Abstract
Toxigenic types of Clostridium perfringens are important causes of enteric disease in domestic animals, although type E is putatively rare, appearing as an uncommon cause of enterotoxemia of lambs, calves, and rabbits. We report here two geographically distinct cases of type E enterotoxemia in calves, and diagnostic findings which suggest that type E may play a significant role in enteritis of neonatal calves. The cases had many similarities, including a history of diarrhea and sudden death, abomasitis, and hemorrhagic enteritis. In both cases, anaerobic cultures of abomasum yielded heavy growth of C. perfringens genotype E. Four percent of > 1000 strains of C. perfringens from cases of enteritis in domestic animals were type E, and all (n=45) were from neonatal calves with hemorrhagic enteritis. Furthermore, type E isolates represented nearly 50% of all isolates submitted from similar clinical cases in calves. Commercial toxoids available in North America have no label claims for efficacy against type E infections. Consideration should be given to type E-associated enteritis when planning for the health care of calves.
Keywords: Clostridium perfringens, Iota toxin, Bovine neonatal enteritis, Hemorrhagic enteritis
1. Introduction
Clostridium perfringens is an important cause of enteric disease in domestic animals [1], [2], [3], [4], [5]. Its virulence is based largely upon toxinogenesis [6], [7], and production of four so-called major toxins is the basis for division of the species into types [6], [8], [9], [10], [11] (Table 1 ).
Table 1.
Diseases produced by toxigenic types of C. perfringens
| Toxin type | Diseases | Major toxins |
|---|---|---|
| A | Myonecrosis, food poisoning, necrotic enteritis in fowl, enterotoxemia in cattle and lambs, necrotizing enterocolitis in piglets; possibly equine colitis, canine hemorrhagic gastroenteritis | Alpha |
| B | Dysentery in newborn lambs, chronic enteritis in older lambs (“pine”), hemorrhagic enteritis in neonatal calves and foals, hemorrhagic enterotoxemia in adult sheep | Alpha, beta, epsilon |
| C | Enteritis necroticans (pigbel) in humans, necrotic enteritis in fowl, hemorrhagic or necrotic enterotoxemia in neonatal pigs, lambs, calves, goats, foals, acute enterotoxemia (“struck”) in adult sheep | Alpha, beta |
| D | Enterotoxemia in lambs (“pulpy kidney”) and calves, enterocolitis in neonatal and adult goats, possibly enterotoxemia in adult cattle | Alpha, epsilon |
| E | Enterotoxemia likely in calves and lambs, enteritis in rabbits; host range and disease type unclear | Alpha, iota |
Type E is a putatively uncommon cause of enterotoxemia of lambs, calves, and rabbits [12] (Table 1). Iota enterotoxemia in calves and lambs was reported 50 years ago in Britain, and accounts published since that time have been of hemorrhagic, necrotic enteritis of calves [13] and of detection of type E organisms and iota toxin in ovine or bovine intestines at post mortem [14]. Suspected type E-induced disease in rabbits must be differentiated from that caused by C. spiroforme [3], [15]. Strains of type E are distinguished from other toxinotypes by their production of iota toxin, which consists of two non-covalently associated components and ADP-ribosylates actin at Arg-177 [16], [17], [18]. Little is known of the pathogenesis of type E infections, although it is assumed that, in keeping with the pattern set by isolates of other toxin types, iota toxin plays an important role.
2. Case reports
Case one: Fixed and fresh tissues from a 2-week-old male Angus calf were submitted by a veterinarian in Wisconsin. The history included diarrhea and sudden death, and necropsy findings of hyperemia and edema involving the abomasum and small intestine. Microscopic examination of abomasum revealed mild multifocal mucosal hemorrhage and acute inflammation of the submucosal layer (Fig. 1 ). The submucosa was edematous and contained aggregates of neutrophils.
Fig. 1.
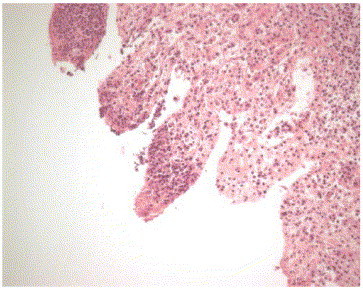
Affected ileum from Case one. Tissue is autolysed, but necrotic leukocytes are evident in lamina propria.
Fluorescent antibody examinations were negative for rotavirus, coronavirus, and BVD virus. Attempts at virus isolation yielded negative results, as did electron microscopic examination of intestinal contents. Direct fecal smears were negative for cryptosporidia. Anaerobic cultures of abomasum confirmed a heavy growth of C. perfringens, which was determined by PCR analysis to be genotype E; PCR results also revealed the presence of the genes for enterotoxin (cpe) and beta2 toxin (cpb2) [10], [11], [19] (Fig. 2 ). No significant organisms were found in small intestine, colon, or lymph node.
Fig. 2.
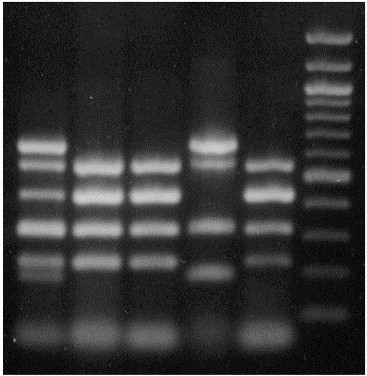
PCR genotyping of type E isolates. Lane 1: standards (combined PCR amplification products from genotyping of types B and E control strains; from top down are bands indicating amplification of portions of genes for epsilon, beta2, iota A, and alpha toxins, enterotoxin, and beta toxin); Lane 2: Type E isolate from Case one; Lane 3: Type E isolate from Case two; Lane 4: Type B control; Lane 5: Type E control; Lane 6: standards (1 kbp ladder).
Case two: Fixed and fresh tissues from an 11-day-old crossbred female calf were submitted by a veterinarian in Nebraska. The calf was one of 70 in the herd, of which about 10% were affected and three had died. In the subject animal, death followed 3 days of scouring, and the veterinarian suspected a coronaviral infection.
Necropsy revealed jejunal hemorrhage, which was especially notable from the serosal surface. Streaks of inflammation were seen on the mucosal surface of the spiral colon, and mesenteric lymph nodes were edematous and inflamed.
Microscopic examination of tissues revealed mild autolytic change in segments of jejunum, ileum, and colon. Some areas of ileum appeared necrotic, with inflammation of some villous tips, and lymphadenitis was evident.
Fluorescent antibody examinations of intestine were negative for rotavirus and coronavirus, as was electron microscopic examination. Direct fecal smears were negative for cryptosporidia. No significant bacteria were found in lymph nodes or colon, but heavy growth of C. perfringens was obtained upon anaerobic culture of intestine. The isolate was determined by PCR analysis to be genotype E; PCR results were also positive for cpe and cpb2 [10], [11], [19] (Fig. 2).
In both cases, findings were consistent with C. perfringens type E enteritis.
3. Discussion
As noted, infection by C. perfringens type E has been a rare diagnosis since its first description more than 50 years ago. Diagnoses in rabbits may, in fact, have been infections by C. spiroforme, an organism which is very different from C. perfringens but which produces a toxin quite similar to iota toxin [3]. An important point illustrated by the cases presented here is that, without bacteriologic culture and genotyping, these infections, if attributed to C. perfringens, would likely have been assumed to be caused by organisms of type A or C.
In fact, strains of type E are not uncommon in certain niches. Examination of 1113 strains of C. perfringens from cases of enteritis in domestic animals revealed 45 type E strains (4%), all from different herds. The origin of these isolates was uniformly from neonatal calves with hemorrhagic enteritis (and most experiencing sudden death), which is consistent with the findings of others [15], [19]. Samples submitted to us for diagnostic screening are not necessarily random or representative; nevertheless, the fact that all 45 type E isolates identified in this study originated from a single host type and condition is notable, since these type E isolates represented 46.9% of all isolates submitted from similar clinical cases in calves. More rigorous epidemiologic and diagnostic pursuit of similar cases is perhaps warranted.
It is also important to note that currently available commercial toxoids will likely offer little or no protection against type E infections. Thus, disease could occur even in the face of faithful use of an immunoprophylactic product directed against other genotypes. It is tempting to speculate on a role for type E strains in so-called vaccine breaks.
The finding of cpe in isolates from these cases is consistent with previous work by ourselves and others [19], [20], [21], [22], in which the gene could be amplified from strains of type E, but enterotoxin (CPE) was not expressed. Silent cpe sequences, found near the iota toxin genes on episomal DNA, were highly conserved among type E isolates, but contained nine nonsense and two frameshift mutations and lacked the initiation codon, promoters, and ribosome binding site. This is remarkable, given that sequencing of cpe from eight different isolates revealed 100% sequence homology [19], [23], [24], [25]. These strains were apparently not clonal; location of cpe sequences, with iap and ibp, on episomal DNA and lack of isolate-specific mutations suggests recent wide distribution among C. perfringens isolates.
Detection of cpb2 sequences by PCR analysis is of uncertain importance. CPB2 has been associated with enteric disease in pigs [26], horses [27], and feedlot cattle [28]. We have found cpb2 in 12.9% of bovine isolates across-the-board, and in 30.1% of isolates from cattle with enteritis (unpublished data). However, there is no direct experimental evidence for a role in pathogenesis, in cattle or any other species. Furthermore, we have found that cpb2 is expressed by only ∼50% of all bovine isolates; cpb2 is silent in all type E isolates we have examined.
In sum, consideration should be given to type E-associated enteritis when planning for the health care of calves. Greater attention to bacteriologic culture and genotyping as part of diagnostic approaches will provide more information on the true importance of this problem. Development of immunoprophylactic products is desirable, but may not be considered financially viable.
Acknowledgements
The authors gratefully acknowledge Connie Gates, Jane Christopher-Hennings, and Dawn M. Bueschel for technical assistance. Supported in part by funds from Boehringer Ingelheim Vetmedica and USDA-Hatch.
References
- 1.Johnson S, Gerding D.N. Enterotoxemic infections. In: Rood J.I, McClane B.A, Songer J.G, Titball R.W, editors. The clostridia: molecular biology and pathogenesis. Academic Press; London, UK: 1997. pp. 207–223. [Google Scholar]
- 2.McClane B.A. Clostridium perfringens. In: Doyle M.P, Beuchat L.R, Montville T.J, editors. Food microbiology: fundamentals and frontiers. ASM Press; Washington, DC: 1997. pp. 305–326. [Google Scholar]
- 3.Songer J.G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Songer J.G. Clostridial disease of animals. In: Rood J.I, McClane B.A, Songer J.G, Titball R.W, editors. The clostridia: molecular biology and pathogenesis. Academic Press; London, UK: 1997. pp. 153–184. [Google Scholar]
- 5.Stevens D.L. Necrotizing clostridial soft tissue infections. In: Rood J.I, McClane B.A, Songer J.G, Titball R.W, editors. The clostridia: molecular biology and pathogenesis. Academic Press; London, UK: 1997. pp. 141–152. [Google Scholar]
- 6.McDonel J.L. Toxins of Clostridium perfringens types A, B, C, D and E. In: Dorner F, Drews H, editors. Pharmacology of bacterial toxins. Pergamon Press; Oxford, UK: 1986. pp. 477–517. [Google Scholar]
- 7.Rood J, Cole S.T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991;55:621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daube G, China B, Simon P, Hvala K, Mainil J. Typing of Clostridium perfringens by in vitro amplification of toxin genes. J Appl Bacteriol. 1994;77:650–655. doi: 10.1111/j.1365-2672.1994.tb02815.x. [DOI] [PubMed] [Google Scholar]
- 9.Daube G, Simon P, Limbourg B, Manteca C, Mainil J, Kaeckenbeeck A. Hybridization of 2659 Clostridium perfringens isolates with gene probes for seven toxins (alpha, beta, epsilon, iota, theta, mu, and enterotoxin) and for sialidase. Am J Vet Res. 1996;57:496–501. [PubMed] [Google Scholar]
- 10.Meer R.R, Songer J.G. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am J Vet Res. 1997;58:702–705. [PubMed] [Google Scholar]
- 11.Songer J.G, Meer R.R. Genotyping of Clostridium perfringens by polymerase chain reaction is a useful adjunct to diagnosis of clostridial enteric disease in animals. Anaerobe. 1996;2:197–203. [Google Scholar]
- 12.Kokai-Kun J.F, Songer J.G, Czeczulin J.R, Chen F, McClane B.A. Comparison of Western immunoblots and gene detection assays for identification of potentially enterotoxigenic isolates of Clostridium perfringens. J Clin Microbiol. 1994;32:2533–2539. doi: 10.1128/jcm.32.10.2533-2539.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart B, Hooper P.T. Enterotoxemia of calves due to Clostridium welchii type E. Aust Vet J. 1967;43:360–363. doi: 10.1111/j.1751-0813.1967.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 14.Sterne M, Thomson A. The isolation and identification of clostridia from pathological conditions of animals. Bull Off Int Epizoot. 1963;59:1487–1489. [Google Scholar]
- 15.Borriello S.P, Carman R.J. Clostridial diseases of the gastrointestinal tract in animals. In: Borriello S.P, editor. Clostridia in gastrointestinal disease. CRC Press; Boca Raton, FL: 1985. pp. 195–222. [Google Scholar]
- 16.Carman R.J, Perelle S, Popoff M.R. Binary toxins from Clostridium spiroforme and Clostridium perfringens. In: Rood J.I, McClane B.A, Songer J.G, Titball R.W, editors. The clostridia: molecular biology and pathogenesis. Academic Press; London, UK: 1997. pp. 359–368. [Google Scholar]
- 17.Perelle S, Gibert M, Boquet P, Popoff M.R. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect Immun. 1993;61:5147–5156. doi: 10.1128/iai.61.12.5147-5156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Damme J, Jung M, Hofmann F, Just I, Vandekerckhove J, Aktories K. Analysis of the catalytic site of the actin ADP-ribosylating Clostridium perfringens iota toxin. FEBS Lett. 1996;380:291–295. doi: 10.1016/0014-5793(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 19.Billington S.J, Wieckowski E.U, Sarker M.R, Bueschel D.M, Songer J.G, McClane B.A. Clostridium perfringens type E animal enteritis isolates with highly conserved, silent enterotoxin gene sequences. Infect Immun. 1998;66:4531–4536. doi: 10.1128/iai.66.9.4531-4536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Dupuy B, Daube G, Popoff M.R, Cole S.T. Clostridium perfringens urease genes are plasmid borne. Infect Immun. 1997;65:2313–2320. doi: 10.1128/iai.65.6.2313-2320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibert M, Jolivet-Reynaud C, Popoff M.R, Jolivet-Renaud C. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene. 1997;203:65–73. doi: 10.1016/s0378-1119(97)00493-9. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay J.A. Clostridium perfringens type A enterotoxin (CPE): more than just explosive diarrhea. Crit Rev Microbiol. 1996;22:257–277. doi: 10.3109/10408419609105482. [DOI] [PubMed] [Google Scholar]
- 23.Collie R.E, Kokai-Kun J.F, McClane B.A. Phenotypic characterization of enterotoxigenic Clostridium perfringens isolates from nonfoodborne human gastrointestinal diseases. Anaerobe. 1998;4:69–79. doi: 10.1006/anae.1998.0152. [DOI] [PubMed] [Google Scholar]
- 24.Cornillot E, Saint-Joanis B, Daube G, Katayama S, Granum P.E, Carnard B, Cole S.T. The enterotoxin gene (cpe) of Clostridium perfringens can be chromosomal orplasmid-borne. Mol Microbiol. 1995;15:639–647. doi: 10.1111/j.1365-2958.1995.tb02373.x. [DOI] [PubMed] [Google Scholar]
- 25.Czeczulin J.R, Hanna P.C, McClane B.A. Cloning, nucleotide sequencing, expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun. 1993;61:3429–3439. doi: 10.1128/iai.61.8.3429-3439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibert M, Perelle S, Daube G, Popoff M.R. Clostridium spiroforme toxin genes are related to C. perfringens iota toxin genes but have a different genomic localization. Syst Appl Microbiol. 1997;20:337–347. [Google Scholar]
- 27.Herholz C, Miserez R, Nicolet J, Frey J, Popoff M, Gibert M, Gerber H, Straub R. Prevalence of beta2-toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol. 1999;37:358–361. doi: 10.1128/jcm.37.2.358-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manteca C, Daube G, Jauniaux T, Linden A, Pirson V, Detilleux J, Ginter A, Coppe P, Kaeckenbeeck A, Mainil J.G. A role for the Clostridium perfringens beta2 toxin in bovine enterotoxaemia? Vet Microbiol. 2002;86:191–202. doi: 10.1016/S0378-1135(02)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


