Abstract
There is currently a lack of pathologic data on the novel coronavirus (severe acute respiratory syndrome coronavirus 2) pneumonia, or coronavirus disease 2019 (COVID-19), from autopsy or biopsy. Two patients who recently underwent lung lobectomies for adenocarcinoma were retrospectively found to have had COVID-19 at the time of the operation. These two cases thus provide important first opportunities to study the pathology of COVID-19. Pathologic examinations revealed that apart from the tumors, the lungs of both patients exhibited edema, proteinaceous exudate, focal reactive hyperplasia of pneumocytes with patchy inflammatory cellular infiltration, and multinucleated giant cells. Hyaline membranes were not prominent. Because both patients did not exhibit symptoms of pneumonia at the time of operation, these changes likely represent an early phase of the lung pathology of COVID-19 pneumonia.
Keywords: Coronavirus, COVID-19 pneumonia, Pathology, SARS-CoV-2
Since December 2019, the outbreak of a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), infection (coronavirus disease 2019 [COVID-19]) that started in Wuhan, Hubei Province, People’s Republic of China,1 , 2 has spread to all parts in the People’s Republic of China, other parts of Asia such as Japan and Thailand, Australia, Europe, and North America. The number of confirmed cases in the People’s Republic of China has reached 42,700, including 1017 deaths, as of February 11, 2020 (new reference, website). Although patients initially present with fever with or without respiratory symptoms, various degrees of pulmonary abnormalities develop later in all patients, and these can be seen on chest computed tomography (CT) imaging.1 , 3 Most patients only have a common, mild form of illness, but approximately 15% to 20% fall in the severe group, meaning they require assisted oxygenation as part of treatment.3 The severe group has a high mortality rate and is associated with older age, underlying diseases such as diabetes, and medical procedures (such as patients who were infected in a hospital setting while undergoing an operation for other indications).
Although there have been several studies describing clinical features and characteristic radiographic findings (mainly chest CT scans),1 , 3 no pathologic studies have been conducted on the basis of autopsies or biopsies. Some of the reasons for the lack of autopsies and biopsies include suddenness of the outbreak, vast patient volume in hospitals, shortage of health care personnel, and high rate of transmission, which makes invasive diagnostic procedures less of a clinical priority.
Fortunately and unfortunately, we encountered two patients who underwent an operation for malignancy and were later found to have been infected with SARS-CoV-2. The operation overlapped in time with the infection, which allowed us to obtain the necessary specimens to examine the histopathology of COVID-19 pneumonia.
Case Presentation
Case 1
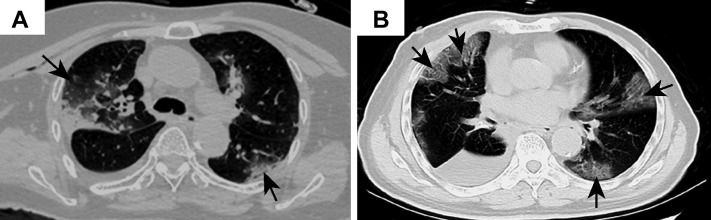
Case 1 was a female patient aged 84 years, who was admitted for treatment evaluation of a tumor measuring 1.5 cm in the right middle lobe of the lung. The tumor was discovered on a chest CT scan at an outside hospital. She had a medical history of hypertension for 30 years and type II diabetes. On day 6 of hospitalization, an enhanced chest CT scan was performed that confirmed an irregular solid nodule in the right middle lobe and bilateral ground-glass opacity (GGO). At that time, the significance of the latter findings was unknown. Her general condition was good, with no fever or respiratory symptoms, and with clear lung sounds on auscultation bilaterally. She underwent presurgical tests and preparations. On day 12, a thoracoscopic resection of the right middle lobe was performed without event. On day 13 (postoperative day 1), a repeat CT scan revealed postresection changes and bilateral GGO in the lower lobes of the lungs (Fig. 1 A). White blood cell (WBC) count was 12.49 × 1012/liter, whereas lymphocyte count decreased to 0.4 × 109/liter and the differential to 5%. There was a slight wheezing sound on auscultation on the right side. On day 16, the patient experienced some difficulty in breathing, chest tightness, wheezing, and dry cough. She received a diagnosis “suggestive of viral pneumonia,” with intermittent peripheral capillary oxygen saturation between 72% and 88%. On day 24, she was transferred to a special isolation ward owing to a pharyngeal swab test result that was positive for the 2019 novel coronavirus (2019-nCoV). Laboratory specimens drawn on the previous day (day 23) revealed the following: increased WBC count to 33.52 × 109/liter; increased neutrophils to 89.80%; decreased lymphocytes to 1.90%; decreased eosinophils to 0%; increased neutrophil count to 30.10 × 109/liter; decreased lymphocyte count to 0.65 × 109/liter; increased monocyte count to 2.50 × 109/liter; decreased eosinophil count to 0.01 × 109/liter; and increased basophil count to 0.26 × 109/liter.
Figure 1.
Representative images of chest computed tomography scan. (A) Case 1: image on postoperative day 1 revealing changes in the right lung and increased ground-glass opacities bilaterally (arrows); (B) case 2: foci of ground-glass opacity seen bilaterally (arrows).
Despite comprehensive treatment, including antibiotics, assisted oxygenation, and other supportive care, the patient’s condition deteriorated. Her peripheral capillary oxygen saturation (SpO2) decreased to 62.6% and heart rate to 40 bpm. A do-not-resuscitate order was given. She went into coma on day 27 and died on day 29. She did not manifest fever during the hospital stay.
Subsequent clinical information confirmed that she was exposed to another patient in the same room who was subsequently found to be infected with the 2019-nCoV.
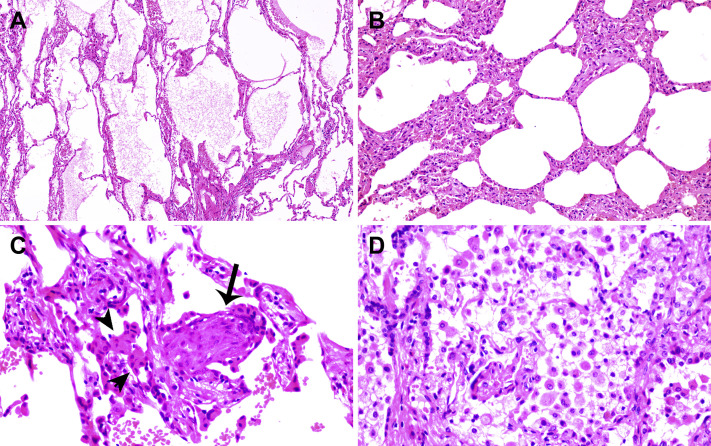
The right middle lobe resection specimen was delivered to the surgical pathology laboratory and processed according to the routine biosafety standards. Hematoxylin and eosin–stained sections were reviewed. A firm area of 1.5 cm in diameter was identified grossly, which in the histologic diagnosis was consistent with typical adenocarcinoma, with half exhibiting a lepidic and half an acinar pattern (not revealed). Sections away from the tumor, as found in Figure 2 , revealed evident alveolar damage, including alveolar edema and proteinaceous exudates (Fig. 2 A). Prominent inspissated spherical secretions or globules were also noted (Fig. 2 B). There was vascular congestion but patchy and mild inflammatory infiltration. Focal fibrin clusters mixed with mononuclear inflammatory cells and multinucleated giant cells were noted in the airspaces (Fig. 2 C). No significant neutrophil infiltration was present in the tissue. There was patchy and severe pneumocyte hyperplasia and interstitial thickening, indicating an ongoing reparative process. Suspected viral inclusions were also noted in some of these cells (Fig. 2 D).
Figure 2.
Histologic changes from case 1. (A) Proteinaceous exudates in alveolar spaces, with granules; (B) scattered large protein globules (arrows); (C) intra-alveolar fibrin with early organization, mononuclear inflammatory cells, and multinucleated giant cells; (D) hyperplastic pneumocytes, some with suspected viral inclusions (arrow).
Case 2
Case 2 was a male patient aged 73 years, who presented for elective surgery for lung cancer. Nine months earlier, a nodule was discovered radiologically in the right lower lobe of the lung during a health examination. He had a medical history of hypertension for 20 years, which had been adequately managed. A diagnosis of adenocarcinoma was made in a subsequent needle biopsy. The patient was admitted 1 week after the biopsy to the thoracic tumor ward, where he underwent a right lower lobe lung resection with lymph node dissection 3 days after admission. He recovered well and was discharged on day 6 postoperationally. A chest CT scan was performed on postoperative day 2, which revealed postoperative changes and patchy GGO in the right upper lobe. On retrospective re-examination of the images, the patient was diagnosed as “suspect for atypical viral pneumonia.” A fever developed in the patient on postoperative day 9 (38.2°C), with dry cough, chest tightness, and muscle pain. A nucleic acid test for 2019-nCoV came back positive. Other laboratory specimens were significant for decreased lymphocyte count. He was readmitted to the infectious disease ward. A repeat chest CT scan revealed additional foci of GGO in the bilateral upper lobes, consistent with viral pneumonia (Fig. 1 B, case 2). Tests for influenza virus and other infectious agents were negative. He underwent treatment for novel coronavirus pneumonia. He gradually recovered and was discharged after 20 days of treatment in the infectious disease ward.
On pathologic examination of the resected lobectomy specimen, a 1.2-cm gray-white nodule adjacent to the pleura was identified, which was poorly demarcated from the adjacent nontumor lung parenchyma. Histopathologic diagnosis of the tumor was that of adenocarcinoma, pT1bN0 (28 lymph nodes all negative). The resection margins were negative as well. Histologically, the surrounding lung parenchyma was patchy but with evident proteinaceous and fibrin exudates (Fig. 3 A). There was diffuse thickening of alveolar walls (Fig. 3 B), consisting of proliferating interstitial fibroblasts and type II pneumocyte hyperplasia. Focal fibroblast plug (arrow) and multinucleated giant cells (arrowheads) were seen in the airspaces (Fig. 3 C), indicating varying degrees of proliferative phase of diffuse alveolar damage. Some areas had abundant alveolar macrophages along with type II pneumocyte hyperplasia (Fig. 3 D).
Figure 3.
Histologic changes of coronavirus disease 2019 pneumonia in case 2. (A) Evident proteinaceous and fibrin exudate; (B) diffuse expansion of alveolar walls and septa owing to fibroblastic proliferations and type II pneumocyte hyperplasia, consistent with early diffuse alveolar damage pattern; (C) plugs of proliferating fibroblasts or “fibroblast balls” in the interstitium (arrow); (D) abundant macrophages infiltrating airspaces and type II pneumocyte hyperplasia.
Discussion
To our knowledge, the pathologic findings reported here represent the first for SARS-CoV-2 pneumonia or COVID-19. At the time of manuscript preparation, no autopsies had been performed on patients with COVID-19. Data on lung biopsies performed for COVID-19 are similarly lacking.
Pathologic findings from these two patients were edema and prominent proteinaceous exudates, vascular congestion, and inflammatory clusters with fibrinoid material and multinucleated giant cells. Reactive alveolar epithelial hyperplasia was seen in case 1, and fibroblastic proliferation (fibroblast plugs) in case 2 is indicative of early organization. No prominent neutrophil infiltration was seen. The significance of the large protein globules is not entirely clear, as these were described in patients with SARS but could also represent a nonspecific change with aging. More cases with sufficient controls are necessary to further clarify this change.
The two cases reported here represent “accidental” sampling of COVID-19, in which surgeries were performed for tumors in the lungs at a time when the superimposed infections were not recognized. These provided the first opportunities for studying the pathology of COVID-19. For case 1, the operation was performed 6 days after the CT findings of early GGO signs, meaning the pathologic changes of the non–tumor lung parenchyma indeed represent at least the peripheral part of COVID-19 pneumonia, as the imaging changes were more prominent toward the lower lobes. For case 2, as recognized later, the patient was unknowingly placed in the same room with patients who were positive for SARS-CoV-2 infection; the status of infection was not known to anyone at the time. He developed early lung lesions on a chest CT scan performed to evaluate the result of the operation. However, owing to a lack of sufficient knowledge about the new infection, the lesions were recognized only retrospectively as representing COVID-19 pneumonia.
The differential diagnoses of COVID-19 pneumonia might include, but is not limited to, acute or chronic pneumonia resulting from other infections. Comprehensive clinical analyses of the epidemiologic status, CT scan, and nucleic acid test can easily exclude such possibilities. As for the original SARS, SARS-COV-19 shares high genetic homology with SARS-CoV. Therefore, the International Committee on Taxonomy of Viruses recently renamed the 2019-nCoV to SARS-CoV-2 and the disease as COVID-19. Compared with pathologic findings in a cohort of autopsy cases of SARS, the two cases presented here also exhibited exudative and proliferative phases of acute lung injury, such as edema, inflammatory infiltrate, type II pneumocyte hyperplasia, and organization, but without obvious hyaline membrane formation and other long-term processes, such as squamous metaplasia.4, 5, 6 Of note, the pathologic changes seen in our two cases preceded the development of clinical symptoms and likely represent an earlier phase of the disease. Future studies of autopsies may add to the current findings.
Although case 1 patient was never febrile, her complete blood count profile, especially from postoperative day 1, revealed high WBC counts and lymphocytopenia, which is consistent with COVID-19. This may be a good clue for early diagnosis in the future. Case 2 developed a fever a few days after the CT findings, suggesting a delay in symptom development in these patients. During the earlier days of the outbreak, there had been limitations in both capacity and turnaround time for the nucleic acid test, which had further caused delay in confirming the diagnosis of COVID-197 in many patients. It seems that the time for the early lung lesions or COVID-19 to become severe enough to cause clinical symptoms is rather long. Even among patients with fever, the typically used pharyngeal swab polymerase chain reaction test may be negative, owing to the absence of viruses in the upper respiratory tract despite the presence of pneumonia. However, radiographic changes can occur early (chest CT scan is mostly employed in the People’s Republic of China during the current outbreak). Therefore, during an epidemic season, it is prudent to carefully evaluate any lung infiltration for GGO, and an appropriate serology test must be performed to rule out potential infection.7
These two incidents also typify a common scenario during the earlier phase of the SARS-CoV-2 outbreak, during which a significant number of health care providers became infected in hospitals in Wuhan, and patients in a same room were cross-infected, as they were exposed to unknown transmission sources. Because of this, it is important to practice “universal precaution” in surgical pathology laboratories and regard all fresh specimens as potentially infectious. In the People’s Republic of China, most surgical specimens are received already fixed in formalin. However, for larger specimens, the center of a specimen may not be sufficiently fixed and still pose potential risk for infection. Therefore, proper personal protective equipment with surgical masks or N95 respirators is worn all the time in the gross room. Fortunately, thus far, to our knowledge, no cases of pathologists being infected by COVID-19 had occurred.
It would be beneficial if reverse-transcriptase polymerase chain reaction or immunohistochemical stains, or both could be performed on these two cases to further confirm the presence of the viruses that may be associated with pneumonia. Unfortunately, these tests are currently under development, and adaptation to tissue specimens is not yet available. Nevertheless, we believe that it is imperative to report the findings of routine histopathology for better understanding of the mechanism by which the SARS-CoV-2 causes lung injury in the unfortunate tens of thousands of patients in Wuhan and worldwide.
Acknowledgments
The authors thank Jian Xu and Bei Qi for excellent technical and logistic assistance. This study was approved by the Ethic Consensus Committee of the Zhongnan Hospital of Wuhan University in the People’s Republic of China. Data on accumulated number of cases and deaths were from the website of the National Health Commission of the People’s Republic of China. Update on the epidemic of novel coronavirus (2019-nCoV)-infected pneumonia (2020-01-26) (EB/OL) is available at http://www.nhc.gov.cn/xcs/yqtb/202001/3882fdcdbfdc4b4fa4e3a829b62d518e.shtml.
Footnotes
Drs. Tian and Hu contributed equally to this work.
Disclosure: The authors declare no conflict of interest.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [e-pub ahead of print]. JAMA. https://doi.org/10.1001/jama.2020.1585, accessed February 7, 2020. [DOI] [PMC free article] [PubMed]
- 4.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks T.J., Chong P.Y., Chui P. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholls J.M., Poon L.L., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao SY, Wu Y, Liu H. Evolving status of the 2019 novel coronavirus infection: proposal of conventional serologic assays for disease diagnosis and infection monitoring [e-pub ahead of print]. J Med Virol. https://doi.org/10.1002/jmv.25702, accessed February 7, 2020. [DOI] [PMC free article] [PubMed]