Abstract
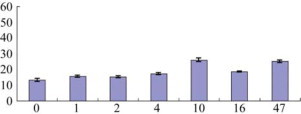
The present study was designed to determine the effects of copy number variations (CNVs) of squalene synthase 1(SQS1) gene on the mevalonate (MVA) pathway. SQS1 gene from G. uralensis (GuSQS1) was cloned and over-expressed in Pichia pastoris GS115. Six recombinant P. pastoris strains containing different copy number of GuSQS1 were constructed. HPLC was used to assay the level of ergosterol in all transgenic P. pastoris strains containing GuSQS1. HPLC analysis showed that the contents of ergosterol in all of the transgenic P. pastoris containing GuSQS1 were higher than that in the negative control. And with the increase of copy number of GuSQS1, the content of ergosterol showed an increasing-decreasing-increasing pattern. The contents of ergosterol in 10-copy-GuSQS1 P. pastoris and 47-copy-GuSQS1 P. pastoris were significantly higher than that in the rest recombinant P. pastoris strains. In conclusion, the CNVs of GuSQS1 influence the content of secondary metabolites in the MVA pathway. The present study provides a basis for over-expressing GuSQS1 and increasing the content of glycyrrhizin in G. uralensis cultivars.
Key Words: Glycyrrhiza uralensis, GuSQS1, Over-expression, Pichia pastoris, Copy number variations
Six recombinant P. pastoris strains containing different copy number of GuSQS1 gene were obtained in this paper. HPLC analysis showed that the content of ergosterol in all recombinant P. pastoris containing GuSQS1 gene were higher than that in negative control. And with the increase of copy number of GuSQS1 gene, the content of ergosterol was presented in an increasing-decreasing-increasing pattern.
Footnotes
Available online 20 May 2015
Research funding This work was supported by National Natural Science Fundation of China (No. 81373909).
These authors have no conflict of interest to declare.
References
- 1.Zeng L, Li SH, Lou ZC. Morphological and histological studies of Chinese licorice [J] Acta Pharm Sin. 1988;23:200–208. [PubMed] [Google Scholar]
- 2.State Pharmacopoeia Committee . Pharmacopoeia of China, Part 1 [M] Chemical Industry Press; Beijing: 2010. pp. 80–81. [Google Scholar]
- 3.Cherng JM, Lin HJ, Hsu YH. A quantitative bioassay for HIV-1 gene expression based on UV activation effect of glycyrrhizic acid [J] Antiviral Res. 2004;62(1):27–36. doi: 10.1016/j.antiviral.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hoever G, Bzltina L, Michaelis M. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus [J] J Med Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 5.Hyung KK, Yoonkyung P, Hee NK. Antimicrobial mechanism of b-glycyrrhetinic acid isolated from licorice. Glycyrrhiza glabra [J] Biotechnol Lett. 2002;24(22):1899–1902. [Google Scholar]
- 6.Shibata S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice [J] Yakugaku Zasshi. 2000;120(10):849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 7.Van Rossum TG, Vulto AG. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double blind, randomized, placebo-controlled phase I/II trial [J] J Gastroenterol Hepatol. 1999;14(11):1093–1099. doi: 10.1046/j.1440-1746.1999.02008.x. [DOI] [PubMed] [Google Scholar]
- 8.Wei SL, Wang WQ, Wang JY. Preliminary study in glycyrrhizin content and its influencing factors of wild and cultivated in different region of China [J] China J Chin Mat Med. 2012;37(10):1341–1345. [PubMed] [Google Scholar]
- 9.Hayashi H, Fukui H, Tabata M. Examination of triterpenoids produced by callus and cell suspension cultures of Glycyrrhiza glabra [J] Plant Cell Rep. 1988;7(7):508–511. doi: 10.1007/BF00272743. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Huang P, Inoue K. Up-regulation of soyasaponin biosynthesis by methyl Jasmonate in cultured cells of Glycyrrhiza glabra [J] Plant Cell Physiol. 2003;44(4):404–411. doi: 10.1093/pcp/pcg054. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Sakai T, Fukui H. Formation of soyasaponins in licorice cell suspension cultures [J] Phytochemistry. 1990;29(10):3127–3129. [Google Scholar]
- 12.Ayabe S, Takano H, Fujita T. Triterpenois biosynthesis in tissue cultures of Glycyrrhiza glaba var. glanduliera [J] Plant Cell Rep. 1990;9(4):181–184. doi: 10.1007/BF00232175. [DOI] [PubMed] [Google Scholar]
- 13.Paulet LC, Cairo A, Pavia PB. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase [J] Plant Mol Biol. 2006;62(4-5):683–695. doi: 10.1007/s11103-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Yang B, Lu BB. Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing Putrescine N-methyltransferase is methyl jasmonate-dependent [J] Planta. 2007;225(4):887–896. doi: 10.1007/s00425-006-0402-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang N, Chen HH. Cloning and characterization of two cDNA sequences coding squalene synthase involved in glycyrrhizic acid biosynthesis in Glycyrrhiza uralensis [J] Lect Notes Electric Engineer. 2013;269:329–342. [Google Scholar]
- 16.Liu DJ. Researches on the correlation between CNVs of HMGR, SQS1, β-AS gene and origin and morphology of Glycyrrhiza uralensis [D]. Dissertation. Beijing University of Chinese Medicine; 2011. pp. 8–11. [Google Scholar]
- 17.Lu HY, Liu JM, Zhang HC. Ri-mediated transformation of Glycyrrhiza uralensis with a squalene synthase gene (GuSQS1) for production of glycyrrhizin [J] Plant Mol Biol Rep. 2008;26(1):1–11. [Google Scholar]
- 18.Lee MH, Jeong JH, Seo JW. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene [J] Plant Cell Physiol. 2004;45(8):976–984. doi: 10.1093/pcp/pch126. [DOI] [PubMed] [Google Scholar]
- 19.Seo JW, Jeong JH, Shin CG. Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation [J] Phytochemistry. 2005;66(8):869–877. doi: 10.1016/j.phytochem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Mirjalili MH, Moyano E, Bonfill M. Overexpression of the Arabidopsis thaliana squalene synthase gene in Withania coagulans hairy root cultures [J] Biologia plantarum. 2011;55(2):357–360. [Google Scholar]
- 21.Kribii R, Arro M, Arco AD. Cloning and characterization of the Arabidopsis thaliana SQS1 gene encoding squalene synthase involvement of the C-terminal region of the enzyme in the channeling of squalene through the sterol pathway [J] Eur J Biochem. 1997;249(1):61–69. doi: 10.1111/j.1432-1033.1997.00061.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi H, Huang P, Inoue K. Up-regulation of soyasaponin biosynthesis by methyl Jasmonate in cultured cells of Glycyrrhiza glabra [J] Plant Cell Physiol. 2003;44(4):404–411. doi: 10.1093/pcp/pcg054. [DOI] [PubMed] [Google Scholar]
- 23.Uchida H, Yamashita H, Kajikawa M. Cloning and charaterization of a squalene synthase gene from a petroleum plant, Euphorbia tirucalli L. [J] Planta. 2009;229(6):1243–1252. doi: 10.1007/s00425-009-0906-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Yoon YH, Kim HY. Cloning and expression of squalene synthase cDNA from hot pepper (Capsicum annuum L.) [J] Mol Cells. 2002;13(3):436–443. [PubMed] [Google Scholar]
- 25.Zhang HC, Liu JM, Lu HY. Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment [J] Plant Cell Rep. 2009;28(8):1205–1213. doi: 10.1007/s00299-009-0721-3. [DOI] [PubMed] [Google Scholar]
- 26.Kang TW, Jeon YJ, Jang E. Copy number variations (CNVs) identified in Korean Individuals [J] BMC Genomics. 2008;9(1):492–499. doi: 10.1186/1471-2164-9-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palta P, Kaplinski L, Nagirnaja L. Haplotype phasing and inheritance of copy number variants in nuclear families [J] PLoS One. 2015;10(4):e0122713. doi: 10.1371/journal.pone.0122713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redon R, Ishikawa S, Fitch KR. Global variation in copy number in the human genome [J] Nature. 2006;444(7118):444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckmann JS, Estivill X, Antonarakis SE. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability [J] Nat Rev Genet. 2007;8(8):639–646. doi: 10.1038/nrg2149. [DOI] [PubMed] [Google Scholar]
- 30.Stranger BE, Forrest MS, Dunning M. Relative impact of nucleotide and copy number variation on gene expression phenotypes [J] Science. 2007;315(5813):848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastings PJ, Lupski JR, Rosenberg SM. Mechanisms of change in gene copy number [J] Nat Rev Genet. 2009;10(8):551–564. doi: 10.1038/nrg2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ausubel FM, Kinston RE, Seidman JG. Short Protocols in Molecular Biology [M] 4th ed. Science Press; Beijing: 2005. p. 25. [Google Scholar]
- 33.Zhu XQ. Researches on the influences of CNVs of functional genes HMGR, SQS and β-AS on their expression in Glycyrrhiza uralensis [D] Beijing University of Chinese Medicine; 2012. pp. 40–41. [Google Scholar]
- 34.Waterham HR, Digan ME, Koutz PJ. Isolation of the Pichia Pastoris glyceradehyde-3-phosphate dehydrosgenasee gene and regulation and use of its promoter [J] Gene. 1997;186(1):37–44. doi: 10.1016/s0378-1119(96)00675-0. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Liu DJ, Liu CS. Mechanism of genuineness of liquorice Glycyrrhiza uralensis based on CNVs of HMGR, SQS1 and β-AS gene [J] Acta Pharm Sin. 2012;47(2):250–255. [PubMed] [Google Scholar]
- 36.Liu Y, Xu QX, Wang XY. Researches on the influence of 3-hydroxy-3-methylglutary-coenzyme A reductase gene polymorphism on catalytic efficiency of its encode enzyme in Glycyrrhiza uralensis [J] China J Chin Mat Med. 2012;37(24):3784–3788. [PubMed] [Google Scholar]
- 37.Liu Y, Xu QX, Wang XY. Analysis on correlation between 3-hydroxy-3-methylglutary-coenzyme A reductase gene polymorphism of Glycyrrhiza uralensis and content of glycyrrhizic acid [J] China J Chin Mat Med. 2012;37(24):3789–3792. [PubMed] [Google Scholar]
- 38.Liu Y, Zhang N, Wang XY. Researches on influence of squalene synthase gene polymorphism on catalytic efficiency of its encode enzyme in Glycyrrhiza uralensis [J] China J Chin Mat Med. 2012;37(24):3777–3783. [PubMed] [Google Scholar]
- 39.Larkin PJ, Miller JAC, Allen RS. Increasing morphinan alkaloid production by over-expressing codeinone reductase in transgenic Papaver somniferum [J] Plant Biotechnol J. 2007;5(1):26–37. doi: 10.1111/j.1467-7652.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 40.White PJ. The regulation of K+ influx into roots of rye (secale cereale L.) seedlings by negative feedback via the K+ flux from shoot to root in the phloem [J] J Exp Bot. 1997;48(12):2063–2073. [Google Scholar]


