Abstract
Feline orofacial pain syndrome (FOPS) is a pain disorder of cats with behavioural signs of oral discomfort and tongue mutilation. This report describes the findings from a case series of 113 cats including 100 Burmese. FOPS is suspected to be a neuropathic pain disorder and the predominance within the Burmese cat breed suggests an inherited disorder, possibly involving central and/or ganglion processing of sensory trigeminal information. The disease is characterised by an episodic, typically unilateral, discomfort with pain-free intervals. The discomfort is triggered, in many cases, by mouth movements. The disease is often recurrent and with time may become unremitting — 12% of cases in this series were euthanased as a consequence of the condition. Sensitisation of trigeminal nerve endings as a consequence of oral disease or tooth eruption appears to be an important factor in the aetiology — 63% of cases had a history of oral lesions and at least 16% experienced their first sign of discomfort during eruption of permanent teeth. External factors can also influence the disease as FOPS events could be directly linked to a situation causing anxiety in 20% of cats. FOPS can be resistant to traditional analgesics and in some cases successful management required anti-convulsants with an analgesic effect.
Feline orofacial pain syndrome (FOPS) is a condition characterised by signs of acute oral discomfort and mutilation. It was first recognised in the early 1990s 1 and has been described predominantly in Burmese cats. 2 Affected cats are most commonly presented with exaggerated licking and chewing movements, and pawing at the mouth. More severe cases have mutilation of tongue, lips and buccal mucosa (Figs 1 and 2). Neurological examination of affected cats is unremarkable; in particular there are no apparent motor or sensory trigeminal deficits. Discomfort appears to be confined to one side of the oral cavity and lips. 3 The cat remains alert and can be distracted, although with considerable difficulty in some cases. 3 The cat may be anorexic/unwilling to eat. Diagnosis is by elimination of other causes of oral pain or trigeminal nerve dysfunction. Preliminary studies have suggested oral lesions and environmental stress can precipitate the condition and the authors have previously hypothesised that the disease is most likely a neuropathic pain disorder analogous to trigeminal neuralgia and/or glossodynia in humans. 3 The discomfort appears to be relieved by anti-epileptic drugs, speculated to be because of their allodynic rather than anti-convulsant effect. In this retrospective study, clinical details of 113 cats diagnosed with FOPS were collated and reviewed with the objective of better understanding the syndrome and with the aims of suggesting what management regimes were more likely to be effective and identifying which factors should be further investigated.
Fig 1.
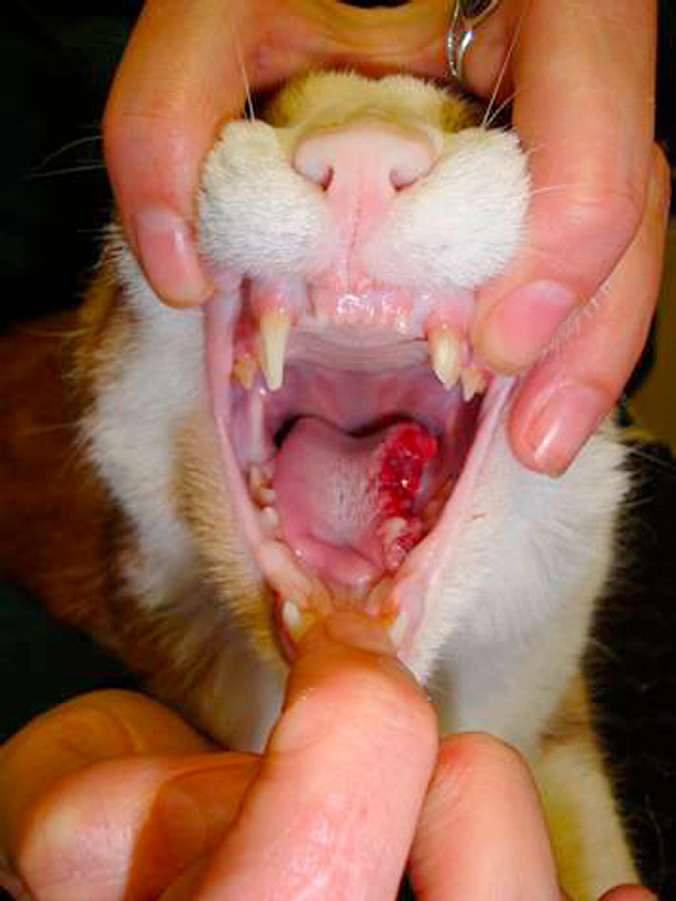
A 5-year-old domestic shorthair cat that presented with left sided tongue mutilation. In cases of severe tongue mutilation surgical repair may be required and the cat may also need to be fed via a naso-oesophageal or oesophageal feeding tube until the tongue lesions have healed. In this case the FOPS was thought to be triggered by a fractured upper canine with exposed pulp cavity. The damaged tooth was removed and the pain was managed with a reducing course of gabapentin.
Fig 2.
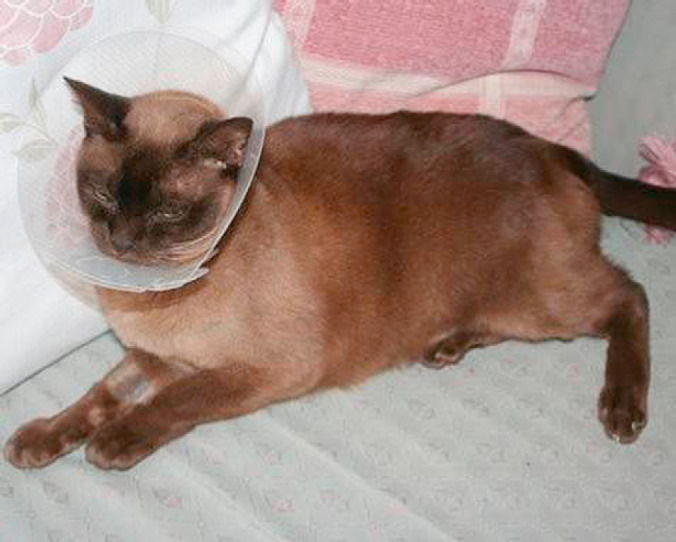
A Burmese cat with FOPS. Until discomfort can be controlled, mutilation should be prevented by using an Elizabethan collar and/or paw bandaging. ‘Soft claws’ (http://www.spuk.com) are an additional method of controlling self-mutilation. However, the underlying discomfort should also be addressed. Merely preventing the cat from mutilation without attempting to prevent the discomfort is, in the authors' opinion, unethical. Photo courtesy of Judith Cornish-Trestrail and Christine Stalker.
Materials and methods
The details of the cats in this case series were collated from clinical cases presenting to the authors CR, SH, DG-M, NJ and also from distant cases from veterinary surgeons or owners that contacted the above authors for advice via telephone, fax or email. All of the cases had long-term follow-up of at least a year after the initial episode. Cases were included if there was: (1) mutilation to the face or tongue or (2) repeated signs of oral discomfort without oral lesions or other pathology. Cases were excluded if they showed signs of mouth discomfort without mutilation where there was an obvious predisposing cause, eg, jaw neoplasia, and/or where the signs of discomfort resolved after having dental treatment alone.
Information was collected on signalment; age at the first episode; whether the condition had resolved; whether the cat had a recurrent problem; the coexistence of dental disease or environmental stress, and whether the episodes of discomfort could be triggered by any particular event, eg, eating. Diagnostic tests with results, treatments tried and outcomes were also documented and the pedigrees of the cats (if available) were evaluated.
Results
Signalment
Details on 113 cats were collected comprising 100 Burmese cats, one Burmese cross, one Burmilla, six domestic shorthair, two Siamese, one British Shorthair, one Somali and one unknown. The colour of the Burmese cats was recorded in 67/100 cats and was distributed as follows: 20 brown (30%), 15 lilac (22%), 11 blue (16%), seven chocolate (10%), six red (9%), four cream (6%) three brown tortie (4%), one chocolate tortie (1%). A request was made to the Burmese Cat Club and the Governing Council of the Cat Fancy for details on colour for registered Burmese kittens; however, this information is not available. The mean age of cats was 10.5 years (median 11 years; range 0.5–22 years). Data on age were missing for four cats. There were 50 female cats (two entire, three entire at the time of the first of two or more FOPS episodes but subsequently neutered, and 45 neutered) contrasting with 59 males (five recorded as entire, three entire at the time of the first of two or more FOPS episodes and subsequently neutered, and 51 neutered). This gave an overall female to male ratio of 46:54. A sex predisposition proportion test was not significant (P=0.444). There were missing data on gender for four cases.
FOPS events – age and recurrence
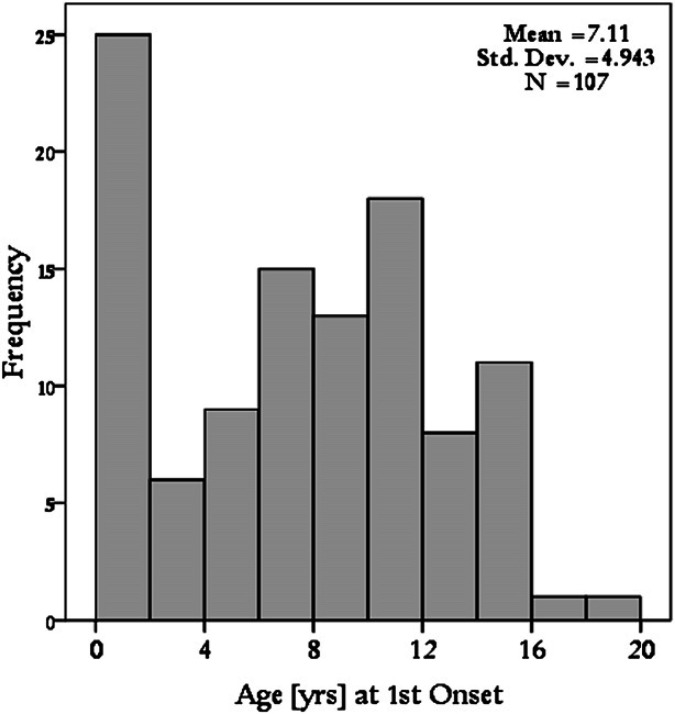
The mean age of cats at the first FOPS event was 7.1 years (median 7.5 years; range 0.1–19 years). The data on age were not normally distributed indicating a peak in immature cats. Nineteen of the cats were 6 months or less at the time of the first FOPS event; all of these cats were Burmese. When cats aged less than 12 months where removed from the dataset a bell shaped Gaussian sampling distribution (ie, normal) was observed (Fig. 3). Seventy-five of 113 cats were reported as having recurrent or ongoing FOPS problems compared to 26 cats that had one episode (which had either responded to treatment or spontaneously improved). For 12 cases, data regarding recurrence were missing. Of the cats documented to have recurrent problems, the mean number of repeat FOPS events was 3 with a range of 1–10. The mean age of the second episode was 8.2 years (range 0.9–15 years, median 8.3). The mean time between episodes was 2.01 years with a standard deviation of 2.07.
Fig 3.
Histogram demonstrating age at first onset of episode of FOPS in 107 cats. There is a peak in immature cats. In older cats there is a bell shaped Gaussian sampling distribution.
Triggers of oral discomfort
Mouth movements appeared to trigger discomfort for 33/113 cats; 28 cats were described as having signs of distress following eating, in addition to four cats following eating, drinking and grooming and one cat following grooming only. Two owners felt that strong perfumes could precipitate discomfort; one owner claimed that sunshine was a trigger and another thought cold could be a factor.
Risk factors for disease
Table 1 details the breakdown for risk factors for disease. For 32/113 cats no apparent predisposing cause for the FOPS was identified. Interestingly, two of these cats (Burmese) also had feline hyperaesthesia syndrome which is another poorly understood condition with behavioural signs of discomfort.
Table 1.
FOPS breakdown of precipitating causes 113 cats.
| Precipitating cause | Number of cats | |
|---|---|---|
| Oral lesions only | 57 | |
| Oral lesions and stress | 14 | As separate events – 11 |
| During single episode – 3 | ||
| Stress only | 10 | |
| No cause identified | 32 | |
Oral lesions
Seventy-one of 113 cats had evidence of oral lesions in addition to the behavioural signs of discomfort. Forty-eight of 71 had periodontal disease which ranged from generalised gingivitis to odontoclastic resorption lesions and endodontic disease secondary to periodontitis. Three cats had mouth ulcers thought to be associated with herpes- or calicivirus infection (two cats) and primary vaccination (one cat). Two cats had evidence of recent lost of a permanent tooth and 18 cats had erupting permanent teeth. Fourteen cats had more than one possible predisposing cause, ie, stress and dental disease.
Environmental stress
One or more FOPS events could be directly linked to a situation causing anxiety in 24 cats. In 14/24 cats this was related to social incompatibility, eg, living in a multi-cat household or following the introduction of a new kitten. In 8/24, the FOPS event could be related to another event causing distress ranging from a cattery stay, having builders in their environment, the death of their primary carer and moving house. Two of 24 cats had a FOPS event related to a veterinary hospital stay. Four of the cats with stress as a precipitating factor had had their first episode of FOPS when teething.
Hereditary tendency
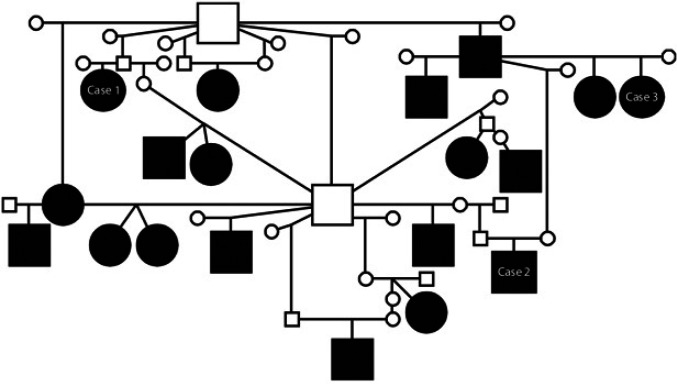
Pedigrees were available from 46 Burmese cats (12 brown, four brown tortie, three chocolate, two cream, 11 blue, eight lilac, three red and three undisclosed colour). Dates of birth of affected cats in this series ranged from 1984 to 2008. Analysis revealed common ancestors on both the paternal and maternal sides of all the affected cats' pedigrees. Some cats were obviously very closely related (Fig. 4) a–c and many Burmese breeders made anecdotal comments regarding affected litters or relatives suggesting a hereditary tendency. However, information on affected or unaffected relatives is currently too incomplete to make conclusions about the mode of inheritance.
Fig 4.
Familial relationship between 19 Burmese cats with FOPS. Circle – female, square – male; black fill – affected with FOPS; small unfilled circle or square – clinical status unknown. A DNA collection programme has been established in collaboration with the DNA archive for Companion Animals, Manchester (for information see http://www.veterinary-neurologist.co.uk/fops.htm). The spectrum of presentation with FOPS can vary as illustrated with related cases 1–3 (see foot notes a–c).
Diagnostic tests
All cases had a clinical examination including oral examination. Serum biochemistry and haematology were preformed in 19 cats and were unremarkable in 17; two cats had an elevated alanine transferase with normal bile acids and one cat's haematology had a mild inflammatory pattern. Retroviral tests were obtained in four cats and were negative in three; one Burmese was feline immunodeficiency virus positive. Toxoplasma antibody titres were obtained in three cats and all were negative. Feline coronavirus antibody titre was obtained in one Somali cat and was low positive. Magnetic resonance imaging (MRI) of the head (including brain, trigeminal nerve roots, ears, tongue and other soft tissue) together with cerebrospinal fluid analysis in two Burmese cats was normal. Electromyography of the tongue, masticatory muscles and pharynx was normal in one domestic shorthair cat and the tongue was biopsied in three Burmese cats. Histopathology of the biopsies revealed inflammation and granulation tissue which in all cases was interpreted as being secondary to self trauma. Dental radiographs were obtained and used in planning of dental treatment in 10 Burmese. Pathology was identified in seven cats with the remaining three being unremarkable.
Treatment
Table 2 provides a broad illustration of the effectiveness of the most commonly used drugs.
Table 2.
Most common drugs used to treat FOPS and the anecdotal effectiveness.
| Drug | Number of cases | % Effective | % Partially effective | % Not effective | % Unknown |
| NSAIDs | 18 | 33 | 6 | 50 | 11 |
| Corticosteroids | 17 | 41 | 24 | 24 | 11 |
| Antibiotics | 12 | 17 | 8 | 67 | 8 |
| ABS/antinfla | 21 | 43 | 0 | 38 | 19 |
| Phenobarbital | 16 | 88 | 6 | 6 | 0 |
| Diazepam | 15 | 86 | 7 | 7 | 0 |
| Opioids | 14 | 29 | 21 | 36 | 14 |
| Amitriptyline | 7 | 28 | 28 | 44 | 0 |
ABS=antibiotics; antinfla=anti-inflammatory drugs.
Dental treatment
Fifty-three cats were reported as having dental treatment. The nature and the extent of the work, for example the number of tooth extractions, were not detailed in the majority of cases. In 35/53 cats, the signs of FOPS were improved following dental treatment, however, for nine cats this improvement was not sustained. For a further 11 cats the signs of FOPS were not improved following dental treatment and one cat was worse. For four cats the outcome was not stated. One cat was improved after the first dental procedure and not after subsequent dental therapy. For two cats the clinical signs of FOPS started immediately following dental procedures, having not been present before. Only 1/43 cats was described as having dental disease (gingivitis) but did not have dental treatment. This cat was managed with corticosteroids and was ultimately euthanased.
Non-steroidal ant-inflammatory drugs (NSAIDs)
Eighteen cats received NSAIDs (meloxicam, ketoprofen or carprofen) with (six) or without (12) dental procedures. In six cats this was reported as being effective in controlling pain. In one cat that also had dental treatment, these drugs appeared to give partial relief. For two cats the effect of the NSAIDs was not stated and for nine cats NSAIDs were ineffective (including five of the cats which also had dental work).
Corticosteroids
Seventeen cats received corticosteroids (prednisolone, methylprednisolone or dexamethasone) without other medication. In seven cats it was reported as effective at controlling pain. In four cats there was an initial improvement but this was not sustained. For two cats the effect was not stated and in four cats corticosteroids were ineffective.
Antibiotics
Twelve cats received antibiotics alone (or following a dental procedure) and these were reported as being effective in two cats, giving an unsustained improvement in one cat, and were ineffective in eight cats. The effect was not stated for one cat.
Combination anti-inflammatory and antibiotic treatment
Twenty-one cats were treated with antibiotics in combination with either corticosteroids or NSAIDs. This was deemed effective for controlling signs of FOPS for nine cats but was ineffective for eight cats. For four cases the effect was not stated.
Opioids
Opioids (buprenorphine, pethidine or butorphanol) were used in 14 cases and were deemed ineffective for five cats, to have some effect for three cats and were effective for four cats. The effect was not stated for two cases.
Adjuvant analgesics (anti-epileptic drugs and amitriptyline)
An adjuvant analgesic is a drug that has a primary non-pain indication but which may be analgesic in certain circumstances, for example, anti-epileptic drugs and amitriptyline. Sixteen cats received phenobarbital alone (14 cats) or following a dental procedure (two cats) and this drug was reported as being effective in alleviating signs of FOPS in 14 cats, partially effective in one cat and ineffective in one cat. The cat for which phenobarbital was ineffective was receiving a dose of 1 mg/kg twice daily compared to at least 2 mg/kg twice daily for the other cats. Fifteen cats received diazepam alone (14 cats) or following a dental procedure (one cat) and this drug was reported as effective in 13 cats, resulting in some improvement for one cat but no improvement in one case. Gabapentin and carbamazepine were used in single cases and were reported as being effective for alleviating signs of pain in those cats. Amitriptyline was used in seven cases and was reported as ineffective for three cats, effective for two cats and having some effect for two cats.
Other drugs/management
The anti-histamine chlorpheniramine was used in four cats. For two cases this was stated as effective, however, the drug was given in combination with diazepam so the true effect could not be determined. For one case the effect was not stated and for one cat it was thought to have some effect. Selegiline was used in four cases and was reported as having no benefit for two cases and a partial benefit for two cases. Clomipramine (Clomicalm; Novartis Animal Health) was prescribed for one cat and was ineffective in preventing signs of FOPS. Megestrol (Ovarid; Schering Plough Animal Health) was used in two cats and was seemingly effective in controlling signs with withdrawal resulting in a recurrence of signs in both cats. Interestingly for both cats ‘stress’ was cited as a trigger for the episodes. Lidocaine sprayed on buccal mucosa was ineffective for one cat. Acepromazine was reported as effective for one case, however, the ability to accurately conclude an effect is doubted because it would not be possible to determine if this was because the cat was too sedated to express the behaviour or because the pain was controlled. Feline facial pheromone F3 (Feliway; Ceva Animal Health) was deemed to be helpful for three cats and unhelpful for one. Homeopathy was tried in one cat and was ineffective. Finally, one owner found the signs improved after fish was eliminated from the cat's diet and two owners claimed signs improved after they stopped using perfumed products in the house. There were six cats described as having an ongoing problem but not receiving any medication. Three of these cats were ultimately euthanased. For two of the other cases the owners did not consider the signs sufficiently severe to warrant medication although at least one of those cats had signs on a daily basis. The remaining case was required to wear a permanent Elizabethan collar as her owners considered this ‘kinder’ than permanent medication.
Outcome
In all 12/113 cats were euthanased with FOPS being cited as the main/only reason for the euthanasia. One further cat was euthanased after developing diabetes mellitus and coma thought to be as a consequence of long-term corticosteroids prescribed for FOPS. Eleven of the euthanased cats were Burmese and the others were a domestic shorthair and a Somali cat. The mean age at euthanasia was 13.4 years (median 14.5 years; range 10–17.9 years) and all of the euthanased cats were described as having ongoing or recurrent FOPS. Of the remaining cats, 33/113 were described as having ongoing signs of FOPS or required permanent medication to control signs. Of these 33 cats, 25 had a history of one or more previous episodes of FOPS. By contrast 53/113 were successfully managed for their single or multiple episodes and medication could be withdrawn at least between episodes. For 14 cats the outcome was unknown.
For the teething kittens, the signs of pain resolved and medication could be withdrawn when the teeth (typically the canines) had fully erupted. Ten of these 18 kittens were documented to have recurrent problems and of these four eventually required permanent medication. The remaining eight kittens were only followed to a maximum age of 2.2 years old so it is possible that the number of cats with recurrent problems may actually be higher with a longer period of follow-up. Of the 48 cats originally presented with dental disease 22 were described as having a complete and sustained resolution of FOPS. By comparison 21 cats ultimately developed persistent discomfort requiring medication (12 cats) or were euthanased because of the condition (nine cats). For 5/48 cats with dental disease the final outcome was unknown. Of the 21/48 cats with dental disease with continuing discomfort, two cats (including one euthanased cat) had ongoing issues from social incompatibility (‘multi-cat’ household) and for one cat the signs appeared to be precipitated by a stressful event in this case a veterinary hospital stay for unrelated condition.
Discussion
This study has many inherent problems not least because the information collected is anecdotal (especially regarding treatment) and likely to be biased towards more severe cases because owners/veterinary surgeons are more likely to seek advice from a specialist if the case is challenging. Another criticism of this study is that the majority of cases were not followed though their entire lifetime and, therefore, important clinical information about recurrence and subsequent success (or not) of treatment is probably missing. Finally, in a disease where the diagnosis is made by elimination, it is not possible to be certain that all the cats in the series definitely had FOPS. Therefore, firm conclusions about the incidence, presentation, pathophysiology and management of FOPS cannot be made from this study. However, some broad suggestions can be offered which can hopefully be used as the basis for more formal studies.
The study found an overwhelming predilection to the Burmese cat (88% of cases). FOPS was also described in a Burmese cross and a Burmilla, a breed where the ancestry can be traced back to Burmese and Chinchilla cats. This predisposition to a single purebred cat variety suggests a hereditary tendency and indeed many of the cats appeared closely related as is illustrated in Fig. 4. Currently there is not enough information to determine the mode of inheritance. Any age of cat can be affected with FOPS and the age at first presentation ranged from 0.1 to 19 years. However, many affected cats first had signs when erupting permanent teeth and these cats often re-developed the syndrome as mature cats. It is possible that more of the cats were affected as kittens. Several Burmese breeders discussed the condition with the authors and were not only aware of the disease but reported that it was frequently necessary to bandage the paws of kittens whilst teething to prevent severe mutilation. Many considered it a benign problem and unlikely to recur and, therefore, would not necessarily pass this information on to prospective owners.
The reader is advised caution in interpretation of the results on treatment as the information on response is very anecdotal and within treatment groups the drug and dosing regimes varied greatly. In many cases the length of time of treatment was not stated. However, there was a suggestion that anti-epileptic drugs (phenobarbital, diazepam, carbamazepine and gabapentin) appeared to be more effective than anti-inflammatory drugs or opioids for appeasing the discomfort of FOPS, at least in challenging cases. For example, phenobarbital (P=0.007) and diazepam (P=0.010) had a significantly higher success rate than corticosteroids.
This would, therefore, suggest that where licensed opioids and NSAIDs are ineffective for an individual FOPS case then adding in or switching to unlicensed anti-epileptic drugs would be a reasonable management alternative. FOPS does not have the characteristics of a seizure disorder so it is perhaps more likely that the anti-epileptic drugs were effective because FOPS is a condition of neuropathic pain. Neuropathic pain is a clinical syndrome of pain due to abnormal somatosensory processing in the peripheral and/or central nervous system (CNS). 4 Unlike physiological and inflammatory pain it serves no beneficial purpose to the animal and can be regarded as a disease in itself. Discomfort ranges from spontaneous pain; paraesthesia (a spontaneous or evoked abnormal but not unpleasant sensation); dysaesthesia (a spontaneous or evoked unpleasant abnormal sensation usually described as burning); allodynia (pain from a stimulus that is not normally painful, eg, touch), or hyperpathia (increased pain from stimuli which are normally painful). Neuralgia is a pain in the distribution of a nerve or nerves and in FOPS the signs suggest discomfort is confined to the oral cavity and lips. The trigeminal nerve provides sensory information for these areas; however, there is no apparent discomfort elsewhere in the trigeminal nerve distribution, eg, nose or eyes. Trigeminal sensory loss is not apparent although the possibility of subtle deficits has not been ruled out especially given the extent of mutilation in some cases. In all cases the discomfort of FOPS was described as unilateral or worse on one side.
The pathophysiology of neuropathic pain is complex and incompletely understood. 4,5 There are four pivotal phenomena intrinsic to its development; (1) peripheral activation, ie, sensitisation and/or excitation of peripheral sensory neurons, 6 (2) central sensitisation, ie, the process of ‘wind up’ and the resulting transcriptional changes in spinal and medullary dorsal horn neurons leading to altered synaptic neurotransmitter levels and number of receptors, 4,7 (3) central disinhibition, ie, an imbalance between the excitatory and inhibitory sides of the nervous system, 4,8 and (4) phenotypic change of mechanoreceptive Aβ-fibres (light touching) to produce substance P so that input from them is perceived as pain. 9 The authors hypothesise that Burmese and possibly other cats prone to FOPS may have a dysfunction of central and/or ganglion processing of sensory trigeminal information. Signs of FOPS seem to be precipitated in many cases when the endings of the trigeminal nerves are damaged/sensitised, eg, with teething or dental disease. This study found that 63% of the cats had oral lesions, including dental disease, oral ulceration and eruption of permanent teeth. However, it should be considered that there is a high incidence of periodontal disease in the feline population; one study reported an incidence of 72%, with purebred cats being more predisposed, 10 ie, a direct association cannot be proved at this time although anecdotally the signs of FOPS improved in many cases following resolution of the oral lesions.
FOPS can be compared to human conditions of neuropathic facial pain such as trigeminal neuralgia and glossodynia (burning mouth syndrome). Human trigeminal neuralgia is characterised by severe pain in the distribution of the trigeminal nerve, usually the mandibles and/or maxilla. People with this condition describe attacks of pain which typically last only seconds but may occur repeatedly within a short period of time. 11 The attacks are often, but not always, precipitated by mild sensory stimulation of so called trigger zones (allodynia) which may be located anywhere within the territory of the affected trigeminal nerve. Classical antecedent stimuli include light touch, draughts of wind, and facial movement such as eating and drinking. 11 This study found that 33 cats had signs of FOPS triggered by mouth movements including eating, drinking and/or grooming. Other anecdotal triggers were also reported. Human trigeminal neuralgia tends to occur in bouts over a period of weeks or months with subsequent spontaneous remission that may last months or years. Over time however, the attacks usually become more frequent and pain more sustained. 11 In this study 46/113 cats were reported as having ongoing and persistent disease and in 38/46 cats there had been a history of previous recurrent bouts. The prognosis is poorer for this group (P=0.047) and 13/46 were euthanased either because of FOPS or because of the effects of medication for FOPS.
A more unusual human facial pain syndrome, part of the spectrum of trigeminal neuralgia, is glossodynia (burning mouth syndrome). 12 This is described as a burning or prickling sensation of the oral mucosa, most commonly at the front of the tongue in the absence of physical abnormalities of the oral mucosa. 13–15 In many of the affected cats, caudal tongue discomfort seems to be the primary problem and many cats were presented with mutilation injuries to the tongue. Unlike typical trigeminal neuralgia the cause of glossodynia is often enigmatic. For trigeminal neuralgia the majority of cases in humans can be shown to be due to demyelination of the trigeminal sensory fibres within the nerve root or brain stem. The most common cause is compression of the CNS nerve root by an overlying artery or vein. 11 Familial trigeminal neuralgia is also described although it is rare. 16–19 By contrast glossodynia is considered to have a multifactorial aetiology and most cases are described as ‘idiopathic’. It is often linked to anxiety disorders – although this may be the effect rather that the cause. 13,20–22
Conditions of neuropathic pain can be greatly influenced by many internal and external factors. 23 Examples of external factors include social variables, housing and other external stress factors. Genetic influences on homeostatic adaptation mechanisms to stress may also be important. 23 The current study found that for 24 cats, an FOPS event could be directly linked to a situation causing anxiety. This equates to approximately one in five cats. This figure may be artificially low as anxiety or stress influences on disease are easily overlooked if appropriate questions are not asked during history taking. The most important queries are to ascertain if the five essential feline resources – food, water, resting places, latrines and points of entry and exit into the territory – are appropriately distributed. The cat should also have a private area(s) and the ability to hide and gain access to a high vantage point in order to control anxiety. The most common ‘stress’ contributing to FOPS was social, ie, other cats in the immediate environment suggesting that individuals with poor social coping strategies may be more vulnerable. Removing the cat from its core territory, eg, moving house or a stay in a cattery could also precipitate the disease. Psychosocial stress can promote a relative resistance to glucocorticoids with increased sympathetic and decreased parasympathetic activity together with increased production and release of pro-inflammatory mediators. This dysregulation of the stress/inflammatory pathways promotes alterations in brain circuitry that modulates mood, pain and the stress response. Over time, these functional changes probably promote disruptions in neurotrophic support and disturbances of glia–neuronal communication. These changes, in turn, have been associated with the related processes of central sensitisation in pain disorders which may account for the progressive and self-perpetuating nature of the disease especially when inadequately treated. 24
Conditions of neuropathic pain are a challenge to treat and experience in veterinary medicine is limited. 25–27 However, knowledge gained from humans and laboratory animal models, including experimental feline models of trigeminal neuralgia, 28–30 can suggest a rational approach. Several anti-epileptic drugs have an anti-allodynic effect 31 and are reported by human patients to be particularly effective for neuropathic pain that is burning and lancinating in nature. 5 However, the anti-convulsants do not all have the same site of action and an area for further study is to ascertain which drugs are most efficacious and safe. Determining the nature of the suspected mutation in the Burmese cat and, therefore, the site of action for potential pharmacotherapy is an important future goal. In this study phenobarbital was chosen for treating many of the cats because its pharmacokinetics and toxicity have been studied in this species. 32,33 Diazepam was also used; however, the authors prefer to avoid this drug because of the risk of idiosyncratic hepatitic failure. 34 Phenobarbital and diazepam exert their pharmacological effects via an action at the gamma-aminobutyric acid (GABA) receptor, in addition to other sites including the excitatory neurotransmitter glutamate (phenobarbital), calcium channels (phenobarbital and diazepam), sodium channels (diazepam) and voltage dependant potassium currents (phenobarbital). 35 GABAergic neurons and ionotropic GABA(A) receptors are found in the dorsal horn 36 and trigeminal ganglion 37 where they control the propagation of pain signals from the periphery to higher CNS areas. 36,38 There is also evidence that GABAergic neurons in the rostral ventromedial medulla are involved in a pain-control system that ‘descends’ from the brain onto the spinal cord. 39 Recent evidence indicates that diminished GABA(A) medicated inhibitory control is a major factor in chronic pain syndromes. 36 GABA receptor agonists display anti-nociceptive properties in a variety of animal models of pain 38 although the side effects of such agents, in particular sedation, limit their usefulness. 40
Clinical and experimental data indicate that changes in the expression of voltage-gated sodium channels in trigeminal ganglia and trigeminal subnucleus caudalis play a key role in the pathogenesis of trigeminal neuralgia and that drugs that antagonise these channels are potentially therapeutic. 31,41,42 Recent work has demonstrated that there is abnormal expression of voltage-gated sodium channels in trigeminal neuralgia. 43 Gain-of-function mutations in SCN9A, the gene which encodes the voltage-gated sodium channel Na(v)1.7, leads to dorsal root and trigeminal ganglion neuron hyperexcitability and is associated with rare inherited neuropathic pain syndromes. 44–46 This has lead to the proposal that some cases of trigeminal neuralgia may result from a channelopathy. 43 This is also a theoretical possibility with FOPS. Stress may also influence sodium channels, as a stressful environment may contribute to permanent sympathetic hyperactivity which will induce sodium channel up-regulation and sympathetic sprouting in dorsal root ganglia through nerve growth factor over-expression. 47
Tricyclic anti-depressants, eg, amitriptyline, 48 and some anti-convulsants, eg, phenytoin, carbamazepine and oxcarbazepine, antagonise sodium channels. 42 Typically these drugs are first-line therapy for neuropathic pain and trigeminal neuralgia in humans. 41,42 Amitriptyline may have suitable pharmacokinetics for the treatment of FOPS as this drug can be useful in some stress related behavioural disorders 49 and recurrent idiopathic cystitis. 50 However, it is not yet established whether amitriptyline will be effective for feline neuropathic pain and the current study did not suggest that this drug was more effective than others tried (given that there was no significant difference between amitriptyline and corticosteroids (P=0.669)). However, phenobarbital (P=0.011) and diazepam (P=0.014) were found to be significantly more effective than amitriptyline in this small sample. Somnolence, weight gain, decreased grooming, and transient cystic calculi are reported as possible adverse effects of amitriptyline treatment. 50
The anti-convulsant phenytoin is less appropriate for cats as the slow hepatic metabolism increases the risk of hepatotoxicity and other adverse effects. 51,52 Carbamazepine and oxcarbazepine, the most common drugs for treating human trigeminal neuralgia, have been used successfully in feline models of neuropathic trigeminal pain 28,29 and may be alternatives to phenobarbital. The pharmacokinetics and toxicity of these drugs when used therapeutically have not been investigated.
Gabapentin is a drug that was originally developed as an anti-convulsant but clinically has been more useful for the treatment of neurogenic pain in people 53 including refractory cases of trigeminal neuralgia. 54 Gabapentin is thought to influence ‘wind up’ by preventing the release of the excitatory neurotransmitter glutamate in the dorsal horn via interaction with the α2delta subunit of voltage-gated calcium channels. 53,54 It has been used as a monotherapy and in combination with carbamazepine in a feline model of trigeminal neuralgia where the combination therapy was apparently more effective 30 Again the pharmacokinetics and toxicity of these drugs when used therapeutically have not been investigated. Pregabalin has emerged as an effective drug for neuropathic pain in humans and has been used in management of trigeminal neuralgia. 55 It is a structural (but not functional) analogue of GABA, which is also thought to exert its pharmacodynamic effect by modulating voltage-gated calcium channels resulting in a reduction of glutamate and substance P release. 56 The pharmacokinetics and potential toxicity in cats are currently unknown; however, anecdotal reports of its use for epilepsy have been documented. 57 It is also possible that other anti-epileptic drugs, eg, levetiracetam 58 and topiramate 59,60 may be useful for FOPS as monotherapy or in combination with other drugs.
Conclusion
A feline neuropathic pain condition is described characterised by episodic, typically unilateral, oral and/or tongue discomfort, triggered in many cases by mouth movements. Burmese cats are predisposed and an inherited disorder affecting trigeminal sensory processing is suspected. A history of first signs during eruption of permanent teeth is common and the disease is often recurrent and with time may become unremitting. Oral lesions may be an important predisposing cause. Any oral disease should be identified and dental radiographs to detect more subtle lesions are recommended. Environmental factors may also influence the disease and the history should be explored for possible contributory factors. Identification of social incompatibility in a multi-cat household is a key step. For analgesia, if licensed products such as a combination of NSAIDs and opioids are ineffective, then (unlicensed) adjuvant drugs useful for the treatment of neuropathic pain may be beneficial. Based on the experiences in this paper phenobarbital (dose rate 2–3 mg/kg twice daily) is a reasonable first choice for treatment and can be given by the oral or intramuscular route. Further studies are necessary to establish which drugs are most effective for treating this disease and other anti-epileptic drugs such as carbamazepine or gabapentin may prove to be more appropriate. Periodic monitoring of liver function and drug serum concentrations is recommended for cats treated with anti-epileptic drugs.
Acknowledgements
The authors would like to thank the many veterinary surgeons, owners and Burmese breeders who took the time to provide information on cases. We also appreciate the contribution of Douglas Paterson who collated some of the case information and Lisa Milella who performed the dental procedures on case 1 (Fig. 4).
Footnotes
Case 1: A 6.8-year-old neutered female Burmese cat originally presented with acute onset orofacial discomfort aged 5 months. Oral examination revealed she was erupting her permanent canine teeth. Diagnostic tests including MRI were unremarkable. There was a poor response to meloxicam (Metacam; Boehringer Ingelheim) and buprenorphine but a rapid alleviation signs following intramuscular phenobarbital at 3 mg/kg. She was maintained on oral phenobarbital at 3 mg/kg twice daily for 1 month and then this was gradually withdrawn over a 2-week period. The cat was represented with signs of FOPS and required further course of phenobarbital when 5.5 years old. The precipitating cause was thought to be oral ulceration. The cat was represented at 6.8 years old following a recurrence of FOPS which had been controlled by phenobarbital for the previous 3 weeks. On dental examination with radiographs she was found to have a generalised grade 2 gingivitis (bleeding on probing) with a number of ‘missing’ teeth and type 1 tooth resorption lesions (Feline TR) on a premolar and molar tooth. In addition, gingival recession and horizontal bone loss were present on oral and radiographic examination in a number of teeth. The cat had an upper incisor root remnant removed and surgical extractions of all cheek teeth distal to the canines. Following this the phenobarbital was successfully withdrawn but the cat had two further episodes of FOPS over the next 15 months. The first was associated with a cat show and responded to meloxicam and the second was associated with a stay in a cattery and was managed with phenobarbital which was has been successfully withdrawn for 3 months.
Case 2: A 10-year-old male neutered Burmese cat that lived in a multi-cat household was presented with severe tongue mutilation. The cat had a history suggesting social incompatibility; 8 days before presentation he had been involved in a fight with another cat and had received minor head skin wounds as a consequence. These subsequently healed and at presentation he had no evidence of skin or dental disease. He was subsequently managed with behavioural modification to deal with social stress. This involved ensuring that the distribution of essential resources, such as food, water, latrines and resting places, was sufficient to allow each cat within the household to have free and immediate access to those resources at all times, without having to interact with another cat. There have been no further episodes of mutilation, however, he continues to have episodes of discomfort which the owner relates to continuing distress from another cat.
Case 3: A 6-year-old female Burmese cat with recurrent facial mutilation with episodes at 5 months old (associated with eruption of permanent teeth) and also at 2, 3, 5 and 6 years old. Signs eventually became persistent and the owner was using a permanent buster collar to prevent mutilation. On removal of the collar, signs of mutilation recurred, usually within minutes, with an apparent trigger of grooming. There were no signs of dental disease, diagnostic tests were normal and she had no behavioural signs of anxiety. She was prescribed phenobarbital at 2 mg/kg twice daily and the dose was increased to 4 mg/kg twice daily to achieve a serum phenobarbital concentration of approximately 120 μmol/l. This controlled clinical signs and after 3 months an attempt was made to wean the phenobarbital. However, signs recurred and it has proved necessary to maintain permanent medication.
References
- 1.Roche G.M. Irritation from erupting teeth, Vet Rec 134, 1994, 360. [DOI] [PubMed] [Google Scholar]
- 2.Heath S., Rusbridge C., Johnson N., Gunn-Moore D. Orofacial pain syndrome in cats, Vet Rec 149, 2001, 660. [PubMed] [Google Scholar]
- 3.Rusbridge C., Heath S.E., Johnson N.W., Gunn-Moore D.A. Feline orofacial pain syndrome. Proceedings of the 15th annual symposium of the European society of veterinary neurology, Philadelphia, J Vet Intern Med 17, 2002, 246. [Google Scholar]
- 4.Woolf C.J., Salter M.W. Neuronal plasticity: increasing the gain in pain, Science 288, 2000, 1765–1788. [DOI] [PubMed] [Google Scholar]
- 5.Costigan M., Woolf C.J. Pain: molecular mechanisms, J Pain 1 (3 suppl), 2000, 35–44. [DOI] [PubMed] [Google Scholar]
- 6.Stein C., Clark J.D., Oh U., et al. Peripheral mechanisms of pain and analgesia, Brain Res Rev 60, 2009, 90–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coste J., Voisin D.L., Miraucourt L.S., Dallel R., Luccarini P. Dorsal horn NK1-expressing neurons control windup of downstream trigeminal nociceptive neurons, Pain 137, 2008, 340–351. [DOI] [PubMed] [Google Scholar]
- 8.Yaksh T.L. Behavioral and anatomic correlates of the tactile-evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists, Pain 37, 1989, 111–123. [DOI] [PubMed] [Google Scholar]
- 9.Neumann S., Doubell T.P., Leslie T., Woolf C.J. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons, Nature 384, 1996, 360–364. [DOI] [PubMed] [Google Scholar]
- 10.Lommer M.J., Verstraete F.J. Radiographic patterns of periodontitis in cats: 147 cases (1998–1999), J Am Vet Med Assoc 218, 2001, 230–234. [DOI] [PubMed] [Google Scholar]
- 11.Love S., Coakham H.B. Trigeminal neuralgia: pathology and pathogenesis, Brain 124, 2001, 2347–2360. [DOI] [PubMed] [Google Scholar]
- 12.Marbach J.J. Medically unexplained chronic orofacial pain. Temporomandibular pain and dysfunction syndrome, orofacial phantom pain, burning mouth syndrome and trigeminal neuralgia, Med Clin North Am 83, 1999, 691–710. [DOI] [PubMed] [Google Scholar]
- 13.Reiss M., Reiss G. Burning mouth syndrome–etiology, differential diagnostical aspects and therapy, Ther Umsch 61, 2004, 308–312. [DOI] [PubMed] [Google Scholar]
- 14.Pinto A., Stoopler E.T., DeRossi S.S., Sollecito T.P., Popovic R. Burning mouth syndrome: a guide for the general practitioner, Gen Dent 51, 2003, 458–461. [PubMed] [Google Scholar]
- 15.Muzyka B.C., De Rossi S.S. A review of burning mouth syndrome, Cutis 64, 1999, 29–35. [PubMed] [Google Scholar]
- 16.Braga F.M., Bonatelli A.D., Suriano I., Canteras M. Familial trigeminal neuralgia, Surg Neurol 26, 1986, 405–408. [DOI] [PubMed] [Google Scholar]
- 17.Coffey R.J., Fromm G.H. Familial trigeminal neuralgia and Charcot–Marie–Tooth neuropathy. Report of two families and review, Surg Neurol 35, 1991, 49–53. [DOI] [PubMed] [Google Scholar]
- 18.Fleetwood I.G., Innes A.M., Hansen S.R., Steinberg G.K. Familial trigeminal neuralgia. Case report and review of the literature, J Neurosurg 95, 2001, 513–517. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick D.B. Familial trigeminal neuralgia: case report, Neurosurgery 24, 1989, 758–761. [DOI] [PubMed] [Google Scholar]
- 20.Miyaoka H., Kamijima K., Katayama Y., Ebihara T., Nagai T. A psychiatric appraisal of ‘glossodynia’, Psychosomatics 37, 1996, 346–348. [DOI] [PubMed] [Google Scholar]
- 21.Eli I., Baht R., Littner M.M., Kleinhauz M. Detection of psychopathologic trends in glossodynia patients, Psychosom Med 56, 1994, 389–394. [DOI] [PubMed] [Google Scholar]
- 22.Kuğu N., Akyüz G., Doğan O. Burning mouth syndrome and depression: a case report, Turk Psikiyatri Derg 13, 2002, 232–237. [PubMed] [Google Scholar]
- 23.Vissers K., De Jongh R., Hoffmann V., Heylen R., Crul B., Meert T. Internal and external factors affecting the development of neuropathic pain in rodents. Is it all about pain?, Pain Pract 3, 2003, 326–342. [DOI] [PubMed] [Google Scholar]
- 24.Maletic V., Raison C.L. Neurobiology of depression, fibromyalgia and neuropathic pain, Front Biosci 14, 2009, 5291–5338. [DOI] [PubMed] [Google Scholar]
- 25.Cashmore R.G., Harcourt-Brown T.R., Freeman P.M., Jeffery N.D., Granger N. Clinical diagnosis and treatment of suspected neuropathic pain in three dogs, Aust Vet J 87, 2009, 45–50. [DOI] [PubMed] [Google Scholar]
- 26.Mathews K.A. Neuropathic pain in dogs and cats: if only they could tell us if they hurt, Vet Clin North Am Small Anim Pract 38, 2008, 1365–1414. [DOI] [PubMed] [Google Scholar]
- 27.Rusbridge C., Jeffery N.D. Pathophysiology and treatment of neuropathic pain associated with syringomyelia, Vet J 175, 2008, 164–172. [DOI] [PubMed] [Google Scholar]
- 28.Sessle B.J., Greenwood L.F.J. Effects of trigeminal tractotomy and of carbamazepine on single trigeminal sensory neurons in cats, J Dent Res 54 (Spec No B), 1975, B201–B206. [DOI] [PubMed] [Google Scholar]
- 29.Kiguchi S., Ichikawa K., Kojima M. Suppressive effects of oxcarbazepine on tooth pulp-evoked potentials recorded at the trigeminal spinal tract nucleus in cats, Clin Exp Pharmacol Physiol 28, 2001, 169–175. [DOI] [PubMed] [Google Scholar]
- 30.Gilron I., Flatters S.J. Gabapentin and pregabalin for the treatment of neuropathic pain: a review of laboratory and clinical evidence, Pain Res Management 11 (suppl A), 2006, 16A–29A. [Google Scholar]
- 31.Tremont-Lukats I.W., Megeff C., Backonja M.M. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy, Drugs 60, 2000, 1029–1052. [DOI] [PubMed] [Google Scholar]
- 32.Bunch S.E. Hepatotoxicity associated with pharmacologic agents in dogs and cats, Vet Clin North Am Small Anim Pract 23, 1993, 659–670. [DOI] [PubMed] [Google Scholar]
- 33.Cochrane S.M., Parent J.M., Black W.D., Allen D.G., Lumsden J.H. Pharmacokinetics of phenobarbital in the cat following multiple oral administration, Can J Vet Res 54, 1990, 309–312. [PMC free article] [PubMed] [Google Scholar]
- 34.Center S.A., Elston T.H., Rowland P.H., et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats, J Am Vet Med Assoc 209, 1996, 618–625. [PubMed] [Google Scholar]
- 35.Hannah J.A., Sills G.J., Brodie M. Established and new antiepileptic drugs: an overview. Aldenkamp A.P., Dreifuss F.E., Renier W., Suurmeijer T.P.B.M. Epilepsy in children and adolescents, 1st edn, 1995, CRC Press, 101–129. [Google Scholar]
- 36.Zeilhofer H.U., Möhler H., Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists, Trends Pharmacol Sci 30, 2009, 397–402. [DOI] [PubMed] [Google Scholar]
- 37.Hayasaki H., Sohma Y., Kanbara K., Maemura K., Kubota T., Watanabe M. A local GABAergic system within rat trigeminal ganglion cells, Eur J Neurosci 23, 2006, 745–757. [DOI] [PubMed] [Google Scholar]
- 38.Price T.J., Hargreaves K.M., Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat, Brain Res 1112, 2006, 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato G., Yasaka T., Katafuchi T., et al. Direct GABAergic and glycinergic inhibition of the substantia gelatinosa from the rostral ventromedial medulla revealed by in vivo patch-clamp analysis in rats, J Neurosci 26, 2006, 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enna S.J., McCarson K.E. The role of GABA in the mediation and perception of pain, Adv Pharmacol 54, 2006, 1–27. [DOI] [PubMed] [Google Scholar]
- 41.Prasad S., Galetta S. Trigeminal neuralgia: historical notes and current concepts, Neurologist 15, 2009, 87–94. [DOI] [PubMed] [Google Scholar]
- 42.Wood J.N., Boorman J.P., Okuse K., Baker M.D. Voltage-gated sodium channels and pain pathways, J Neurobio 61, 2004, 55–71. [DOI] [PubMed] [Google Scholar]
- 43.Siqueira S.R., Alves B., Malpartida H.M., Teixeira M.J., Siqueira J.T. Abnormal expression of voltage-gated sodium channels Nav1.7. Nav1.3 and Nav1.8 in trigeminal neuralgia, Neuroscience, 2009. Aug 20, (Epub ahead of print) [DOI] [PubMed]
- 44.Dib-Hajj S.D., Cummins T.R., Black J.A., Waxman S.G. From genes to pain: Na v 1.7 and human pain disorders, Trends Neurosci 30, 2007, 555–563. [DOI] [PubMed] [Google Scholar]
- 45.Dib-Hajj S.D., Yang Y., Waxman S.G. Genetics and molecular pathophysiology of Na(v)1.7-related pain syndromes, Adv Genet 63, 2008, 85–110. [DOI] [PubMed] [Google Scholar]
- 46.Estacion M., Dib-Hajj S.D., Benke P.J., et al. NaV1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders, J Neurosci 28, 2008, 11079–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez-Lavin M., Solano C. Dorsal root ganglia, sodium channels, and fibromyalgia sympathetic pain, Med Hypotheses 72, 2009, 64–66. [DOI] [PubMed] [Google Scholar]
- 48.Wang G.K., Russell C., Wang S.Y. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain, Pain 110, 2004, 166–174. [DOI] [PubMed] [Google Scholar]
- 49.Sawyer L.S., Moon-Fanelli A.A., Dodman N.H. Psychogenic alopecia in cats: 11 cases (1993–1996), J Am Vet Med Assoc 214, 1999, 71–74. [PubMed] [Google Scholar]
- 50.Chew D.J., Buffington C.A., Kendall M.S., DiBartola S.P., Woodworth B.E. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats, J Am Vet Med Assoc 213, 1998, 1282–1286. [PubMed] [Google Scholar]
- 51.Schwartz-Porsche D., Kaiser E. Feline epilepsy, Probl Vet Med 1, 1989, 628–649. [PubMed] [Google Scholar]
- 52.Kamali F., Ball D.E., McLaughlin W.S., Seymour R.A. Phenytoin metabolism to 5-(4-hydroxyphenyl)-5-phenylhydantoin (HPPH) in man, cat and rat in vitro and in vivo, and susceptibility to phenytoin-induced gingival overgrowth, J Periodontal Res 34, 1999, 145–153. [DOI] [PubMed] [Google Scholar]
- 53.Krafft R.M. Trigeminal neuralgia, Am Fam Physician 77, 2008, 1291–1296. [PubMed] [Google Scholar]
- 54.Guerrero-Figueroa R., Escobar-Juyo A., Caballero-García G., Blanco-Castillo I.P. The effect of gabapentin in bucco-facial allodynia. Experimental correlation of the trigeminal nerve, Rev Neurol 12, 1999, 1147–1153. [PubMed] [Google Scholar]
- 55.Obermann M., Yoon M.S., Sensen K., Maschke M., Diener H.C., Katsarava Z. Efficacy of pregabalin in the treatment of trigeminal neuralgia, Cephalalgia 28, 2008, 174–181. [DOI] [PubMed] [Google Scholar]
- 56.Hamandi K., Sander J.W. Pregabalin: a new antiepileptic drug for refractory epilepsy, Seizure 15, 2006, 73–78. [DOI] [PubMed] [Google Scholar]
- 57.Bailey K. Smith, Dewey C.W. The seizuring cat. Diagnostic work-up and therapy, J Feline Med Surg 11, 2009, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jorns T.P., Johnston A., Zakrzewska J.M. Pilot study to evaluate the efficacy and tolerability of levetiracetam (Keppra) in treatment of patients with trigeminal neuralgia, Eur J Neurol 16, 2009, 740–744. [DOI] [PubMed] [Google Scholar]
- 59.Domingues R.B., Kuster G.W., Aquino C.C. Treatment of trigeminal neuralgia with low doses of topiramate, Arq Neuropsiquiatr 65, 2007, 792–794. [DOI] [PubMed] [Google Scholar]
- 60.Siniscalchi A., Gallelli L., Marigliano N.M., Orlando P., De Sarro G. Use of topiramate for glossodynia, Pain Med 8, 2007, 531–534. [DOI] [PubMed] [Google Scholar]