Abstract
Recent progress in lung biology includes the description of a series of pulmonary stem and progenitor cells involved in homeostasis and regeneration of the respiratory system. Moreover, the contribution of extrapulmonary stem cells to healthy and pathological lung tissue has been observed and the developmental biology of such processes should provide important hints for understanding maintenance and repair of adult lung structure and function. Despite such remarkable advances, the phenotypic and especially the functional characterization of these stem and progenitor cells, and their derivatives, along with an understanding of the molecular cues and pathways underlying differentiation into specific respiratory lineages is still in its infancy. Accordingly, the role of endogenous and extrapulmonary stem cells in normal tissue repair and pathogenesis is still largely mysterious and added basic knowledge is required in order to explore their potential for novel regenerative therapies. This review provides an overview of the current state of the art in adult lung stem cell biology including technical aspects of isolation, characterization and differentiation, and a discussion of perspectives for future regenerative therapies.
Keywords: Stem cells, Respiratory, Pulmonary, Lung, Regeneration
1. Introduction
In contrast to other organs and tissue types such as heart, bone and cartilage, where stem cell research has already entered the clinical arena, corresponding developments in the respiratory system are only beginning. One of the main reasons for this lag compared to other organs is the lack of clear clinical perspectives. In the heart for instance, a variety of clinical studies involving stem cell-based myocardial regeneration after ischemic infarction have already been performed along with efforts to reconstruct congenital malformations by means of tissue engineering. Another explanation for the delayed development of regenerative therapies for respiratory disorders is the lack of suitable animal models that provide an unambiguous read out for the functionality of transplanted cells or for the regeneration of injured lung tissue.
Nevertheless, the importance of stem cell biology for understanding basic lung biology and for the treatment of respiratory diseases has now been recognized. Considerable efforts are currently underway to identify, characterize and understand pulmonary stem cells, and to explore the potential of extrapulmonary stem cells for therapeutic lung regeneration. Additionally, technological aspects including the identification of key differentiation factors and the analysis of molecular differentiation pathways are under study.
2. Biology and function of the lung
The lung is a complex organ composed of more than 60 different cell types [1]. Functionally, trachea and lung represent a tree of epithelial tubes for the conduction of air to the numerous primary gas exchange units, where circulating blood is oxygenated. Whereas bronchi and bronchioles are responsible for air conduction, the alveoli form the air/blood interface (Fig. 1 ).
Fig. 1.
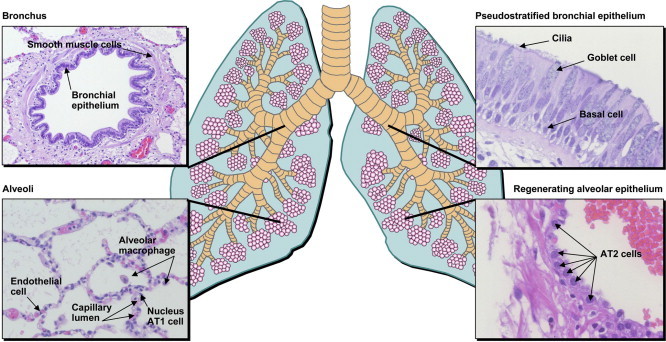
Morphology and histology of the lung.
Extension of the lung during inhalation occurs due to contraction of the diaphragm. During exhalation, the diaphragm relaxes and the airways and alveoli are able to contract due to elastic fibers in their walls. In addition, a thin layer of liquid lining the alveoli exerts surface tension, tending to collapse the lungs. On the other hand, surfactant, a mixture of different lipids and certain proteins, greatly decreases surface tension thereby reducing considerably the effort required during inhalation and extension of the lung.
Besides immigrated and resident immune cells, the lung contains fibroblasts as major components of the lung mesenchyme. Additionally, smooth muscle and endothelial cells serve as functional cell types in the pulmonary-vascular system. The lumenal widths of bronchi and bronchioles can be regulated by smooth muscle cells, and in the bronchi imperfect cartilage rings provide stability.
Different types of epithelium form the inner surface of the bronchi, bronchioles and alveoli. Pseudo-stratified epithelium with mucosal goblet cells, ciliated epithelial cells, basal cells and Clara cells cover the conducting airways of the lung. Clustered neuroendocrine cells are located predominantly within the large proximal airways as neuroendocrine bodies [2]. While goblet cells produce mucins that protect the respiratory epithelium, the ciliated cells are responsible for the transportation of mucus, microorganisms and foreign particles, and for respiratory fluid removal for optimal gas exchange [3]. Clara cells, dome-shaped with short microvilli, were originally described by their namesake, Max Clara, and are non-mucous and non-ciliated secretory cells. One of the main functions of secretory epithelial cells is to protect the bronchiolar (and tracheal) epithelium by releasing a small variety of products, including Clara cell secretory protein (CCSP) and components of the lung surfactant. They are also responsible for detoxifying harmful substances and may serve as progenitors of ciliated cells of the bronchiolar epithelium [4], [5], [6]. The majority of Clara cells are located in the respiratory bronchioles, which represent the transition from the conducting portion to the respiratory portion of the lung, the alveoli. These narrow channels are lined by a simple ciliated cuboidal epithelium. In contrast, the alveolar walls consist of a single layer of epithelium without ciliated epithelial cells. Ninety-five percent of the alveolar surface is covered by type I alveolar epithelial (AT1) cells. These are flattened, non-dividing cells that contain very limited numbers of organelles and form the air interface of the alveoli. In contrast, the more compact type II alveolar epithelial (AT2) cells contribute only to a minor extent to the alveolar surface. AT2 cells hold central functions: (i) production of surfactant. Surfactant decreases the surface tension within the lung thereby preventing a collapse of the alveoli, and fulfills specific immunological functions. (ii) Ion transport between alveolar fluid and interstitial tissue for alveolar fluid clearance [3]. (iii) AT2 cells represent progenitor cells, able to develop into AT1 cells [7], [8], [9] and (iv) exert certain immunological functions for instance by expressing Toll-like receptors [10], [11], by releasing or responding to cytokines [12], [13], [14], [15], [16] or by producing complement components [17], [18], [19].
3. Regenerative therapies for treatment of lung disorders
Pulmonary diseases are one of the leading causes of death worldwide. Statistics published by the World Health Organization indicate that of the 50.5 million deaths registered worldwide in 1990, 9.4 million (18.7%) were due to respiratory diseases. An increase in these numbers is predicted by 2020 to 11.9 million of a total of 68.3 million deaths worldwide caused by lung diseases. Besides infectious diseases such as pneumonia and tuberculosis, smoke-induced chronic obstructive pulmonary diseases (COPD), cancers of the trachea, bronchus and lung tissues play a major role. Further severe pulmonary diseases that frequently result in terminal lung failure with parenchymal, bronchial, or pulmonary-vascular pathologies include genetic disorders such as α-1-antitrypsin deficiency, which results in emphysema similar to COPD, and cystic fibrosis, vascular diseases (e.g. primary pulmonary hypertension) or interstitial lung disease that starts with inflammation of the bronchioles, alveoli or capillaries, and ends in pulmonary fibrosis.
At present, allogeneic lung transplantation is considered to be the only approach to treat patients with terminal pulmonary failure. However, continuing donor organ shortage results in a 30% preoperative lethality of potential lung transplant (LTx) recipients on waiting lists. Moreover, in spite of significant progress in surgical technique (e.g. minimal invasive technique) and therapy of acute rejection as well as management of pulmonary infections, postoperative survival times after LTx are still significantly shorter when compared to those after transplantation of other parenchymal organs [20]. Clearly, alternative therapeutic approaches are urgently needed. At this point, the question arises, how can stem cell-based approaches be helpful for treating severe lung disorders?
Stem cell-based therapies of genetic disorders are probably simpler to establish than treatment of other respiratory diseases. Indeed, numerous recent studies have focused on the application of gene therapy to the correction of hereditary lung disorders, including attempts to treat alpha-1-antitrypsin deficiency [21], cystic fibrosis [22], [23], [24] or surfactant protein deficiency [25].
Since efficient pulmonary in vivo gene transfer with stable long term expression is still difficult to achieve [26], genetic in vitro engineering of suitable stem cells with subsequent specific in vitro differentiation and intravenous or intra-tracheal delivery is a promising alternative. Another option would be allogeneic transplantation of unaffected cells. However, as long as no immunologically matched cells are available or induction of immunological tolerance is not a clinical reality, pharmacological immunosuppression would be required just as for organ transplantation.
Several hurdles have to be overcome before approaches aimed at correcting gene deficiencies using genetically modified stem cell derivatives can be realized. These include general technological and safety aspects of therapeutic gene transfer, the stem cell source, expansion, specific differentiation and potential purification of stem cell progeny, and last but not least the mode of delivery.
Although in vitro gene transfer into stem cells is easier to achieve than efficient in vivo gene transfer into pulmonary target cells, this does not guarantee stable long term expression at an optimal level. In particular if integrating vectors (e.g. gamma retroviral or lentiviral ones) are applied, the risk of malignant transformation [27] has to be considered and ideally, appropriate cell clones should be selected. Importantly, a suitable stem cell source has to be identified that can be easily isolated or generated, cultured and expanded. Whether the optimal stem cell type, such as an exogenous adult stem cell (e.g. from bone marrow), an endogenous lung stem cell, which is more difficult to isolate especially in case of autologous cells, or a pluripotent stem cell (e.g. an induced pluripotent stem cell [28], [29]), can be easily collected mainly depends on the target disease. For example, for treatment of patients with cystic fibrosis or surfactant protein B (SP-B) deficiency, stem cell derived bronchial [30] or alveolar epithelial cells have to be generated. Efforts are already underway to differentiate functional type II alveolar epithelial (AT2) cells [31], [32], [33], the exclusive natural producers of SP-C [34], for future therapy of surfactant protein C (SP-C) deficiency. For treatment of alpha-1-antitrypsin deficiency, several cell types such as hepatocytes as the principle natural source of alpha-1-antitrypsin, AT2 cells and alveolar macrophages [35], [36] are likely candidates.
While alpha-1-antitrypsin deficiency in early stages may be treatable by transplantation of transgenic stem cell derivatives, this possibility appears less likely in advanced stage cases after massive development of emphysema. This issue leads to the general question whether advanced stages of lung diseases that affect the fragile structure of the lung can be reversed by regenerative therapies.
Circulating blood cells including those derived from bone marrow contribute to repair of LPS- [37], [38], elastase- [39], [40], irradiation- [41], [42] naphthalene- [43] or bleomycin- [44], [45] mediated acute lung injuries, thus preventing pathological consequences including emphysema or fibrosis. However, evidence for reversal of manifested pulmonary emphysema or fibrosis is scarce [46], [47].
In cases of emphysema, the alveoli undergo continuous damage especially caused by digestive neutrophil-derived elastase. This finally leads to an almost complete and irreversible loss of alveolar microstructure (Fig. 2 ), a dramatic reduction of lung surface, and progressive shortness of breath. In addition, the patient experiences great difficulties in exhaling due to a reduced elasticity of the lung tissue, and the bronchial tubes may collapse, trapping air in the lungs. At least in a genetic mouse model, it has been demonstrated that wild type bone marrow transplantation can revert mild emphysema [46]. Another study reported that systemic administration of adrenomedullin, a potent vasodilator peptide, resulted in limited regeneration of alveoli and vasculature in elastase-induced established emphysema [40]. Although at this point mechanisms of the above findings are largely unknown, and although it appears unlikely that final stages of emphysema can be reverted using stem cell therapies, there is hope that prior to substantial manifestation of emphysema reversion of the disease may be possible applying novel regenerative technologies.
Fig. 2.
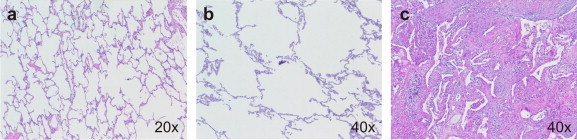
Histology of normal lung tissue (a), advanced stages of emphysema (b) and pulmonary fibrosis (c).
In contrast to emphysema, interstitial lung disease (ILD), which is a general term that includes a variety of chronic lung disorders, does not only lead to destruction of the alveolar epithelium but to scarring (or fibrosis) of the interstitium (Fig. 2). In general, ILD appears to start with an inflammation affecting the bronchioles, the alveoli or capillaries of the lung. Inflammation of these parts of the lung may heal or may lead to necrosis or apoptosis of alveolar epithelium [48] and irreparable scarring of the lung tissue with abnormal accumulation of fibroblasts, and deposition of collagen and other extracellular matrix components in the interstitial and alveolar spaces. ILD can also be initiated by epithelial injury and abnormal wound repair in the absence of prior inflammation [49], [50]. Apparently, AT1 cells are more sensitive to injury than AT2 cells, and lung injuries that are restricted to AT1 cells are generally repairable by persisting AT2 cells [51]. In contrast, additional loss of AT2 cells as a source for new AT1 cells leads to irreversible loss of alveoli and progressing scar formation [52]. Probably, ILD is triggered by epithelial damage and depends on further injury-related immunological processes [49]. Similarly, a special form of pulmonary fibrosis in allografts, the so-called bronchiolitis obliterans syndrome, is triggered by ischemia reperfusion injury and depends additionally on alloreactive immunological processes [53], [54]. Some ILDs have known causes including occupational and environmental exposures, sarcoidosis, and connective tissue or collagen diseases such as rheumatoid arthritis and systemic sclerosis. Some (idiopathic) ILDs have unknown causes. An effective therapeutic regimen has yet to be identified and developed.
Attempts to reverse alveolar damage and to prevent lung fibrosis through cell transplantation and induction of AT2 cell proliferation have been reported, including application of isolated alveolar epithelial cells [47] or keratinocyte growth factor (KGF), which has been shown to specifically promote mitogenesis in AT2 cells, thereby reducing the degree of lung injury [55], [56]. Likewise, mesenchymal stem cells (MSCs) have been transplanted for inhibition of fibrosis [44], [45]. According to these studies, intra-tracheal and even intravenous application of AT2 cells or MSCs inhibited developing fibrosis. Notably, a low proportion of donor cells were detected in the injured lung tissue, only, and their differentiation into pulmonary phenotypes was not demonstrated unequivocally since epithelial characteristics of the donor derived cells were not demonstrated, or merely single marker proteins such as aquaporin 5 or SP-C were analyzed [44], [45]. In addition, cell fusion of donor cells with resident bronchioalveolar cells could not be excluded [45]. Hence, the observed inhibition of fibrosis is more likely based on paracrine effects. Clearly, concepts of treating fibrosis with bone marrow or blood-derived stem and progenitor cells, and especially with mesenchymal stem cells, have been challenged by recent findings that fibrotic tissue in ILD does not exclusively arise from resident fibroblasts or by epithelial–mesenchymal-transition (EMT) [57], [58], but also from blood and bone marrow derived circulating cells [59], [60], [61], [62], [63], [64], [65], [66], [67].
Although recent studies [44], [45], [47] suggest that stem cells may inhibit the progress of bleomycin-induced fibrosis, it is doubtful whether such treatments will ever be able to reverse advanced scar formation. Nevertheless, similar to emphysema, early disease stages may be treatable using stem cell-based strategies.
Despite numerous studies, the role of adult extrapulmonary (stem) cells in lung injury, repair and remodeling is still unclear and controversial. In particular, it is contentious whether circulating extrapulmonary cells are able to adopt the fate of respiratory epithelial cells [68]. Recent studies suggested that such cells are able to support pathological mechanisms, moreover also the development of pulmonary diseases can be suppressed, while induction of pulmonary regeneration has been reported, too. Obviously, even minor technical details can determine whether a certain treatment protocol is able to inhibit progressing pulmonary diseases and to promote regeneration of injured lung tissue. Even if a certain stem cell type, for instance MSCs, does not efficiently differentiate into a functional cell type of interest, for example type II alveolar epithelial cells, alternative mechanisms such as cell fusion or paracrine effects may provide the basis for development of novel regenerative therapies that act via activation of endogenous regeneration potential.
4. Identification and characterization of endogenous respiratory stem and progenitor cells
The respiratory system can be divided into two anatomically distinct regions – the conducting airways including trachea, bronchi and bronchioli, and the gas-exchanging airspaces, the alveoli. The existence of region-specific stem or progenitor cells along the murine epithelial pulmonary tree has been demonstrated. Historically, basal cells [69], [70], Clara cells [71], [72] and type II alveolar epithelial cells [7], [9], [73] have been considered as stem or progenitor cells of the conducting and respiratory compartments of the lung.
In the trachea, stem cells may be localized in different niches. IB4-lectinpos basal cells [74], [75] and cells in the submucosal gland ducts [76] appear to hold stem cell function. These cell types were identified by: i) differentiation potential of labeled cells in vitro [77] or after inoculation in de-epithelialized rat tracheas and transplantation in nude mice [78], [79], [80], [81], ii) high cytokeratin expression as a marker for a primitive phenotype [76] or iii) bromodeoxyuridine (BrdU) label retention documenting extended slow turnover of so-called label-retaining cells (LRCs)[76]. Multipotency of cytokeratin 14 expressing basal cells was demonstrated in a mouse model of naphthalene injury using a bitransgenic ligand-regulated Cre-loxP reporter approach in which expression of an ubiquitously expressed LacZ reporter was dependent on activation of the cytokeratin 14 promoter [74]. Tagged clusters of basal cells were multipotent and capable of generating basal, ciliated and secretory cells, other cells were unipotent and formed progeny with the morphology of basal cells, only. Thus, basal cells may consist of varied subpopulations with different differentiation potential that cannot currently be distinguished. Basal cells can be isolated according to the following protocol:
-
1.
After surgical isolation, the trachea should be stored on ice in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen Corporation Products, Karlsruhe, Germany), with antibiotics added, if necessary.
-
2.
Incubate the trachea with 0.05% protease for 24–48 h at 4 °C. Important: as activity of proteases usually varies, it is necessary to pretest different types/lots of proteases.
-
3.
Transfer the resulting cell suspension into a falcon tube and rinse the tracheal lumen with stop-medium (DMEM + 20% fetal calf serum, FCS). Transfer this stop-medium to the falcon tube. After pelleting, the cells are washed once with DMEM.
-
4.
Resuspend the cells in Bronchial Epithelial Growth Medium (BEGM, Lonza GmbH, Wuppertal, Germany) and plate in tissue culture flasks (3 × 106 cells per T25 flask).
-
5.
After 48 h, remove medium with non-adherent cells. The remaining cells contain ciliated epithelium as well as basal cells.
-
6.
After 1 week, cells should be almost confluent. For passaging, cells are incubated with trypsin. By passaging, ciliated cells should be eliminated and the majority of remaining adherent cells represent basal cells.
Similar to the trachea, different stem cell types exist in the bronchial epithelium. The non-ciliated columnar Clara cells [71], [82], [83], [84], [85], [86], [87] have historically been considered as stem cells of the conducting airways. Similar to basal cells, labeled Clara cells have been inoculated in de-epithelialized rat tracheal grafts and gave rise to Clara and ciliated cells but not to basal and mucous cells [88], indicating that Clara cells are probably not the universal stem cells of the bronchial compartment. Although not confirmed, one report suggested that in turn ciliated cells have the potential to become Clara cells [89]. Others inoculated primary bronchial epithelial cells, which were not characterized in detail and may contain Clara cells as well as other cell types, after retroviral labeling in denuded rat tracheas [90], [91], [92]. In this model, lineage analyses demonstrated the existence of stem/progenitor cells with either limited or oligopotent capacity of differentiation. Single clones developed basal, goblet and ciliated epithelial morphologies, a subset of clones was able to develop mucosal glands [91].
A mixed population of human bronchial epithelial cells including Clara cells can be isolated according to Engelhardt et al. [92] as follows:
-
1.
Soak and rinse human bronchial tissue in Modified Eagle’s medium (MEM) with 10 μg/ml DNAse and 0.5 mg/ml dithiothreitol, and with antibiotics added, if necessary, for 4–12 h at 4 °C.
-
2.
Transfer the tissue to the same medium supplemented with 0.1% protease 14 and incubate for an additional 30–34 h at 4 °C.
-
3.
Add FCS to a final concentration of 10% and remove cells by agitation and blunt scraping.
-
4.
After pelleting, the cells are washed two times in Ham’s F12 containing 10% FCS.
-
5.
Finally, resuspend the cells in Ham’s F12 hormonally defined medium containing 1 μM hydrocortisone, 10 μg/ml insulin, 30 nM thyroxine, 5 μg/ml transferrin, 25 ng/ml epidermal growth factor, 3.75 μg/ml endothelial cell growth supplement, 10 ng/ml cholera toxin and additional antibiotics.
-
6.
Cells are plated in tissue culture plates at a density of 2 × 106 cells per 100 mm plate.
-
7.
Typically, cultures can be harvested on day 4 by trypsination.
Another cell type with a distribution very similar to Clara cells is pulmonary neuroendocrine cells (PNECs) [93]. PNECs, identified based on their expression of calcitonin gene-related peptide [94], are principally derived from endoderm and not from neural crest [95]. In several studies Clara cells were ablated, either using naphthalene [83], [85], [86] or a conditional transgenic approach expressing herpes simplex thymidine kinase under control of the Clara cell secretory protein promoter [83], [87]. Napthalene is metabolized by P450-2F2 and -2B2 isoenzymes, expressed principally within Clara cells, resulting in the production of the highly toxic naphthalene 1 R 2 S epoxide [96], [97]. According to Hong et al. [83], PNECs function as self-renewing population of the neuroendocrine bodies (NEBs) [87], but are not able to contribute to the formation of new Clara cells and ciliated epithelium [83]. In contrast, a subpopulation of Clara cells that are deficient in xenobiotic metabolizing enzymes, resistant to naphthalene and specifically maintained in the NEB environment are capable of regenerating Clara cell depleted epithelium [83]. Similar naphthalene-resistant Clara-like cells could be found at the junction between conducting and respiratory epithelium, the bronchioalveolar duct junction (BADJ) [82], [83], [86], [87].
Other early studies detected a small population of undifferentiated epithelial cells that labeled with tritiated thymidine but could not be distinguished at this time from slow cycling Clara cells by any of the known markers [72]. Already in 1988, it had been discussed whether there may be a pluripotent epithelial stem cell common to anatomically and functionally discrete segments of the distal respiratory system, namely bronchioli and alveoli [98]. Moreover, it had been proposed that these cells were located in a transitional zone between respiratory bronchioli and alveoli [99]. This bronchioalveolar stem cell (BASC) has now been identified as the normal counterpart to SP-C/CCSP double positive cells in adenomas [100] and is located at the BADJ [84]. These cells are also naphthalene-resistant and may be identical to cells that have been described earlier [72], [82], [83], [86], [87]. In contrast to Clara cells and AT2 cells, murine BASCs are positive for the membrane-bound stem cell markers CD34, the classical hematopoietic stem cell marker that is also present on endothelial cells [101], and Sca-1, a stem cell marker that can be found not only on murine bone marrow and resident stem cells of different tissue types, but also on endothelial cells including pulmonary ones [102]. Interestingly, anti mouse Sca-1 has been used to isolate human cardiac stem cells (A. Smits, Utrecht, pers. communication) although no Sca-1 homolog has been identified in humans. Expression of both markers has enabled the flow cytometrical purification and characterization of BASCs. BASCs can be isolated from murine lung tissue as follows:
-
1.
After anesthesia mice are perfused with 10 mL PBS, followed by intra-tracheal instillation of 1 mL dispase (Becton Dickinson, Heidelberg, Germany, 50 U/mL) and 1 mL 1% low melting point agarose.
-
2.
Subsequently, lungs are minced on ice and incubated in 0.001% DNAse (Sigma–Aldrich) and 2 μg/mL collagenase/dispase (Roche, Grenzach–Wyhlen, Germany) in PBS for 45 min at 37 °C.
-
3.
The resulting crude cell suspension is filtered through 100 and 40 μm cell strainers (Fisher Scientific) and centrifuged at 800 rpm, 5 min at 4 °C.
-
4.
Cells are resuspended in red blood cell lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 4 min, washed in DME/10% fetal bovine serum (FBS), and resuspended in PBS/10% FBS (PF10) at 1 × 106 cells/100 μL.
-
5.
For FACSorting, cells are incubated 15 min at 4 °C with 1 μL of the appropriate antibodies (anti-Sca-1, anti-CD45, anti-PECAM, anti-CD34) for surface marker staining.
Isolated BASCs are not ciliated, and can be cultured on feeder cells similar to embryonic stem cells (ESCs) [84] forming clonal colonies.
Immunocytological analyses demonstrated the presence of CCSP and SP-C, and lack of expression of CD45 and PECAM. Importantly, in vitro differentiation experiments on Matrigel demonstrated their multilineage capacity leading to the formation of CCSPpos, SP-Cpos and aquaporin 5pos cells.
Interestingly, Ling et al. described Oct-4pos resident stem cells in the neonatal murine and the adult human lung that are located at the bronchioalveolar junction similar to BASCs [103]. Oct-4 is a typical marker for embryonic stem cells but has now also been reported in tissue resident stem cells [104], [105], [106]. In this context it should be noted that analysis of Oct-4 expression in adult tissue and stem cells critically depends on the discrimination of Oct-4 isoforms and pseudogenes as described in detail by Liedtke et al. [107]. Oct-4 expressing pulmonary stem cells appears to be the main target for SARS (severe acute respiratory syndrome) infection [103]. For isolation of these cells, lung tissue was minced and enzymatically digested resulting in a mixed cell population containing stem cell colonies with epithelial morphology. Obviously, cell growth was supported by pulmonary mesenchymal cells similar to ESCs that depend on embryonic feeder cells under standard culture conditions. In culture, these cells also express SSEA-1, another ESC marker. Whether SSEA-1 is also expressed in vivo has not been analyzed. In addition, these cells express Sca-1 similarly to BASCs, and Cytokeratin-7, a marker for epithelium, as well as CCSP and CyP450, both Clara cell markers, and appear to represent Clara/alveolar epithelial stem cells [103]. Differentiation experiments resulted in expression of AT2 (SP-C) and AT1 (aquaporin 5) markers. Thus, Ling et al. apparently described the same cells as Kim et al. [84]. Although in another publication the distribution of Oct-4pos/CD34pos (as already mentioned, no protein that corresponds to the murine Sca-1 is known in humans) and SARS-permissive cells was found to be extended to the alveoli of the adult human lung, and although these cells may not express SSEA1, cytokeratin, SP-C and SP-A in vivo, it is likely that they represent the human counterpart of BASCs [108].
As already mentioned above, AT2 cells are historically considered as stem/progenitor cells of the alveolar epithelium capable of differentiating into AT1 cells. A recent report suggested that AT2 cells contain several subpopulations with E-cadherinpos, damage resistant cells representing a putative progenitor subpopulation with high telomerase activity [109].
Murine AT2 cells can be isolated using the following protocol according to Rice et al. [110]:
-
1.
Inject 0.2 ml Nembutal intraperitoneal for anesthesia of mice.
-
2.
After opening the abdominal cavity, exsanguinate the mice by severing the inferior vena cava and the left renal artery. After isolation, the trachea can be cannulated with a 20-gauge luer stub adapter. Then the diaphragm has to be cut, and the chest plate and thymus have to be removed.
-
3.
With the use of a 21-gauge needle fitted on a 10-ml syringe, lungs can be perfused with 10–20 ml 0.9% saline via the pulmonary artery.
-
4.
Subsequently, dispase (3 ml) has to be rapidly instilled through the cannula in the trachea followed by 0.5 ml agarose (45 °C).
-
5.
Lungs should be covered immediately with ice for 2 min to gel the agarose. Then lungs can be removed from the animals and incubated in 1 ml dispase for 45 min (25 °C).
-
6.
Lungs are then transferred to a 60-mm culture dish containing 7 ml of HEPES-buffered DMEM and 100 U/ml DNAse I, and lung tissue is gently teased from the bronchi.
-
7.
Subsequently, the cell suspension is filtered through progressively smaller cell strainers (100 and 40 μm) and nylon gauze (20 μm).
-
8.
Cells can be collected by centrifugation at 130g for 8 min (4 °C) and placed on prewashed 100-mm tissue culture plates that have been coated for 24–48 h at 4 °C with 42 μg CD45 and 16 μg CD32 in PBS. After incubation for 1–2 h at 37 °C, AT2 cells can be gently panned from the plate and collected by centrifugation.
-
9.
Finally, AT2 cells are resuspended in Bronchial Epithelial Growth Medium (BEGM, Lonza GmbH) supplemented with 10 ng/ml keratinocyte growth factor (KGF, Amgen Inc., Thousand Oaks, CA). Cells are cultured in six well plates coated with 1 ml of Matrigel that had been diluted with BEGM (1:2, BEGM/Matrigel). Type II cells are seeded into each well at a density of 5 × 105 cells/cm2.
Recently, side population (SP) cells have been identified in the lung [111], [112], [113], [114], [115] similar to bone marrow [116] and other organs. Independent of origin and localization, SP cells are defined by their ability to efflux Hoechst dyes such as Hoechst 33342 [116]. This phenotype is based on the expression of the multidrug resistance-like (MDR) Bcrp1/ABCG2 transporter [117]. SP cells can be detected by FACS after excitation by a UV-laser resulting in a characteristic cell population with low blue and red fluorescence emission. As the uptake of the Hoechst dyes is an active biological process, great attention to staining conditions is necessary to obtain optimal resolution of the profile. Analysis and sorting of lung SP cells is possible based on following protocol:
The Hoechst concentration, staining time, and staining temperature are all CRITICAL. Likewise, subsequent to the staining process, the cells should be maintained at 4 °C in order to prevent further dye efflux. Pulmonary SP cells can be collected from enzyme-digested lungs and by bronchoalveolar lavage (BAL).
-
1.
For lung digestion, mince freshly isolated lung tissue with a razor blade and incubate the minced tissue with 0.1% collagenase (Roche Diagnostics, Indianapolis, IN), 2.4 U/ml dispase (Roche Diagnostics), and 2.5 mM CaCl2 at 37 °C for 1.5 h. After adding an equal volume DMEM + 20% FBS to stop the enzymatic digestion, cell suspensions are sequentially filtered through 70- and 40-μm filters to remove nonspecific debris, and then resuspended at a concentration of 1 × 106 cells per ml in pre-warmed DMEM + 5% FBS. Mix well.
-
2.
BAL cells can be collected according to published protocols [118]: in brief, after cannulation of the trachea, lungs are insufflated with 1 ml of PBS. Wash fluid is removed and placed into a collection tube. This process has to be repeated until each lung has been lavaged with a total of 5 ml of PBS. To remove nonspecific debris, cell suspensions are sequentially filtered through 70- and 40-μm filters and then resuspended at a concentration of 1 × 106 cells/ml in pre-warmed DMEM + 5% FBS.
-
3.
Ensure that a water bath is precisely at 37 °C. The medium needs to be pre-warmed beforehand.
-
4.
Add Hoechst dye to a final concentration of 5 μg/ml.
-
5.
Mix the cells well, and place in the 37 °C water bath for 90 min exactly. Tubes should be mixed several times during incubation.
-
6.
After 90 min, spin down the cells at 4 °C and resuspend in ice-cold PBS.
-
7.
All further proceedings should be carried out at 4 °C to prohibit leakage of the Hoechst dye. Add 2 μg/ml of 7-AAD to the suspended cells and mix about 5 min before FACS analysis. This will allow for the discrimination of dead versus live cells as 7-AAD permeates only cells that do not have an intact membrane.
-
8.
Flow cytometry analysis of Hoechst-stained cells can be performed on a triple laser flow cytometer (MoFlo; Cytomation, Fort Collins, CO). An argon multiline UV (333–363 nm) laser is necessary to excite Hoechst dye. Fluorescence emission can be collected with a 405/30 band pass filter (Hoechst blue) and a 660 ALP (Hoechst red). A second 488-nm argon laser has to be used to excite 7-AAD.
Lung SP cells can be sub-divided into CD45pos and CD45neg cells. According to Summer et al. [114], ∼60–75% of murine lung SP cells express CD45 [113], [114]. CD45pos SP cells exhibit a small, round phenotype with scant cytoplasm and large nuclear to cytoplasmic ratio similar to bone marrow SP cells. In contrast, CD45neg SP cells are larger with abundant cytoplasm. Hematoxylin staining demonstrated extensive granularity. CD34 was detected on murine CD45pos cells only, whereas Sca-1 was present on the majority of both subpopulations, which may indicate overlap of side population cells with BASCs [84]. Furthermore, all CD45pos cells and about 40% of the CD45neg side population cells were CD31pos suggesting the possibility that lung SP cells play a role in homeostasis of the pulmonary vasculature [113]. A recent study compared clonogenicity of SP preparations derived from different lung compartments under different culture conditions [115]. Clonogenicity was compromised by previous bleomycin treatment and varied significantly among different preparations and different media. Whereas expression of the lung epithelial markers SP-C and CCSP was almost undetectable in SP preparations, vimentin mRNA was highly enriched suggesting a mesenchymal phenotype with wound-repair potential [115]. These results are in contrast to previous studies [82], [113]; most likely, this is based on technical reasons. In particular, drawbacks of the Hoechst efflux technique concerning the parallel purification of different stem cell types have been emphasized [115]. As determined by whole bone marrow transplantations, at least a considerable proportion of CD45pos and CD45neg lung side population cells are bone marrow derived [114]. However, recent results do not exclude that a fraction of side population cells exist in the adult lung that has been formed already during embryogenesis.
Finally, a very recent study investigating the characteristics of plastic-adherent cells from bronchioalveolar lavages of pulmonary allotransplants provided the first evidence of a pulmonary mesenchymal stem cell able to differentiate into adipocytes, chondrocytes and osteocytes [119]. The detection of donor derived Y-chromosomes in these cells as well as their long pulmonary persistence for more than one decade strongly suggest that these MSCs do not represent circulating “passenger MSCs” that have been co-transplanted with the lung but true resident MSCs.
Plastic-adherent MSCs from bronchioalveolar lavages or minced lung tissue can be isolated based on standard protocols for MSC isolation:
-
1.
Perfom lung digestion or alveolar lavage as described above for isolation of SP cells.
-
2.
Centrifuge for 5 min 600g and discard supernatant.
-
3.
In case of lung tissue, add 10 ml 0.2% sodium chloride for lysis of erythrocytes and incubate for exactly 1 min, then add 10 ml 1.6% sodium chloride and centrifuge 5 min 600g; discard supernatant.
-
4.
Resuspend the cell pellet in PBS with 2% FCS (important: FCS lot should be pretested for culture of MSCs) and 1 mM EDTA. Then, carefully overlay one volume of percoll working solution (GE Healthcare Life Sciences) with two volumes of the cell suspension.
-
5.
Centrifuge 30 min at room temperature and 300g, without brake!
-
6.
Remove mononuclear cell layer with a Pasteur pipette, dilute up to 50 ml with PBS with 2% FCS and 1 mM EDTA. Centrifuge 5 min 600g at 4 °C.
-
7.
Discard supernatant and resuspend the cell pellet in MSCGM medium (Lonza GmbH, Wuppertal, Germany). Plate the cell suspension in a small tissue culture flask. Cell adherence can take up to one week, thereafter medium can be changed to remove non-adherent cells.
Resulting adherent MSCs should strongly express CD73 (SH3/SH4), CD90 (Thy-1), and CD105 (SH2). Moreover, they should be negative for the hematopoietic lineage markers CD14, CD34, and CD45.
In conclusion, a considerable number of resident respiratory stem cells have been described that need further characterization as to their roles in lung homeostasis and repair, their differentiation and potential overlap between different subtypes. At present, it is still not always clear whether indeed stem or progenitor cells have been identified, and further work is necessary to achieve a clear characterization and classification of the numerous cell types contributing to lung homeostasis and repair. Although significant progress in the field has been achieved, further improvement and development of methods for purification of resident stem cells and functional testing in vitro is mandatory. To date, lung stem cell purification has frequently been limited by the fact that molecular markers for respiratory stem cells are intracellular proteins that are not restricted to specific levels of the stem cell hierarchy. Thus, identification of additional markers, biochemical activities or biophysical properties enabling discrimination and purification of distinct stem cell types would greatly advance further analysis of mechanisms underlying stem cell-based lung repair and the development of regenerative therapies.
Importantly, not much is known about differences between murine and human pulmonary stem cells. Considering the fact that major differences between rodents and humans have been observed concerning postnatal/adult pulmonary growth and proliferation kinetics [120], the relevance of mouse models for the development of regenerative therapies may be limited. Whether resident pulmonary stem cells can finally act as a basis to induce endogenous regeneration after injury or whether some of these cells can be isolated, expanded and differentiated for cell therapeutic approaches remains to be demonstrated.
5. Analyzing the role of extrapulmonary (stem) cells in lung homeostasis, regeneration and pathology
The contribution of circulating extrapulmonary (stem) cells to respiratory repair/regeneration and tissue homeostasis has been investigated in animal models [37], [41], [42], [43], [121], [122], [123], [124]. Bone marrow contains classical hematopoietic as well as other types of stem cells including mesenchymal stem cells [125] and so-called MAPCs [123]. Recent studies suggest that bone marrow derived cells have the capacity to produce not only hematopoietic phenotypes but can also differentiate into non-hematopoietic derivatives including muscle [126], heart [127], [128], brain [129] and liver [121]. Although many of these studies are controversial and contradictory reports demonstrate that certain findings are based on technical artifacts [130], [131], [132], [133], [134], [135], it is clear that the commitment of bone marrow stem cells is not as strict as initially thought.
A series of studies were conducted in mouse models of cellular transplantation after lethal irradiation [38], [41], [42], [46], [68], [121], [122], [123], [124]. For instance, Krause et al. reported a robust contribution of donor cells to bronchial and alveolar epithelium after transplantation of single bone marrow stem cells, reaching 3.74% for bronchial and 20.20% for AT2 cells after 11 months [121]. The same group observed significantly less contribution to alveolar epithelium after lethal irradiation and whole bone marrow transplantation with a range of 0.6–8.1% of donor AT2 cells [42]. No donor derived AT2 cells but AT1 cells and significant numbers of donor derived interstitial fibroblasts (between 5% and 20% in isolated cells) have been detected in a parabiotic mouse model [41]. Similarly, another study did not find any donor derived AT2 cells, but AT1 cells, and additionally donor-derived CD34pos CD45neg cells that may represent endothelial cells [38]. Cytokeratinpos epithelial as well as CD34pos endothelial cells of donor origin were observed by Ishizawa et al. after elastase-induced emphysema [39]. This was not the case in a model of compensatory lung growth: bone marrow transplantation after lethal irradiation did result in bone marrow engraftment and vascular differentiation within the bone marrow, but not in contribution to pulmonary endothelium [124].
Further studies aimed to investigate more specifically the differentiation potential of diverse populations of bone marrow stem cells. Side population cells [111], plastic-adherent bone marrow [43], [61], [124], MAPCs [123] or MSCs [44], [45], [136] were applied intra-tracheally [37], [43] or systemically [45], [68], [124], [136]. Some studies observed significant differentiation of plastic-adherent bone marrow/MSCs into bronchial and alveolar epithelium [43], [45]. Furthermore, it was demonstrated that bone marrow derived cells can also promote pathogenic events: obviously, circulating fibroblasts or fibroblast precursors from bone marrow contribute to pulmonary fibrosis [61].
Although many of the above studies provided important findings and stimulated further research in this field, the significance of mouse models with respect to human disease is clearly limited and transplantation of whole bone marrow, specific bone marrow stem cell types as well as induced mobilization obviously has experimental limitations. Importantly, some careful studies raised severe doubts on the pulmonary differentiation potential of bone marrow derived cells [40], [46], [68], [137], [138].
The heterogeneous results underscore that the pulmonary differentiation potential of bone marrow is far from being well characterized or even understood. Technical difficulties associated with evaluating pulmonary engraftment, in particular the ability to distinguish by immunohistochemical methods differentiated organ cells from hematopoietic or mesenchymal marrow-derived cells may account for the described discrepancies. Notably, fusion of bone marrow cells with resident somatic ones can result in donor cells with pulmonary phenotypes, and discrimination is possible, only, using sophisticated transgenic approaches. Additionally, there is no evidence so far that the observed donor-derived cells expressing certain pulmonary markers are phenotypically or functionally comparable to the corresponding native cell type.
In addition, specific effects of the different animal model may be responsible for the divergent results. For instance, homing as well as pulmonary integration and differentiation is apparently dependent on pathological processes and tissue injury. Studies performing cell transplantation after induced lung injury [37], [38], [41], [43], [44], [45], [61] demonstrated enhanced cell engraftment, differentiation and regeneration compared to non-injured lungs.
With respect to the limited significance of animal models for regeneration of the human respiratory system, studies on human tissue are of special importance. A number of recent papers that analyzed not only the presence and frequency but also the phenotype of recipient cells in transplanted solid organs indicated that circulating cells enter the lung and are able to develop into different pulmonary cell types [139], [140], [141], [142], [143]. Most studies combined Y-chromosome-specific fluorescence in situ hybridization (FISH) with immunohistology for cytokeratin or SP-C. Another study used a more sophisticated approach combining immunostaining with laser microdissection and short tandem repeat PCR. Dependent on the approach and the analyzed grafts, 0–25% of donor/recipient chimerism was observed in bronchi, alveoli and glands. An initial study did not detect any donor/recipient chimerism in epithelia of bronchi or alveoli, or in the pulmonary blood vessels of the donor lung [139], which is in accordance with another study demonstrating only minor recipient contribution to the donor epithelium [143]. Later studies reported low levels of chimerism in respiratory epithelium between 2% and 8% [141], [142]. Much higher levels [up to 42%] were observed in pulmonary endothelium [141]. In contrast, another study that did not use FISH but performed short tandem repeat PCR of laser microdissected cells observed epithelial chimerism in bronchi, alveoli and glands of up to 25% [140]. Since recent findings are so far inconsistent, results should be judged with caution. At present, it is unclear whether technical details or differences in the analyzed donor grafts account for the reported differences. Moreover, it is still a matter of debate whether the potential contribution of extrapulmonary cells to respiratory epithelium and endothelium indicate replacement of lost cell populations during normal tissue homeostasis or is related to allogeneic immune reactions, inflammation and corresponding tissue damage.
Other studies analyzed patients who received bone marrow transplantation [140], [142], [144]. Mattson et al. as well as Albera et al. investigated pulmonary donor chimerism after bone marrow transplantation by FISH/immunohistology. Both studies suggested that circulating cells, which contribute to pulmonary epithelium, are bone marrow derived [142], [144]. In contrast, Kleeberger et al. denied contribution of bone marrow cells to pulmonary epithelium [140].
In conclusion, and similar to existing animal studies, studies on transplant patients provide divergent results. The respiratory differentiation potential of extrapulmonary stem cells is controversial and the usefulness of such cells for the development of regenerative therapies for the treatment of lung diseases is unclear. Clearly, the development of improved and novel molecular, histological and transgenic techniques is urgently needed to allow an unequivocal analysis of the contribution of adult stem cells to pulmonary pathogenesis and regeneration.
6. Concluding remarks
The identification of different resident pulmonary stem cells and the accumulating evidence for a pulmonary differentiation potential of certain extrapulmonary stem cells raises hope that stem cell biology and technology will eventually turn cell therapy into a useful treatment for a variety of lung diseases. Nonetheless, compared to other organ systems such as bone and heart, research on pulmonary regeneration and development of regenerative therapies targeting respiratory disorders is only beginning. Similar to other fields of stem cell research, the pulmonary differentiation potential of adult stem and progenitor cells is currently highly controversial. Further work in transgenic models as well as intensified research on human stem cells is urgently needed to clarify whether adult stem cells will be clinically useful and whether pluripotent stem cell types such as spermatogonial, embryonic or induced pluripotent stem cells will play a role. Further problems in the field include the lack of suitable animal models that closely mirror important human diseases and enable proof of functionality of stem cell derivatives. The fact that the most common lung diseases result in emphysema or fibrosis, both characterized by continuing pathological alteration of the natural tissue morphology and finally leading to almost complete destruction of the functional lung architecture, poses further challenges. At present, it is difficult to imagine that advanced stages of such diseases can be reversed using stem cell-based technologies. Thus, while pulmonary stem cell biology is exciting and in many ways promising, our meager understanding of these processes is limiting rapid therapeutic advances.
Acknowledgments
Histological images were kindly provided by Florian Länger, Institute of Pathology, Hannover Medical School. The author is grateful to Ina Gruh, LEBAO, for revising the manuscript.
References
- 1.Stone K.C., Mercer R.R., Gehr P., Stockstill B., Crapo J.D. Am. J. Respir. Cell Mol. Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 2.Bannister L.H. Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery. Churchill Livingstone; 1995. [Google Scholar]
- 3.Folkesson H.G., Matthay M.A. Am. J. Respir. Cell Mol. Biol. 2006;35:10–19. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth P.J., Muller B. Pathol. Res. Pract. 1999;195:487–493. doi: 10.1016/S0344-0338(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 5.Evans M.J., Johnson L.V., Stephens R.J., Freeman G. Lab. Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 6.Evans M.J., Shami S.G., Cabral-Anderson L.J., Dekker N.P. Am. J. Pathol. 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- 7.Adamson I.Y., Bowden D.H. Lab. Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- 8.Danto S.I., Shannon J.M., Borok Z., Zabski S.M., Crandall E.D. Am. J. Respir. Cell Mol. Biol. 1995;12:497–502. doi: 10.1165/ajrcmb.12.5.7742013. [DOI] [PubMed] [Google Scholar]
- 9.Evans M.J., Cabral L.J., Stephens R.J., Freeman G. Am. J. Pathol. 1973;70:175–198. [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong L., Medford A.R., Uppington K.M., Robertson J., Witherden I.R., Tetley T.D., Millar A.B. Am. J. Respir. Cell Mol. Biol. 2004;31:241–245. doi: 10.1165/rcmb.2004-0078OC. [DOI] [PubMed] [Google Scholar]
- 11.Droemann D., Goldmann T., Branscheid D., Clark R., Dalhoff K., Zabel P., Vollmer E. Histochem. Cell Biol. 2003;119:103–108. doi: 10.1007/s00418-003-0497-4. [DOI] [PubMed] [Google Scholar]
- 12.Homer R.J., Zheng T., Chupp G., He S., Zhu Z., Chen Q., Ma B., Hite R.D., Gobran L.I., Rooney S.A., Elias J.A. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L52–L59. doi: 10.1152/ajplung.00438.2001. [DOI] [PubMed] [Google Scholar]
- 13.Thorley A.J., Ford P.A., Giembycz M.A., Goldstraw P., Young A., Tetley T.D. J. Immunol. 2007;178:463–473. doi: 10.4049/jimmunol.178.1.463. [DOI] [PubMed] [Google Scholar]
- 14.Vanderbilt J.N., Mager E.M., Allen L., Sawa T., Wiener-Kronish J., Gonzalez R., Dobbs L.G. Am. J. Respir. Cell Mol. Biol. 2003;29:661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- 15.Witherden I.R., Vanden Bon E.J., Goldstraw P., Ratcliffe C., Pastorino U., Tetley T.D. Am. J. Respir. Cell Mol. Biol. 2004;30:500–509. doi: 10.1165/rcmb.4890. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A.K., Fernandez L.G., Awad A.S., Kron I.L., Laubach V.E. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L105–L113. doi: 10.1152/ajplung.00470.2006. [DOI] [PubMed] [Google Scholar]
- 17.Strunk R.C., Eidlen D.M., Mason R.J. J. Clin. Invest. 1988;81:1419–1426. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman B.L., Despins A.W., Kreutzer D.L. J. Immunol. 1990;145:592–598. [PubMed] [Google Scholar]
- 19.Zhao Y.X., Andoh A., Shimada M., Takaya H., Hata K., Fujiyama Y., Bamda T. Int. J. Mol. Med. 2000;5:415–419. doi: 10.3892/ijmm.5.4.415. [DOI] [PubMed] [Google Scholar]
- 20.Trulock E.P., Christie J.D., Edwards L.B., Boucek M.M., Aurora P., Taylor D.O., Dobbels F., Rahmel A.O., Keck B.M., Hertz M.I. J. Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld M.A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L.E., Paakko P.K., Gilardi P., Stratford-Perricaudet L.D., Perricaudet M. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 22.Rosenecker J., Huth S., Rudolph C. Curr. Opin. Mol. Ther. 2006;8:439–445. [PubMed] [Google Scholar]
- 23.Sueblinvong V., Suratt B.T., Weiss D.J. Clin. Chest Med. 2007;28:361–379. doi: 10.1016/j.ccm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang G., Bunnell B.A., Painter R.G., Quiniones B.C., Tom S., Lanson N.A., Jr., Spees J.L., Bertucci D., Peister A., Weiss D.J., Valentine V.G., Prockop D.J., Kolls J.K. Proc. Natl. Acad. Sci. USA. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yei S., Bachurski C.J., Weaver T.E., Wert S.E., Trapnell B.C., Whitsett J.A. Am. J. Respir. Cell Mol. Biol. 1994;11:329–336. doi: 10.1165/ajrcmb.11.3.8086169. [DOI] [PubMed] [Google Scholar]
- 26.T. Lee, K.W. Southern, Cochrane Database Syst. Rev., CD005599, 2007. [DOI] [PubMed]
- 27.Li Z., Dullmann J., Schiedlmeier B., Schmidt M., von Kalle C., Meyer J., Forster M., Stocking C., Wahlers A., Frank O., Ostertag W., Kuhlcke K., Eckert H.G., Fehse B., Baum C. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin, Thomson J.A. Science. 2007 doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 30.Coraux C., Nawrocki-Raby B., Hinnrasky J., Kileztky C., Gaillard D., Dani C., Puchelle E. Am. J. Respir. Cell Mol. Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 31.Rippon H.J., Polak J.M., Qin M., Bishop A.E. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- 32.Wang D., Haviland D.L., Burns A.R., Zsigmond E., Wetsel R.A. Proc. Natl. Acad. Sci. USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkler M.E., Mauritz C., Groos S., Kispert A., Menke S., Hoffmann A., Gruh I., Schwanke K., Haverich A., Martin U. Cloning Stem Cells. 2008 doi: 10.1089/clo.2007.0075. [DOI] [PubMed] [Google Scholar]
- 34.Wohlford-Lenane C.L., Durham P.L., Snyder J.M. Am. J. Respir. Cell Mol. Biol. 1992;6:225–234. doi: 10.1165/ajrcmb/6.2.225. [DOI] [PubMed] [Google Scholar]
- 35.Perlmutter D.H., Cole F.S., Kilbridge P., Rossing T.H., Colten H.R. Proc. Natl. Acad. Sci. USA. 1985;82:795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutten A., Venembre P., Seta N., Hamelin J., Aubier M., Durand G., Dehoux M.S. Am. J. Respir. Cell Mol. Biol. 1998;18:511–520. doi: 10.1165/ajrcmb.18.4.2772. [DOI] [PubMed] [Google Scholar]
- 37.Gupta N., Su X., Popov B., Lee J.W., Serikov V., Matthay M.A. J. Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 38.Yamada M., Kubo H., Kobayashi S., Ishizawa K., Numasaki M., Ueda S., Suzuki T., Sasaki H. J. Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 39.Ishizawa K., Kubo H., Yamada M., Kobayashi S., Numasaki M., Ueda S., Suzuki T., Sasaki H. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 40.Murakami S., Nagaya N., Itoh T., Iwase T., Fujisato T., Nishioka K., Hamada K., Kangawa K., Kimura H. Am. J. Respir. Crit. Care Med. 2005;172:581–589. doi: 10.1164/rccm.200409-1280OC. [DOI] [PubMed] [Google Scholar]
- 41.Abe S., Boyer C., Liu X., Wen F.Q., Kobayashi T., Fang Q., Wang X., Hashimoto M., Sharp J.G., Rennard S.I. Am. J. Respir. Crit. Care Med. 2004;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- 42.Theise N.D., Henegariu O., Grove J., Jagirdar J., Kao P.N., Crawford J.M., Badve S., Saxena R., Krause D.S. Exp. Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 43.Wong A.P., Dutly A.E., Sacher A., Lee H., Hwang D.M., Liu M., Keshavjee S., Hu J., Waddell T.K. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz L.A., Gambelli F., McBride C., Gaupp D., Baddoo M., Kaminski N., Phinney D.G. Proc. Natl. Acad. Sci. USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rojas M., Xu J., Woods C.R., Mora A.L., Spears W., Roman J., Brigham K.L. Am, J Respir. Cell Mol. Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi Y., Oyaizu H., Taketani S., Minamino K., Yamaguchi K., Shultz L.D., Iwasaki M., Tomita M., Suzuki Y., Nakano K., Koike Y., Yasumizu R., Sata M., Hirama N., Kubota I., Fukuhara S., Ikehara S. Stem Cells. 2006;24:2071–2077. doi: 10.1634/stemcells.2005-0575. [DOI] [PubMed] [Google Scholar]
- 47.Serrano-Mollar A., Nacher M., Gay-Jordi G., Closa D., Xaubet A., Bulbena O. Am. J. Respir. Crit. Care Med. 2007;176:1261–1268. doi: 10.1164/rccm.200610-1491OC. [DOI] [PubMed] [Google Scholar]
- 48.Uhal B.D., Joshi I., True A.L., Mundle S., Raza A., Pardo A., Selman M. Am. J. Physiol. 1995;269:L819–L828. doi: 10.1152/ajplung.1995.269.6.L819. [DOI] [PubMed] [Google Scholar]
- 49.Strieter R.M. Chest. 2005;128:526S–532S. doi: 10.1378/chest.128.5_suppl_1.526S. [DOI] [PubMed] [Google Scholar]
- 50.Zuo F., Kaminski N., Eugui E., Allard J., Yakhini Z., Ben-Dor A., Lollini L., Morris D., Kim Y., DeLustro B., Sheppard D., Pardo A., Selman M., Heller R.A. Proc. Natl. Acad. Sci. USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomashefski J.F., Jr. Clin. Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 52.Uhal B.D. Am. J. Physiol. 1997;272:L1031–L1045. doi: 10.1152/ajplung.1997.272.6.L1031. [DOI] [PubMed] [Google Scholar]
- 53.Belperio J.A., Keane M.P., Burdick M.D., Lynch J.P., 3rd, Xue Y.Y., Berlin A., Ross D.J., Kunkel S.L., Charo I.F., Strieter R.M. J. Clin. Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher A.J., Wardle J., Dark J.H., Corris P.A. J. Heart Lung Transplant. 2002;21:1206–1212. doi: 10.1016/s1053-2498(02)00450-3. [DOI] [PubMed] [Google Scholar]
- 55.Panos R.J., Bak P.M., Simonet W.S., Rubin J.S., Smith L.J. J. Clin. Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano T., Deterding R.R., Simonet W.S., Shannon J.M., Mason R.J. Am. J. Respir. Cell Mol. Biol. 1996;15:433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 57.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Am. J. Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain R., Shaul P.W., Borok Z., Willis B.C. Am. J. Respir. Cell Mol. Biol. 2007;37:38–47. doi: 10.1165/rcmb.2006-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brocker V., Langer F., Fellous T.G., Mengel M., Brittan M., Bredt M., Milde S., Welte T., Eder M., Haverich A., Alison M.R., Kreipe H., Lehmann U. Am. J. Respir. Crit. Care Med. 2006;173:1276–1282. doi: 10.1164/rccm.200509-1381OC. [DOI] [PubMed] [Google Scholar]
- 60.Dunsmore S.E., Shapiro S.D. J. Clin. Invest. 2004;113:180–182. doi: 10.1172/JCI20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hashimoto N., Jin H., Liu T., Chensue S.W., Phan S.H. J. Clin. Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Epperly M.W., Guo H., Gretton J.E., Greenberger J.S. Am. J. Respir. Cell Mol. Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 63.Garantziotis S., Steele M.P., Schwartz D.A. J. Clin. Invest. 2004;114:319–321. doi: 10.1172/JCI22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenberger J.S., Epperly M.W., Gretton J., Jefferson M., Nie S., Bernarding M., Kagan V., Guo H.L. Curr. Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 65.Ishii G., Sangai T., Sugiyama K., Ito T., Hasebe T., Endoh Y., Magae J., Ochiai A. Stem Cells. 2005;23:699–706. doi: 10.1634/stemcells.2004-0183. [DOI] [PubMed] [Google Scholar]
- 66.Lama V.N., Phan S.H. Proc. Am. Thorac. Soc. 2006;3:373–376. doi: 10.1513/pats.200512-133TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips R.J., Burdick M.D., Hong K., Lutz M.A., Murray L.A., Xue Y.Y., Belperio J.A., Keane M.P., Strieter R.M. J. Clin. Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang J.C., Summer R., Sun X., Fitzsimmons K., Fine A. Am. J. Respir. Cell Mol. Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breuer R., Zajicek G., Christensen T.G., Lucey E.C., Snider G.L. Am. J. Respir. Cell Mol. Biol. 1990;2:51–58. doi: 10.1165/ajrcmb/2.1.51. [DOI] [PubMed] [Google Scholar]
- 70.Evans M.J., Cox R.A., Shami S.G., Wilson B., Plopper C.G. Am. J. Respir. Cell Mol. Biol. 1989;1:463–469. doi: 10.1165/ajrcmb/1.6.463. [DOI] [PubMed] [Google Scholar]
- 71.Evans M.J., Cabral-Anderson L.J., Freeman G. Lab. Invest. 1978;38:648–653. [PubMed] [Google Scholar]
- 72.Plopper C.G., Nishio S.J., Alley J.L., Kass P., Hyde D.M. Am. J. Respir. Cell Mol. Biol. 1992;7:606–613. doi: 10.1165/ajrcmb/7.6.606. [DOI] [PubMed] [Google Scholar]
- 73.Evans M.J., Cabral L.J., Stephens R.J., Freeman G. Exp. Mol. Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 74.Hong K.U., Reynolds S.D., Watkins S., Fuchs E., Stripp B.R. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 75.Schoch K.G., Lori A., Burns K.A., Eldred T., Olsen J.C., Randell S.H. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L631–L642. doi: 10.1152/ajplung.00112.2003. [DOI] [PubMed] [Google Scholar]
- 76.Borthwick D.W., Shahbazian M., Krantz Q.T., Dorin J.R., Randell S.H. Am. J. Respir. Cell Mol. Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 77.Moller P.C., Partridge L.R., Cox R.A., Pellegrini V., Ritchie D.G. Tissue Cell. 1989;21:195–198. doi: 10.1016/0040-8166(89)90064-5. [DOI] [PubMed] [Google Scholar]
- 78.Inayama Y., Hook G.E., Brody A.R., Cameron G.S., Jetten A.M., Gilmore L.B., Gray T., Nettesheim P. Lab. Invest. 1988;58:706–717. [PubMed] [Google Scholar]
- 79.Inayama Y., Hook G.E., Brody A.R., Jetten A.M., Gray T., Mahler J., Nettesheim P. Am. J. Pathol. 1989;134:539–549. [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson N.F., Hubbs A.F. Am. J. Respir. Cell Mol. Biol. 1990;3:579–585. doi: 10.1165/ajrcmb/3.6.579. [DOI] [PubMed] [Google Scholar]
- 81.Liu J.Y., Nettesheim P., Randell S.H. Am. J. Physiol. 1994;266:L296–L307. doi: 10.1152/ajplung.1994.266.3.L296. [DOI] [PubMed] [Google Scholar]
- 82.Giangreco A., Reynolds S.D., Stripp B.R. Am. J. Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong K.U., Reynolds S.D., Giangreco A., Hurley C.M., Stripp B.R. Am. J. Respir. Cell Mol. Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 84.Kim C.F., Jackson E.L., Woolfenden A.E., Lawrence S., Babar I., Vogel S., Crowley D., Bronson R.T., Jacks T. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 85.Peake J.L., Reynolds S.D., Stripp B.R., Stephens K.E., Pinkerton K.E. Am. J. Pathol. 2000;156:279–286. doi: 10.1016/S0002-9440(10)64728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds S.D., Giangreco A., Power J.H., Stripp B.R. Am. J. Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds S.D., Hong K.U., Giangreco A., Mango G.W., Guron C., Morimoto Y., Stripp B.R. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 88.Brody A.R., Hook G.E., Cameron G.S., Jetten A.M., Butterick C.J., Nettesheim P. Lab. Invest. 1987;57:219–229. [PubMed] [Google Scholar]
- 89.Park K.S., Wells J.M., Zorn A.M., Wert S.E., Laubach V.E., Fernandez L.G., Whitsett J.A. Am. J. Respir. Cell Mol. Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zepeda M.L., Chinoy M.R., Wilson J.M. Somat. Cell Mol. Genet. 1995;21:61–73. doi: 10.1007/BF02255823. [DOI] [PubMed] [Google Scholar]
- 91.Engelhardt J.F., Schlossberg H., Yankaskas J.R., Dudus L. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- 92.Engelhardt J.F., Yankaskas J.R., Wilson J.M. J. Clin. Invest. 1992;90:2598–2607. doi: 10.1172/JCI116155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Lommel A., Bolle T., Fannes W., Lauweryns J.M. Arch. Histol. Cytol. 1999;62:1–16. doi: 10.1679/aohc.62.1. [DOI] [PubMed] [Google Scholar]
- 94.McCormack D.G., Salonen R.O., Barnes P.J. Life Sci. 1989;45:2405–2412. doi: 10.1016/0024-3205(89)90004-0. [DOI] [PubMed] [Google Scholar]
- 95.Warburton D., Wuenschell C., Flores-Delgado G., Anderson K. Biochem. Cell Biol. 1998;76:971–995. [PubMed] [Google Scholar]
- 96.Plopper C.G., Macklin J., Nishio S.J., Hyde D.M., Buckpitt A.R. Lab. Invest. 1992;67:553–565. [PubMed] [Google Scholar]
- 97.Plopper C.G., Suverkropp C., Morin D., Nishio S., Buckpitt A. J. Pharmacol. Exp. Ther. 1992;261:353–363. [PubMed] [Google Scholar]
- 98.Tyler N.K., Hyde D.M., Hendrickx A.G., Plopper C.G. Am. J. Anat. 1988;182:215–223. doi: 10.1002/aja.1001820303. [DOI] [PubMed] [Google Scholar]
- 99.Emura M. In vitro cellular & developmental biology––animal. 1997;33:3–14. doi: 10.1007/s11626-997-0015-4. [DOI] [PubMed] [Google Scholar]
- 100.Jackson E.L., Willis N., Mercer K., Bronson R.T., Crowley D., Montoya R., Jacks T., Tuveson D.A. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fina L., Molgaard H.V., Robertson D., Bradley N.J., Monaghan P., Delia D., Sutherland D.R., Baker M.A., Greaves M.F. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 102.Kotton D.N., Summer R.S., Sun X., Ma B.Y., Fine A. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L990–L996. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- 103.Ling T.Y., Kuo M.D., Li C.L., Yu A.L., Huang Y.H., Wu T.J., Lin Y.C., Chen S.H., Yu J. Proc. Natl. Acad. Sci. USA. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kues W.A., Petersen B., Mysegades W., Carnwath J.W., Niemann H. Biol. Reprod. 2005;72:1020–1028. doi: 10.1095/biolreprod.104.031229. [DOI] [PubMed] [Google Scholar]
- 105.Beltrami A.P., Cesselli D., Bergamin N., Marcon P., Rigo S., Puppato E., D’Aurizio F., Verardo R., Piazza S., Pignatelli A., Poz A., Baccarani U., Damiani D., Fanin R., Mariuzzi L., Finato N., Masolini P., Burelli S., Belluzzi O., Schneider C., Beltrami C.A. Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 106.Hajdu M., Luttun A., Pelacho B., Burns T.C., Chase L., Gutierrez-Perez M., Jiang Y., Lenvik T., Vas V., Uher F., Sebestyen A., Verfaillie C. Oncol. Res. 2007;13:302–310. doi: 10.1007/BF02940309. [DOI] [PubMed] [Google Scholar]
- 107.Liedtke S., Enczmann J., Waclawczyk S., Wernet P., Kogler G. Cell Stem Cell. 2007;1:364–366. doi: 10.1016/j.stem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y., Chan V.S., Zheng B., Chan K.Y., Xu X., To L.Y., Huang F.P., Khoo U.S., Lin C.L. J. Exp. Med. 2007;204:2529–2536. doi: 10.1084/jem.20070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reddy R., Buckley S., Doerken M., Barsky L., Weinberg K., Anderson K.D., Warburton D., Driscoll B. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L658–L667. doi: 10.1152/ajplung.00159.2003. [DOI] [PubMed] [Google Scholar]
- 110.Rice W.R., Conkright J.J., Na C.L., Ikegami M., Shannon J.M., Weaver T.E. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L256–L264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- 111.Abe S., Lauby G., Boyer C., Rennard S.I., Sharp J.G. Cytotherapy. 2003;5:523–533. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- 112.Dubin P.J., Kolls J.K. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L475–L476. doi: 10.1152/ajplung.00149.2004. [DOI] [PubMed] [Google Scholar]
- 113.Summer R., Kotton D.N., Sun X., Ma B., Fitzsimmons K., Fine A. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 114.Summer R., Kotton D.N., Sun X., Fitzsimmons K., Fine A. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L477–L483. doi: 10.1152/ajplung.00020.2004. [DOI] [PubMed] [Google Scholar]
- 115.Reynolds S.D., Shen H., Reynolds P.R., Betsuyaku T., Pilewski J.M., Gambelli F., Di Giuseppe M., Ortiz L.A., Stripp B.R. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- 116.Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. J. Exp. Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou S., Schuetz J.D., Bunting K.D., Colapietro A.M., Sampath J., Morris J.J., Lagutina I., Grosveld G.C., Osawa M., Nakauchi H., Sorrentino B.P. Nat. Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 118.Hessel E.M., Cruikshank W.W., Van Ark I., De Bie J.J., Van Esch B., Hofman G., Nijkamp F.P., Center D.M., Van Oosterhout A.J. J. Immunol. 1998;160:2998–3005. [PubMed] [Google Scholar]
- 119.Lama V.N., Smith L., Badri L., Flint A., Andrei A.C., Murray S., Wang Z., Liao H., Toews G.B., Krebsbach P.H., Peters-Golden M., Pinsky D.J., Martinez F.J., Thannickal V.J. J. Clin. Invest. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thurlbeck W.M. Pathobiol. Annu. 1975;5:1–34. [PubMed] [Google Scholar]
- 121.Krause D.S., Theise N.D., Collector M.I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S.J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 122.Grove J.E., Lutzko C., Priller J., Henegariu O., Theise N.D., Kohn D.B., Krause D.S. Am. J. Respir. Cell Mol. Biol. 2002;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- 123.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R., Reyes M., Lenvik T., Lund T., Blackstad M., Du J., Aldrich S., Lisberg A., Low W.C., Largaespada D.A., Verfaillie C.M. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 124.Voswinckel R., Ziegelhoeffer T., Heil M., Kostin S., Breier G., Mehling T., Haberberger R., Clauss M., Gaumann A., Schaper W., Seeger W. Circ. Res. 2003;93:372–379. doi: 10.1161/01.RES.0000087643.60150.C2. [DOI] [PubMed] [Google Scholar]
- 125.Phinney D.G., Prockop D.J. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 126.Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 127.Badorff C., Brandes R.P., Popp R., Rupp S., Urbich C., Aicher A., Fleming I., Busse R., Zeiher A.M., Dimmeler S. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 128.Balsam L.B., Wagers A.J., Christensen J.L., Kofidis T., Weissman I.L., Robbins R.C. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 129.Meletis K., Frisen J. J. Cell Biol. 2001;155:699–702. doi: 10.1083/jcb.200110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wagers A.J., Sherwood R.I., Christensen J.L., Weissman I.L. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 131.Alvarez-Dolado M., Pardal R., Garcia-Verdugo J.M., Fike J.R., Lee H.O., Pfeffer K., Lois C., Morrison S.J., Alvarez-Buylla A. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 132.Gruh I., Beilner J., Blomer U., Schmiedl A., Schmidt-Richter I., Kruse M.L., Haverich A., Martin U. Circulation. 2006;113:1326–1334. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 133.Klose R., Kemter E., Bedke T., Bittmann I., Kelsser B., Endres R., Pfeffer K., Schwinzer R., Wolf E. Transplantation. 2005;80:222–230. doi: 10.1097/01.tp.0000164817.59006.c2. [DOI] [PubMed] [Google Scholar]
- 134.Nygren J.M., Jovinge S., Breitbach M., Sawen P., Roll W., Hescheler J., Taneera J., Fleischmann B.K., Jacobsen S.E. Nat. Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 135.Ying Q.L., Nichols J., Evans E.P., Smith A.G. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 136.Sueblinvong V., Loi R., Eisenhauer P.L., Bernstein I.M., Suratt B.T., Spees J.L., Weiss D.J. Am. J. Respir. Crit. Care Med. 2007 doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Loi R., Beckett T., Goncz K.K., Suratt B.T., Weiss D.J. Am. J. Respir. Crit. Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kotton D.N., Fabian A.J., Mulligan R.C. Am. J. Respir. Cell Mol. Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yousem S.A., Sonmez-Alpan E. Chest. 1991;99:275–279. doi: 10.1378/chest.99.2.275. [DOI] [PubMed] [Google Scholar]
- 140.Kleeberger W., Versmold A., Rothamel T., Glockner S., Bredt M., Haverich A., Lehmann U., Kreipe H. Am. J. Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suratt B.T., Cool C.D., Serls A.E., Chen L., Varella-Garcia M., Shpall E.J., Brown K.K., Worthen G.S. Am. J. Respir. Crit. Care Med. 2003;168:318–322. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- 142.Albera C., Polak J.M., Janes S., Griffiths M.J., Alison M.R., Wright N.A., Navaratnarasah S., Poulsom R., Jeffery R., Fisher C., Burke M., Bishop A.E. Tissue Eng. 2005;11:1115–1121. doi: 10.1089/ten.2005.11.1115. [DOI] [PubMed] [Google Scholar]
- 143.Zander D.S., Baz M.A., Cogle C.R., Visner G.A., Theise N.D., Crawford J.M. Transplantation. 2005;80:206–212. doi: 10.1097/01.tp.0000165095.39320.50. [DOI] [PubMed] [Google Scholar]
- 144.Mattsson J., Jansson M., Wernerson A., Hassan M. Transplantation. 2004;78:154–157. doi: 10.1097/01.tp.0000132326.08628.74. [DOI] [PubMed] [Google Scholar]


