Abstract
Veterinarians practicing emergency medicine and/or working with exotic animals must be well versed in the pathophysiology of shock because many exotic pets present with an acute crisis or an acute manifestation of a chronic process causing poor organ perfusion. This article discusses the pathophysiology of shock and the systemic inflammatory response syndrome, which may lead to organ dysfunction, organ failure, sepsis, and death. The physiology of perfusion, perfusion measurements, categories of shock, and altered function of the immune system, gastrointestinal barrier, and coagulation system are discussed. Veterinarians providing emergency care to patients with shock must also be aware of comorbidities.
Keywords: Shock, Physiology, Critical care, Exotic animals
Key points
-
•
Most of the literature discussing shock, systemic inflammation, and sepsis relates to experimental studies using small mammals such as rodents and rabbits; there is a paucity of research of these syndromes in other species, such as ferrets, birds, and reptiles.
-
•
Although small mammals, experimentally, have a similar autonomic response to dogs during induced hemorrhage and shock states, this response is not noted in the clinical setting, in which decompensated shock is more commonly reported.
-
•
Poor perfusion affects every major organ system, but particular attention should be given to the immune system, coagulation system, cardiovascular system, gastrointestinal tract, and kidneys.
-
•
Knowing how these systems are affected helps explain to clinicians why patients with shock can later become septic or coagulopathic.
-
•
To ensure the best outcomes in patients who have shock or systemic inflammatory response syndrome, early recognition and intervention by provision of supplemental oxygen and fluid resuscitation is essential.
Definitions
-
1.
Shock is the clinical expression of dysoxia. Dysoxia is lack of oxygen supply to, or use by, the cells, limiting energy production.1 Poor perfusion and diminished oxygen delivery to the tissues with resulting signs of pallor, tachycardia, tachypnea, and altered pulse quality is another way to describe shock.
-
2.
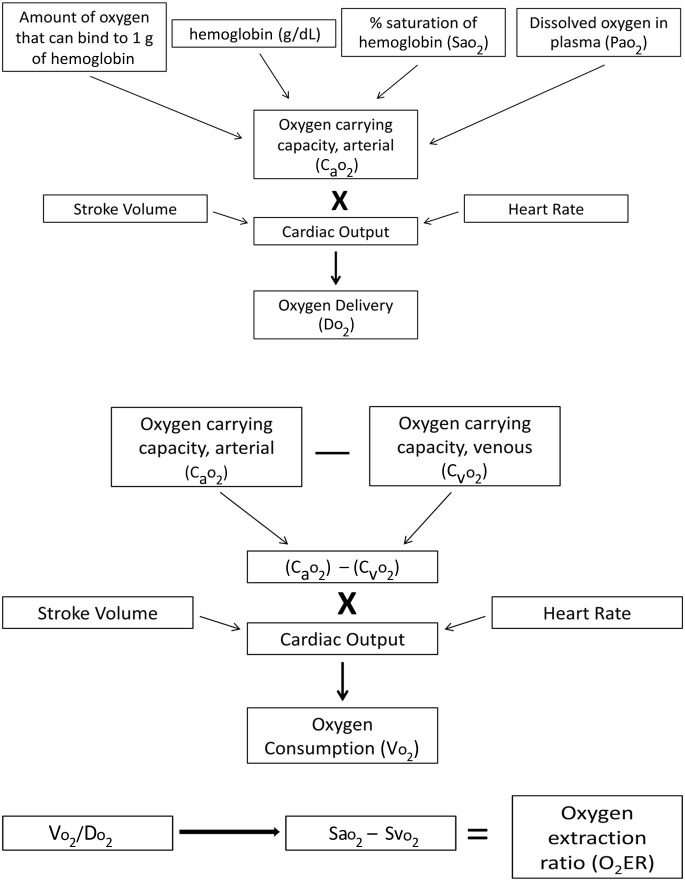
Oxygen delivery (Do 2) is the amount of oxygen delivered to tissues as determined by cardiac output and oxygen carrying capacity.2 It is described best by the equation CO × Cao 2, in which CO is cardiac output (the heart rate multiplied by stroke volume) and Ca o 2 is the oxygen carrying capacity of arterial blood, which is determined by the saturation of oxygen bound to hemoglobin, the amount of hemoglobin, and the small amount of dissolved oxygen in the serum (Fig. 1 ).
-
3.
Oxygen consumption (Vo 2) is the amount of oxygen used by the tissues after being delivered and off-loaded from hemoglobin.3 It can be determined by using the equation CO × (Ca o 2 – Cv o 2) which is cardiac output multiplied by the difference between arterial oxygen content (Ca o 2) and venous oxygen content (Cv o 2).
-
4.
Systemic inflammatory response syndrome (SIRS) is a condition characterized by changes in heart rate, body temperature, and respiration rate with either leukocytosis or leukopenia.4 SIRS can be caused by trauma; organ inflammation, such as pancreatitis; infection; and environmental stressors, like heat stroke.5 Criteria for SIRS have been described for humans and extrapolated for small animal veterinary medicine.6 SIRS has been described experimentally using similar criteria in rabbits7 and in rodents.8 SIRS in reptiles, amphibians, and other exotic species has not been characterized to the author’s knowledge.
-
5.
Sepsis is defined as SIRS secondary to infection. An infection is a pathologic process caused by the invasion of microorganisms that cause disease of normally sterile tissue or fluid.9 The term has been used since Hippocrates’ time to describe putrefaction and decomposition.10 Many clinicians use terminology such as sepsis syndrome, bacteremia, and SIRS interchangeably with sepsis. Note that SIRS can occur without infection.
-
6.
Multiple organ dysfunction syndrome is a condition whereby disease, injury, lack of perfusion, and so forth cause at least 1 vital organ (heart, lung, kidney, nervous system) to no longer function normally. Failure of multiple organs (>1) is multiple organ failure (MOF).11
Fig. 1.
Perfusion dynamics.
Physiology of perfusion
To provide oxygen to tissues and their cells, there are 3 basic requirements: functional cardiovascular system comprising a pump (heart) and responsive vasculature, the ability of the fluid pumped to carry oxygen (red blood cells, normally working hemoglobin, and saturation of the hemoglobin), and a way for the oxygen to become bound to hemoglobin through a normally working respiratory system.
Oxygen delivery (Do 2) is the amount of oxygen carried to the tissues in blood by hemoglobin. It is determined by cardiac output and oxygen carrying capacity (see Fig. 1). In rats, normal Do 2 is approximately 1.8 ± 0.2 mL O2/min/kg.12 In rabbits, the normal Do 2 reference range is 27.1 ± 2.7 mL/kg/min.13
Determination of oxygen delivery is more difficult in reptiles because of the various species and slow heart rates. Instead of Do 2, researchers sometimes measure oxygen pulse (OP), which was used in several tissue oxygenation studies summarized by Gleeson and Bennett.14 OP is the amount of oxygen delivered per heartbeat, which differs among turtles, snakes, and lizards according to their body temperature, with values ranging between 1 and 4 mL O2 × 10−5/g/beat. In turtles, as body temperature increased, so did OP,15 whereas in lizards16 and snakes17 OP decreased with increasing body temperature. Therefore, species differences and habitat affect emergency treatment such as warming or not warming a reptile in shock.
Oxygen use by the tissues, termed Vo 2, has a direct association with the amount of oxygen being delivered and extracted by cells. The ratio of arterial oxygen delivery to oxygen consumption is the oxygen extraction ratio (o 2ER) (see Fig. 1). Approximately 20% to 30% of the oxygen delivered to tissues is extracted in mammals under normal conditions.18 During periods of increased oxygen need caused by changes in metabolism, such as fever, the tissues can extract more oxygen. This ability also occurs during times when there is diminished oxygen delivery caused by hypovolemia, bradycardia, dyshemoglobinemia, pulmonary disease, and so forth. Therefore, the cells, and specifically the mitochondria of cells, can still produce enough ATP despite diminished hemoglobin saturation and lowered cardiac output by extracting more oxygen.
During exercise, acute hypoxia, and acute anemia, Do 2 is maintained by increasing cardiac output.19 However, once a critical point is reached, the tissues can no longer extract enough oxygen unless more is delivered. For example, you are stocking up on water because of an impending hurricane. You fill up a whole shopping cart with bottles of water. Because you have a big family, you need more than 1 cart-full, so you fill another cart. Soon, the bottled water shelves become sparse. The only way you can extract more water (ie, put more in the cart) is if more is delivered and stocked on the shelves.
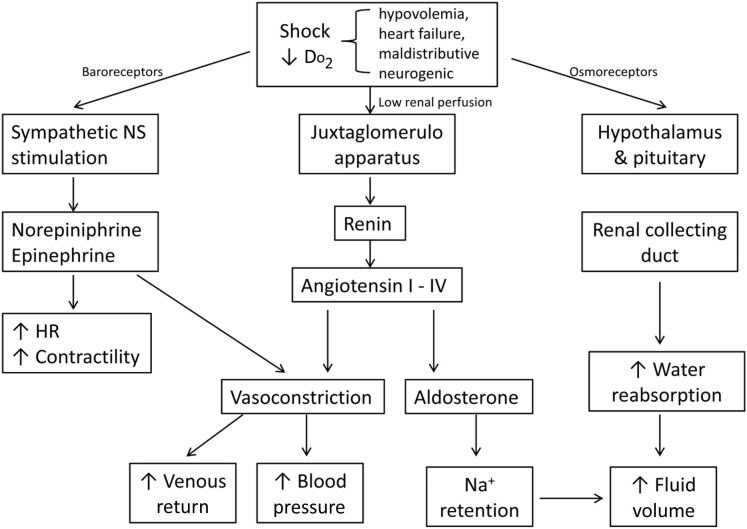
During states in which the tissues and cells cannot extract enough oxygen and more must be delivered, anaerobic metabolism ensues. This critical point is termed oxygen supply dependency.20 It is at this point that symptoms of shock are seen; a response that tries to increase oxygen delivery. Physiologic changes that occur include tachycardia, tachypnea, and vasoconstriction of peripheral and splanchnic vessels. This condition is shown in Fig. 2 ; once the Do 2 cannot be maintained at greater than or equal to twice the level of Vo 2, biochemical changes associated with shock, such as hyperlactatemia and acidemia, ensue. Not only does inadequate Do 2 result from hypovolemia, pulmonary disease, and dyshemoglobinemia but it can also be caused by altered cellular function, as seen with sepsis and mitochondrial dysfunction in the critically ill. During such insults, the cell’s machinery, cell membrane, protein receptors, and genes become dysfunctional, leading to apoptosis and loss of integrity.21
Fig. 2.
Compensatory mechanisms of shock.
Nonmammalian species such as reptiles and birds have different values for Do 2, Vo 2, and o 2ER because of slower or faster heart rates, hemoglobin concentration, and saturation of hemoglobin. Reptiles, depending on the species, balance oxygen delivery during exercise differently. Iguanas maintain Do 2 primarily by increasing heart rate because stroke volume decreases by 20% from a resting state during exercise. However, Varanus spp such as monitor lizards increase stroke volume and heart rate to normalize oxygen delivery during exercise.22 Reptiles also tend to have lower oxygen saturation of arterial blood. Most reptiles have Sao 2 of 70% to 90%, with turtles at the higher end of the range and lizards at the lower end.23 Reptiles differ from birds and mammals in their higher reliance on anaerobic metabolism, which can make evaluation of the importance of oxygenation and perfusion more challenging. Another feature that affects reptile oxygen dynamics is blood shunting. Reptilian hearts (other than those of the Crocodylia) have an incomplete interventricular septum. In these animals, both left-to-right shunting and right-to-left shunting can occur. With intracardiac left-to-right shunting, areas of the myocardium that are normally oxygen poor receive more oxygen.24 Reduction of right-to-left shunting of deoxygenated blood leads to an increase in oxygen carrying capacity. It is thought that alteration of the shunt fraction is controlled by changes in physiologic conditions. This change, along with a right-shift to the oxygen dissociation curve, maintains oxygen delivery in reptiles that have been studied.25 Studies in turtles have shown right-to-left shunting facilitates warming while left-to-right shunting improves systemic oxygen transport. Left-to-right shunting is partly responsible for increasing myocardial oxygenation, which is needed for the tachycardic response to hemorrhage.26
Pathophysiology of shock
During episodes of decreased tissue oxygenation, or shock, cells try to maintain homeostasis by altering their metabolism to create ATP anaerobically via glycolysis. It is thought that, in mammals, the stimulus for anaerobic glycogenolysis is not oxygen deficiency but stimulation of sarcolemmal Na+/K+/ATPase secondary to stress hormone release (ie, epinephrine).27 Stimulation of the sympathetic nervous system (SNS) occurs through baroreceptor-mediated response to poor cardiac output and decreased tissue oxygen delivery.28 The increase of sympathetic activity results in tachycardia, increased contractility, and vasoconstriction. Fluid shifts from the interstitial and extravascular space to the intravascular space occur following the Starling law, especially during episodes of diminished hydrostatic pressure.28 Activation of the rennin-angiotensin-aldosterone system (RAAS) also occurs because of reduced renal perfusion and also sympathetic system–induced vasoconstriction of the splanchnic vasculature. RAAS activation results in sodium and water retention, vasoconstriction, more upregulation of the sympathetic response, and release of antidiuretic hormone (ADH).29 The purpose of RAAS activation is to increase effective circulating volume, increase cardiac output, and shunt blood to the heart and brain. A summary of compensatory mechanisms is outlined in Fig. 2.
Shock stages
Compensatory Shock
The stimulation of the SNS and release of RAAS and ADH result in classic clinical signs of dull mentation, pallor, and tachycardia. During this stage, capillary refill time may be normal and blood pressure is preserved.30
Decompensatory Shock
If perfusion, effective circulating volume, cardiac output, and/or oxygen delivery do not improve from compensatory mechanisms, additional symptoms of prolonged capillary refill, poor pulse quality, and hypotension occur. As poor perfusion persists, the eventual result of this phase is unresponsive hypotension.31 This final phase of decompensatory shock results from hemodynamic collapse of the autoregulatory escape mechanism. As organs are starved of oxygen, fluid, and nutrients, arterioles dilate to increase flow. This process is a global phenomenon that leads to systemic hemodynamic collapse and death.32 Intervention (fluid therapy, oxygen supplementation, pressor drugs, transfusion, and so forth) does not reverse this reflexive mechanism.
Systemic inflammatory response syndrome
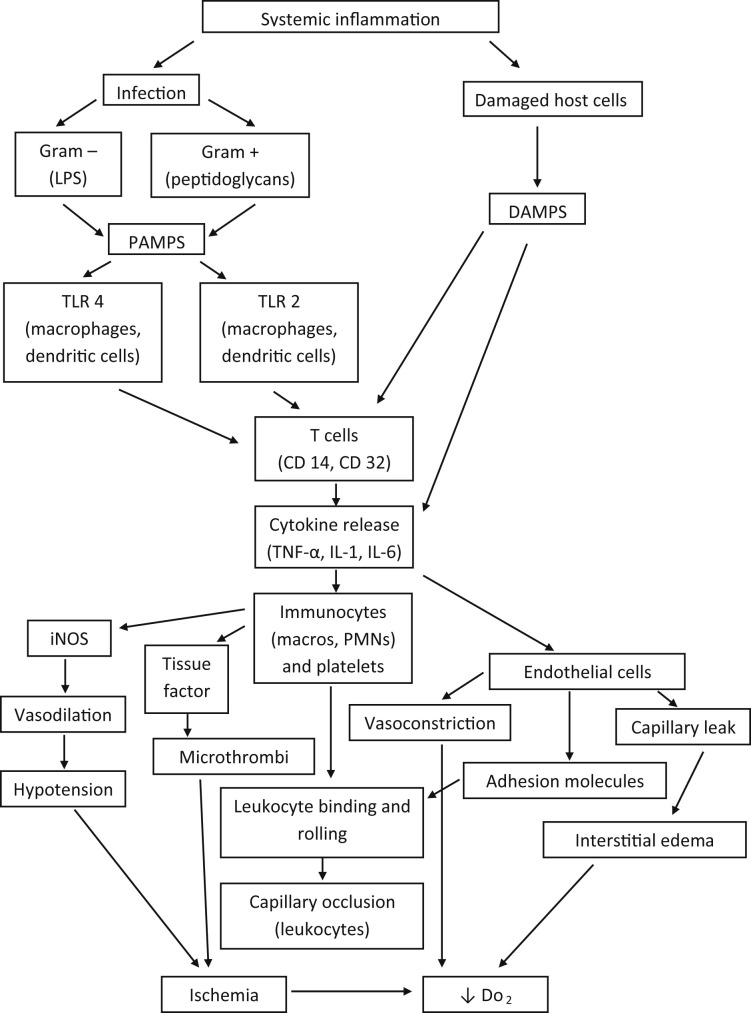
As previously defined, SIRS is a state in which there is clinical and clinicopathologic evidence of systemic inflammation, characterized by tachycardia, tachypnea, hypothermia or hyperthermia, and leukocytosis or leukopenia. Insults causing SIRS, such as trauma, neoplasia, immune-mediated disease, can progress to cause shock, multiple organ dysfunction, and MOF.33 The underlying cause of SIRS is the immune system’s lack of balance between the activators of inflammation and inhibitors of inflammation. If the balance is tipped in either direction, organ damage occurs. Injury to organs occurs from activated leukocytes’ release of damaging cytoplasmic granules, microcirculatory ischemia from leukocyte obstruction, altered apoptosis, and production of reactive oxygen species.34 Infection causing sepsis is the classic example of SIRS and results in the production of inflammatory mediators, called cytokines, such as tumor necrosis factor-alpha, interleukin-1, and interleukin-6. Cytokines are produced by immunocytes, especially B lymphocytes, T lymphocytes, and macrophages.35 There are more than 20 different cytokines and many more non–immunocyte-produced inflammatory proteins, such as platelet activating factor, interferons, and products of the arachidonic acid cascade.36 These inflammatory mediators bind to receptors of endothelial cells, other immunocytes such as neutrophils, and mast cells. Effects of cytokine binding include alteration of vascular permeability, increased chemotaxis of immunocytes, and activation of the coagulation cascade.
To limit this robust inflammatory response, many of the same cells that produce inflammatory cytokines also produce antiinflammatory molecules known as the compensatory antiinflammatory response syndrome (CARS). Antiinflammatory mediators include interleukin 10, Transforming growth factor-beta, and interleukin-13.37 SIRS and CARS are balanced to produce enough inflammation to respond to an insult, such as an infection or a neoplasm, whereby the inflammatory mediators attack the infectious agent/neoplastic cell and activate the complement cascade while sparing noninfected/nonneoplastic tissue. If the inflammatory response overwhelms the counteractive antiinflammatory response, SIRS progresses, causing organ damage, cellular apoptosis, and a hypermetabolic state. In contrast, if the antiinflammatory response is too forceful, a syndrome of immunoparalysis develops, increasing the likelihood of dying of something like a simple infection.38
Pathogen recognition through receptor activation is a key factor in how the immune system responds to infection and targets the organism. Receptors against pathogens are expressed by immunocytes and other cells, such as the endothelium. Targets of pathogen recognition are called pathogen-associated molecular patterns (PAMPs). These signaling molecules are produced by microbial pathogens and are structures that give the pathogen the ability to survive and cause disease. The classic example of a PAMP is lipopolysaccharide from gram-negative bacteria. PAMPs bind to many different receptors, the largest class being Toll-like receptors, of which there are more than 10 described in the literature.39 Once bound to a pattern recognition receptor, internal signaling pathways result in a change of cellular metabolism, protein production, or other cell activation, such as chemotaxis. PAMP production is influenced by genetic factors and aging. In dogs, one study noted that the cytokine response to PAMPs alters with age and is likely a contributing factor to morbidity in geriatric dogs with gram-negative sepsis.40
Danger-associated molecular patterns (DAMPs) are similar to PAMPs. These molecules also can initiate and perpetuate the inflammatory response by binding to many of the same receptors of PAMPs found on leukocytes. The main difference is that DAMPs are proteins and molecules from host cells, not the pathogen. DAMPs constitute molecules from the plasma membrane, endoplasmic reticulum, nucleus, and cytosol from apoptotic cells and damaged cells.41 For this reason, noninfectious sources of injury, such as trauma, pancreatitis, and heat stroke, can lead to SIRS.
Another organ system heavily involved in the pathophysiology of SIRS is the endothelial system. Activation of endothelial cells from trauma, infection, or inflammation results in expression of receptors (P and L selectins, endothelial cell immunoglobulin adhesion molecules).42 Leukocytes, namely neutrophils, bind to expressed endothelial receptors, adhering to the wall of the vessel lumen. As this process continues with more neutrophils binding and rolling, microvascular flow can be disrupted, causing changes in capillary integrity and vascular tone.43 In addition, cytokines cause changes in vascular tone, resulting in contraction of arteries but not venules. This process, mediated by tumor necrosis factor-alpha, interleukin-6, and other cytokines, modifies the blood supply to ischemic and inflamed tissues.44 Interstitial edema, especially that of the pulmonary parenchyma, occurs as the result of cytokines and inducible nitric oxide which is a potent vasodilator. Several studies have found that acute lung inflammation, known as acute respiratory distress syndrome, is in part caused by activation of immunocytes, their production of cytokines, and the response by the pulmonary microvascular system.45, 46 This type of edema, termed capillary leak syndrome, not only affects the lungs but also other organ systems, causing interstitial edema, which makes it more difficult to deliver oxygen from the capillaries to their target cells. As this process progresses, multiple organ system failure ensues.47
The coagulation system is also altered during the SIRS cascade. Tissue factor expression by monocytes, and its expression from activated platelets and endothelial cell microparticles, results in thrombin formation. Thrombin formation leads to additional thrombin activation and the final formation of cross-linked fibrin.48 Fibrin and activated platelets form thrombi in the microvasculature, disrupting blood flow and oxygen delivery. Protection against thrombin formation is overwhelmed during states of SIRS, exhausting stores of endogenous anticoagulants such as activated protein C, antithrombin, and protein S. As this process becomes more systemic, no longer isolated to the site of inflammation or infection, disseminated intravascular coagulopathy (DIC) occurs. DIC is an independent predictor of mortality and its diagnosis is often a poor prognostic sign.49
An overview of some of the pathways of SIRS, with resulting effects on target organs, is provided in Fig. 3 .
Fig. 3.
SIRS cascade. IL, interleukin; iNOS, inducible nitric oxide; Macro, macrophage; PAMPS, pathogen-associated molecular pattern; PMNs, polymorphonuclear leukocytes; TNF-α, tumor necrosis factor-alpha.
Clinically, 2 common effects of poor perfusion and the subsequent sympathetic response are alteration of the gastrointestinal tract and the renal system. When there is significant hypovolemia, trauma, or sepsis with resultant sympathetic-induced vasoconstriction, the splanchnic vasculature is affected,50 leading to ischemic injury and loss of the protective gastrointestinal barrier, which is primarily sustained because of normal capillary mucosal blood flow. Once the barrier is compromised, commensal pathogenic bacteria have the ability to increase in number and translocate. Primarily, translocation occurs via lymphatic vessels but can also occur through the blood supply via the portal system.51 This process is one reason whereby patients who present for trauma or hypovolemic shock from an inflammatory, noninfectious process can become septic without being inoculated with bacteria.
The main effect of poor perfusion on the renal system is a decrease in glomerular filtration rate (GFR). This phenomenon is now termed acute kidney injury, in contrast with the older terminology of acute renal failure.52 However, traditional biochemical methods of estimating GFR using blood urea nitrogen and creatinine concentrations do not alert clinicians to the injury until 75% of functioning nephrons are no longer working. In humans, sepsis accounts for 50% of acute kidney injury and some degree of acute kidney injury is documented in up to 20% of persons hospitalized in intensive care units.53 Acute kidney injury has been described in birds with both viral and bacterial infections.54 Small mammals, mainly rodents, have served as models of acute kidney injury secondary to sepsis for some time. It is well recognized that sepsis and septic shock induced through cecal ligation result in renal injury.55 Although chronic renal failure is more commonly reported in reptiles, acute renal injury has been reported and is more likely to occur because of injected medications to the hind limbs. The reptile renal-portal system allows circulation from the hind limbs and tail to traverse directly to the kidneys.
Species differences of shock and systemic inflammatory response syndrome
Mammals
Overall, there is a paucity of clinical data regarding shock and SIRS in the clinical setting. Much of the shock research on pocket pets, rabbits, and rodents are controlled experiments. Rodents and rabbits have served as research animals in this field of study for many decades, and there are numerous reports inducing shock, sepsis, and SIRS in rats, mice, and rabbits.56, 57, 58 In rats, typical increases in heart rate and sympathetic response occur during sepsis and shock, characterized by tachycardia and decreased mean arterial pressure.59 Rats also develop hypothermia associated with shock, similar to dogs, cats, and humans.60 Hamsters have been used to study sepsis and, similar to rats in shock, develop hypotension and leukocyte activation with vessel margination.61 Similarly, in a ferret model of SARS –coronavirus, hyperthermia associated with viremic sepsis progressing to hypothermia associated with decompensatory shock was described. That same study also showed leukopenia in moribund ferrets on day 2 after infection.62 One study of rabbits that underwent experimental hemorrhagic shock by removing 26% of blood volume noted tachycardia, hypotension, and significantly decreased cardiac output versus controls.63 That same study, by Chalmers and colleagues,63 which compared normal rabbits with adrenalectomized rabbits, noted that the sympathetic response in normal animals minimized reductions in CO and blood pressure. Contrasting this research, clinical reports note the difficulty in resuscitating hypotensive rabbits and small mammals because of vagal stimulation, which becomes active simultaneously with sympathetic activity.64 Rabbits in an uncontrolled hemorrhage shock study required 123 to 133 mL/kg of crystalloid fluid in attempts to control hypotension.65 The aforementioned dose is significantly higher than crystalloid shock dose recommendations for lagomorphs in the literature, which are 15 mL/kg or initial boluses of 3 to 5 mL/kg.66
Reptiles
There is limited experimental research on hypovolemia and its effects on cardiovascular measurements in reptiles. Reptiles have vascular adaptations allowing blood shunting, known as the renal-portal system. During periods of dehydration and prolonged hypovolemia, blood from their hind limbs and tail traverses to the afferent renal portal veins, preserving renal perfusion.67 One study noted that, during acute hemorrhage, snakes were able to maintain their blood volume by restoring up to 90% of deficit within 2 hours of hemorrhage by shifting extravascular fluid to the intravascular space.68 Although snakes are able to restore their circulating volume after hemorrhage, their autonomic response is minor and does not allow compensation of hypotension.69 Because of their body configuration, environmental temperature effects on metabolism, and cardiovascular anatomy (3-chambered heart), snakes have highly variable blood pressure. Depending on their habitat (arboreal vs terrestrial vs aquatic), systemic blood pressures in snakes correspond with gravitational stress. Arboreal snakes have higher arterial pressures than aquatic snakes. Blood pressure in these species also depends on body mass, with larger snakes having higher blood pressure.70
Turtles also have temperature and habitat influences on their cardiovascular system. Turtles that underwent controlled hemorrhage had significantly increased heart rates and hypotension. In that same study, turtles were able to restore prehemorrhage blood volume 2 hours after hemorrhage, which is similar to snakes.71 The ability of turtles to recover from hemorrhage is not highly dependent on vasoconstriction but depends on increased heart rate. Baroreceptor response occurs in these species, resulting in tachycardia and increased contractility. Catecholamine effects on their vasculature causes accelerated resorption of interstitial fluid versus increased pressure.72
Lizards, specifically the green iguana, have undergone similar controlled hemorrhage studies to determine cardiovascular responses. Progressive hemorrhage resulted in tachycardia, decreased femoral blood flow, and hypotension. Changes in body position affect arterial pressure, and passive, head-up tilting induce reflexive cardiovascular changes to regulate blood pressure.73 Hemorrhage and subsequent clot formation takes significantly longer in iguanas than in mammals. Higher temperatures have been shown to improve blood clotting in iguanas.74
Birds
Much of the scientific literature discussing hypotension and shock in avian species uses poultry as models. One article compared fluid types for resuscitation in leghorn chickens. That study only used colloidal-type fluids (hetastarch) and a hemoglobin-based oxygen carrier (HBOC) (Hemospan) to resuscitate chickens that had undergone experimentally induced hemorrhage of 50% of their blood volume. Surprisingly, there were no significant differences found between those two fluids and autotransfusion with respect to heart rate, respiratory rate, and systolic blood pressure.75 In a similar study by Lichtenberger and colleagues,76 mallard ducks underwent controlled hemorrhage and fluid resuscitation with either crystalloid (Plasmalyte A), colloid (Hetastarch), or an HBOC (Oxyglobin). Tachycardia was not noted until 25% to 45% of blood volume was lost. There was no statistical difference in mortality among the different types of fluid. However, Oxyglobin is no longer commercially available.
Birds tolerate hypovolemia secondary to hemorrhage very well. Clinical signs of anemia and shock are not seen until 50% to 60% of blood volume is lost. In pigeons, loss of 60% of blood volume resulted in no clinical signs and their hematocrits returned to normal within 7 days. The ability of birds to tolerate such large volumes of blood loss is thought to be caused by 3 adaptations. There is rapid extravascular fluid resorption from muscles that have a large capillary surface area, which then replenishes the intravascular deficit. In addition, birds have blunted autonomic response, resulting in fewer clinical signs of tachycardia, tachypnea, and hypothermia. The ability to mobilize large numbers of immature red blood cells is a third adaptation to hemorrhagic shock that birds possess.77 This unique response to hemorrhage was also noted in a study that compared the hemodynamics of hemorrhage in chicks with that of rats. Controlled hemorrhage caused significant reduction of mean arterial pressure and cardiac output in rats, by 23% and 43% respectively. In the chicks, mean arterial pressure was only reduced by 15% and cardiac output reduced by 4% because of their ability to maintain stroke volume. The investigators concluded that chicks were able to maintain mean arterial pressure independently of changes in peripheral vascular tone.78
Summary
In conclusion, most of the literature discussing shock, systemic inflammation, and sepsis relates to experimental studies using small mammals such as rodents and rabbits. There is a paucity of research of these syndromes in other species such as ferrets, birds, and reptiles. Although small mammals, experimentally, have a similar autonomic response to dogs during induced hemorrhage and shock states, that response is not noted in the clinical setting, in which decompensated shock is more commonly reported. Poor perfusion affects every major organ system, but particular attention should be given to the immune system, coagulation system, cardiovascular system, gastrointestinal tract, and kidneys. Knowing how these systems are affected helps to explain to clinicians why patients with shock can later become septic or coagulopathic. To ensure the best outcomes in patients who have shock or SIRS, early recognition and intervention by provision of supplemental oxygen and fluid resuscitation is essential. Treatment to combat shock in exotic species is discussed elsewhere in this issue.
Footnotes
Disclosure: The author has nothing to disclose.
References
- 1.Marino P. The ICU book. Lippincott Williams & Wilkins; Philadelphia: 2007. Tissue oxygenation; pp. 193–207. [Google Scholar]
- 2.Mazzaferro E. Oxygen therapy. In: Silverstein D.C., Hopper K., editors. Small animal critical care medicine. 2nd edition. Elsevier; St Louis (MO): 2014. pp. 77–80. [Google Scholar]
- 3.Klabunde RE. Cardiovascular physiology concepts. Available at: http://www.cvphysiology.com/CAD/CAD003.htm. Accessed November 9, 2015.
- 4.Marino P. The ICU book. Lippincott Williams & Wilkins; Philadelphia: 2007. Inflammation and infection in the ICU; pp. 737–747. [Google Scholar]
- 5.Rangel-Frausto M.S., Pittet D., Costigan M. The natural history of the systemic inflammatory response syndrome: a prospective study. JAMA. 1995;273(2):117. [PubMed] [Google Scholar]
- 6.Byers CG. Principles of fluid therapy in sepsis & SIRS in dogs & cats. In: American College of Veterinary Internal Medicine (ACVIM) Proceedings 2014. Nashville (TN): 2014.
- 7.Jackubrovsky J., Brozman M., Chorváth D. A comparative study of the morphology of the systemic anaphylactic reaction (SAR) and the shock reaction induced by antigen-antibody complexes in rabbits. Virchows Arch A Pathol Anat Histol. 1981;394(1–2):97–108. doi: 10.1007/BF00431668. [DOI] [PubMed] [Google Scholar]
- 8.Jackubrovsky J., Brozman M. Morphology of haemorrhagic shock, systemic immunocomplex reaction and systemic anaphylactic reaction. A comparative study. Czech Med. 1984;7(4):215–237. [PubMed] [Google Scholar]
- 9.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 10.Geroulanos S., Douka E.T. Historical perspective of the word “sepsis”. Intensive Care Med. 2006;32:2077. doi: 10.1007/s00134-006-0392-2. [DOI] [PubMed] [Google Scholar]
- 11.Osterbur K., Mann F.A., Kuroki K. Multiple organ dysfunction syndrome in humans and animals. J Vet Intern Med. 2014;28:1141–1151. doi: 10.1111/jvim.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmunds N.J., Marshall J.M. Vasodilatation, oxygen delivery and oxygen consumption in rat hindlimb during systemic hypoxia: roles of nitric oxide. J Physiol. 2001;532(1):251–259. doi: 10.1111/j.1469-7793.2001.0251g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuyama S., Hayakawa K. Vasodilating prostaglandin E1 does not reproduce interleukin-1B-induced oxygen metabolism abnormalities in rabbits. Acute Med Surg. 2015;2:40–47. doi: 10.1002/ams2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleeson T.T., Bennett A.F. Respiratory and cardiovascular adjustments to exercise in reptiles. In: Raymond G., editor. Circulation, respiration, and metabolism: current comparative approaches. Springer-Verlag; Berlin: 1985. pp. 34–35. [Google Scholar]
- 15.Gatten R.E., Jr. Effects of temperature and activity on aerobic and anaerobic metabolism and heart rate in the turtles Pseudemys scripta and Terrapene ornate. Comp Biochem Physiol. 1974;8A:619–648. doi: 10.1016/0300-9629(74)90606-9. [DOI] [PubMed] [Google Scholar]
- 16.Bennet A.F. Blood physiology and oxygen transport during activity in two lizards, Varanus gouldii and Sauromalus hispidus. Comp Biochem Physiol. 1973;46A:673–690. doi: 10.1016/0300-9629(73)90120-5. [DOI] [PubMed] [Google Scholar]
- 17.Dmi’el R., Borut A. Thermal behavior, heat exchange and metabolism in the desert snake Spalerosophis cliffordi. Physiol Zool. 1972;223(3):510–516. [Google Scholar]
- 18.McLellan S.A., Walsh T.S. Oxygen delivery and haemoglobin. Crit Care Pain. 2004;4(4):123–126. [Google Scholar]
- 19.Bartlett R.H. Critical care physiology. Little, Brown and Co; 1996. Oxygen kinetics; pp. 1–23. [Google Scholar]
- 20.Bakker J., Vincent J.-L. The oxygen supply dependency phenomenon is associated with increased blood lactate levels. J Crit Care. 1991;6(3):152–159. [Google Scholar]
- 21.Rocha L.L., Pessoa C.M., Corrêa T.D. Current concepts on hemodynamic support and therapy in septic shock. Rev Bras Anestesiol. 2015;65(5):395–402. doi: 10.1016/j.bjan.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson T.T. Metabolic recovery from exhaustive activity by a large lizard. J Appl Physiol Respir Environ Exerc Physiol. 1980;48(4):689–694. doi: 10.1152/jappl.1980.48.4.689. [DOI] [PubMed] [Google Scholar]
- 23.Plough F.H. Summary of oxygen transport characteristics of reptilian blood. Smithson Herp Inf Serv. 1979;45:1–18. [Google Scholar]
- 24.Farmer C.G., Hicks J.W. The intracardiac shunt as a source of myocardial oxygen in the turtle, Trachemys scripta. Integr Comp Biol. 2002;42:208–215. doi: 10.1093/icb/42.2.208. [DOI] [PubMed] [Google Scholar]
- 25.Wood S.C. Effect of O2 affinity on arterial PO2 in animals with central vascular shunts. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1360–1364. doi: 10.1152/jappl.1982.53.6.1360. [DOI] [PubMed] [Google Scholar]
- 26.Farmer C.G., Hicks J.W. The intracardiac shunt as a source of myocardial oxygen in a turtle, Trachemys scripta. Integr Comp Biol. 2002;42:208–215. doi: 10.1093/icb/42.2.208. [DOI] [PubMed] [Google Scholar]
- 27.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care. 2006;12(4):315–321. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 28.Hopper K., Silverstein D., Bateman S. Shock syndromes. In: Dibartola, editor. Fluid, electrolyte and acid-base disorders in small animal practice. 4th edition. Elsevier; St Louis (MO): 2012. pp. 557–561. [Google Scholar]
- 29.De Laforcade A., Silverstein D. Shock. In: Silverstein D., Hopper K., editors. Small animal critical care medicine. 2nd edition. Elsevier; St Louis (MO): 2015. pp. 26–27. [Google Scholar]
- 30.Garreston S., Malberti S. Understanding hypovolaemic, cardiogenic and septic shock. Nurs Stand. 2007;50(21):46–55. doi: 10.7748/ns2007.08.21.50.46.c4608. [DOI] [PubMed] [Google Scholar]
- 31.Bonanno F.G. Clinical pathology of the shock syndromes. J Emerg Trauma Shock. 2011;4(2):233–243. doi: 10.4103/0974-2700.82211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raffe M.R., Wingfield W. Hemorrhage and hypovolemia. In: Raffe M.R., Wingfield W., editors. The veterinary ICU book. Teton NewMedia; Jackson (WY): 2002. p. 455. [Google Scholar]
- 33.De Laforcade A. Systemic inflammatory response syndrome. In: Silverstein D., Hopper K., editors. Small animal critical care medicine. 2nd edition. Elsevier; St Louis (MO): 2015. pp. 30–31. [Google Scholar]
- 34.Binkowska A.M., Michalak G., Słotwiński R. Current views on the mechanisms of immune responses to trauma and infection. Cent Eur J Immunol. 2015;40(2):206–216. doi: 10.5114/ceji.2015.52835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund F.E. Cytokine-producing B lymphocytes – key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J.-M., An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adib-Conquy M., Cavaillon J.M. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 38.Ward N.S., Casserly B., Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29(4):617–625. doi: 10.1016/j.ccm.2008.06.010. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis D.H., Chan D.L., Pinheiro D. The immunopathology of sepsis: pathogen recognition, systemic inflammation, the compensatory anti-inflammatory response, and regulatory T cells. J Vet Intern Med. 2012;26:457–482. doi: 10.1111/j.1939-1676.2012.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deischel S.J., Kerl M.E., Chang C.H. Age-associated changes to pathogen-associated molecular pattern-induced inflammatory mediator production in dogs. J Vet Emerg Crit Care (San Antonio) 2010;20(5):494–502. doi: 10.1111/j.1476-4431.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 41.Krysko D.V., Agostinis P., Krysko O. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32(4):157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Granger N.D., Senchenkova E. Inflammation and the microcirculation. Morgan and Claypool Life Sciences; San Rafael (CA): 2010. Leukocyte-endothelial cell adhesion. Chapter 7. [PubMed] [Google Scholar]
- 43.Hatakeyama N., Matsuda N. Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharm Des. 2014;20(4):5766–5778. doi: 10.2174/138161282036140912122809. [DOI] [PubMed] [Google Scholar]
- 44.Iversen P.O., Nicolaysen A., Kvernebo K. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflugers Arch. 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- 45.Abraham E., Araroli J., Carmody A. Cutting edge: HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 46.Abraham E., Bursten S., Shenkar R. Phosphatidic acid signaling mediates lung cytokine expression and lung inflammatory injury after hemorrhage in mice. J Exp Med. 1995;181(2):569–575. doi: 10.1084/jem.181.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su J., Zhan Y., Hu W. The current opinions of capillary leak syndrome. J Clin Diag. 2015;5:14–19. [Google Scholar]
- 48.Choi G., Schultz M.J., Levi M. The relationship between inflammation and the coagulation system. Swiss Med Wkly. 2006;136:139–144. doi: 10.4414/smw.2006.11059. [DOI] [PubMed] [Google Scholar]
- 49.Gando S., Kameue T., Nanzaki S. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb Haemost. 1996;75(2):224–228. [PubMed] [Google Scholar]
- 50.Fink M.P. Gastrointestinal mucosal injury in experimental models shock, trauma, and sepsis. Crit Care Med. 1991;19(5):627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Magnotti L.J., Upperman J.S., Xu D.Z. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228(4):518–524. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IRIS Guidelines. Available at: www.iris-kidney.com.
- 53.Wan L., Bagshaw S.M., Langenberg C. Pathophysiology of septic acute kidney injury: what do we really know. Crit Care Med. 2008;36(4):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 54.Eisbruch A., Zevin D., Djaldetti M. Acute renal failure due to combined infection with psittacosis and salmonellosis. Harefuah. 1981;100(10):460–461. [PubMed] [Google Scholar]
- 55.Zhou F., Zhi-yon P., Bishop J.V. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42(4):e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowie H.G., Stevenson J.A. A standardized method for the production of hemorrhagic shock in the rat. Can J Biochem Physiol. 1955;33(3):436–437. [PubMed] [Google Scholar]
- 57.Millican R.C., Tabor H., Rosenthal S.M. Traumatic shock in mice: comparison of survival rates following therapy. Am J Physiol. 1952;170(1):187–195. doi: 10.1152/ajplegacy.1952.170.1.179. [DOI] [PubMed] [Google Scholar]
- 58.Fine J., Rutenbug S., Schweinburg F.B. The role of the reticulo-endothelial system in hemorrhagic shock. J Exp Med. 1959;110:547–569. doi: 10.1084/jem.110.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palsson J., Ricksten S.E., Delle M. Changes in renal sympathetic nerve activity during experimental septic and endotoxin shock in conscious rats. Circ Shock. 1988;24(2):133–141. [PubMed] [Google Scholar]
- 60.Stoner H.B., Little R.A. Studies on the mechanism of shock. The effect of catecholamines on the temperature response to injury in the rat. Br J Exp Pathol. 1969;50:107–124. [PMC free article] [PubMed] [Google Scholar]
- 61.Villela N.R., Maia Teixeira dos Santos A.O., de Miranda M.L. Fluid resuscitation therapy in endotoxemic hamsters improves survival and attenuates capillary perfusion deficits and inflammatory responses by a mechanism related to nitric oxide. J Transl Med. 2014;12:232. doi: 10.1186/s12967-014-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu Y.-K., Ali G.D., Jia F. The SARS-COV ferret model in an infection-challenge study. Virology. 2008;374(1):151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chalmers J.P., Korner P.I., White S.W. The effects of haemorrhage in the unanaesthetized rabbit. J Physiol. 1967;189:367–391. doi: 10.1113/jphysiol.1967.sp008174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lichtenberger M. Monitoring and treatment of hypovolemic shock in small mammals. In: Proceedings of the SCIVAC. Rimini (Italy): 2007. p. 323–4.
- 65.Rezende-Neto J.B., Rizoli S.B., Andrade M.V. Rabbit model of uncontrolled hemorrhagic shock and hypotensive resuscitation. Braz J Med Biol Res. 2010;43(12):1153–1159. doi: 10.1590/s0100-879x2010007500127. [DOI] [PubMed] [Google Scholar]
- 66.Lennox AM. Emergency and critical care in exotic companion mammals. SCIVAC International Congress. 2012. Rimini (Italy).
- 67.Holz P.H. The reptilian renal portal system – a review. Bull Assoc Reptil Amphib Vet. 1999;9(1):4–9. [Google Scholar]
- 68.Smits A.W., Lillywhite H.B. Maintenance of blood volume in snakes: transcapillary shifts of extravascular fluids during acute hemorrhage. J Comp Physiol B. 1985;155(3):305–310. doi: 10.1007/BF00687472. [DOI] [PubMed] [Google Scholar]
- 69.Lillywhite H.B., Pough F.H. Control of arterial pressure in aquatic sea snakes. Am J Physiol. 1983;244(1):R66–R73. doi: 10.1152/ajpregu.1983.244.1.R66. [DOI] [PubMed] [Google Scholar]
- 70.Mosley CA. Anatomic and physiologic considerations for reptile anesthesia. In: Proceedings of the North American Veterinary Conference. Orlando (FL): 2006. p. 1643–5.
- 71.Smits A., Kozumbowski M.M. Partitioning of body fluids and cardiovascular responses to circulatory hypovolaemia in the turtle, Pseudemys scripta elegans. J Exp Biol. 1985;116:237–250. doi: 10.1242/jeb.116.1.237. [DOI] [PubMed] [Google Scholar]
- 72.Millard R.W., Moalli R. Baroreflex sensitivity in an amphibian, Rana catesbeiana, and a reptilian, Pseudemys scripta elegans. J Exp Zool. 1980;213:283–288. doi: 10.1002/jez.1402130216. [DOI] [PubMed] [Google Scholar]
- 73.Hohnke L.A. Regulation of arterial blood pressure in the common green iguana. Am J Physiol. 1975;228(2):386–391. doi: 10.1152/ajplegacy.1975.228.2.386. [DOI] [PubMed] [Google Scholar]
- 74.Kubalek S., Mischke R., Fehr M. Investigations on blood coagulation in the green iguana (Iguana iguana) J Vet Med A Physiol Pathol Clin Med. 2002;49:210–216. doi: 10.1046/j.1439-0442.2002.00431.x. [DOI] [PubMed] [Google Scholar]
- 75.Wernick M.B., Steinmetz H.W., Martin-Jurado O. Comparison of fluid types for resuscitation in acute hemorrhagic shock and evaluation of gastric luminal and transcutaneous PCO2 in Leghorn chickens. J Avian Med Surg. 2013;27(2):109–119. doi: 10.1647/2012-018. [DOI] [PubMed] [Google Scholar]
- 76.Lichtenberger M., Orectt C., Cray C. Comparison of fluid types for resuscitation after acute blood loss in mallard ducks (Anas platyrhynchos) J Vet Emerg Crit Care (San Antonio) 2009;19(5):467–472. doi: 10.1111/j.1476-4431.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 77.Morrisey JK. Practical hematology and transfusion medicine in birds. Western Veterinary Conference. 2013, Indianapolis (IN).
- 78.Ploucha J.M., Fink G.D. Hemodynamics of hemorrhage in the conscious rat and chicken. Am J Physiol. 1986;251(5):R846–R850. doi: 10.1152/ajpregu.1986.251.5.R846. [DOI] [PubMed] [Google Scholar]