Graphical abstract
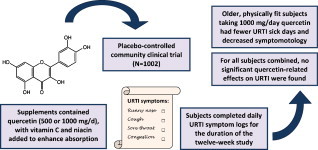
Abbreviations: BMI, body mass index; SARS-CoV, severe acute respiratory syndrome-associated coronavirus; URTI, upper respiratory tract infection; WURSS, Wisconsin Upper Respiratory Symptom Survey
Keywords: Flavonoid, Quercetin supplementation, Upper respiratory tract infection
Abstract
Quercetin in culture with target cells and pathogens exerts anti-pathogenic activities against a wide variety of viruses and bacteria. A few small-scale human quercetin supplementation studies have produced conflicting results regarding quercetin's effects on upper respiratory tract infection rates, and little is known regarding the appropriate human dose. The purpose of this randomized, double-blinded, placebo-controlled trial was to measure the influence of two quercetin doses (500 and 1000 mg/day) compared to placebo on upper respiratory tract infection (URTI) rates in a large community group (N = 1002) of subjects varying widely in age (18–85 years). Subjects ingested supplements for 12 weeks and logged URTI symptoms on a daily basis using the Wisconsin Upper Respiratory Symptom Survey (WURSS). No significant group differences were measured for URTI outcomes for all subjects combined, or when analyzing separately by gender, body mass index, and age categories. Regression analysis revealed that the strongest interaction effect with group status was self-reported fitness level. A separate analysis of subjects 40 years of age and older rating themselves in the top half of the entire group for fitness level (N = 325) showed lower URTI severity (36% reduction, P = 0.020) and URTI total sick days (31% reduction, P = 0.048) for the Q-1000 group compared to placebo. In summary, for all subjects combined, quercetin supplementation over 12 weeks had no significant influence on URTI rates or symptomatology compared to placebo. A reduction in URTI total sick days and severity was noted in middle aged and older subjects ingesting 1000 mg quercetin/day for 12 weeks who rated themselves as physically fit.
1. Introduction
Of all human illnesses, upper respiratory tract infections (URTI) occur most frequently [1]. These acute infections of the nose, paranasal sinuses, pharynx, larynx, trachea, and bronchi are exemplified by the common cold. Viruses (predominantly rhinovirus, influenza virus, coronavirus, adenovirus, parainfluenza virus, and respiratory syncytial virus) are the causal agents of most URTIs, with rhinovirus accounting for the largest number of cases [2]. According to the National Institute of Allergy and Infectious Diseases, the average adult has 2–4 colds each year, with young children suffering 6–10 [3]. Because of their high incidence, non-influenza-related viral respiratory infections impart an estimated $40 billion burden in direct and indirect costs on the U.S. economy [4].
Prevention and treatment of URTI through supplementation with herbs, plant extracts, and isolated plant molecules is an active area of research, but clinical trials have typically led to mixed and confusing results [5], [6], [7]. Quercetin, an antioxidant flavonoid found in fruits and vegetables, has been shown to exert strong anti-viral activities when cultured with target cells and causal agents of URTI such as severe acute respiratory syndrome-associated coronavirus (SARS-CoV) and several adenoviruses [8], [9]. Quercetin has also been reported to influence immune function via upregulation of IFN-γ [10]. However, nearly all the known quercetin-related effects come from in vitro study designs.
Given the in vitro anti-pathogenic and immunomodulatory effects of quercetin, a series of studies have been conducted to determine whether quercetin supplementation offsets exercise-induced immune perturbations and URTI risk. One such study investigated the effects of quercetin on infection rates in mice using a viral-challenge study design. The data from this study indicated that 12.5 mg/kg quercetin feedings for 7 days prior to inoculation with an LD50 dose of A/Puerto Rico/8/34 (H1N1) influenza virus offset the exercise-induced increase in morbidity, symptom severity, and mortality [11]. However, this study did not examine measures of immune function, so it was unclear whether this effect was due to direct anti-viral activities or to augmentation of immune function by quercetin. A double-blind, placebo-controlled study with 40 cyclists, in which immune function was analyzed in parallel with incidence and severity of URTI, was conducted by our research team [12]. The results of this trial showed that supplementation with 1000 mg/day quercetin for 3 weeks significantly increased plasma quercetin levels and reduced self-reported incidence of URTI during the 2-week period following 3 days of exhaustive exercise. Immune dysfunction, inflammation, and oxidative stress, however, were not affected by quercetin, suggesting that quercetin may have exerted these effects via direct anti-viral activities, at least within the context of the study design.
Based on these study results, we hypothesized that long-term quercetin supplementation would reduce URTI incidence and symptomatology in the general community, especially among high-risk groups such as young adult females and other individuals with risk factors for URTI such as obesity, poor physical fitness, or high levels of mental stress [13], [14], [15], [16]. A large number of subjects were recruited to allow investigation of quercetin's potential influence on URTI rates in various subgroups differing in gender, age, body mass index, physical fitness, and recent exposure to stressful events. Because the in vitro anti-viral properties of quercetin have been shown to be potentiated by ascorbate [17], and because unpublished data from Quercegen Pharma indicates that absorption of quercetin is enhanced by addition of vitamin C and niacin, the supplementation formula used in this study was a combination of quercetin (500 or 1000 mg/day), vitamin C (500 or 1000 mg/day), and niacin (20 or 40 mg/day).
2. Study design and methods
2.1. Subjects
Male and female subjects (N = 1023), 18–85 years of age, were recruited via mass advertising from the community. Half of the subjects were studied during a 12-week period from January to April 2008, and the second half from August to November 2008. Subjects had to be noninstitutionalized, and women were excluded if pregnant or lactating. No other exclusion criteria were employed, and both diseased and nondiseased subjects were admitted into the study, with monitoring of disease status and medication usage. Written informed consent was obtained from each subject and the Appalachian State University institutional review board approved all experimental procedures. During recruitment, subjects were stratified by gender (∼40% male and 60% female), age (40% young adult 18–40 years of age, 40% middle-aged 41–65, and 20% elderly 65 and over), and body mass index (BMI) groups (33% normal 18.5–24.9, 33% overweight 25–29.9, and 33% obese ≥30 kg/m2) to ensure representation of these various subgroups. Subjects agreed to avoid any other supplements containing quercetin, and they also agreed to make no major changes in their dietary or exercise habits for the duration of the study; no other restrictions were placed on diet, supplement usage, or medications.
2.2. Research design
Subjects were randomized to one of three groups: Q-500 (500 mg quercetin/day), Q-1000 (1000 mg quercetin/day), or placebo. Supplements were administered using double-blinded procedures. Subjects ingested two soft chew supplements twice daily (upon awakening, and between 2:00 p.m. and the last meal of the day) during the 12-week study period. Supplements were prepared by Nutravail Technologies (Chantilly, VA, USA) with Quercegen Pharma (Newton, MA, USA) and were soft, individually wrapped chews (5.3 g/piece) that contained either 125 or 250 mg quercetin, 125 or 250 mg vitamin C (ascorbic acid and sodium ascorbate), 5 or 10 mg niacin (nicotinamide), and 20 kcal of sugars in a carnauba wax, soy lecithin, corn starch, glycerine, and palm oil base colored with FD&C yellow #5 and #6. Placebo supplements were prepared exactly the same way minus the quercetin, vitamin C, and niacin. Previous studies performed in our lab used the same or similar supplement and placebo formulas [18], [19]. Compliance to the supplementation regimen was monitored by having all subjects complete an adherence survey each month; subjects indicating non-compliance were dropped from the study.
Subjects started supplementing after the first blood sample and continued for 12 weeks. Subjects completed a monthly log to verify physical activity and diet status, change in disease status and medication use, gastrointestinal (constipation, heartburn, bloating, diarrhea, nausea, vomiting), skin (rash, dryness, flushing), allergy, and mental (energy, headache, stress, focus/concentration) symptoms.
Two weeks prior to the first lab visit for the study, subjects provided demographic and lifestyle habit information using a survey posted on SurveyMonkey.com (Portland, OR USA). Information on dietary patterns was obtained through a semi-quantitative food frequency questionnaire for food groups including fruit, vegetables, cereals, meat, dairy, and fat. Exercise habits were assessed through answers to categorical questions dealing with both leisure-time and work activities. Physical fitness levels were self-reported using a ten-point Likert scale, and subjects were grouped into fitness tertiles according to their self-reported levels, with 1–4 corresponding to low fitness, 5–7 to medium fitness, and 8–10 to high fitness. Recent exposure to stressful events was assessed through answers to the question, “During the past month, would you say that you experienced (‘a lot,’ ‘moderate,’ ‘relatively little,’ or ‘almost no’) stress?” Subjects were grouped into stress tertiles based on their answers, with subjects answering “relatively little” or “almost no” stress being grouped together in the low stress tertile.
Subject height was measured with a stadiometer, and body mass was determined using a Tanita scale (Tanita, Arlington Heights, IL, USA). Subjects donated blood samples before and after the 12-week supplementation period in the morning (7:00–9:00 a.m.) after an overnight fast. Blood samples were taken from an antecubital vein with subjects in a seated position. Once separated, plasma samples were immediately flash-frozen in liquid nitrogen and stored at −80 °C.
2.3. Plasma quercetin
Total plasma quercetin (quercetin and its primary conjugates) from heparin-treated blood samples was measured as previously described [19], [20].
2.4. Upper respiratory tract infection
The Wisconsin Upper Respiratory Symptom Survey (WURSS) was used to assess URTI incidence and symptomatology [21]. The construct validity of the WURSS has been supported by measures of reliability, responsiveness, importance to patients, and convergence [21]. The WURSS-21 includes 10 items assessing symptoms, nine items assessing functional impairments, and one item assessing global severity and global change. Subjects filled in the one-page WURSS-21 at the end of each day in the study. From the responses recorded during the 84-day study, an URTI severity score was calculated by summing the daily URTI global severity score (0 = not sick, 1 = very mild URTI to 7 = severe). An URTI symptom score for the 84-day period was calculated by summing all 10 symptom scores for each day's entry (0 = do not have this symptom, 1 = very mild to 7 = severe), and an URTI daily activity score was calculated by summing all nine functional impairment entries (0 = not at all, 1 = very mildly to 7 = severely).
2.5. Statistical methods
All statistical analyses were performed using SPSS PC v.16.0 software (SPSS Inc., Chicago, IL, USA). URTI outcomes were compared between groups (Q-1000, Q-500, and placebo) and within groups by gender, BMI categories (normal weight <25 kg/m2, overweight 25–29.9 kg/m2, and obese ≥30 kg/m2), and age categories (<40, 40–59, and ≥60 years). Data are expressed as means ± SEM, and were compared between groups using one-way ANOVA, with a Tukey post hoc analysis. Stepwise linear regression analysis was used to determine the effect of age, BMI, and other demographic and lifestyle factors on URTI outcome measures. Plasma quercetin data were analyzed using a 3 (group) × 2 (time) repeated measures ANOVA, between groups design, with post hoc analysis using Bonferroni adjusted independent t-tests that contrasted pre-to-post-supplementation changes of Q-500 and Q-1000 with placebo. For all tests, P < 0.05 was considered significant.
3. Results
3.1. Subject characteristics
Subjects ranged in age from 18 to 85 years, with a large variance in body mass and composition (Table 1 ). Of the 1023 subjects recruited into the study, 1002 completed the trial. Among the 21 dropouts (7 from the placebo group, 6 from Q-500, and 8 from Q-1000), 12 failed to take the supplement and/or adhere to testing procedures, and 9 reported adverse symptoms from taking the supplement. No changes in body mass and composition were noted over the course of the study, and interaction effects indicated no group differences for any subject characteristic (data not shown). Thirty-seven percent of subjects reported past or current history for one or more chronic diseases: hypertension (19%), arthritis (16%), cancer (6%), cardiovascular disease (4%), diabetes (4%); with no group differences in chronic disease status (χ 2 0.087; P = 0.958).
Table 1.
Subject characteristics (N = 1002)a.
| Placebo (N = 335) | Q-500 (N = 334) | Q-1000 (N = 333) | All (N = 1002) | |
|---|---|---|---|---|
| (M = 123; F = 212) | (M = 138; F = 196) | (M = 134; F = 199) | (M = 395; F = 607) | |
| Age (year) | ||||
| Males | 43.8 ± 1.5 | 45.3 ± 1.2 | 45.5 ± 1.4 | 46.0 ± 0.5 |
| Females | 47.4 ± 1.1 | 47.2 ± 1.1 | 45.2 ± 1.1 | (range 18–85) |
| Weight (kg) | ||||
| Males | 84.8 ± 1.4 | 85.7 ± 1.2 | 88.1 ± 1.5 | 77.2 ± 0.6 |
| Females | 71.2 ± 1.1 | 71.6 ± 1.2 | 71.4 ± 1.3 | (42.7–157.5) |
| Height (m) | ||||
| Males | 1.77 ± 0.06 | 1.78 ± 0.04 | 1.77 ± 0.06 | 1.70 ± 0.03 |
| Females | 1.64 ± 0.05 | 1.65 ± 0.05 | 1.64 ± 0.04 | (1.39–2.02) |
| BMI (kg/m2) | ||||
| Males | 27.0 ± 0.4 | 26.9 ± 0.4 | 28.1 ± 0.4 | 26.7 ± 0.2 |
| Females | 26.4 ± 0.4 | 26.2 ± 0.4 | 26.4 ± 0.5 | (16.7–52.7) |
| Education (year) | 15.5 ± 0.2 | 15.5 ± 0.2 | 15.8 ± 0.2 | 15.6 ± 0.1 |
| Marital status | 34% single | 32% single | 33% single | 33% single |
| 53% married | 60% married | 55% married | 56% married | |
| 13% other | 8% other | 12% other | 11% other | |
Data are presented as mean ± SEM.
3.2. Plasma quercetin
Plasma quercetin in both quercetin groups (500 and 1000 mg/day) increased significantly above placebo levels (Group × time interaction effect, P < 0.001) (Fig. 1 ). Monthly symptom logs revealed no group differences over time for gastrointestinal, skin, allergy, or mental symptoms. No significant correlations were found between plasma quercetin levels and dietary variables (data not shown).
Fig. 1.
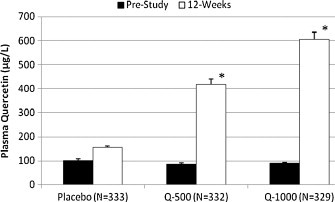
Plasma quercetin levels before and after 12-week supplementation with placebo, 500 mg/day, or 1000 mg/day quercetin. *P < 0.05 compared to placebo.
3.3. Upper respiratory tract infections
The quercetin–URTI relationships for all outcomes measured did not differ between the first and second cohorts of this study, and thus data are combined for this analysis. For all subjects combined, no significant group differences were measured for total number of days with URTI symptoms, URTI severity, or URTI symptom scores during the 12-week study period (Table 2 ). A separate analysis of subjects 40 years of age and older rating themselves in the top half of the entire group for fitness level (N = 325) showed lower URTI severity (36% reduction, P = 0.020) and URTI total sick days (31% reduction, P = 0.048) for the Q-1000 group compared to placebo (Fig. 2A and B). Analyses for all other age, gender, BMI, and fitness combinations showed no significant group effects.
Table 2.
URTI data for all subjects and subgroups during 12 weeks supplementationa.
| URTI variable | Placebo (N = 317) | Q-500 (N = 315) | Q-1000 (N = 315) | 1-Way ANOVA F-probability |
|---|---|---|---|---|
| All subjects | ||||
| URTI, # days | 8.4 ± 0.5 | 8.5 ± 0.5 | 8.5 ± 0.5 | 0.982 |
| URTI severity | 19.3 ± 1.3 | 18.3 ± 1.2 | 19.8 ± 1.3 | 0.670 |
| URTI symptom | 108 ± 8.0 | 108 ± 8.3 | 111 ± 8.0 | 0.946 |
| Gender | ||||
| Males | (N = 117) | (N = 137) | (N = 131) | |
| URTI, # days | 7.1 ± 0.9 | 7.0 ± 8.1 | 7.7 ± 0.8 | 0.794 |
| URTI severity | 17.2 ± 2.1 | 14.8 ± 1.6 | 17.1 ± 1.7 | 0.537 |
| URTI symptom | 92.0 ± 11.5 | 77.5 ± 10.2 | 98.8 ± 12.0 | 0.375 |
| Females | (N = 200) | (N = 178) | (N = 184) | |
| URTI, # days | 9.2 ± 0.7 | 9.5 ± 0.8 | 9.2 ± 0.7 | 0.930 |
| URTI severity | 20.6 ± 1.6 | 20.8 ± 1.7 | 21.8 ± 1.8 | 0.873 |
| URTI symptom | 118 ± 10.7 | 129 ± 12.0 | 120 ± 10.7 | 0.753 |
| Age (years) | ||||
| <40 | (N = 107) | (N = 103) | (N = 115) | |
| URTI, # days | 8.6 ± 0.9 | 10.3 ± 1.0 | 11.1 ± 0.9 | 0.129 |
| URTI severity | 20.6 ± 2.2 | 21.2 ± 2.0 | 26.2 ± 2.2 | 0.124 |
| URTI symptom | 123 ± 13.2 | 112 ± 12.2 | 147 ± 14.0 | 0.165 |
| 40–59 | (N = 133) | (N = 130) | (N = 140) | |
| URTI, # days | 9.0 ± 0.9 | 9.1 ± 0.8 | 7.7 ± 0.7 | 0.398 |
| URTI severity | 21.1 ± 2.1 | 21.1 ± 2.1 | 18.4 ± 1.8 | 0.544 |
| URTI symptom | 113 ± 13.4 | 129 ± 14.6 | 105 ± 11.8 | 0.427 |
| 60–85 | (N = 77) | (N = 82) | (N = 60) | |
| URTI, # days | 7.2 ± 1.1 | 5.2 ± 0.9 | 5.5 ± 1.1 | 0.303 |
| URTI severity | 14.5 ± 2.2 | 10.2 ± 1.9 | 10.9 ± 2.5 | 0.299 |
| URTI symptom | 78.5 ± 14.4 | 67.7 ± 14.8 | 57.4 ± 14.2 | 0.617 |
| BMI (kg/m2) | ||||
| <25 | (N = 143) | (N = 149) | (N = 137) | |
| URTI, # days | 8.6 ± 0.8 | 8.7 ± 0.8 | 9.6 ± 0.8 | 0.617 |
| URTI severity | 20.2 ± 1.9 | 18.2 ± 1.7 | 20.3 ± 1.8 | 0.639 |
| URTI symptom | 114 ± 11.7 | 106 ± 10.8 | 109 ± 11.4 | 0.873 |
| 25–29.9 | (N = 90) | (N = 89) | (N = 104) | |
| URTI, # days | 8.5 ± 1.1 | 8.3 ± 1.1 | 7.3 ± 0.8 | 0.615 |
| URTI severity | 20.7 ± 2.7 | 17.7 ± 2.3 | 18.2 ± 2.1 | 0.636 |
| URTI symptom | 113 ± 16.9 | 95.2 ± 14.8 | 108 ± 14.1 | 0.713 |
| ≥30 | (N = 84) | (N = 77) | (N = 74) | |
| URTI, # days | 8.0 ± 0.9 | 8.3 ± 1.0 | 8.5 ± 1.1 | 0.939 |
| URTI severity | 16.3 ± 1.9 | 19.1 ± 2.7 | 21.3 ± 3.0 | 0.391 |
| URTI symptom | 93.8 ± 13.8 | 126 ± 20.4 | 118 ± 18.1 | 0.381 |
Data are presented as mean ± SEM.
Fig. 2.
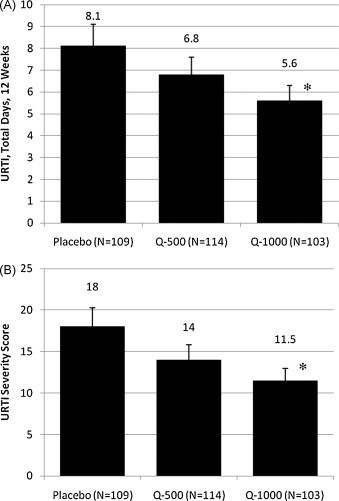
Effects of quercetin supplementation on total number of (A) URTI sick days and (B) URTI symptom severity score for subjects 40 years of age and older who rated themselves in the top half of the group for fitness. *P < 0.05 compared to placebo.
For all subjects combined, a decrease in sick days and symptomatology (severity and symptom scores) was related to the male gender (all P < 0.001) and increasing age (all P < 0.001) but not to BMI (Table 2). Measures of healthy lifestyles, such as increased self-reported fitness level and daily fruit intake, were significantly related to fewer URTI sick days and decreased symptomatology (Table 3 ). Finally, subjects who reported high stress during the month prior to the study experienced significantly more URTI sick days and increased symptomatology than did those subjects reporting low or moderate stress (Table 3). For subjects in the high stress group (N = 129), quercetin supplementation at 1000 mg/day tended to decrease the number of URTI sick days and symptomatology (P = 0.099).
Table 3.
URTI data for all subjects according to tertiles of lifestyle characteristicsa.
| URTI variable | Lifestyle characteristics |
F-Probability | ||
|---|---|---|---|---|
| Low fitnessb (N = 143) | Medium fitness (N = 467) | High fitness (N = 338) | ||
| URTI, # days | 10.6 ± 0.8 | 8.5 ± 0.4 | 7.5 ± 0.5* | 0.0043 |
| URTI severity | 25.9 ± 2.1 | 18.7 ± 1.0* | 16.4 ± 1.1* | 0.0001 |
| URTI symptom | 142 ± 12.8 | 101 ± 6.3* | 87.7 ± 7.1* | 0.0004 |
| URTI variable | Lifestyle characteristics |
F-Probability | ||
|---|---|---|---|---|
| High stressc (N = 129) | Medium stress (N = 464) | Low stress (N = 355) | ||
| URTI, # days | 9.9 ± 0.9 | 9.0 ± 0.5* | 7.2 ± 0.5* | 0.0036 |
| URTI severity | 22.2 ± 2.1 | 21.2 ± 1.1* | 15.0 ± 1.0* | 0.0001 |
| URTI symptom | 125 ± 12.7 | 116 ± 6.6* | 76.3 ± 6.2* | <0.0001 |
| URTI variable | Lifestyle characteristics |
F-Probability | ||
|---|---|---|---|---|
| Low fruitd (N = 262) | Medium fruit (N = 343) | High fruit (N = 343) | ||
| URTI, # days | 10.6 ± 0.8 | 8.5 ± 0.4 | 7.5 ± 0.5* | 0.0220 |
| URTI severity | 25.9 ± 2.1 | 18.7 ± 1.0 | 16.4 ± 1.1* | 0.0001 |
| URTI symptom | 142 ± 12.8 | 101 ± 6.3 | 87.7 ± 7.1* | 0.0372 |
P < 0.05 compared to the first tertile.
Data are presented as mean ± SEM.
Fitness tertiles were based on a 10 pt Likert scale: low (1–4), medium (5–7), high (8–10).
Stress: low (“relatively little” or “almost no” stress), medium (“moderate”), high (“a lot”).
Fruit intake: low (0 or 1 serving/day), medium (2 servings/day), high (3 or more servings/day).
4. Discussion
These data indicate that supplementation with 500 or 1000 mg/day quercetin with the addition of 500 or 1000 mg/day vitamin C and 20 or 40 mg/day niacin for 12 weeks significantly increased plasma quercetin levels, did not result in adverse symptoms, and had no effect on total number of URTI sick days, URTI severity, or URTI symptom scores. The lack of a quercetin-related effect on URTI remained, even when adjusting for gender, age, and BMI. Although URTI measures were not globally affected by quercetin supplementation, total URTI sick days and symptom severity were reduced by about one-third in middle-aged and older subjects rating themselves in the top half of the group for fitness.
Prior research performed in our lab found that cyclists who supplemented with 1000 mg/day quercetin before, during, and after exercising heavily for three consecutive days experienced a significant reduction in URTI incidence compared to placebo [12]. This protective effect was only noted during the 2-week period following the bouts of exhaustive exercise, when the athletes were at increased risk of URTI because of exercise-induced immune perturbations, leading us to the hypothesis that a quercetin-related effect on URTI would be most likely found in high-risk groups. In the present study, however, a significant reduction in URTI duration and severity associated with quercetin supplementation was experienced only in subjects over 40 who rated themselves as physically fit. This was a surprising finding, because these older and fitter subjects were already at low risk for URTI [14], [15]. Contrary to our hypothesis, no effect of quercetin supplementation on URTI was found in high-risk groups, although a trend toward a beneficial effect was noted in subjects who reported high levels of mental stress during the month prior to the beginning of the study.
It should be noted that the current study design cannot distinguish whether the reduction in total number of URTI days and symptom severity was due to quercetin, vitamin C and niacin, or a synergistic effect between the three components. However, the authors consider it unlikely that the protective effect noted can be attributed to the vitamin C or niacin included in the supplements. Vitamin C has not been shown to reduce risk of URTI in the general community [22]. Furthermore, the dosages of niacin used in the two supplementation formulas were relatively low (100 and 200% of U.S. RDA, respectively) and niacin has not been linked to URTI risk.
Several differences in study design should be pointed out when comparing the results of this trial to previous studies. First, the exercise studies previously performed by our research team were designed to study the effects of quercetin supplementation on URTI incidence during times of acute, rather than chronic, stress. Also, subjects rating themselves at high physical fitness levels in the current study not only exercised more frequently, but also ate more servings of fruit per day and had reduced BMI levels compared to subjects in the lower fitness tertiles; thus, the physical fitness rating has broader implications with regard to overall habits. Quercetin supplementation added to the beneficial influence of age and physical fitness level on reduced URTI symptomatology, and this synergism deserves further exploration.
Quercetin has been shown to exert strong anti-pathogenic effects against several causal agents of URTI in culture studies [8], [9]. However, in the present study, quercetin supplementation had no global effect on total number of URTI sick days or symptomatology. This finding may be explained by the potential reduction in quercetin's anti-viral activities due to metabolic biotransformation following ingestion. Quercetin is present in fruits and vegetables as water-soluble quercetin glycosides. Chen et al. [9] demonstrated that one such glycoside, quercetin-3-β-galactoside, inhibits a protease essential for viral replication of SARS-CoV when studied in vitro. When quercetin glycosides are consumed, however, the sugar moiety is cleaved off in the small intestine, releasing the lipid-soluble aglycone form of quercetin [23]. Chiang et al. [8] used aglycone quercetin in their culture studies and found that it also exhibited strong anti-viral effects, inhibiting early viral replication of adenoviruses-3, 8, and 11 in a dose-responsive manner. However, aglycone quercetin is either methylated, sulphated, or glucuronidated during phase II metabolism in the liver, and it is in these conjugate forms that quercetin is predominately found in the blood compartment [23]. One study found that 3-methylquercetin exhibited strong anti-viral activity against polio virus [17], but further work is needed to examine whether quercetin exerts anti-pathogenic activities in its other conjugate forms. Thus, although in vitro studies have pointed to the potential value of quercetin as an anti-viral agent, the affectivity of quercetin conjugates requires further study. Quercetin's in vivo role in augmenting immune function also appears limited, as we have reported in previous studies [12], [24].
In this study, increased number of days with URTI and overall symptomatology were related to the female gender, younger age, and self-reported high levels of mental stress, low fruit intake, and low physical fitness levels, but not to BMI. These findings are consistent with published studies linking URTI risk to age, gender, and exposure to stressful life events [13], [14] but are counter to some studies linking URTI risk to obesity [16]. Several randomized trials and epidemiological studies have compared URTI incidence in groups across the continuum of self-selected physical activity and fitness levels, and in agreement with our data, reported 25–50% reductions among the most active and physically fit groups [25], [26], [27]. Limited data support a moderate reduction in URTI risk with higher fruit intake especially when combined with vegetables [28].
In summary, quercetin supplementation in doses of 500 and 1000 mg/day for 12 weeks significantly increased plasma quercetin levels with no reported side effects, but was not found to decrease total number of URTI sick days or reduce symptomatology for all subjects. Contrary to our hypothesis, the strongest quercetin-URTI related effects were seen in a relatively low-risk group: older subjects who rated themselves as physically fit. Subjects in this group ingesting 1000 mg/day quercetin experienced a one-third reduction in total number of URTI sick days and URTI severity compared to placebo. Future work is needed to elucidate the potential connection between quercetin affectivity and exercise status.
Role of the funding source
This work was supported by grants from Coca-Cola and Quercegen Pharma. Coca-Cola and Quercegen Pharma were involved in designing the study, but they had no role in the collection, analysis, and interpretation of data; nor were they involved in the writing of the report or the decision to submit the paper for publication.
Conflict of interest
S. A. Heinz, D. A. Henson, M. D. Austin, and F. Jin declare no conflicts of interest. D. C. Nieman holds a position on the science advisory board for Quercegen Pharma.
Acknowledgements
The authors would like to thank the staff and students of the Human Performance Laboratory at Appalachian State University for their assistance with this project.
References
- 1.Monto A.S. Epidemiology of viral respiratory infections. Am J Med. 2002;112:4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 2.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Allergy and Infectious Diseases. The common cold fact sheet. http://www3.niaid.nih.gov/topics/commonCold (accessed 12 March 2010).
- 4.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Schoop R., Klein P., Suter A., Johnston S.L. Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther. 2006;28:174–183. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Linde K, Barrett B, Bauer R, Melchart D, Woelkart K. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews 1, CD000530, 2006. Retrieved from http://www.cochrane.org/reviews/en/ab000530.html (accessed 12 March 2010). [DOI] [PubMed]
- 7.Predy G.N., Goel V., Lovlin R., Donner A., Stitt L., Basu T.K. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial. Can Med Assoc J. 2005;173:1043–1048. doi: 10.1503/cmaj.1041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang L.C., Chiang W., Liu M.C., Lin C.C. In vitro antiviral activities of Caesalpiniapulcherrima and its related flavonoids. J Antimicrob Chemother. 2003;52:194–198. doi: 10.1093/jac/dkg291. [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Li J., Luo C., Liu H., Xu W., Chen G. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: structure–activity relationship studies reveal salient pharmacophore features. Bioorg Med Chem. 2006;14:8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair M.P., Kandaswami C., Mahajan S., Chadha K.C., Chawda R., Nair H. The flavonoid, quercetin, differentially regulates Th-1 (IFNγ) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta. 2002;1593:29–36. doi: 10.1016/s0167-4889(02)00328-2. [DOI] [PubMed] [Google Scholar]
- 11.Davis J., Murphy E., McClellan J., Carmichael M., Gangemi J. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol: Regul Integr Comp Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- 12.Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S., Tyrrell D.A.J., Smith A.P. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 14.Monto A.S., Sullivan K.M. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110:145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieman D.C., Henson D.A., Gusewitch G., Warren B.J., Dotson R.C., Butterworth D.E. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 16.van Wayenburg C.A.M., Lemiengre M.B.T., van Reenen-Schimmel A.H., Bor J.H.J., Bakx J.C., van Staveren W.A. Encounters for common illnesses in general practice increased in obese patients. Fam Pract. 2008;25(Suppl 1):i93–i98. doi: 10.1093/fampra/cmn059. [DOI] [PubMed] [Google Scholar]
- 17.Vrijsen R., Everaert L., Boeyé A. Antiviral activity of flavones and potentiation by ascorbate. J Gen Virol. 1988;69:1749–1751. doi: 10.1099/0022-1317-69-7-1749. [DOI] [PubMed] [Google Scholar]
- 18.Nieman D.C., Henson D.A., Davis J.M., Dumke C.L., Gross S.J., Jenkins D.P. Quercetin ingestion does not alter cytokine changes in athletes competing in the western states endurance run. J Interferon Cytokine Res. 2007;27:1003–1012. doi: 10.1089/jir.2007.0050. [DOI] [PubMed] [Google Scholar]
- 19.Nieman D.C., Henson D.A., Maxwell K.R., Williams A.S., McAnulty S.R., Jin F. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc. 2009;41:1467–1475. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 20.Nieman D.C., Henson D.A., Davis J.M., Murphy E.A., Jenkins D.P., Gross S.J. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol. 2007;103:1728–1735. doi: 10.1152/japplphysiol.00707.2007. [DOI] [PubMed] [Google Scholar]
- 21.Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas R.M., Hemilä H., Chalker E., Treacy B. Cochrane review: vitamin C for preventing and treating the common cold. Evidence-Based Child Health: Cochrane Rev J. 2008;3:672–720. doi: 10.1002/14651858.CD000980.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth K., Piskula M.K. Food content, processing, absorption and metabolism of onion flavonoids. Crit Rev Food Sci Nutr. 2007;47:397–409. doi: 10.1080/10408390600846291. [DOI] [PubMed] [Google Scholar]
- 24.Heinz SA, Henson DA, Nieman DC, Austin Md, Jin F. Twelve-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity, or granulocyte phagocytosis in female human subjects. Br J Nutr, in press. [DOI] [PubMed]
- 25.Chubak J., McTiernan A., Sorensen B., Wener M.H., Yasui Y., Velasquez M. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119:937–942. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Matthews C.E., Ockene I.S., Freedson P.S., Rosal M.C., Merriam P.A., Hebert J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Nieman D.C., Nehlsen-Cannarella S.L., Markoff P.A., Balk-Lamberton A.J., Yang H., Chritton D.B.W. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 28.Li L., Werler M.M. Fruit and vegetable intake and risk of upper respiratory tract infection in pregnant women. Public Health Nutr. 2010;13:276–282. doi: 10.1017/S1368980009990590. [DOI] [PMC free article] [PubMed] [Google Scholar]


