Abstract
Early diagnosis of pneumococcal pneumonia facilitates appropriate antibiotic therapy. The urinary antigen test (UAT) is known to be useful for the diagnosis of pneumococcal pneumonia. This study aimed to evaluate the usefulness of UAT in the 13-valent pneumococcal conjugated vaccine (PCV13) era. Community-acquired pneumonia (CAP) cases aged ≥19 years were reviewed retrospectively. This study evaluated the utility of Streptococcus pneumoniae UAT (BinaxNOW® assay) for diagnosis of pneumococcal CAP, and the relation of the UAT positive rate to age, comorbidities, pneumonia severity, and pneumococcal serotypes. Among 752 microbiologically identified CAP cases, S. pneumoniae (36.7%) was the most common isolate, and of those cases, 56.4% were positive for UAT. UAT positivity varied by pneumococcal serotype (serotype 3, 50%; 9V/9A, 85%; 11A/11E, 54%; 14, 36.4%; 19A, 50%; and 23F, 37.5%), and was significantly increased since 2012, two years after introduction of PCV13. The positive rate of UAT was significantly related to CRP level (P = 0.007) and lobar pneumonia (P = 0.006), but not to age, co-morbidities or prior antibiotic therapy. In conclusion, urinary antigen detection varied depending on the S. pneumoniae serotype. In the PCV13 era, the serotype distribution of pneumococcal pneumonia may be changing, and the clinical usefulness of UAT needs to be monitored. The positive rate of UAT may be influenced by a localized bacterial burden and host reactions.
Keywords: Streptococcus pneumonia, Pneumococcal infections, Pneumonia, Diagnosis
1. Introduction
Community-acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide. Streptococcus pneumoniae is the most common cause of CAP [1] and may be the leading cause of pneumonia of unknown etiology [2]. Therefore, early diagnosis of pneumococcal pneumonia would facilitate appropriate antibiotic therapy, while accurate diagnosis will lead to a better understanding of the disease burden.
As for the diagnosis of pneumococcal pneumonia, traditional microbiologic procedures showed low sensitivity, so culture-proven pneumococcal pneumonia might represent only some portion of whole pneumococcal pneumonia cases [2], [3]. The time spent between specimen collection and performance of microbiologic studies or previous antibiotics use may be a part of explanation. There are also other reasons such as challenges with bacterial growth on media, antibiotics decreases yield and etc. S. pneumoniae urinary antigen test (Binax NOW® S. pneumoniae assay) is known as a useful technique because of rapidity (∼15min), simplicity, reasonable specificity in adults, and the ability to detect pneumococcal pneumonia even after antibiotic administration and enhancing the diagnosis of pneumococcal pneumonia [4]. Even though urinary antigen test (UAT) is not possible in children due to a high rate of nasopharyngeal carriage of bacteria, studies in adults have shown better results, with a reported sensitivity of 74%–75% and specificity of 94%–97% [5], [6], [7], [8]. Although previous studies have supported the usefulness of UAT, there has been little published research about the effect of patient- and disease-specific factors (such as patient age, comorbidity, and pneumonia severity etc.) on the diagnostic utility of UAT.
Pneumococci are divided into serotypes based on their capsular polysaccharide structure, which is considered to be the most important virulence factor [9], and there are serotype-specific differences in nasopharyngeal carriage prevalence and disease incidence [10], [11]. These observations gave rise to the hypothesis that the utility of UAT would vary depending on the pneumococcal serotypes, and that there would be a change in the positive rate of UAT in accordance with changes in the distribution of pneumococcal serotypes that may have occurred since the introduction of the 13-valent pneumococcal conjugated vaccine (PCV13).
This study aimed to evaluate the proportion of pneumococcal pneumonia among hospitalized CAP and the usefulness of S. pneumoniae UAT for the diagnosis of pneumococcal pneumonia in the PCV13 era. The utility of S. pneumoniae UAT was further evaluated in relation to patient age, comorbidities, disease severity, and S. pneumoniae serotypes.
2. Patients and methods
2.1. Study design
Medical records from January 1st, 2007 through to December 31st, 2013 were examined to select the adult cases aged 19 years or older, who had a discharge diagnosis of pneumonia (ICD10 codes J09–J18.9) at a 1000-bed teaching hospital in Seoul, Korea. The clinical, radiological, and microbiological findings of the selected records were re-evaluated to determine whether the patients fulfilled the criteria of CAP as described below. The microbiologic etiology of CAP was retrospectively assessed with conventional laboratory techniques (sputum, pleura, blood culture, and respiratory virus PCR) and UAT.
From those patients hospitalized with CAP, pneumococcal pneumonia cases were selected for further evaluation. Because the overall number of cases was too small to evaluate certain planned analyses, especially in terms of S. pneumoniae serotypes, missed cases were added through the microbiologic database; during this stage of the study, we also enrolled outpatients if their condition met the definition of pneumococcal CAP (Fig. 1 ). We evaluated the baseline characteristics of included cases and examined the temporal change in UAT results, which might reflect the alteration of S. pneumoniae serotype distribution in CAP patients due to vaccine pressure following the introduction of the PCV13 into pediatric use. For this analysis, we defined three time periods relative to the introduction of PCV13: the period 2007–2009 was defined as the pre-PCV13 period, 2010–2011 as the period immediately after pediatric PCV13 use began (transition period), and 2012–2013 as the period 2 years after pediatric PCV13 use began (post-PCV13 period). Additionally, we analyzed the positive rate of UAT in relation to patient age, comorbidities, disease severity, and S. pneumoniae serotypes.
Fig. 1.
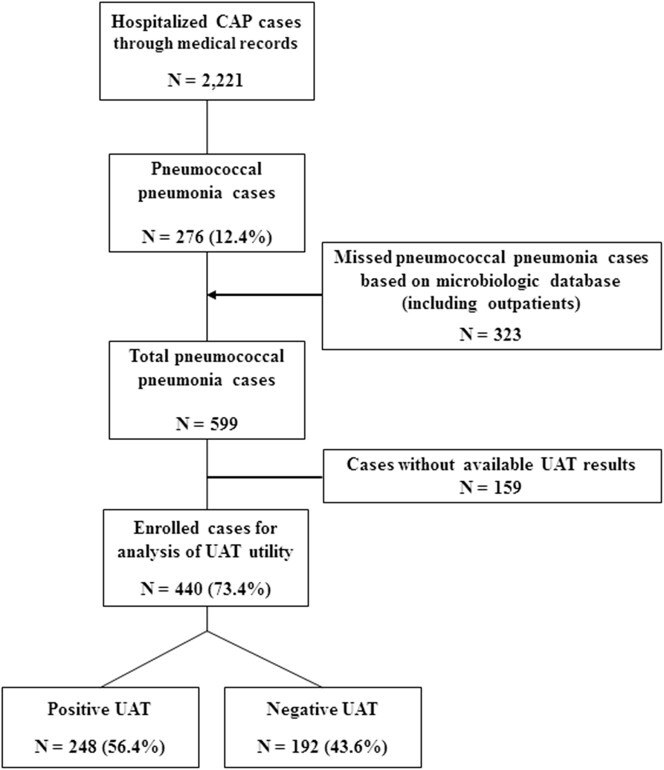
Study flowchart (CAP, community-acquired pneumonia; UAT, urinary antigen test).
Demographic and clinical data were collected from the hospital medical records, including age, sex, date of admission, previous use of antibiotics, smoking, alcohol consumption, comorbidities, complications, C-reactive protein (CRP), procalcitonin, chest X-ray findings, CURB-65 score, 30-day mortality and etc. The definitions of each medical condition were described previously [12]. Pneumococcal serotypes were determined for the available isolates.
2.2. Case definitions
CAP was diagnosed based on the following definitions: (a) an acute pulmonary infiltrate evident on chest radiographs and consistent with pneumonia within 48 h after admission; (b) confirmatory findings on clinical examination; and (c) acquisition of infection outside a hospital [13]. Patients with healthcare-associated or hospital-acquired pneumonia were excluded [14].
The patients with CAP were determined to have pneumococcal pneumonia if the bacterium S. pneumoniae was isolated from their blood, pleural effusion, or adequate lower respiratory specimens, or if the UAT was positive in cases whose adequately obtained sputum showed the predominance of gram-positive diplococci. Adequate lower respiratory specimens included transbronchial aspirates, broncho-alveolar-lavage (BAL) specimens, and sputum specimens with a Gram stain of adequate quality (>25 WBCs and <10 squamous epithelial cells per low-power field).
2.3. Serotyping of pneumococcal isolates
All available pneumococcal isolates were re-identified in the research laboratory by assaying colony morphology, optochin susceptibility, and bile solubility. Following re-identification, all optochin-sensitive, bile-soluble isolates were serotyped by monoclonal antibodies and/or PCR using the previously-described multi-bead serotyping assay [15].
2.4. Statistical analysis
We performed descriptive analysis and comparisons to examine demographics and clinical characteristics. To calculate the positive rate of UAT and relate this to patient- and disease-specific factors, cases were classified into two groups: those with positive UAT results, and those with negative UAT results. We used the χ 2 test or Fisher's exact test to compare the proportions of categorical variables between the two groups, and Student's t test to compare continuous variables between groups. Clinically relevant factors and factors showing significant inter-group differences by univariate analysis were included in a multivariate logistic regression analysis. Data were analyzed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). P values of <0.05 were considered statistically significant.
3. Results
A total of 2221 cases with hospitalized CAP were included, and the most commonly identified pathogen was S. pneumoniae, accounting for 276 (12.4%) cases (Table 1 ). After the exclusion of CAP cases caused by an unidentified pathogen, pneumococcal pneumonia represented 36.7% of remaining cases, followed by respiratory viruses and Staphylococcus aureus (Table 1). Notably, the proportion of pneumococcal pneumonia among CAP cases declined significantly after 2010, when pediatric PCV13 was introduced, representing 15.5% of cases (126 of 814 cases) in 2007–2009, 10.3% (58 of 561 cases) in 2010–2011, and 10.9% (92 of 846 cases) in 2012–2013 (P = 0.004).
Table 1.
Etiologic distribution of community acquired pneumonia.
| Cases, no. | Proportion (%) among all CAP cases | Proportion (%) among microbiologically identified cases | |
|---|---|---|---|
| Streptococcus pneumoniae | 276 | 12.42 | 36.7 |
| Staphylococcus aureus | 109 | 4.90 | 14.5 |
| Haemophilus influenzae | 105 | 4.72 | 14 |
| Klebsiella pneumoniae | 105 | 4.72 | 14 |
| Escherichia coli | 17 | 0.76 | 2.26 |
| Pseudomonas aeruginosa | 83 | 3.73 | 11 |
| Enterobacter spp. | 18 | 0.81 | 2.39 |
| Moraxella catarrhalis | 24 | 1.08 | 3.19 |
| Other Streptococcus spp. | 9 | 0.40 | 1.2 |
| Other Klebsiella spp. | 6 | 0.27 | 0.8 |
| Other Pseudomonas spp. | 5 | 0.22 | 0.66 |
| Acinetobacter spp. | 7 | 0.31 | 0.93 |
| Serratia marcescens | 6 | 0.27 | 0.8 |
| Stenotrophomonas maltophilia | 4 | 0.18 | 0.53 |
| Mycoplasma pneumoniae | 5 | 0.22 | 0.66 |
| Other bacteriaa | 12 | 0.54 | 1.6 |
| Respiratory virusesb | 179 | 8.05 | 23.8 |
| Undetermined | 1469 | 66.14 | – |
| Totalc | 2221 | 100% | 100% |
CAP, Community-acquired pneumonia.
Other bacteria (no. of cases): Aeromonas caviae (2), Pantoea agglomerans (1), Burkholderia cepacia (1), Morganella morganii (2), CMV (2), Sphingomonas paucimobilis (1), Alcaligenes xylosoxidans ssp. denitrificans (1), Raoultella planticola (1), Citrobacter freundii (1).
Respiratory viruses (no. of cases): Rhinovirus (37), influenza A/B (31), Parainfluenza (21), Metapneumovirus (19), RSV (19), Adenovirus (16), Coronavirus OC43/HKU1 (15), Coronavirus 229E/NL63 (11), Enterovirus (11).
The total count was smaller than the sum of the cases because of polymicrobial infection in some cases.
3.1. Characteristics of pneumococcal pneumonia
A total of 599 pneumococcal pneumonia cases were enrolled. Their baseline characteristics are summarized in Table 2 . Pneumococcal pneumonia was more common in males (67.1%) and the elderly with the mean age of 65.5 years. The overall 30-day mortality rate was 7.5%, increasing with age: 4.5% in those aged 19–50 years, 20.5% in those aged 51–64 years, and 75% in those aged 65 years or older (P = 0.054). Bacteremia occurred in 4.4% of cases, and bacteremic cases showed a significantly higher 30-day mortality rate than those without bacteremia (22.7% vs. 6.9%, P = 0.019). Only 324 (54.1%) cases had data available for the S. pneumoniae serotype. Among the cases with known serotypes, serotype 3 (14.2%) was most common, followed by serotypes 19A (11.1%), 11A/11E (10.8%), and 19F (9.3%) (Table 3 ).
Table 2.
Baseline characteristics of pneumococcal pneumonia cases.
| Baseline demographic and clinical characteristics (total no. = 599) | ||
|---|---|---|
| Male sex – no. (%) | 402 (67.1) | |
| Age (year) – mean (range) | 65.45 (19–96) | |
| Age group – no. (%) | 19–50 years | 86 (14.4) |
| 51–64 years | 158 (26.4) | |
| >65 years | 355 (59.3) | |
| Prior antibiotics use | 68 (11.4) | |
| Bacteremia – no. (%) | 23 (4.4) | |
| 30-day mortality – no. (%) | 44 (7.5) | |
| CXR | Interstitial | 235 (39.2) |
| Lobar | 130 (21.7) | |
| Bronchopneumonia | 234 (39.1) | |
| C-reactive protein – mean (range) | 116.27 (0.24–470.59) | |
| Risk group – no. (%) | Lowa | 238 (39.7) |
| Moderateb | 255 (42.6) | |
| Highc | 106 (17.7) | |
| CURB-65 score | 0–1 | 449 (75) |
| 2–5 | 150 (25) | |
| Comorbidity | Splenectomy | 0 |
| Hematologic malignancy | 3 (0.5) | |
| Multiple myeloma | 2 (0.3) | |
| Chemotherapy | 58 (9.7) | |
| Chronic renal failure | 6 (1.0) | |
| Nephrotic syndrome | 2 (0.3) | |
| Transplantation | 0 (0) | |
| Steroid | 52 (8.7) | |
| HIV | 2 (0.3) | |
| Diabetes | 109 (18.2) | |
| Chronic liver disease | 9 (1.5) | |
| Chronic pulmonary disease | 116 (19.4) | |
| Chronic cardiovascular disease | 36 (6.0) | |
| Cerebrovascular disease | 24 (4.0) | |
| Neurodegenerative disease | 15 (2.5) | |
| Neuromuscular diseases | 2 (0.3) | |
| Complication | Empyema | 2 (0.3) |
| Lung abscess | 0 | |
| ARDS | 2 (0.3) | |
| HUS | 0 | |
| Smoking – no. (%) | 138 (23.9) | |
| Alcohol – no. (%) | 85 (14.5) | |
HIV, human immunodeficiency virus; ARDS, acute respiratory distress syndrome; HUS, hemolytic uremic syndrome.
Conditions that did not satisfy criteria for high or moderate risk.
Moderate risk was defined by the presence of one or more of the following: (1) diabetes mellitus, (2) chronic liver disease, (3) chronic pulmonary disease, such as asthma or chronic obstructive lung disease, (4) chronic cardiovascular disease, such as heart failure, cardiomyopathy, or other chronic condition affecting cardiac function, (5) current smoking, or (6) heavy alcohol use, which was defined as alcohol use for more than 5 days a week or having a diagnosis of alcoholism.
High risk was defined by the presence of one or more of the following: (1) splenic dysfunction including post-splenectomy status, (2) hematologic malignancy, such as multiple myeloma, leukemia, or lymphoma, (3) a condition affecting the bone marrow or lymphatic system, (4) solid organ or stem cell transplantation, (5) chronic renal disease (6) HIV infection, (7) high-dose corticosteroid use (≥20 mg/day of prednisone or equivalent) lasting two or more weeks, or (8) treatment with a recombinant human immunomodulator.
Table 3.
Serotype distribution among pneumococcal pneumonia cases.
| Serotype | Total cases (%) | Chronological change of serotype, no. (%) |
||
|---|---|---|---|---|
| 2007–2009 year | 2010–2011 year | 2012–2013 year | ||
| 3 | 46 (14.2) | 27 (17.3) | 14 (10.2) | 5 (16.1) |
| 4 | 5 (1.5) | 3 (1.9) | 2 (1.5) | 0 (0) |
| 5 | 2 (0.6) | 2 (1.3) | 0 (0) | 0 (0) |
| 6A | 20 (6.2) | 9 (5.8) | 11 (8.0) | 0 (0) |
| 6B | 14 (4.3) | 6 (3.8) | 7 (5.1) | 1 (3.2) |
| 6C | 5 (1.5) | 0 (0) | 2 (1.5) | 3 (9.7) |
| 6D | 9 (2.8) | 3 (1.9) | 5 (3.6) | 1 (3.2) |
| 7F | 1 (0.3) | 1 (0.6) | 0 (0) | 0 (0) |
| 8 | 2 (0.6) | 2 (1.3) | 0 (0) | 0 (0) |
| 9 | 10 (3.1) | 5 (3.2) | 3 (2.2) | 2 (6.5) |
| 10A/39 | 4 (1.2) | 2 (1.3) | 2 (1.5) | 0 (0) |
| 11A/11E | 35 (10.8) | 13 (8.3) | 20 (14.6) | 2 (6.5) |
| 12F | 1 (0.3) | 1 (0.6) | 0 (0) | 0 (0) |
| 14 | 16 (4.9) | 10 (6.4) | 5 (3.6) | 1 (3.2) |
| 15B | 8 (2.7) | 4 (2.6) | 4 (2.9) | 0 (0) |
| 17F/17A | 1 (0.3) | 0 (0) | 1 (0.7) | 0 (0) |
| 18C | 2 (0.6) | 1 (0.6) | 1 (0.7) | 0 (0) |
| 19A | 36 (11.1) | 15 (9.6) | 17 (12.4) | 4 (12.9) |
| 19F | 30 (9.3) | 12 (7.7) | 17 (12.4) | 1 (3.2) |
| 20 | 8 (2.5) | 3 (1.9) | 5 (3.6) | 0 (0) |
| 22 | 6 (1.9) | 1 (0.6) | 4 (2.9) | 1 (3.2) |
| 23F | 12 (3.7) | 7 (4.5) | 5 (3.6) | 0 (0) |
| NT | 51 (15.7) | 29 (18.6) | 12 (8.8) | 10 (32.3) |
| PCV13 VT | 193 (59.6) | 98 (62.8) | 81 (59.1) | 14 (45.2) |
| PPV23 VT | 235 (72.5) | 114 (73.1) | 104 (76) | 17 (54.8) |
| Total | 324 (100) | 156 | 137 | 31 |
NT, nontypeable; VT, vaccine type.
PCV13 VT: 13-valent pneumococcal conjugate vaccine, vaccine type.
PPV23 VT: 23-valent pneumococcal polysaccharide vaccine, vaccine type.
3.2. Clinical usefulness of the urinary antigen test
Using conventional culture techniques, an etiological diagnosis of S. pneumoniae was achieved in 191 (8.6%) cases, and an additional 85 (3.8%) cases were diagnosed using UAT. When we analyzed the temporal change of UAT results, we found that the UAT positive rate had significantly increased over time between 2007 and 2013, and showed an especially sudden increase in the 2012–2013 periods. The UAT positive rates according to the time period were: 53.4% (94 of 176 cases) in 2007–2009, 51.1% (70 of 137 cases) in 2010–2011, and 66.1% (84 of 127 cases) in 2012–2013 (P = 0.029). There was no difference between the pre-PCV13 period (2007–2009) and the period immediately after pediatric PCV13 use (2010–2011) (P = 0.684), however, there was a significant difference between the pre-PCV13 period and the period 2 years after pediatric PCV13 introduction (2012–2013) (P = 0.026) (Fig. 2 ). Additionally, we analyzed the temporal change of causative serotype by collecting only available isolates as listed in Table 3, the proportion of PCV13 serotypes tended to decrease in the post-PCV 13 period, 2 years after the introduction of PCV13, compared with pre-PCV13 period (P = 0.067), although the difference was not significant. After introduction of PCV13, serotype 6A, 19F and 23F decreased, while serotype 6C rather increased. Serotype 3 and 19A was frequently isolated across the periods (Table 3). The positive rate of UAT varied diversely among cases with different pneumococcal serotypes (Fig. 3 ). The UAT positive rates by serotype were as follows: serotype 3, 50%; serotype 9V/9A, 83.3%; serotype 11A/11E, 59.1%; serotype 14, 36.3%; serotype 19A, 50%; serotype 19F, 46%; serotype 20, 75%; serotype 23F, 37.5%; serotype 4, 40%; and non-typeable, 55.3%. The positive rate of UAT was 49.2% (65 of 132 cases) in PCV13 serotypes and 53.5% (53 of 99 cases) in non-PCV13 serotypes, and these two groups were not significantly different in their UAT positive rates (P = 0.518).
Fig. 2.
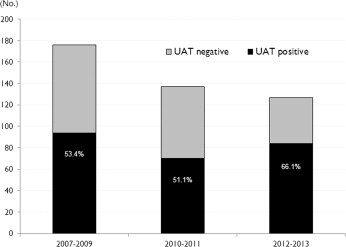
Temporal change of positive rate of urinary antigen test.
Fig. 3.
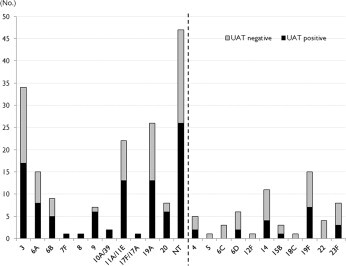
The positive rates of urinary antigen test according to the serotypes; serotypes are divided depending on positive rate (50% or more on left side versus below 50% on right side).
3.3. Clinical and laboratory factors influencing the results of the urinary antigen test
We evaluated the utility of the S. pneumoniae UAT among the 440 of 599 cases (73.4%) who underwent UAT. Pneumococcal urinary antigen was detected for 248 of 440 cases (56.4%). The baseline characteristics of these cases are summarized in Table 4 . Cases showing positive UAT results had a significantly higher mean CRP level than those showing negative UAT results (P = 0.007). It was difficult to determine the relationship between UAT results and procalcitonin, because only a limited number of cases had available data for procalcitonin (26% of cases), but considering only data from 2013, from which period 81.5% of cases had available data, there was no significant difference in the UAT positive rate according to procalcitonin level (P = 0.390). Cases with lobar pneumonia and diabetes had significantly higher UAT positive rates (P = 0.006 and P = 0.034, respectively). The positive rate of UAT was not influenced by age, sex, comorbidities, or complications, except for diabetes. Prior antibiotic use also did not affect the UAT positive rate (P = 0.296).
Table 4.
Comparison of baseline characteristics between urinary antigen test-positive and urinary antigen test-negative groups.
| Positive UAT (n = 248) |
Negative UAT (n = 192) |
p-Value | ||
|---|---|---|---|---|
| Male – no. (%) | 159 (64.1) | 134 (69.8) | 0.210 | |
| Age – mean (SD) | 66.22 (13.8) | 65.99 (13.9) | 0.867 | |
| Age – no. (%) | 0.514 | |||
| 19–50 | 30 (12.1) | 29 (15.1) | ||
| 51–65 | 66 (26.6) | 44 (22.9) | ||
| >65 | 152 (61.3) | 119 (62) | ||
| Prior antibiotic use, no. (%) | 34 (13.7) | 20 (10.4) | 0.296 | |
| Bacteremia, no. (%) | 12 (5.0) | 5 (2.8) | 0.269 | |
| 30d mortality, no. (%) | 19 (7.9) | 11 (5.7) | 0.380 | |
| CXR, no. (%) | Interstitial | 87 (35.1) | 76 (39.6) | 0.332 |
| Lobar | 72 (29.0) | 34 (17.7) | 0.006 | |
| bronchopneumonia | 89 (35.9) | 82 (42.7) | 0.145 | |
| C-reactive protein (mg/L) | mean(range) | 134.35 (104.4) | 108.53 (89.3) | 0.007 |
| Risk group, no. (%) | 0.330 | |||
| Low | 81 (32.7) | 75 (39.1) | ||
| Moderate | 115 (46.4) | 84 (43.8) | ||
| High | 52 (21.0) | 33 (17.2) | ||
| CURB-65 score, no. (%) | 0.275 | |||
| 0–1 | 173 (69.8) | 143 (74.5) | ||
| 2–5 | 75 (30.2) | 49 (25.5) | ||
| Comorbidity, no. (%) | Splenectomy | 0 | 0 | |
| Hematologic malignancy | 0 | 2 (1.0) | 0.190 | |
| Multiple myeloma | 2 (0.5) | 0 | 0.507 | |
| Chemotherapy | 30 (12.1) | 16 (8.3) | 0.201 | |
| Chronic renal failure | 2 (0.8) | 1 (0.5) | 1.000 | |
| Nephrotic syndrome | 1 (0.4) | 1 (0.5) | 1.000 | |
| Transplantation | 0 | 0 | ||
| Steroid | 27 (10.9) | 19 (9.9) | 0.736 | |
| HIV | 1 (0.4) | 0 | 1.000 | |
| Diabetes | 53 (21.4) | 26 (13.5) | 0.034 | |
| Chronic liver disease | 5 (2.0) | 1 (0.5) | 0.238 | |
| Chronic pulmonary disease | 45 (18.1) | 45 (23.4) | 0.172 | |
| Chronic cardiovascular disease | 17 (6.9) | 8 (4.2) | 0.227 | |
| Smoking | 59 (24.4) | 53 (28.3) | 0.354 | |
| Alcohol | 45 (18.5) | 27 (14.4) | 0.251 | |
| Cerebrovascular disease | 10 (4.0) | 7 (3.6) | 0.835 | |
| Neurodegenerative disease | 5 (2.0) | 4 (2.1) | 1.000 | |
| Neuromuscular diseases | 0 | 1 (0.5) | 0.436 | |
| Complication, no. (%) | Empyema | 0 | 1 (0.5) | 0.436 |
| Lung abscess | 0 | 0 | ||
| ARDS | 2 (0.8) | 0 | 0.507 | |
| HUS | 0 | 0 | ||
HIV, human immunodeficiency virus; ARDS, acute respiratory distress syndrome; HUS, hemolytic uremic syndrome.
3.4. Multivariate analysis
On multivariate analysis, lobar pneumonia (odds ratio [OR] 1.78, 95% CI, 1.046–3.034), and CRP (OR 1.002, 95% CI, 1.000–1.005) were significantly associated with differences in the UAT positive rate. In addition, although the difference was not significant, the positive rate of UAT showed a trend towards being increased in the period 2012–2013 compared with the period 2007–2009 (before PCV13 introduction) (OR 1.535, 95% CI, 0.913–2.579).
4. Discussion
In this study, the proportion of microbiologically identified pneumococcal pneumonia among hospitalized CAP was 8.6% (191 cases) and 12.4% (276 cases) without and with inclusion of UAT results, respectively. These findings show a relative increase of the diagnostic yield for S. pneumoniae of 44.5% (additional 85 cases to 191 cases) similar to previous studies which showed relative increases of diagnostic yield in the range of 39–50% [16], [17]. With the exception of unidentified pathogens, S. pneumoniae was isolated from 36.7% of cases, showing it to be the most common etiologic pathogen of hospitalized CAP. Thus, the clinical importance of S. pneumonia UAT should be emphasized. The BinaxNOW® S. pneumoniae assay (Alere, Waltham, MA, USA) is a UAT that detects C-polysaccharide (also known as teichoic acid). This test was commercialized in 2003 (when it was approved by the Food and Drug Administration, USA) [9].
This study found that between the introduction of PCV13 in 2010 and 2013, the proportion of pneumococcal pneumonia among total CAP cases decreased significantly. The similar temporal trend in pediatric Invasive pneumococcal disease (IPD) following the introduction of PCV13 was found in many industrialized countries [18], [19], although no efficacy data is available yet in Korea. Based on such findings, we presumed that the decrease of the proportion of pneumococcal pneumonia might be due to the herd effect from pediatric vaccination. During the studied periods, the pneumococcal vaccine rate in adults was estimated to be 9.7%, and PCV13 coverage rate was less than 1% [20]; however, the pediatric vaccination rate of PCV13 was 70.4%, and the rate was even higher when incomplete vaccinations were included (Korean Centers for Disease Control and Prevention, unpublished data). Several studies have shown a significant change in the serotypes of S. pneumoniae with nasopharyngeal carriage within 2 years after introduction of PCV13 [21], [22], even in areas with less vaccination coverage (69.3% vaccinated with at least 1 dose of PCV13) [21]. The herd effect of pediatric pneumococcal vaccination on reducing adult disease has been demonstrated in multiple clinical settings [23], [24].
The positive rate of UAT was also compared between the time periods before and after the introduction of PCV13. Considering that the positive rate of UAT increased significantly after 2012, and PCV13 serotypes showed a decreasing tendency, we presumed that the positive rate of UAT might have been altered in relation to the increase in non-typeable serotypes after the introduction of PCV13 (Table 3). The herd effect of the PCV7 even exceeded the direct protective benefit for immunized children [25], and this effect was especially significant after 2 years from vaccine introduction [26], [27]. According to a previous study, which showed an altered frequency of patient serotypes after PCV7 introduction [27], the change became remarkable only 2 years after PCV7 introduction; an increase of non-typeable serotypes occurred first, and then an increase of non-vaccine types arose later. The temporal change in the UAT positive rate, which increased significantly in the post-PCV13 era, 2 years after the introduction of PCV13, may be related to increased non-typeable pneumococci, decreased capsule production and a capsular serotype shift. Indeed, PCV13 serotypes rather decreased, while the proportion of non-typeable serotypes was significantly increased since 2012: 18.6% (29 of 156 cases) in 2007–2009 versus 8.8% (12 of 137 cases) in 2010–2011 versus 32.3% (10 of 31 cases) in 2012–2013 (P = 0.002). Further large-scale studies are required to better clarify the relationship between capsular serotype shift and UAT results.
Pneumococci are divided into serotypes based on the capsular polysaccharides they produce. Since different pneumococcal serotypes have varying tendencies toward nasopharyngeal colonization or invasiveness [10], [11], the hypothesis was formed that urinary antigen detection would vary by serotype. Previous studies have suggested that capsular polysaccharide serotypes with less metabolic cost (i.e. having fewer carbon atoms per saccharide unit) will be more heavily encapsulated. Such lower metabolic cost capsular polysaccharide serotypes include 3, 6A, 6B, 14, 19A, 19F, and 23F [28]. Theoretically, heavily encapsulated serotypes with a low metabolic cost might be able to decrease C-polysaccharide exposure, thereby raising suspicion about serotype-specific differences in urinary antigen detection. Contrary to our initial hypothesis, serotype 3 showed a urinary antigen detection rate of 50%, similar to the overall positive rate. The other pneumococcal serotypes also did not show any clear trend toward a urinary antigen detection rate differing from the overall positive rate, as shown in Fig. 3. The positive rates of UAT were variable by pneumococcal serotypes. The results suggest that other factors besides capsule thickness, such as the amount of C-polysaccharide amount, nasopharyngeal carriage rate, or vaccine pressure may influence the urinary antigen detection rate.
In this study, the positive rate of UAT was rather low (56.4%) compared to previous reports [5], [6], [7], [8]. Because the sensitivity of UAT has been reported slightly lower (52–78%) for non-bacteremic pneumococcal pneumonia than for bacteremic cases [9], [29], the lower detection rate of urinary antigen in this study than in previous studies may be partly explained by the effect of the fewer bacteremic episodes (4.4%) in this study, compared with previous studies [8]. Although a number of studies have demonstrated the utility of UAT, there has been little information about the effects of either host or microbiologic factors on the utility of UAT, particularly in terms of serotypes. This study, which analyzed the positive rate of UAT according to several demographic or clinical factors, demonstrated that a higher CRP level was significantly related with a higher positive rate of UAT. Cases showing lobar pneumonia were also associated with a higher UAT positive rate. The C-polysaccharide of S. pneumoniae is a major ligand for CRP, and binding of CRP to its ligands activates the classical complement pathway and stimulates phagocytosis [30]. In addition, pneumococcal C-polysaccharide is known to trigger inflammatory cytokine release, including interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor-α, which in turn induce the production of CRP [31]. Considering these data together, we thought it likely that a localized bacterial burden and host reactions, such as the acute phase response, may influence the urinary antigen detection. Indeed, several previous studies showed that bacteremia was associated with a higher detection of urinary antigen [8], [32], [33]. Here, the positive rate of UAT showed only a non-significant tendency to increase with bacteremia, perhaps reflecting the relatively small number of bacteremic cases included. In the present study, the UAT results were not influenced by previous antibiotics use, which is similar to the findings of previous studies [32], [34].
This study has some limitations. First, this study was conducted as a retrospective design. Although it is important to know the vaccination status of cases in order to analyze the results, we have little information owing to limited medical records. Second, limited numbers of pneumococcal isolates were available for serotyping, so statistical power was low for the evaluation of significant differences in the UAT positive rate among capsular serotypes.
In summary, UAT is a useful technique for the detection of S. pneumoniae antigen in urine samples and increases the diagnostic yield for pneumococci in patients with CAP irrespective of prior antibiotics therapy. The results of UAT might be influenced by localized bacterial burden and host reactions. In addition, we have shown that the results of UAT differ according to the S. pneumoniae serotypes causing pneumococcal pneumonia. In the era of PCV13, the S. pneumoniae serotype distribution may be changing, and the clinical usefulness of UAT needs to be monitored prospectively.
Acknowledgments
This work was supported by a Korea University grant number K1503011.
Conflict of interest
We have no potential conflicts to declare.
References
- 1.Woodhead M.A., Macfarlane J.T., McCracken J.S., Rose D.H., Finch R.G. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1:671–674. doi: 10.1016/s0140-6736(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-González A., Falguera M., Nogués A., Rubio-Caballero M. Is streptococcus pneumoniae the leading cause of pneumonia of unknown etiology? A microbiologic study of lung aspirates in consecutive patients with community-acquired pneumonia. Am J Med. 1999;106:385–390. doi: 10.1016/s0002-9343(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez F., Masiá M., Rodríguez J.C., Ayelo A., Soldán B., Cebrián L., et al. Evaluation of the immunochromatographic binax now assay for detection of streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in spain. Clin Infect Dis. 2003;36:286–292. doi: 10.1086/345852. [DOI] [PubMed] [Google Scholar]
- 4.Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C., et al. Infectious Diseases Society of A and American Thoracic S. Infectious diseases society of america/american thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinclair A., Xie X., Teltscher M., Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by streptococcus pneumoniae. J Clin Microbiol. 2013;51:2303–2310. doi: 10.1128/JCM.00137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulware D.R., Daley C.L., Merrifield C., Hopewell P.C., Janoff E.N. Rapid diagnosis of pneumococcal pneumonia among hiv-infected adults with urine antigen detection. J Infect. 2007;55:300–309. doi: 10.1016/j.jinf.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horita N., Miyazawa N., Kojima R., Kimura N., Inoue M., Ishigatsubo Y., et al. Sensitivity and specificity of the streptococcus pneumoniae urinary antigen test for unconcentrated urine from adult patients with pneumonia: a meta-analysis. Respirology. 2013;18:1177–1183. doi: 10.1111/resp.12163. [DOI] [PubMed] [Google Scholar]
- 8.Said M.A., Johnson H.L., Nonyane B.A., Deloria-Knoll M., O'Brien K.L., Agedd Adult Pneumococcal Burden Study Team, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J.Y., Eun B.W., Nahm M.H. Diagnosis of pneumococcal pneumonia: current pitfalls and the way forward. Infect Chemother. 2013;45:351–366. doi: 10.3947/ic.2013.45.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briles D.E., Crain M.J., Gray B.M., Forman C., Yother J. Strong association between capsular type and virulence for mice among human isolates of streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe Z.B., Thomsen R.W., Riis A., Valentiner-Branth P., Christensen J.J., Lambertsen L., et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6:e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J.Y., Choi J.Y., Lee J.S., Bae I.G., Kim Y.K., Sohn J.W., et al. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013;13:202. doi: 10.1186/1471-2334-13-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodí M., Rodríguez A., Solé-Violán J., Gilavert M.C., Garnacho J., Blanquer J., et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: Impact of adherence to infectious diseases society of america guidelines on survival. Clin Infect Dis. 2005;41:1709–1716. doi: 10.1086/498119. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society and Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 15.Yu J., Lin J., Kim K.H., Benjamin W.H., Jr., Nahm M.H. Development of an automated and multiplexed serotyping assay for streptococcus pneumoniae. Clin Vaccine Immunol. 2011;18:1900–1907. doi: 10.1128/CVI.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genné D., Siegrist H.H., Lienhard R. Enhancing the etiologic diagnosis of community-acquired pneumonia in adults using the urinary antigen assay (binax now) Int J Infect Dis. 2006;10:124–128. doi: 10.1016/j.ijid.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Huijts S.M., Pride M.W., Vos J.M., Jansen K.U., Webber C., Gruber W., et al. Diagnostic accuracy of a serotype-specific antigen test in community-acquired pneumonia. Eur Respir J. 2013;42:1283–1290. doi: 10.1183/09031936.00137412. [DOI] [PubMed] [Google Scholar]
- 18.Picazo J., Ruiz-Contreras J., Casado-Flores J., Giangaspro E., García-de-Miguel M.J., Hernández-Sampelayo T., et al. Impact of introduction of conjugate vaccines in the vaccination schedule on the incidence of pediatric invasive pneumococcal disease requiring hospitalization in Madrid 2007 to 2011. Pediatr Infect Dis J. 2013;32:656–661. doi: 10.1097/INF.0b013e31827e8594. [DOI] [PubMed] [Google Scholar]
- 19.Plosker G.L. 13-valent pneumococcal conjugate vaccine: a review of its use in infants, children, and adolescents. Paediatr Drugs. 2013;15:403–423. doi: 10.1007/s40272-013-0047-z. [DOI] [PubMed] [Google Scholar]
- 20.Song J.Y., Lee J.S., Wie S., Kim H.Y., Lee J., Seo Y.B., et al. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2014;22:229–234. doi: 10.1128/CVI.00673-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen R., Levy C., Bingen E., Koskas M., Nave I., Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012;31:297–301. doi: 10.1097/INF.0b013e318247ef84. [DOI] [PubMed] [Google Scholar]
- 22.Dagan R., Patterson S., Juergens C., Greenberg D., Givon-Lavi N., Porat N., et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 23.Grabenstein J.D., Weber D.J. Pneumococcal serotype diversity among adults in various countries, influenced by pediatric pneumococcal vaccination uptake. Clin Infect Dis. 2014;58:854–864. doi: 10.1093/cid/cit800. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien K.L., Millar E.V., Zell E.R., Bronsdon M., Weatherholtz R., Reid R., et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–1220. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control Prevention Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–897. [PubMed] [Google Scholar]
- 26.Lexau C.A., Lynfield R., Danila R., Pilishvili T., Facklam R., Farley M.M., et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 27.Song JY, Cheong HJ, Choi MJ, Jeon JH, Kang SH, Jeong EJ, et al. Serotype 6d pneumococcal infections in South Korea from 2004–2011. 2014 Annual Meeting of the Korean Society of Infectious Diseases and the Korean Society for Chemotherapy. Jeju, Republic of Korea; 2014.
- 28.Song J.H., Dagan R., Klugman K.P., Fritzell B. The relationship between pneumococcal serotypes and antibiotic resistance. Vaccine. 2012;30:2728–2737. doi: 10.1016/j.vaccine.2012.01.091. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch D.R., Laing R.T., Mills G.D., Karalus N.C., Town G.I., Mirrett S., et al. Evaluation of a rapid immunochromatographic test for detection of streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39:3495–3498. doi: 10.1128/JCM.39.10.3495-3498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 31.Simpson S.Q., Singh R., Bice D.E. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-alpha production by murine macrophages. Am J Respir Cell Mol Biol. 1994;10:284–289. doi: 10.1165/ajrcmb.10.3.8117447. [DOI] [PubMed] [Google Scholar]
- 32.Rosón B., Fernández-Sabé N., Carratalà J., Verdaguer R., Dorca J., Manresa F., et al. Contribution of a urinary antigen assay (binax now) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis. 2004;38:222–226. doi: 10.1086/380639. [DOI] [PubMed] [Google Scholar]
- 33.Tateda K., Kusano E., Matsumoto T., Kimura K., Uchida K., Nakata K., et al. Semi-quantitative analysis of streptococcus pneumoniae urinary antigen: kinetics of antigen titers and severity of diseases. Scand J Infect Dis. 2006;38:166–171. doi: 10.1080/00365540500400944. [DOI] [PubMed] [Google Scholar]
- 34.Porcel J.M., Ruiz-González A., Falguera M., Nogués A., Galindo C., Carratalá J., et al. Contribution of a pleural antigen assay (binax now) to the diagnosis of pneumococcal pneumonia. Chest. 2007;131:1442–1447. doi: 10.1378/chest.06-1884. [DOI] [PubMed] [Google Scholar]


