderness, headaches, Hot flashes, loss of libido, vaginal dryness, weight gain |
| Ixabepilone | Ixempra | Epithilone chemotherapy | Metastatic | Interferes cell division | Abdominal, bone, join and muscle pain; constipation; diarrhea; fatigue; hair loss; hand-foot syndrome; headache; heartburn; insomnia; irregular periods; nausea; skin problems; taste changes; vomiting; watery eyes; weakness; weight loss |
| Lapatinib | Tykerb | HER2 inhibitor | HER2+ | Reduces tumour cell growth | Diarrhea, fatigue, insomnia, liver problems, loss of appetite, mouth or throat sores, nausea, neuropathy, rash, vomiting |
| Larotrectinib | Vitrakvi | Tyrosine receptor kinase inhibitor | – | Inhibits cell growth | Fatigue, nausea, dizziness, vomiting, liver problem, cough, constipation, diarrhea, Infection, fever, abdominal pain |
| Letrozole | Femara | Aromatase inhibitor | ER+, PR+, Early and advanced stages | Lowers estrogen amount | Body and joint pain, nausea, mood swings, depression, headache, weakened bones, fatigue, difficulty breathing, high cholesterol |
| Leucovorin | Folinic acid, Wellcovorin, Citrovorum factor | Folic acid (B9 Vitamin) | Combination | Protects healthy cells | Allergic reaction, nausea, vomiting, diarrhea, mouth sores, seizures, fainting |
| Leuprolide | Lupron | HT-LHRH agent | ER+, PR+, early stage | Reduces estrogen amount | Hot flashes, mood swings, loss of libido, osteoporosis |
| Lipoosomal doxorubicin | Doxil | Anthracycline | Advanced stages | Damages genetic material | Hand-foot syndrome, hair loss, irregular periods, leukopenia, loss of appetite, low platelet counts, vomiting, nail changes, mouth sores, nausea |
| Megestrol | Megace | HT-Progestin | ER+, PR+, Advanced stage | Suppress the effects of estrogen | Loss of libido, vaginal bleeding, insomnia, gas, rash |
| Methotrexate or amethopterin | Mexate, Folex, Rheumatrex | Antimetabolite | Early and advanced stage | False building blocks | Diarrhea, hair changes, irregular periods, rash, joint pain, loss of appetite, nausea, mouth sores, swelling in the feet and legs, vomiting |
| Mitoxantrone | Novantrone | Anthracycline | Advanced stage | Damages genetic material | Back pain, constipation, diarrhea, fatigue, hair loss, headache, heartburn, irregular periods, leukopenia, loss of appetite, mouth sores, nail changes, nausea, neutropenia, vomiting, weakness |
| Mutamycin | Mitomycin | Antibiotic | Advanced stage | Damages genetic material | Fatigue, hair loss, irregular periods, low white blood cell counts, mouth sores, nausea, susceptibility to infection, vomiting |
| Neratinib | Nerlynx | HER inhibitor | HER2+, early stage | Inhibits cell growth | Abdominal pain, diarrhea, fatigue, liver problems, mouth sores, nausea, rash, vomiting |
| Olaparib | Lynparza | PARP inhibitor | HER2- | PARP inhibition | Anemia, nausea, fatigue, vomiting, leukopenia, upper respiratory infections, cold and flu symptoms, diarrhea, bone and joint pain, changes in sense of taste, headache, decreased appetite, constipation, mouth sores |
| Paclitaxel | Taxol | Taxane | Early and advanced stages | Interferes with cell division | Allergic reactions, diarrhea, hair loss, irregular periods, leukopenia, mouth sores, neuropathy, susceptibility to infection, vomiting, weakness |
| Palbociclib | Ibrance | CDK 4/6 inhibitor | ER+, PR+, HER2-, Advanced stage | Controls cell division | Anemia, fatigue, nausea, neuropathy, mouth sores, hair loss, Diarrhea, vomiting, weakness, decreased appetite, neutropenia, infections, blood clots |
| Pamidronate | Aredia | Biophosphonate | Bone complications | Limits bone damage by osteoclasts | Anemia, bone pain, fatigue, fever, loss of appetite, nausea, osteonecrosis of the jaw, vomiting, |
| Pegfilgrastim | Neulasta, Neulastim | GCSF | Combination | Make more neutrophils | Stomach, left shoulder, bone, joint and muscle pain, spleen rupture, fever, breathing problems, rash, itching, headache, weakness, constipation, vomiting, swelling |
| Pertuzumab | Perjeta | HER2 inhibitor | HER2+, early satge | Reduces growth | Birth defects, diarrhea, fatigue, hair loss, nausea, neutropenia, peripheral neuropathy, rash |
| Raloxifene | Evista | SERMs | ER+, PR+ | Reduces ER amount | Blood clots, depression, hot flashes, irregular periods, joint pain, mood swings, trouble sleeping, vaginal discharge or bleeding, weight gain |
| Ribolcicilib | Kisqali | CDK 4/6 inhibitor | HER2- | Controls cell division | Back pain, constipation, diarrhea, fatigue, hair loss, headache, liver problems, nausea, neutro- and leuko-penia, vomiting |
| Talazoparib | Talzenna | PARP inhibitor | HER2-, Advanced stage | PARP inhibition | Fatigue, anemia, nausea, neutropenia, headache, thrombocytopenia, vomiting, hair loss, diarrhea, decreased appetite |
| Tamoxifen | Nolvadex, Tamofen, Tamone, Soltamox | SERM | ER+, PR+, Advanced stage | Binds to estrogen receptor | Blood clots, depression, endometrial cancer, weight gain, irregular periods, hot flashes, vaginal discharge or bleeding, |
| Thiotepa | Thioplex | Alkylating agent | Early and advanced stages | Damages genetic material | Leukopenia, neutropenia, nausea, vomiting, loss of appetite, fatigue, hair changes, irregular period |
| Toremifene | Fareston | SERM | ER+, PR+ | Binds to ER | Hot flashes, nausea, weight gain, skin rashes, headache |
| Trastuzumab | Herceptin Hylecta | HER2 inhibitor | HER2+, Early and advanced stages | Reduces cell growth and enzyme helps body in using herceptin | Body pain, chills, cough, diarrhea, fatigue, fever, headache, heart problems, infection, insomnia, joint pain, lung problems, muscle pain, nausea, rash, upper respiratory tract infection, weakening of the heart muscle |
| Trastuzumab-dkst | Ogivri | HER2 inhibitor | HER2+, early satge | Reduces growth | Diarrhea, low white blood cell counts, lung problems, peripheral neuropathy, weakening of the heart muscle and other heart problems |
| Trastuzumab-dttb | Ontruzant | HER2 inhibitor | HER2+, early satge | Reduces growth | Chills, cough, diarrhea, fever, headache, infection, insomnia, nausea, rash |
| Trastuzumab-pkrb | Herzuma | HER2 inhibitor | HER2+ | Reduces tumor cell growth | Headache, diarrhea, nausea, chills, fever, infection, insomnia, cough, rash, low white blood cell count |
| Trastuzumab-qyyp | Trazimera | HER2 inhibitor | HER2+ | Reduces tumor cell growth | Headache, diarrhea, nausea, chills, fever, infection, insomnia, cough, rash |
| Triptorelin | Trelstar | HT-LHRH | ER+, PR+, Early stage | Reduces amount of estrogen | Hot flashes, mood swings, loss of libido, osteoporosis |
| Vincristine | Oncovin, Vincasar PES, Vincrex | Vinca alkaloid | Advanced stage | Interefers with genes | Nausea, vomiting, Diarrhea, constipation, hair changes, neuropathy, fatigue, muscle and abdominal cramps, irregular periods |
| Vinorelbine | Navelbine | Vinca alkaloid | Advanced stage | Interfere with genes | Diarrhea, hair loss, irregular periods, leukopenia, mouth sores, nausea, neuropathy, vomiting |
| Zoledronic acid | Zometa | Bisphosphonate | Combination | Limits osteoclast activity | Bone pain, nausea, vomiting, fever, fatigue, constipation, diarrhea, loss of appetite, watery eyes, heartburn, mouth sores, depression, vaginal discharge, hand-foot syndrome, hair changes, osteonecrosis of the jaw |
Abbreviations: CDK: cyclin-dependent kinase; TNBC: triple negative breast cancer; PD-L1: programmed death-ligand 1; VEGF: vascular endothelial growth factor; HER: human epidermal growth factor receptor 2; ERD: estrogen receptor downregulator; SERM: Selective estrogen receptor modulator; ER: estrogen receptor; PR: progesterone receptor; LHRH: luteinizing hormone-releasing hormone; HT: hormone therapy.
We have previously reviewed the overall process involved in cancer drug development [25,26]. The drug development for breast cancer like other cancer types is a multi-step process involving the designing, synthesis, characterization, testing for efficacy and toxicities, and approval. Overall, the process is lengthy, involving huge investment of money. It is estimated that the development of a new drug usually requires 15–18 years of time and approximately 2–3 billion dollars. On the top of all this, nearly 90% of the drugs fail during the clinical trial stage due to unexpected lack of appropriate efficacy, unacceptable side effects or regulatory norms [25,26]. Therefore, the approaches to expedite the drug development process are required. One approach might be finding new uses for old clinically approved drugs (Drug repositioning).
3. Drug repositioning
The drug repositioning (aka drug repurposing, drug reprofiling, drug re-tasking, drug redesigning, drug resorting, drug reindication, indication switching, therapeutic switching) can be defined as exploring the new uses for an old clinically approved drug, with reduced risk, time and cost. Drug repositioning circumvents the usual route of substantially higher rates, slow pace and side effects etc. [[27], [28], [29]]. It is becoming an attractive concept for pharmaceutical industries. It is estimated that the repositioning for drug development requires much lesser time (3–5 years) and money ($0.3 billion) with at least known and approved side effects; and higher probability of success [28,[30], [31], [32], [33]]. Repositioning of old drugs offer rapid transition from bench to bedside as already approved by FDA and other regulatory norms, passed through the clinical trials and are approved for human use. These drugs have well defined pharmacodynamics, pharmacokinetics, dose, side effects, metabolic profiles and targeted molecular pathway etc [29,34]. Here, the clinical development can directly start from the phase II trial to assess the efficacy for new indication/disease target. The successful example of drug repositioning includes Sildenafil (Viagra) and Thalidomide. Viagra was originally launched for hypertension and angina pectoris patients by Pfizer (1980). However, the patients developed penile erections as side effects in phase I clinical trial. The drug was later developed for erectile dysfunctions. In 2012, the total sale of Viagra was 2.05 billion dollars [35]. Similarly, the sedative thalidomide was serendipitously repurposed for erythema nodosum leprosum (ENL) in 1964 and for multiple myeloma and other cancers in 1999 [[36], [37], [38], [39]].
4. Strategies for drug repositioning
Recent advances in genomics, proteomics, transcriptomics and metabolomics have provided vast and deep knowledge about the molecular and metabolic alterations that occurs in cancers. The fundamentals of drug repurposing through the integration of system biology and bioinformatics depends on basic concepts i.e. activity based and in silico drug discovery [40]. Activity based drug repurposing is the experimental approach where the drug candidate is evaluated for the anti-cancer activity directly. The drug that is structurally similar would tend to have similar target, shared biological activity and indications [41]. In the two diseases where same metabolic pathway or signaling is affected, then the drugs that target the specific pathway can be used in both of the diseases irrespective of their structural dissimilarity [26,42]. The drug that shows strong efficiency for any off-target (side effect) in one disease can be explored further i.e. the off-target binding and effect can be used as the novel indication of that drug in some other disease [[43], [44], [45]]. In silico drug repurposing approach is based on the association and/or overlaps in the disease-gene-drug paradigm. The large data about drug-disease, gene expression (microarrays) or protein-protein interactions or gene-protein interactions or signaling pathway mapping, signature matching, genome-wide association studies (GWAS) can be used for providing drug-disease gene association networking by the systematic integration and coordination of computation and bioinformatics, modeling (such as docking for structural modeling), experimentation, statistics, machine learning etc. [[46], [47], [48], [49]]. The various data resources are available that can be used for bio-informatics based exploration followed by experimental validation for any drug repurposing possibilities in any disease. The similar gene expression profile for drug phenotype in patients with different diseases as mapped by connectivity map (cMap), Library Integrated Network based Cellular Signatures (LINCS), expression signatures may have similar therapeutic applications [[50], [51], [52]]. Other important public data resources that are used for network-based drug repositioning includes gene set enrichment analysis (GSEA) for drug-drug similarity network [53]; DrugBank [54], online mendelian inheritance in men (OMIM) [55] and GEO [56] for predicting drug-disease network; KEGG [57], STRING [58], BioGrid [59], HAPPI [60] and Reactome [61] for pathway and/or protein-protein interactions; STITCH drug-gene/protein database [62,63], TTD therapeutic target database [64], SFINX for drug-drug interactions [65]; and SIDER for drug side effects [66]. Also, the recently reported ‘Drug Repurposing from Control System theory (DeCoST)’ is a comprehensive platform for drug repurposing that encompasses various limitations in the previous databases like variation in number of copies of gene of interest, mutations, lack of reference for normal range of gene expression etc. in different diseases [67]. Vigorously exploited ‘network-based approach’ by integrating the mentioned resources for identifying potential target, pathway and drug has been reported as the most potential way for drug repositioning [68].
5. Drugs repurposed for breast cancer treatment
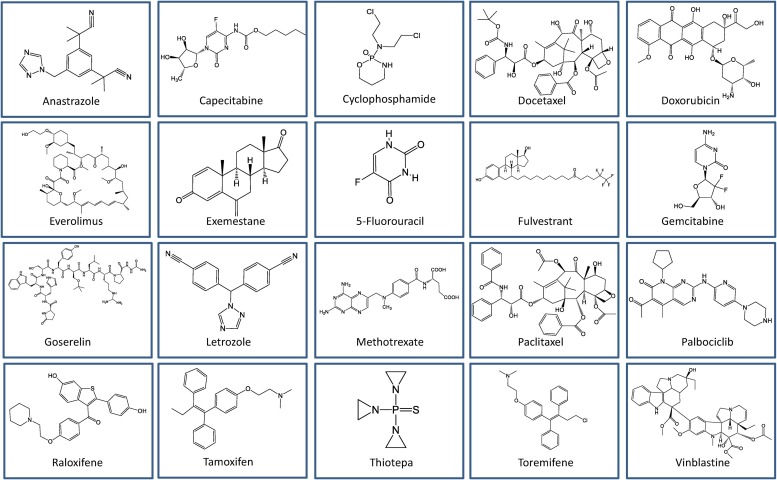
In the following section, we discuss the clinically approved drugs that were originally used for diseases other than breast cancer. However, these drugs are now being used or explored for breast cancer therapy. The clinically approved repositioned drugs for breast cancer are grouped based on their mode of action (Table 2 ). These drugs are diverse in terms of their chemical structures (Fig. 1 ).
Table 2.
A list of repositioned drugs approved for breast cancer treatment.
| Drug |
Breast cancer stage | Mechanism | Original indication | ||
|---|---|---|---|---|---|
| Category | Chemical name | Commercial name | |||
| Alkylating agent | Cyclophosphamide | Cytoxan, Clafen, Neosar | Early and advanced | Inhibits DNA replication by damaging genetic material of the cell | As immuno-modulator in autoimmune diseases |
| Thiotepa | Thioplex, Tespa, Thiophosphoamide, TSPA, Tepadina | Early and advanced | Immunosuppressant | ||
| Anthracyclins | Doxorubicin | Adriamycin, Caelyx, Rubex | Advanced | DNA intercalation | Antibiotic from Streptomyces peucetius bacterium |
| Antimetabolite | Capecitabine | Xeloda | Metastatic and advanced | False building block incorporation during cell growth | Colon cancer |
| Fluorouracil | Adrucil, Carac | Early, advanced and metastatic | Keratoacanthomas, actinic keratosis, and skin warts | ||
| Gemcitabine | Gemzar | Metastatic and advanced | Anti-viral drug | ||
| Methotrexate | Mexate, Folex, Rheumatrex | Early and advanced | Leukemia | ||
| CDK 4/6 inhibitor | Palbociclib, Palbonix | Ibrance | ER+, PR+, HER2-, advanced | Interferes with cell cycle | CDK 4/6 inhibitor |
| HT-SERM | Tamoxifen | Nolvadex, Apo-Tamox, Tamifen, Soltamox | ER+, PR+, advanced | Binds to ER | Albright syndrome, ovulation induction |
| Toremifene | Fareston | ER+, PR+ | Infertility with an ovulatory disorders | ||
| Raloxifene | Evista | ER+, PR+ | Osteoporosis in postmenopausal women | ||
| HT-Aromatase inhibitor | Exemestane | Aromasin | ER+, PR+ | Lowers estrogen amount | Ovulation induction |
| Letrozole | Femara | ER+, PR+, early and advanced | Ovulation induction | ||
| Anastrazole | Arimidex | ER+, PR+, advanced | Ovulation induction | ||
| HT-SERD | Fulvestrant | Faslodex | ER+, PR+, HER2-, advanced | ER degradation | Antiestrogen |
| HT-LHRH agent | Goserelin | Zoladex | ER+, PR+, early | Reduces amount of estrogen | Prostate cancer, uterine fibroids, assisted reproduction |
| mTOR inhibitor | Everolimus, Votubia, Evertor | Afinitor | HER2+, HER2- | Interferes with mTOR kinase | Immunosuppressant during organ transplants, wound healing |
| Mitotic inhibitor | Docetaxel | Taxotere | Early and advanced | Interferes with cell division | Hormone-refractory prostate cancer |
| Paclitaxel | Taxol, Onxol | Early and advanced | Ovarian cancer, atrial restenosis | ||
| Vinblastine | Velban, Velsar, Adria, Velbe | Advanced | Interferes with genes | Hodgkin lymphoma, non-Hodgkin's lymphoma, histiocytosis | |
Abbreviations: CDK: cyclin-dependent kinase; ERD: ER down-regulator; LHRH: luteinizing hormone-releasing hormone; SERD: selective estrogen receptor degrader; SERM: selective estrogen receptor modulator; ER: estrogen receptor; PR: progesterone receptor, HER: human epidermal growth factor receptor.
Fig. 1.
Chemical structure of clinically approved drugs repositioned for breast cancer treatment.
5.1. Alkylating agents
These are DNA damaging agent that alkylate the guanine base thereby rendering them unable to bind to complementary strand. This leads to inhibition of DNA replication and cancer cell growth. The alkylating agents affect all phases of cell cycle. Cyclophosphamide is a known immuno-modulator that inhibits the suppressive regulatory T cells and enhances the effector T cells in tumor microenvironment. It shows the biphasic effect i.e. at low doses it imparts the immuno-suppressive function and at higher doses it functions as an alkylating agent leading to the death of tumour as well as the lymphoid cells [69,70]. Usually 600 mg/m² cyclophosphamide is intravenously (IV) administered to breast cancer patients in combination with other chemotherapeutics (Docetaxel, Paclitaxel, Doxorubicin). The recommended regimens for robustly healthy breast cancer patients include 6 cycles of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or 4 cycles of adriamycin and cyclophosphamide (AC) [71,72].
Thiotepa is a derivative of N,N',N‿-triethylenephosphoramide (TEPA) that was launched (1953) as immunosuppressive drug for transplantation in hematological diseases [73]. Subsequently, the drug was recommended for solid tumors in 1959 [74] and for breast cancer (0.3 to 0.4 mg/kg IV repeated every 1–4 weeks) in 1963 [75]. Thiotepa in combination with Vinblastine, Adriamycin, and Halotestin (VATH) was effective in patients with relapse after adjuvant therapy and in metastatic breast cancer patients [76].
5.2. Anthracyclins
Anthracyclins are antibiotics that intercalates between the adjacent base pairs of DNA in such a manner that it forms anthracyclin-DNA-TopoisomeraseII ternary complex. The ternary complex disrupts the resealing activity of enzyme leading to inhibition of DNA and RNA synthesis in highly replicating cancer cells [77,78]. Some of the common anthracyclins are doxorubicin, daunorubicin, epirubicin and idarubicin. These are generally extracted from Streptomyces bacterium. Approved for medical use in 1974, doxorubicin (14-hydroxylated version of Daunorubicin) [79] was originally extracted from Streptomyces peucetius [80]. There was a long lapse of 20 years between first clinical usages of anthracyclins to their proven relevance in breast cancer treatment. However, once established the chemotherapeutic regimens that contained doxorubicin [usual dose: 60–75 mg/m² intravenously (IV) every 21 days] were used regularly with dose-intense and dose-dense schedules. Some of the regimens containing doxorubicin are AC (adriamycin, cyclophosphamide), TAC (taxotere, AC) and FAC (5-fluorouracil, AC) [81]. Unlike doxil (the PEGylated form of doxorubucin), myocet is the non-PEGylated liposomal version of doxorubicin that is approved for treatment of metastatic breast cancer in combination with cyclophosphamide in Europe and Canada [82].
5.3. Antimetabolites
Antimetabolites are the chemo-drugs that interfere with the metabolic pathways in the cancer cells primarily by acting as structural analogues to important cellular metabolites. 5-Flourouracil (5-FU) is a pyrimidine antimetabolite, an uracil analogue that inhibits the activity of thymidylate synthetase by converting 5-FU to FdUMP and FUTP, instead of uracil to thymidine conversion. Therefore, 5-FU not only inhibits the DNA synthesis in the cell but also inhibits RNA synthesis due to FdUTP incorporation in RNA; leading to highly toxic effects on the growth of rapidly multiplying cells like cancer cells. Klein and colleagues introduced 5-FU for medical use in 1962 for the treatment of self-healing, squamous cell tumors of skin i.e. keratoacanthomas (KAs) [83]. 5-FU was subsequently used for other cancer types including breast cancer [84]. General drug dosage of 5-FU is 500 mg/m2 or 600 mg/m2 IV on day 1 and day 8 of every 28-day cycle (six cycles in total). Lokich et al (1985) first introduced the infusion of 5-FU in combination with methotrexate (M) for breast cancer [85]. Since then various regimes of combination therapy containing 5-FU (F) with other drugs like doxorubicin (A), cisplatin (C), cyclophosphamide (CX) and epirubicin (E) (ECF, CMF, CAF) have been continuously used for treating metastatic breast cancer [86]. The other combinations such as 5-FU with paclitaxel in 1998 [87] and high dose 5-FU/paclitaxel with doxorubicin [88] were found successful for breast cancer treatment.
Methotrexate is a folate/folic acid antagonist that binds to the dihydrofolate reductase (DHFR) enzyme and inhibits the synthesis of building blocks of DNA and RNA itself. Folic acid is converted to dihydrofolate by DHFR, which is then reduced to tetrahydrofolate (THDF) for further action by thymidine synthetase. Methotrexate binding to DHFR decreases the synthesis of purines and pyrimidines in the cells that inhibits the cell cycle progression in S-phase. Methotrexate was discovered in 1940s as a substitute to folic acid, because the cases with decreased leukemic cell count (acute lymphoblastic leukemia) due to dietary deficiency of folic acid were being presented in clinics [89,90]. Methotrexate was first shown to remit breast cancer in 1951 by Jane C Wright [91]. In 1960s and 1970s, methotrexate was being used as single cytotoxic agent for advanced breast cancer treatment [92]. However, Gianni and Bonadonna group (1976) showed the first treatment with 12 cycles of CMF combination in 400 mastectomised women with advanced cancer and positive lymph nodes and reported only 5.3% treatment failure with acceptable toxicity [93,94]. In fact, CMF regimen was the first ever schedule for breast cancer treatment. Further, CMF was found to be equally effective even at 6 cycles. Immunosuppressive activity of Methotrexate has been explored for its clinical use in autoimmune diseases like rheumatoid arthritis [95]. General dosage of CMF regimen is defined as 6–8 cycles of cyclophosphamide (600 mg/m2); methotrexate (40 mg/m2), and fluorouracil (600 mg/m2) at 14-day intervals.
Capecitabine, a pro-drug for 5-FU, is another pyrimidine antimetabolite. The conversion of capecitabine to 5-FU requires the three systematic enzymatic reaction cascades through intestine, liver and tumour cells. The enzyme that catalyzes the last step of conversion is highly expressed in tumour cells as compared to the normal cells. Therefore, the tumour selective conversion of capecitabine to 5-FU prevents the systemic exposure of body to 5-FU [96]. Also, capecitabine is easier to administer and safer than 5-FU with better efficacy. The capecitabine was first used against colon cancer in 1998 [97]. Capecitabine (1250 mg/m2 given orally twice daily for 14 days followed by a 7-day rest period in a 21-day cycle for 8 cycles) is generally used to treat paclitaxel or docetaxel resistant advanced and metastatic breast cancers [98]. It is used either alone or in combination with cabazitaxel [99], vinorelbine [100] or ixabepilone [101].
Gemcitabine is a pro-drug that gets tri-phosphorylated inside the cell (dFdCTP) by sequential enzyme catalyzed reactions. This dFdCTP masquerades as an analogue of cytidine and gets incorporated in the newly synthesized DNA generating irreparable error that inhibits DNA replication leading to cell death [102,103]. Gemcitabine was primarily manufactured in 1980 by Larry Hertel's group at Eli Lilly and Company to be used as an anti-viral drug against enteroviruses [Coxsackievirus B3 (CVB3)]. The drug was also used against CVB3, EV71, human rhinoviruses (HRVs), human immunodeficiency virus (HIV), hepatitis C virus (HCV), poliovirus, influenza virus, ZIKV and MERS-CoV [104,105]. It was then pre-clinically tested for its anti-tumour attribute. The drug was approved by FDA for pancreatic cancer in 1995 [106] and for non-small lung cancer in 1998 [107]. Finally, Gemcitabine was approved for metastatic breast cancer in 2004 in combination with paclitaxel [108,109]. After anthracycline-containing adjuvant chemotherapy failure, gemcitabine (1250 mg/m² IV infusion over 30 min on Days 1 and 8 of each 21-day cycle) and paclitaxel (175 mg/m² on Day 1 as a 3 h infusion before gemcitabine) combination is used as the first-line of treatment against metastatic cancer. Other combinations such as gemcitabine/vinorelbine (GemVin), gemcitabine/cisplatin (GemCis), gemcitabine/capecitabine (GemCap) have also shown increased response rate and overall survival in pretreated metastatic breast cancer patients [110].
5.4. CDK4/6 inhibitor
Palbociclib is a CDK4/6 inhibitor that halts the progression of cells from G1- to S-phase. Palbociclib (125 mg, 28-day cycle with aromatase inhibitor) is used as the targeted therapy against ER+/HER2− advanced and metastatic breast cancer in conjunction with hormone therapy. The open label PALOMA (Palbociclib: Ongoing Trials in the Management of Breast Cancer) clinical trials were designed recently with either aromatase inhibitor (letrozole) (PALOMA-1 or PALOMA-2 Trials) [111,112] or Fulvestrant hormone therapies (PALOMA-3 trails) [113]. An improvement in the overall survival of metastatic breast cancer patients was reported in PALOMA-3 phase III clinical trials. Inhibiting CDK4/6 activity delays the resistant to hormone therapy and significantly improves the progression free survival (PFS) of patients [114,115].
5.5. Hormone therapy or endocrine therapy
The hormonal therapy or endocrine therapy is usually given for 5–10 years. This therapy either directly targets the hormone (estrogen and/or progesterone) production or negatively regulates the functional effects in hormone sensitive breast cancer patients (ER+ and/or PR+).
Selective estrogen receptor modules (SERMs) serve as anti-estrogens by binding to the hormone receptors as antagonists. The widely used SERMs that have been repositioned as breast cancer drugs are tamoxifen (1977), toremifene (1997) and raloxifene (2007). Tamoxifen is the oldest SERM that has been in use for more than 30 years for early stage breast cancer treatments in pre-and post-menopausal women. Toremifene and raloxifene are equally effective but safer alternatives of tamoxifen that are used in only post-menopausal women with advanced breast cancer [116,117]. Multiple Outcomes Raloxifene Evaluation (MORE) clinical trial was an osteoporosis treatment trial in postmenopausal women, with secondary objective of evaluating the effects on breast cancer risk reduction. MORE lead to the designing of further clinical trials such as Continuing Outcomes Relevant to Evista® (CORE), Raloxifene Use for The Heart (RUTH), and Study of Tamoxifen and Raloxifene (STAR) [118].
Aromatase inhibitor hormone therapy is administered only in post-menopausal women to treat ER + early and/or late stage breast cancer [22]. It acts on the aromatase enzyme that still produces estrogen hormone in fat tissue of post-menopausal women or women without active ovaries. Thus, the aromatase inhibitor reduces the amount of estrogen in post-menopausal women with breast cancer that would otherwise feed the breast cancer cells for further growth. The aromatase inhibitors (anastrazole, exemestane and letrozole) were initially used for ovary stimulation and induction of ovulation in infertile females or polycystic ovary syndrome. Aromatase inhibitors can be used as neoadjuvant or adjuvant therapy, mostly alone or in combination, which were introduced as an alternate to tamoxifen in postmenopausal patients [119].
Selective estrogen receptor degrader (SERD) such as fulvestrant, are pure anti-estrogens that blocks estrogen receptor and degrades the receptor without any agonist effect [120]. Fulvestrant was first used in 2002 as a ‘SERD hormone therapy’ against HR+ HER2− advanced and metastatic breast cancer in post-menopausal women that were resistant to other hormone therapy [121]. It is used in combination with CDK4/6 inhibitors like palbociclib (PALOMA-3) and ribociclib (MONALEESA-3) and anti-PI3K/AKT/mTOR pathway drugs such as pictilisib (FERGI) and buparlisib (BELLE-2 and BELLE-3) [122].
Luteinizing hormone releasing hormone (LHRH) analogs interfere with the signaling mechanism that activates the estrogen synthesis in ovaries causing temporary menopause. In 1987, goserelin was used for the assisted reproduction and prostate cancer treatment. Goserelin was then approved for the treatment of pre-menopausal women with hormone sensitive breast cancers in 1989. It is used alone or in combination with other hormone therapies [123]. Goserelin is currently under Phase II clinical trial as an additional drug into the standard neoadjuvant therapy for TNBC patients. The goserelin phase II trial is expected to complete by 2023 [124].
5.6. mTOR kinase inhibitor
Everolimus is an mTOR kinase inhibitor that inhibits the PI3K/AKT/mTOR signaling pathway. Everolimus was originally approved for renal cancer in 2009, as immunosuppressant during renal transplants in 2010 and for pancreatic cancer in 2011. A phase III clinical trial ‘Breast Cancer Trial of Oral Everolimus-2 (BOLERO-2)’ that included everolimus in combination with exemestane was successfully completed in 2012 leading to the approval of everolimus by US FDA for the treatment of HR+, HER2− advanced metastatic cancers that are resistant to letrozole or anastrazole [125,126].
5.7. Mitotic inhibitor
Mitotic inhibitors terminate the cell division or mitosis by disrupting the microtubule dynamics. This leads to G2/M phase cell cycle arrest or inhibition of spindle formation. Some of the common examples of mitotic inhibitor include docetaxel, paclitaxel and vinblastine. While docetaxel and paclitaxel induces G2/M cell cycle arrest, vinblastine is known to inhibit spindle formation.
Docetaxel and paclitaxel are used as neoadjuvant or adjuvant therapy as single agent or in combination with other chemotherapeutic agents for the treatment of early, advanced and metastatic breast cancer in pre- and postmenopausal women. Paclitaxel was isolated from pacific yew in 1971. It was used as drug for arterial restenosis. Docetaxel and paclitaxel were initially used as therapeutics in prostate and ovarian cancer (1992) respectively. Thereafter, docetaxel (75 mg/m² IV 1 h after doxorubicin and cyclophosphamide 3 Weeks x 6 cycles) and paclitaxel (175 mg/m² IV over 3 h 3 weeks 4 times with doxorubicin-containing regimen) were repositioned as chemotherapy adjunct in breast cancer treatment regimen [[127], [128], [129]]. Taxanes are now used as the part of standard chemotherapy in metastatic breast cancer. Numerous combinatorial chemotherapy containing taxanes are used in routine practice for treating breast cancer by clinicians around the globe [130].
Vinblastine is a naturally occurring Vinca alkaloid found in white flowered periwinkle, Vinca rosea. This was discovered by Robert Noble and Charles T Beer in 1958. The discovery of vinblastine is a beautiful example of serendipity in drug development. The group rather aimed to evaluate the anti-diabetic effect of extract in rats and observed Pseudomonas mediated septicemia that was accompanied with the rapid WBC fall and granulocytopenia. Further study in this direction showed peripheral granulocytopenia and leukopenia in Vinca rosea extract treated rats. Finally, they reported carcinostatic activity of Vinca rosea extract/vinblastine in rats with transplantable mammary adenocarcinoma and sarcoma [131,132]. Vinblastine was approved for lymphoma in 1965. Also, since 1980s, vinblastine (2–4 mg/mm2 IV once weekly or every other week) in combination with mitomycin or MVP (mitomycin C, vinblastine and cisplatin) has been used as chemotherapy against advanced and metastatic breast cancer [133,134].
6. Future perspectives and challenges
With a drastic increase in the number of new cases, the cost of the cancer treatment is rising even at a higher speed. This is because the disease demands molecular dissection at gene and protein level to find newer strategies, appropriate targets and corresponding drugs. This requires a lot of time and the use of high-end techniques by pharmaceutical companies thereby increasing the overall financial investments and eventually the cost of the drug [135]. The antibody-based immunotherapies or cell-based CART therapy, DC vaccine, ACT types of drug treatment might prove promising. However, these therapies are unaffordable by most patients in developed as well as developing countries. This necessitates the development of drugs with higher efficacy, lower side effects and practically lower cost. One of the smart ways is to repurpose an old, existing and approved drug for a newer indication. The advantage of this approach is that the approved drugs have existing information about molecular targets, off- targets, modes of action, safety level and side effects. This would not only save a lot of time that is otherwise required for discovery, designing, clinical trials and approvals of a new drug but also reduce the overall cost of anti-cancer drug. This will cut the cost involved in preclinical development and phase I trial. Further, the old drugs would be off-patent and hence cheaper than the initial costs.
In this article, we discussed in detail the advantages of drug repurposing for breast cancer treatment. We provided several drugs that have been successfully repurposed for breast cancer treatment. The triple negative breast cancer (TNBC) is highly heterogeneous, aggressive and complex form of breast cancer without expression of ER, PR, or HER2 receptors. TNBC is untreatable with regular hormone therapy. However, the combination of chemotherapies and the drug repositioning approach has offered promising outcomes by preclinical studies.
Flunarizine, a N-Ras inhibitor, has been approved for migraine or vertigo. Flunarizine has also shown promise in TNBC mouse models by inducing autophagy [135]. A recent publication showed that the combination of Metformin and Hemin, used for Type 2 diabetes or porphyria respectively, could inhibit the growth of breast cancer cells. Bioinformatics tools revealed that the BACH1 expression is significantly elevated in TNBC and the Hemin sensitizes the TNBC to metformin-mediated degradation of BACH1 [136]. The completion of repurposing drug in oncology (ReDO) project has provided evidence for the new uses of 6 drugs for breast cancer. Originally these were discovered for indications other than breast cancer [137]. These drugs include mebendazole (anti-helminthic), cimitedine (anti-acid), nitroglycerins (heart attack preventing), itraconazole (anti-fungal), and diclofenac (anti-inflammatory).
Other drugs such as L-NMMA (tilarginine acetate, a nitric oxide synthase inhibitor) [138], pro-viral integration moloney virus kinase (PIM)-1 inhibitors (olaparib) [139], L-asparginase [140], and fenofibrate [141] are being repurposed for breast cancer. These drugs were originally used in patients with cardiogenic shock, against viral infection, leukemia, helminthic infection, and in patients with high serum cholesterol and triglycerides, respectively.
Implementation of network pharmacology in examining the potential of natural herbs i.e. ayurvedic formulations and traditional chinese medicines (TCM) are also clinching the attention of researchers for anticancer drug repositioning. These are based on multi-targeted synergistic drug approach instead of one target-one drug approach and thus appear to be promising for breast cancer therapy.
The integration of super-computation, simulations, network pharmacology and bioinformatics can detect the target and efficacy of herbs. Triphala is the mixture of at least 1500 ayurvedic formulations meant for the treatment of many diseases. The bioactive-target-pathway-cancer type networking revealed the link of triphala with 24 cancer types including breast cancer through 13 bioactive and 17 targets. This information can be explored further for either bioactive- or target-based drug repositioning and/or new drug development.
However, some challenges and concerns associated with drug repurposing for breast cancer therapy require thorough consideration. The breast tumor heterogeneity, poorly defined molecular signatures, and poorly identified drug dosage provides a roadblock to the drug repurposing. Moreover, the current strategies often ignore tumor grade. It is crucial to search the new therapeutic strategies for certain very stringent and hard to treat molecular subgroups of breast cancers such as basal subtype, TNBC, MBC and tumors resistant to standard treatment therapy. The molecular alterations in TNBC, MBC, and resistant tumor types should also be thoroughly investigated. Aforementioned BACH1 inhibition by targeting the energy generating mechanism of rapidly growing cells is indeed the novel approach against otherwise resistant- breast cancer. Understanding and targeting the tumour microenvironment instead of tumor itself appears more promising in detailing the heterogeneity in breast cancer patients. The overlapping area that consists of tumour and normal cells in tumor microenvironment should be thoroughly characterized at the molecular level [142,143]. Identifying the molecular basis of super-responders and non-responders also holds valuable insight for deeper exploration [144]. Another area that needs attention while strategizing drug repositioning for breast cancer is personalized medicine i.e. to extend the repositioning strategy to treat upto single patient design, as the patients under same molecular subgroup often presents further variations leading to unexpected response. Structurally similar drugs may, at times, target functionally dissimilar protein hence pathway driven repurposing strategy is better for multi-targeted diseases like cancer. The orphan drugs generally have short or no patent and thus repurposing them is associated with lower cost. Repurposing the off-patent drugs eventually blocks the patenting rights on repurposed drug and hence future investors [145]. Inclusion and exclusion criteria for the selection of trial group for repurposed drug is very crucial as different physiological responses are anticipated in comparison to the original group. For example, the pregnant women (first trimester) with cancer are excluded for thalidomide treatment due to risk of Amelia and phocomelia [146]. Drug companies that expand the repositioning in similar therapeutic areas, such as reusing ovarian cancer drug as breast cancer drug, had success rate of 67% in comparison to 33% success rate when explored in different area [147]. A comprehensive, large-scale data mining and research is required before jumping on to actual repurposing procedure in order to save the time and finances because smallest of errors in computation or simulations can mislead the entire study. Faultless selection of most relevant pharmacological target using most appropriate database (TCGA and METABRIC) for data mining and accurate repurposing strategy is indispensable for the successful drug repositioning for breast cancer. Repurposed drugs mainly include the Phase III clinical trial, which still is the most challenging phase due to the longest duration, huge investments and inclusion of largest number of patients as compared to the Phase I and Phase II clinical trials. The repurposed drug for breast cancers usually does not work in monotherapy but as poly-pharmacology/combinations. The PARP inhibitors are synthetic lethal with BRAC2 mutation [148]. Hence, initially proving the repurposed drug efficacy as single drug agent becomes difficult. The toxicity of repurposed drug in pretreated patients or in combination therapy is unknown. For example, Valproate-doxorubicin treatment caused toxicity-induced death of two patients in a group of 16 cancer patients [149]. The overall success rate of development of new drugs (6%) and repositioning of drugs does not vary much as the ultimate efficacy remains same [150]. Also, the repositioned drug anyhow requires clinical re-assessment for optimizing its efficacy and cytotoxicity if the route of administration or drug dosage is different from the older indication; thereby increasing the overall cost of repositioning scheme. Nonetheless, the animal model used for drug testing does not represent the exact patient phenotype and hence is less predictive of efficacy in real [151]. Despite many benefits of drug repositioning, much attention is required to lower the drug dosage and toxicity without mitigating efficacy and resolve above discussed limitations for cost effective and more efficient drug development.
7. Concluding remarks
In this article, we have comprehensively explained the current scenario of repurposed drugs for breast cancer. We have discussed in detail the need and strategies of drug repositioning in breast cancer and several classical examples of drugs that have been repositioned as breast cancer chemotherapy. The futuristic potential of non-cancer drugs that are under investigations for breast cancer as well as the challenges and bottlenecks of drug repositioning were also discussed. We thus conclude that comprehensive approach of selecting the most appropriate gene-protein-pathway-target-drug modeling via integration of system biology and bioinformatics holds the high potential of providing more efficient, safer and cost-effective chemotherapeutics for treatment of even the most stringent forms of breast cancer (metastatic and triple negative). The scientists, researchers and clinicians must continue to work together for extension of the present pharmacological databases, multi-omics and bioinformatics tools. We hope that this will lead to the development of novel drugs and eventually fighting the deadly breast cancer.
Declaration of Competing Interest
None.
Acknowledgements
This work was supported in part by funds from the Science and Engineering Research Board (ECR/2016/000034), and University Grants Commission [30-112/2015 (BSR)] to SCG. SSV was supported by a fellowship from DBT New Delhi (DBT/2017/BHU/786).
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yalaza M., Inan A., Bozer M. Male breast cancer. J. Breast Health. 2016;12(1):1–8. doi: 10.5152/tjbh.2015.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hondermarck H., Vercoutter‿Edouart A.S., Révillion F., Lemoine J., El‿Yazidi‿Belkoura I., Nurcombe V., Peyrat J.P. Proteomics of breast cancer for marker discovery and signal pathway profiling. PROTEOMICS: Int. Ed. 2001;1(10):1216–1232. doi: 10.1002/1615-9861(200110)1:10<1216::AID-PROT1216>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Onitilo A.A., Engel J.M., Greenlee R.T., Mukesh B.N. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 2009;7(1–2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigelt B., Reis-Filho J.S. Molecular profiling currently offers no more than tumour morphology and basic immunohistochemistry. Breast Cancer Res. 2010;12(Suppl. 4):S5. doi: 10.1186/bcr2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waks A.G., Winer E.P. Breast cancer treatment: a review. Jama. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 7.Banin Hirata B.K., Oda J.M., Losi Guembarovski R., Ariza C.B., de Oliveira C.E., Watanabe M.A. Molecular markers for breast cancer: prediction on tumor behavior. Dis. Markers. 2014;2014 doi: 10.1155/2014/513158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue K., Fry E.A. Novel molecular markers for breast cancer. Biomark. Cancer. 2016;8:25–42. doi: 10.4137/BIC.S38394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maughan K.L., Lutterbie M.A., Ham P.S. Treatment of breast cancer. Am. Fam. Physician. 2010;81(11):1339–1346. [PubMed] [Google Scholar]
- 10.Tong C.W.S., Wu M., Cho W.C.S., To K.K.W. Recent advances in the treatment of breast cancer. Front. Oncol. 2018;8:227. doi: 10.3389/fonc.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nounou M.I., ElAmrawy F., Ahmed N., Abdelraouf K., Goda S., Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer: Basic Clin. Res. 2015;9(Suppl. 2):17–34. doi: 10.4137/BCBCR.S29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houssami N., Cuzick J., Dixon J.M. The prevention, detection, and management of breast cancer. Med. J. Aust. 2006;184(5):230–234. doi: 10.5694/j.1326-5377.2006.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 13.Davies C., Pan H., Godwin J., Gray R., Arriagada R., Raina V., Abraham M., Medeiros Alencar V.H., Badran A., Bonfill X., Bradbury J., Clarke M., Collins R., Davis S.R., Delmestri A., Forbes J.F., Haddad P., Hou M.F., Inbar M., Khaled H., Kielanowska J., Kwan W.H., Mathew B.S., Mittra I., Muller B., Nicolucci A., Peralta O., Pernas F., Petruzelka L., Pienkowski T., Radhika R., Rajan B., Rubach M.T., Tort S., Urrutia G., Valentini M., Wang Y., Peto R., G. Adjuvant Tamoxifen: Longer Against Shorter Collaborative Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhankhar R., Vyas S.P., Jain A.K., Arora S., Rath G., Goyal A.K. Advances in novel drug delivery strategies for breast cancer therapy. Artif. Cells Blood Substitutes Immobilization Biotechnol. 2010;38(5):230–249. doi: 10.3109/10731199.2010.494578. [DOI] [PubMed] [Google Scholar]
- 15.Matsen C.B., Neumayer L.A. Breast cancer: a review for the general surgeon. JAMA Surg. 2013;148(10):971–979. doi: 10.1001/jamasurg.2013.3393. [DOI] [PubMed] [Google Scholar]
- 16.Tsang R.Y., Finn R.S. Beyond trastuzumab: novel therapeutic strategies in HER2-positive metastatic breast cancer. Br. J. Cancer. 2012;106(1):6–13. doi: 10.1038/bjc.2011.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swain S.M., Im Y.H., Im S.A., Chan V., Miles D., Knott A., Clark E., Ross G., Baselga J. Safety profile of Pertuzumab with Trastuzumab and Docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist. 2014;19(7):693–701. doi: 10.1634/theoncologist.2014-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsimaki P., Aaltonen A., Mantyla E. Toxicity of antiestrogens. Breast J. 2002;8(2):92–96. doi: 10.1046/j.1524-4741.2002.08204.x. [DOI] [PubMed] [Google Scholar]
- 19.Puhalla S., Brufsky A., Davidson N. Adjuvant endocrine therapy for premenopausal women with breast cancer. Breast. 2009;18(Suppl. 3):S122–30. doi: 10.1016/S0960-9776(09)70286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thurlimann B., Senn H.J., Panel m. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robson M., Im S.A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., Wu W., Goessl C., Runswick S., Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 22.Telli M.L., Hellyer J., Audeh W., Jensen K.C., Bose S., Timms K.M., Gutin A., Abkevich V., Peterson R.N., Neff C., Hughes E., Sangale Z., Jones J., Hartman A.R., Chang P.J., Vinayak S., Wenstrup R., Ford J.M. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res. Treat. 2018;168(3):625–630. doi: 10.1007/s10549-017-4624-7. [DOI] [PubMed] [Google Scholar]
- 23.Neuhouser M.L., Smith A.W., George S.M., Gibson J.T., Baumgartner K.B., Baumgartner R., Duggan C., Bernstein L., McTiernan A., Ballard R. Use of complementary and alternative medicine and breast cancer survival in the Health, eating, activity, and lifestyle study. Breast Cancer Res. Treat. 2016;160(3):539–546. doi: 10.1007/s10549-016-4010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akram M., Siddiqui S.A. Breast cancer management: past, present and evolving. Indian J. Cancer. 2012;49(3):277–282. doi: 10.4103/0019-509X.104486. [DOI] [PubMed] [Google Scholar]
- 25.Prasad S., Gupta S.C., Aggarwal B.B. Serendipity in cancer drug discovery: rational or coincidence? Trends Pharmacol. Sci. 2016;37(6):435–450. doi: 10.1016/j.tips.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S.C., Sung B., Prasad S., Webb L.J., Aggarwal B.B. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013;34(9):508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Paul S.M., Mytelka D.S., Dunwiddie C.T., Persinger C.C., Munos B.H., Lindborg S.R., Schacht A.L. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010;9(3):203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 28.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 29.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., Norris A., Sanseau P., Cavalla D., Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 30.Jadamba E., Shin M. A systematic framework for drug repositioning from integrated omics and drug phenotype profiles using pathway-drug network. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/7147039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534(7607):314–316. doi: 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- 32.Dimasi J.A. New drug development in the United States from 1963 to 1999. Clin. Pharmacol. Ther. 2001;69(5):286–296. doi: 10.1067/mcp.2001.115132. [DOI] [PubMed] [Google Scholar]
- 33.Oprea T.I., Bauman J.E., Bologa C.G., Buranda T., Chigaev A., Edwards B.S., Jarvik J.W., Gresham H.D., Haynes M.K., Hjelle B., Hromas R., Hudson L., Mackenzie D.A., Muller C.Y., Reed J.C., Simons P.C., Smagley Y., Strouse J., Surviladze Z., Thompson T., Ursu O., Waller A., Wandinger-Ness A., Winter S.S., Wu Y., Young S.M., Larson R.S., Willman C., Sklar L.A. Drug repurposing from an academic perspective. Drug Discov. Today Therapeutic Strategies. 2011;8(3–4):61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heckman-Stoddard B.M., DeCensi A., Sahasrabuddhe V.V., Ford L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639–1647. doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boolell M., Allen M.J., Ballard S.A., Gepi-Attee S., Muirhead G.J., Naylor A.M., Osterloh I.H., Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8(2):47–52. [PubMed] [Google Scholar]
- 36.McBride W.G. Thalidomide embryopathy. Teratology. 1977;16(1):79–82. doi: 10.1002/tera.1420160113. [DOI] [PubMed] [Google Scholar]
- 37.D’Amato R.J., Loughnan M.S., Flynn E., Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singhal S., Mehta J., Desikan R., Ayers D., Roberson P., Eddlemon P., Munshi N., Anaissie E., Wilson C., Dhodapkar M., Zeddis J., Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 39.Ning Y.M., Gulley J.L., Arlen P.M., Woo S., Steinberg S.M., Wright J.J., Parnes H.L., Trepel J.B., Lee M.J., Kim Y.S., Sun H., Madan R.A., Latham L., Jones E., Chen C.C., Figg W.D., Dahut W.L. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010;28(12):2070–2076. doi: 10.1200/JCO.2009.25.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shim J.S., Liu J.O. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int. J. Biol. Sci. 2014;10(7):654–663. doi: 10.7150/ijbs.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oprea T.I., Tropsha A., Faulon J.L., Rintoul M.D. Systems chemical biology. Nat. Chem. Biol. 2007;3(8):447–450. doi: 10.1038/nchembio0807-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyer A., Pasquier E., Tomasini P., Ciccolini J., Greillier L., Andre N., Barlesi F., Mascaux C. Drug repurposing in malignant pleural mesothelioma: a breath of fresh air? Eur. Respir. Rev. 2018;27(147) doi: 10.1183/16000617.0098-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oprea T.I., Mestres J. Drug repurposing: far beyond new targets for old drugs. AAPS J. 2012;14(4):759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Napolitano F., Zhao Y., Moreira V.M., Tagliaferri R., Kere J., D’Amato M., Greco D. Drug repositioning: a machine-learning approach through data integration. J. Cheminform. 2013;5(1):30. doi: 10.1186/1758-2946-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Zheng S., Chen B., Butte A.J., Swamidass S.J., Lu Z. A survey of current trends in computational drug repositioning. Brief. Bioinf. 2016;17(1):2–12. doi: 10.1093/bib/bbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H., Zhang H., Zhang Z., Cao Y., Tang W. Network-based inference methods for drug repositioning. Comput. Math. Methods Med. 2015;2015 doi: 10.1155/2015/130620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garraway L.A., Verweij J., Ballman K.V. Precision oncology: an overview. J. Clin. Oncol. 2013;31(15):1803–1805. doi: 10.1200/JCO.2013.49.4799. [DOI] [PubMed] [Google Scholar]
- 48.Zou D., Ma L., Yu J., Zhang Z. Biological databases for human research. Genomics, Proteomics Bioinf. 2015;13(1):55–63. doi: 10.1016/j.gpb.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreeger P.K., Lauffenburger D.A. Cancer systems biology: a network modeling perspective. Carcinogenesis. 2010;31(1):2–8. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamb J., Crawford E.D., Peck D., Modell J.W., Blat I.C., Wrobel M.J., Lerner J., Brunet J.P., Subramanian A., Ross K.N., Reich M., Hieronymus H., Wei G., Armstrong S.A., Haggarty S.J., Clemons P.A., Wei R., Carr S.A., Lander E.S., Golub T.R. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 51.Lamb J. The Connectivity Map: a new tool for biomedical research. Nat. Rev. Cancer. 2007;7(1):54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- 52.Iorio F., Tagliaferri R., di Bernardo D. Identifying network of drug mode of action by gene expression profiling. J. Comput. Biol. 2009;16(2):241–251. doi: 10.1089/cmb.2008.10TT. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Law V., Knox C., Djoumbou Y., Jewison T., Guo A.C., Liu Y., Maciejewski A., Arndt D., Wilson M., Neveu V., Tang A., Gabriel G., Ly C., Adamjee S., Dame Z.T., Han B., Zhou Y., Wishart D.S. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42(Database issue):D1091–7. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamosh A., Scott A.F., Amberger J.S., Bocchini C.A., McKusick V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–7. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., Yefanov A., Lee H., Zhang N., Robertson C.L., Serova N., Davis S., Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K.P., Kuhn M., Bork P., Jensen L.J., von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43(Database issue):D447–52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatr-Aryamontri A., Breitkreutz B.J., Heinicke S., Boucher L., Winter A., Stark C., Nixon J., Ramage L., Kolas N., O’Donnell L., Reguly T., Breitkreutz A., Sellam A., Chen D., Chang C., Rust J., Livstone M., Oughtred R., Dolinski K., Tyers M. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41(Database issue):D816–23. doi: 10.1093/nar/gks1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J.Y., Pandey R., Nguyen T.M. HAPPI-2: a comprehensive and high-quality map of human annotated and predicted protein interactions. BMC Genomics. 2017;18(1):182. doi: 10.1186/s12864-017-3512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croft D., O’Kelly G., Wu G., Haw R., Gillespie M., Matthews L., Caudy M., Garapati P., Gopinath G., Jassal B., Jupe S., Kalatskaya I., Mahajan S., May B., Ndegwa N., Schmidt E., Shamovsky V., Yung C., Birney E., Hermjakob H., D’Eustachio P., Stein L. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39(Database issue):D691–7. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhn M., von Mering C., Campillos M., Jensen L.J., Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36(Database issue):D684–8. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhn M., Szklarczyk D., Franceschini A., von Mering C., Jensen L.J., Bork P. STITCH 3: zooming in on protein-chemical interactions. Nucleic Acids Res. 2012;40(Database issue):D876–80. doi: 10.1093/nar/gkr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu F., Shi Z., Qin C., Tao L., Liu X., Xu F., Zhang L., Song Y., Liu X., Zhang J., Han B., Zhang P., Chen Y. Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012;40(Database issue):D1128–36. doi: 10.1093/nar/gkr797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson M.L., Bottiger Y., Bastholm-Rahmner P., Ovesjo M.L., Veg A., Eiermann B. Evaluation of usage patterns and user perception of the drug-drug interaction database SFINX. Int. J. Med. Inform. 2015;84(5):327–333. doi: 10.1016/j.ijmedinf.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Kuhn M., Letunic I., Jensen L.J., Bork P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44(D1):D1075–9. doi: 10.1093/nar/gkv1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen T.M., Muhammad S.A., Ibrahim S., Ma L., Guo J., Bai B., Zeng B. DeCoST: a new approach in drug repurposing from control system theory. Front. Pharmacol. 2018;9:583. doi: 10.3389/fphar.2018.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pujol A., Mosca R., Farres J., Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol. Sci. 2010;31(3):115–123. doi: 10.1016/j.tips.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 69.de Jonge M.E., Huitema A.D., Rodenhuis S., Beijnen J.H. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005;44(11):1135–1164. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 70.Abu Eid R., Razavi G.S., Mkrtichyan M., Janik J., Khleif S.N. Old-school chemotherapy in immunotherapeutic combination in cancer, a low-cost drug repurposed. Cancer Immunol. Res. 2016;4(5):377–382. doi: 10.1158/2326-6066.CIR-16-0048. [DOI] [PubMed] [Google Scholar]
- 71.Singh J.C., Mamtani A., Barrio A., Morrow M., Sugarman S., Jones L.W., Yu A.F., Argolo D., Smyth L.M., Modi S., Schweber S., Boafo C., Patil S., Norton L., Baselga J., Hudis C.A., Dang C. Pathologic complete response with neoadjuvant doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab and Pertuzumab in patients with HER2-positive early stage breast cancer: a single center experience. Oncologist. 2017;22(2):139–143. doi: 10.1634/theoncologist.2016-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakatsukasa K., Koyama H., Oouchi Y., Imanishi S., Mizuta N., Sakaguchi K., Fujita Y., Fujiwara I., Kotani T., Matsuda T., Fukuda K., Morita M., Kawakami S., Kadotani Y., Konishi E., Yanagisawa A., Taguchi T. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer. 2017;24(1):63–68. doi: 10.1007/s12282-016-0666-7. [DOI] [PubMed] [Google Scholar]
- 73.Sykes M.P., Karnofsky D.A., Philips F.S., Burchenal J.H. Clinical studies on triethylenephosphoramide and diethylenephosphoramide, compounds with nitrogen‿mustard‿like activity. Cancer. 1953;6(1):142–148. [Google Scholar]
- 74.Kim K.-W., Roh J.K., Wee H.-J., Kim C. Springer; 2016. Cancer Drug Discovery. [Google Scholar]
- 75.Lyons A., Edelstyn G. Thiotepa in treatment of advanced breast cancer. Br. Med. J. 1962;2(5315):1280–1283. doi: 10.1136/bmj.2.5315.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perloff M., Hart R.D., Holland J.F. Vinblastine, adriamycin, thiotepa, and halotestin (VATH): therapy for advanced breast cancer refractory to prior chemotherapy. Cancer. 1978;42(6):2534–2537. doi: 10.1002/1097-0142(197812)42:6<2534::aid-cncr2820420605>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 77.Gewirtz D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 78.Binaschi M., Bigioni M., Cipollone A., Rossi C., Goso C., Maggi C.A., Capranico G., Animati F. Anthracyclines: selected new developments, Current medicinal chemistry. Anticancer Agents Med. Chem. 2001;1(2):113–130. doi: 10.2174/1568011013354723. [DOI] [PubMed] [Google Scholar]
- 79.T.A.S.o.H.-S . 2016. Pharmacists, Doxorubicin Hydrochloride.https://www.ashp.org/ [Google Scholar]
- 80.Ravina E. John Wiley & Sons; 2011. The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. [Google Scholar]
- 81.Brayfield A. Pharmaceutical Press; 2017. Martindale: The Complete Drug Reference. [Google Scholar]
- 82.M.C. Support . 2009. Liposomal Doxorubicin (Caelyx, Myocet)macmillan.org.uk/information-and-support/treating/chemotherapy/drugs-and-combination-regimens/individual-drugs/liposomal-doxorubicin.html [Google Scholar]
- 83.Moore A.Y. Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J. Dermatolog. Treat. 2009;20(6):328–335. doi: 10.3109/09546630902789326. [DOI] [PubMed] [Google Scholar]
- 84.Ansfield F.J., Schroeder J.M., Curreri A.R. Five years clinical experience with 5-fluorouracil. Jama. 1962;181:295–299. doi: 10.1001/jama.1962.03050300015003. [DOI] [PubMed] [Google Scholar]
- 85.Lokich J.J., Phillips D., Green R., Paul S., Sonneborn H., Zipoli T.E., Curt G. 5-Fluorouracil and methotrexate administered simultaneously as a continuous infusion. A phase I study. Cancer. 1985;56(10):2395–2398. doi: 10.1002/1097-0142(19851115)56:10<2395::aid-cncr2820561009>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 86.Cameron D.A., Gabra H., Leonard R.C. Continuous 5-fluorouracil in the treatment of breast cancer. Br. J. Cancer. 1994;70(1):120–124. doi: 10.1038/bjc.1994.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klaassen U., Wilke H., Harstrick A., Seeber S. Fluorouracil-based combinations in the treatment of metastatic breast cancer. Oncology. 1998;12(Suppl. 1):31–35. [PubMed] [Google Scholar]
- 88.Zoli W., Ulivi P., Tesei A., Fabbri F., Rosetti M., Maltoni R., Giunchi D.C., Ricotti L., Brigliadori G., Vannini I., Amadori D. Addition of 5-fluorouracil to doxorubicin-paclitaxel sequence increases caspase-dependent apoptosis in breast cancer cell lines. Breast Cancer Res. 2005;7(5):R681–9. doi: 10.1186/bcr1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cronstein B.N., Bertino J.R. Springer Science & Business Media; 2000. Methotrexate. [Google Scholar]
- 90.Jolivet J., Cowan K.H., Curt G.A., Clendeninn N.J., Chabner B.A. The pharmacology and clinical use of methotrexate. N. Engl. J. Med. 1983;309(18):1094–1104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- 91.Wright J.C., Prigot A., Wright B., Weintraub S., Wright L.T. An evaluation of folic acid antagonists in adults with neoplastic diseases: a study of 93 patients with incurable neoplasms. J. Med. Assoc. 1951;43(4):211–240. [PMC free article] [PubMed] [Google Scholar]
- 92.Fracchia A.A., Farrow J.H., Adam Y.G., Monroy J., Knapper W.H. Systemic chemotherapy for advanced breast cancer. Cancer. 1970;26(3):642–649. doi: 10.1002/1097-0142(197009)26:3<642::aid-cncr2820260323>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 93.Bonadonna G., Brusamolino E., Valagussa P., Rossi A., Brugnatelli L., Brambilla C., De Lena M., Tancini G., Bajetta E., Musumeci R., Veronesi U. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N. Engl. J. Med. 1976;294(8):405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 94.Bonadonna G., Moliterni A., Zambetti M., Daidone M.G., Pilotti S., Gianni L., Valagussa P. 30 years’ follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330(7485):217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.N.I.f.H.a.C . 2009. Excellence, Rheumatoid Arthritis. The Management of Rheumatoid Arthritis in Adults.https://www.nice.org.uk/guidance/ng100 [Google Scholar]
- 96.Tabata T., Katoh M., Tokudome S., Nakajima M., Yokoi T. Identification of the cytosolic carboxylesterase catalyzing the 5′-deoxy-5-fluorocytidine formation from capecitabine in human liver. Drug Metab. Dispos. 2004;32(10):1103–1110. doi: 10.1124/dmd.104.000554. [DOI] [PubMed] [Google Scholar]
- 97.Miwa M., Ura M., Nishida M., Sawada N., Ishikawa T., Mori K., Shimma N., Umeda I., Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur. J. Cancer. 1998;34(8):1274–1281. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 98.Pivot X., Asmar L., Buzdar A.U., Valero V., Hortobagyi G. A unified definition of clinical anthracycline resistance breast cancer. Br. J. Cancer. 2000;82(3):529–534. doi: 10.1054/bjoc.1999.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Villanueva C., Awada A., Campone M., Machiels J.P., Besse T., Magherini E., Dubin F., Semiond D., Pivot X. A multicentre dose-escalating study of cabazitaxel (XRP6258) in combination with capecitabine in patients with metastatic breast cancer progressing after anthracycline and taxane treatment: a phase I/II study. Eur. J. Cancer. 2011;47(7):1037–1045. doi: 10.1016/j.ejca.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Fumoleau P., Delgado F.M., Delozier T., Monnier A., Gil Delgado M.A., Kerbrat P., Garcia-Giralt E., Keiling R., Namer M., Closon M.T. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J. Clin. Oncol. 1993;11(7):1245–1252. doi: 10.1200/JCO.1993.11.7.1245. [DOI] [PubMed] [Google Scholar]
- 101.Sparano J.A., Vrdoljak E., Rixe O., Xu B., Manikhas A., Medina C., Da Costa S.C., Ro J., Rubio G., Rondinon M., Perez Manga G., Peck R., Poulart V., Conte P. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 2010;28(20):3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alvarellos M.L., Lamba J., Sangkuhl K., Thorn C.F., Wang L., Klein D.J., Altman R.B., Klein T.E. PharmGKB summary: gemcitabine pathway. Pharmacogenet. Genomics. 2014;24(11):564–574. doi: 10.1097/FPC.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006;17(Suppl. 5):v7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 104.Plunkett W., Huang P., Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6(Suppl. 6):7–13. doi: 10.1097/00001813-199512006-00002. [DOI] [PubMed] [Google Scholar]
- 105.Lee K., Kim D.E., Jang K.S., Kim S.J., Cho S., Kim C. Gemcitabine, a broad-spectrum antiviral drug, suppresses enterovirus infections through innate immunity induced by the inhibition of pyrimidine biosynthesis and nucleotide depletion. Oncotarget. 2017;8(70):115315–115325. doi: 10.18632/oncotarget.23258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.King R.S. Gemcitabine. New first-line therapy for pancreatic cancer. Cancer Pract. 1996;4(6):353–354. [PubMed] [Google Scholar]
- 107.Takada M., Negoro S., Kudo S., Furuse K., Nishikawa H., Takada Y., Kamei T., Niitani H., Fukuoka M. Activity of gemcitabine in non-small-cell lung cancer: results of the Japan gemcitabine group (A) phase II study. Cancer Chemother. Pharmacol. 1998;41(3):217–222. doi: 10.1007/s002800050731. [DOI] [PubMed] [Google Scholar]
- 108.Spielmann M., Llombart-Cussac A., Kalla S., Espie M., Namer M., Ferrero J.M., Dieras V., Fumoleau P., Cuvier C., Perrocheau G., Ponzio A., Kayitalire L., Pouillart P. Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology. 2001;60(4):303–307. doi: 10.1159/000058524. [DOI] [PubMed] [Google Scholar]
- 109.Mason J.S., Taylor J.B., Triggle D.J. Elsevier; 2007. Comprehensive Medicinal Chemistry II.: Computer-assisted Drug Design. [Google Scholar]
- 110.Stemmler H.J., diGioia D., Freier W., Tessen H.W., Gitsch G., Jonat W., Brugger W., Kettner E., Abenhardt W., Tesch H., Hurtz H.J., Rosel S., Brudler O., Heinemann V. Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer. Br. J. Cancer. 2011;104(7):1071–1078. doi: 10.1038/bjc.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 112.Rugo H.S., Finn R.S., Dieras V., Ettl J., Lipatov O., Joy A.A., Harbeck N., Castrellon A., Iyer S., Lu D.R., Mori A., Gauthier E.R., Bartlett C.H., Gelmon K.A., Slamon D.J. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019;174(3):719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masuda N., Inoue K., Nakamura R., Rai Y., Mukai H., Ohno S., Hara F., Mori Y., Hashigaki S., Muramatsu Y., Nagasawa T., Umeyama Y., Huang X., Iwata H. Palbociclib in combination with fulvestrant in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: PALOMA-3 subgroup analysis of Japanese patients. Int. J. Clin. Oncol. 2019;24(3):262–273. doi: 10.1007/s10147-018-1359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., Iwata H., Harbeck N., Loibl S., Andre F., Puyana Theall K., Huang X., Giorgetti C., Huang Bartlett C., Cristofanilli M. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 115.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., Iwata H., Harbeck N., Zhang K., Theall K.P., Jiang Y., Bartlett C.H., Koehler M., Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 116.Vogel C.L., Johnston M.A., Capers C., Braccia D. Toremifene for breast cancer: a review of 20 years of data. Clin. Breast Cancer. 2014;14(1):1–9. doi: 10.1016/j.clbc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 117.Provinciali N., Suen C., Dunn B.K., DeCensi A. Raloxifene hydrochloride for breast cancer risk reduction in postmenopausal women. Expert Rev. Clin. Pharmacol. 2016;9(10):1263–1272. doi: 10.1080/17512433.2016.1231575. [DOI] [PubMed] [Google Scholar]
- 118.Martino S., Cauley J.A., Barrett-Connor E., Powles T.J., Mershon J., Disch D., Secrest R.J., Cummings S.R., Investigators C. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J. Natl. Cancer Inst. 2004;96(23):1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 119.Pistelli M., Mora A.D., Ballatore Z., Berardi R. Aromatase inhibitors in premenopausal women with breast cancer: the state of the art and future prospects. Curr. Oncol. 2018;25(2):e168–e175. doi: 10.3747/co.25.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carlson R.W. The history and mechanism of action of fulvestrant. Clin. Breast Cancer. 2005;6(Suppl. 1):S5–8. doi: 10.3816/cbc.2005.s.008. [DOI] [PubMed] [Google Scholar]
- 121.Mehta R.S., Barlow W.E., Albain K.S., Vandenberg T.A., Dakhil S.R., Tirumali N.R., Lew D.L., Hayes D.F., Gralow J.R., Linden H.M. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N. Engl. J. Med. 2019;380(13):1226–1234. doi: 10.1056/NEJMoa1811714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nathan M.R., Schmid P. A review of fulvestrant in breast cancer. Oncol. Ther. 2017;5(1):17–29. doi: 10.1007/s40487-017-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheer S.M., Plosker G.L., Simpson D., Wagstaff A.J. Goserelin: a review of its use in the treatment of early breast cancer in premenopausal and perimenopausal women. Drugs. 2005;65(18):2639–2655. doi: 10.2165/00003495-200565180-00011. [DOI] [PubMed] [Google Scholar]
- 124.Carbognin L., Furlanetto J., Vicentini C., Nortilli R., Pilotto S., Brunelli M., Pellini F., Pollini G.P., Bria E., Tortora G. Neoadjuvant strategies for triple negative breast cancer:’ state-of-the-art’ and future perspectives. Anticancer Agents Med. Chem. 2015;15(1):15–25. doi: 10.2174/1871520614666141019191616. [DOI] [PubMed] [Google Scholar]
- 125.Royce M.E., Osman D. Everolimus in the treatment of metastatic breast cancer. Breast Cancer: Basic Clin. Res. 2015;9:73–79. doi: 10.4137/BCBCR.S29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baselga J., Campone M., Piccart M., Burris H.A., 3rd, Rugo H.S., Sahmoud T., Noguchi S., Gnant M., Pritchard K.I., Lebrun F., Beck J.T., Ito Y., Yardley D., Deleu I., Perez A., Bachelot T., Vittori L., Xu Z., Mukhopadhyay P., Lebwohl D., Hortobagyi G.N. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chan J.M., Rhee J.-W., Drum C.L., Bronson R.T., Golomb G., Langer R., Farokhzad O.C. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid–polymeric nanoparticles. Proc. Natl. Acad. Sci. 2011;108(48):19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sparano J.A. Taxanes for breast cancer: an evidence-based review of randomized phase II and phase III trials. Clin. Breast Cancer. 2000;1(1):32–40. doi: 10.3816/CBC.2000.n.002. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 129.Nabholtz J.M., Gligorov J. The role of taxanes in the treatment of breast cancer. Expert Opin. Pharmacother. 2005;6(7):1073–1094. doi: 10.1517/14656566.6.7.1073. [DOI] [PubMed] [Google Scholar]
- 130.Crown J., O’Leary M., Ooi W.S. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist. 2004;9(Suppl. 2):24–32. doi: 10.1634/theoncologist.9-suppl_2-24. [DOI] [PubMed] [Google Scholar]
- 131.Noble R.L., Beer C.T., Cutts J.H. Role of chance observations in chemotherapy: vinca rosea. Ann. N. Y. Acad. Sci. 1958;76(3):882–894. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]
- 132.Radford J.A., Knight R.K., Rubens R.D. Mitomycin C and vinblastine in the treatment of advanced breast cancer. Eur. J. Cancer Clin. Oncol. 1985;21(12):1475–1477. doi: 10.1016/0277-5379(85)90241-x. [DOI] [PubMed] [Google Scholar]
- 133.Sedlacek S.M. First-line and salvage therapy of metastatic breast cancer with mitomycin/vinblastine. Oncology. 1993;50(Suppl. 1):16–21. doi: 10.1159/000227243. [DOI] [PubMed] [Google Scholar]
- 134.Urruticoechea A., Archer C.D., Assersohn L.A., Gregory R.K., Verrill M., Mendes R., Walsh G., Smith I.E., Johnston S.R. Mitomycin C, vinblastine and cisplatin (MVP): an active and well-tolerated salvage regimen for advanced breast cancer. Br. J. Cancer. 2005;92(3):475–479. doi: 10.1038/sj.bjc.6602367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zheng Z.Y., Li J., Li F., Zhu Y., Cui K., Wong S.T., Chang E.C., Liao Y.H. Induction of N-Ras degradation by flunarizine-mediated autophagy. Sci. Rep. 2018;8(1):16932. doi: 10.1038/s41598-018-35237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee J., Yesilkanal A.E., Wynne J.P., Frankenberger C., Liu J., Yan J., Elbaz M., Rabe D.C., Rustandy F.D., Tiwari P., Grossman E.A., Hart P.C., Kang C., Sanderson S.M., Andrade J., Nomura D.K., Bonini M.G., Locasale J.W., Rosner M.R. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019;568(7751):254–258. doi: 10.1038/s41586-019-1005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pantziarka P., Verbaanderd C., Sukhatme V., Rica Capistrano I., Crispino S., Gyawali B., Rooman I., Van Nuffel A.M., Meheus L., Sukhatme V.P., Bouche G. ReDO_DB: the repurposing drugs in oncology database. E Cancer Med. Sci. 2018;12:886. doi: 10.3332/ecancer.2018.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Firger J. Nitric oxide inhibitors hit target for triple-negative breast cancer. J. Natl. Cancer Inst. 2015;107(8) doi: 10.1093/jnci/djv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao X., Liu X., Lu Y., Wang Y., Cao W., Liu X., Hu H., Wang H. PIM1 is responsible for IL-6-induced breast cancer cell EMT and stemness via c-myc activation. Breast Cancer. 2019;26(5):663–671. doi: 10.1007/s12282-019-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Knott S.R.V., Wagenblast E., Khan S., Kim S.Y., Soto M., Wagner M., Turgeon M.O., Fish L., Erard N., Gable A.L., Maceli A.R., Dickopf S., Papachristou E.K., D’Santos C.S., Carey L.A., Wilkinson J.E., Harrell J.C., Perou C.M., Goodarzi H., Poulogiannis G., Hannon G.J. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554(7692):378–381. doi: 10.1038/nature25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lian X., Wang G., Zhou H., Zheng Z., Fu Y., Cai L. Anticancer properties of fenofibrate: a repurposing use. J. Cancer. 2018;9(9):1527–1537. doi: 10.7150/jca.24488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mejia-Pedroza R.A., Espinal-Enriquez J., Hernandez-Lemus E. Pathway-based drug repositioning for breast cancer molecular subtypes. Front. Pharmacol. 2018;9:905. doi: 10.3389/fphar.2018.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cagan R., Meyer P. Rethinking cancer: current challenges and opportunities in cancer research. Dis. Model. Mech. 2017;10(4):349–352. doi: 10.1242/dmm.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prasad V., Vandross A. Characteristics of exceptional or super responders to cancer drugs. Mayo Clin. Proc. 2015;90(12):1639–1649. doi: 10.1016/j.mayocp.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Morello L. More cuts loom for US science. Nature. 2013;501(7466):147–148. doi: 10.1038/501147a. [DOI] [PubMed] [Google Scholar]
- 146.Matthews S.J., McCoy C. Thalidomide: a review of approved and investigational uses. Clin. Ther. 2003;25(2):342–395. doi: 10.1016/s0149-2918(03)80085-1. [DOI] [PubMed] [Google Scholar]
- 147.Neuberger A., Oraiopoulos N., Drakeman D.L. Renovation as innovation: is repurposing the future of drug discovery research? Drug Discov. Today. 2019;24(1):1–3. doi: 10.1016/j.drudis.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 148.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scherpereel A., Berghmans T., Lafitte J.J., Colinet B., Richez M., Bonduelle Y., Meert A.P., Dhalluin X., Leclercq N., Paesmans M., Willems L., Sculier J.P., P. European Lung Cancer Working Valproate-doxorubicin: promising therapy for progressing mesothelioma. A phase II study. Eur. Respir. J. 2011;37(1):129–135. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 150.Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 151.Nowak-Sliwinska P., Scapozza L., Altaba A.R.I. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. et Biophys. Acta. Rev. Cancer. 2019;1871(2):434–454. doi: 10.1016/j.bbcan.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]