Abstract
Given the mode of transmission of Middle East respiratory syndrome (MERS), healthcare workers (HCWs) in contact with MERS patients are expected to be at risk of MERS infections. We evaluated the prevalence of MERS coronavirus (CoV) immunoglobulin (Ig) G in HCWs exposed to MERS patients and calculated the incidence of MERS-affected cases in HCWs. We enrolled HCWs from hospitals where confirmed MERS patients had visited. Serum was collected 4 to 6 weeks after the last contact with a confirmed MERS patient. We performed an enzyme-linked immunosorbent assay (ELISA) to screen for the presence of MERS-CoV IgG and an indirect immunofluorescence test (IIFT) to confirm MERS-CoV IgG. We used a questionnaire to collect information regarding the exposure. We calculated the incidence of MERS-affected cases by dividing the sum of PCR-confirmed and serology-confirmed cases by the number of exposed HCWs in participating hospitals. In total, 1169 HCWs in 31 hospitals had contact with 114 MERS patients, and among the HCWs, 15 were PCR-confirmed MERS cases in study hospitals. Serologic analysis was performed for 737 participants. ELISA was positive in five participants and borderline for seven. IIFT was positive for two (0.3%) of these 12 participants. Among the participants who did not use appropriate personal protective equipment (PPE), seropositivity was 0.7% (2/294) compared to 0% (0/443) in cases with appropriate PPE use. The incidence of MERS infection in HCWs was 1.5% (17/1169). The seroprevalence of MERS-CoV IgG among HCWs was higher among participants who did not use appropriate PPE.
Keywords: Healthcare personnel, IgG, Incidence, Middle East respiratory syndrome, Personal protective equipment
Introduction
Middle East respiratory syndrome (MERS) is an emerging infectious disease, first described in Saudi Arabia [1], [2] and mainly found within the Middle Eastern region [3]. Only a few cases have been reported outside the Middle East [4], [5], [6], and no epidemic event outside the Middle East was seen before 2015. However, that year, the largest single-nation outbreak outside of Saudi Arabia occurred in South Korea over 45 days, with 186 confirmed MERS patients including 38 deaths (http://www.mers.go.kr/mers/html/jsp/Menu_C/list_C4.jsp) [7]. Because the main mode of transmission of MERS is respiratory droplet and most MERS transmission occurs in the nosocomial setting, healthcare workers (HCWs) in contact with confirmed MERS patients are at high risk of MERS infections [3], [8], [9]. In South Korea, among the 186 laboratory-confirmed MERS patients, 39 cases (21.0%) occurred in medical professionals or HCWs [9], [10].
The spectrum of clinical manifestations of MERS was diverse, and some patients, including a number of affected HCWs, showed relatively mild symptoms. Therefore, it was suspected that asymptomatic or undetected MERS infection may present in some of the HCWs who had been involved in managing confirmed MERS patients. One previous study reported that 25% of MERS coronavirus (CoV) PCR-positive patients were asymptomatic, and among these, 64% were HCWs [3]. Moreover, the period in which MERS-CoV is present in respiratory specimens is unknown because the virus shedding mechanism is still ambiguous even in confirmed MERS patients, and the PCR positivity rate of asymptomatic patients remains unknown. Therefore, we aimed to evaluate the seroprevalence of MERS-CoV IgG in HCWs exposed to MERS patients and to calculate the incidence of MERS affected cases in HCWs. Furthermore, we aimed to identify risk factors of MERS infection in HCWs.
Methods
Population
We enrolled HCWs from participating hospitals where confirmed MERS patients had visited or been treated. The participating HCWs included doctors, nurses, nursing assistants, radiologic technologists, patient transporters and patient caregivers. Others were also included in the study if they had had direct contact with confirmed MERS patients. This study did not use mandatory surveillance, and only those who agreed to participate in the study were enrolled. HCWs who were already diagnosed with PCR-confirmed MERS were not included in serologic assay but were included in calculating the incidence.
Definitions
We included as participants only individuals who had been in direct contact with confirmed MERS patients. Direct contact was defined as any of the following: (a) sharing conversations with a confirmed MERS patient within a 2 m reach, (b) staying with a patient in a closed room for longer than 5 minutes or (c) direct contact with respiratory or gastrointestinal secretions from a patient. Environmental factors and air circulation conditions were not considered because these varied markedly among the hospitals.
Study hospitals were divided into two groups. MERS-referral hospitals are those to which PCR-confirmed MERS patients were referred for management, whereas MERS-affected hospitals are those where patients suspected to have MERS had visited before confirmation of their diagnosis. That is, patients who had fever and respiratory symptoms visited MERS-affected rather than MERS-referral hospitals, and if MERS was confirmed by means of MERS-CoV PCR, these patients were transferred to designated MERS-referral hospitals. In some of the hospitals initially visited by patients, suspected patients were admitted and managed after laboratory confirmation of MERS. These hospitals, serving as a single stop for patients, were defined as MERS-affected in this study.
Some participating HCWs were quarantined or under contact surveillance after contact with a confirmed MERS patient. The decision between quarantine and contact surveillance was made by national Epidemic Intelligence Service officers dispatched to specific hospitals according to national guidelines. In brief, if the HCW was a close contact with MERS patients without appropriate protection, the patient was placed under quarantine. If casual contact occurred, the patient was placed under contact surveillance [11]. HCWs who were quarantined were confined at home or in a quarantine facility for 14 days. If respiratory symptoms or fever developed in quarantined HCWs, MERS-CoV PCR in respiratory specimen was performed twice in a 48-hour period, according to national guidelines, in each institution [11]. HCWs who were placed under contact surveillance were monitored daily for fever and respiratory symptoms for 14 days but were not prohibited from working in hospitals.
The definition of appropriate personal protective equipment (PPE) was drawn from previous recommendations (http://www.cdc.gov/coronavirus/mers/downloads/MERS-Infection-Control-Guidance-051414.pdf) [11], [12], [13]. Appropriate PPE was defined as use of all of the following: (a) N95 respirator or powered air-purifying respirator (PAPR), (b) isolation gown (coverall), (c) goggles or face shield and (d) gloves. If any part of the PPE was missing, it was considered to be exposure without appropriate PPE.
We defined aerosol-generating procedures (AGP) as follows: suction of airway, application of high-flow O2 instrument, bronchoscopy, intubation and/or cardiopulmonary resuscitation. In cases in which AGP were performed, only PAPR, not an N95 respirator, was considered appropriate PPE [11].
Sample collection and survey
We collected the serum of participants to identify the presence of MERS-CoV IgG. Further, we used a questionnaire survey to gather information regarding the HCWs' demographic characteristics and extent of exposure. The survey questionnaire was based on a World Health Organization questionnaire (http://www.who.int/csr/disease/coronavirus_infections/Healthcare_MERS_Seroepi_Investigation_27Jan2014.pdf).
Serum was collected 4 to 6 weeks after the last contact with confirmed MERS patients.
Laboratory procedures
We performed an enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Lübeck, Germany) to screen for the presence of MERS-CoV IgG. In cases in which the optical density of the ELISA exceeded a predefined cutoff value (>50% of the reference value), we performed an indirect immunofluorescence test (IIFT) (Euroimmun) to confirm MERS-CoV IgG and quantify antibody titres. The cutoff ELISA values were 80% of the reference value for a positive and 50% for borderline result. Serum was diluted 100-fold, according to the protocol suggested by the manufacturer. Antibody titre measurement was conducted by twofold dilution from 1:100 to 1:3200.
Statistical analysis
The data were analysed by SPSS 20.0 software (IBM SPSS, Chicago, IL, USA). We compared MERS-referral and MERS-affected hospitals using the chi-square test and the Mann-Whitney U test. All tests were two sided, and a p value of 0.05 or less was considered significant.
Incidence was calculated as follows: incidence of MERS-infected cases = (number of PCR-confirmed MERS cases in participating hospitals + number of serology-confirmed MERS cases in participating hospitals)/total number of MERS exposed HCWs in participating hospitals.
Study approval
All participants enrolled onto the study voluntarily, and written informed consent was acquired before participation. The study protocol was approved by the institutional review board of Ewha Womans University Mokdong Hospital in Seoul, South Korea (EUMC 2015-07-002). The trial was submitted to Clinicaltrials.gov under identifier NCT02497885.
Results
Baseline characteristics
Eighteen MERS-affected hospitals and 13 MERS-referral hospitals participated in the study (Fig. 1 ). A total of 114 cases of MERS were managed in the participating hospitals. A total 1169 HCWs had contact with MERS patients in study hospitals, of whom 603 were in MERS-affected hospitals and 566 were in MERS-referral hospitals. Among these, 15 were diagnosed as having PCR-confirmed MERS during quarantine, all of whom were in MERS-affected hospitals. Four hundred seventeen HCWs did not agree to participate in the study. Therefore, 737 HCWs were enrolled onto the study (Fig. 2 ). Of these participants, doctors accounted for 19.4%, nurses 69.1% and radiologic technologists 2.3% (Table 1 ). In MERS-affected hospitals, 62.4% of participants were quarantined, whereas only 2.5% of participants in MERS-referral hospitals were quarantined. The baseline characteristics of participants are shown in Table 1.
Fig. 1.
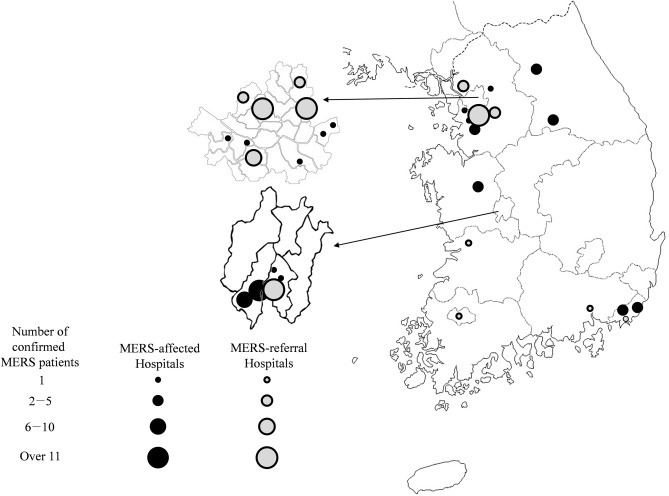
Geographic distribution of the participating 31 hospitals in South Korea. MERS, Middle east respiratory syndrome.
Fig. 2.
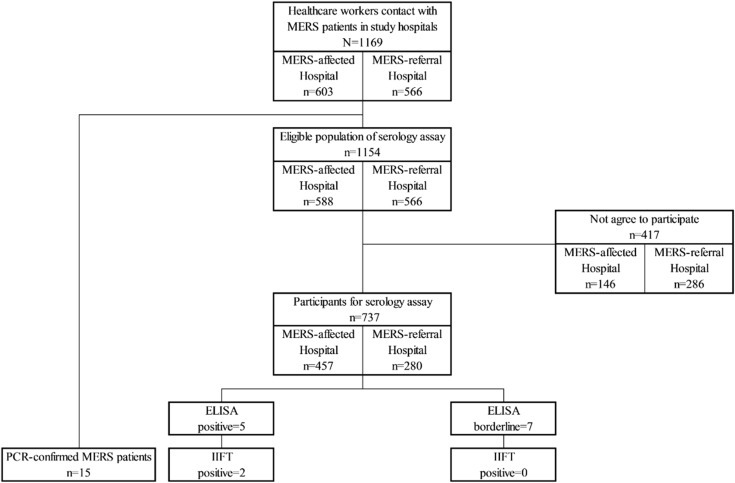
STROBE flowchart of participating population. ELISA, enzyme-linked immunosorbent assay; IIFT, indirect immunofluorescence test; MERS, Middle east respiratory syndrome.
Table 1.
Baseline characteristics of enrolled participants
| Characteristic | Total (n = 737) | MERS-referral hospital (n = 280) | MERS-affected hospital (n = 457) | p |
|---|---|---|---|---|
| Sex, male, n (%) | 160 (21.7%) | 51 (18.2%) | 109 (23.9%) | 0.072 |
| Age, mean (±SD), range | 33.0 (±8.5), 18–67 | 33.8 (±8.6), 18–58 | 32.6 (±8.5), 22–67 | 0.037 |
| Occupation, n (%) | ||||
| Doctor | 143 (19.4%) | 46 (16.4%) | 97 (21.2%) | |
| Nurse | 509 (69.1%) | 201 (71.8%) | 308 (67.4%) | |
| Nursing assistant | 13 (1.8%) | 3 (1.1%) | 10 (2.2%) | |
| Radiologic technologist | 17 (2.3%) | 3 (1.1%) | 14 (3.1%) | |
| Patient transporter | 12 (1.6%) | 0 | 12 (2.6%) | |
| Clerical officer/security guard | 8 (1.1%) | 1 (0.4%) | 7 (1.5%) | |
| Other | 35 (4.7%) | 26 (9.3%) | 9 (2.0%) | |
| Doctor, department, n (%)a | ||||
| Medical | 108 (76.6%) | 42 (93.3%) | 66 (68.8%) | |
| Surgical | 9 (6.4%) | 2 (4.4%) | 7 (7.3%) | |
| Emergency medicine | 23 (16.3%) | 0 | 23 (24.0%) | |
| Location of exposure, n (%)b | ||||
| Emergency room | 79 (10.7%) | 9 (3.2%) | 70 (15.3%) | <0.001 |
| Ward | 411 (55.8%) | 232 (82.9%) | 179 (39.2%) | <0.001 |
| Intensive care unit | 186 (25.2%) | 41 (14.6%) | 145 (31.7%) | <0.001 |
| Outpatient department | 40 (5.4%) | 5 (1.9%) | 35 (7.7%) | <0.001 |
| Quarantine, n (%) | ||||
| No | 340 (46.1%) | 236 (84.3%) | 104 (22.8%) | <0.001 |
| Yes | 292 (39.6%) | 7 (2.5%) | 285 (62.4%) | |
| Active surveillance, n (%) | 105 (14.2%) | 37 (13.2%) | 68 (14.9%) | |
MERS, Middle East respiratory syndrome.
One participant, a doctor of Oriental medicine, is excluded from department classification.
Some participants were exposed at multiple sites.
ELISA and IIFT
The ELISA result was positive in five (0.7%) of 737 participants and borderline in seven (0.9%) of 737 participants. The IIFT was positive in two among the 12 participants who showed borderline or positive results on the ELISA (0.3% of the total) (Table 2 ). Quantitative IIFT showed that the titre of antibody was 1:400 and 1:800, respectively.
Table 2.
Participants' laboratory results for ELISA and IIFT by serum
| Characteristic | Total (n = 737) | MERS-referral hospital (n = 280) | MERS-affected hospital (n = 457) | p |
|---|---|---|---|---|
| ELISA | ||||
| OD 50–79% | 7 (0.9%) | 2 (0.7%) | 5 (1.2%) | |
| OD >80% | 5 (0.7%) | 1 (0.4%) | 4 (0.9%) | 0.655 |
| IIFT positive | 2 (0.3%) | 0 | 2 (0.4%) | 0.528 |
ELISA, enzyme-linked immunosorbent assay; IIFT, indirect immunofluorescence test; MERS, Middle East respiratory syndrome; OD, optical density.
Calculation of incidence
We found two seropositive cases among 737 participants. Therefore, seroprevalence of MERS-CoV IgG among HCWs exposed to MERS patients who were asymptomatic or symptomatic with negative MERS-CoV PCR was 0.3%. On the basis of the 15 cases of PCR-confirmed MERS cases in our study hospitals, we assumed that at least 17 HCWs were affected by MERS and that the incidence was at least 1.5% (17/1169). Five of these cases were men and 12 were women.
Symptoms reported by participants and extent of exposure
Overall, 221 (30.0%) of 737 participants reported one or more symptoms within 4 weeks of contact with PCR-confirmed MERS patient. Generalized symptoms (177/737, 24.0%), including fever (82/737, 11.1%), fatigue (82/737, 11.1%) and myalgia (68/737, 9.2%), were frequently reported. Respiratory symptoms were reported in 13.6% and gastrointestinal symptoms in 7.5% of participants.
Total duration of contact with MERS patients, and mean duration of contact with MERS patients in a day were both significantly longer in MERS-referral hospitals. Two hundred ninety-four participants had been exposed to one or more PCR-confirmed MERS patients without at least one form of appropriate PPE. Exposure to AGP without PAPR occurred in 122 participants (Table 3 ).
Table 3.
Extent of exposure to MERS-confirmed patients among enrolled participants
| Characteristic | Total (n = 737) | MERS-referral hospital (n = 280) | MERS-affected hospital (n = 457) | p |
|---|---|---|---|---|
| Duration of contact with MERS patientsa | ||||
| ≤3 d | 284 (43.0%) | 13 (5.5%) | 271 (64.2%) | <0.001 |
| 4–7 d | 89 (13.5%) | 13 (5.5%) | 76 (18.0%) | |
| 8–14 d | 90 (13.6%) | 61 (25.6%) | 29 (6.9%) | |
| 15–30 d | 107 (16.2%) | 65 (27.3%) | 42 (10.0%) | |
| >31 d | 4 (0.9%) | 86 (36.1%) | 4 (0.9%) | |
| Mean duration of contact with MERS patients per day, hb | ||||
| ≤0.5 | 221 (33.5%) | 39 (14.4%) | 182 (46.7%) | <0.001 |
| 0.5–1 | 105 (15.9%) | 47 (17.4%) | 58 (14.9%) | |
| 1–2 | 86 (13.0%) | 53 (19.6%) | 33 (8.5%) | |
| 2–6 | 113 (17.1%) | 80 (29.6%) | 33 (8.5%) | |
| 6–12 | 121 (18.3%) | 44 (16.3%) | 77 (19.7%) | |
| >12 | 14 (2.1%) | 7 (2.6%) | 7 (1.8%) | |
| Hospitals in contact with case with superspreading eventc | 255 (34.6%) | 56 (20.0%) | 199 (43.5%) | <0.001 |
| Exposure without appropriate PPE | 294 (39.9%) | 53 (18.9%) | 241 (52.7%) | <0.001 |
| Exposure without PAPR during aerosol-generation procedured | 122 (16.6%) | 47 (16.8%) | 75 (16.4%) | 0.894 |
MERS, Middle East respiratory syndrome; PAPR, powered air-purifying respirator; PPE, personal protective equipment.
Data were missing for 42 and 35 participants in MERS-referral and MERS-affected hospitals, respectively.
Data were missing for 10 and 67 participants in MERS-referral and MERS-affected hospitals, respectively.
Case of superspreading event (confirmed MERS patient who infected more than five people).
Not all 737 participants were exposed to aerosol-generating procedures.
Among the participants who on even one occasion did not use appropriate PPE, 0.7% (2/294) were seropositive compared to 0 among those who used it appropriately every time. Among participants who were exposed to AGP, 0.8% (1/122) were seropositive among those who had been exposed without PAPR even once, whereas 0.2% (1/615) were seropositive among those who had been exposed only with PAPR (Table 4 ).
Table 4.
Use of personal protective equipment and seropositivity in MERS-exposed healthcare workersa
| Extent of exposure | Seropositive (n = 2) | Seronegative (n = 735) | p |
|---|---|---|---|
| Exposure without appropriate PPE | |||
| Yes | 2 (0.7%) | 292 (99.3%) | 0.159 |
| Never | 0 | 443 (100%) | |
| Exposure without PAPR during aerosolized procedure | |||
| Yes | 1 (0.8%) | 121 (99.2%) | 0.304 |
| Never or do not perform such procedures | 1 (0.2%) | 614 (99.8%) | |
MERS, Middle East respiratory syndrome; PAPR, powered air-purifying respirator; PPE, personal protective equipment.
Percentages in parentheses are proportion of each serostatus according to exposure status.
Discussion
In this study, we evaluated the seroprevalence of MERS-CoV among HCWs who had had contact with MERS patients. We found two asymptomatic or subclinical MERS infection in HCWs, both of whom were exposed without appropriate PPE. Overall prevalence of MERS-CoV seropositivity was 0.3% (2/737); among the participants who did not use appropriate PPE, 0.7% (2/294) were seropositive. Considering 15 PCR-confirmed MERS cases among HCWs in study hospital, the incidence of MERS affected cases among 1169 exposed HCWs was at least 1.5%.
MERS-CoV seroprevalence among populations other than confirmed MERS patients are limited. Recently it was reported that seroprevalence of MERS-CoV IgG among the general population of Saudi Arabia was 0.15%, and that of the high-risk population was 2.3 to 3.6% [14]. This suggests that a number of cases of asymptomatic or mild infection may be present in the high-risk population. However, there are not sufficient MERS-CoV IgG seroprevalence data among HCWs with which we can compare our results. In severe acute respiratory syndrome–affected areas in 2003, seroprevalence among HCWs by using a confirmatory test ranged from 0 to 1.04% [15], suggesting that undetected or asymptomatic cases were present after the epidemic. Our study found a similar proportion of MERS subclinical infection among HCWs.
To prevent MERS infection in HCWs, use of PPE is emphasized. In general, isolation gown and gloves are recommended as a contact precaution, and surgical mask is recommended as a droplet precaution [16]. Although MERS is known to be transmitted by droplet and by direct contact, use of appropriate PPE, including an N95 respirator and an isolation gown, has been emphasized in preventing MERS infection (http://www.cdc.gov/coronavirus/mers/downloads/MERS-Infection-Control-Guidance-051414.pdf) [11], [12], [13]. In our study, only participants who were exposed to MERS patients without appropriate PPE had IgG antibody against MERS-CoV. This was also found in PCR-confirmed MERS-infected HCWs. Among the 39 PCR-confirmed MERS-infected HCWs (http://www.mers.go.kr/mers/html/jsp/Menu_C/list_C4.jsp) [10], we reviewed the 15 patients who were affiliated with our study's participating hospitals (data not shown). We found that 14 of these patients were exposed without using an N95 respirator. Therefore, in our participating hospitals, almost all MERS-infected HCWs were related to not using appropriate PPE. There were two exceptional cases; they had used isolation gowns and N95 respirators following the US Centers for Disease Control and Prevention guidelines (http://www.cdc.gov/coronavirus/mers/downloads/MERS-Infection-Control-Guidance-051414.pdf), but they were eventually infected with MERS. Both were exposed to AGP, intubation of a MERS patient (seropositive case in our study) and cardiopulmonary resuscitation of a MERS patient (PCR-confirmed MERS case). Although the fitting test was not performed in either case, we guess that the N95 respirator is less efficient in AGP for high-virus-burden patients. Consequently, appropriate use of PPE is important in protection of MERS, and when performing AGP, more efficient respirators might be necessary [11].
In previous studies, the presence of MERS-CoV IgG was confirmed by a neutralizing assay such as a plaque reduction neutralization test [14] or microneutralization assay [17]. Müller et al. [14] reported that only 10% of samples that received positive ELISA results for antibody to S1 antigen were positive in the neutralization assay. In their study, however, the IIFT was well correlated with the neutralization assay. Their report showed that ELISA alone was useful in screening for the presence of MERS-CoV IgG but not in confirming it, whereas the IIFT could substitute the neutralization assay. In another recent report, the correlation of MERS-CoV ELISA and neutralization assay was strong in PCR-confirmed MERS patients [18]. Therefore, our protocol, composed of screening by MERS-CoV S1 ELISA and confirming by IIFT, may be robust to detect truly seropositive samples.
Our study has several limitations. Firstly, the optimal timing of serum collection for MERS testing is unknown. It is unknown how long serum antibodies persist in MERS-infected patients. Moreover, recent studies have shown that confirmed MERS patients with mild symptoms had only borderline serum IgG levels 32 days after diagnosis [19]. Thus, some of the negative ELISA results in our participants may have been falsely negative.
Secondly, because this work was for research purposes only, participation was not mandatory but voluntary. Therefore, not all HCWs exposed to MERS patients were enrolled. Approximately 48 hospitals and 61 clinics in South Korea were affected by MERS, and 30 hospitals were designated as MERS-referral hospitals. Among these institutions, only 31 hospitals participated in this study, and in these hospitals, only 63.0% of HCWs (75.2% in MERS-affected hospitals and 49.5% in MERS-referral hospitals) in participating institutions who had been potentially exposed to confirmed MERS patients participated.
Thirdly, the estimated incidence of MERS-affected HCWs could be underestimated by two reasons. Firstly, because 35.7% (417/1169) of HCWs did not agree to be enrolled onto the study, some seropositive cases may be missing. Secondly, two hospitals where large clustered cases developed did not participate in our study, and the incidence of MERS-affected cases in these hospitals may be higher than others. These hospitals included one that was visited by the first Korean MERS-infected patient and another at which the largest superspreading event occurred. In those hospitals, HCWs were not prepared for such a high risk of infection, and appropriate PPE was not used during the management of patients in the early period of outbreak. Therefore, seroprevalence would be expected to be higher in those hospitals than others.
In conclusion, the seroprevalence of MERS-CoV IgG in HCWs after contact with MERS patients in participating hospitals was found to be 0.3%, and among the participants who did not use appropriate PPE, it was 0.7%. The calculated incidence of MERS-affected cases in HCWs was at least 1.5%. The seroprevalence of MERS-CoV IgG was higher among participants who did not use appropriate PPE.
Transparency Declaration
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2015P7400200). All authors report no conflicts of interest relevant to this article.
Editor: M. Paul
References
- 1.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 2.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 3.Oboho I.K., Tomczyk S.M., Al-Asmari A.M., Banjar A.A., Al-Mugti H., Aloraini M.S. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermingham A., Chand M.A., Brown C.S., Aarons E., Tong C., Langrish C. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17:20290. [PubMed] [Google Scholar]
- 5.Bialek S.R., Allen D., Alvarado-Ramy F., Arthur R., Balajee A., Bell D. First confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in the United States, updated information on the epidemiology of MERS-CoV infection, and guidance for the public, clinicians, and public health authorities—May 2014. MMWR Morb Mortal Wkly Rep. 2014;63:431–436. [PMC free article] [PubMed] [Google Scholar]
- 6.Sridhar S., Brouqui P., Parola P., Gautret P. Imported cases of Middle East respiratory syndrome: an update. Travel Med Infect Dis. 2015;13:106–109. doi: 10.1016/j.tmaid.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J.Y. An outbreak of Middle East respiratory syndrome coronavirus infection in South Korea, 2015. Yonsei Med J. 2015;56:1174–1176. doi: 10.3349/ymj.2015.56.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Tawfiq J.A., Perl T.M. Middle East respiratory syndrome coronavirus in healthcare settings. Curr Opin Infect Dis. 2015;28:392–396. doi: 10.1097/QCO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korea Centers for Disease Control and Prevention Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6:269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.Y., Song J.Y., Yoon Y.K., Choi S.-H., Song Y.G., Kim S.-R. Middle East respiratory syndrome infection control and prevention guidelines for healthcare facilities. Infect Chemother. 2015;47:278–302. doi: 10.3947/ic.2015.47.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breakwell L., Pringle K., Chea N., Allen D., Allen S., Richards S. Lack of transmission among close contacts of patient with imported case of Middle East respiratory syndrome into the United States, 2014. Emerg Infect Dis. 2015;21:1128–1134. doi: 10.3201/eid2107.150054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korea Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention . Korea Ministry of Health and Welfare; Sejong: 2015. Guidelines on Middle East respiratory syndrome. [Google Scholar]
- 14.Muller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung G.M., Lim W.W., Ho L.M., Lam T.H., Ghani A.C., Donnelly C.A. Seroprevalence of IgG antibodies to SARS-coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect. 2006;134:211–221. doi: 10.1017/S0950268805004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S., Perera R., Choe P., Lau E., Choi S., Chun J. Comparison of serological assays in human Middle East respiratory syndrome (MERS)-coronavirus infection. Euro Surveill. 2015;20:30042. doi: 10.2807/1560-7917.ES.2015.20.41.30042. [DOI] [PubMed] [Google Scholar]
- 19.Park W.B., Perera R., Choe P.G., Lau E., Choi S.J., Chun J.Y. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]


