Abstract
Clin Microbiol Infect 2011; 17(Suppl. 6): E1–E59
Abstract
This document is an update of Guidelines published in 2005 and now includes scientific publications through to May 2010. It provides evidence‐based recommendations for the most common management questions occurring in routine clinical practice in the management of adult patients with LRTI. Topics include management outside hospital, management inside hospital (including community‐acquired pneumonia (CAP), acute exacerbations of COPD (AECOPD), acute exacerbations of bronchiectasis) and prevention. Background sections and graded evidence tables are also included. The target audience for the Guideline is thus all those whose routine practice includes the management of adult LRTI.
Keywords: Antibiotic, community‐acquired pneumonia, exacerbation of COPD, guidelines, lower respiratory tract infection
Introduction
In 2005 the European Respiratory Society (ERS), in collaboration with The European Society for Clinical Microbiology and Infectious Diseases (ESCMID), published guidelines on the management of lower respiratory tract infections (LRTI) in adults [1]. This document was based on published scientific literature up to the end of 2002. We have now updated these guidelines to include publications to May 2010. The Taskforce responsible for guideline development has been sponsored by the ERS and ESCMID. Members of the Taskforce are members of the sponsoring ERS and/or ESCMID.
Our objective is to provide evidence‐based recommendations for the most common management questions occurring in routine clinical practice in the management of adult patients with LRTI. The target audience for the guidelines is thus all those whose routine practice includes the management of adult LRTI.
This document begins with definitions and background sections on microbial cause, resistance and pharmacokinetics/pharmacodynamics, with conventional referencing. The guideline section captures management outside hospital, management inside hospital (including community‐acquired pneumonia (CAP), acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and acute exacerbations of bronchiectasis) and prevention. The guidelines are about the management of infection. This means that for conditions such as AECOPD, aspects of management that are unreleated to infection (e.g. use of steroids or bronchodilators) are not included. It contains the graded recommendations but also the background information for each recommendation, with details about each new cited reference and the evidence grades. Because this is an update, original data and publications have usually not been repeated and the reader is referred to the original publication [1] for this. As this is an update using the same methodologies, the layout of the document, including text, recommendations and evidence tables, is the same as in 2005.
Methods
Using the same search filter as for the 2005 document (this is described in detail in the previous publication [1] and website documents—http://www.ersnet.org; http://www.escmid.org) we identified relevant manuscripts in PubMed published from July 2002 to May 2010. Thereby we retrieved 15 261 titles and loaded them into an electronic database. From these, 1677 titles were identified as potentially relevant publications by the expert panel members. The same process of evidence appraisal and grading (Appendix 1) and recommendation development and grading (Appendix 2) as in the 2005 document was used.
The document takes each clinical question for which there was a recommendation in the 2005 guidelines and presents new information when available, followed by a new recommendation. In some circumstances, because of lack of new evidence, or sometimes even in the presence of new evidence, the recommendation is unchanged from 2005. Where this is the case it is indicated.
In some parts of the guidelines new questions and recommendations have been added to cover relevant areas not included in the 2005 guidelines (e.g. aspiration pneumonia).
LRTI Definitions
The guidelines are to be used to guide the management of adults with lower respiratory tract infection (LRTI). As will be seen in the following text, this diagnosis, and the other clinical syndromes within this grouping, can be difficult to identify accurately. In the absence of agreed definitions of these syndromes, these guidelines are to be used when, in the opinion of a clinician, an LRTI syndrome is present. The following are put forward as definitions to guide the clinician, but it will be seen in the ensuing text that some of these labels will always be inaccurate. These definitions are pragmatic and based on a synthesis of available studies. They are primarily meant to be simple to apply in clinical practice, and this might be at the expense of scientific accuracy. These definitions are not mutually exclusive, with lower respiratory tract infection being an umbrella term that includes all others, which can also be used for cases that cannot be classified into one of the other groups. No new evidence has been identified that would lead to a change in the clinical definitions, which are therefore unchanged from the 2005 publication.
Since the publication of the 2005 guidelines the term health care‐associated pneumonia (HCAP) has been put forward to capture groups of patients with pneumonia, some acquired outside hospital, expected to be caused by similar pathogens, but different from those usually found in community‐acquired LRTI. In the opinion of the taskforce members the evidence base does not support the use of this term as being clinically relevant in Europe at the present time. HCAP is therefore not covered further in this document [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17].
Lower respiratory tract infection
An acute illness (present for 21 days or less), usually with cough as the main symptom, with at least one other lower respiratory tract symptom (sputum production, dyspnoea, wheeze or chest discomfort/pain) and no alternative explanation (e.g. sinusitis or asthma).
Acute Bronchitis (AB)
An acute illness, occurring in a patient without chronic lung disease, with symptoms including cough, which may or may not be productive and associated with other symptoms or clinical signs that suggest LRTI and no alternative explanation (e.g. sinusitis or asthma).
Influenza
An acute illness, usually with fever, together with the presence of one or more of headache, myalgia, cough or sore throat.
Suspected community‐acquired pneumonia (CAP)
An acute illness with cough and at least one of new focal chest signs, fever >4 days or dyspnoea/tachypnoea, and without other obvious cause.
Definite community‐acquired pneumonia (CAP)
As above, but supported by chest radiograph findings of lung shadowing that is likely to be new. In the elderly, the presence of chest radiograph shadowing accompanied by acute clinical illness (unspecified) without other obvious cause.
Acute exacerbation of COPD (AECOPD)
An event in the natural course of the disease characterized by a worsening of the patient’s baseline dyspnoea, cough and/or sputum beyond day‐to‐day variability sufficient to warrant a change in management. If chest radiograph shadowing, consistent with infection, is present the patient is considered to have CAP.
Acute exacerbation of bronchiectasis (AEBX)
In a patient with features suggestive of bronchiectasis, an event in the natural course of the disease characterized by a worsening in the patient’s baseline dyspnoea, and/or cough and/or sputum beyond day‐to‐day variability sufficient to warrant a change in management. If chest radiograph shadowing, consistent with infection, is present the patient is considered to have CAP.
Background
What new information is available about the microbiological causes of LRTI?
Wide variations between studies regarding the frequency of each microorganism can be explained by several factors, including differences in studied populations (e.g. age range or other risk factors), geographical area, studied samples and microbiological methods; for example, some studies focused on bacterial agents and others on viruses and intracellular bacteria. Supplementing traditional diagnostic methods with new technology‐based methods could achieve higher microbial yield [18].
-
1
In the majority of studies of LRTI there is a large proportion of cases with no pathogen identified, either because the appropriate tests were not performed (as is usually the rule in outpatients) or the organism was missed. Age >70 years, renal and cardiac co‐morbid illnesses and non alveolar infiltrates were independently associated with a higher proportion of unknown aetiology in 204 patients hospitalized for CAP [19].
-
2
On the other hand, multiple organisms may be found in adults, as already described in youngsters. Paediatric studies have found polymicrobial infections in CAP: dual viral infection is present in 0–14%, dual bacterial infection in 0–14%, and mixed viral‐bacterial infection in 3–30% [20].
In hospitalized adult non‐immunocompromised patients, polymicrobial CAP occurred in 6–26% [21, 22, 23, 24, 25, 26, 27, 28]. Gutierrez et al. [21] report two or more pathogens at all ages, and as well in inpatients and outpatients, the most frequent combinations being those of bacteria with an atypical organism (29%) and two bacteria (29%); patients with mixed pneumonia are likely to have more co‐morbidities and a more altered outcome. Angeles Marcos et al. [23] found that the most frequent co‐pathogens were S. pneumoniae and C. pneumoniae, and the most frequent combinations S. pneumoniae and either influenza or parainfluenza virus, and influenza virus with C. pneumoniae. De Roux et al. [29] reported that in the 10% of patients with mixed CAP, S. pneumoniae was the most prevalent microorganism; the most frequent combination was S. pneumoniae with H. influenzae; influenza virus A and S. pneumoniae was the most frequent association in the mixed pyogenic pneumonia group. Among the 17% of patients with mixed infections, Song et al. found 73% of patients with two different pathogens, 13% with three different pathogens and 13% with four different pathogens. The most frequent combination was S. pneumoniae with C. pneumoniae (15%). Mixed infections were found in 25% of patients with pneumococcal CAP [28]. Jennings et al. [27] found that polymicrobial infections involving bacterial and viral pathogens occurred in 15% of patients with CAP and might be associated with severe pneumonia. Johansson et al. found two or more pathogens in 35% of patients with CAP with a determined aetiology, most commonly S. pneumoniae together with a respiratory virus [18]. Evidence of concurrent bacterial infection was found in lung tissue specimens from 22 (29%) of the 77 US patients with fatal cases of confirmed 2009 pandemic influenza A (H1N1), including 13% caused by S. pneumoniae [30].
Table 1 summarizes the microbiological aetiologies of LRTI in the community. Studies have investigated the microbiological causes of CAP in outpatients (Table 2) and patients admitted to hospital (Table 3) or to the intensive care unit (Table 4). Most studies of mild infections suggest that microbial aetiologies in outpatients are similar to those in hospitalized patients [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57].
Table 1.
Aetiology of lower respiratory tract infection in the community (%). (Blank boxes indicate organism not sought)
| Reference | n | SP | HI | MC | SA | MP | CS | CPne | CB | Virus | Influenza |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Boldy et al. [91] | 42 | 3.0 | 3.0 | 3.0 | 0 | 8.0 | 0 | 0 | 21.0 | 10.0 | |
| Creer et al. 2006 [65] | 80 | 18.8 | 6.3 | 1.2 | 1.2 | 61.3 | 23.8 | ||||
| Everett [92] | 187 | 6.0 | 2.0 | 0 | 6.0 | 4.0 | |||||
| Fransen and Wolontis [93] | 78 | 8.0 | 3.0 | 3.0 | 3.0 | 20.0 | 12.0 | ||||
| Graffelman et al. [94] | 145 | 6.2 | 9.0 | 2.1 | 9.0 | 1.3 | 39.0 | 30.3 | |||
| Holm et al. [95] | 364 | 6 | 4 | 1 | <1 | 3 | <1 | 24 | 10 | ||
| Hopstaken et al. [96] | 247 | 2.9 | 13.8 | 2.9 | |||||||
| Macfarlane et al. [97] | 206 | 30.0 | 8.0 | 2 | 1.0 | 0.5 | 0.5 | 8.0 | 5.0 | ||
| Macfarlane et al. [98] | 316 | 17.1 | 9.8 | 2.2 | 7.3 | 17.4 | 19.3 | 7.3 | |||
| Shaw and Fry [99] | 40 | 16.0 | 14.0 | 10.0 | 5.0 | 3.0 | 0 | 11.0 | 11.0 | ||
| Range | 3–30 | 3–14 | 1–3 | 1–10 | 0.5–9 | 0–3 | 0–0.5 | 6–61 | 4–30 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative bacilli; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CPne, Chlamydophila pneumoniae; CPsi, Chlamydophila psittaci; CB, Coxiella burnetii.
Table 2.
Aetiology of community‐acquired pneumonia in the community (%). (Blank boxes indicate organism not sought)
| Reference | n | SP | HI | LP | MC | SA | GNEB | MP | CS | CPne | CPsi | CB | Virus | Influenza |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almirall et al. [100] | 105 | 12.4 | 0 | 2.9 | 0 | 0 | 7.6 | 15.2 | 15.2 | 0 | 0 | 11.4 | 0 | |
| Almirall et al. [31] | 232 | 11.6 | 0.4 | 2.2 | 0 | 0.4 | 3.9 | 9.5 | 0 | 2.2 | 14.2 | 8. 2 | ||
| Beovic et al. [101] | 109 | 13.8 | 3.6 | 1.8 | 2.7 | 0.9 | 24.8 | 21.1 | 0.9 | |||||
| Berntsson et al. [102] | 54 | 9.3 | 11.1 | 0 | – | – | 37.0 | 3.7 | – | 3.7 | 0 | 13.0 | 7.4 | |
| Blanquer et al. [103] | 48 | 12.5 | 0 | 12.5 | 0 | 0 | 12.5 | – | – | 0 | 0 | 20.8 | 14.6 | |
| BTS et al. [104] | 67 | 6.0 | 0 | 0 | 0 | 0 | 0 | 3.0 | 28.0 | 10.0 | ||||
| Dulake and Selkon [105] | 36 | 19.0 | 14.0 | 0 | 0 | 2.0 | 0 | 2 | 2 | |||||
| Foy et al. [106] | 2256 | 12.0 | 20.0 | 25.0 | 8.0 | |||||||||
| Holm et al. [95] | 48 | 15 | 4 | 0 | 0 | 2 | 0 | 8 | 0 | 13 | 4 | |||
| Jokinen et al. [42] | 304 | 41 | 4 | 3 | 10 | 12 | 10 | 1 | 9 | 2 | ||||
| Marrie et al. [49] | 149 | 22.8 | 10.7 | 2.7 | 2.7 | |||||||||
| Marrie et al. [107] | 507 | 5.9 | 4.9 | 15 | 12 | |||||||||
| Melbye et al. [108] | 36 | 11.1 | 0 | 0 | – | – | 13.9 | 8.3 | 0 | – | 33.3 | 19.4 | ||
| Michetti et al. [52] | 119 | 0 | 0 | 3.4 | 0 | 0 | 32.8 | 16.0 | 6.7 | 9.2 | 0 | 5.9 | 3.4 | |
| Miyashita et al. [109] | 106 | 12.3 | 4.7 | 1.9 | 0.9 | 27.4 | 1.9 | |||||||
| Wattanathum et al. [25] | 98 | 13.3 | 1 | 8.2 | 29.6 | 36.7 | ||||||||
| Woodhead et al. [110] | 236 | 36.0 | 10.0 | 0.5 | 0 | 1.0 | 1.0 | 1.0 | 1.0 | 0 | 13.0 | 8.0 | ||
| Range | 0–36 | 0–14 | 0–13 | 0–3 | 0–1 | 0–1 | 1–33 | 1–16 | 7–37 | 0–9 | 0–3 | 2–33 | 0–19 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CPne, Chlamydophila pneumoniae; CPsi, Chlamydophila psittaci; CB, Coxiella burnetii.
Table 3.
Aetiology of community‐acquired pneumonia in adults admitted to hospital (%). (Blank boxes indicate organism not sought)
| Reference | n | SP | HI | LP | SA | MC | GNEB | PA | MP | CS | CPne | CPsi | CB | Virus | Influenza |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angeles Marcos et al. [23] | 198 | 29.3 | 5.1 | 3.0 | 2.5 | 0 | 2.0 | 1.0 | 1.5 | 0.5 | 0.5 | 0 | 1.0 | 23.2 | 8.1 |
| Arancibia et al. [111] | 559 | 13.8 | 5.0 | 5.2 | 10.7 | 7.0 | 1.8 | 9.5 | 7.7 | 0.2 | 1.6 | 2.8 | |||
| Aubertin et al. [112] | 274 | 12.4 | 3.3 | 10.6 | 2.2 | 0.0 | 2.9 | 8.8 | – | – | 2.6 | 0.7 | 2.6 | 0.0 | |
| Ausina et al. [113] | 207 | 39.1 | 1.0 | 6.3 | 0.5 | 0.0 | 2.9 | 16.9 | – | – | 6.3 | 2.4 | 3.9 | 2.4 | |
| Berntsson et al. [114] | 127 | 54.3 | 3.9 | 0.8 | 0.8 | 0.0 | 0.0 | 14.2 | – | – | 2.4 | 0.0 | 18.1 | 12.6 | |
| Blanquer et al. [103] | 462 | 14.7 | 1.9 | 13.9 | 1.7 | 0.0 | 3.2 | 3.5 | – | – | 0.2 | 0.6 | 13.0 | 7.8 | |
| Blasi et al. [33] | 207 | 7.7 | 2.4 | 4.8 | 3.9 | 1.0 | 5.3 | 8.2 | 10.1 | 10.1 | 0.0 | 0.0 | – | – | |
| Bohte et al. [34] | 334 | 26.9 | 7.8 | 2.4 | 1.2 | 1.5 | 3.3 | 5.7 | – | – | – | 0.3 | 8.1 | 4.2 | |
| BTS [104] | 453 | 34.0 | 5.7 | 2.0 | 0.9 | 0.0 | 0.9 | 17.9 | – | – | 2.9 | 1.1 | 7.1 | 7.1 | |
| Burman et al. [115] | 196 | 32.1 | 4.6 | 2.0 | 1.5 | 1.5 | 1.0 | 8.7 | – | – | 3.1 | 0.0 | 21.9 | 8.7 | |
| Charles et al. [116] | 885 | 14 | 5 | 3 | 1 | 1 | 2 | 2 | 9 | 2 | 15 | 8 | |||
| de Roux et al. [22] | 338 | 41 | 14.5 | 10 | 12 | 18 | 12 | ||||||||
| Ewig et al. [19] | 204 | 19 | 6 | 5 | 2 | 1 | 6.5 | 4 | 2 | 10.5 | 10 | 0.5 | 3 | 3 | |
| Falco et al. [117] | 400 | 21.0 | 3.3 | 7.5 | 0.0 | 0.0 | 2.0 | 2.3 | – | – | 2.8 | 0.0 | – | – | |
| Falguera et al. [118] | 660 | 34 | 2 | 5 | 2 | 3 | 9 | 16 | 11 | 1 | 4 | 5 | 4 | ||
| Garbino et al. [119] | 318 | 12.6 | 6 | 4.4 | 1.6 | 1.6 | 7.5 | 5.3 | |||||||
| GarciaVidal et al. [58] | 1634 | 26 | 7 | 7 | <1 | 1 | 1 | 2 | 1 | 1 | <1 | <1 | |||
| Ginesu et al. [37] | 520 | 10.8 | 32.9 | 0.4 | 0.9 | ||||||||||
| Gomez et al. [38] | 342 | 12.6 | 5.6 | 1.5 | 0.0 | 0.3 | 0.0 | 3.2 | 6.1 | 6.1 | 0.0 | 0.0 | – | – | |
| Gutierrez et al. [120] | 493a | 16.8 | 1.8 | 4.3 | 0.4 | 0.2 | 3.2 | 2.2 | 7.7 | 6.1 | 0.4 | 4.1 | 2.8 | ||
| Holmberg [121] | 147 | 46.9 | 9.5 | 2.7 | 0.7 | 2.0 | 0.0 | 5.4 | – | – | 1.4 | 0.0 | 10.9 | 10.2 | |
| Hone et al. [122] | 50 | 20.0 | 16.0 | 4.0 | 0.0 | 2.0 | 2.0 | 4.0 | – | – | 0.0 | 0.0 | 20.0 | 10.0 | |
| Huang et al. [123] | 389b | 3.1 | 20.6 | 0.5 | 1.5 | 0.3 | 6.2 | 10.8 | 4.4 | ||||||
| Jennings et al. [27] | 304 | 31 | 11 | 4 | 2 | 3 | 31 | 10 | |||||||
| Johansson et al. [18] | 184 | 38 | 5 | 1 | 2 | 4 | 8 | 29 | 8 | ||||||
| Johnstone et al. [67] | 193 | 7 | 1 | <1 | 1 | 2 | 3 | 2 | 2 | 0 | 0 | 15 | 4 | ||
| Leesik et al. [124] | 439 | 10.5 | 1.1 | 2.0 | 5.7 | 18.0 | 3.2 | 3.0 | 2.5 | ||||||
| Levy et al. [125] | 116 | 25.9 | 11.2 | 4.3 | 2.6 | 0.9 | 6.9 | 3.4 | – | – | 0.9 | 0.0 | 4.3 | – | |
| Logroscino et al. [46] | 613 | 5.9 | 3.6 | 2.8 | 1.1 | 0.8 | 3.9 | 3.3 | 4.2 | – | – | 3.1 | – | ||
| Lorente et al. [47] | 114 | 35.1 | 0.9 | 1.8 | 2.6 | 0.0 | 2.6 | 9.6 | 1.8 | – | 0.9 | – | – | ||
| Macfarlane et al. [126] | 127 | 75.6 | 3.1 | 15.0 | 2.4 | 0.0 | 0.8 | 2.4 | – | – | 5.5 | 0.8 | 8.7 | 5.5 | |
| Marrie et al. [127] | 539 | 2.2–8.1 | |||||||||||||
| McNabb et al. [128] | 80 | 50.0 | 6.3 | 1.3 | 3.8 | 0.0 | 1.3 | 0.0 | – | – | 0.0 | 0.0 | 6.3 | 6.3 | |
| Menendez et al. [51] | 184 | 23.9 | 1.6 | 0.5 | 0.0 | 0.0 | 1.6 | 14.1 | 0.5 | 0.0 | 1.1 | 1.6 | 1.6 | ||
| Michetti et al. [52] | 60 | 8.3 | 6.7 | 11.7 | 1.7 | 1.7 | 3.3 | 8.3 | 6.7 | 1.7 | 0.0 | 1.7 | 1.7 | ||
| Miyashita et al. [109] | 400 | 26.3 | 13 | 1.5 | 3.3 | 3.5 | 4 | 2 | 9.3 | 1.3 | 0.5 | 3 | |||
| Ortqvist et al. [129] | 277 | 46.2 | 3.6 | 3.6 | 0.7 | 1.1 | 1.4 | 9.7 | 1.1 | 0.0 | 1.1 | 0.0 | 15.5 | 2.5 | |
| Ostergaard and Andersen 1993 [130] | 254 | 13.8 | 6.3 | 3.1 | 0.4 | 0.8 | 2.0 | 3.9 | – | – | 1.2 | 0.0 | – | – | |
| Pareja et al. [131] | 165 | 7.3 | 1.8 | 2.4 | 2.4 | 0.0 | 27.3 | 10.3 | – | – | 1.2 | 10.9 | 18.2 | 13.3 | |
| Ruf et al. [132] | 442 | 15.4 | 2.5 | 3.8 | 2.7 | 0.0 | 2.5 | 9.3 | – | – | 3.2 | 0.0 | 8.8 | 4.1 | |
| Ruiz et al. [54] | 395 | 16.5 | 6.3 | 4.3 | 1.8 | 1.0 | 6.3 | 3.3 | 3.8 | 0.5 | 2.8 | 9.9 | 5.8 | ||
| Saito et al. [26] | 232c | 24.6 | 18.5 | 3.9 | 3.4 | 2.2 | 1.7 | 0.4 | 5.2 | 8.7 | 6.5 | 2.2 | 0.9 | 16.4 | |
| Schneeberger et al. [133] | 159 | 11.3 | 10.6 | 2.5 | 3.8 | 3.8 | 8.2 | 12 | 3 | ||||||
| Socan et al. [55] | 211 | 5.7 | 0.9 | 2.8 | 0.5 | 0.0 | 1.9 | 5.7 | 18.0 | 0.9 | 0.5 | 24.2 | – | ||
| Sohn et al. [134] | 126 | 13.5 | 0.8 | 2.4 | 0.8 | 12.5 | 3.1 | 6.3 | 7.1 | 7.1 | 0 | ||||
| Song et al. [28] | 955 | 12 | 6 | 1 | 2 | 1 | 6 | 3 | 6 | 6 | |||||
| Sopena et al. [56] | 330 | 20.3 | 2.1 | 13.9 | 0.6 | 0.0 | 0.3 | 1.5 | 15.8 | 0.0 | 1.2 | – | – | ||
| Steinhoff et al. [57] | 237 | 8.6 | 5.1 | 6.3 | 7.7 | 6.3 | |||||||||
| Wattanathum et al. [25] | 147 | 22.4 | 2.7 | 5.4 | 3.4 | 17.7 | 0.7 | 6.8 | 16.3 | ||||||
| White et al. [135] | 210 | 11.4 | 1.9 | 1.4 | 3.8 | 0.0 | 1.4 | 14.3 | – | – | 1.4 | 2.9 | 14.8 | 12.4 | |
| Range | 3–76 | 1–21 | 1–14 | 0–4 | 0–4 | 0–33 | 0–12 | 0–18 | 0–16 | 0–18 | 0–6 | 0–11 | 1–24 | 0–13 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; PA, Pseudomonas aeruginosa; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CPne, Chlamydophila pneumoniae; CPsi, Chlamydophila psittaci; CB, Coxiella burnetii.
a26.8% were outpatients.
b36.2% were outpatients.
c16% were outpatients.
Table 4.
Aetiology of community‐acquired pneumonia in adults admitted to an ICU (%). (Blank boxes indicate organism not sought)
| Reference | n | SP | HI | LP | SA | GNEB | MP | CS | CPsi | CB | Virus | Influenza |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkhayer et al. [136] | 18 | 16.7 | 0 | 11.1 | 5.6 | 0 | 0 | 5.6 | 0 | 16.7 | 0 | |
| Almirall et al. [137] | 58 | 17.2 | 1.7 | 8.6 | 0 | 6.9 | 0 | 1.7 | 0 | 1.75 | – | |
| BTS [138] | 60 | 18.3 | 11.7 | 11.7 | 5 | 3.3 | 6.7 | 0 | 0 | 0 | 8.3 | 5.0 |
| El Solh et al. [36] | 57 | 14 | 7 | 9 | 7 | 14 | 2 | |||||
| Gowardman and Trent [39] | 32 | 18.4 | 9.2 | 11.6 | ||||||||
| Hirani and Macfarlane 1997 [41] | 57 | 17.5 | 0 | 15.8 | 12.3 | 1.8 | 0 | 5.3 | 0 | 10.5 | 8.8 | |
| Leroy et al. [139] | 299 | 26.8 | 8.7 | 0 | 19.1 | 15.1 | 0.7 | 1.7 | 0 | – | – | |
| Moine et al. [140] | 132 | 32.6 | 10.6 | 3.0 | 3.8 | 10.6 | 0.8 | 0.8 | 1.5 | 5.35 | 1.5 | |
| Olaechea et al. [53] | 262 | 11.5 | 3.8 | 8.0 | 3.8 | 3.1 | 3.1 | 1.5 | 0 | 1.95 | – | |
| Ortqvist et al. [141] | 53 | 17.0 | 1.0 | 9.0 | 0 | 7.0 | 0 | 2.0 | 0 | 0 | ||
| Pachon et al. [142] | 67 | 17.9 | 3.0 | 10.4 | 1.5 | 6.0 | 0 | 0 | 0 | 0 | 1.5 | 1.5 |
| Paganin et al. [143] | 112 | 42.9 | 0.9 | 1.8 | 1.8 | 26.8 | ||||||
| Rello et al. [144] | 58 | 22.4 | 0 | 13.8 | 0 | 8.6 | 0 | 0 | 0 | 0 | 1.75 | 1.7 |
| Rello et al. [145] | 204 | 20.1 | 5.3 | 11.2 | 2.4 | 5.8 | 0.9 | |||||
| Sorensen et al. [146] | 36 | 33.3 | 8.33 | 8.3 | 8.3 | 2.8 | 0 | 0 | 0 | 0 | 13.9 | 2.8 |
| Torres et al. [147] | 92 | 15.2 | 0 | 14.1 | 1.1 | 9.8 | 6.5 | 0 | 0 | 0 | – | – |
| Woodhead et al. [148] | 50 | 32 | 0 | 30.0 | 10 | 0 | 2 | 0 | 0 | 0 | 8.0 | 4.0 |
| Range | 12–43 | 0–12 | 0–30 | 0–19 | 0–27 | 0–7 | 0–2 | 0–6 | 0–2 | 0–17 | 0–9 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CPsi, Chlamydophila psittaci; CB, Coxiella burnetii.
In the community and on the regular ward, extracellular bacteria, especially Streptococcus pneumoniae (S. pneumoniae), are in first place, followed by Haemophilus influenzae (H. influenzae), Staphylococcus aureus (S. aureus) and Moraxella catarrhalis. Among intracellular bacilli, Mycoplasma pneumoniae (M. pneumoniae) is the most common, followed in frequency by Legionella and Chlamydia species, with viruses being involved in up to 60% of community‐acquired LRTI and 30% of CAP. In the intensive care unit, S. aureus, Gram‐negative bacilli and Legionella spp. might be more frequently encountered. Recurrence of CAP is more likely when Gram‐negative bacteria are involved, and less likely if Legionella spp. are involved [58].
Originally a nosocomial pathogen, methicillin‐resistant S. aureus (MRSA) disseminated during the last decade in the community (community‐acquired MRSA, CA‐MRSA). Methicillin resistance is mediated by the mecA gene that has been associated with the Panton‐Valentine leukocidin (PVL) toxin, which creates lytic pores in the cell membranes of neutrophils and induces the release of neutrophil chemotactic factors that promote inflammation and tissue destruction. New PVL‐positive clones may be arising and disseminating in the community [59]. MRSA has emerged as an infectious agent of increasing frequency associated with skin and soft‐tissue infections in the community setting. However, CA‐MRSA can also lead to severe pulmonary infections, including necrotizing and haemorrhagic pneumonia, pneumothorax, pneumopyothorax, empyema, ventilatory failure and septicaemia [60, 61, 62, 63].
Coxiella burnetii, a Gram‐negative intracellular bacterium, and a potential bioterrorism agent, is responsible for Q fever, which may have a wide variety of clinical manifestations, including flu‐like syndrome, pneumonia and long‐lasting fatigue syndrome. C. burnetii is present worldwide, cattle, sheep and goats being the most common reservoirs. Q fever occurs as endemic cases or as outbreaks in endemic areas. Outbreaks have ocurred in Europe in recent decades including Switzerland, Spain, the UK, Germany and most recently, the Netherlands repeatedly since 2007, with more than 4000 notified cases [64].
The importance of viruses as causal agents has been confirmed in LRTI [65] and CAP [22, 23, 66]. In the majority of aetiological CAP studies looking for viruses and bacteria, viruses are the most common aetiological agents after S. pneumoniae [23, 67].
Sporadic viral pneumonias that occurred in recent years were due to new virus, avian influenza virus, hantavirus and coronavirus. Avian influenza virus A/H5N1 infections increase the risk of a pandemic, are much more severe than routine seasonal influenza, and are associated with severe illness and a >50% mortality rate, especially in people aged 10–39 years [68, 69]. The hantavirus pulmonary syndrome was recognized in 1983, but was retrospectively identified using serological testing in patients who had a similar illness in 1959 [70]. The syndrome can result from several hantaviruses, such as Sin Nombre virus. Avoidance of areas where infected rodents live is the only preventive measure. An outbreak of severe acute respiratory syndrome (SARS) was reported in 2002, mainly in Asian countries and Canada [71, 72]. New viruses belonging to the coronaviridae family were found to be responsible.
In the spring of 2009, an outbreak of severe pneumonia was reported in conjunction with the concurrent isolation of novel swine‐origin influenza A (H1N1) subtype viruses, which have rarely predominated since the 1957 pandemic, with features of the epidemic similar to those of past influenza pandemics. The new influenza virus was affecting a younger population, suggesting relative protection for persons who were exposed to H1N1 strains during childhood before the 1957 pandemic [73]. Severe pneumonias were reported in conjunction with the novel influenza A (H1N1) subtype virus. Pneumonias were due to the virus and to superinfection by S. pneumoniae or Staphylococcus.
Microorganisms isolated in hospitalized elderly patients with CAP are shown in (Table 5). There are large variations, depending on the elderly threshold, where patients live and comorbidities. However, Gutierez et al. [74] found that age has a strong influence on the incidence of CAP caused by the main microbial pathogens; ageing is associated with a higher risk of acquiring pneumonia by S. pneumoniae, influenza virus and Chlamydia species. Ingarfield et al. [75] emphasize that enterobacteriacae accounted for more than 25% of isolates in patients older than 65 years.
Table 5.
Microorganisms isolated in hospitalized elderly patients with community‐acquired pneumonia (CAP) (%). (Blank boxes indicate organism not sought)
| Reference | n | Patients | SP | HI | LP | MC | SA | GNEB | MP | CS | CB | Virus | Influenza | Aspiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El‐Solh et al. [36] | 57 | ≥80 years Home | 14 | 7 | 9 | 4 | 7 | 17 | 2 | 2 | 2 | |||
| El‐Solh et al. [36] | 47 | ≥80 years Nursing Home | 9 | 2 | 0 | 2 | 29 | 20 | 0 | 0 | ||||
| Fernandez‐Sabéet al. [149] | 305 | ≥80 years Home | 23 | 5 | 1 | 3 | 0.7 | 0.3 | 0 | 8 | 10 | |||
| Flamaing 2003 [66] | 165 | ≥80 years Home & Nursing Home | 3.6 | 1.2 | 4.2 | 0.6 | 30.9 | 26.1 | ||||||
| Gutierrez et al. [21] | 136 | ≥75 years Home | 19.1 | 0.7 | 1.5 | 0 | 0 | 6.6 | 2.2 | 3.7 | 3.7 | 2.2 | ||
| Huang et al. [123] | 126 | ≥60 years | 2.4 | 14.3 | 0.8 | 0.8 | 2.4 | 12.7 | 7.1 | 6.3 | ||||
| Jokinen et al. [42] | 140 | ≥60 years Home | 48 | 4 | 3 | 3 | 13 | 12 | 0 | |||||
| Riquelme et al. [150] | 101 | ≥65 years Home | 18.8 | 3 | 1 | 3 | 8.9 | 5.9 | ||||||
| Saito et al. [26] | 114 | ≥65 years Home | 28. | 20.2 | 2.6 | 3.5 | 3.5 | 7.9 | 1.8 | 9.6 | 0.9 | 13.2 | ||
| Zalacain 2003 [151] | 503 | ≥65 years Home & Nursing Home | 19.5 | 5.4 | 3.8 | 0.6 | 1.6 | 4.4 | 2.0 | 2.6 | 2.2 | 1.2 | 0.6 | |
| Range | 2–48 | 2–20 | 0–9 | 0–4 | 7–29 | 3–20 | 0–7 | 2–13 | 0–6 | 0–31 | 0–26 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; LP, Legionella pneumophila; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CB, Coxiella burnetii.
Table 6 provides microbiological aetiologies of airway infection in patients with COPD exacerbation, as found in studies using various methods. Recent studies of the microbiology of acute exacerbations of chronic bronchitis found an influence of the baseline level of lung function on pathogens (typical and atypical bacteria and/or virus) found in respiratory secretion samples [76, 77, 78, 79, 80, 81, 82, 83]. P. aeruginosa should be suspected in patients who have been treated with antibiotics and in those not vaccinated against influenza [84]. Both short‐term colonization followed by clearance and long‐term persistence of P. aeruginosa are observed. While serum antibody responses do not mediate clearance of P. aeruginosa, mucoid strains persist in the airways [85].
Table 6.
Aetiology of exacerbations in patients with COPD (%). (Blank boxes indicate organism not sought)
| Reference | Sample | n | SP | HI | MC | SA | GNEB | PA | MP | CS | CPne | CPsi | CB | Virus | Influenza | PI | RV | Adv | RSV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alamoudi [78] | Sputum | 139 | 4 | 12 | 25 | 9 | 12 | ||||||||||||
| Beaty et al. [152] | Serology | 44 | 4.5 | ||||||||||||||||
| Carilli et al. [153] | Serology | 46 | 8.7 | 8.7 | 4.3 | 0 | 17.4 | ||||||||||||
| Eadie et al. [154] | Serology | 47 | 4.3 | 2.1 | 23.4 | 0 | |||||||||||||
| Eller et al. [76] | Sputum | 211 | 9 | 7.6 | 4 | 7.1 | 18.9 | 6.6 | |||||||||||
| Erkan et al. [155] | Sputum, Serology | 75 | 5 | 35 | 6 | 1 | 9 | 17 | |||||||||||
| Fagon et al. [156] | PSB | 54 | 8 | 26 | 3.5 | 4.5 | 6 | 3.5 | |||||||||||
| Groenewegen and Wouters 2003 [157] | Sputum | 171 | 14.0 | 22.2 | 2.9 | 2.3 | 7.6 | ||||||||||||
| Gump et al. [158] | Serology | 116 | 27.6 | 42.24 | 10.3 | 21.6 | 6.9 | 0.8 | 33.6 | 12.9 | 7.8 | 3.4 | 4.3 | ||||||
| Hutchinson et al. [81] | Sputum, Swab, Serology | 148 | 5 | 11 | 2 | 2 | 7 | 6 | 1 | 2 | 1 | 1 | 23 | 2 | 1 | 18 | 1 | 1 | |
| Karnak et al. [159] | Serology | 38 | 34.0 | 34.0 | |||||||||||||||
| Ko et al. [160] | Sputum | 418 | 4.0 | 23.1 | 2.0 | 1.2 | 5.2 | 6.3 | |||||||||||
| Ko et al. [83] | Sputum, Swab, Serology | 643 | 4 | 10 | 3 | 0 | 4 | 4 | 0 | 0 | 5 | 1 | 2 | ||||||
| Lamy et al. [161] | Serology | 49 | 2.0 | 28.6 | 24.5 | 6.1 | |||||||||||||
| Lieberman et al. [162] | Serology | 62 | 11.3 | 11.3 | |||||||||||||||
| McManus 2008 [79] | Sputum | 136 | 37 | 2 | 24 | 7 | 2 | ||||||||||||
| McNamara et al. [163] | Serology | 42 | 9.5 | 0 | 0 | 42.8 | 11.9 | ||||||||||||
| Miravitlles et al. [77] | Sputum | 91 | 10 | 22 | 9 | 7 | 15 | ||||||||||||
| Mogulkoc et al. [164] | Serology Sputum | 49 | 8.2 | 8.2 | 6.1 | 6.1 | 22.4 | 22.4 | |||||||||||
| Monsòet al. [165] | PSB | 29 | 10.3 | 34.5 | 6.9 | 6.9 | |||||||||||||
| Murphy et al. [166] | Sputum | 104 | 10 | ||||||||||||||||
| Papi et al. [167] | Sputum | 64 | 12.5 | 14.1 | 10.9 | 6.3 | 4.7 | 6.3 | 48.4 | 10.9 | 3 | 27 | 6 | ||||||
| Roche et al. [80] | Sputum | 200 | 8 | 26 | 6 | 6 | 9 | ||||||||||||
| Rohde et al. [168] | Sputum Nasal lavage | 85 | 56 | 20 | 7 | 25 | 15 | ||||||||||||
| Rosell et al. [169] | PSB | 86 | 7 | 30 | 7 | 0 | 16 | 9 | |||||||||||
| Ross et al. [170] | Serology | 125 | 0 | 0 | 0 | 0 | 10.4 | 1.6 | 3.2 | ||||||||||
| Seemungal et al. [171] | Serology Culture | 168 | 0 | 0.6 | 0.6 | 5.4 | 0.6 | 23.2 | |||||||||||
| de Serres et al. [172] | Sputum, Swab, Serology | 108 | 4 | 5 | 4 | 10 | 8 | 7 | 1 | 32 | 9 | 6 | 3 | 7 | |||||
| Soler et al. [173] | PSB | 50 | 8.0 | 22.0 | 8.0 | 8.0 | 18.0 | 18.0 | 14.0 | 2.0 | 2.0 | 12.0 | 10.0 | ||||||
| Range | 8–28 | 0–42 | 3–11 | 4–22 | 5–19 | 0–18 | 0–10 | 0–34 | 0.34 | 12–49 | 0–29 | 0–25 | 0–43 | 0–17 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; PA, Pseudomonas aeruginosa; MP, Mycoplasma pneumoniae; CS, Chlamydia species (all); CPne, Chlamydophila pneumoniae; CPsi, Chlamydophila psittaci; CB, Coxiella burnetii; PI, Para‐influenza; RI, Rhino‐virus; RSV, Respiratory syncytial virus.
The microbiological pattern of airway infection may also differ between pneumonic and non‐pneumonic hospitalized exacerbations of COPD, as shown in a prospective study of 240 patients. Identification of a pathogen was more frequent in pneumonic cases (96% vs. 71%), in which S. pneumoniae and viruses were more frequent (43% and 78% vs. 18% and 46%, respectively) [86]. Respiratory viruses are more frequently found in induced sputum of hospitalized patients with COPD exacerbations than in control stable COPD subjects (47% vs. 10%), the most frequent viruses being rhinovirus, influenza, parainfuenza and RSV. However, if exacerbations of chronic bronchitis and/or COPD may be due to viral and/or bacterial infection, such infections may occur without exacerbation [87]. Finally, bacterial exacerbations of COPD could be related to the appearance of new strains of S. pneumoniae, H. influenzae or M. catarrhalis in the colonized airways [88].
Only a few studies assessed the microbiological pattern of airway colonization in bronchiectasis, and no study has investigated the microbiological aetiology of exacerbations. The main results for steady state bronchiectasis are provided in Table 7; they highlight the high frequency of Pseudomonas infection, particularly in the case of impaired lung function.
Table 7.
Microorganisms isolated in inpatients with non‐cystic fibrosis bronchiectasis (%). (Blank boxes indicate organism not sought)
| Reference | Sample | n | SP | HI | MC | SA | GNEB | PA | MP | NTM |
|---|---|---|---|---|---|---|---|---|---|---|
| Angrill et al. [89] | PSB | 75 | 8 | 32 | 3 | 18 | 15 | 4 | – | |
| Chan et al. [174] | Sputum | 32 | – | 19 | – | 53 | 34 | – | – | |
| Ho et al. [90] | Sputum | 100 | 6 | 10 | 5 | 38 | 33 | 2 | 3 | |
| King et al. [175] | Sputum | 89 | 7 | 47 | 8 | 4 | 3 | 12 | 2 | 2 |
| Nicotra et al. [176] | Sputum | 123 | 10.6 | 30.1 | 2.4 | 7.3 | 44 | 30.9 | – | 22.8 |
| O’Donnell et al. [177] | Sputum | 349 | – | – | – | – | 25 | – | – | |
| Range | 6–11 | 10–32 | 3–7 | 18–53 | 15–33 | 2–4 | 3–23 |
SP, Streptococcus pneumoniae; HI, Haemophilus influenzae; MC, Moraxella catarrhalis; SA, Staphylococcus aureus; GNEB, Gram‐negative enteric bacilli; PA, Pseudomonas aeruginosa; MP, Mycoplasma pneumoniae; NTM, non‐tuberculous Mycobacteria.
In a 2‐year prospective study of 77 patients with clinically stable bronchiectasis, multivariate analysis found that early diagnosis of the disease (before 14 years of age), reduced FEV1 (<80% predicted) and varicose‐cystic bronchiectasis are risk factors for bronchial colonization with pathogenic bacteria, mainly H. influenzae and P. aeruginosa (odds ratio: 3.92, 3.91 and 4.80, respectively) [89]. In a study of 100 patients with steady‐state bronchiectasis, the presence of P. aeruginosa in the sputum was associated with a lower FEV1/FVC ratio (60% vs. 72% in the absence of a pathogenic microorganism) and higher volume of daily sputum production (1–6 score: 3 vs. 1) [90]. In that study, FEV1/FVC <60% and high sputum output were independently associated with an increased risk of sputum isolation of P. aeruginosa (odds ratio: 3.1 and 4.7, respectively).
Conclusion
There has been no major change in causative pathogens for LRTI. More information is available about the frequency of polymicrobial infections, including viral infections. PVL‐producing Staphylococcus aureus has emerged as a new cause, often of severe CAP, but currently remains uncommon.
What information is available about the frequency of antimicrobial resistance in these settings
Streptococcus pneumoniae. Beta‐lactams: The prevalence of resistance to penicillin and other drugs among pneumococci has considerably complicated the empirical treatment of respiratory tract infections. Worryingly, the majority of resistant isolates are resistant to multiple classes of antimicrobials, which has a serious impact on many first‐line antimicrobial therapies.
The mechanism of resistance to penicillin and other β‐lactams is due to alterations of penicillin‐binding proteins (PBP). PBPs interact with β‐lactams enzymatically by forming a covalent complex via the active‐site serine. The loss of affinity for the PBPs affects all β‐lactams, although this may vary substantially depending on the drug. The affinity for a given β‐lactam is different for different PBPs, and conversely, one PBP has distinct affinities for different β‐lactams. Therefore point mutations reducing the affinity for one β‐lactam do not necessarily affect the affinity for another compound [178]. However, National Committee for Clinical Laboratory Standards (NCCLS) guidelines state that a pneumococcal isolate that is susceptible to penicillin can be considered susceptible to other β‐lactams. It is generally accepted that the MICs of amoxicillin and extended‐spectrum cephalosporins are usually equal to or two to four times lower than the MIC of benzylpenicillin. However, pneumococci resistant to amoxicillin and or extended‐spectrum cephalosporins with the MICs of these agents equal to or 1 dilution higher than the MIC of penicillin have been identified [179].
Pneumococci with decreased susceptibility to penicillin have a much higher rate of resistance to other classes of antibiotics, as has been mentioned above. Carbapenems, imipenem, meropenem and ertapenem, are the most active β‐lactams available against PRSP. Among parenteral cephalosporins, those with good activity are cefotaxime, ceftriaxone, cefepime and cefpirome. It is important to note that other parenteral third‐generation cephalosporins are considerably less active, for example ceftizoxime and ceftazidime; the latter has been linked to a poor clinical response [180].
Amoxicillin remains the most active of all oral β‐lactams, and among cephalosporins, cefditoren and cefpodoxime are most active, then cefuroxime and cefprozil. The use of cefuroxime in cases of bacteraemic pneumococcal pneumonia caused by penicillin non‐susceptible strains has been linked to an increased mortality [181].
The prevalence of penicillin‐resistant Streptococcus pneumoniae (PRSP) and multidrug‐resistant SP varies between regions. Data on the prevalence of antibiotic resistance among Streptococcus pneumoniae has been regularly produced by the EARSS project, a European‐wide network of national surveillance systems, providing reference data on antimicrobial resistance for public health purposes. This network receives funding from the European Commission (http://www.earss.rivm.nl).
In 2008, 1152 (10%) of the 11 584 invasive S. pneumoniae isolates reported by 32 countries were non‐susceptible to penicillin (Fig. 1). Penicillin non‐susceptible S. pneumoniae (PNSP) shows a heterogeneous picture in Europe. Most northern European countries had levels of non‐susceptibility below 5%, but Finland (11%, n = 642) and Ireland (23%, n = 441) reported relatively high levels. High levels of PNSP, above 25%, were mainly reported from southern and eastern Europe, Cyprus (43%, n = 14), France (30%, n = 557), Hungary (27%, n = 166), Malta (47%, n = 17) and Turkey (34%, n = 97). The level of penicillin non‐susceptibility in Finland and Ireland has risen significantly from 2005. The two countries with the highest levels of PNSP in 2007 (France and Israel) showed significant decreasing rates of PNSP during the past years. Lithuania and Norway (the latter only significantly for the laboratories reporting consistently in the last 4 years) also showed decreasing trends for PNSP. In Belgium, the proportions of PNSP as well as PRSP continued to decrease significantly in 2008. In Croatia, Hungary, Ireland and Turkey a significant increase was also observed, but only for the percentage of fully resistant isolates (see Fig. 1).
Figure 1.
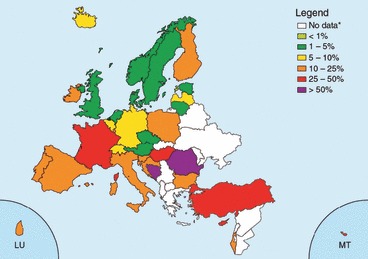
Streptococcus pneumoniae: proportion of invasive isolates non‐susceptible to penicillin (PNSP) in 2008. *These countries did not report any data or reported <10 isolates.
The changes in the distribution of serotypes compared with 2007 were small. Serogroups 1 and 19 were still the most prevalent ones, whereas serogroup 7 and serogroup 3 became slightly more prevalent, and serogroup 14 became less prevalent in the population. The highest resistance proportions were identified in serogroups 1, 6, 9, 14, 19F and 33, of which all but 1 and 33 are included in the seven‐conjugate vaccine.
Another recent survey of interest was performed in eastern and southern Mediterranean countries. Over a 36‐month period, from 2003 to 2005, the ARMed project collected 1298 susceptibility test results of invasive isolates of S. pneumoniae from blood and spinal fluid cultures routinely processed within 59 participating laboratories situated in Algeria, Cyprus, Egypt, Jordan, Lebanon, Malta, Morocco, Tunisia and Turkey. Overall, 26% (335) of isolates were reported as non‐susceptible to penicillin, with the highest proportions being reported from Algeria (44%) and Lebanon (40%) [182].
In the US, the incidence of invasive pneumococcal disease due to penicillin‐resistant 19A isolates increased from 6.7% to 35% between 1998 and 2005 (p <0.0001). Of 151 penicillin‐resistant 19A isolates, 111 (73.5%) belonged to the rapidly emerging clonal complex 320, which is related to multidrug‐resistant Taiwan (19F)‐14 [183]. The importance of these findings is the high levels of penicillin resistance among strains with this serotype (amoxicillin MIC, ≥4 mg/L; cefotaxime MIC, ≥2 mg/L), and their frequent multiresistance, precluding the use of any oral β‐lactam for the treatment of infections caused by these resistant strains.
Of special concern, is the increase in some European countries of MDR strains of serotype 19A, particularly in Spain and France [184].
The new susceptibility breakpoints for S. pneumoniae, published by the Clinical and Laboratory Standards Institute (CLSI) in January 2008, were the result of a re‐evaluation that showed clinical response to penicillin was being preserved in clinical studies of pneumococcal infection, despite reduced susceptibility response in vitro. Antimicrobial susceptibility breakpoints are currently established based on (i) the pharmacokinetic and pharmacodynamic properties of an antimicrobial agent and (ii) data correlating individual MIC results with patient outcomes. Under the former criteria, susceptible, intermediate and resistant MIC breakpoints for penicillin were ≤0.06, 0.12–1 and ≥2 mg/L, respectively, for all pneumococcal isolates, regardless of clinical syndrome or route of penicillin administration. Those breakpoints remain unchanged for patients without meningitis who can be treated with oral penicillin (e.g. for outpatient pneumonia). For patients without meningitis who are treated with intravenous penicillin, the new breakpoints are ≤2, 4 and ≥8 mg/L, respectively.
The changes in penicillin breakpoints for S. pneumoniae have the potential to allow clinicians to increase use of penicillin to treat penicillin‐susceptible non‐meningitis pneumococcal infections, instead of using broader‐spectrum antimicrobials. Its use is encouraged to prevent the spread of antimicrobial‐resistant S. pneumoniae and also the spread of methicillin‐resistant Staphylococcus aureus and Clostridium difficile, which can result from use of broader‐spectrum antimicrobials [185]. In accordance with the penicillin breakpoints, the doses of suitable β‐lactam agents for the treatment of hospitalized patients with pneumonia when Streptococcus pneumoniae is suspected are: penicillin G 2 g (3.2 mU) i.v. Q4 h should be adequate for strains with a penicillin MIC of ≤8 mg/L; dose to be adjusted for renal impairment; ceftriaxone 1 g i.v. or i.m. Q 12 h or cefotaxime 2 g i.v. Q6 h, should be adequate for strains with a MIC of ≤8 mg/L [186].
The new formulation of amoxicillin‐clavulanic acid (2 g/125 q12 h) available in some European countries, is able to eradicate amoxicillin‐resistant strains (MICs, 4–8 mg/L), as shown in two recent randomized clinical trials (RCTs) [187].
Macrolides: In the EARSS database 10 982 (95%) invasive S. pneumoniae isolates had susceptibility results for erythromycin in 2008. From the 32 countries reporting data, 1655 (15%) isolates were reported as non‐susceptible to erythromycin. Three countries reported erythromycin non‐susceptibility below 5% (Czech Republic (n = 243), Estonia (n = 53) and Bulgaria (n = 24)). On the other hand, five countries reported non‐susceptibility proportions above 25%, namely Italy (27%, n = 154), Turkey (29%, n = 97), France (31%, n = 557), Hungary (32%, n = 158) and Cyprus (29%, n = 14). A very pronounced increase of erythromycin resistance was reported from Turkey (10% in 2005 vs.29% in 2008) and from Ireland, only significant for the selected laboratories. The proportion of isolates non‐susceptible to erythromycin in Belgium, France and the UK continued to decrease, and now also Germany, the Netherlands and Norway have reported significant decreasing rates with respect to this (see Fig. 2).
Figure 2.
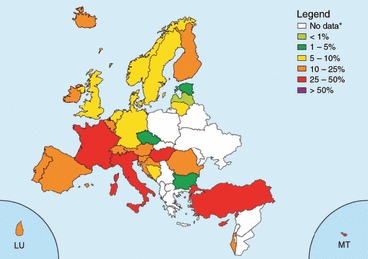
Streptococcus pneumoniae: proportion of invasive isolates non‐susceptible to erythromycin in 2008. From EARSS. *These countries did not report any data or reported <10 isolates.
In another survey, during the same time period, the highest proportions of pneumococci that were not susceptible to erythromycin were reported from Malta (46%) and Tunisia (39%) [182].
Macrolide resistance in S. pneumoniae occurs by two main mechanisms: target‐site modification or efflux of the drug out of the cell. The most common form of target‐site modification is a specific adenine residue on the 23S rRNA (A2058) that is dimethylated by an rRNA methylase. The predominant methylase responsible for macrolide resistance in S. pneumoniae is encoded by erm (B). This methylation is thought to lead to conformational changes in the ribosome, resulting in decreased binding of all macrolide, lincosamide and streptogramin antibacterials (the so‐called MLSB phenotype). The pneumococci harbouring erm (B) gene exhibits highs to very high levels of resistance to all macrolides, with a MIC90 of both clarithromycin and azithromycin of 256 mg/L or more [188, 189].
Macrolide efflux is mediated by the product of the mef (A) gene, which usually causes MICs lower than the erm (B) isolates (MICs of 1–32 mg/L) and retains susceptibility to clindamycin (the so‐called M‐phenotype) [190]. Much more rarely, mutations at different positions in domains V and II of 23S rRNA and in genes that encode the ribosomal proteins L4 and L22 have been identified as a cause of macrolide resistance [191].
Although it is not surprising that highly resistant strains (MIC, ≥16 mg/mL) may lead to clinical failure, the relevance of low‐level resistance (MIC, 0.5–8 mg/mL) has been brought into question. Early this decade, a matched case‐control study of patients with bacteraemic pneumococcal infections showed that breakthrough bacteraemia with an erythromycin‐resistant isolate occurred in 18 (24%) of 76 patients taking a macrolide compared with none of the 136 matched patients with bacteraemia with an erythromycin‐susceptible isolate [192]. These results established that macrolide resistance among pneumococci, including low level erythromycin‐resistant isolates (M phenotype), is a cause of failure of outpatient pneumonia therapy. A more recent population‐based case‐control study from Toronto has confirmed these results [193].
Macrolide resistance contributes to an increased risk of macrolide failure, irrespective of the underlying resistance mechanism or the degree of elevation in erythromycin MIC. Therefore, it would be wise to avoid empirical macrolide therapy when a patient is at risk of being infected with a macrolide‐resistant pathogen, either as a result of patient‐specific characteristics or the overall rate of resistance in the community. Clinical parameters associated with macrolide resistance among pneumococci include macrolide exposure within the previous 3 months, recent use of a penicillin or trimethroprim–sulphamethoxazole, extremes of age, HIV infection and exposure to siblings colonized with resistant isolates [194].
The issue of whether the outcome of bacteraemic pneumococcal pneumonia is improved with the use of combination antibiotic therapy vs. monotherapy is still not resolved. The mechanism for the potential benefit of combining a macrolide with a β‐lactam is uncertain, and may be multifactorial, such as providing cover for atypical pathogens, unrecognized polymicrobial infection, and/or additional cover for drug‐resistant infections, synergy between these two classes of agents, and immunomodulatory properties of the macrolides. Macrolides, at sub‐MICs, but not other classes of antibiotic, subvert the production of pneumolysin, even in the presence of (and irrespective of the mechanism of) macrolide resistance in S. pneumoniae [195].
Fluoroquinolones: Resistance to quinolones occurs in a stepwise fashion, with mutations being observed first in either parC or gyrA leading to decreased fluoroquinolone susceptibility. Strains usually become fully resistant with the addition of a mutation in the other target gene (either gyrA or parC) [196]. Mutations in parE and gyrB and efflux pump are less important mechanisms of resistance.
Emergence of resistance during the course of antimicrobial therapy is most likely to develop from strains that already carry one quinolone resistance determining region (QRDR) as they require only one additional mutation in one of the other target genes to become resistant. The concept of mutant prevention concentration reflects the concentration that prevents the growth of first‐step mutants. Based on their potential for restricting the selection of resistant mutants, not all fluoroquinolones are equal and can be classified accordingly; their ability to prevent the selection of mutants is in descending order: moxifloxacin, trovafloxacin, gatifloxacin, grepafloxacin and levofloxacin [197].
Fluoroquinolone resistance among S. pneumoniae remains rare in Europe. The use of older agents and incorrect dosing are the main drivers of resistance. The Alexander Project reported fluoroquinolone resistance among pneumococci of <1% in 2001 in northern and southern Europe (http://www.alexandernetwork.com). The PROTEKT study identified no quinolone‐resistant isolates in northern Europe and only 1.3% of S. pneumoniae from southern Europe were resistant to levofloxacin (http://www.protekt.org.). However, the prevalence of first‐step mutants is largely unknown. More recent surveys suggest that the prevalence of resistance to levofloxacin and 8‐methoxi fluoroquinolones (moxifloxacin, gatifloxacin) in southern Europe, specifically in Italy and Spain, appears to be around 2–3% [198].
Tetracyclines and other agents: In many countries of the world chloramphenicol, co‐trimoxazole and tetracyclines have reached such a level and prevalence of resistance that they are no longer a good option for empirical therapy in RTI of pneumococcal aetiology. Thus, resistance to trimethoprim‐sulphamethoxazole is reported in approximately 35% of isolates. Tetracycline resistance in pneumococi remains relatively high in some European countries. However, no recent comprehensive surveillance data on tetracycline resistance are available. Early this decade, among invasive isolates, up to 11.5% were reported to be resistant to tetracycline, and among non‐invasive isolates, the prevalence of tetracycline resistance can be as high as 42% in southern Europe. In other European countries, recent studies have shown low resistant rates of tetracycline resistance. Thus, in the UK and Ireland, out of 1388 invasive isolates, only 4% were resistant, and among 5810 respiratory isolates, 7.6% were resistant [199].
Haemophilus influenzae. Beta‐lactams: β‐Lactamase production is the primary mechanism of resistance among H. influenzae and is a well‐known predictor of treatment failure in community‐acquired respiratory tract infections. This can be overcome with the use of β‐lactamase‐stable cephalosporins or β‐lactam plus β‐lactamase‐inhibitor combinations. In addition, H. influenzae isolates carrying amino acid substitutions in the ftsI gene (encoding PBP 3) are phenotypically recognized as β‐lactamase negative ampicillin resistant (BLNAR), which leads to the loss of susceptibility to aminopenicillin and some cephalosporins.
In Europe, resistance rates of Haemophilus influenzae against β‐lactams, in spite of large inter‐regional differences, seem to decline due to a decreasing number of BL‐producing strains. In a recent surveillance study of antibiotic resistance in H. influenzae, the mean prevalence of β‐lactam producers was 7.6%, with a range of 0.7–17.6% [200]. Although rare, β‐lactamase‐negative ampicillin‐resistant (BLNAR) and β‐lactamase‐positive amoxicillin/clavulanate‐resistant (BLPACR) H. influenzae are of concern where they exist.
Macrolides: Azithromycin is the most active of these agents against H. influenzae, with a MIC four‐ to eightfold lower than erythromycin (azithromycin MICs, <0.25–4 mg/L). On the other hand, the existence of efflux pumps leads to loss of susceptibility to macrolides in more than 98% of H. influenzae strains [201]. It appears that the vast majority (>98%) of H. influenzae strains have a macrolide efflux mechanism, with a few of these being hyper‐resistant (1.3%; azithromycin MICs >4 mg/L) due to one or several ribosomal mutations. Occasional hypersusceptible strains (1.8%; azithromycin MICs <0.25 mg/L) are found without any underlying mechanism of resistance and appear to be the only truly macrolide‐susceptible variants of H. influenzae.
The prevalence of resistance is based on the use of pharmacokinetic/pharmacodynamic breakpoints; large discrepancies are observed in terms of susceptibility, by use of CLSI breakpoints. So, for instance, the rate of susceptibility to clarithromycin can shift from >99% to 5% (by use of the PK/PD breakpoints).
Fluoroquinolones and other agents: Fluoroquinolone resistance remains rare with H. Influenzae.
Prevalence of tetracycline resistance: few recent data are available. A survey in the UK and Ireland showed a significant though slow downward trend (p <0.00008) in tetracycline non‐susceptibility, which reduced from 3.5% in 1999/2000 to 1.2% in 2006/2007 and dipped as low as 0.9% in 2004/2005 [202].
In Greece, resistance to tetracycline increased from 1.6% in 1996 to 38% in 2005 [203].
Resistance to other orally administered agents, such as trimethoprim‐sulphamethoxazole (TMP‐SMX) and chloramphenicol, is well known. The overall frequencies of resistance to TMP‐SMX remain around 18% in a recent survey in the US [204].
Moraxella catarrhalis. The susceptibility of M. catarrhalis has changed little since 1999. It is interesting to note that, despite almost universal β‐lactamase prevalence, resistance to other antibacterial agents has not developed in M. catarrhalis. Clinicians should assume that all isolates of M. catarrhalis are resistant to amoxicillin, ampicillin, piperacillin and penicillin. Two types of β‐lactamases can be found that are phenotypically identical: the BRO‐1 and BRO‐2 types. Both enzymes are readily inactivated by β‐lactamase inhibitors, and all isolates are still susceptible to amoxicillin in combination with clavulanic acid. Other enzyme‐stable β‐lactams, macrolides and tetracyclines are still very active against M. catarrhalis, but rates of TMP‐SMX resistance as high as 50% have been occasionally reported.
Mycoplasma pneumoniae. M. pneumoniae is inhibited by tetracyclines, macrolides, ketolides and fluoroquinolones, with little variation in MICs among clinical isolates [205, 206]. Other agents that are active at the bacterial ribosome, such as streptogramins, chloramphenicol and aminoglycosides, may also show in vitro inhibitory activity against M. pneumoniae but are not normally used for therapeutic purposes against this organism. Clindamycin is active in vitro but its in vivo activity has never been demonstrated. Due to the lack of a cell wall, mycoplasmas are resistant to all β‐lactams and glycopeptides. Sulphonamides, trimethoprim, polymixins, nalidixic acid and rifampin are also inactive [207]. As tetracyclines and fluoroquinolones are not approved for use in children, macrolides are generally considered the treatment of choice for M. pneumoniae infections in both adults and children.
Since 2000, the emergence of macrolide resistance has been reported mainly in Asia. In Japan, several recent studies reported that macrolide‐resistant M. pneumoniae isolates have been spreading since 2000, with prevalence increasing up to 30.6% according to these studies [208, 209, 210]. The A2058G mutation in domain V of 23S rRNA is the most frequent substitution associated with macrolide resistance in clinical isolates.
Data regarding current resistance patterns for M. pneumoniae in European adult and adolescent patients with CAP are limited. Macrolide resistance rates of 3.0% in Germany have been recently reported [211]. In France, among M. pneumoniae‐positive specimens collected before 2005, no macrolide‐resistant M. pneumoniae isolate was detected. In contrast, among 51 samples collected between 2005 and 2007, five (9.8%) yielded a resistant genotype, suggesting a recent increase in macrolide‐resistant M. pneumoniae isolates in France [212]. These emerging data suggest that the epidemiological monitoring of macrolide resistance in this species has become necessary in Europe.
Staphylococcus aureus. In the European setting, S. aureus remains an unusual primary cause of CAP [213], although it is an important cause of pneumonia and death following influenza [214]. The role of CA‐MRSA is even more poorly defined, although emergent in Europe [215]. Infections due to CA‐MRSA have symptom onset before or within 48 h of admission to hospital and patients have no significant previous healthcare contact. CAP, which is due to CA‐MRSA, classically presents in a young, previously healthy, individual with rapidly progressive, severe respiratory disease. The aggressive nature of CA‐MRSA, due to toxin production, causes massive destruction in previously normal lungs.
CA‐MRSA is usually only resistant to the β‐lactams and susceptible to most other antibiotic classes. This difference in the laboratory findings may indicate that the patient has a CA‐MRSA isolate as opposed to an HA‐MRSA isolate. However, with time, CA‐MRSA is likely to acquire the resistance genes that will make it more difficult to differentiate from HA‐MRSA by routine antimicrobial susceptibility testing.
Because S. aureus is an uncommon cause of CAP, it does not need to be covered routinely by the empirical CAP treatment. However, the severity associated with S. aureus pneumonia reinforces the importance of performing routine blood and respiratory cultures in pneumonia patients.
Clindamycin and linezolid markedly suppress the formation of PVL, α‐haemolysin and toxic shock syndrome toxin 1 by suppressing translation but not transcription. Nafcillin, on the other hand, stimulates toxin production, whereas toxin levels with use of vancomycin are comparable to those in control samples not exposed to antibiotics.
As suppression of toxin production may correlate with improved outcome, vancomycin alone may not be the optimal treatment for pneumonia caused by toxin‐producing CA‐MRSA. Although it has not been established that the combination of a bactericidal agent with a toxin‐suppressing agent, such as clindamycin or linezolid, is associated with improved outcome, it is the general feeling that vancomycin should not be used as a single agent in the treatment of CA‐MRSA CAP.
In severe infections there are limited trial data to support the use of one regimen over another and recommendations are largely based on expert advice. Adjunctive therapy, such as intravenous immunoglobulin, has been successful in some case reports, but its real contribution is unknown.
What new information is available about the clinical relevance of antimicrobial resistance in this setting?
The pattern of antimicrobial resistance varies between European countries. Changes in the prevalence of antibiotic resistance among the main respiratory pathogens in Europe have been reported; continued surveillance of antimicrobial resistance in all common pathogens is essential.
-
1
In pneumococci, erythromycin MICs >0.5 mg/L predict clinical failure. The prevalence of resistance in many countries compromises the efficacy of macrolides in the treatment of pneumococcal infection. The prevalence of resistance will dictate the need to reassess current recommendations for the treatment of CAP.
-
2
Adequate choice and dosing of selected β‐lactams is still useful in the treatment of extrameningeal pneumococcal infections. There are no documented failures in patients with extrameningeal infections due to penicillin‐resistant strains treated with adequate doses of penicillins and third‐generation cephalosporins. Penicillin, 2 g (3.2 mU) i.v. Q 4 h, should be adequate for strains with a penicillin MIC of ≤8 mg/L; adjust dose for renal impairment; ceftriaxone 1 g i.v. or i.m. Q 12 h or cefotaxime 2 g i.v. Q 6 h, should be adequate for strains with a MIC of ≤8 mg/L. A new formulation of Amox/Clav (2 g/125 Q 12 h) eradicated amoxicillin‐resistant strains (MICs, 4–8 mg/L) in two RCTs. Oral cephalosporins are not adequate for the treatment of infection caused by strains with penicillin MICs >2 mg/L.
-
3
Fluoroquinolones are highly active and efficacious against respiratory pathogens; they should be used in well‐defined circumstances. If the prevalence of first‐step mutants is low, the use of the most potent FQ is a logical choice if resistance has to be avoided/delayed. Previous exposure to an FQ in the recent past precludes the use of a member of this class for the empirical treatment of CAP.
-
4
Macrolides show, at best, only modest activity against H. influenzae. The existence of efflux pumps leads to loss of susceptibility to this class in more than 98% of H. influenzae strains.
-
5
Among ‘atypicals’, antibiotic resistance is rare and very seldom responsible for clinical failures.
-
6
Macrolide resistance in Mycoplasma pneumoniae is rising in Japan; there is a need for European local surveillance studies.
-
7
The role of CA‐MRSA in CAP is poorly defined, although emergent in Europe. CA‐MRSA is usually only resistant to the β‐lactams and susceptible to most other antibiotic classes. The antibiotic treatment of CA‐MRSA pneumonia is not known. As suppression of toxin production may correlate with improved outcome, vancomycin alone may not be the optimal treatment for pneumonia. Thus, the combination of a bactericidal agent with a toxin‐suppressing agent, such a clindamycin or linezolid, has been suggested as the optimal choice.
-
8
In vivo selection of resistance means that proper use of antimicrobials is essential.
What new information is available about antimicrobial pharmacokinetics and pharmacodynamics
The only new information is about the need for high levofloxacin doses (750 mg OD) in the treatment of Pseudomonas and Klebsiella [216, 217]. Two other new studies do now alter the current guideline recommendations [218, 219].
Management Outside Hospital
Introduction
Lower respiratory tract infection is a broad description of a group of disease entities, encompassing acute bronchitis, pneumonia and exacerbations of chronic lung disease. In primary care it is very difficult to differentiate between those different diseases without doing extensive additional diagnostic tests. Patients can present with cough, dyspnoea, tachypnoea, fever, pain in the chest, wheezing and auscultatory abnormalities. There is huge overlap in presentation between the different lower respiratory diseases mentioned above and it is neither feasible nor cost‐efficient to do a full diagnostic work‐up in all patients. Therefore an empirical and pragmatic approach is warranted. The statements and recommendations below are based on primary care studies, expert opinion and consensus among members of the working group.
Diagnosis
When should aspiration pneumonia be considered?
Recommendation: Aspiration pneumonia should be considered in patients with difficulties with swallowing who show signs of an acute LRTI. In these patients a chest X‐ray should be performed [C3].
No new information. Recommendation not changed.
When should left ventricular failure be considered?
Recommendation: Left ventricular failure should be considered in patients above 65, with either orthopnoea, displaced apex beat and/or a history of myocardial infarction, hypertension or atrial fibrillation.
Low serum levels of atrial natriuretic peptide (Brain Natriucetic Peptide <40 pg/mL) or N‐terminal pro‐BNP <150 pg/mg) make the presence of left ventricular failure unlikely [C3].
New information. Recommendation not changed.
A number of new studies on the diagnosis of cardiac failure in primary care were found, but none involving patients with a cough. The presence of hypertension and atrial fibrillation is associated with cardiac failure, and levels of BNP and NT‐proBNP were found to have diagnostic value for detecting cardiac failure [220, 221, 222].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Aspromonte et al. [220] | To evaluate whether BNP measurement associated with echocardiography could effectively stratify patients with new symptoms | CSS | 4A+ |
| Mikkelsen et al. [221] | To assess diagnostic accuracy of cardiac peptides in detecting any left ventricular dysfunction (LVD) in patients referred from primary care with suspected HF before institution of medical therapy | CSS | 4A+ |
| Fuat et al. [222] | To test and compare the diagnostic accuracy and utility of B‐type natriuretic peptide (BNP) and N‐terminal B‐type natriuretic peptide (NT proBNP) in diagnosing heart failure due to left ventricular systolic dysfunction in patients with suspected heart failure referred by GPs to one‐stop diagnostic clinics | CSS | 4A+ |
When should pulmonary embolism be considered?
Recommendation: Pulmonary embolism should be considered in patients with one of the following characteristics: a history of DVT or pulmonary embolism, immobilization in the past 4 weeks, or malignant disease [C3].
No new information. Recommendation not changed.
When should chronic airway disease be considered?
Recommendation: In patients with a persistent cough and at least two of the following, wheezing (either as sign or as symptom), previous consultations for wheezing or cough, dyspnoea, prolonged expiration, a smoking history and symptoms of allergy, lung‐function tests should be considered to assess the presence of chronic airway disease. In elderly patients who smoke and present with a cough, COPD should be considered [B1].
One relevant study indicated that smoking and age >60 years in combination with a cough is clearly related to the presence of COPD [223]. One literature review was recently published that gave a critical report on six studies on the detection of COPD. The following signs and symptoms were mentioned at least three times in those studies: dyspnoea, wheezing (complaint), previous consultation for wheezing or cough, self‐reported COPD, age, smoking, wheezing (sign), prolonged expirium and forced expiration time. The review concluded that variation and weaknesses in study designs warranted further studies [224].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Van Schayck et al. [223] | To investigate the effectiveness of case finding of patients at risk of developing chronic obstructive pulmonary disease | CSS | 4A+ |
| Broekhuizen et al. [224] | To review the literature on detection of COPD in patients with cough in primary care | MA | 1C? |
How to differentiate between pneumonia and other respiratory tract infections
Recommendation: A patient should be suspected of having pneumonia when one of the following signs and symptoms are present: new focal chest signs, dyspnoea, tachypnoea, pulse rate >100, fever >4 days. In patients with a suspected pneumonia a test for serum‐level of C‐reactive protein (CRP) can be done. A CRP level of <20 mg/L at presentation, with symptoms for >24 h, makes the presence of pneumonia highly unlikely, a level of >100 mg/L makes pneumonia likely.
In the case of persisting doubt after CRP testing, a chest X‐ray should be considered to confirm or reject the diagnosis [B1].
Two new studies on the diagnostic value of signs, symptoms and CRP [225, 226] both showed that a combination of signs, symptoms and CRP does have diagnostic value in detecting and mainly ruling out pneumonia. Two new studies on the isolated diagnostic value of CRP confirmed the diagnostic value of CRP [227, 228].
On the other hand, two reviews on the value of CRP in this field conclude that CRP has no clear diagnostic value in primary care. The review by van der Meer et al., however, found excellent positive and negative predictive values, with a ROC curve with area under the curve of 0.80. Falk et al. concluded in their review that the isolated use of CRP will not be very useful in primary care but state nevertheless in their discussion that when a physician is in doubt about the presence of pneumonia, CRP could be helpful to rule out the disease [229, 230].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Flanders et al. [227] | To evaluate the performance of a rapid, bedside whole blood C‐reactive protein test as a diagnostic test for pneumonia in adults | PCS | 4A+ |
| Hopstaken et al. [225] | To assess the diagnostic value of symptoms, signs, erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP) for pneumonia | PCS | 4A+ |
| Van de Meer et al. [229] | To evaluate the diagnostic accuracy of C‐reactive protein in detecting radiologically proved pneumonia and to evaluate how well it can discriminate between bacterial and viral infections of the lower respiratory tract. | MA | 1A? |
| Graffelman et al. [226] | To assess the diagnostic value of signs, symptoms and CRP in detecting pneumonia | PCS | 3B+ |
| Holm et al. [228] | To evaluate the diagnostic value of CRP and procalcitonine in detecting pneumonia | PCS | 3A+ |
| Falk and Fahey [230] | To assess the diagnostic value of CRP in detecting pneumonia | MA | 1A? |
Should the primary care physician test for a possible microbiological aetiology of LRTI?
Recommendation: Microbiological tests such as cultures and Gram stains are not recommended [B1].
‘Biomarkers to assess the presence of a bacterial pathogen are not recommended in primary care’ [A1].
A new systematic review and two observational studies underlined these recommendations [94, 228, 229]. New information. Recommendation not changed.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Van de Meer et al. [229] | To evaluate the diagnostic accuracy of C‐reactive protein in detecting radiologically proved pneumonia and to evaluate how well it can discriminate between bacterial and viral infections of the lower respiratory tract | MA | 1A+ |
| Graffelman et al. [94] | To evaluate the diagnostic value of medical history, physical examinations and additional tests in discriminating between viral and bacterial infections in patients with acute cough | PCS | 3B+ |
| Holm et al. [228] | To evaluate the diagnostic value of CRP and PCT in discriminating between bacterial and viral lower respiratory tract infections | PCS | 3A+ |
Prognosis
How should the risk of complications be assessed in a primary care patient with LRTI?
Recommendation: Patients with an elevated risk of complications should be monitored carefully and referral should be considered. In patients over 65 years of age the following characteristics are associated with a complicated course: presence of COPD, diabetes or heart failure, previous hospitalization in the past year, taking oral glucosteroids, antibiotic use in the previous month, general malaise, absence of upper respiratory symptoms, confusion/diminished consciousness, pulse >100, temperature >38, respiratory rate >30, blood pressure <90/60 and when the primary care physician diagnoses pneumonia [A3]. In patients under 65 the working group thinks that diabetes, a diagnosis of pneumonia and possibly also asthma are risk factors for complications. For all age groups, serious conditions such as active malignant disease, liver and renal disease and other disorders that are relatively rare in primary care but affect immunocompetence do also increase the risk of complications [C3].
Several studies have been published, mainly on prognosis in the elderly. Some of the findings mentioned above are not yet validated externally.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Hak et al. [231] | To determine prognostic factors for complications of LRTI among elderly patients in primary care | RCS | 4A+ |
| Seppa et al. [232] | To determine which information can be used to assess the severity of LRTI in primary care | PCS | 3A+ |
| Bauer et al. [233] | To validate the CURB, CRB and CRB‐65 scores for the prediction of death from community‐acquired pneumonia (CAP) | PCS | 3A+ |
| Bont et al. [234] | To study predictors of complications of lower respiratory tract infections in elderly patients | PCS | 3A+ |
| Bont et al. [235] | To validate the CRB‐65 rule for elderly patients in primary care | PCS | 3A+ |
| Bont [236] | To develop a prediction model for lower respiratory tract infections in elderly patients in primary care | PCS | 3A+ |
Treatment
Should symptomatic acute cough be treated?
Recommendation: Cough suppressants, expectorants, mucolytics, antihistamines, inhaled corticosteroids and bronchodilators should not be prescribed in acute LRTI in primary care [A1].
One new updated Cochrane review on cough medication concluded that there is no clear benefit from interventions [237] Some of the studies in this review did report some beneficial effects from expectorants and antitusive agents, but these studies were small and suffered from methodological flaws. The Cochrane review on the use of bronchodilators in acute cough showed no beneficial effects [238]. One new RCT on the effects of inhaled fluticasone in patients with acute cough showed a small effect on symptom severity in the second week of disease. The clinical relevance of this small effect is, however, doubtful [239].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Smith et al. [237] | To assess the effects of oral over‐the‐counter cough preparations for acute cough. | MA | 1A− |
| Smucny et al. [238] | To determine whether beta2‐agonists improve the symptoms of acute bronchitis in patients who do not have underlying pulmonary disease. | MA | 1A+ |
| Ponsioen et al. [239] | To investigate the short‐term effects of an inhaled steroid (fluticasone propionate (FP)) on cough | RCT | 2A+ |
When should antibiotic treatment be considered in patients with LRTI?
Recommendation: Antibiotic treatment should be prescribed in patients with suspected or definite pneumonia (see How to differentiate between pneumonia and other respiratory tract infections?) [C1].
Antibiotic treatment should be considered for patients with LRTI and serious co‐morbidity such as:
-
1
selected exacerbations of COPD (see section ‘Exacerbations of chronic obstructive pulmonary disease’);
-
2
cardiac failure;
-
3
insulin‐dependent diabetes mellitus; or
-
4
a serious neurological disorder (stroke, etc.) [C3].
There is one new update of a Cochrane review on the effects of antibiotics in acute bronchitis, including one large new trial on the effects of antimicrobial therapy: no new conclusions on the overall effects on the average adult patient with acute bronchitis [240, 241]. Recommendations for subgroups are based on consensus.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Little et al. [241] | To estimate the effectiveness of three prescribing strategies and an information leaflet for acute lower respiratory tract infection | RCT | 2A+ |
| Smith [240] | To assess the effects of antibiotic treatment for patients with a clinical diagnosis of acute bronchitis | MA | 1A+ |
What are the indications for antibiotic treatment of acute exacerbations of chronic obstructive lung disease (COPD)?
Recommendation: An antibiotic should be given in exacerbations of COPD in patients with all three of the following symptoms: increased dyspnoea, sputum volume and sputum purulence. In addition, antibiotics should be considered for exacerbations in patients with severe COPD [C1].
New information. Recommendation not changed.
A new Cochrane review concluded that antibiotic treatment has beneficial effects in moderately and severely ill patients with increased cough and purulence of sputum. However, the authors state that their conclusions are somewhat weakened by the considerable differences in methodology and settings between studies. The three studies in outpatients indicate that there is only a potentially beneficial effect in patients with three Anthonisen criteria [242].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Ram et al. [242] | To conduct a systematic review of the literature estimating the value of antibiotics in the management of acute COPD exacerbations | MA | 1A+ |
Which antibiotics should be used in patients with LRTI?
Recommendation: Amoxicillin or tetracycline should be used as antibiotic of first choice based on least chance of harm and wide experience in clinical practice. In case of hypersensitivity a tetracycline or macrolide such as azithromycin, clarithromycin, erythromycin or roxithromycin is a good alternative in countries with low pneumococcal macrolide resistance. National/local resistance rates should be considered when choosing a particular antibiotic. When there are clinically relevant bacterial resistance rates against all first‐choice agents, treatment with levofloxacin or moxifloxacin may be considered [C1].
No clear preferences between available antibiotics can be given based on short‐term benefits or frequency of side‐effects. Clinical trials assessing the effects of antibiotics in primary care do vary considerably both in quality and methods regarding their reports on side‐effects and adverse events in subjects. Equally, it is not really possible to compare tendencies to evoke bacterial resistance or rare, but important, side‐effects. All available antibiotic agents that are active against respiratory pathogens do cause bacterial resistance. In the following recommendations the newer broad‐spectrum antibiotics are reserved for second‐choice escape medication when the traditional well‐known agents cannot be used. Two new reviews support these recommendations [243, 244].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Bjerre et al. [243] | To summarize the evidence currently available from randomized controlled trials (RCTs) concerning the efficacy of alternative antibiotic treatments for CAP in ambulatory patients above 12 years of age | MA | 1A+ |
| Mills et al. [244] | To systematically compare beta lactam antibiotics with antibiotics active against atypical pathogens in the management of community‐acquired pneumonia | MA | 1A+ |
Is antiviral treatment useful in patients with LRTI?
Recommendation: The empirical use of antiviral treatment in patients suspected of having influenza is usually not recommended [B1]. Only in high‐risk patients who have typical influenza symptoms (fever, muscle ache, general malaise and respiratory tract infection), for <2 days and during a known influenza epidemic, can antiviral treatment can be considered [A1].
New information. Recommendation not changed [245, 246].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Cooper et al. [245] | To review the clinical effectiveness of oseltamivir and zanamivir for the treatment and prevention of influenza A and B | MA | 1A+ |
| Jefferson et al. [246] | To review the evidence of efficacy, effectiveness and safety of registered antivirals against naturally occurring influenza in healthy adults | MA | 1A+ |
How should patients with LRTI be monitored?
Recommendation: A patient should be advised to return if the symptoms take longer than 3 weeks to disappear.
A clinical effect of antibiotic treatment should be expected within 3 days and patients should be instructed to contact their doctor if this effect is not noticeable. Seriously ill patients, meaning those with suspected pneumonia and elderly patients with relevant co‐morbidity, should be followed‐up 2 days after the first visit.
‘All patients or persons in their environment should be advised to contact their doctor again if fever exceeds 4 days, dyspnoea gets worse, patients stop drinking or consciousness is decreasing’ [C3].
No new information. Recommendation rephrased.
When should patients with LRTI be referred to hospital?
Recommendation: In the following categories of patients, referral to hospital should be considered:
-
1
Severely ill patients with suspected pneumonia (the following signs and symptoms are especially relevant here: tachypnoea, tachycardia, hypotension and confusion).
-
2
Patients with pneumonia who fail to respond to antibiotic treatment.
-
3
Elderly patients with pneumonia and elevated risk of complications, notably those with relevant co‐morbidity (diabetes, heart failure, moderate and severe COPD, liver disease, renal disease or malignant disease).
-
4
Patients suspected of pulmonary embolism.
-
5
Patients suspected of malignant disease of the lung [C3].
These recommendations are based on consensus in the working group. There are no studies comparing different referral strategies.
Management Inside Hospital
Community‐acquired pneumonia Who should be admitted to hospital?
Recommendation: The decision to hospitalize remains a clinical decision. However, this decision should be validated against an objective tool of risk assessment. The CRB‐65 is most practical in its simplicity. In patients meeting a CRB‐65 of one or more (except age ≥65 as the only criterion met), hospitalization should be seriously considered [A3]. Biomarkers (e.g. CRP or PCT) have a significant potential to improve severity assessment but have not been sufficiently evaluated for the decision to hospitalize [A3].
Most recent publications have shown that the CURB‐score and its modifications (particularly CRB‐65 score) are comparable to the Pneumonia Severity Index index in terms of prediction of death from pneumonia in both outpatients and inpatients [233, 247, 248, 249, 250, 251, 252, 253, 254]. Moreover, the CURB‐65 has been shown to outperform generic sepsis and early warning scores [255]. In view of its simplicity and the absence of any laboratory and radiographic criterion, which may not be easily available in general practice, the CRB‐65 score is recommended as tool of choice in the assessment of pneumonia severity. Systolic blood pressure is the best haemodynamic predictor; diastolic pressure may be neglected [256]. The priority of clinical judgement and the need to consider non‐clinical factors for decision making about treatment settings is reinforced [257, 258, 259]. In patients residing in nursing homes, a predefined clinical pathway can help to reduce hospitalization by about 50%, with comparable clinical outcomes [260].
Biomarkers (C‐reactive protein (CRP)) [228, 261, 262, 263, 264], procalcitonin (PCT) [228, 263, 265, 266], D‐dimer [267], carboxy‐terminal provasopressin (CT‐proAVO, copeptin) [268], midregional proatrial natriuretic peptide (MR‐pro‐ANP) [266, 269, 270], midregional proadrenomedullin (MR‐ADM) [271, 272], and triggering receptor expressed on myeloid cells (TREM‐1) [273], as well as the adrenal response [274, 275], as an alternative or additional tool for the assessment of pneumonia severity, have recently gained much attention. It appears that all of them seem to have a significant potential to predict mortality. Some data suggest that predictive tools and biomarkers do not reflect identical processes and that biomarkers may improve predictions based on clinical parameters [276, 277]. However, the optimal use of clinical assessment, including severity scores and biomarkers, remains to be established. Currently, CRP and PCT are best available and may be implemented as an additional severity tool; however, the evidence is still limited. Among all biomarkers investigated, pro‐ADM seems most promising [271, 272].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| References | Objective | Design | Evidence |
|---|---|---|---|
| Predictive tools | |||
| Aujesky et al. [250] | Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia | PCS | 3A+ |
| Barlow et al.[255] | The CURB65 pneumonia severity score outperforms generic sepsis and early warning scores in predicting mortality in community‐acquired pneumonia | RCS | 4B+ |
| Bauer et al. [233] | CRB‐65 predicts death from CAP | PCS | 3A+ |
| Busing et al. [252] | A prospective comparison of severity scores for identifying patients with severe community‐acquired pneumonia: reconsidering what is meant by severe pneumonia | PCS | 3A+ |
| Capelastegui et al. [251] | Validation of a predictive rule for the management of community‐acquired pneumonia | PCS | 3A+ |
| Chalmers et al. [267] | Systolic blood pressure is superior to other haemodynamic predictors of outcome in community‐acquired pneumonia | PCS | 3A+ |
| Ewig et al. [248] | Validation of predictive rules and indices of severity for community‐acquired pneumonia | PCS | 3A+ |
| Ewig et al. [249] | New perspectives on community‐acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality | PCS | 3A+ |
| Labarere et al. [257] | Factors associated with the hospitalization of low‐risk patients with community‐acquired pneumonia in a cluster‐randomized trial | PCS | 3A+ |
| Lim et al. [247] | Defining community‐acquired pneumonia severity on presentation to hospital: an international derivation and validation study | PCS | 3A+ |
| Loeb et al. [260] | Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial | RCT | 2A+ |
| Man et al. [253] | Prospective comparison of three predictive rules for assessing severity of community‐acquired pneumonia in Hong Kong | PCS | 3A+ |
| Marrie and Huang [258] | Admission is not always necessary for patients with community‐acquired pneumonia in risk classes IV and V diagnosed in the emergency room | PCS | 3A+ |
| Myint et al. [254] | Severity assessment criteria recommended by the British Thoracic Society (BTS) for community‐acquired pneumonia (CAP) and older patients. Should SOAR (systolic blood pressure, oxygenation, age and respiratory rate) criteria be used in older people? A compilation study of two prospective cohorts | PCS | 3A+ |
| Biomarkers | |||
| Chalmers et al. [267] | Admission D‐dimer can identify low‐risk patients with community‐acquired pneumonia | PCS | 3A+ |
| Christ‐Crain et al. [271] | Pro‐adrenomedullin to predict severity and outcome in community‐acquired pneumonia | PCS | 3A+ |
| Christ‐Crain et al. [274] | Free and total cortisol levels as predictors of severity and outcome in community‐acquired pneumonia | PCS | 3B+ |
| Christ‐Crain et al. [269] | Use of B‐type natriuretic peptide in the risk stratification of community‐acquired pneumonia | PCS | 3B+ |
| Hirakata et al. [261] | Comparison of usefulness of plasma procalcitonin and C‐reactive protein measurements for estimation of severity in adults with community‐acquired pneumonia | RCS | 4C− |
| Hohenthal et al. [262] | Utility of C‐reactive protein in assessing the disease severity and complications of community‐acquired pneumonia | RCS | 4B+ |
| Holm et al. [228] | Procalcitonin vs. C‐reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care | PCS | 3B+ |
| Huang et al. [272] | Midregional proadrenomedullin as a prognostic tool in community‐acquired pneumonia | PCS | 3A+ |
| Menendez et al. [263] | Biomarkers improve mortality prediction by prognostic scales in community‐acquired pneumonia | PCS | 3A+ |
| Okimoto et al. [265] | Procalcitonin and severity of community‐acquired pneumonia | RCS | 4C− |
| Kruger et al. [266] | Pro‐atrial natriuretic peptide and pro‐vasopressin to predict severity and prognosis in community‐acquired pneumonia: results from the German competence network CAPNETZ | PCS | 3A+ |
| Kruger et al. [276] | Procalcitonin predicts patients at low risk of death from community‐acquired pneumonia across all CRB‐65 classes | PCS | 3A+ |
| Kruger et al. [268] | C‐terminal provasopressin (copeptin) in patients with community‐acquired pneumonia—influence of antibiotic pretreatment: results from the German competence network CAPNETZ | PCS | 3A+ |
| Prat et al. [270] | Midregional pro‐atrial natriuretic peptide as a prognostic marker in pneumonia | PCS | 3A+ |
| Salluh et al. [275] | Adrenal response in severe community‐acquired pneumonia: impact on outcomes and disease severity | PCS | 3A+ |
| Tejera et al. [273] | Prognosis of community‐acquired pneumonia (CAP): value of triggering receptor expressed on myeloid cells‐1 (TREM‐1) and other mediators of the inflammatory response | PCS | 3B− |
| Thiem et al. [264] | C‐reactive protein, severity of pneumonia and mortality in elderly, hospitalised patients with community‐acquired pneumonia | RCS | 4B+ |
Who should be considered for ICU admission?
Recommendation: Findings reflecting acute respiratory failure, severe sepsis or septic shock and radiographic extension of infiltrates, as well as severely decompensated co‐morbities, should prompt consideration of admission to the ICU or an intermediate care unit [A3].
The predictive potential of rules for the prediction of ICU admission depends on local facilities. Therefore, it appears that severity criteria should be used to indicate the need for intensive care treatment rather than care in a special unit.
The presence of at least two of systolic blood pressure <90 mmHg, severe respiratory failure (PaO2/FIO2 < 250), involvement of >two lobes on chest radiograph (multilobar involvement), or one of requirement for mechanical ventilation or requirement for vasopressors >4 h (septic shock), indicates severe CAP. Alternatively, the presence of several minor criteria as provided in the last IDSA/ATS update may indicate severe CAP [A3].
Both rules should increase the attention given to the recognition of patients with unstable courses of pneumonia in order to avoid delayed transfer to the ICU.
External validation of the modified ATS rule as well as other rules (e.g. the IDSA/ATS rule [278] and SMART‐COP rule [279, 280]) has resulted in two important insights. First, no rule is able to account for all important severity criteria, which could justify ICU admission without substantial loss of specificity. Second, the decision to admit to the ICU is usually not exclusively based on clinical criteria but also depends on the local settings and facilities [279, 281, 282, 283]. Therefore, it appears that criteria for ICU admission should be used as indicators for the need for intensified treatment (i.e. monitoring and treatment for acute respiratory failure and/or severe sepsis) rather than as advice for ICU admission.
Whereas no score has been shown to be consistently superior to others, scores relying on so‐called ‘minor criteria’ should be preferred, at least for clinical use, because they avoid relying on tautological ‘major criteria’. Pneumonia severity rules such as CRB‐65/CURB‐65 and PSI are not useful for identifying patients with severe pneumonia.
In view of a worse prognosis in patients with a delayed transfer to the ICU as compared with direct transfer patients, close monitoring within intensified treatment should be offered to patients at risk of progressive disease. However, there is still a major need for predictors of patients who will deteriorate. The recently developed REA‐ICU index still awaits validation in independent cohorts and different settings [284]. Currently, close monitoring of patients at risk within intensified treatment is the best measure to identify those patients.
Consecutive measurements of CRP and assessment of oxygenation may be used during follow‐up to assess treatment response [285, 286].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Brown et al. [281] | Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community‐acquired pneumonia | PCS | 3B+ |
| Bruns et al. [285] | Usefulness of consecutive C‐reactive protein measurements in follow‐up of severe community‐acquired pneumonia | PCS | 3B+ |
| Charles et al. [279] | SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia | PCS | 3A+ |
| Marrie and Shariatzadeh [282] | Community‐acquired pneumonia requiring admission to an intensive care unit: a descriptive study | RCS | 4A+ |
| Phua et al. [278] | Validation and clinical implications of the IDSA/ATS minor criteria for severe community‐acquired pneumonia | PCS | 3A+ |
| Renaud et al. [284] | Association between timing of intensive care unit admission and outcomes for emergency department patients with community‐acquired pneumonia | PCS | 3A+ |
| Wu et al. [286] | Early evolution of arterial oxygenation in severe community‐acquired pneumonia: a prospective observational study | PCS | 3B− |
What is the value of blood cultures in the diagnosis of community‐acquired pneumonia?
Recommendation: Two sets of blood cultures should be performed in all patients with CAP who require hospitalization [A3].
New information. Recommendation not changed.
S. pneumoniae is identified in approximately 60% of positive blood cultures [287, 288] and Haemophilus influenzae in various percentages from 2% to 13%. Other organisms are recovered in diminishing order of frequency from 14% to 2% and 1%: Gram‐negative aerobes, streptococci (S. pyogenes and other), Staphylococcus aureus and mixtures of organisms [287]. For most of the latter it is difficult to decide whether they were present in the bloodstream or are skin contaminants.
In a retrospective observational cohort study of 684 hospitalized patients admitted via the Emergency Department for treatment of pneumonia [289], only 3.4% had true positive blood cultures. Combining the results of this study with six other studies, only 2.2% of >3000 patients had antibiotics changed based on positive blood cultures. This study demonstrates the limited utility of blood cultures in CAP patients. However, it did not include many patients at risk of multidrug‐resistant pathogens.
From a systematic review of 15 studies with a total of 3898 adult patients admitted with CAP, it was concluded that blood cultures rarely alter empirical antibiotic therapy, and even when there is a change, it is mostly not likely to impact patient outcome [290]. The findings of this systematic review do not support obtaining blood cultures in all adults hospitalized with CAP.
However, also in this systematic review, most investigations excluded immunocompromized or other high‐risk groups, which could have biased results against blood culture utility. It would be prudent therefore not to generalize the findings.
In addition, all 15 studies included in this review were observational. Most did not prospectively require blood cultures in all patients admitted with CAP. Several studies did not explicitly require two sets of blood cultures or that blood cultures be done prior to antibiotics, so they may not have revealed the maximum utility of blood cultures. Methicilin‐resistant Staphylococcus aureus (MRSA) previously confined to nosocomial infections has become more prevalent in the community, causing community‐associated MRSA infections, including CAP [61, 291]. During recent years healthy young people without traditional risk factors for S. aureus disease present increasingly with severe MRSA CAP associated with high mortality. Many strains contain toxin and Panton‐Valentine leucocidine genes. Specimens including blood cultures should be obtained for diagnostic and antimicrobial drug susceptibility testing in order to target therapy.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Bradley [291] | Role of Staphylococcus aureus in CAP | MA | 1A+ |
| Hageman et al. [61] | Role of Staphylococcus aureus in CAP | RCS | 4A+ |
| Beneson et al. [289] | Selective use of blood cultures in emergency department pneumonia patients | RCS | 4A+ |
| Asfhar et al. [290] | Blood cultures for community‐acquired pneumonia: are they worthy of two quality measures? A systematic review | SR | 1A+ |
What other invasive techniques for normally sterile specimens can be useful in the laboratory diagnosis of pneumonia?
Recommendation:
-
1
(a) Thoracentesis: diagnostic thoracentesis should be performed in hospitalized patients with CAP when a significant pleural effusion is present [A3].
No new information. Recommendation not changed.
-
2
(b) Transthoracic needle aspiration (TNA): because of the inherent potential adverse effects, TNA can be considered ONLY on an individual basis for some severely ill patients with a focal infiltrate in whom less invasive measures have been non‐diagnostic [A3].
No new information. Recommendation not changed.
-
3
(c) Bronchoscopic protected specimen brush (PSB) and bronchoalveolar lavage (BAL)) and quantitative endotracheal aspirates (QEA): BAL should be the preferred technique in non‐resolving pneumonia [A3].
Bronchoscopic sampling of the lower respiratory tract can be considered in intubated patients and selected non‐intubated patients, where gas exchange status allows [A3].
New information. Recommendation not changed.
El Sohl studied nursing home patients requiring mechanical ventilation for suspected pneumonia and evaluated quantitative endotracheal aspirates in comparison with PSB and BAL specimens [292]. This study shows that QEA correlate well with quantitative bronchoscopic PSB and BAL. Diagnostic accuracy was most favourable at 104 CFU/mL and may be a reliable alternative to PSB or BAL in patients admitted from nursing homes requiring ventilation when bronchoscopic procedures are not feasible or available.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| El Sohl et al. [292] | Diagnostic yield of quantitative endotracheal aspirates in patients with severe nursing home‐acquired pneumonia | PCS | 3A+ |
What is the value of sputum examination?
Recommendation: Gram strain: should be performed when a purulent sputum sample can be obtained from patients with CAP and processed in a timely manner. The presence of a predominant bacterial morphotype allows inference of the aetiologic bacterial species and interpretation of the results of sputum culture [A3].
New information. Recommendation not changed.
Acceptable sputum specimens can be obtained with some effort from approximately 25% of patients after inhalation of hypertonic saline to induce secretion and cough [293].
The value of the Gram stain of acceptable sputum specimens depends on the presence of a predominant bacterial morphotype [294, 295, 296]. In a retrospective cohort study [297], sputum examination was used as a diagnostic tool in a minority of the patients, without noticeable benefit in the clinical management of CAP inpatients.
The study of Anevlavis is the first reported study to have such an amount of information concerning operating characteristics and the diagnostic value of sputum Gram stain in 1390 patients with bacteraemic CAP [298]. The sensitivity of sputum Gram stain was 82% for pneumococcal pneumonia, 76% for staphylococcal pneumonia and 79% for Haemophilus influenzae pneumonia, with specificities ranging from 93% to 96%. Data from this study suggest that a properly collected and read Gram stain provides a simple, readily available, rapid and inexpensive test result and can be a dependable test for the early aetiological diagnosis of bacterial pneumonia in bacteraemic patients.
Infection by Aspergillus spp. can be distinguished from colonization by the presence of hyphae in respiratory specimens but the diagnosis of aspergillosis is still based on the detection of circulating antigens in serum [299].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study. CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Lagerstrom et al. [293] | Good quality sputum specimens can be obtained after inhalation of hypertonic saline | PCS | 3A+ |
| Garcia Vazquez et al. [294] | The value of the presence of predominant morphotype in sputum for aetiological diagnosis of CAP confirmed | PCS | 3A+ |
| Van der Eerden et al. [295] | The value of the presence of predominant morphotype in sputum for aetiological diagnosis of CAP confirmed | PCS | 3A+ |
| Musher et al. [296] | The value of the presence of predominant morphotype in sputum for aetiological diagnosis of CAP confirmed | PCS | 3A+ |
| Uffredi et al. [299] | Diagnosis of aspergillus CAP in sputum | RCS | 4B+ |
| Signori et al. [297] | Sputum examination in the clinical management of community‐acquired pneumonia | RCS | 4A+ |
| Anevlavis et al. [298] | A prospective study of the diagnostic utility of sputum Gram stain in pneumonia | PCS | 3A+ |
Recommendation: Culture: a culture from a purulent sputum specimen of a bacterial species compatible with the morphotype observed in the Gram stain, which is processed correctly, should be considered for confirmation of the species identification and antibiotic susceptibility testing [B3].
No new information. Recommendation not changed.
Sensitivity and specificity of sputum cultures are reduced by contamination with flora colonizing the upper respiratory tract. The value of sputum cultures in establishing a bacterial cause of LRTI depends on how the specimens are collected and processed and on whether a predominant bacterial morphotype has been observed in the Gram stain.
What can antigen tests offer in the diagnosis of community‐acquired pneumonia?
Recommendation: The immunochromatographic urinary antigen test for S. pneumoniae should be performed in patients admitted to the hospital for reasons of illness severity. This test should also be considered whenever a pleural fluid sample is obtained in the setting of a parapneumonic effusion [A3].
Urine L. pneumophila serogroup 1 antigen detection should be performed in patients admitted to the hospital for reasons of severity and in other patients where this infection is clinically or epidemiologically suspected [A3]. L. pneumophila serogroup 1 antigen detection in urine is the most rapid method for diagnosing or excluding the infection. A negative test makes legionella unlikely, but does not exclude legionella infection [A3].
The value of the S. pneumoniae urinary antigen test in adults has a sensitivity of 65–100% and a specificity of 94%; however, weak positive results should be interpreted with caution. There is a relationship between the degree of the S. pneumoniae urinary antigen test positivity and the pneumonic severity index [300]. Therefore and for cost saving, the test could be applied in a sequential manner with reservation of the test for high‐risk patients for whom demonstrative results of a sputum Gram stain are unavailable [301, 302, 303, 304, 305, 306, 307, 308, 309, 310]. An S. pneumoniae type specific urinary antigen identifies the serotype involved [311].
Also in the prospective cohort study reported by Kobashi the pneumococcal urinary immunochromatographic test (ICT) [312] increased the diagnostic yield for pneumococcal pneumonia in patients with CAP and was particularly useful for diagnosing patients with poor quality sputum in whom antibiotic treatment nevertheless had to be selected. In this study, the authors were able to establish the clinical impact of the rapidity and simplicity of the ICT test for pneumococcal pneumonia. Pneumonia caused by S. pneumoniae also appeared to be treated safely and effectively with high‐dose penicillin based on positive results of the urinary antigen test in the retrospective study reported by Oka [313]. Even compared with PCR on blood samples the Binax NOW S. pneumonia urinary antigen test is a more sensitive and rapid test for the early diagnosis of bacteraemic pneumococcal pneumonia [314]. Persistence of S. pneumoniae antigenuria following diagnosis of pneumococcal pneumonia is normal and can be prolonged, especially if concentrated urine is used [315].
The effect of pretreatment with antibiotics resulted in contradictory reports: a lower detection rate in one study [295] and an increased detection rate if the test is performed 24–48 h after initiation of antibiotic treatment [316]. The urinary antigen test may also be carried out on pleural fluid with a sensitivity and specificity of 79% and 94%, respectively [307], and on serum samples with a sensitivity of 50% in bacteraemic patients and 40% in non‐bacteraemic patients [317]. The ICT test performed on pleural fluid samples augments the standard diagnostic methods of blood and pleural fluid cultures, even in the case of prior antibiotic therapy, and enhances the ICT urinary antigen test: it may provide additional information beyond that obtained by the measurement of urine samples alone and vice versa [318]. Therefore this test should be considered whenever a pleural fluid sample is obtained in the setting of a parapneumonic effusion, particularly when the urinary antigen test is not contributory.
Vaccination does not result in a positive urinary antigen test [161]. Urinary antigen detection is currently the most helpful rapid test for the diagnosis of Legionella infection. The immunochromatographic format is better suited for single specimens, and produces a result within minutes. In one report different urinary antigen tests have an identical sensitivity [319]; in a second report the results of the tests differ when performed on unconcentrated urine samples but are identical when performed on concentrated urine specimens [320]. In the study by Olsen, the Binax test had a significantly higher sensitivity than the Biotest kit both for L. pneumophila serogroup one species and for non‐L. pneumophila species or non‐serogroup 1 L. pneumophila [321]. New Legionella antigen tests have been developed and are becoming available. They show performances comparable to that of the Binax NOW test and could be an alternative for the detection of L. pneumophila antigen in urine from patients suspected of having a Legionella pneumonia [322, 323].
Since the urinary antigen test has been introduced, early diagnosis and treatment has helped to improve the outcomes and case fatality rate of cases involved in outbreaks of Legionellosis [324].
In Legionella infection there also exists a relationship between the degree of positivity of the urinary antigen test and the severity of the disease [325]. A positive result of the urinary antigen test, as demonstrated in the CAPNETZ study [326], is associated with a more severe clinical course and leads to a potential relevant under‐recognition of species other than L. pneumophila.
Rapid antigen tests on respiratory specimens for the diagnosis of influenza virus infection in adult patients are too insensitive and consequently of limited value for confirming the diagnosis when influenza is clinically suspected in adults, according to one study [327].
However, the study by Falsey [328] clearly showed that rapid influenza testing leads to reduction in antibiotic use in hospitalized adults.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study. CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Gutierrez et al. [301] | Value of S. pneumoniae urinary antigen test (UAT) | PCS | 3B+ |
| Smith et al. [302] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Marcos et al. [303] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Roson et al. [304] | Value of S. pneumoniae UAT. Proposal to apply S. pneumoniae UAT in high‐risk patients without demonstrative Gram stain result | PCS | 3A+ |
| Ishida et al. [305] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Stralin et al. [306] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Andreo et al. [307] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Ercis et al. [308] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Genne et al. [309] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Lasocki et al. [310] | Value of S. pneumoniae UAT | RCS | 4A+ |
| Leeming et al. [311] | S. pneumoniae serotype specific EIA on urine sample | PCS | 3A+ |
| Van der Eerden et al. [295] | Value of S. pneumoniae UAT | PCS | 3A+ |
| Korsgaard et al. [316] | S. pneumoniae UAT more positive after antibiotic treatment | PCS | 3A+ |
| Andreo et al. [307] | S. pneumoniae UAT applicable on BAL | PCS | 3A+ |
| Dominguez et al. [317] | S. pneumoniae UAT applied on serum | PCS | 3A+ |
| Ortega et al. [300] | Relation between UAT and PSI | PCS | 3A+ |
| Vazquez et al. [329] | S . pneumoniae UAT not positive after S. pneumoniae vaccination | PCS | 3A+ |
| Dirven et al. [319] | Sensitivity of three UATs similar | PCS | 3A+ |
| Guerrero et al. [320] | Sensitivity of UATs different on unconcentrated samples, identical on concentrated samples | PCS | 3A+ |
| Blazques et al. [325] | Positivity of Legionella UAT related to severity of disease | PCS | 3A+ |
| Kobashi et al. [312] | Evaluating the use of a Streptococcus pneumoniae urinary antigen detection kit for the management of community‐acquired pneumonia in Japan | PCS | 3A+ |
| Oka et al. [313] | The efficacy of high‐dose penicillin for community‐acquired pneumonia diagnosed by pneumococcal urine antigen test | RCS | 4A+ |
| Smith et al. [314] | Diagnosis of Streptococcus pneumoniae infections in adults with bacteraemia and community‐acquired pneumonia: clinical comparison of pneumococcal PCR and urinary antigen detection | PCS | 3A+ |
| Andreo et al. [315] | Persistence of Streptococcus pneumoniae urinary antigen excretion after pneumococcal pneumonia | PCS | 3A+ |
| Porcel et al. [318] | Contribution of a pleural antigen assay (Binax NOW) to the diagnosis of pneumococcal pneumonia | PCS | 4A+ |
| Olsen et al. [321] | Comparison of the sensitivity of the Legionella urinary antigen EIA kits from Binax and Biotest with urine from patients with infections caused by less common serogroups and subgroups of Legionella | RCS | 4A+ |
| Blanco et al. [322] | Detection of Legionella antigen in non‐concentrated and concentrated urine samples by a new immunochromatographic assay | RCS | 4A+ |
| Diederen et al. [323] | Evaluation of the Oxoid Xpect Legionella test kit for detection of Legionella pneumophila serogroup 1 antigen in urine | RCS | 4A+ |
| Alvarez et al. [324] | Impact of the Legionella urinary antigen test on epidemiological trends in community outbreaks of legionellosis in Catalonia, Spain, 1990–2004 | PCS | 3A+ |
| Von Baum et al. [326] | Community‐acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia | PCS | 3A+ |
| Steininger et al. [327] | Near‐patient assays for diagnosis of influenza virus infection in adult patients | RCS | 4A+ |
| Falsey [328] | Impact of rapid diagnosis on management of adults hospitalized with influenza | RCS | 4A+ |
What can serological tests offer in the diagnosis of pneumonia?
Recommendation: Serology for infections caused by M. pneumoniae, C. pneumoniae and Legionella is more useful in epidemiological studies than in the routine management of the individual patient. If aetiological diagnosis of the atypical agents is considered in the management of the individual patient (e.g. in patients not responding to betalactam therapy), serological tests should not be performed as the only routine diagnostic test [A3]. A combination of IgM antibody detection and PCR may be the most sensitive approach [A3].
Many test formats for the detection of Mycoplasma pneumoniae, Chlamydophila pneumonia and Legionella pneumophila antibodies have been proposed. Several studies illustrate a lack of standardization of antigens of Mycoplasma pneumoniae [330, 331, 332]. In one study 6/12 and 9/12 of PCR‐documented M. pneumoniae infections were diagnosed in acute and convalescent phase sera, respectively [333]. In another study anti‐M. pneumoniae IgM antibodies were detected in 7–25% (depending on the test applied) of acute sera and IgG antibodies in 41–63% of convalescent sera [330]. Although IgM detection in the acute phase shows a moderate sensitivity, provided a specific test is used, a combination of IgM antibody detection and PCR may be the most sensitive approach to diagnose Mycoplasma pneumoniae infections, as demonstrated in the study by Martinez [334] and in the CAPNETZ study [335]. Also for acute LRTI due to C. pneumoniae a combination of PCR detection and specific single serum IgM measurement seems recommended [336].
Also the recent study on Legionella antibody detection confirms that the diagnosis cannot be based on one serum sample from the patient. As serology based on paired sera in most cases cannot be confirmed until rather late in the course of the disease, it is advisable to use other diagnostic tests in combination with serology [337].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study. CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Beersma et al. [330] | Lack of standardization of antigens for M. pneumoniae serology of CAP. Variations in antibody detection depending on test applied | PCS | 3A+ |
| Talkington et al. [331] | Lack of standardization of antigens for M. pneumoniae serology of CAP | PCS | 3A+ |
| Templeton et al. [333] | Serology detects 50% and 66.6% of cases in acute and convalescent phases, respectively | PCS | 3A+ |
| Nir‐Paz et al. [332] | Lack of standardization of antigens for M. pneumoniae serology of CAP | PCS | 3A+ |
| Martinez et al. [334] | Detection of Mycoplasma pneumoniae in adult community‐acquired pneumonia by PCR and serology | PCS | 3A+ |
| Von Baum et al. [335] | Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community‐acquired pneumonia (CAPNETZ) | PCS | 3A+ |
| Hvidsten et al. [336] | Chlamydophila pneumoniae diagnostics: importance of methodology in relation to timing of sampling | RCS | 4A+ |
| Elverdal et al. [337] | Comparison and evaluation of four commercial kits relative to an in‐house immunofluorescence test for detection of antibodies against Legionella pneumophila | RCS | 4A+ |
Are amplification tests useful for the diagnosis of LRTI?
Recommendation: Where available, application of quantitative molecular tests for the detection of Streptococcus pneumoniae, both in sputum and in blood, may be valuable in CAP patients in whom antibiotic therapy has been initiated and may be a useful tool for severity assessment Application of molecular tests for the detection of influenza and RSV should be considered during the winter season and for the detection of atypical pathogens, provided the tests are validated and the results can be obtained sufficiently rapidly to be therapeutically relevant [A3].
Qualitative Nucleic Acid Amplification Tests (NAATs) for S. pneumoniae on pleural fluid, peripheral blood or sputum add little to the existing diagnostic tests in sputum and are unable to distinguish colonization from infection.
In a recent prospective study, real‐time quantitative PCR (RQ‐PCR) was evaluated on sputum samples from patients with CAP admitted to the hospital: the yield from RQ‐PCR was almost twice as high as that from sputum culture in patients with proven pneumococcal aetiology. These figures suggest that in hospital‐treated CAP patients, sputum PCR is a more sensitive method for detecting S. pneumoniae than sputum culture and the previously chosen cut‐off level corresponding to 105 CFU/mL is confirmed [338]. Especially when antibiotic treatment has been initiated, RQ‐PCR, together with urine antigen detection, was the best method for identifying S. pneumoniae.
The detection of S. pneumoniae specific targets by real‐time PCR assays, such as Spn9802 or lytA in plasma, is also useful for the rapid detection of bacteraemic pneumococcal pneumonia [339]. Detection of bacterial DNA load in whole blood supports the diagnosis of S. pneumoniae infection in patients with CAP [340]. Bacterial load is associated with the likelihood of death, the risk of septic shock, and the need for mechanical ventilation. High genomic bacterial load for S. pneumoniae may be a useful tool for severity assessment [341].
The ompP6‐based real‐time PCR for the detection of Haemophilus influenzae is both sensitive and specific for the detection of Haemophilus influenzae in respiratory secretions. Quantification facilitates discrimination between disease‐causing H. influenzae strains and commensal colonization [342].
Quantitative PCR assays have also been shown to be useful in the diagnosis of CAP cases caused by L. pneumophila, although they had lower sensitivity than the urinary antigen test. Both RQ‐PCR and antigen testing should be considered complementary in the diagnostic armamentarium for Legionellosis. High bacterial loads determined by RQ‐PCR in LRT samples were useful for predicting disease severity, which may be an advantage of these techniques and therefore warrant further investigation [343].
NAATs for M. pneumoniae, C. pneumoniae, L. pneumophila and B. pertussis, preferably in sputum, have been further validated [333, 344].
The addition of an L. pneumophila‐specific PCR to a urinary antigen test is useful in patients with suspected Legionnaires’ disease who produce sputum and might allow the early detection of a significant number of additional patients [345].
For the detection of M. pneumoniae CAP or LRTI cases, PCR was less sensitive than serology in one study [334] but superior to serology, especially during the early phases of infection, in another study [346]. Data analysis of different studies indicates that no single available test was reliable for the identification of M. pneumoniae in CAP. A combination of serology and PCR proved to be the most reliable approach for identification of M. pneumoniae [334, 335, 347].
Also for acute LRTI caused by C. pneumoniae a combination of PCR detection and specific single serum IgM measurement seems recommended [336].
The use of a Bordetella pertussis specific PCR in combination with single‐serum serology [348] or the combination of culture and PCR increases the sensitivity for pertussis diagnosis [349].
The results of a recent study confirm previous findings that the addition of PCR‐based methods to the conventional microbial techniques improves the yield of aetiological agents significantly and indicate that PCR is not only more rapid than conventional methods, but also more sensitive, both in aetiological diagnosis of CAP [18] and for the detection of respiratory viruses in LRTI [350, 351, 352, 353], allowing clinicians to initiate optimal symptomatic treatment and rational use of antibiotics, adequate antiviral therapy where indicated and optimal infection control.
Previously unknown viruses have been discovered: several coronaviruses, human metapneumovirus and bocavirus. They are detected in CAP by NAATs. Reports on infection by a mixture of several viruses or infection by a mixture of viruses and bacteria exist. Systematic comprehensive studies are awaited to define the clinical importance of these viral and mixed infections.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Templeton et al. [333] | AT for M. pneumoniae and Bordetella pertussis validated | PCS | 3A+ |
| Raty et al. [344] | AT for M. pneumoniae validated | PCS | 3A+ |
| Johansson et al. [338] | Quantitative detection of Streptococcus pneumoniae from sputum samples with real‐time quantitative polymerase chain reaction for aetiological diagnosis of community‐acquired pneumonia | PCS | 3A+ |
| Abdeldaim et al. [339] | Usefulness of real‐time PCR for lytA, ply and Spn9802 on plasma samples for the diagnosis of pneumococcal pneumonia | PCS | 3A+ |
| Peters et al. [340] | Streptococcus pneumoniae DNA load in blood as a marker of infection in patients with community‐acquired pneumonia | RCS | 4A+ |
| Rello et al. [341] | Severity of pneumococcal pneumonia associated with genomic bacterial load | PCS | 3A+ |
| Abdeldaim et al. [342] | Detection of Haemophilus influenzae in respiratory secretions from pneumonia patients by quantitative real‐time polymerase chain reaction | PCS | 3A+ |
| Maurin et al. [343] | Quantitative real‐time PCR tests for diagnostic and prognostic purposes in cases of legionellosis | RCS | 4A+ |
| Diederen et al. [345] | Utility of real‐time PCR for diagnosis of Legionnaires’ disease in routine clinical practice | RCS | 4A+ |
| Martinez et al. [334] | Detection of Mycoplasma pneumoniae in adult community‐acquired pneumonia by PCR and serology | PCS | 3A+ |
| Nilsson et al. [346] | Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection | PCS | 3A+ |
| Thurman et al. [347] | Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks | PCS | 3A+ |
| Von Baum et al. [335] | Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community‐acquired pneumonia (CAPNETZ). BMC Infect Dis 9:62 | PCS | 3A+ |
| Hvidsten et al. [336] | Chlamydophila pneumoniae diagnostics: importance of methodology in relation to timing of sampling. Clin Microbiol Infect 15:42–49 | RCS | 4A+ |
| Andréet al. [348] | Comparison of serological and real‐time PCR assays to diagnose Bordetella pertussis infection | PCS | 3A+ |
| Sotir et al. [349] | Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county‐wide outbreak focused on adolescents and adults | PCS | 3A+ |
| Johansson et al. [18] | Aetiology of community‐acquired pneumonia: increased microbiological yield with new diagnostic methods | PCS | 3A+ |
| Mahony et al. [350] | Development of a respiratory virus panel test for detection of 20 human respiratory viruses by use of multiplex PCR and a fluid microbead‐based assay | PCS | 3A+ |
| Van de Pol et al. [351] | Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses and adenoviruses with real‐time PCR in samples from patients with respiratory symptoms | PCS | 3A+ |
| Ginocchio et al. [352] | Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak | RCS | 4A+ |
| Caram et al. [353] | Respiratory syncytial virus outbreak in a long‐term care facility detected using reverse transcriptase polymerase chain reaction: an argument for real‐time detection methods | PCS | 3A+ |
What classification should be used for treatment?
Recommendation: Antimicrobial treatment has to be empirical and should follow an approach according to the individual risk of mortality. The assessment of severity according to mild, moderate and severe pneumonia implies a decision about the most appropriate treatment setting (ambulatory, hospital ward or ICU) [A4]. Antimicrobial treatment should be initiated as soon as possible [A3].
The guidance for empirical initial antimicrobial treatment should follow three basic considerations and overall ten criteria.
-
1
(A) Prognostic assessment
-
1
The assessment of age: patients aged ≥65 years are subdivided into those with moderate/good ability and those who are severely disabled. Ideally, this assessment should follow an established score (e.g. ADL score). Roughly, severely disabled patients may be defined as bedridden.
-
2
The assessment of general prognosis: patients with pneumonia as an expected terminal event of severe co‐morbidity should be managed along principles of palliative medicine.
(B) Assessment of correct grouping
-
3
Previous hospitalizations and antimicrobial treatment: patients with hospitalizations <3 months ago and those with repeated recent antimicrobial treatments should be classified as nosocomial pneumonia and treated accordingly.
-
4
Risk factors for severe immunosuppression (i.e. at risk of opportunistic pathogens): these patients should be managed following the guidelines for immunocompromised patients.
-
6
(C) Assessment of factors determining selection of antimicrobial treatment.
-
5
Severity: although severity has only a minor impact on microbial patterns, broad combination treatment is mandatory in order to cover all potential pathogens and prevent excess mortality due to treatment failure.
-
6
Co‐morbidity: co‐morbidities may have an independent bearing on potential underlying pathogens.
-
7
Residence: nursing home residence as such may not alter microbial patterns. Such risk should be assessed individually.
-
8
Aspiration: may be witnessed or suspected; may correspond to gross or silent aspiration.
-
9
Regional and local patterns of microbial prevalence and resistance.
-
10
Considerations of tolerability and toxicity of antimicrobial agents in the individual patient.
When should antibiotics be administered after diagnosis of pneumonia?
Recommendation: Antibiotic treatment should be initiated immediately after diagnosis of CAP [C3]. In patients with CAP and septic shock, delay must not be more than 1 h after diagnosis [A1].
As a consequence of studies suggesting an adverse prognostic effect of delayed antimicrobial treatment, immediate timely administration of antibiotics has been advocated in patients with CAP and suggested as an indicator of quality. Although early antibiotic treatment has been confirmed as advantageous by some authors [354], it has been heavily challenged. Some studies failed to confirm such a disadvantage of delayed antibiotic treatment [355, 356]; others have questioned this practice in view of the questionable feasibility of such a policy [357], a high rate of misdiagnosis and overtreatment [358, 359]. The American Academy of Emergency Medicine recommended that measurement of time to first antibiotic dose in CAP be discontinued [360]. Not all authors confirm misdiagnosis and overtreatment along with reporting antibiotic timing [358].
A distinct diagnosis of pneumonia seems mandatory before initiation of antibiotic treatment. It appears that the prognostic relevance of antibiotic timing is highest in patients at a higher risk of death.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Berjohn et al. [354] | Treatment and outcomes for patients with bacteraemic pneumococcal pneumonia. Medicine | PCS | 3A+ |
| Bruns et al. [356] | Time to first antibiotic related to composite endpoint of clinical instability, ICU admission and death | RCS | 4B− |
| Fee and Weber [357] | Identification of 90% of patients ultimately diagnosed with community‐acquired pneumonia within 4 h of emergency department arrival may not be feasible | RCS | 4C− |
| Kanwar et al. [359] | Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side‐effects of the 4‐h antibiotic administration rule | PCS | 3B− |
| Cheng and Buising [355] | Delayed administration of antibiotics and mortality in patients with community‐acquired pneumonia | PCS | 3A+ |
| Friedberg et al. [358] | Reporting hospitals’ antibiotic timing in pneumonia: adverse consequences for patients? | RCS | 4A+ |
| Pines et al. [360] | The measurement of time to first antibiotic dose for pneumonia in the emergency department: a white paper and position statement prepared for the American Academy of Emergency Medicine | Expert opinion | 6C? |
What initial empirical treatments are recommended? Treatment options for hospitalized patients with community‐acquired pneumonia (no need for intensive care treatment) (in alphabetical order) [C4]:
Recommendation:
| Aminopenicillin ± macrolidea,b |
| Aminopenicillin/β‐lactamase inhibitora± macrolideb |
| Non‐antipseudomonal cephalosporin Cefotaxime or ceftriaxone ± macrolideb |
| Levofloxacina Moxifloxacina,c |
| Penicillin G ± macrolide |
aCan be applied as sequential treatment using the same drug.
bNew macrolides preferred to erythromycin.
cWithin the fluoroquinolones, moxifloxacin has the highest antipneumococcal activity.
In patients at risk of GNEB, particularly strains with ESBL, but without risk (or after exclusion of) of P. aeruginosa, ertapenem may be used.
Several publications have demonstrated that low‐level pneumococcal resistance to penicillin is not associated with adverse outcomes in the treatment of patients with community‐acquired pneumonia. Resistance to macrolides may be relevant in patients with moderate to severe pneumonia [361, 362]. Therefore, the choice of antimicrobial agents should be based on considerations of allergy, intolerance, previous use of penicillins, macrolides or quinolones, cost and potential adverse effects rather than pencillin resistance.
Several retrospective studies suggest the superiority of a β‐lactam‐macrolide combination therapy in hospitalized patients, particularly those with more severe disease [363, 364, 365]. However, definite conclusions cannot be made from the present data [366]. Therefore, it appears that combination treatment should be restricted to patients with higher risk classes. As a rule of thumb, the more severely the patient presents, the stronger is the recommendation for such combination treatment.
There is a new formulation of amoxicillin‐clavulanic acid available (2000/125 instead of 875–1000/125), which offers the advantage of higher penicillin dosing [187, 367, 368, 369]. This may be particularly advantageous in patients with pneumococcal pneumonia resistant (low‐level) to penicillin [187].
Respiratory quinolones are now established treatment options [363, 370, 371, 372, 373, 374, 375, 376, 377, 378, 379]. However, the potential small superiority of respiratory quinolones as compared with penicillin and macrolides must be balanced against concerns of selection pressure and cost [374]. Of note, because of the absence of pneumococcal coverage, ciprofloxacin is contraindicated in the treatment of community‐acquired pneumonia.
The EMEA has limited the use of oral moxifloxacin. Although it was stated that ‘the benefits continue to outweigh its risks’, it is stated that it should only be prescribed when other antibiotics cannot be used or have failed. This recommendation was made mainly in view of an increased risk of adverse hepatic reactions. There is no evidence from the literature that moxifloxacin should be considered differently to levofloxacin in this regard. Moreover, there is evidence that liver toxicity is higher in amoxicillin‐clavulanic acid than in respiratory quinolones [380].
Two additional agents have been investigated in patients with CAP: tigecycline [370, 376, 377, 381] and ertapenem [382, 383, 384]. However, there are concerns about low serum levels of tigecycline at standard dosage, which might be hazardous in bacteraemic pneumonia. Ertapenem seems to be an attractive choice in patients at risk of Gram‐negative enterobacteriaceae (GNEB) infection, particularly with ESBL‐producing strains, but not in those at risk of Pseudomonas aeruginosa infection [385, 386, 387, 388].
Regular coverage of atypical pathogens may not be necessary in non‐severe hospitalized patients [244, 389, 390, 391].
Treatment options for patients with severe community‐acquired pneumonia [c4] (ICU or intermediate care):
Recommendation:
| No risk factors for P. aeruginosa |
| Non‐antipseudomonal cephalosporin III + macrolidea or moxifloxacin or levofloxacin ± non‐antipseudomonal cephalosporin III |
| Risk factors for P. aeruginosa |
| Antipseudomonal cephalosporinb or acylureidopenicillin/β‐lactamase inhibitor or carbapenem (meropenem preferred, up to 6 g possible, 3 × 2 in 3‐h infusion) PLUS Ciprofloxacinc OR PLUS Macrolidea + aminoglycoside (gentamicin, tobramycin or amikacin) |
aNew macrolides preferred to erythromycin.
bCeftazidime has to be combined with penicillin G for coverage of S. pneumoniae.
cLevofloxacin 750 mg/24 h or 500 mg twice daily is an alternative and also covers Gram‐positive bacteria if treatment is empirical.
No controlled trials are available for patients treated in the ICU or meeting predictive rules for severe CAP.
Combination treatment offers an advantage over monotherapy by expanding the antimicrobial coverage [392, 393, 394] and probably by immunomodulation (macrolides, quinolones). Therefore, it should be the treatment of choice. However, respiratory quinolones may be used as monotherapy in severe pneumonia without septic shock [395, 396, 397, 398, 399, 400, 401].
The incidence of CAP through P. aeruginosa seems to be low [388]. In patients with risk factors for P. aeruginosa, meropenem offers advantages over imipenem because of the option to increase the dose significantly up to 3 × 2 g [402]. Patients at risk of CAP through P. aeruginosa always should be treated by two antipseudomonal drugs in order to reduce the chance of inadequate treatment. After pathogen isolation and susceptibility testing, combination treatment may be de‐escalated to monotherapy.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Alvarez‐Lerma [395] | Levofloxacin in the treatment of pneumonia in intensive care unit patients | RCS | 4B+ |
| Erard et al. [396] | Full‐course oral levofloxacin for treatment of hospitalized patients with community‐acquired pneumonia | RCT | 2A+ |
| Van Bambeke and Tulkens [380] | Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes | MA | 1A+ |
| Frei et al. [389] | Impact of atypical coverage for patients with community‐acquired pneumonia managed on the medical ward: results from the United States Community‐Acquired Pneumonia Project | RCS | 4A+ |
| Mills et al. [244] | Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community‐acquired pneumonia: meta‐analysis | MA | 1A+ |
| Portier et al. [372] | Moxifloxacin monotherapy compared with amoxicillin‐clavulanate plus roxithromycin for non‐severe community‐acquired pneumonia in adults with risk factors | RCT | 2A+ |
| Querol‐Ribelles et al. [373] | Levofloxacin vs. ceftriaxone plus clarithromycin in the treatment of adults with community‐acquired pneumonia requiring hospitalization | PCS | 3C− |
| Salkind et al. [374] | Fluoroquinolone treatment of community‐acquired pneumonia: a meta‐analysis | MA | 1A+ |
| File et al. [403] | Double‐blind, randomized study of the efficacy and safety of oral pharmacokinetically enhanced amoxicillin‐clavulanate (2000/125 mg) vs. those of amoxicillin‐clavulanate (875/125 mg), both given twice daily for 7 days, in the treatment of bacterial community‐acquired pneumonia in adults | RCT | 2A+ |
| File et al. [187] | Efficacy of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate (2000/125 mg) in adults with community‐acquired pneumonia caused by Streptococcus pneumoniae, including penicillin‐resistant strains | MA | 1B+ |
| Garcia et al. [392] | Lower mortality among patients with community‐acquired pneumonia treated with a macrolide plus a beta‐lactam agent vs. a beta‐lactam agent alone | RCS | 4C− |
| Petitpretz et al. [368] | The efficacy and safety of oral pharmacokinetically enhanced amoxycillin‐clavulanate 2000/125 mg, twice daily, vs. oral amoxycillin‐clavulanate 1000/125 mg, three times daily, for the treatment of bacterial community‐acquired pneumonia in adults | RCT | 2A+ |
| Siquier et al. [369] | Efficacy and safety of twice‐daily pharmacokinetically enhanced amoxicillin/clavulanate (2000/125 mg) in the treatment of adults with community‐acquired pneumonia in a country with a high prevalence of penicillin‐resistant Streptococcus pneumoniae | RCT | 2A+ |
| Bergallo et al. [381] | Safety and efficacy of intravenous tigecycline in the treatment of community‐acquired pneumonia: results from a double‐blind randomized phase 3 comparison study with levofloxacin | RCT | 2A+ |
| Ortiz‐Ruiz et al. [382] | Ertapenem vs. ceftriaxone for the treatment of community‐acquired pneumonia in adults: combined analysis of two multicentre randomized, double‐blind studies | RCT | 2A+ |
| Yakovlev et al. [384] | Ertapenem vs. cefepime for initial empirical treatment of pneumonia acquired in skilled‐care facilities or in hospitals outside the intensive care unit | RCT | 2A+ |
| Martinez [393] | Monotherapy vs. dual therapy for community‐acquired pneumonia in hospitalized patients | Expert opinion | 4A+ |
| Torres et al. [400] | Moxifloxacin monotherapy is effective in hospitalized patients with community‐acquired pneumonia: the MOTIV study—a randomized clinical trial | RCT | 2A+ |
| Von Baum et al. [388] | Community‐acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors | RCS | 4A+ |
| Vetter et al. [383] | A prospective, randomized, double‐blind multicentre comparison of parenteral ertapenem and ceftriaxone for the treatment of hospitalized adults with community‐acquired pneumonia | RCT | 2A+ |
| Torres et al. [378] | Effectiveness of oral moxifloxacin in standard first‐line therapy in community‐acquired pneumonia | RCT | 2A+ |
| Dartois et al. [370] | Tigecycline vs. levofloxacin for the treatment of community‐acquired pneumonia: European experience | RCT | 2A+ |
| File [367] | Gemifloxacin once daily for 5 days vs. 7 days for the treatment of community‐acquired pneumonia: a randomized, multicentre, double‐blind study | RCT | 2A+ |
| Lin et al. [371] | An open‐label, randomized comparison of levofloxacin and amoxicillin/clavulanate plus clarithromycin for the treatment of hospitalized patients with community‐acquired pneumonia | PCS | 3B+ |
| Lodise et al. [363] | Comparison of beta‐lactam and macrolide combination therapy vs. fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community‐acquired pneumonia | PCS | 3A+ |
| Murcia et al. [386] | Clinical response to ertapenem in severe community‐acquired pneumonia: a retrospective series in an elderly population | RCS | 4C− |
| Paladino et al. [387] | Once‐daily cefepime vs. ceftriaxone for nursing home‐acquired pneumonia | PCS | 3A+ |
| Schein et al. [375] | A comparison of levofloxacin and moxifloxacin use in hospitalized community‐acquired pneumonia (CAP) patients in the US: focus on length of stay | RCT | 3A+ |
| Tanaseanu et al. [376] | Integrated results of two phase 3 studies comparing tigecycline and levofloxacin in community‐acquired pneumonia | PCS | 3A+ |
| Tanaseanu et al. [377] | Efficacy and safety of tigecycline vs. levofloxacin for community‐acquired pneumonia | PCS | 3A+ |
| Lui et al. [390] | Role of ‘atypical pathogens’ among adult hospitalized patients with community‐acquired pneumonia | PCS | 3A+ |
| Metersky et al. [364] | Antibiotics for bacteraemic pneumonia: improved outcomes with macrolides but not fluoroquinolones | RCS | 4A+ |
| Paul et al. [366] | The need for macrolides in hospitalized community‐acquired pneumonia: propensity analysis | PCS | 3A+ |
| Vardakas et al. [379] | Respiratory fluoroquinolones for the treatment of community‐acquired pneumonia: a meta‐analysis of randomized controlled trials | SMA | 1A+ |
| Iannini et al. [361] | A case series of macrolide treatment failures in community‐acquired pneumonia | RCS | 4A+ |
| Rodriguez et al. [399] | Combination antibiotic therapy improves survival in patients with community‐acquired pneumonia and shock | PCS | 3A+ |
| Tessmer et al. [365] | Impact of intravenous β‐lactam/macrolide vs. β‐lactam monotherapy on mortality in hospitalized patients with community‐acquired pneumonia | RCS | 4C− |
| Kothe et al. [385] | Outcome of community‐acquired pneumonia: influence of age, residence status and antimicrobial treatment | PCS | 3B+ |
| Katz et al. [397] | Safety and efficacy of sequential i.v. to p.o. moxifloxacin vs. conventional combination therapies for the treatment of community‐acquired pneumonia in patients requiring initial i.v. therapy | PCS | 3A+ |
| Lode et al. [398] | Sequential i.v./p.o. moxifloxacin treatment of patients with severe community‐acquired pneumonia | RCT | 2A+ |
| Martinez et al. [404] | Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteraemic pneumococcal pneumonia | RCS | 4C− |
| Romanelli et al. [402] | Carbapenems in the treatment of severe community‐acquired pneumonia in hospitalized elderly patients: a comparative study against standard therapy | PCS | 3A+ |
| Rzeszutek et al. [362] | A review of clinical failures associated with macrolide‐resistant Streptococcus pneumoniae | MA | 1A+ |
| Shefet et al. [391] | Empirical antibiotic coverage of atypical pathogens for community‐acquired pneumonia in hospitalized adults | MA | 1A+ |
| Wasserfallen et al. [401] | Cost‐effectiveness of full‐course oral levofloxacin in severe community‐acquired pneumonia | RCT | 2A+ |
| Weiss and Tillotson [405] | The controversy of combination vs. monotherapy in the treatment of hospitalized community‐acquired pneumonia | MA | 1A+ |
What is the recommended treatment for specific identified pathogens? Treatment for specific identified pathogens:
Recommendation:
| Pathogen | Recommended treatment |
|---|---|
| Highly resistant S. pneumoniae (>8 mg/dL) | Levofloxacin Moxifloxacin Vancomycin, teicoplanin Linezolid |
| MSSA | Flucloxacillin Cephalosporin II Clindamycin Levofloxacin Moxifloxacin |
| MRSA | Vancomycin, teicoplanin, ± rifampin Linezolid (Clindamycin if sensitive) |
| Ampicillin‐resistant H. influenzae | Aminopenicillin plus β‐lactamase inhibitor Levofloxacin Moxifloxacin |
| Mycoplasma pneumoniae | Doxycycline Macrolide Levofloxacin Moxifloxacin |
| Chlamydophila pneumoniae | Doxycycline Macrolide Levofloxacin Moxifloxacin |
| Legionella spp. | Levofloxacin Moxifloxacin (most data availabe for levofloxacin) Macrolide (azithromycin preferred) ± rifampicin |
| Coxiella burnetii | Doxycycline Levofloxacin Moxifloxacin |
| Acinetobacter baumanii | Third‐generation cephalosporin + aminoglycoside Ampicillin‐sulbactam |
No experience in pneumonia for tigecycline.
There is still no convincing evidence that discordant treatment of penicillin‐resistant pneumococci negatively affects clinical outcome [186, 406, 407, 408, 409]. Thus, pencillin may still be used as a targeted treatment in pneumococci resistant up to MIC 4 mg/L.
Recent publications have confirmed that respiratory quinolones, particularly levofloxacin, offer advantages over macrolide treatment for Legionella infection. If a macrolide is used, azithromycin is the preferred drug. The superiority of levofloxacin and azithromycin is most relevant in patients with severe Legionellosis [410, 411, 412].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Aspa et al. [406] | Drug‐resistant pneumococcal pneumonia: clinical relevance and related factors | PCS | 3A+ |
| Bonnard et al. [407] | Community‐acquired bacteraemic pneumococcal pneumonia in adults: effect of diminished penicillin susceptibility on clinical outcome | PCS | 3B? |
| Falco et al. [408] | Influence of penicillin resistance on outcome in adult patients with invasive pneumococcal pneumonia: is penicillin useful against intermediately resistant strains? | PCS | 3A+ |
| Lujan et al. [409] | Prospective observational study of bacteraemic pneumococcal pneumonia: effect of discordant therapy on mortality | PCS | 3B? |
| Plouffe et al. [410] | Azithromycin in the treatment of Legionella pneumonia requiring hospitalization | PCS | 3A+ |
| Sabria et al. [411] | Fluoroquinolones vs. macrolides in the treatment of Legionnaires disease | PCS | 3A+ |
| Yu et al. [412] | Levofloxacin efficacy in the treatment of community‐acquired legionellosis | MA | 1A+ |
| Peterson et al. [186] | Penicillins for treatment of pneumococcal pneumonia: does in vitro resistance really matter? | MA | 1A+ |
What should be the duration of treatment?
Recommendation: The duration of treatment should generally not exceed 8 days in a responding patient [C2]. Biomarkers, particularly PCT, may guide shorter treatment duration.
The focus of recent studies dealing with treatment duration has been the assessment of post‐discharge outcomes. European authors report a declining duration of hospitalization (and therefore i.v. treatment) [413]. Co‐morbidity, particularly cardiopulmonary and neurological conditions, has been associated with rehospitalizations but not treatment failures due to inadequately short (i.v.) treatment duration [414]. On the other hand, ongoing clinical inflammation despite clinical recovery has been described [415]. However, it is improbable that the level of inflammation can be influenced by prolonged treatment duration.
Most patients with hospitalized non‐severe pneumonia are appropriately treated with 7 days of antibiotics. Although there is only one study addressing treatment duration in nosocomial pneumonia, it appears reasonable to believe that treatment duration for severe pneumonia should not be different from nosocomial pneumonia. According to this study, 8 days appears to be comparable to 15 days of treatment. However, in the presence of P. aeruginosa and other non‐fermenters, clinicians must be aware of an increased risk of relapses [416].
Recently, biomarkers have been described as useful tools to safely reduce antibiotic treatment duration. Biomarkers can guide treatment duration by the application of predefined stopping rules for antibiotics [417, 418, 419]. It has been shown that such rules work even in most severe cases, including pneumonia with septic shock, and even if clinicians are allowed to overrule the predefined stopping rule [420, 421].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Bouadma et al. [420] | Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomized controlled trial | RCT | 2A+ |
| Capelastegui et al. [413] | Declining length of hospital stay for pneumonia and post‐discharge outcomes | PCS | 3B− |
| Chastre et al. [416] | Comparison of 8 vs. 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA 2003; 290(19):2588–2598. Ref ID: 4116 | RCT | 2A+ |
| Christ‐Crain et al. [417] | Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial | RCT | 2B+ |
| El Moussaoui et al. [422] | Comparison of 3 days with 8 days of intravenous amoxicillin | RCT | 2A+ |
| Jasti et al. [414] | Causes and risk factors for rehospitalization of patients hospitalized with community‐acquired pneumonia | PCS | 3A+ |
| Kristoffersen et al. [418] | Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission—a randomized trial | RCT | 2A+ |
| Nobre et al. [421] | Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial | RCT | 2A+ |
| Schuetz et al. [419] | Effect of procalcitonin‐based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial | RCT | 2A+ |
| Yende et al. [415] | Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis | PCS | 3A+ |
When should i.v. treatment be used and when should the switch to oral occur?
Recommendation: In ambulatory pneumonia, treatment can be applied orally from the beginning [A3]. Some carefully selected hospital inpatients may also be candidates for exclusively oral treatment.
In hospitalized patients, sequential treatment should be considered in all patients except the most severely ill. The optimal time to switch to oral treatment is also unknown; this decision should be guided by the resolution of the most prominent clinical features at admission [A3]. In most patients it is probably not necessary to observe patients in hospital after having switched to oral treatment [A3]. Switch to oral treatment after reaching clinical stability is also safe in patients with severe pneumonia [A2].
The efficacy and safety of early switch therapy has been confirmed by several studies and meta‐analyses [423, 424]. Hospitalized patients with non‐severe pneumonia, no sepsis and no reason for impaired intestinal absorption are candidates for oral treatment from the beginning [425]. Switch therapy is safe and may be guided by an algorithm [426] or pathway [427]. The routine practice of in‐hospital observation after the switch from i.v. to oral antibiotics for patients with CAP may be avoided [428]. Also in patients with severe pneumonia, switch to oral antimicrobial treatment after 3 days of intravenous treatment and treatment response is safe [429].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Athanassa et al. [423] | Early switch to oral treatment in patients with moderate to severe community‐acquired pneumonia: a meta‐analysis | MA | 1A+ |
| Lee and Lindstrom [424] | Early switch to oral antibiotics and early discharge guidelines in the management of community‐acquired pneumonia | PCS | 3B+ |
| Marras et al. [425] | Efficacy of exclusively oral antibiotic therapy in patients hospitalized with non‐severe community‐acquired pneumonia: a retrospective study and meta‐analysis | RCS + MA | 4B + 1B+ |
| Nathan et al. [428] | In‐hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med 2006; 119: 512–517. Ref ID: 603 | PCS | 3A+ |
| Oosterheert et al. [429] | Effectiveness of early switch from intravenous to oral antibiotics in severe community‐acquired pneumonia: multicentre randomized trial. BMJ 2006; 333: 1193–1196. Ref ID: 32 | RCT | 2A+ |
| Shindo et al. [427] | Implication of clinical pathway care for community‐acquired pneumonia in a community hospital: early switch from an intravenous beta‐lactam plus a macrolide to an oral respiratory fluoroquinolone | RCS | 4A+ |
| van der Eerden et al. [426] | Evaluation of an algorithm for switching from i.v. to p.o. therapy in clinical practice in patients with community‐acquired pneumonia | PCS | 3A+ |
Which additional therapies are recommended?
Recommendation: All patients should be subject to early mobilization [A3].
Low molecular heparin should be given in patients with acute respiratory failure [A3]. The use of non‐invasive ventilation is not yet standard care but can be considered, particularly in patients with COPD [B3] and ARDS [A3].
The treatment of severe sepsis and septic shock is confined to supportive measures [A3].
Steroids are not recommended in the treatment of pneumonia [A3].
Early mobilization has been shown to be associated with better outcome. For the purpose of the study, early mobilization was defined as movement out of bed with change from horizontal to upright position for at least 20 min during the first 24 h of hospitalization, with progressive movement each subsequent day during hospitalization [430].
Several studies indicate that non‐invasive ventilation (NIV) may also work in patients with pneumonia, particularly in patients with COPD [431, 432]. Non‐invasive ventilation has been shown to reduce intubation in patients with ARDS in 54% of treated cases [433]. It may be feasible and also effective in do‐not‐intubate patients [434] and, therefore, may be an option even in palliative care.
Despite one promising controlled trial [435], two meta‐analyses show that at present steroids cannot be recommended in the treatment of patients with CAP [436, 437].
One meta‐analysis failed to find an effect for the following interventions: activated protein C, non‐invasive mechanical ventilation, anticoagulants, immunoglobulin, granulocyte‐colony‐stimulating factor, statins, probiotics, chest physiotherapy, antiplatelet drugs, over‐the‐counter cough medications, beta(2)‐agonists, inhaled nitric oxide and angiotensin‐converting enzyme inhibitors [438].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Antonelli et al. [433] | A multiple‐centre survey on the use in clinical practice of non‐invasive ventilation as a first‐line intervention for acute respiratory distress syndrome | PCS | 3A+ |
| Bulow and Thorsager [434] | Non‐invasive ventilation in do‐not‐intubate patients: 5‐year follow‐up on a 2‐year prospective, consecutive cohort study | RCS | 4B+ |
| Confalonieri et al. [435] | Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomized study | RCT | 2C− |
| Confalonieri et al. [431] | Acute respiratory failure in patients with severe community‐acquired pneumonia. A prospective randomized evaluation of non‐invasive ventilation | RCT | 2B+ |
| Ferrer et al. [432] | Non‐invasive ventilation in severe hypoxaemic respiratory failure: a randomized clinical trial | RCT | 2B+ |
| Gorman et al. [436] | Corticosteroid treatment of severe community‐acquired pneumonia. | MA | 1A+ |
| Mundy et al. [430] | Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest 2003; 124(3):883–889. Ref ID: 4438 | PCS | 3A+ |
| Salluh et al. [437] | The role of corticosteroids in severe community‐acquired pneumonia: a systematic review | MA | 1A+ |
| Siempos et al. [438] | Adjunctive therapies for community‐acquired pneumonia: a systematic review | MA | 1A+ |
When should aspiration pneumonia be suspected?
Recommendation: There is no agreed definition. Aspiration pneumonia should be suspected in those with CAP which either:
-
1
follows an episode of witnessed aspiration; or
-
2
occurs in the presence of risk factors for aspiration, including reduced consciousness level and dysphagia due to mechanical or neurological upper digestive tract dysfunction [C3].
Evidence Table
MA, meta‐analysis (or systematic review), RCT, randomized controlled trial, PCS, prospective cohort study, RCS, retrospective cohort study, CCS, case‐control study, CSS, cross‐sectional study, SR, systematic review
Table of evidences
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Adams et al. [439] | To assess role of lipid laden macrophages in diagnosis | PCS | 3B+ |
| Chen et al. [440] | Study of pneumonia in patients with dementia | RCS | 2B+ |
| DeToledo et al. [441] | To assess AP frequency after epileptic fits | RCS | 4A+ |
| El Solh et al. [6] | BAL study of microbiology of nursing home patients | PCS | 4A+ |
| Kadowaki et al. [442] | Antibiotic trial | RCT | 2B+ |
| Leroy et al. [44] | Study of ICU admissions | RCS | 4B+ |
| Mier et al. [443] | PSB study of ICU admissions | PCS | 3B+ |
| Mylotte et al. [444] | To compare features of aspiration pneumonia (AP) with aspiration pneumonitis | RCS | 4A+ |
| Reza et al. [445] | To compare features of AP in patients from long‐term care facilities and the community | RCS | 4A+ |
| Teramoto et al. [446] | To identify frequency of aspiration pneumonia in hospitalized adults with CAP | PCS | 3B? |
What empirical antibiotic treatment is recommended for aspiration pneumonia?
Recommendation:
| Hospital ward, admitted from home | ICU or admitted from nursing home |
|---|---|
| Oral or i.v. β‐lactam/β‐lactamase inhibitor or Clindamycin or i.v. cephalosporin + oral metronidazole or moxifloxacin | Clindamycin + cephalosporin or Cephalosporin + metronidazole |
Studies (mainly of clindamycin vs. a comparator antibiotic) have mainly included only small numbers of patients (<40 per treatment arm) and do not reach consistent conclusions regarding the superiority of one antibiotic regime over another [442, 447, 448, 449, 450, 451]. In one larger open RCT, clinical response was identical in those treated with moxifloxacin and those treated with ampicillin–sulbactam, but a significant difference could have been missed due to lack of blinding and because target recruitment was not achieved [452]. Our recommendation is based on knowledge of likely causative pathogens [6, 44, 443] and the antibiotic regimes used in these studies.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
Table of evidences
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Allewelt et al. [447] | Ampicillin/sulbactam vs. clindamycin + cephalosporin | RCT | 2B− |
| Bartlett and Gorbach [448] | Penicillin G vs. clindamycin in anaerobic infection | RCT | 3B+ |
| El Solh et al. [6] | BAL study of microbiology of NH patients | PCS | 4A+ |
| Fernandez Sabe et al. [449] | Co‐amoxiclav in anerobic infection | PCS | 3B+ |
| Gudiol et al. [450] | Clindamycin vs. penicillin | RCT | 2B+ |
| Kadowaki et al. [442] | Ampiciilin/sulbactam vs. clindamycin vs. panipenem/betamiprom | RCT | 2B− |
| Leroy et al. [44] | Study of ICU admissions | PCS | 4B+ |
| Mier et al. [443] | PSB study of ICU admissions | PCS | 3B+ |
| Ott et al. [452] | Comparison of moxifloxacin vs. ampicillin/sulbactam | RCT | 2C− |
| Perlino [451] | Clindamycin vs. metronidazole | RCT | 3B− |
How should response be assessed and should chest radiograph be repeated?
Recommendation: Response to treatment should be monitored by simple clinical criteria, including body temperature, respiratory and haemodynamic parameters. The same parameters should be applied to judge suitability for hospital discharge [A3]. Complete response, including radiographic resolution, requires longer time periods. C‐reactive protein should be measured on days 1 and 3/4, especially in those with unfavourable clinical parameters. The same clinical parameters should be applied to judge suitability for hospital discharge [A3]. Discharge decisions should be based on robust markers of clinical stabilization [A3].
Repeated daily measurement of the PSI found a rising PSI to be related to mortality in one study, but is not practical in routine practice [453].
A number of studies have used C‐reactive protein levels on admission [454] and repeated measurements after admission, both for all admissions [262, 285, 454, 455] and those admitted to the ICU [456], to predict clinical outcome. Measurement of CRP on day 3 [285, 454] or day 4 [262, 455] appears to be most useful. Failure of CRP to fall by 50% by day 4 was associated with fivefold increase in mortality, ventilation and complications [455].
Procalcitonin may also be useful but has not been sufficiently studied to make a recommendation [454].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Bruns et al. [285] | To study relation between day 3 and 7 CRP levels and inappropriate antibiotic therapy | PCS | 3C+ |
| Chalmers et al. [455] | To study CRP as severity predictor in CAP | PCS | 3A+ |
| Chen et al. [453] | To assess value of repeated PSI measurement as mortality predictor | PCS | 3A+ |
| Coelho et al. [456] | To study relationship between CRP and clinical course of CAP on the ICU | PCS | 3A+ |
| Hohenthal et al. [262] | To study relationship between daily CRP and complications in CAP | PCS | 3A+ |
| Menendez et al. [454] | Study of relationship between cytokines and treatment failure | PCS | 3A+ |
How should the non‐responding patient be assessed?
Recommendation: Two types of treatment failures, non‐responding pneumonia and slowly resolving pneumonia, should be differentiated [A3]. Non‐responding pneumonia occurring in the first 72 h of admission is usually due to antimicrobial resistance or an unusually virulent organism or a host defence defect. Non‐response after 72 h is usually due to a complication. The evaluation of non‐responding pneumonia depends on the clinical condition. There are no trials of different approaches to the non‐responding patient to guide this recommendation. In unstable patients, full reinvestigation followed by a second empirical antimicrobial treatment regimen should be carried out. The latter may be withheld in stable patients. Slowly resolving pneumonia should be reinvestigated according to clinical needs, the condition of the patient and his/her individual risk factors [C3].
Exacerbations of chronic obstructive pulmonary disease Which hospitalized patients with COPD exacerbations should receive antibiotics?
Recommendation:
-
1
Patients with all three of the following symptoms: increased dyspnoea, sputum volume and sputum purulence (a type I Anthonisen exacerbation) [A2].
-
2
Patients with only two of the above three symptoms (a type II Anthonisen exacerbation) when increased purulence of sputum is one of the two cardinal symptoms [A2].
-
3
Patients with a severe exacerbation that requires invasive or non‐invasive mechanical ventilation [A2].
-
4
Antibiotics are generally not recommended in Anthonisen type II without purulence and type III patients (one or less of the above symptoms) [A2].
New information. Recommendation not changed.
Fever is not observed in 30% of exacerbations [457]. The relationship between purulence and bacterial growth is confirmed in one study [458]. In addition, a bronchoscopic study found that referred purulence by the patient had a sensitivity of 89.5%, a specificity of 76%, a predictive positive value of 77% and a negative predictive value of 89% to detect bacteria in protected specimen brush bronchoscopic samples in COPD hospitalized patients with exacerbation [459].
However, small studies found a weak association between sputum purulence and bacterial load [460] or bacterial growth [461]. In this later study Gram stain of sputum was the best indicator of bacterial infection. Randomized‐controlled trials are needed to clarify which COPD exacerbated patients requiring hospitalization would benefit from antibiotics. Biomarkers such as procalcitonin may help to detect those exacerbations requiring antibiotics but the information available comes from a single‐centre randomized study [462].
In one case–control study of AECOPD, viruses were found in an important percentage of AECOPD patients requiring hospitalization [168]. In one study focusing on Mycoplasma pneumoniae, this microorganism was involved in 32% of hospitalizations [463].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Allegra et al. [458] | To study the relationship between objective purulence and the presence of bacteria | PCS | 3B+ |
| Lieberman et al. [457] | To study the frequency of fever in exacerbations | PCS | 3C+ |
| Rohde et al. [168] | A case control study to investigate the role of different viruses on acute exacerbations of chronic obstructive pulmonary disease (AECOPD) | CCS | 3C+ |
| Lieberman et al. [463] | To study the role of Mycoplasma pneumoniae in hospitalized patients with AECOPD | PCS | 2C+ |
| Soler et al. [459] | To study the association between purulence and bacterial bronchoscopic samples | PCS | 3A+ |
| Brusse‐Keizer et al. [460] | To study the association between sputum colour and sputum bacterial load | PCS | 3C− |
| Burley et al. [461] | To study the association between symptoms and Gram stain and sputum bacterial growth | PCS | 3B+ |
| Stolz et al. [462] | To study the value of procalcitonin to decrease the use of antibiotics in exacerbated COPD | RCT | 2A+ |
What stratification of patients with COPD exacerbation is recommended in order to direct treatment?
Recommendation:
-
1
Group A: admitted to hospital without risk factors for P. aeruginosa infection [A3].
-
2
Group B: admitted to hospital with risk factors for P. aeruginosa [A3].
New information. Recommendation reworded, but not changed.
It is confirmed that P. aeruginosa is associated with a small percentage of exacerbations that need hospitalization [157, 160]. P. aeruginosa exacerbations seem to be independent of the bronchial bacterial load [169]. P. aeruginosa represented the 17% of isolated microorganisms in 328 out of 494 episodes in Taiwan. The isolation of P. aeruginosa was associated with poorer outcome [464]. In a case‐control study the isolation of multidrug‐resistant microorganisms in AECOPD, including P. aeruginosa, was associated with higher mortality [465].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Groenewegen and Wouters [157] | To study bacterial infections in COPD exacerbated patients that need hospitalization | PCS | 3C+ |
| Ko et al. [160] | To study sputum microbiology in AECOPD | PCS | 4C+ |
| Rosell et al. [169] | To study the microbiological determinants of AECOPD | MA | 1A+ |
| Lin et al. [464] | To study the microbiology of AECOPD | PCS | 3B+ |
| Montero et al. [465] | To study the association between multi‐resistant P. aeruginosa and outcome of AECOPD | CCS | 4b+ |
What are the risk factors for P. aeruginosa?
Recommendation:
P. aeruginosa should be considered in the presence of at least two of the following.
-
1
Recent hospitalization [A3].
-
2
Frequent (>4 courses per year) or recent administration of antibiotics (last 3 months) [A3].
-
3
Severe disease (FEV1 < 30%) [A3].
-
4
Oral steroid use (>10 mg of prednisolone daily in the last 2 weeks) [A3].
One study has investigated [84] the risk factors for P. aeruginosa. Prior use of antibiotics was a risk factor for P. aeruginosa infection (OR 6.06). Influenza vaccination was a protective factor (OR 0.15). We do not know the negative predictive value of this finding. A study of 193 patients with acute exacerbation identified the following variables as independent predictors of Gram‐negative bacilli and P. aeruginosa infection: FEV1 < 35% of predicted value, systemic steroid use and prior antibiotic therapy within the preceding 3 months. The negative predictive value of this rule was 89% [466]. A recent study from Garcia Vidal et al. [467] found that the risk factors for P. aeruginosa in the initial sputum were the BODE index, admissions in the previous year, systemic steroid treatment and previous isolation of P. aeruginosa.
However, in a very large retrospective study P. aeruginosa was found independently of the severity (uncomplicated AECOPD vs. complicated AECOPD; 6% vs. 9.4%) [468].
Despite the fact that recommendations for treating Pseudomonas aeruginosa remain unchanged in these guidelines, some members of the panel disagreed about covering Pseudomonas aeruginosa as initial empirical treatment in patients at risk. The rationale behind this disagreement lies in the studies that consider that P. aeruginosa is a colonizer and not a pathogen.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Monsóet al. [84] | To study the risk factors for bacterial exacerbations | PCS | 3C+ |
| Lode et al. [466] | To study the risk factors for bacterial aetiology in AECOPD | PCS | 3B+ |
| Garcia‐Vidal et al. [467] | To study the risk factors for P. aeruginosa isolation in AECOPD | PCS | 3B+ |
| Kahn et al. [468] | To study the entry microbiological criteria in antibiotic trials of AECOPD | RCT | 4B+ |
Which microbiological investigations are recommended for the hospitalized patient with COPD exacerbation?
Recommendation: Sputum cultures or endotracheal aspirates (in mechanically ventilated patients) should be obtained and are a good alternative to bronchoscopic procedures for evaluation of the bacterial burden by potential pathogenic microorganisms [A3].
Recommendation modified.
Is there new information about pathogens associated with COPD? Most bacterial isolates from patients with COPD are Streptococcus pneumoniae and Haemophilus influenzae, but Moraxella catarrhalis has recently been shown to be associated with approximately 10% of all exacerbations of COPD [166]. In the case of H. influenzae, it is now clear that patients can be colonized by an identical strain of H. influenzae over extended periods of time despite intermittent cultures being negative for the colonizing (or any other) H. influenzae strain [469]. Over the course of the lifetime of a COPD patient, the flora associated with exacerbations does change. In severe cases with FEV1 < 50% of normal, Gram‐negative flora, including P. aeruginosa, become increasingly important as associated pathogens [458]. Acquisition of a new strain of P. aeruginosa is associated with exacerbations [85, 458].
Is there a causal relationship between infections and exacerbations of COPD? Purulent sputum is almost always associated with significantly positive cultures [458]. A causal relationship between infections and exacerbations of COPD has not been established but the association between the two is very strong. In a prospective analysis of COPD patients with exacerbations requiring hospitalization, Papi et al. [167] showed that the frequency of isolation from sputum of bacteria and viruses was much higher during exacerbations than during stable periods, and that eosinophilia within sputum was higher in viral infections. Furthermore, in a longitudinal study of bacterial load in sputum amongst patients with COPD, the FEV1 decline was mirrored by an increase in sputum bacterial load [470]. A recent detailed longitudinal study found that quantitative counts of established sputum flora do not greatly change between stable and exacerbation periods in COPD patients [471]. Sethi et al. [472] have demonstrated that during exacerbations of COPD caused by H. influenzae there is a specific immune response to the infecting strain of H. influenzae.
Does PSI sampling increase the diagnostic yield over other respiratory tract samples? In patients with cystic fibrosis, PSI sampling does not increase the culturable yield of P. aeruginosa over regular sputum sampling [473].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Aaron et al. [473] | To determine if PSI of biofilms of cystic fibrosis patients yields additional P. aeruginosa isolates cf sputum culture | PCS | 3A? small numbers |
| Allegra et al. [458] | Colorimetric and detailed microbiological assessment of sputum in a large sample of COPD patients of varying clinical severity | PCS | 3A+ |
| Murphy et al. [166] | To establish causal link viz AECOPD and Moraxella in a longitudinal cohort with molecular microbiological testing of sputum and serology | PCS | 3A? there have been previous studies that found little evidence of moraxella involvement |
| Murphy et al. [469] | Longitudinal molecular analysis of H. influenzae isolates from sputum | PCS | 3A+ |
| Papi et al. [167] | Prospective study of diagnostic yield from sputa from patients during and after exacerbations of COPD | PCS | 3A+ |
| Sethi et al. [472] | Longitudinal study of COPD patients including detailed analysis of serological responses during exacerbations | PCS | 3A+ |
| Wilkinson et al. [470] | Correlation of sputum bacterial load with clinical features and severity of longitudinal series of COPD patients | PCS | 3A+ |
| Sethi et al. [471] | Detailed longitudinal study of quantitative sputum counts comparing stable and exacerbation periods | PCS | 3A+ |
| Murphy et al. [85] | Acquisition of a new stain of P. aeruginosa is associated with exacerbations of COPD | PCS | 3A+ |
Which initial antimicrobial treatments are recommended for patients admitted to hospital with COPD exacerbation?
Recommendation:
-
1
In patients without risk factors for P. aeruginosa several options for antibiotic treatment are available. The selection of one or other antibiotic should depend on the severity of the exacerbation, local pattern of resistance, tolerability, cost and potential compliance. Amoxicillan‐clavulanic acid is recommended, while levofloxacin and moxifloxacin are alternatives [A2].
-
2
In patients with risk factors for P. aeruginosa, ciprofloxacin (or levofloxacin 750 mg/24 h or 500 mg twice daily) is the antibiotic of choice when the oral route is available. When parenteral treatment is needed ciprofloxacin, or a β‐lactam with antipseudomonal activity, are the options available. The addition of aminoglycosides is optional [A2].
-
3
The use of the oral or intravenous route should be guided by the stability of the clinical condition and the severity of exacerbation. Switch (intravenous to oral) should be done by day 3 of admission if the patient is clinically stable [A3].
Oral gemifloxacin and levofloxacin (750 mg/24 h) over 5 days may be used to effectively treat AECOPD patients that require hospitalization [474, 475]. This information comes from two randomized clinical trials that compare these two quinolones with standard treatments (10 days) in hospitalized and non‐hospitalized patients with AECB.
A meta‐analysis of randomized controlled‐trials (including six studies on hospitalized AECOPD patients), comparing what they called first‐line (amoxicillin, ampicillin, trimetroprim‐sulphamethoxazol) with second‐line antibiotics (amoxicillin‐clavulanic acid, macrolides, second‐ or third‐generation cephalosporins) for AECOPD, showed that first‐line antibiotics were associated with lower treatment success compared with second‐line antibiotics (mainly macrolides and amoxicillin‐clavulanate; OR., 0.51) [476]
| Preferred | Alternative | |
|---|---|---|
| Without risk factors for P. aeruginosa | Co‐amoxiclav | Levofloxacin Moxifloxacin |
| + Risk factors for P. aeruginosa | Ciprofloxacina | Piperacillin/tazobactam i.v. |
aLevofloxacin 750 mg/24 h or 500 mg twice daily is an alternative.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Wilson et al. [474] | A randomized open label study comparing oral gemifloxacin for 5 days with ceftriaxone i.m./cefuroxime orally 10 days in hospitalized patients with AECB | RCT | 2A+ |
| Martínez et al. [475] | A randomized trial comparing 5 days of levofloxacin (750 mg/24 h) with amoxicillin–clavulanic acid for 10 days | RCT | 2C+ |
| Dimopoulos et al. [476] | A meta‐analysis comparing first‐line with second‐line antibiotics in AECOPD | SR | 1A+ |
How should the non‐responding patient with COPD exacerbation be assessed?
Recommendation:
-
1
After close re‐evaluation of non‐infectious causes of failure (i.e. inadequate medical treatment, embolisms, cardiac failure, other) a careful microbiological reassessment, as mentioned in the section on microbiological diagnosis, should be considered [C3].
-
2
Change to an antibiotic with good coverage against P. aeruginosa, S. pneumoniae resistant to antibiotics and non‐fermenters, and subsequent adjustment of the new antibiotic treatment according to microbiological results, should be considered for treatment in cases of failure [C3].
New information. Recommendation not changed.
In one study colonization by non‐fermenting GNB, mainly Pseudomonas aeruginosa, was significantly associated with non‐invasive mechanical ventilation failure in patients with AECOPD admitted to the ICU [477].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Ferrer et al. [477] | To study microbiological determinants associated with NIMV failure in AECOP | PCS | 3A+ |
Exacerbations of bronchiectasis General recommendations for exacerbations of bronchiectasis.
Recommendation:
-
1
Periodic surveillance of colonization should be considered [B3].
-
2
Antibiotic treatment should be given to patients with exacerbations [B3].
-
3
Obtaining a sputum sample for culture before starting antibiotic treatment should be carried out in most cases and particularly in those requiring hospitalization [B3].
-
4
For empirical antibiotic treatment patients should be stratified according to the potential risk of Pseudomonas spp infection [B3] (see section What are the risk factors for P. aeruginosa? above).
-
5
Empirical antibiotics should be adjusted or modified according to sputum culture results [A3].
New information. Recommendation not changed.
The combination of ciprofloxacin and inhaled tobramycin may improve microbiological and clinical outcome. However, in 50% of patients treated with inhaled tobramycin wheezing was observed [478].
Prolonged antibiotic therapy has shown small benefit in modifying the outcome of purulent bronchiectasis [479] [B2].
What antibiotics are recommended for exacerbations of bronchiectasis? [C4]. The risk of P. aeruginosa infection should be considered. No validated risk factors are available; however, risk appears to be related to recent antibiotic therapy or hospitalization, serious disease or prior isolation of Pseudomonas species [89]
Recommendation:
| Oral treatment | Parenteral treatment | |
|---|---|---|
| No risk of Pseudomonas spp. | Amoxicillin‐clavulanate Moxifloxacin Levofloxacin | |
| Risk of Pseudomonas spp.a | Ciprofloxacinb | Ceftazidime, or carbapenem, or piperacillin‐tazobactam |
aUse the same criteria mentioned for chronic obstructive pulmonary disease exacerbation.
bLevofloxacin 750 mg/24 h or 500 mg twice daily is an alternative.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence level |
|---|---|---|---|
| Evans et al. [479] | To identify the role of prolonged antibiotic therapy in modifying the outcome of purulent bronchiectasis | MA | 1B− |
| Bilton et al. [478] | To study the effect of adding inhaled tobramycin solution to oral ciprofloxacin for the treatment of acute exacerbations of non‐CF bronchiectasis in patients with P. aeruginosa infection | RCT | 2A− |
| Angrill et al. [89] | To investigate the incidence, diagnostic yield of non‐invasive and bronchoscopic techniques, and risk factors for airway colonization in patients with bronchiectasis in a stable clinical situation | PCS | 3B+ |
Prevention
Prevention by methods other than vaccination Does oral immunization with bacterial extracts prevent LRTI?
Recommendation: In patients with chronic bronchitis (CB) or COPD H. influenzae oral vaccine [B1] or bacterial extracts (OM‐85 BV) [B2] should not be given.
New information [480, 481, 482, 483]. Recommendation not changed.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study, CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Cogo et al. [480] | Prophylaxis of AE of COPD by a sublingual vaccine | CCS | 3C+ |
| Foxwell et al. [481] | Cochrane: H. influenza oral vaccine for the prevention of AE of COPD | MA | 1A+ |
| Steurer‐Stey et al. [482] | BronchVaxom: meta‐analysis | MA | 1B− |
| Tricarico et al. [483] | Oral bacterial (mechanical lysis) sublingual | RCT | 2A+ |
What is the role of prophylactic antibiotic therapy in chronic bronchitis or COPD?
Recommendation: In patients with CB or COPD, oral or parenteral antibiotics should not be given for prevention [A1].
New information [484, 485]. The PULSE study investigated whether a pulsed therapy with moxifloxacin every 8 weeks for 5 days over a 6‐month period was able to prolong the time to the next exacerbation in COPD patients in comparison to placebo. The study was negative, although there was some trend that patients with purulent sputum showed a prolongation of the time to the next acute exacerbation [487].
Recommendation not changed.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Black et al. [484] | Prophylatic antibiotics for chronic bronchitis | MA | 1A+ |
| Smucny et al. [485] | Antibiotics for acute bronchitis | MA | 1A− |
| Sethi et al. [487] | Proof‐of‐concept study evaluates whether intermittent pulsed moxifloxacin treatment (5 days/8 weeks) could reduce the frequency of these exacerbations | RCT | 2A− |
What is the role of prophylactic antibiotic therapy in patients with COPD or bronchiectasis?
(a) COPD
Recommendation: The use of nebulized antibiotics or intermittent long‐term macrolide therapy is not recommended in COPD patients in general [C4].
The use of nebulized antibiotics for the prevention of LRTI has only been studied in small groups of patients with COPD.
One randomized clinical trial has investigated the use of erythromycin (2 × 250 mg/day) over 12 months in COPD patients, with the aim of reducing moderate to severe exacerbations in these patients [486]. In total, 109 outpatients have been included in the trial: 69 (63%) male; 52 (48%) current smokers; mean (SD) age, 67.2 (8.6) years; FEV1, 1.32 (0.53) L; FEV1% predicted, 50 (18%). Thirty‐eight (35%) of the patients had three or more exacerbations in the year before recruitment, with no differences between treatment groups. There was a total of 206 moderate to severe exacerbations; 125 occurred in the placebo arm. Ten in the placebo group and nine in the macrolide group withdrew. Generalized linear modelling showed that the rate ratio for exacerbations for the macrolide‐treated patients compared with placebo‐treated patients was 0.648 (95% confidence interval, 0.489, 0.859; p 0.003) and that these patients had shorter‐duration exacerbations compared with those on placebo.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Seemungal et al. [486] | To study the efficacy of adding 2 × 250 mg erythromycin to the existing treatment regimen in patients with COPD | RCT | 2A+ |
(b) Bronchiectasis—nebulized antibiotics
Recommendation: There is not enough evidence to recommend the use of nebulized antibiotics (tobramycin) in non‐CF‐bronchiectasis [C2].
Nebulized tobramycin has been used with some success in cystic fibrosis patients. In non‐CF‐bronchiectasis patients, only small studies have been done. One found no effect [488] and one [489] found a decrease of hospital admission and some clinical improvement. Clear evidence for a recommendation to use inhaled tobramycin could not be drawn from these studies.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review [C4].
(c) Bronchiectasis—macrolides
Recommendation: There is not enough evidence to recommend the use of intermittent long‐term macrolide therapy in non‐CF‐bronchiectasis in general [C2].
Use of intermittent macrolide therapy has been successful in patients with CF and patients following lung transplantation. The number of studies investigating non‐CF‐bronchiectasis patients is low. Besides some letters, case reports and very small studies [490], one retrospective study has been published. This study investigated prophylaxis with 3× azithromycin/week in bronchiectasis patients. A reduction of acute exacerbations of 50% has been observed, as well as an increase of FEV1 [491].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Cymbala et al. [490] | To study the efficacy of the addition of 6‐months’ twice‐weekly azithromycin to the existing treatment regimen in patients with bronchiectasis | RCT | 2A+ |
| Anwar et al. [491] | To study the effects of long‐term low‐dose azithromycin in patients with non‐CF bronchiectasis | RCS | 4C+ |
Does antibiotic treatment of upper respiratory tract infections prevent LRTI?
Recommendation: Antibiotics should not be given as treatment for URTI to prevent LRTI [A1].
No new information. Recommendation not changed.
Does treatment with inhaled steroids or long‐acting beta‐2‐agonists or long‐acting anti‐muscarinics prevent LRTI?
Recommendation: Inhaled steroids [B1] or long‐acting beta‐2‐agonists [C4] or long‐acting anti‐muscarinics [C4] should not be used to prevent LRTI (this does not mean that they might not prevent exacerbations of COPD, which is an issue beyond the scope of this document).
No new information. Recommendation not changed.
Does regular physiotherapy prevent LRTI?
Recommendation: Physiotherapy should not be used as a preventive measure against LRTI [C4].
No new information. Recommendation not changed.
Do antiviral substances prevent influenza virus infection?
Recommendation: Prevention of influenza by antiviral substances should only be considered in special situations (for example in outbreaks in closed communities during influenza seasons) [A1]. In the case of seasonal influenza outbreaks or a pandemic situation, the national recommendations should be followed.
New information [492]. Recommendation not changed.
Evidence Table
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Nordstrom et al. [492] | Oseltamivir prevents pneumonia, and decreases the use of antibiotics in patients with ILD | CCS | 3B+ |
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
Are oral mucolytics useful for the prevention of LRTI?
Recommendation:
In patients with bronchiectasis, oral mucolytics should not be used for prevention of LRTI [B1]. Prescription of oral mucolytics through the winter months should be considered for those who have frequent or prolonged exacerbations, or those who are repeatedly admitted to hospital with exacerbations of COPD and for whom inhaled corticosteroids (ICS) are not prescribed [B1].
Although it has been shown that oral mucolytics prevent acute exacerbations in patients with chronic bronchitis (1A+), it has not been shown that these substances prevent infection in the general population. However, there is some evidence that individuals who have frequent or prolonged exacerbations, or those who are repeatedly admitted to hospital with exacerbations of COPD and for whom inhaled corticosteroids (ICS) are not prescribed, may benefit from a prescription of oral mucolytics through the winter months (1A+).
A third systematic review in a row has shown at least some effect of oral mucolytics in selected patients (severe COPD, frequent exacerbations, no ICS) [493].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Poole et al. [493] | Mucolytics for CB or COPD | MA | 1A+ |
Is there evidence that homeopathic substances prevent LRTI?
Recommendation: Homeopathic substances should not be used as a preventive measure against LRTI [C4].
New information [494, 495, 496]. Recommendation not changed.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
| Douglas et al. [494] | Vitamin C against common cold | MA | 1A− |
| Barrett et al. [495] | Ecchinacea for common cold | RCT | 2A− |
| McElhaney et al. [496] | Extract of the roots of North American ginseng (Panax quinquefolium) | RCT | 2A+ |
| Heimer et al. [497] | Vitamin C to prevent common cold | MA | 1B− |
Oral care in nursing homes.
Recommendation: Intensified oral care in nursing home residents should be considered as a preventive measure to reduce the incidence of pneumonia and the risk of death from pneumonia in these patients [B1].
Since the last version of these recommendations one meta‐analysis and two intervention trials have investigated the question of intensified oral care in nursing home patients in relation to the prevention of LRTI or pneumonia.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Sjogren et al. [498] | A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes | MA | 1B+ |
| Awano et al. [499] | To study the risk of death from pneumonia in relation to dental status | CCS | 4C+ |
| Bassim et al. [500] | To study the risk of mortality from pneumonia with oral hygiene care | CCS | 3B+ |
Are there commonly used medications decreasing the risk of LRTI or CAP? Since the last version of these recommendations a variety of commonly used drugs has been investigated with regard to their potential to decrease the risk of LRTI or CAP. These drugs are: inhaled steroids in COPD patients, and ACE‐inhibitors or statins in the general population.
Inhaled steroids in COPD patients: Inhaled steroids might decrease the risk of acute exacerbation in subgroups of COPD patients, but they do not decrease the risk of LRTI. In fact they seem to increase the risk of LTRI/CAP in COPD patients.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review
| Reference | Objective | Design | Evidence |
| Singh et al. [501] | Randomized controlled trials of any inhaled corticosteroid vs. a control treatment for COPD, with at least 24 weeks of follow‐up and reporting of pneumonia as an adverse event were included | MA | 1A+ |
| Almirall et al. [502] | 1336 patients with confirmed CAP were matched to control subjects by age, sex and primary centre over 1 year. Multivariable analysis confirmed inhalation therapy (particularly containing steroids and using plastic pear‐spacers) as independent risk factors | CCS | 4B+ |
| Ernst et al. [503] | To study the effect of inhaled corticosteroids on pneumonia hospitalization | CCS | 4B+ |
| Drummond et al. [504] | To study the effect of inhaled corticosteroids on pneumonia mortality | MA | 1A+ |
| Sin et al. [505] | Pooled patient data from seven clinical trials of inhaled budesonide for the risk of pneumonia | MA | 1A− |
Statin use in the general population and the risk of CAP and death from CAP: The use of statins and/or ACE inhibitors in the general population has been investigated with regard to their potential to decrease the risk of CAP or CAP‐related death.
The use of statins and/or ACE inhibitors might decrease the risk of CAP or CAP‐related death in the general population. There are many more data for statins then for ACE inhibitors.
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review.
| Reference | Objective | Design | Evidence |
|---|---|---|---|
| Mortensen et al. [506] | Effect of current statin use and ACE inhibitor use on 30‐day mortality of patients hospitalized for pneumonia | CCS | 4B+ |
| Chalmers et al. [507] | To study effects of statin use on mortality in those admitted to hospital with pneumonia | CCS | 3B+ |
| Dublin et al. [508] | Case‐control study of statin use in pneumonia | CCS | 4B− |
| Schlienger et al. [509] | Current statin users had a significantly reduced risk of fatal pneumonia | CCS | B4+ |
| Thomsen et al. [510] | To study mortality in pneumonia in current statin users | CCS | 4B+ |
| Tleyjeh et al. [511] | To study statin use to prevent infection | SR | 1A+ |
Recommendations for influenza vaccination Should influenza vaccine be used to prevent LRTI?
Recommendation:
-
1
Influenza vaccine should be given yearly to persons at increased risk of complications due to influenza [A2]. Vaccination should be given to immunocompetent adults belonging to one, or more, of the following categories: age >65 years, institutionalization, chronic cardiac diseases, chronic pulmonary diseases, diabetes mellitus, chronic renal diseases, haemoglobinopathies, and women who will be in the second or third trimester of pregnancy during the influenza season [6].
-
2
Repeated vaccinations are safe and do not lead to a decreased immune response [B1].
-
3
In adults, inactivated, rather than live attenuated, vaccine should be used [A1].
-
4
Yearly vaccination should be carried out for health care personnel, especially in settings where elderly persons or other high‐risk groups are treated [B2].
-
5
General vaccination of all healthy adults should not be carried out in the absence of robust cost‐effectiveness data for vaccination [B1]
In the elderly (>65 years of age) and in high‐risk adults, irrespective of age, new studies have confirmed that seasonal influenza vaccination is effective in prevention of severe complications or death due to influenza [512, 513, 514, 515]. As most of these results are based on non‐controlled studies, they may result in either a too pessimistic or too optimistic view of the effectiveness of vaccination. The latter, based on ‘healthy user biases’, has been shown in several recent studies [515, 516, 517, 518].
A recent Cochrane analysis was unable to reach clear conclusions about the effects of the influenza vaccine in the elderly [519], but it must be emphasized that a lack of evidence does not equal a lack of effectiveness. So far, there is unfortunately only one randomized controlled study of high quality [520]. This study clearly demonstrated that the vaccine was effective in prevention of clinical and laboratory verified influenza in the elderly, but was not powered to detect effects on complications. However, based on that study it is reasonable to assume that the vaccine will also prevent severe influenza and its complications, which is in accordance with the findings of a large majority of well‐performed observational studies.
The specific age‐limit of ≥65 years of age for recommendation of general seasonal influenza vaccination used in these guidelines is based on the fact that most trials have used this cut‐off for the inclusion of patients and/or the analysis of the results. In some countries general vaccination is recommended also for some age groups below 65 years (e.g. in the USA, where vaccination is recommended for all persons aged 50–64 years because persons in this age group have an increased prevalence of high‐risk conditions and low vaccination rates) [521].
Yearly vaccinations with the seasonal influenza vaccine do not lead to a decreasing immune response or protection, or to more frequent effects than those seen after primary vaccination. Two new studies have confirmed that the inactivated injectable influenza vaccine is superior to the live attenuated vaccine in healthy adults [522, 523] However, although the seasonal influenza vaccine prevents respiratory illnesses in healthy adults [523, 524], a revision of the Cochrane analysis by Demicheli et al. [525] indicates that vaccination is of only limited clinical value in this group of patients.
Systematic reviews indicate that vaccination of health care personnel against influenza may reduce influenza‐like illness and all‐cause mortality of elderly people in long‐term hospitals, but have not demonstrated an effect on specific outcomes, such as laboratory‐proven influenza, pneumonia or deaths from pneumonia [526, 527].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review.
| Refernece | Objective | Study design | Evidence level |
|---|---|---|---|
| Demicheli et al. [525] | To study the effectiveness of influenza vaccination in persons 14–64 years of age | SR | 1A+ |
| Hak et al. [512] | To study the effectiveness of influenza vaccination in adult and elderly high‐risk persons | CCS | 4A+ |
| Hak et al. [513] [2] | To study the effectiveness of influenza vaccination in adults 18–64 with COPD | CCS | 4A+ |
| Jackson et al. [516] | To study the effectiveness of influenza vaccination in elderly persons | RCS | 4A+ |
| Thomas et al. [526] | To study the effectiveness of influenza vaccination of health‐care workers in order to protect elderly persons | SR | 1A− |
| Squarcione et al. [528] | To study the immunogenicity and reactogenicity of inactivated influenza vaccine in older persons | RCT | 2C− |
| Wang et al. [522] | To study live attenuated vs. inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel | PCS | 3B+ |
| Monto et al. [523] | Comparative efficacy of inactivated and live attenuated influenza vaccines in healthy adults | RCT | 2A+ |
| Nichol et al. [524] | To study the effectiveness of influenza vaccination in prevention of influenza‐like illness | PCS | 3B+ |
| Schembri et al. [514] | To study the effectiveness of influenza vaccination in prevention of all‐cause mortality in elderly persons | RCS | 4A+ |
| Örtqvist et al. [515] | To study the effectiveness of influenza vaccination in prevention of all‐cause mortality in elderly persons | PCS | 3A+ |
| Eurich et al. [517] | To study the effectiveness of influenza vaccination in prevention of all‐cause mortality in elderly persons | PCS | 3A+ |
| Jackson et al. [518] | To study the effectiveness of influenza vaccination in prevention of community‐acquired pneumonia in immunocompetent elderly people | CCS | 4A+ |
| Jefferson et al. [519] | To study vaccines for preventing influenza in the elderly | SR | 1B− |
| Thomas et al. [527] | To study the effect of influenza vaccination for healthcare workers who work with the elderly | SR | 1B− |
Recommendations for pneumococcal vaccination Should pneumococcal vaccine be used to prevent LRTI?
Recommendation:
-
1
The 23‐valent polysaccharide pneumococcal vaccine prevents invasive pneumococcal disease in older persons and in other high‐risk groups and should be given to all adult persons at risk of pneumococcal disease [A1].
-
2
Risk factors for pneumococcal disease are: age >65 years, institutionalization, dementia, seizure disorders, congestive heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, history of a previous pneumonia, chronic liver disease, diabetes mellitus, functional or anatomical asplenia, and chronic cerebrospinal fluid leakage [B3]. Although smoking seems to be a significant risk factor in otherwise healthy younger adults, measures aimed at reducing smoking and exposure to environmental tobacco smoke should be preferred in this group.
-
3
Revaccination, once, and not earlier than 5 years after primary vaccination, should be performed in asplenic patients and can be considered in the elderly and other high‐risk groups [B3].
-
4
There are not enough data to give any recommendations concerning the use of conjugate pneumococcal vaccine in adults.
The immunogenicity of the 23‐valent polysaccharide pneumococcal vaccine (PPV) is generally good, but may be poor in some elderly patients or in persons with some underlying illnesses (e.g. bronchiectasis) [529]. It is also important to stress that the PPV includes 23 antigens and that a person can develop a pneumococcal disease from one of these serotypes, despite responding to all the others [530]. Previously, there have been conflicting data concerning whether a pneumococcal conjugate vaccine (PCV) could result in a superior immune response in elderly patients or high‐risk adults, compared with PPV [531], but a couple of recent studies do indicate that this may be the case [532, 533]. The drawbacks of PCV, however, are the limited number of serotypes, the much higher price, and the lack of data on efficacy (although a large RCT is underway).
Vaccination of children with PCV may be of benefit also for adults. Since the start of vaccination of children with PCV in the USA in 2000 a marked reduction of IPD has been noted both in the vaccinated cohorts and in adults [534, 535]. This ‘herd immunity’ effect has been most marked in the age groups of parents (20–39 years of age) and grandparents (above 65 years of age). Concerning other outcomes, the herd effect is less clear, with one study indicating a decrease of all‐cause pneumonia and pneumococcal pneumonia in adults 18–39 years of age [536], while another showed no impact at all for all‐cause pneumonia in adults [537].
The efficacy of PPV in adults, including the elderly, has been evaluated in eight meta‐analyses/systematic reviews (MA/SR) of randomized controlled trials (RCTs). The three most recent reviews have also included a systematic review of observational studies of invasive pneumococcal disease (IPD) [538, 539, 540, 541]. During the last 2 years, one double‐blind randomized controlled trial and some other studies on the effectiveness of PPV have been published [542, 543, 544, 545, 546, 547, 548, 549, 550].
The MA/SRs of RCTs have shown strong evidence of PPV efficacy in prevention of invasive pneumococcal disease (IPD) in healthy adults, including the elderly (40–75% protective efficacy), while the effect against IPD may be somewhat poorer in persons with chronic illnesses. The estimates of protection against IPD from SRs of observational studies have been consistent, homogenous and compatible with those of RCTs [538, 539, 540, 541]. Reports of significant reductions in the incidence of IPD in the elderly after the introduction of large‐scale vaccination programmes from two European countries support the effectiveness of the vaccine against IPD [551, 552].
There is very limited evidence that PPV prevents all‐cause pneumonia in the elderly or in other risk groups. However, a recent double‐blind randomized controlled trial among about 1000 nursing home residents in Japan demonstrated that PPV was associated with a reduction of the incidence of all‐cause pneumonia by 45% and of pneumococcal pneumonia by 64% [546]. There was also a significant higher death rate among persons with pneumococcal pneumonia in the placebo group, 35% (13/37) vs. 0% (0/14). This study supports earlier findings from recent cohort studies indicating that PPV is associated with a reduction of pneumonia overall, pneumococcal pneumonia, hospitalization for pneumonia and death due to pneumonia [544, 545, 547, 553, 554]. In contrast, some other cohort studies have found no protection against all‐cause pneumonia or hospitalization for pneumonia [543, 550].
In an open RCT, performed in adults with COPD, a high degree of protection against CAP due to S. pneumoniae or unknown aetiology was seen in persons <65 years of age, and especially in those with severe functional obstruction (FEV1 < 40%) [542]. In contrast, pneumococcal vaccination did not alter significantly the risk of overall CAP in a cohort study of older adults with chronic respiratory diseases [548].
In European studies, vaccination with PPV of the elderly has not been cost‐saving, but shown moderate to good cost‐effectiveness in preventing hospital admission for IPD [555, 556]. The most recent study [556] indicated that pneumoccoocal vaccination would be cost neutral if it was 75–89% efficacious against IPD or 28–38% against pneumococcal pneumonia in the elderly. If the vaccine efficacy against IPD was 50% the net cost for society would be £2500 per year of life saved. Using data on the effect of herd immunity from the USA, it has been estimated that it would be cost‐effective from an adult point of view to vaccinate children in the UK with four doses of the seven‐valent conjugate vaccine [557].
The safety and immunogenicity of one revaccination with pneumococcal vaccine has been confirmed by several studies [558, 559, 560]. In a large randomized controlled trial patients who previously had received one dose of PPV were randomized to receive PPV or PCV, in four different dosages [558]. Local side‐effects were common, but usually mild. The frequency of local reactions in the PCV group depended on the dose given, and in the highest dosage group the risk of a reaction was comparable to that of PPV. In a prospective cohort study of 61 elderly persons (median age 75 years) revaccinated on average 5.3 years after the primary vaccination, significant increases of the geometric mean antibody concentration and geometric mean antibody fold increase were seen, although to lower levels than after primary vaccination [559]. Thirty‐six of 61 (59%) of patients responded with a fold increase >2, to two or more of six serotypes. Early revaccination may lead to a short‐lived antibody rise, which could be due to an immunological suppression or tolerance [532]. However, this suppressive effect seems to wane after some years and in studies where revaccination has been performed after 5 years or more persons have responded well [559, 560].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review.
| Reference | Objective | Study design | Evidence level |
|---|---|---|---|
| Van Kessel et al. [529] | To study immunogenicity of PPV in patients with bronchiectasis | PCS | 3C− |
| Abraham‐Van Parijs et al. [531] | To compare immunogenicity of PPV and PCV in healthy and high‐risk adults | SR | 1B− |
| Kyaw et al. [534] | To study the effectiveness of reducing IPD in adults by vaccination of children with conjugate pneumococcal vaccine | RCS | 4C+ |
| Whitney et al. [535] | To study the effectiveness of reducing IPD in adults by vaccination of children with conjugate pneumococcal vaccine | RCS | 4C+ |
| Melegaro and Edmunds [538] | To study the efficacy of PPV in adults above 50 years of age | SR | 1A− |
| Dear et al. [539] | To study the efficacy of PPV in adults | SR | 1A− |
| Conaty et al. [540] | To study the efficacy of PPV in adults | SR | 1A− |
| Alfageme et al. [542] | To study the efficacy of PPV in adults with COPD | RCT | 2C+ |
| Jackson et al. [543] | To study the efficacy of PPV in adults above 65 years of age | RCS | 4B+ |
| Christenson et al. [544] | To study the efficacy of PPV in adults above 65 years of age | PCS | 3B+ |
| Vila‐Córcoles et al. [545] | To study the efficacy of PPV in adults above 65 years of age | PCS | 3B+ |
| Fisman et al. [553] | To study the efficacy of PPV in adults | RCS | 4C+ |
| Mykietiuk et al. [554] | To study the efficacy of PPV in adults | PCS | 3C+ |
| Melegaro et al. [555] | To study cost‐effectiveness of PPV in elderly persons | RCS | 5B+ |
| Mangtani et al. [556] | To study cost‐effectiveness of PPV in elderly persons | RCS | 5B+ |
| McIntosh et al. [557] | To study cost‐effectiveness in adults after vaccination of children with PCV | RCS | 5B+ |
| Jackson et al. [558] | To compare safety of PPV and PCV in elderly persons | RCT | 2A+ |
| Törling et al. [559] | To study the immune response to revaccination with PPV in elderly persons | PCS | 3A+ |
| Sisk [26] | To study cost‐effectiveness of PPV in adults 50–54 years of age | RCS | 5B+ |
| Andrews [27] | To study the efficacy of PPV in adults above 65 years of age | PCS | 4C+ |
| Pepper [28] | To study cost‐effectiveness of PPV in healthy adults | RCS | 5B+ |
| Ortqvist et al. [530] | To study response to specific serotypes causing failure of 23‐valent pneumococcal polysaccharide vaccine in the elderly | CCS | 4A+ |
| Musher et al. [532] | To study the initial and subsequent response to pneumococcal polysaccharide and protein‐conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia | PCS | 3B+ |
| de Roux et al. [533] | To compare pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults | RCT | 2B+ |
| Grijalva et al. [536] | To study pneumonia admissions after routine childhood immunization with pneumococcal conjugate vaccine in the USA | RCS | 4B+ |
| Nelson et al. [537] | To study impact of the introduction of pneumococcal conjugate vaccine on rates of community‐acquired pneumonia in children and adults | RCS | 4A+ |
| Moberley et al. [541] | Systematic review of vaccines for preventing pneumococcal infection in adults | SR | 1A+ |
| Maruyama et al. [546] | To study the efficacy of 23‐valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents | RCT | 2A+ |
| Christenson et al. [547] | To study the effect of influenza and pneumococcal vaccines in elderly people | PCS | 3C+ |
| Ochoa‐Gondar et al. [548] | To study the effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases | PCS | 3B− |
| Lee et al. [549] | To study the impact of pneumococcal vaccination on pneumonia rates in adult patients with COPD and asthma | RCS | 4B+ |
| Skull et al. [550] | To study whether influenza and/or pneumococcal vaccine prevents hospitalization because of community‐acquired pneumonia in the elderly | CCS | 4B− |
| Johnstone et al. [561] | To study the effect of pneumococcal vaccination in hospitalized adults with community‐acquired pneumonia | PCS | 3B+ |
| Waites et al. [560] | To study the effects of revaccination of adults with spinal cord injury using the 23‐valent pneumococcal polysaccharide vaccine | PCS | 3A+ |
Recommendations for implementation.
Recommendation: Active interventions should be used to enhance vaccination with either, or both, of the vaccines and is effective and needed to in order to achieve an adequate vaccination coverage of the targeted population [A1].
New studies have confirmed that different types of interventions (e.g. patient reminders or standing orders) are effective for increasing vaccination of the targeted population against influenza and pneumococcal disease [562, 563, 564, 565].
Evidence Table
MA, meta‐analysis (or systematic review); RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; CCS, case‐control study; CSS, cross‐sectional study; SR, systematic review.
| Reference | Objective | Study design | Evidence level |
|---|---|---|---|
| Dexter et al. [562] | To study the effectiveness of different methods to increase vaccine coverage in adults eligible for vaccination | RCT | 2C+ |
| Jacobson et al. [563] | To study the effectiveness of different methods to increase vaccine coverage in adults of all age groups | SR | 1A+ |
| de Hart et al. [564] | To study the effectiveness of different methods to increase vaccine coverage in elderly persons | PCS | 3C+ |
| Jha et al. [565] | To study performance measures, vaccinations and pneumonia rates among high‐risk patients in Veterans Administration health care | RCS | 4C+ |
Appendix 1
Evidence grades (∼hierarchy of methods)
-
1
= Systematic reviews and meta‐analyses (of study types under grade 2 or 3)
-
2
= Randomized trials
-
3
= Prospective cohort
-
4
= Case‐control, cross‐sectional, retrospective cohort
-
5
= Case reports
-
6
= Expert opinion, consensus
Suffix for evidence grades 1–6.
-
1
A = low risk of biased results (flaws very unlikely for both blinding and follow‐up)
-
2
B = moderate risk of biased results (flaws unlikely for both blinding and follow‐up)
-
3
C = high risk of biased results (flaws likely for either or both blinding and follow‐up)
Suffix for evidence grades 1A–6C.
-
1
+ = determinant‐outcome relation of interest clearly established
-
1
i.e. the numerical results from the study unequivocally support a positive answer to the research question
-
1
-
2
− = determinant‐outcome relation of interest clearly not established
-
1
i.e. the numerical results from the study are unequivocally not supportive of a positive answer to the research question
-
1
-
3
? = determinant‐outcome relation of interest unclear
Appendix 2
Recommendation grading
Grades
-
1
A = Consistent evidence ‐>Clear outcome
-
2
B = Inconsistent evidence ‐>Unclear outcome
-
3
C = Insufficient evidence ‐>Consensus
Suffix for recommendation grades A–C
-
1
For studies of diagnosis and treatment (including prevention and harm)
-
1
1. = Systematic review (SR) or meta‐analysis (MA) of RCTs
-
2
2. = 1 RCT or more (>1: no SR or MA yet)
-
3
3. = 1 cohort study or more (>1: no SR or MA yet)
-
4
4. = Else
-
1
-
2
For studies of prognosis and aetiology
-
1
1. = SR or MA of cohort studies
-
2
2. = 1 cohort study or more (>1: no SR or MA yet)
-
3
3. = Else
-
1
References
- 1. Woodhead M, Blasi F, Ewig S et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 2005; 26: 1138–1180. [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society; Infectious Disease Society of North America . Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 3. Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health‐care‐associated pneumonia: results from a large US database of culture‐positive pneumonia. Chest 2005; 128: 3854–3862. [DOI] [PubMed] [Google Scholar]
- 4. Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care‐associated pneumonia and community‐acquired pneumonia: a single‐center experience. Antimicrob Agents Chemother 2007; 51: 3568–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Solh AA, Pietrantoni C, Bhat A, Bhora M, Berbary E. Indicators of potentially drug‐resistant bacteria in severe nursing home‐acquired pneumonia. Clin Infect Dis 2004; 39: 474–480. [DOI] [PubMed] [Google Scholar]
- 6. El‐Solh AA, Pietrantoni C, Bhat A et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med 2003; 167: 1650–1654. [DOI] [PubMed] [Google Scholar]
- 7. Carratala J, Mykietiuk A, Fernandez‐Sabe N et al. Health care‐associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 2007; 167: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 8. Garb JL, Brown RB, Garb JR, Tuthill RW. Differences in etiology of pneumonias in nursing home and community patients. JAMA 1978; 240: 2169–2172. [PubMed] [Google Scholar]
- 9. Lim WS, Macfarlane JT. A prospective comparison of nursing home acquired pneumonia with community acquired pneumonia. Eur Respir J 2001; 18: 362–368. [DOI] [PubMed] [Google Scholar]
- 10. Marrie TJ, Blanchard W. A comparison of nursing home‐acquired pneumonia patients with patients with community‐acquired pneumonia and nursing home patients without pneumonia. J Am Geriatr Soc 1997; 45: 50–55. [DOI] [PubMed] [Google Scholar]
- 11. Meehan TP, Chua‐Reyes JM, Tate J et al. Process of care performance, patient characteristics, and outcomes in elderly patients hospitalized with community‐acquired or nursing home‐acquired pneumonia. Chest 2000; 117: 1378–1385. [DOI] [PubMed] [Google Scholar]
- 12. Naughton BJ, Mylotte JM, Ramadan F, Karuza J, Priore RL. Antibiotic use, hospital admissions, and mortality before and after implementing guidelines for nursing home‐acquired pneumonia. J Am Geriatr Soc 2001; 49: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 13. Shindo Y, Sato S, Maruyama E et al. Health‐care‐associated pneumonia among hospitalized patients in a Japanese community hospital. Chest 2009; 135: 633–640. [DOI] [PubMed] [Google Scholar]
- 14. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic‐resistant bacteria by select risk factors for health care‐associated pneumonia. Arch Intern Med 2008; 168: 2205–2210. [DOI] [PubMed] [Google Scholar]
- 15. Venditti M, Falcone M, Corrao S, Licata G, Serra P. Outcomes of patients hospitalized with community‐acquired, health care‐associated, and hospital‐acquired pneumonia. Ann Intern Med 2009; 150: 19–26. [DOI] [PubMed] [Google Scholar]
- 16. Webster D, Chui L, Tyrrell GJ, Marrie TJ. Health care‐associated Staphylococcus aureus pneumonia. Can J Infect Dis Med Microbiol 2007; 18: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zilberberg MD, Shorr AF, Micek ST, Mody SH, Kollef MH. Antimicrobial therapy escalation and hospital mortality among patients with health‐care‐associated pneumonia: a single‐center experience. Chest 2008; 134: 963–968. [DOI] [PubMed] [Google Scholar]
- 18. Johansson N, Kalin M, Tiveljung‐Lindell A, Giske CG, Hedlund J. Etiology of community‐acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010; 50: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ewig S, Torres A, Angeles Marcos M et al. Factors associated with unknown aetiology in patients with community‐acquired pneumonia. Eur Respir J 2002; 20: 1254–1262. [DOI] [PubMed] [Google Scholar]
- 20. Korppi M. Mixed microbial aetiology of community‐acquired pneumonia in children. APMIS 2002; 110: 515–522. [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez F, Masia M, Rodriguez JC et al. Community‐acquired pneumonia of mixed etiology: prevalence, clinical characteristics, and outcome. Eur J Clin Microbiol Infect Dis 2005; 24: 377–383. [DOI] [PubMed] [Google Scholar]
- 22. de Roux A, Marcos MA, Garcia E et al. Viral community‐acquired pneumonia in nonimmunocompromised adults. Chest 2004; 125: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 23. Angeles Marcos M, Camps M, Pumarola T et al. The role of viruses in the aetiology of community‐acquired pneumonia in adults. Antivir Ther 2006; 11: 351–359. [PubMed] [Google Scholar]
- 24. Lauderdale TL, Chang FY, Ben RJ et al. Etiology of community acquired pneumonia among adult patients requiring hospitalization in Taiwan. Respir Med 2005; 99: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 25. Wattanathum A, Chaoprasong C, Nunthapisud P et al. Community‐acquired pneumonia in southeast Asia: the microbial differences between ambulatory and hospitalized patients. Chest 2003; 123: 1512–1519. [DOI] [PubMed] [Google Scholar]
- 26. Saito A, Kohno S, Matsushima T et al. Prospective multicenter study of the causative organisms of community‐acquired pneumonia in adults in Japan. J Infect Chemother 2006; 12: 63–69. [DOI] [PubMed] [Google Scholar]
- 27. Jennings LC, Anderson TP, Beynon KA et al. Incidence and characteristics of viral community‐acquired pneumonia in adults. Thorax 2008; 63: 42–48. [DOI] [PubMed] [Google Scholar]
- 28. Song JH, Oh WS, Kang CI et al. Epidemiology and clinical outcomes of community‐acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents 2008; 31: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Roux A, Ewig S, Garcia E et al. Mixed community‐acquired pneumonia in hospitalised patients. Eur Respir J 2006; 27: 795–800. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention (CDC) . Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) – United States, May–August 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 1071–1074. [PubMed] [Google Scholar]
- 31. Almirall J, Bolibar I, Vidal J et al. Epidemiology of community‐acquired pneumonia in adults: a population‐based study. Eur Respir J 2000; 15: 757–763. [DOI] [PubMed] [Google Scholar]
- 32. Anzueto A, Niederman MS, Tillotson GS. Etiology, susceptibility, and treatment of acute bacterial exacerbations of complicated chronic bronchitis in the primary care setting: ciprofloxacin 750 mg b.i.d. versus clarithromycin 500 mg b.i.d. Bronchitis Study Group. Clin Ther 1998; 20: 885–900. [DOI] [PubMed] [Google Scholar]
- 33. Blasi F, Cosentini R, Raccanelli R et al. Emerging pathogens of community‐acquired pneumonia: a two‐year prospective study. J Chemother 1995; 7 (suppl 4): 115–116. [PubMed] [Google Scholar]
- 34. Bohte R, van Furth R, van den Broek PJ. Aetiology of community‐acquired pneumonia: a prospective study among adults requiring admission to hospital. Thorax 1995; 50: 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brandenburg JA, Marrie TJ, Coley CM et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med 2000; 15: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med 2001; 163 (3 Pt 1): 645–651. [DOI] [PubMed] [Google Scholar]
- 37. Ginesu F, Pirina P, Deiola G, Ostera S, Mele S, Fois AG. Etiology and therapy of community‐acquired pneumonia. J Chemother 1997; 9: 285–292. [DOI] [PubMed] [Google Scholar]
- 38. Gomez J, Banos V, Ruiz GJ et al. Prospective study of epidemiology and prognostic factors in community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 1996; 15: 556–560. [DOI] [PubMed] [Google Scholar]
- 39. Gowardman J, Trent L. Severe community acquired pneumonia: a one‐year analysis in a tertiary referral intensive care unit. N Z Med J 2000; 113: 161–164. [PubMed] [Google Scholar]
- 40. Hedlund J, Kalin M, Ortqvist A. Recurrence of pneumonia in middle‐aged and elderly adults after hospital‐treated pneumonia: aetiology and predisposing conditions. Scand J Infect Dis 1997; 29: 387–392. [DOI] [PubMed] [Google Scholar]
- 41. Hirani NA, Macfarlane JT. Impact of management guidelines on the outcome of severe community acquired pneumonia. Thorax 1997; 52: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jokinen C, Heiskanen L, Juvonen H et al. Microbial etiology of community‐acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin Infect Dis 2001; 32: 1141–1154. [DOI] [PubMed] [Google Scholar]
- 43. Jones RN, Croco MA, Kugler KC, Pfaller MA, Beach ML. Respiratory tract pathogens isolated from patients hospitalized with suspected pneumonia: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997). Diagn Microbiol Infect Dis 2000; 37: 115–125. [DOI] [PubMed] [Google Scholar]
- 44. Leroy O, Vandenbussche C, Coffinier C et al. Community‐acquired aspiration pneumonia in intensive care units. Epidemiological and prognosis data. Am J Respir Crit Care Med 1997; 156: 1922–1929. [DOI] [PubMed] [Google Scholar]
- 45. Leroy O, Bosquet C, Vandenbussche C et al. Community‐acquired pneumonia in the intensive care unit: epidemiological and prognosis data in older people. J Am Geriatr Soc 1999; 47: 539–546. [DOI] [PubMed] [Google Scholar]
- 46. Logroscino CD, Penza O, Locicero S et al. Community‐acquired pneumonia in adults: a multicentric observational AIPO study. Monaldi Arch Chest Dis 1999; 54: 11–17. [PubMed] [Google Scholar]
- 47. Lorente ML, Falguera M, Nogues A, Gonzalez AR, Merino MT, Caballero MR. Diagnosis of pneumococcal pneumonia by polymerase chain reaction (PCR) in whole blood: a prospective clinical study. Thorax 2000; 55: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim WS, Macfarlane JT, Boswell TC et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax 2001; 56: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marrie TJ, Peeling RW, Fine MJ, Singer DE, Coley CM, Kapoor WN. Ambulatory patients with community‐acquired pneumonia: the frequency of atypical agents and clinical course. Am J Med 1996; 101: 508–515. [DOI] [PubMed] [Google Scholar]
- 50. Meijer A, Dagnelie CF, de Jong JC et al. Low prevalence of Chlamydia pneumoniae and Mycoplasma pneumoniae among patients with symptoms of respiratory tract infections in Dutch general practices. Eur J Epidemiol 2000; 16: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menendez R, Cordoba J, de La CP et al. Value of the polymerase chain reaction assay in noninvasive respiratory samples for diagnosis of community‐acquired pneumonia. Am J Respir Crit Care Med 1999; 159: 1868–1873. [DOI] [PubMed] [Google Scholar]
- 52. Michetti G, Pugliese C, Bamberga M et al. Community‐acquired pneumonia: is there difference in etiology between hospitalized and out‐patients? Minerva Med 1995; 86: 341–351. [PubMed] [Google Scholar]
- 53. Olaechea PM, Quintana JM, Gallardo MS, Insausti J, Maravi E, Alvarez B. A predictive model for the treatment approach to community‐acquired pneumonia in patients needing ICU admission. Intensive Care Med 1996; 22: 1294–1300. [DOI] [PubMed] [Google Scholar]
- 54. Ruiz M, Ewig S, Marcos MA et al. Etiology of community‐acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med 1999; 160: 397–405. [DOI] [PubMed] [Google Scholar]
- 55. Socan M, Marinic‐Fiser N, Kraigher A, Kotnik A, Logar M. Microbial aetiology of community‐acquired pneumonia in hospitalised patients. Eur J Clin Microbiol Infect Dis 1999; 18: 777–782. [DOI] [PubMed] [Google Scholar]
- 56. Sopena N, Sabria M, Pedro‐Botet ML et al. Prospective study of community‐acquired pneumonia of bacterial etiology in adults. Eur J Clin Microbiol Infect Dis 1999; 18: 852–858. [DOI] [PubMed] [Google Scholar]
- 57. Steinhoff D, Lode H, Ruckdeschel G et al. Chlamydia pneumoniae as a cause of community‐acquired pneumonia in hospitalized patients in Berlin. Clin Infect Dis 1996; 22: 958–964. [DOI] [PubMed] [Google Scholar]
- 58. Garcia‐Vidal C, Carratala J, Fernandez‐Sabe N et al. Aetiology of, and risk factors for, recurrent community‐acquired pneumonia. Clin Microbiol Infect 2009; 15: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 59. Berglund C, Molling P, Sjoberg L, Soderquist B. Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin‐resistant Staphylococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton‐Valentine leukocidin genes. Clin Microbiol Infect 2005; 11: 447–456. [DOI] [PubMed] [Google Scholar]
- 60. Gillet Y, Issartel B, Vanhems P et al. Association between Staphylococcus aureus strains carrying gene for Panton‐Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 2002; 359: 753–759. [DOI] [PubMed] [Google Scholar]
- 61. Hageman JC, Uyeki TM, Francis JS et al. Severe community‐acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006; 12: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lina G, Piemont Y, Godail‐Gamot F et al. Involvement of Panton‐Valentine leukocidin‐producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999; 29: 1128–1132. [DOI] [PubMed] [Google Scholar]
- 63. Davis SL, Perri MB, Donabedian SM et al. Epidemiology and outcomes of community‐associated methicillin‐resistant Staphylococcus aureus infection. J Clin Microbiol 2007; 45: 1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van der HW, Dijkstra F, Schimmer B et al. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 2010; 15: 1–4. [PubMed] [Google Scholar]
- 65. Creer DD, Dilworth JP, Gillespie SH et al. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax 2006; 61: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flamaing J, Engelmann I, Joosten E, Van RM, Verhaegen J, Peetermans WE. Viral lower respiratory tract infection in the elderly: a prospective in‐hospital study. Eur J Clin Microbiol Infect Dis 2003; 22: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community‐acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008; 134: 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beigel JH, Farrar J, Han AM et al. Avian influenza A (H5N1) infection in humans. N Engl J Med 2005; 353: 1374–1385. [DOI] [PubMed] [Google Scholar]
- 69. Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis 2005; 11: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miedzinski L. Community‐acquired pneumonia: new facets of an old disease – Hantavirus pulmonary syndrome. Respir Care Clin N Am 2005; 11: 45–58. [DOI] [PubMed] [Google Scholar]
- 71. Patrick DM, Petric M, Skowronski DM et al. An outbreak of human Coronavirus OC43 infection and serological cross‐reactivity with SARS Coronavirus. Can J Infect Dis Med Microbiol 2006; 17: 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Drosten C, Gunther S, Preiser W et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003; 348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 73. Chowell G, Bertozzi SM, Colchero MA et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361: 674–679. [DOI] [PubMed] [Google Scholar]
- 74. Gutierrez F, Masia M, Mirete C et al. The influence of age and gender on the population‐based incidence of community‐acquired pneumonia caused by different microbial pathogens. J Infect 2006; 53: 166–174. [DOI] [PubMed] [Google Scholar]
- 75. Ingarfield SL, Celenza A, Jacobs IG, Riley TV. The bacteriology of pneumonia diagnosed in Western Australian emergency departments. Epidemiol Infect 2007; 135: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest 1998; 113: 1542–1548. [DOI] [PubMed] [Google Scholar]
- 77. Miravitlles M, Espinosa C, Fernandez‐Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest 1999; 116: 40–46. [DOI] [PubMed] [Google Scholar]
- 78. Alamoudi OS. Bacterial infection and risk factors in outpatients with acute exacerbation of chronic obstructive pulmonary disease: a 2‐year prospective study. Respirology 2007; 12: 283–287. [DOI] [PubMed] [Google Scholar]
- 79. McManus TE, Marley AM, Baxter N et al. Respiratory viral infection in exacerbations of COPD. Respir Med 2008; 102: 1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roche N, Kouassi B, Rabbat A, Mounedji A, Lorut C, Huchon G. Yield of sputum microbiological examination in patients hospitalized for exacerbations of chronic obstructive pulmonary disease with purulent sputum. Respiration 2007; 74: 19–25. [DOI] [PubMed] [Google Scholar]
- 81. Hutchinson AF, Ghimire AK, Thompson MA et al. A community‐based, time‐matched, case‐control study of respiratory viruses and exacerbations of COPD. Respir Med 2007; 101: 2472–2481. [DOI] [PubMed] [Google Scholar]
- 82. Diederen BM, van der Valk PD, Kluytmans JA, Peeters MF, Hendrix R. The role of atypical respiratory pathogens in exacerbations of chronic obstructive pulmonary disease. Eur Respir J 2007; 30: 240–244. [DOI] [PubMed] [Google Scholar]
- 83. Ko FW, Ip M, Chan PK et al. A 1‐year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 2007; 131: 44–52. [DOI] [PubMed] [Google Scholar]
- 84. Monso E, Garcia‐Aymerich J, Soler N et al. Bacterial infection in exacerbated COPD with changes in sputum characteristics. Epidemiol Infect 2003; 131: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Murphy TF, Brauer AL, Eschberger K et al. Pseudomonas aeruginosa in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 853–860. [DOI] [PubMed] [Google Scholar]
- 86. Lieberman D, Lieberman D, Gelfer Y et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest 2002; 122: 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buscho RO, Saxtan D, Shultz PS, Finch E, Mufson MA. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J Infect Dis 1978; 137: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 347: 465–471. [DOI] [PubMed] [Google Scholar]
- 89. Angrill J, Agusti C, de CR et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002; 57: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ho PL, Chan KN, Ip MS et al. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady‐state bronchiectasis. Chest 1998; 114: 1594–1598. [DOI] [PubMed] [Google Scholar]
- 91. Boldy DA, Skidmore SJ, Ayres JG. Acute bronchitis in the community: clinical features, infective factors, changes in pulmonary function and bronchial reactivity to histamine. Respir Med 1990; 84: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Everett MT. Major chest infection managed at home. Practitioner 1983; 227: 1743–1754. [PubMed] [Google Scholar]
- 93. Fransen H, Wolontis S. Infections with viruses, Mycoplasma pneumoniae and bacteria in acute respiratory illness. A study of hospitalized patients, patients treated at home, and healthy subjects. Scand J Infect Dis 1969; 1: 31–37. [DOI] [PubMed] [Google Scholar]
- 94. Graffelman AW, Knuistingh NA, le CS, Kroes AC, Springer MP, van den Broek PJ. A diagnostic rule for the aetiology of lower respiratory tract infections as guidance for antimicrobial treatment. Br J Gen Pract 2004; 54: 20–24. [PMC free article] [PubMed] [Google Scholar]
- 95. Holm A, Nexoe J, Bistrup LA et al. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br J Gen Pract 2007; 57: 547–554. [PMC free article] [PubMed] [Google Scholar]
- 96. Hopstaken RM, Coenen S, Butler CC. Treating patients not diagnoses: challenging assumptions underlying the investigation and management of LRTI in general practice. J Antimicrob Chemother 2005; 56: 941–943. [DOI] [PubMed] [Google Scholar]
- 97. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of aetiology and outcome of adult lower‐respiratory‐tract infections in the community. Lancet 1993; 341: 511–514. [DOI] [PubMed] [Google Scholar]
- 98. Macfarlane J, Holmes W, Gard P et al. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax 2001; 56: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shaw AB, Fry J. Acute infections of the chest in general practice. Br Med J 1955; ii: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Almirall J, Morato I, Riera F et al. Incidence of community‐acquired pneumonia and Chlamydia pneumoniae infection: a prospective multicentre study. Eur Respir J 1993; 6: 14–18. [PubMed] [Google Scholar]
- 101. Beovic B, Bonac B, Kese D et al. Aetiology and clinical presentation of mild community‐acquired bacterial pneumonia. Eur J Clin Microbiol Infect Dis 2003; 22: 584–591. [DOI] [PubMed] [Google Scholar]
- 102. Berntsson E, Lagergard T, Strannegard O, Trollfors B. Etiology of community‐acquired pneumonia in out‐patients. Eur J Clin Microbiol 1986; 5: 446–447. [DOI] [PubMed] [Google Scholar]
- 103. Blanquer J, Blanquer R, Borras R et al. Aetiology of community acquired pneumonia in Valencia, Spain: a multicentre prospective study. Thorax 1991; 46: 508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. The British Thoracic Society and the Public Health Laboratory Service . Community‐acquired pneumonia in adults in British hospitals in 1982–1983: a survey of aetiology, mortality, prognostic factors and outcome. Q J Med 1987; 62: 195–220. [PubMed] [Google Scholar]
- 105. Dulake C, Selkon J. The incidence of pneumonia in the UK – preliminary findings from Newcastle and London. R Soc Med Int Congr Symp Ser 1989; 27: 87–94. [Google Scholar]
- 106. Foy HM, Cooney MK, McMahan R, Grayston JT. Viral and mycoplasmal pneumonia in a prepaid medical care group during an eight‐year period. Am J Epidemiol 1973; 97: 93–102. [DOI] [PubMed] [Google Scholar]
- 107. Marrie TJ, Poulin‐Costello M, Beecroft MD, Herman‐Gnjidic Z. Etiology of community‐acquired pneumonia treated in an ambulatory setting. Respir Med 2005; 99: 60–65. [DOI] [PubMed] [Google Scholar]
- 108. Melbye H, Berdal BP, Straume B, Russell H, Vorland L, Thacker WL. Pneumonia – a clinical or radiographic diagnosis? Etiology and clinical features of lower respiratory tract infection in adults in general practice. Scand J Infect Dis 1992; 24: 647–655. [DOI] [PubMed] [Google Scholar]
- 109. Miyashita N, Fukano H, Mouri K et al. Community‐acquired pneumonia in Japan: a prospective ambulatory and hospitalized patient study. J Med Microbiol 2005; 54 (Pt 4): 395–400. [DOI] [PubMed] [Google Scholar]
- 110. Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987; 1: 671–674. [DOI] [PubMed] [Google Scholar]
- 111. Arancibia F, Bauer TT, Ewig S et al. Community‐acquired pneumonia due to gram‐negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 2002; 162: 1849–1858. [DOI] [PubMed] [Google Scholar]
- 112. Aubertin J, Dabis F, Fleurette J et al. Prevalence of legionellosis among adults: a study of community‐acquired pneumonia in France. Infection 1987; 15: 328–331. [DOI] [PubMed] [Google Scholar]
- 113. Ausina V, Coll P, Sambeat M et al. Prospective study on the etiology of community‐acquired pneumonia in children and adults in Spain. Eur J Clin Microbiol Infect Dis 1988; 7: 342–347. [DOI] [PubMed] [Google Scholar]
- 114. Berntsson E, Blomberg J, Lagergard T, Trollfors B. Etiology of community‐acquired pneumonia in patients requiring hospitalization. Eur J Clin Microbiol 1985; 4: 268–272. [DOI] [PubMed] [Google Scholar]
- 115. Burman LA, Trollfors B, Andersson B et al. Diagnosis of pneumonia by cultures, bacterial and viral antigen detection tests, and serology with special reference to antibodies against pneumococcal antigens. J Infect Dis 1991; 163: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 116. Charles PG, Whitby M, Fuller AJ et al. The etiology of community‐acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 2008; 46: 1513–1521. [DOI] [PubMed] [Google Scholar]
- 117. Falco V, Fernandez dS, Alegre J, Ferrer A, Martinez Vazquez JM. Legionella pneumophila. A cause of severe community‐acquired pneumonia. Chest 1991; 100: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 118. Falguera M, Pifarre R, Martin A, Sheikh A, Moreno A. Etiology and outcome of community‐acquired pneumonia in patients with diabetes mellitus. Chest 2005; 128: 3233–3239. [DOI] [PubMed] [Google Scholar]
- 119. Garbino J, Sommer R, Gerber A et al. Prospective epidemiologic survey of patients with community‐acquired pneumonia requiring hospitalization in Switzerland. Int J Infect Dis 2002; 6: 288–293. [DOI] [PubMed] [Google Scholar]
- 120. Gutierrez F, Masia M, Rodriguez JC et al. Epidemiology of community‐acquired pneumonia in adult patients at the dawn of the 21st century: a prospective study on the Mediterranean coast of Spain. Clin Microbiol Infect 2005; 11: 788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Holmberg H. Aetiology of community‐acquired pneumonia in hospital treated patients. Scand J Infect Dis 1987; 19: 491–501. [DOI] [PubMed] [Google Scholar]
- 122. Hone R, Haugh C, O’Connor B, Hollingsworth J. Legionella: an infrequent cause of adult community acquired pneumonia in Dublin. Ir J Med Sci 1989; 158: 230–232. [DOI] [PubMed] [Google Scholar]
- 123. Huang HH, Zhang YY, Xiu QY et al. Community‐acquired pneumonia in Shanghai, China: microbial etiology and implications for empirical therapy in a prospective study of 389 patients. Eur J Clin Microbiol Infect Dis 2006; 25: 369–374. [DOI] [PubMed] [Google Scholar]
- 124. Leesik H, Ani U, Juhani A, Altraja A. Microbial pathogens of adult community‐acquired pneumonia in Southern Estonia. Medicina (Kaunas) 2006; 42: 384–394. [PubMed] [Google Scholar]
- 125. Levy M, Dromer F, Brion N, Leturdu F, Carbon C. Community‐acquired pneumonia. Importance of initial noninvasive bacteriologic and radiographic investigations. Chest 1988; 93: 43–48. [DOI] [PubMed] [Google Scholar]
- 126. Macfarlane JT, Finch RG, Ward MJ, Macrae AD. Hospital study of adult community‐acquired pneumonia. Lancet 1982; 2: 255–258. [DOI] [PubMed] [Google Scholar]
- 127. Marrie TJ, Peeling RW, Reid T, De CE. Chlamydia species as a cause of community‐acquired pneumonia in Canada. Eur Respir J 2003; 21: 779–784. [DOI] [PubMed] [Google Scholar]
- 128. McNabb WR, Shanson DC, Williams TD, Lant AF. Adult community‐acquired pneumonia in central London. J R Soc Med 1984; 77: 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ortqvist A, Hedlund J, Grillner L et al. Aetiology, outcome and prognostic factors in community‐acquired pneumonia requiring hospitalization. Eur Respir J 1990; 3: 1105–1113. [PubMed] [Google Scholar]
- 130. Ostergaard L, Andersen PL. Etiology of community‐acquired pneumonia. Evaluation by transtracheal aspiration, blood culture, or serology. Chest 1993; 104: 1400–1407. [DOI] [PubMed] [Google Scholar]
- 131. Pareja A, Bernal C, Leyva A, Piedrola G, Maroto MC. Etiologic study of patients with community‐acquired pneumonia. Chest 1992; 101: 1207–1210. [DOI] [PubMed] [Google Scholar]
- 132. Ruf B, Schurmann D, Horbach I, Fehrenbach FJ, Pohle HD. The incidence of legionella pneumonia: a 1‐year prospective study in a large community hospital. Lung 1989; 167: 11–22. [DOI] [PubMed] [Google Scholar]
- 133. Schneeberger PM, Dorigo‐Zetsma JW, van der ZA, van BM, van Opstal JL. Diagnosis of atypical pathogens in patients hospitalized with community‐acquired respiratory infection. Scand J Infect Dis 2004; 36: 269–273. [DOI] [PubMed] [Google Scholar]
- 134. Sohn JW, Park SC, Choi YH et al. Atypical pathogens as etiologic agents in hospitalized patients with community‐acquired pneumonia in Korea: a prospective multi‐center study. J Korean Med Sci 2006; 21: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. White RJ, Blainey AD, Harrison KJ, Clarke SK. Causes of pneumonia presenting to a district general hospital. Thorax 1981; 36: 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Alkhayer M, Jenkins PF, Harrison BD. The outcome of community acquired pneumonia treated on the intensive care unit. Respir Med 1990; 84: 13–16. [DOI] [PubMed] [Google Scholar]
- 137. Almirall J, Mesalles E, Klamburg J, Parra O, Agudo A. Prognostic factors of pneumonia requiring admission to the intensive care unit. Chest 1995; 107: 511–516. [DOI] [PubMed] [Google Scholar]
- 138. The British Thoracic Society Research Committee and The Public Health Laboratory Service . The aetiology, management and outcome of severe community‐acquired pneumonia on the intensive care unit. Respir Med 1992; 86: 7–13. [DOI] [PubMed] [Google Scholar]
- 139. Leroy O, Santre C, Beuscart C et al. A five‐year study of severe community‐acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med 1995; 21: 24–31. [DOI] [PubMed] [Google Scholar]
- 140. Moine P, Vercken JB, Chevret S, Chastang C, Gajdos P. Severe community‐acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community‐Acquired Pneumonia in the Intensive Care Unit. Chest 1994; 105: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 141. Ortqvist A, Sterner G, Nilsson JA. Severe community‐acquired pneumonia: factors influencing need of intensive care treatment and prognosis. Scand J Infect Dis 1985; 17: 377–386. [DOI] [PubMed] [Google Scholar]
- 142. Pachon J, Prados MD, Capote F, Cuello JA, Garnacho J, Verano A. Severe community‐acquired pneumonia. Etiology, prognosis, and treatment. Am Rev Respir Dis 1990; 142: 369–373. [DOI] [PubMed] [Google Scholar]
- 143. Paganin F, Lilienthal F, Bourdin A et al. Severe community‐acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J 2004; 24: 779–785. [DOI] [PubMed] [Google Scholar]
- 144. Rello J, Quintana E, Ausina V, Net A, Prats G. A three‐year study of severe community‐acquired pneumonia with emphasis on outcome. Chest 1993; 103: 232–235. [DOI] [PubMed] [Google Scholar]
- 145. Rello J, Bodi M, Mariscal D et al. Microbiological testing and outcome of patients with severe community‐acquired pneumonia. Chest 2003; 123: 174–180. [DOI] [PubMed] [Google Scholar]
- 146. Sorensen J, Forsberg P, Hakanson E et al. A new diagnostic approach to the patient with severe pneumonia. Scand J Infect Dis 1989; 21: 33–41. [DOI] [PubMed] [Google Scholar]
- 147. Torres A, Serra‐Batlles J, Ferrer A et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991; 144: 312–318. [DOI] [PubMed] [Google Scholar]
- 148. Woodhead MA, Macfarlane JT, Rodgers FG, Laverick A, Pilkington R, Macrae AD. Aetiology and outcome of severe community‐acquired pneumonia. J Infect 1985; 10: 204–210. [DOI] [PubMed] [Google Scholar]
- 149. Fernandez‐Sabe N, Carratala J, Roson B et al. Community‐acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore) 2003; 82: 159–169. [DOI] [PubMed] [Google Scholar]
- 150. Riquelme R, Torres A, El‐Ebiary M et al. Community‐acquired pneumonia in the elderly: a multivariate analysis of risk and prognostic factors. Am J Respir Crit Care Med 1996; 154: 1450–1455. [DOI] [PubMed] [Google Scholar]
- 151. Zalacain R, Torres A, Celis R et al. Community‐acquired pneumonia in the elderly: Spanish multicentre study. Eur Respir J 2003; 21: 294–302. [DOI] [PubMed] [Google Scholar]
- 152. Beaty CD, Grayston JT, Wang SP, Kuo CC, Reto CS, Martin TR. Chlamydia pneumoniae, strain TWAR, infection in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144: 1408–1410. [DOI] [PubMed] [Google Scholar]
- 153. Carilli AD, Gohd RS, Gordon W. A virologic study of chronic bronchitis. N Engl J Med 1964; 270: 123–127. [DOI] [PubMed] [Google Scholar]
- 154. Eadie MB, Stott EJ, Grist NR. Virologic studies in chronic bronchitis. Br Med J 1966; 2: 671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Erkan L, Uzun O, Findik S, Katar D, Sanic A, Atici AG. Role of bacteria in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2008; 3: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Fagon JY, Chastre J, Trouillet JL et al. Characterization of distal bronchial microflora during acute exacerbation of chronic bronchitis. Use of the protected specimen brush technique in 54 mechanically ventilated patients. Am Rev Respir Dis 1990; 142: 1004–1008. [DOI] [PubMed] [Google Scholar]
- 157. Groenewegen KH, Wouters EF. Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study. Respir Med 2003; 97: 770–777. [DOI] [PubMed] [Google Scholar]
- 158. Gump DW, Phillips CA, Forsyth BR, McIntosh K, Lamborn KR, Stouch WH. Role of infection in chronic bronchitis. Am Rev Respir Dis 1976; 113: 465–474. [DOI] [PubMed] [Google Scholar]
- 159. Karnak D, Beng‐sun S, Beder S, Kayacan O. Chlamydia pneumoniae infection and acute exacerbation of chronic obstructive pulmonary disease (COPD). Respir Med 2001; 95: 811–816. [DOI] [PubMed] [Google Scholar]
- 160. Ko FW, Ng TK, Li TS et al. Sputum bacteriology in patients with acute exacerbations of COPD in Hong Kong. Respir Med 2005; 99: 454–460. [DOI] [PubMed] [Google Scholar]
- 161. Lamy ME, Pouthier‐Simon F, Debacker‐Willame E. Respiratory viral infections in hospital patients with chronic bronchitis. Observations during periods of exacerbation and quiescence. Chest 1973; 63: 336–341. [DOI] [PubMed] [Google Scholar]
- 162. Lieberman D, Ben Yaakov M, Lazarovich Z, Ohana B, Boldur I. Chlamydia pneumoniae infection in acute exacerbations of chronic obstructive pulmonary disease: analysis of 250 hospitalizations. Eur J Clin Microbiol Infect Dis 2001; 20: 698–704. [DOI] [PubMed] [Google Scholar]
- 163. McNamara MJ, Phillips IA, Williams OB. Viral and Mycoplasma pneumoniae infections in exacerbations of chronic lung disease. Am Rev Respir Dis 1969; 100: 19–24. [DOI] [PubMed] [Google Scholar]
- 164. Mogulkoc N, Karakurt S, Isalska B et al. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med 1999; 160: 349–353. [DOI] [PubMed] [Google Scholar]
- 165. Monso E, Ruiz J, Rosell A et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med 1995; 152 (4 Pt 1): 1316–1320. [DOI] [PubMed] [Google Scholar]
- 166. Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med 2005; 172: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Papi A, Bellettato CM, Braccioni F et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006; 173: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 168. Rohde G, Wiethege A, Borg I et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case‐control study. Thorax 2003; 58: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rosell A, Monso E, Soler N et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med 2005; 165: 891–897. [DOI] [PubMed] [Google Scholar]
- 170. Ross CA, McMichael S, Eadie MB, Lees AW, Murray EA, Pinkerton I. Infective agents and chronic bronchitis. Thorax 1966; 21: 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Seemungal T, Harper‐Owen R, Bhowmik A et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1618–1623. [DOI] [PubMed] [Google Scholar]
- 172. De SG, Lampron N, La FJ et al. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol 2009; 46: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Soler N, Torres A, Ewig S et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157 (5 Pt 1): 1498–1505. [DOI] [PubMed] [Google Scholar]
- 174. Chan TH, Ho SS, Lai CK et al. Comparison of oral ciprofloxacin and amoxycillin in treating infective exacerbations of bronchiectasis in Hong Kong. Chemotherapy 1996; 42: 150–156. [DOI] [PubMed] [Google Scholar]
- 175. King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow‐up study in adult bronchiectasis. Respir Med 2007; 101: 1633–1638. [DOI] [PubMed] [Google Scholar]
- 176. Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995; 108: 955–961. [DOI] [PubMed] [Google Scholar]
- 177. O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998; 113: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 178. Spratt BG, Pardee AB. Penicillin‐binding proteins and cell shape in E. coli . Nature 1975; 254: 516–517. [DOI] [PubMed] [Google Scholar]
- 179. Doit C, Loukil C, Fitoussi F, Geslin P, Bingen E. Emergence in france of multiple clones of clinical Streptococcus pneumoniae isolates with high‐level resistance to amoxicillin. Antimicrob Agents Chemother 1999; 43: 1480–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Carratala J, Marron A, Fernandez‐Sevilla A, Linares J, Gudiol F. Treatment of penicillin‐resistant pneumococcal bacteremia in neutropenic patients with cancer. Clin Infect Dis 1997; 24: 148–152. [DOI] [PubMed] [Google Scholar]
- 181. Yu VL, Chiou CC, Feldman C et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis 2003; 37: 230–237. [DOI] [PubMed] [Google Scholar]
- 182. Borg MA, Tiemersma E, Scicluna E et al. Prevalence of penicillin and erythromycin resistance among invasive Streptococcus pneumoniae isolates reported by laboratories in the southern and eastern Mediterranean region. Clin Microbiol Infect 2009; 15: 232–237. [DOI] [PubMed] [Google Scholar]
- 183. Moore MR, Gertz RE Jr, Woodbury RL et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 184. Ardanuy C, Rolo D, Fenoll A, Tarrago D, Calatayud L, Linares J. Emergence of a multidrug‐resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J Antimicrob Chemother 2009; 64: 507–510. [DOI] [PubMed] [Google Scholar]
- 185. Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. CLSI document M100‐S18. Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 186. Peterson LR. Penicillins for treatment of pneumococcal pneumonia: does in vitro resistance really matter? Clin Infect Dis 2006; 42: 224–233. [DOI] [PubMed] [Google Scholar]
- 187. File TM, Garau J, Jacobs MR, Wynne B, Twynholm M, Berkowitz E. Efficacy of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate (2000/125 mg) in adults with community‐acquired pneumonia caused by Streptococcus pneumoniae, including penicillin‐resistant strains. Int J Antimicrob Agents 2005; 25: 110–119. [DOI] [PubMed] [Google Scholar]
- 188. Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother 1995; 39: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Syrogiannopoulos GA, Grivea IN, Tait‐Kamradt A et al. Identification of an erm(A) erythromycin resistance methylase gene in Streptococcus pneumoniae isolated in Greece. Antimicrob Agents Chemother 2001; 45: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Johnston NJ, De Azavedo JC, Kellner JD, Low DE. Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae . Antimicrob Agents Chemother 1998; 42: 2425–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Farrell DJ, Douthwaite S, Morrissey I et al. Macrolide resistance by ribosomal mutation in clinical isolates of Streptococcus pneumoniae from the PROTEKT 1999‐2000 study. Antimicrob Agents Chemother 2003; 47: 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Lonks JR, Garau J, Gomez L et al. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin‐resistant Streptococcus pneumoniae . Clin Infect Dis 2002; 35: 556–564. [DOI] [PubMed] [Google Scholar]
- 193. Daneman N, McGeer A, Green K, Low DE. Macrolide resistance in bacteremic pneumococcal disease: implications for patient management. Clin Infect Dis 2006; 43: 432–438. [DOI] [PubMed] [Google Scholar]
- 194. Doern GV. Macrolide and ketolide resistance with Streptococcus pneumoniae . Med Clin North Am 2006; 90: 1109–1124. [DOI] [PubMed] [Google Scholar]
- 195. Anderson R, Steel HC, Cockeran R et al. Comparison of the effects of macrolides, amoxicillin, ceftriaxone, doxycycline, tobramycin and fluoroquinolones, on the production of pneumolysin by Streptococcus pneumoniae in vitro. J Antimicrob Chemother 2007; 60: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 196. Pan XS, Ambler J, Mehtar S, Fisher LM. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae . Antimicrob Agents Chemother 1996; 40: 2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Blondeau JM, Zhao X, Hansen G, Drlica K. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae . Antimicrob Agents Chemother 2001; 45: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. de la Campa AG, Ardanuy C, Balsalobre L et al. Changes in fluoroquinolone‐resistant Streptococcus pneumoniae after 7‐valent conjugate vaccination, Spain. Emerg Infect Dis 2009; 15: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Farrell DJ, Felmingham D, Shackcloth J et al. Non‐susceptibility trends and serotype distributions among Streptococcus pneumoniae from community‐acquired respiratory tract infections and from bacteraemias in the UK and Ireland, 1999 to 2007. J Antimicrob Chemother 2008; 62 (suppl 2): ii87–ii95. [DOI] [PubMed] [Google Scholar]
- 200. Jansen WT, Verel A, Beitsma M, Verhoef J, Milatovic D. Longitudinal European surveillance study of antibiotic resistance of Haemophilus influenzae . J Antimicrob Chemother 2006; 58: 873–877. [DOI] [PubMed] [Google Scholar]
- 201. Peric M, Bozdogan B, Jacobs MR, Appelbaum PC. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob Agents Chemother 2003; 47: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Morrissey I, Maher K, Williams L, Shackcloth J, Felmingham D, Reynolds R. Non‐susceptibility trends among Haemophilus influenzae and Moraxella catarrhalis from community‐acquired respiratory tract infections in the UK and Ireland, 1999–2007. J Antimicrob Chemother 2008; 62 (suppl 2): ii97–ii103. [DOI] [PubMed] [Google Scholar]
- 203. Kofteridis DP, Notas G, Maraki S et al. Antimicrobial susceptibilities of 930 Haemophilus influenzae clinical strains isolated from the island of Crete, Greece. Chemotherapy 2008; 54: 492–498. [DOI] [PubMed] [Google Scholar]
- 204. Critchley IA, Brown SD, Traczewski MM, Tillotson GS, Janjic N. National and regional assessment of antimicrobial resistance among community‐acquired respiratory tract pathogens identified in a 2005–2006 U.S. Faropenem surveillance study. Antimicrob Agents Chemother 2007; 51: 4382–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Waites KB, Crabb DM, Bing X, Duffy LB. In vitro susceptibilities to and bactericidal activities of garenoxacin (BMS‐284756) and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother 2003; 47: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Waites KB, Crabb DM, Duffy LB. In vitro activities of ABT‐773 and other antimicrobials against human mycoplasmas. Antimicrob Agents Chemother 2003; 47: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004; 17: 697–728, table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Matsuoka M, Narita M, Okazaki N et al. Characterization and molecular analysis of macrolide‐resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother 2004; 48: 4624–4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Morozumi M, Hasegawa K, Kobayashi R et al. Emergence of macrolide‐resistant Mycoplasma pneumoniae with a 23S rRNA gene mutation. Antimicrob Agents Chemother 2005; 49: 2302–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Morozumi M, Iwata S, Hasegawa K et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community‐acquired pneumonia. Antimicrob Agents Chemother 2008; 52: 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dumke R, von BH, Luck PC, Jacobs E. Occurrence of macrolide‐resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect 2010; 16: 613–616. [DOI] [PubMed] [Google Scholar]
- 212. Peuchant O, Menard A, Renaudin H et al. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real‐time PCR and melting curve analysis. J Antimicrob Chemother 2009; 64: 52–58. [DOI] [PubMed] [Google Scholar]
- 213. Stralin K, Soderquist B. Staphylococcus aureus in community‐acquired pneumonia. Chest 2006; 130: 623. [DOI] [PubMed] [Google Scholar]
- 214. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Nathwani D, Morgan M, Masterton RG et al. Guidelines for UK practice for the diagnosis and management of methicillin‐resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother 2008; 61: 976–994. [DOI] [PubMed] [Google Scholar]
- 216. Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis 2004; 189: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 217. Hutschala D, Skhirtladze K, Zuckermann A et al. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob Agents Chemother 2005; 49: 5107–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Conte JE Jr, Golden JA, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacodynamics of high‐dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int J Antimicrob Agents 2007; 30: 422–427. [DOI] [PubMed] [Google Scholar]
- 219. Bhavnani SM, Forrest A, Hammel JP, Drusano GL, Rubino CM, Ambrose PG. Pharmacokinetics‐pharmacodynamics of quinolones against Streptococcus pneumoniae in patients with community‐acquired pneumonia. Diagn Microbiol Infect Dis 2008; 62: 99–101. [DOI] [PubMed] [Google Scholar]
- 220. Aspromonte N, Feola M, Scardovi AB et al. Early diagnosis of congestive heart failure: clinical utility of B‐type natriuretic peptide testing associated with Doppler echocardiography. J Cardiovasc Med (Hagerstown) 2006; 7: 406–413. [DOI] [PubMed] [Google Scholar]
- 221. Mikkelsen KV, Bie P, Moller JE, Videbaek L, Villadsen HD, Haghfelt T. Neurohormonal activation and diagnostic value of cardiac peptides in patients with suspected mild heart failure. Int J Cardiol 2006; 110: 324–333. [DOI] [PubMed] [Google Scholar]
- 222. Fuat A, Murphy JJ, Hungin AP et al. The diagnostic accuracy and utility of a B‐type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 2006; 56: 327–333. [PMC free article] [PubMed] [Google Scholar]
- 223. Van Schayck CP, Loozen JM, Wagena E, Akkermans RP, Wesseling GJ. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002; 324: 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Broekhuizen BD, Sachs AP, Oostvogels R, Hoes AW, Verheij TJ, Moons KG. The diagnostic value of history and physical examination for COPD in suspected or known cases: a systematic review. Fam Pract 2009; 26: 260–268. [DOI] [PubMed] [Google Scholar]
- 225. Hopstaken RM, Muris JW, Knottnerus JA, Kester AD, Rinkens PE, Dinant GJ. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C‐reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br J Gen Pract 2003; 53: 358–364. [PMC free article] [PubMed] [Google Scholar]
- 226. Graffelman AW, le Cessie S, Knuistingh NA, Wilemssen FE, Zonderland HM, van den Broek PJ. Can history and exam alone reliably predict pneumonia? J Fam Pract 2007; 56: 465–470. [PubMed] [Google Scholar]
- 227. Flanders SA, Stein J, Shochat G et al. Performance of a bedside C‐reactive protein test in the diagnosis of community‐acquired pneumonia in adults with acute cough. Am J Med 2004; 116: 529–535. [DOI] [PubMed] [Google Scholar]
- 228. Holm A, Pedersen SS, Nexoe J et al. Procalcitonin versus C‐reactive protein for predicting pneumonia in adults with lower respiratory tract infection in primary care. Br J Gen Pract 2007; 57: 555–560. [PMC free article] [PubMed] [Google Scholar]
- 229. van der Meer MV, Neven AK, van den Broek PJ, Assendelft WJ. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ 2005; 331: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Falk G, Fahey T. C‐reactive protein and community‐acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Fam Pract 2009; 26: 10–21. [DOI] [PubMed] [Google Scholar]
- 231. Hak E, Bont J, Hoes AW, Verheij TJ. Prognostic factors for serious morbidity and mortality from community‐acquired lower respiratory tract infections among the elderly in primary care. Fam Pract 2005; 22: 375–380. [DOI] [PubMed] [Google Scholar]
- 232. Seppa Y, Bloigu A, Honkanen PO, Miettinen L, Syrjala H. Severity assessment of lower respiratory tract infection in elderly patients in primary care. Arch Intern Med 2001; 161: 2709–2713. [DOI] [PubMed] [Google Scholar]
- 233. Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB‐65 predicts death from community‐acquired pneumonia. J Intern Med 2006; 260: 93–101. [DOI] [PubMed] [Google Scholar]
- 234. Bont J, Hak E, Hoes AW, Schipper M, Schellevis FG, Verheij TJ. A prediction rule for elderly primary‐care patients with lower respiratory tract infections. Eur Respir J 2007; 29: 969–975. [DOI] [PubMed] [Google Scholar]
- 235. Bont J, Hak E, Hoes AW, Macfarlane JT, Verheij TJ. Predicting death in elderly patients with community‐acquired pneumonia: a prospective validation study reevaluating the CRB‐65 severity assessment tool. Arch Intern Med 2008; 168: 1465–1468. [DOI] [PubMed] [Google Scholar]
- 236. Bont J. Lower respiratory tract infections in the elderly; prognostic studies in primary care. Utrecht, The Netherlands: University Medical Center Utrecht, 2008. [Google Scholar]
- 237. Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in ambulatory settings. Chichester, UK: John Wiley & Sons, Ltd., 2010. [DOI] [PubMed] [Google Scholar]
- 238. Smucny J, Becker LA, Glazier R. Beta2‐agonists for acute bronchitis. Chichester, UK: John Wiley & Sons, Ltd., 2010. [Google Scholar]
- 239. Ponsioen BP, Hop WC, Vermue NA, Dekhuijzen PN, Bohnen AM. Efficacy of fluticasone on cough: a randomised controlled trial. Eur Respir J 2005; 25: 147–152. [DOI] [PubMed] [Google Scholar]
- 240. Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Chichester, UK: John Wiley & Sons, Ltd, 2010. [Google Scholar]
- 241. Little P, Rumsby K, Kelly J et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA 2005; 293: 3029–3035. [DOI] [PubMed] [Google Scholar]
- 242. Ram FS, Rodriguez‐Roisin R, Granados‐Navarrete A, Garcia‐Aymerich J, Barnes NC. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 2: CD004403. [DOI] [PubMed] [Google Scholar]
- 243. Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2004; 2: CD002109. [DOI] [PubMed] [Google Scholar]
- 244. Mills GD, Oehley MR, Arrol B. Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis. BMJ 2005; 330: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta‐analyses of randomised controlled trials. BMJ 2003; 326: 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Jefferson T, Demicheli V, Rivetti D, Jones M, Di PC, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet 2006; 367: 303–13. [DOI] [PubMed] [Google Scholar]
- 247. Lim WS, van der Eerden MM, Laing R et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Ewig S, de RA, Bauer T et al. Validation of predictive rules and indices of severity for community acquired pneumonia. Thorax 2004; 59: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ewig S, Birkner N, Strauss R et al. New perspectives on community‐acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax 2009; 64: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Aujesky D, McCausland JB, Whittle J, Obrosky DS, Yealy DM, Fine MJ. Reasons why emergency department providers do not rely on the pneumonia severity index to determine the initial site of treatment for patients with pneumonia. Clin Infect Dis 2009; 49: e100–e108. [DOI] [PubMed] [Google Scholar]
- 251. Capelastegui A, Espana PP, Quintana JM et al. Validation of a predictive rule for the management of community‐acquired pneumonia. Eur Respir J 2006; 27: 151–157. [DOI] [PubMed] [Google Scholar]
- 252. Buising KL, Thursky KA, Black JF et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax 2006; 61: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Man SY, Lee N, Ip M et al. Prospective comparison of three predictive rules for assessing severity of community‐acquired pneumonia in Hong Kong. Thorax 2007; 62: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Myint PK, Kamath AV, Vowler SL, Maisey DN, Harrison BD. Severity assessment criteria recommended by the British Thoracic Society (BTS) for community‐acquired pneumonia (CAP) and older patients. Should SOAR (systolic blood pressure, oxygenation, age and respiratory rate) criteria be used in older people? A compilation study of two prospective cohorts. Age Ageing 2006; 35: 286–291. [DOI] [PubMed] [Google Scholar]
- 255. Barlow G, Nathwani D, Davey P. The CURB65 pneumonia severity score outperforms generic sepsis and early warning scores in predicting mortality in community‐acquired pneumonia. Thorax 2007; 62: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Chalmers JD, Singanayagam A, Hill AT. Systolic blood pressure is superior to other haemodynamic predictors of outcome in community acquired pneumonia. Thorax 2008; 63: 698–702. [DOI] [PubMed] [Google Scholar]
- 257. Labarere J, Stone RA, Scott OD et al. Factors associated with the hospitalization of low‐risk patients with community‐acquired pneumonia in a cluster‐randomized trial. J Gen Intern Med 2006; 21: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Marrie TJ, Huang JQ. Admission is not always necessary for patients with community‐acquired pneumonia in risk classes IV and V diagnosed in the emergency room. Can Respir J 2007; 14: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Seymann G, Barger K, Choo S, Sawhney S, Davis D. Clinical judgment versus the Pneumonia Severity Index in making the admission decision. J Emerg Med 2008; 34: 261–268. [DOI] [PubMed] [Google Scholar]
- 260. Loeb M, Carusone SC, Goeree R et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA 2006; 295: 2503–2510. [DOI] [PubMed] [Google Scholar]
- 261. Hirakata Y, Yanagihara K, Kurihara S et al. Comparison of usefulness of plasma procalcitonin and C‐reactive protein measurements for estimation of severity in adults with community‐acquired pneumonia. Diagn Microbiol Infect Dis 2008; 61: 170–174. [DOI] [PubMed] [Google Scholar]
- 262. Hohenthal U, Hurme S, Helenius H et al. Utility of C‐reactive protein in assessing the disease severity and complications of community‐acquired pneumonia. Clin Microbiol Infect 2009; 15: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 263. Menendez R, Martinez R, Reyes S et al. Biomarkers improve mortality prediction by prognostic scales in community‐acquired pneumonia. Thorax 2009; 64: 587–591. [DOI] [PubMed] [Google Scholar]
- 264. Thiem U, Niklaus D, Sehlhoff B et al. C‐reactive protein, severity of pneumonia and mortality in elderly, hospitalised patients with community‐acquired pneumonia. Age Ageing 2009; 38: 693–697. [DOI] [PubMed] [Google Scholar]
- 265. Okimoto N, Hayashi Y, Ishiga M et al. Procalcitonin and severity of community‐acquired pneumonia. J Infect Chemother 2009; 15: 426–427. [DOI] [PubMed] [Google Scholar]
- 266. Kruger S, Papassotiriou J, Marre R et al. Pro‐atrial natriuretic peptide and pro‐vasopressin to predict severity and prognosis in community‐acquired pneumonia: results from the German competence network CAPNETZ. Intensive Care Med 2007; 33: 2069–2078. [DOI] [PubMed] [Google Scholar]
- 267. Chalmers JD, Singanayagam A, Scally C, Hill AT. Admission D‐dimer can identify low‐risk patients with community‐acquired pneumonia. Ann Emerg Med 2009; 53: 633–638. [DOI] [PubMed] [Google Scholar]
- 268. Kruger S, Ewig S, Kunde J et al. C‐terminal provasopressin (copeptin) in patients with community‐acquired pneumonia – influence of antibiotic pre‐treatment: results from the German competence network CAPNETZ. J Antimicrob Chemother 2009; 64: 159–162. [DOI] [PubMed] [Google Scholar]
- 269. Christ‐Crain M, Breidthardt T, Stolz D et al. Use of B‐type natriuretic peptide in the risk stratification of community‐acquired pneumonia. J Intern Med 2008; 264: 166–176. [DOI] [PubMed] [Google Scholar]
- 270. Prat C, Lacoma A, Dominguez J et al. Midregional pro‐atrial natriuretic peptide as a prognostic marker in pneumonia. J Infect 2007; 55: 400–407. [DOI] [PubMed] [Google Scholar]
- 271. Christ‐Crain M, Morgenthaler NG, Stolz D et al. Pro‐adrenomedullin to predict severity and outcome in community‐acquired pneumonia [ISRCTN04176397]. Crit Care 2006; 10: R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Huang DT, Angus DC, Kellum JA et al. Midregional proadrenomedullin as a prognostic tool in community‐acquired pneumonia. Chest 2009; 136: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Tejera A, Santolaria F, Diez ML et al. Prognosis of community acquired pneumonia (CAP): value of triggering receptor expressed on myeloid cells‐1 (TREM‐1) and other mediators of the inflammatory response. Cytokine 2007; 38: 117–123. [DOI] [PubMed] [Google Scholar]
- 274. Christ‐Crain M, Stolz D, Jutla S et al. Free and total cortisol levels as predictors of severity and outcome in community‐acquired pneumonia. Am J Respir Crit Care Med 2007; 176: 913–920. [DOI] [PubMed] [Google Scholar]
- 275. Salluh JI, Bozza FA, Soares M et al. Adrenal response in severe community‐acquired pneumonia: impact on outcomes and disease severity. Chest 2008; 134: 947–954. [DOI] [PubMed] [Google Scholar]
- 276. Kruger S, Ewig S, Marre R et al. Procalcitonin predicts patients at low risk of death from community‐acquired pneumonia across all CRB‐65 classes. Eur Respir J 2008; 31: 349–355. [DOI] [PubMed] [Google Scholar]
- 277. Huang DT, Weissfeld LA, Kellum JA et al. Risk prediction with procalcitonin and clinical rules in community‐acquired pneumonia. Ann Emerg Med 2008; 52: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Phua J, See KC, Chan YH et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community‐acquired pneumonia. Thorax 2009; 64: 598–603. [DOI] [PubMed] [Google Scholar]
- 279. Charles PG, Wolfe R, Whitby M et al. SMART‐COP: a tool for predicting the need for intensive respiratory or vasopressor support in community‐acquired pneumonia. Clin Infect Dis 2008; 47: 375–384. [DOI] [PubMed] [Google Scholar]
- 280. Chalmers JD, Singanayagam A, Hill AT. Predicting the need for mechanical ventilation and/or inotropic support for young adults admitted to the hospital with community‐acquired pneumonia. Clin Infect Dis 2008; 47: 1571–1574. [DOI] [PubMed] [Google Scholar]
- 281. Brown SM, Jones BE, Jephson AR, Dean NC. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community‐acquired pneumonia. Crit Care Med 2009; 37: 3010–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Marrie TJ, Shariatzadeh MR. Community‐acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore) 2007; 86: 103–111. [DOI] [PubMed] [Google Scholar]
- 283. Riley PD, Aronsky D, Dean NC. Validation of the 2001 American Thoracic Society criteria for severe community‐acquired pneumonia. Crit Care Med 2004; 32: 2398–2402. [DOI] [PubMed] [Google Scholar]
- 284. Renaud B, Santin A, Coma E et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community‐acquired pneumonia. Crit Care Med 2009; 37: 2867–2874. [DOI] [PubMed] [Google Scholar]
- 285. Bruns AH, Oosterheert JJ, Hak E, Hoepelman AI. Usefulness of consecutive C‐reactive protein measurements in follow‐up of severe community‐acquired pneumonia. Eur Respir J 2008; 32: 726–732. [DOI] [PubMed] [Google Scholar]
- 286. Wu CL, Lin FJ, Lee SY et al. Early evolution of arterial oxygenation in severe community‐acquired pneumonia: a prospective observational study. J Crit Care 2007; 22: 129–136. [DOI] [PubMed] [Google Scholar]
- 287. Marrie TJ, Durant H, Yates L. Community‐acquired pneumonia requiring hospitalization: 5‐year prospective study. Rev Infect Dis 1989; 11: 586–599. [DOI] [PubMed] [Google Scholar]
- 288. Gleckman R, DeVita J, Hibert D, Pelletier C, Martin R. Sputum gram stain assessment in community‐acquired bacteremic pneumonia. J Clin Microbiol 1988; 26: 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Benenson RS, Kepner AM, Pyle DN, Cavanaugh S. Selective use of blood cultures in emergency department pneumonia patients. J Emerg Med 2007; 33: 1–8. [DOI] [PubMed] [Google Scholar]
- 290. Afshar N, Tabas J, Afshar K, Silbergleit R. Blood cultures for community‐acquired pneumonia: are they worthy of two quality measures? A systematic review. J Hosp Med 2009; 4: 112–123. [DOI] [PubMed] [Google Scholar]
- 291. Bradley SF. Staphylococcus aureus pneumonia: emergence of MRSA in the community. Semin Respir Crit Care Med 2005; 26: 643–649. [DOI] [PubMed] [Google Scholar]
- 292. El Solh AA, Akinnusi ME, Pineda LA, Mankowski CR. Diagnostic yield of quantitative endotracheal aspirates in patients with severe nursing home‐acquired pneumonia. Crit Care 2007; 11: R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Lagerstrom F, Fredlund H, Holmberg H. Sputum specimens can be obtained from patients with community‐acquired pneumonia in primary care. Scand J Prim Health Care 2004; 22: 83–86. [PubMed] [Google Scholar]
- 294. Garcia‐Vazquez E, Marcos MA, Mensa J et al. Assessment of the usefulness of sputum culture for diagnosis of community‐acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 2004; 164: 1807–1811. [DOI] [PubMed] [Google Scholar]
- 295. van der Eerden MM, Vlaspolder F, de Graaff CS, Groot T, Jansen HM, Boersma WG. Value of intensive diagnostic microbiological investigation in low‐ and high‐risk patients with community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 2005; 24: 241–249. [DOI] [PubMed] [Google Scholar]
- 296. Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram‐stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2004; 39: 165–169. [DOI] [PubMed] [Google Scholar]
- 297. Signori LG, Ferreira MW, Vieira LC, Muller KR, Mattos WL. Sputum examination in the clinical management of community‐acquired pneumonia. J Bras Pneumol 2008; 34: 152–158. [DOI] [PubMed] [Google Scholar]
- 298. Anevlavis S, Petroglou N, Tzavaras A et al. A prospective study of the diagnostic utility of sputum Gram stain in pneumonia. J Infect 2009; 59: 83–89. [DOI] [PubMed] [Google Scholar]
- 299. Uffredi ML, Mangiapan G, Cadranel J, Kac G. Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur J Clin Microbiol Infect Dis 2003; 22: 457–462. [DOI] [PubMed] [Google Scholar]
- 300. Ortega L, Sierra M, Dominguez J et al. Utility of a pneumonia severity index in the optimization of the diagnostic and therapeutic effort for community‐acquired pneumonia. Scand J Infect Dis 2005; 37: 657–663. [DOI] [PubMed] [Google Scholar]
- 301. Gutierrez F, Masia M, Rodriguez JC et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community‐acquired pneumonia in Spain. Clin Infect Dis 2003; 36: 286–292. [DOI] [PubMed] [Google Scholar]
- 302. Smith AL, Fiel SB, Mayer‐Hamblett N, Ramsey B, Burns JL. Susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parenteral antibiotic administration: lack of association in cystic fibrosis. Chest 2003; 123: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 303. Marcos MA, Jimenez de Anta MT, de la Bellacasa JP et al. Rapid urinary antigen test for diagnosis of pneumococcal community‐acquired pneumonia in adults. Eur Respir J 2003; 21: 209–214. [DOI] [PubMed] [Google Scholar]
- 304. Roson B, Fernandez‐Sabe N, Carratala J et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38: 222–226. [DOI] [PubMed] [Google Scholar]
- 305. Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. A 3‐year prospective study of a urinary antigen‐detection test for Streptococcus pneumoniae in community‐acquired pneumonia: utility and clinical impact on the reported etiology. J Infect Chemother 2004; 10: 359–363. [DOI] [PubMed] [Google Scholar]
- 306. Stralin K, Kaltoft MS, Konradsen HB, Olcen P, Holmberg H. Comparison of two urinary antigen tests for establishment of pneumococcal etiology of adult community‐acquired pneumonia. J Clin Microbiol 2004; 42: 3620–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Andreo F, Dominguez J, Ruiz‐Manzano J et al. Usefulness of pneumococcal antigen detection in pleural fluid samples by immunochromatographic assay for diagnosis of pneumococcal pneumonia. Clin Microbiol Infect 2006; 12: 682–684. [DOI] [PubMed] [Google Scholar]
- 308. Ercis S, Ergin A, Sahin GO, Hascelik G, Uzun O. Validation of urinary antigen test for Streptococcus pneumoniae in patients with pneumococcal pneumonia. Jpn J Infect Dis 2006; 59: 388–390. [PubMed] [Google Scholar]
- 309. Genne D, Sommer R, Kaiser L et al. Analysis of factors that contribute to treatment failure in patients with community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 2006; 25: 159–166. [DOI] [PubMed] [Google Scholar]
- 310. Lasocki S, Scanvic A, Le TF et al. Evaluation of the Binax NOW Streptococcus pneumoniae urinary antigen assay in intensive care patients hospitalized for pneumonia. Intensive Care Med 2006; 32: 1766–1772. [DOI] [PubMed] [Google Scholar]
- 311. Leeming JP, Cartwright K, Morris R, Martin SA, Smith MD. Diagnosis of invasive pneumococcal infection by serotype‐specific urinary antigen detection. J Clin Microbiol 2005; 43: 4972–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Kobashi Y, Yoshida K, Miyashita N, Niki Y, Matsushima T. Evaluating the use of a Streptococcus pneumoniae urinary antigen detection kit for the management of community‐acquired pneumonia in Japan. Respiration 2007; 74: 387–393. [DOI] [PubMed] [Google Scholar]
- 313. Oka H, Ueda A, Watanuki Y et al. The efficacy of high‐dose penicillin for community‐acquired pneumonia diagnosed by pneumococcal urine antigen test. J Infect Chemother 2009; 15: 108–112. [DOI] [PubMed] [Google Scholar]
- 314. Smith MD, Sheppard CL, Hogan A et al. Diagnosis of Streptococcus pneumoniae infections in adults with bacteremia and community‐acquired pneumonia: clinical comparison of pneumococcal PCR and urinary antigen detection. J Clin Microbiol 2009; 47: 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Andreo F, Prat C, Ruiz‐Manzano J et al. Persistence of Streptococcus pneumoniae urinary antigen excretion after pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis 2009; 28: 197–201. [DOI] [PubMed] [Google Scholar]
- 316. Korsgaard J, Moller JK, Kilian M. Antibiotic treatment and the diagnosis of Streptococcus pneumoniae in lower respiratory tract infections in adults. Int J Infect Dis 2005; 9: 274–279. [DOI] [PubMed] [Google Scholar]
- 317. Dominguez J, Andreo F, Blanco S et al. Rapid detection of pneumococcal antigen in serum samples for diagnosing pneumococcal pneumonia. J Infect 2006; 53: 21–24. [DOI] [PubMed] [Google Scholar]
- 318. Porcel JM, Ruiz‐Gonzalez A, Falguera M et al. Contribution of a pleural antigen assay (Binax NOW) to the diagnosis of pneumococcal pneumonia. Chest 2007; 131: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 319. Dirven K, Ieven M, Peeters MF, van der ZA, De SK, Goossens H. Comparison of three Legionella urinary antigen assays during an outbreak of legionellosis in Belgium. J Med Microbiol 2005; 54 (Pt 12): 1213–1216. [DOI] [PubMed] [Google Scholar]
- 320. Guerrero C, Toldos CM, Yague G, Ramirez C, Rodriguez T, Segovia M. Comparison of diagnostic sensitivities of three assays (Bartels enzyme immunoassay [EIA], Biotest EIA, and Binax NOW immunochromatographic test) for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 2004; 42: 467–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Olsen CW, Elverdal P, Jorgensen CS, Uldum SA. Comparison of the sensitivity of the Legionella urinary antigen EIA kits from Binax and Biotest with urine from patients with infections caused by less common serogroups and subgroups of Legionella. Eur J Clin Microbiol Infect Dis 2009; 28: 817–820. [DOI] [PubMed] [Google Scholar]
- 322. Blanco S, Lacoma A, Prat C et al. Detection of Legionella antigen in nonconcentrated and concentrated urine samples by a new immunochromatographic assay. Eur J Clin Microbiol Infect Dis 2008; 27: 1249–1251. [DOI] [PubMed] [Google Scholar]
- 323. Diederen BM, Bruin JP, Scopes E, Peeters MF, IJzerman EP. Evaluation of the Oxoid Xpect Legionella test kit for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 2009; 47: 2272–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Alvarez J, Dominguez A, Sabria M et al. Impact of the Legionella urinary antigen test on epidemiological trends in community outbreaks of legionellosis in Catalonia, Spain, 1990–2004. Int J Infect Dis 2009; 13: e365–e370. [DOI] [PubMed] [Google Scholar]
- 325. Blazquez RM, Espinosa FJ, Martinez‐Toldos CM, Alemany L, Garcia‐Orenes MC, Segovia M. Sensitivity of urinary antigen test in relation to clinical severity in a large outbreak of Legionella pneumonia in Spain. Eur J Clin Microbiol Infect Dis 2005; 24: 488–491. [DOI] [PubMed] [Google Scholar]
- 326. von BH, Ewig S, Marre R et al. Community‐acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. Clin Infect Dis 2008; 46: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 327. Steininger C, Redlberger M, Graninger W, Kundi M, Popow‐Kraupp T. Near‐patient assays for diagnosis of influenza virus infection in adult patients. Clin Microbiol Infect 2009; 15: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Falsey AR. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 2007; 28: 171–181. [DOI] [PubMed] [Google Scholar]
- 329. Vazquez EG, Marcos MA, Vilella A, Yague J, Bayas JM, Mensa J. Assessment of a commercial rapid urinary antigen test to detect Streptococcus pneumoniae in patients who received 23‐valent pneumococcal polysaccharide vaccine. Eur J Clin Microbiol Infect Dis 2004; 23: 927–929. [DOI] [PubMed] [Google Scholar]
- 330. Beersma MF, Dirven K, van Dam AP, Templeton KE, Claas EC, Goossens H. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae‐specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard”. J Clin Microbiol 2005; 43: 2277–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Talkington DF, Shott S, Fallon MT, Schwartz SB, Thacker WL. Analysis of eight commercial enzyme immunoassay tests for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin Diagn Lab Immunol 2004; 11: 862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Nir‐Paz R, Michael‐Gayego A, Ron M, Block C. Evaluation of eight commercial tests for Mycoplasma pneumoniae antibodies in the absence of acute infection. Clin Microbiol Infect 2006; 12: 685–688. [DOI] [PubMed] [Google Scholar]
- 333. Templeton KE, Scheltinga SA, Graffelman AW et al. Comparison and evaluation of real‐time PCR, real‐time nucleic acid sequence‐based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae . J Clin Microbiol 2003; 41: 4366–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Martinez MA, Ruiz M, Zunino E, Luchsinger V, Avendano LF. Detection of Mycoplasma pneumoniae in adult community‐acquired pneumonia by PCR and serology. J Med Microbiol 2008; 57 (Pt 12): 1491–1495. [DOI] [PubMed] [Google Scholar]
- 335. von BH, Welte T, Marre R, Suttorp N, Luck C, Ewig S. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community‐acquired pneumonia (CAPNETZ). BMC Infect Dis 2009; 9: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Hvidsten D, Halvorsen DS, Berdal BP, Gutteberg TJ. Chlamydophila pneumoniae diagnostics: importance of methodology in relation to timing of sampling. Clin Microbiol Infect 2009; 15: 42–49. [DOI] [PubMed] [Google Scholar]
- 337. Elverdal P, Jorgensen CS, Uldum SA. Comparison and evaluation of four commercial kits relative to an in‐house immunofluorescence test for detection of antibodies against Legionella pneumophila . Eur J Clin Microbiol Infect Dis 2008; 27: 149–152. [DOI] [PubMed] [Google Scholar]
- 338. Johansson N, Kalin M, Giske CG, Hedlund J. Quantitative detection of Streptococcus pneumoniae from sputum samples with real‐time quantitative polymerase chain reaction for etiologic diagnosis of community‐acquired pneumonia. Diagn Microbiol Infect Dis 2008; 60: 255–261. [DOI] [PubMed] [Google Scholar]
- 339. Abdeldaim G, Herrmann B, Molling P et al. Usefulness of real‐time PCR for lytA, ply, and Spn9802 on plasma samples for the diagnosis of pneumococcal pneumonia. Clin Microbiol Infect 2010; 16: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 340. Peters RP, de Boer RF, Schuurman T et al. Streptococcus pneumoniae DNA load in blood as a marker of infection in patients with community‐acquired pneumonia. J Clin Microbiol 2009; 47: 3308–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Rello J, Lisboa T, Lujan M et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest 2009; 136: 832–840. [DOI] [PubMed] [Google Scholar]
- 342. Abdeldaim GM, Stralin K, Kirsebom LA, Olcen P, Blomberg J, Herrmann B. Detection of Haemophilus influenzae in respiratory secretions from pneumonia patients by quantitative real‐time polymerase chain reaction. Diagn Microbiol Infect Dis 2009; 64: 366–373. [DOI] [PubMed] [Google Scholar]
- 343. Maurin M, Hammer L, Gestin B et al. Quantitative real‐time PCR tests for diagnostic and prognostic purposes in cases of legionellosis. Clin Microbiol Infect 2010; 16: 379–384. [DOI] [PubMed] [Google Scholar]
- 344. Raty R, Ronkko E, Kleemola M. Sample type is crucial to the diagnosis of Mycoplasma pneumoniae pneumonia by PCR. J Med Microbiol 2005; 54 (Pt 3): 287–291. [DOI] [PubMed] [Google Scholar]
- 345. Diederen BM, Kluytmans JA, Vandenbroucke‐Grauls CM, Peeters MF. Utility of real‐time PCR for diagnosis of Legionnaires’ disease in routine clinical practice. J Clin Microbiol 2008; 46: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol 2008; 8: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Thurman KA, Walter ND, Schwartz SB et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis 2009; 48: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 348. Andre P, Caro V, Njamkepo E, Wendelboe AM, Van RA, Guiso N. Comparison of serological and real‐time PCR assays to diagnose Bordetella pertussis infection in 2007. J Clin Microbiol 2008; 46: 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Sotir MJ, Cappozzo DL, Warshauer DM et al. Evaluation of polymerase chain reaction and culture for diagnosis of pertussis in the control of a county‐wide outbreak focused among adolescents and adults. Clin Infect Dis 2007; 44: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 350. Mahony J, Chong S, Merante F et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead‐based assay. J Clin Microbiol 2007; 45: 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. van de Pol AC, van Loon AM, Wolfs TF et al. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real‐time PCR in samples from patients with respiratory symptoms. J Clin Microbiol 2007; 45: 2260–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Ginocchio CC, Zhang F, Manji R et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol 2009; 45: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Caram LB, Chen J, Taggart EW et al. Respiratory syncytial virus outbreak in a long‐term care facility detected using reverse transcriptase polymerase chain reaction: an argument for real‐time detection methods. J Am Geriatr Soc 2009; 57: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Berjohn CM, Fishman NO, Joffe MM, Edelstein PH, Metlay JP. Treatment and outcomes for patients with bacteremic pneumococcal pneumonia. Medicine (Baltimore) 2008; 87: 160–166. [DOI] [PubMed] [Google Scholar]
- 355. Cheng AC, Buising KL. Delayed administration of antibiotics and mortality in patients with community‐acquired pneumonia 89. Ann Emerg Med 2009; 53: 618–624. [DOI] [PubMed] [Google Scholar]
- 356. Bruns AH, Oosterheert JJ, Hustinx WN, Gaillard CA, Hak E, Hoepelman AI. Time for first antibiotic dose is not predictive for the early clinical failure of moderate‐severe community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 2009; 28: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Fee C, Weber EJ. Identification of 90% of patients ultimately diagnosed with community‐acquired pneumonia within four hours of emergency department arrival may not be feasible. Ann Emerg Med 2007; 49: 553–559. [DOI] [PubMed] [Google Scholar]
- 358. Friedberg MW, Mehrotra A, Linder JA. Reporting hospitals’ antibiotic timing in pneumonia: adverse consequences for patients? Am J Manag Care 2009; 15: 137–144. [PMC free article] [PubMed] [Google Scholar]
- 359. Kanwar M, Brar N, Khatib R, Fakih MG. Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4‐h antibiotic administration rule. Chest 2007; 131: 1865–1869. [DOI] [PubMed] [Google Scholar]
- 360. Pines JM, Isserman JA, Hinfey PB. The measurement of time to first antibiotic dose for pneumonia in the emergency department: a white paper and position statement prepared for the American Academy of Emergency Medicine. J Emerg Med 2009; 37: 335–340. [DOI] [PubMed] [Google Scholar]
- 361. Iannini PB, Paladino JA, Lavin B, Singer ME, Schentag JJ. A case series of macrolide treatment failures in community acquired pneumonia. J Chemother 2007; 19: 536–545. [DOI] [PubMed] [Google Scholar]
- 362. Rzeszutek M, Wierzbowski A, Hoban DJ, Conly J, Bishai W, Zhanel GG. A review of clinical failures associated with macrolide‐resistant Streptococcus pneumoniae . Int J Antimicrob Agents 2004; 24: 95–104. [DOI] [PubMed] [Google Scholar]
- 363. Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. Comparison of beta‐lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community‐acquired pneumonia. Antimicrob Agents Chemother 2007; 51: 3977–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest 2007; 131: 466–473. [DOI] [PubMed] [Google Scholar]
- 365. Tessmer A, Welte T, Martus P, Schnoor M, Marre R, Suttorp N. Impact of intravenous {beta}‐lactam/macrolide versus {beta}‐lactam monotherapy on mortality in hospitalized patients with community‐acquired pneumonia. J Antimicrob Chemother 2009; 63: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 366. Paul M, Nielsen AD, Gafter‐Gvili A et al. The need for macrolides in hospitalised community‐acquired pneumonia: propensity analysis. Eur Respir J 2007; 30: 525–531. [DOI] [PubMed] [Google Scholar]
- 367. File TM Jr. The development of pharmacokinetically enhanced amoxicillin/clavulanate for the management of respiratory tract infections in adults. Int J Antimicrob Agents 2007; 30 (suppl 2): S131–S134. [DOI] [PubMed] [Google Scholar]
- 368. Petitpretz P, Chidiac C, Soriano F, Garau J, Stevenson K, Rouffiac E. The efficacy and safety of oral pharmacokinetically enhanced amoxycillin‐clavulanate 2000/125 mg, twice daily, versus oral amoxycillin‐clavulanate 1000/125 mg, three times daily, for the treatment of bacterial community‐acquired pneumonia in adults. Int J Antimicrob Agents 2002; 20: 119–129. [DOI] [PubMed] [Google Scholar]
- 369. Siquier B, Sanchez‐Alvarez J, Garcia‐Mendez E et al. Efficacy and safety of twice‐daily pharmacokinetically enhanced amoxicillin/clavulanate (2000/125 mg) in the treatment of adults with community‐acquired pneumonia in a country with a high prevalence of penicillin‐resistant Streptococcus pneumoniae . J Antimicrob Chemother 2006; 57: 536–545. [DOI] [PubMed] [Google Scholar]
- 370. Dartois N, Castaing N, Gandjini H, Cooper A. Tigecycline versus levofloxacin for the treatment of community‐acquired pneumonia: European experience. J Chemother 2008; 20 (suppl 1): 28–35. [DOI] [PubMed] [Google Scholar]
- 371. Lin TY, Lin SM, Chen HC et al. An open‐label, randomized comparison of levofloxacin and amoxicillin/clavulanate plus clarithromycin for the treatment of hospitalized patients with community‐acquired pneumonia. Chang Gung Med J 2007; 30: 321–332. [PubMed] [Google Scholar]
- 372. Portier H, Brambilla C, Garre M, Paganin F, Poubeau P, Zuck P. Moxifloxacin monotherapy compared to amoxicillin‐clavulanate plus roxithromycin for nonsevere community‐acquired pneumonia in adults with risk factors. Eur J Clin Microbiol Infect Dis 2005; 24: 367–376. [DOI] [PubMed] [Google Scholar]
- 373. Querol‐Ribelles JM, Tenias JM, Querol‐Borras JM et al. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community‐acquired pneumonia requiring hospitalization. Int J Antimicrob Agents 2005; 25: 75–83. [DOI] [PubMed] [Google Scholar]
- 374. Salkind AR, Cuddy PG, Foxworth JW. Fluoroquinolone treatment of community‐acquired pneumonia: a meta‐analysis. Ann Pharmacother 2002; 36: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 375. Schein J, Janagap‐Benson C, Grant R, Sikirica V, Doshi D, Olson W. A comparison of levofloxacin and moxifloxacin use in hospitalized community‐acquired pneumonia (CAP) patients in the US: focus on length of stay. Curr Med Res Opin 2008; 24: 895–906. [DOI] [PubMed] [Google Scholar]
- 376. Tanaseanu C, Bergallo C, Teglia O et al. Integrated results of 2 phase 3 studies comparing tigecycline and levofloxacin in community‐acquired pneumonia 509. Diagn Microbiol Infect Dis 2008; 61: 329–338. [DOI] [PubMed] [Google Scholar]
- 377. Tanaseanu C, Milutinovic S, Calistru PI et al. Efficacy and safety of tigecycline versus levofloxacin for community‐acquired pneumonia. BMC Pulm Med 2009; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Torres A, Muir JF, Corris P et al. Effectiveness of oral moxifloxacin in standard first‐line therapy in community‐acquired pneumonia. Eur Respir J 2003; 21: 135–143. [DOI] [PubMed] [Google Scholar]
- 379. Vardakas KZ, Siempos II, Grammatikos A, Athanassa Z, Korbila IP, Falagas ME. Respiratory fluoroquinolones for the treatment of community‐acquired pneumonia: a meta‐analysis of randomized controlled trials. CMAJ 2008; 179: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Van BF, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Saf 2009; 32: 359–378. [DOI] [PubMed] [Google Scholar]
- 381. Bergallo C, Jasovich A, Teglia O et al. Safety and efficacy of intravenous tigecycline in treatment of community‐acquired pneumonia: results from a double‐blind randomized phase 3 comparison study with levofloxacin. Diagn Microbiol Infect Dis 2009; 63: 52–61. [DOI] [PubMed] [Google Scholar]
- 382. Ortiz‐Ruiz G, Vetter N, Isaacs R, Carides A, Woods GL, Friedland I. Ertapenem versus ceftriaxone for the treatment of community‐acquired pneumonia in adults: combined analysis of two multicentre randomized, double‐blind studies. J Antimicrob Chemother 2004; 53 (suppl 2): ii59–ii66. [DOI] [PubMed] [Google Scholar]
- 383. Vetter N, Cambronero‐Hernandez E, Rohlf J et al. A prospective, randomized, double‐blind multicenter comparison of parenteral ertapenem and ceftriaxone for the treatment of hospitalized adults with community‐acquired pneumonia. Clin Ther 2002; 24: 1770–1785. [DOI] [PubMed] [Google Scholar]
- 384. Yakovlev SV, Stratchounski LS, Woods GL et al. Ertapenem versus cefepime for initial empirical treatment of pneumonia acquired in skilled‐care facilities or in hospitals outside the intensive care unit. Eur J Clin Microbiol Infect Dis 2006; 25: 633–641. [DOI] [PubMed] [Google Scholar]
- 385. Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K. Outcome of community‐acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008; 32: 139–146. [DOI] [PubMed] [Google Scholar]
- 386. Murcia JM, Gonzalez‐Comeche J, Marin A et al. Clinical response to ertapenem in severe community‐acquired pneumonia: a retrospective series in an elderly population. Clin Microbiol Infect 2009; 15: 1046–1050. [DOI] [PubMed] [Google Scholar]
- 387. Paladino JA, Eubanks DA, Adelman MH, Schentag JJ. Once‐daily cefepime versus ceftriaxone for nursing home‐acquired pneumonia. J Am Geriatr Soc 2007; 55: 651–657. [DOI] [PubMed] [Google Scholar]
- 388. von BH, Welte T, Marre R, Suttorp N, Ewig S. Community‐acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J 2010; 35: 598–605. [DOI] [PubMed] [Google Scholar]
- 389. Frei CR, Koeller JM, Burgess DS, Talbert RL, Johnsrud MT. Impact of atypical coverage for patients with community‐acquired pneumonia managed on the medical ward: results from the United States Community‐Acquired Pneumonia Project. Pharmacotherapy 2003; 23: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 390. Lui G, Ip M, Lee N et al. Role of ‘atypical pathogens’ among adult hospitalized patients with community‐acquired pneumonia. Respirology 2009; 14: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 391. Shefet D, Robenshtock E, Paul M, Leibovici L. Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults. Cochrane Database Syst Rev 2005; 2: CD004418. [DOI] [PubMed] [Google Scholar]
- 392. Garcia VE, Mensa J, Martinez JA et al. Lower mortality among patients with community‐acquired pneumonia treated with a macrolide plus a beta‐lactam agent versus a beta‐lactam agent alone. Eur J Clin Microbiol Infect Dis 2005; 24: 190–195. [DOI] [PubMed] [Google Scholar]
- 393. Martinez FJ. Monotherapy versus dual therapy for community‐acquired pneumonia in hospitalized patients. Clin Infect Dis 2004; 38 (suppl 4): S328–S340. [DOI] [PubMed] [Google Scholar]
- 394. Weiss K, Low DE, Cortes L et al. Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults. Can Respir J 2004; 11: 589–593. [DOI] [PubMed] [Google Scholar]
- 395. Alvarez LF. Clinical experience with levofloxacin in the treatment of pneumonia in ICU patients. J Chemother 2004; 16 (suppl 2): 15–17. [PubMed] [Google Scholar]
- 396. Erard V, Lamy O, Bochud PY, Bille J, Cometta A, Calandra T. Full‐course oral levofloxacin for treatment of hospitalized patients with community‐acquired pneumonia. Eur J Clin Microbiol Infect Dis 2004; 23: 82–88. [DOI] [PubMed] [Google Scholar]
- 397. Katz E, Larsen LS, Fogarty CM, Hamed K, Song J, Choudhri S. Safety and efficacy of sequential i.v. to p.o. moxifloxacin versus conventional combination therapies for the treatment of community‐acquired pneumonia in patients requiring initial i.v. therapy. J Emerg Med 2004; 27: 395–405. [DOI] [PubMed] [Google Scholar]
- 398. Lode H, Grossman C, Choudhri S et al. Sequential IV/PO moxifloxacin treatment of patients with severe community‐acquired pneumonia. Respir Med 2003; 97: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 399. Rodriguez A, Mendia A, Sirvent JM et al. Combination antibiotic therapy improves survival in patients with community‐acquired pneumonia and shock. Crit Care Med 2007; 35: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 400. Torres A, Garau J, Arvis P et al. Moxifloxacin monotherapy is effective in hospitalized patients with community‐acquired pneumonia: the MOTIV study – a randomized clinical trial. Clin Infect Dis 2008; 46: 1499–1509. [DOI] [PubMed] [Google Scholar]
- 401. Wasserfallen JB, Erard V, Cometta A, Calandra T, Lamy O. Cost‐effectiveness of full‐course oral levofloxacin in severe community‐acquired pneumonia. Eur Respir J 2004; 24: 644–648. [DOI] [PubMed] [Google Scholar]
- 402. Romanelli G, Cravarezza P, Pozzi A et al. Carbapenems in the treatment of severe community‐acquired pneumonia in hospitalized elderly patients: a comparative study against standard therapy. J Chemother 2002; 14: 609–617. [DOI] [PubMed] [Google Scholar]
- 403. File TM Jr, Lode H, Kurz H, Kozak R, Xie H, Berkowitz E. Double‐blind, randomized study of the efficacy and safety of oral pharmacokinetically enhanced amoxicillin‐clavulanate (2,000/125 milligrams) versus those of amoxicillin‐clavulanate (875/125 milligrams), both given twice daily for 7 days, in treatment of bacterial community‐acquired pneumonia in adults. Antimicrob Agents Chemother 2004; 48: 3323–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Martinez JA, Horcajada JP, Almela M et al. Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2003; 36: 389–395. [DOI] [PubMed] [Google Scholar]
- 405. Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia. Chest 2005; 128: 940–946. [DOI] [PubMed] [Google Scholar]
- 406. Aspa J, Rajas O, Rodriguez de CF et al. Drug‐resistant pneumococcal pneumonia: clinical relevance and related factors. Clin Infect Dis 2004; 38: 787–798. [DOI] [PubMed] [Google Scholar]
- 407. Bonnard P, Lescure FX, Douadi Y et al. Community‐acquired bacteraemic pneumococcal pneumonia in adults: effect of diminished penicillin susceptibility on clinical outcome. J Infect 2005; 51: 69–76. [DOI] [PubMed] [Google Scholar]
- 408. Falco V, Almirante B, Jordano Q et al. Influence of penicillin resistance on outcome in adult patients with invasive pneumococcal pneumonia: is penicillin useful against intermediately resistant strains? J Antimicrob Chemother 2004; 54: 481–488. [DOI] [PubMed] [Google Scholar]
- 409. Lujan M, Gallego M, Fontanals D, Mariscal D, Rello J. Prospective observational study of bacteremic pneumococcal pneumonia: effect of discordant therapy on mortality. Crit Care Med 2004; 32: 625–631. [DOI] [PubMed] [Google Scholar]
- 410. Plouffe JF, Breiman RF, Fields BS et al. Azithromycin in the treatment of Legionella pneumonia requiring hospitalization. Clin Infect Dis 2003; 37: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 411. Sabria M, Pedro‐Botet ML, Gomez J et al. Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest 2005; 128: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 412. Yu VL, Greenberg RN, Zadeikis N et al. Levofloxacin efficacy in the treatment of community‐acquired legionellosis. Chest 2004; 125: 2135–2139. [DOI] [PubMed] [Google Scholar]
- 413. Capelastegui A, Espana PP, Quintana JM et al. Declining length of hospital stay for pneumonia and postdischarge outcomes. Am J Med 2008; 121: 845–852. [DOI] [PubMed] [Google Scholar]
- 414. Jasti H, Mortensen EM, Obrosky DS, Kapoor WN, Fine MJ. Causes and risk factors for rehospitalization of patients hospitalized with community‐acquired pneumonia. Clin Infect Dis 2008; 46: 550–556. [DOI] [PubMed] [Google Scholar]
- 415. Yende S, D’Angelo G, Kellum JA et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med 2008; 177: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Chastre J, Wolff M, Fagon JY et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator‐associated pneumonia in adults: a randomized trial. JAMA 2003; 290: 2588–2598. [DOI] [PubMed] [Google Scholar]
- 417. Christ‐Crain M, Stolz D, Bingisser R et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006; 174: 84–93. [DOI] [PubMed] [Google Scholar]
- 418. Kristoffersen KB, Sogaard OS, Wejse C et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission – a randomized trial. Clin Microbiol Infect 2009; 15: 481–487. [DOI] [PubMed] [Google Scholar]
- 419. Schuetz P, Christ‐Crain M, Thomann R et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 420. Bouadma L, Luyt CE, Tubach F et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375: 463–474. [DOI] [PubMed] [Google Scholar]
- 421. Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008; 177: 498–505. [DOI] [PubMed] [Google Scholar]
- 422. El Moussaoui MR, de Borgie CA, van den BP et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate‐severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332: 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Athanassa Z, Makris G, Dimopoulos G, Falagas ME. Early switch to oral treatment in patients with moderate to severe community‐acquired pneumonia: a meta‐analysis. Drugs 2008; 68: 2469–2481. [DOI] [PubMed] [Google Scholar]
- 424. Lee RW, Lindstrom ST. Early switch to oral antibiotics and early discharge guidelines in the management of community‐acquired pneumonia. Respirology 2007; 12: 111–116. [DOI] [PubMed] [Google Scholar]
- 425. Marras TK, Nopmaneejumruslers C, Chan CK. Efficacy of exclusively oral antibiotic therapy in patients hospitalized with nonsevere community‐acquired pneumonia: a retrospective study and meta‐analysis. Am J Med 2004; 116: 385–393. [DOI] [PubMed] [Google Scholar]
- 426. van der Eerden MM, de Graaff CS, Vlaspolder F, Bronsveld W, Jansen HM, Boersma WG. Evaluation of an algorithm for switching from IV to PO therapy in clinical practice in patients with community‐acquired pneumonia. Clin Ther 2004; 26: 294–303. [DOI] [PubMed] [Google Scholar]
- 427. Shindo Y, Sato S, Maruyama E et al. Implication of clinical pathway care for community‐acquired pneumonia in a community hospital: early switch from an intravenous beta‐lactam plus a macrolide to an oral respiratory fluoroquinolone. Intern Med 2008; 47: 1865–1874. [DOI] [PubMed] [Google Scholar]
- 428. Nathan RV, Rhew DC, Murray C, Bratzler DW, Houck PM, Weingarten SR. In‐hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med 2006; 119: 512–517. [DOI] [PubMed] [Google Scholar]
- 429. Oosterheert JJ, Bonten MJ, Schneider MM et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ 2006; 333: 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest 2003; 124: 883–889. [DOI] [PubMed] [Google Scholar]
- 431. Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto MG. Acute respiratory failure in patients with severe community‐acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med 1999; 160 (5 Pt 1): 1585–1591. [DOI] [PubMed] [Google Scholar]
- 432. Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med 2003; 168: 1438–1444. [DOI] [PubMed] [Google Scholar]
- 433. Antonelli M, Conti G, Esquinas A et al. A multiple‐center survey on the use in clinical practice of noninvasive ventilation as a first‐line intervention for acute respiratory distress syndrome. Crit Care Med 2007; 35: 18–25. [DOI] [PubMed] [Google Scholar]
- 434. Bulow HH, Thorsager B. Non‐invasive ventilation in do‐not‐intubate patients: five‐year follow‐up on a two‐year prospective, consecutive cohort study. Acta Anaesthesiol Scand 2009; 53: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 435. Confalonieri M, Urbino R, Potena A et al. Hydrocortisone infusion for severe community‐acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005; 171: 242–248. [DOI] [PubMed] [Google Scholar]
- 436. Gorman SK, Slavik RS, Marin J. Corticosteroid treatment of severe community‐acquired pneumonia. Ann Pharmacother 2007; 41: 1233–1237. [DOI] [PubMed] [Google Scholar]
- 437. Salluh JI, Povoa P, Soares M, Castro‐Faria‐Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community‐acquired pneumonia: a systematic review. Crit Care 2008; 12: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Adjunctive therapies for community‐acquired pneumonia: a systematic review. J Antimicrob Chemother 2008; 62: 661–668. [DOI] [PubMed] [Google Scholar]
- 439. Adams R, Ruffin R, Campbell D. The value of the lipid‐laden macrophage index in the assessment of aspiration pneumonia. Aust N Z J Med 1997; 27: 550–553. [DOI] [PubMed] [Google Scholar]
- 440. Chen JH, Lamberg JL, Chen YC et al. Occurrence and treatment of suspected pneumonia in long‐term care residents dying with advanced dementia. J Am Geriatr Soc 2006; 54: 290–295. [DOI] [PubMed] [Google Scholar]
- 441. DeToledo JC, Lowe MR, Gonzalez J, Haddad H. Risk of aspiration pneumonia after an epileptic seizure: a retrospective analysis of 1634 adult patients. Epilepsy Behav 2004; 5: 593–595. [DOI] [PubMed] [Google Scholar]
- 442. Kadowaki M, Demura Y, Mizuno S et al. Reappraisal of clindamycin IV monotherapy for treatment of mild‐to‐moderate aspiration pneumonia in elderly patients. Chest 2005; 127: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 443. Mier L, Dreyfuss D, Darchy B et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intensive Care Med 1993; 19: 279–284. [DOI] [PubMed] [Google Scholar]
- 444. Mylotte JM, Goodnough S, Naughton BJ. Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc 2003; 51: 17–23. [DOI] [PubMed] [Google Scholar]
- 445. Reza SM, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc 2006; 54: 296–302. [DOI] [PubMed] [Google Scholar]
- 446. Teramoto S, Fukuchi Y, Sasaki H, Sato K, Sekizawa K, Matsuse T. High incidence of aspiration pneumonia in community‐ and hospital‐acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 2008; 56: 577–579. [DOI] [PubMed] [Google Scholar]
- 447. Allewelt M, Schuler P, Bolcskei PL, Mauch H, Lode H. Ampicillin + sulbactam vs clindamycin +/− cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect 2004; 10: 163–170. [DOI] [PubMed] [Google Scholar]
- 448. Bartlett JG, Gorbach SL. Treatment of aspiration pneumonia and primary lung abscess. Penicillin G vs clindamycin. JAMA 1975; 234: 935–937. [PubMed] [Google Scholar]
- 449. Fernandez‐Sabe N, Carratala J, Dorca J et al. Efficacy and safety of sequential amoxicillin‐clavulanate in the treatment of anaerobic lung infections. Eur J Clin Microbiol Infect Dis 2003; 22: 185–187. [DOI] [PubMed] [Google Scholar]
- 450. Gudiol F, Manresa F, Pallares R et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin‐resistant Bacteroides melaninogenicus. Arch Intern Med 1990; 150: 2525–2529. [PubMed] [Google Scholar]
- 451. Perlino CA. Metronidazole vs clindamycin treatment of anerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med 1981; 141: 1424–1427. [PubMed] [Google Scholar]
- 452. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection 2008; 36: 23–30. [DOI] [PubMed] [Google Scholar]
- 453. Chen CZ, Fan PS, Lin CC, Lee CH, Hsiue TR. Repeated pneumonia severity index measurement after admission increases its predictive value for mortality in severe community‐acquired pneumonia. J Formos Med Assoc 2009; 108: 219–223. [DOI] [PubMed] [Google Scholar]
- 454. Menendez R, Cavalcanti M, Reyes S et al. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax 2008; 63: 447–452. [DOI] [PubMed] [Google Scholar]
- 455. Chalmers JD, Singanayagam A, Hill AT. C‐reactive protein is an independent predictor of severity in community‐acquired pneumonia. Am J Med 2008; 121: 219–225. [DOI] [PubMed] [Google Scholar]
- 456. Coelho L, Povoa P, Almeida E et al. Usefulness of C‐reactive protein in monitoring the severe community‐acquired pneumonia clinical course. Crit Care 2007; 11: R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Lieberman D, Shmarkov O, Gelfer Y, Varshavsky R, Lieberman DV. Prevalence and clinical significance of fever in acute exacerbations of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis 2003; 22: 75–78. [DOI] [PubMed] [Google Scholar]
- 458. Allegra L, Blasi F, Diano P et al. Sputum color as a marker of acute bacterial exacerbations of chronic obstructive pulmonary disease. Respir Med 2005; 99: 742–747. [DOI] [PubMed] [Google Scholar]
- 459. Soler N, Agusti C, Angrill J, Puig DlB, Torres A. Bronchoscopic validation of the significance of sputum purulence in severe exacerbations of chronic obstructive pulmonary disease. Thorax 2007; 62: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Brusse‐Keizer MG, Grotenhuis AJ, Kerstjens HA et al. Relation of sputum colour to bacterial load in acute exacerbations of COPD. Respir Med 2009; 103: 601–606. [DOI] [PubMed] [Google Scholar]
- 461. Burley CJ, Masterton RG, Lovell DP. Indicators of bacterial infection in patients with acute exacerbation of chronic bronchitis for application in clinical trials of antibacterial drugs. J Infect 2007; 55: 226–232. [DOI] [PubMed] [Google Scholar]
- 462. Stolz D, Christ‐Crain M, Bingisser R et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest 2007; 131: 9–19. [DOI] [PubMed] [Google Scholar]
- 463. Lieberman D, Lieberman D, Ben‐Yaakov M et al. Serological evidence of Mycoplasma pneumoniae infection in acute exacerbation of COPD. Diagn Microbiol Infect Dis 2002; 44: 1–6. [DOI] [PubMed] [Google Scholar]
- 464. Lin SH, Kuo PH, Hsueh PR, Yang PC, Kuo SH. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa . Respirology 2007; 12: 81–87. [DOI] [PubMed] [Google Scholar]
- 465. Montero M, Dominguez M, Orozco‐Levi M, Salvado M, Knobel H. Mortality of COPD patients infected with multi‐resistant Pseudomonas aeruginosa: a case and control study. Infection 2009; 37: 16–19. [DOI] [PubMed] [Google Scholar]
- 466. Lode H, Allewelt M, Balk S et al. A prediction model for bacterial etiology in acute exacerbations of COPD. Infection 2007; 35: 143–149. [DOI] [PubMed] [Google Scholar]
- 467. Garcia‐Vidal C, Almagro P, Romani V et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J 2009; 34: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 468. Kahn JB, Khashab M, Ambruzs M. Study entry microbiology in patients with acute bacterial exacerbation of chronic bronchitis in a clinical trial stratifying by disease severity. Curr Med Res Opin 2007; 23: 1–7. [DOI] [PubMed] [Google Scholar]
- 469. Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 266–272. [DOI] [PubMed] [Google Scholar]
- 470. Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 167: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 471. Sethi S, Sethi R, Eschberger K et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176: 356–361. [DOI] [PubMed] [Google Scholar]
- 472. Sethi S, Wrona C, Grant BJ, Murphy TF. Strain‐specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 448–453. [DOI] [PubMed] [Google Scholar]
- 473. Aaron SD, Kottachchi D, Ferris WJ et al. Sputum versus bronchoscopy for diagnosis of Pseudomonas aeruginosa biofilms in cystic fibrosis. Eur Respir J 2004; 24: 631–637. [DOI] [PubMed] [Google Scholar]
- 474. Wilson R, Langan C, Ball P, Bateman K, Pypstra R. Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis. Respir Med 2003; 97: 242–249. [DOI] [PubMed] [Google Scholar]
- 475. Martinez FJ, Grossman RF, Zadeikis N et al. Patient stratification in the management of acute bacterial exacerbation of chronic bronchitis: the role of levofloxacin 750 mg. Eur Respir J 2005; 25: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 476. Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first‐line with second‐line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest 2007; 132: 447–455. [DOI] [PubMed] [Google Scholar]
- 477. Ferrer M, Ioanas M, Arancibia F, Marco MA, de la Bellacasa JP, Torres A. Microbial airway colonization is associated with noninvasive ventilation failure in exacerbation of chronic obstructive pulmonary disease. Crit Care Med 2005; 33: 2003–2009. [DOI] [PubMed] [Google Scholar]
- 478. Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006; 130: 1503–1510. [DOI] [PubMed] [Google Scholar]
- 479. Evans DJ, Bara AI, Greenstone M. Prolonged antibiotics for purulent bronchiectasis. Cochrane Database Syst Rev 2003; 4: CD001392. [DOI] [PubMed] [Google Scholar]
- 480. Cogo R, Ramponi A, Scivoletto G, Rippoli R. Prophylaxis for acute exacerbations of chronic bronchitis using an antibacterial sublingual vaccine obtained through mechanical lysis: a clinical and pharmacoeconomic study. Acta Biomed Ateneo Parmense 2003; 74: 81–87. [PubMed] [Google Scholar]
- 481. Foxwell AR, Cripps AW, Dear KB. Haemophilus influenzae oral whole cell vaccination for preventing acute exacerbations of chronic bronchitis. Cochrane Database Syst Rev 2003; 3: CD001958. [DOI] [PubMed] [Google Scholar]
- 482. Steurer‐Stey C, Bachmann LM, Steurer J, Tramer MR. Oral purified bacterial extracts in chronic bronchitis and COPD: systematic review. Chest 2004; 126: 1645–1655. [DOI] [PubMed] [Google Scholar]
- 483. Tricarico D, Varricchio A, D’Ambrosio S, Ascione E, Motta G. Prevention of recurrent upper respiratory tract infections in a community of cloistered nuns using a new immunostimulating bacterial lysate. A randomized, double‐blind clinical trial. Arzneimittelforschung 2004; 54: 57–63. [DOI] [PubMed] [Google Scholar]
- 484. Black P, Staykova T, Chacko E, Ram FS, Poole P. Prophylactic antibiotic therapy for chronic bronchitis. Cochrane Database Syst Rev 2003; 1: CD004105. [DOI] [PubMed] [Google Scholar]
- 485. Smucny J, Fahey T, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2004; 4: CD000245. [DOI] [PubMed] [Google Scholar]
- 486. Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long‐term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations 86. Am J Respir Crit Care Med 2008; 178: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 487. Sethi S, Jones PW, Theron MS et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 2010; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Drobnic ME, Sune P, Montoro JB, Ferrer A, Orriols R. Inhaled tobramycin in non‐cystic fibrosis patients with bronchiectasis and chronic bronchial infection with Pseudomonas aeruginosa . Ann Pharmacother 2005; 39: 39–44. [DOI] [PubMed] [Google Scholar]
- 489. Barker AF, Couch L, Fiel SB et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000; 162 (2Pt1): 481–485. [DOI] [PubMed] [Google Scholar]
- 490. Cymbala AA, Edmonds LC, Bauer MA et al. The disease‐modifying effects of twice‐weekly oral azithromycin in patients with bronchiectasis. Treat Respir Med 2005; 4: 117–122. [DOI] [PubMed] [Google Scholar]
- 491. Anwar GA, Bourke SC, Afolabi G, Middleton P, Ward C, Rutherford RM. Effects of long‐term low‐dose azithromycin in patients with non‐CF bronchiectasis 232. Respir Med 2008; 102: 1494–1496. [DOI] [PubMed] [Google Scholar]
- 492. Nordstrom BL, Sung I, Suter P, Szneke P. Risk of pneumonia and other complications of influenza‐like illness in patients treated with oseltamivir. Curr Med Res Opin 2005; 21: 761–768. [DOI] [PubMed] [Google Scholar]
- 493. Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 3: CD001287. [DOI] [PubMed] [Google Scholar]
- 494. Douglas RM, Hemila H, D’Souza R, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004; 4: CD000980. [DOI] [PubMed] [Google Scholar]
- 495. Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D’Alessio D. Treatment of the common cold with unrefined echinacea. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 2002; 137: 939–946. [DOI] [PubMed] [Google Scholar]
- 496. McElhaney JE, Goel V, Toane B, Hooten J, Shan JJ. Efficacy of COLD‐fX in the prevention of respiratory symptoms in community‐dwelling adults: a randomized, double‐blinded, placebo controlled trial. J Altern Complement Med 2006; 12: 153–157. [DOI] [PubMed] [Google Scholar]
- 497. Heimer KA, Hart AM, Martin LG, Rubio‐Wallace S. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J Am Acad Nurse Pract 2009; 21: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Sjogren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. J Am Geriatr Soc 2008; 56: 2124–2130. [DOI] [PubMed] [Google Scholar]
- 499. Awano S, Ansai T, Takata Y et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res 2008; 87: 334–339. [DOI] [PubMed] [Google Scholar]
- 500. Bassim CW, Gibson G, Ward T, Paphides BM, Denucci DJ. Modification of the risk of mortality from pneumonia with oral hygiene care. J Am Geriatr Soc 2008; 56: 1601–1607. [DOI] [PubMed] [Google Scholar]
- 501. Singh S, Amin AV, Loke YK. Long‐term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta‐analysis. Arch Intern Med 2009; 169: 219–229. [DOI] [PubMed] [Google Scholar]
- 502. Almirall J, Bolibar I, Serra‐Prat M et al. New evidence of risk factors for community‐acquired pneumonia: a population‐based study. Eur Respir J 2008; 31: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 503. Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007; 176: 162–166. [DOI] [PubMed] [Google Scholar]
- 504. Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta‐analysis. JAMA 2008; 300: 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Sin DD, Tashkin D, Zhang X et al. Budesonide and the risk of pneumonia: a meta‐analysis of individual patient data. Lancet 2009; 374: 712–719. [DOI] [PubMed] [Google Scholar]
- 506. Mortensen EM, Pugh MJ, Copeland LA et al. Impact of statins and angiotensin‐converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J 2008; 31: 611–617. [DOI] [PubMed] [Google Scholar]
- 507. Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community‐acquired pneumonia. Am J Med 2008; 121: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 508. Dublin S, Jackson ML, Nelson JC, Weiss NS, Larson EB, Jackson LA. Statin use and risk of community acquired pneumonia in older people: population based case‐control study. BMJ 2009; 338: b2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a population‐based, nested case‐control study. Pharmacotherapy 2007; 27: 325–332. [DOI] [PubMed] [Google Scholar]
- 510. Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population‐based cohort study of 29,900 patients. Arch Intern Med 2008; 168: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 511. Tleyjeh IM, Kashour T, Hakim FA et al. Statins for the prevention and treatment of infections: a systematic review and meta‐analysis. Arch Intern Med 2009; 169: 1658–1667. [DOI] [PubMed] [Google Scholar]
- 512. Hak E, Buskens E, van Essen GA et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study. Arch Intern Med 2005; 165: 274–280. [DOI] [PubMed] [Google Scholar]
- 513. Hak E, Hoes AW, Grobbee DE et al. Conventional influenza vaccination is not associated with complications in working‐age patients with asthma or chronic obstructive pulmonary disease. Am J Epidemiol 2003; 157: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Schembri S, Morant S, Winter JH, MacDonald TM. Influenza but not pneumococcal vaccination protects against all‐cause mortality in patients with COPD. Thorax 2009; 64: 567–572. [DOI] [PubMed] [Google Scholar]
- 515. Ortqvist A, Granath F, Askling J, Hedlund J. Influenza vaccination and mortality: prospective cohort study of the elderly in a large geographical area. Eur Respir J 2007; 30: 414–422. [DOI] [PubMed] [Google Scholar]
- 516. Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006; 35: 337–344. [DOI] [PubMed] [Google Scholar]
- 517. Eurich DT, Marrie TJ, Johnstone J, Majumdar SR. Mortality reduction with influenza vaccine in patients with pneumonia outside “flu” season: pleiotropic benefits or residual confounding? Am J Respir Crit Care Med 2008; 178: 527–533. [DOI] [PubMed] [Google Scholar]
- 518. Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community‐acquired pneumonia in immunocompetent elderly people: a population‐based, nested case‐control study 181. Lancet 2008; 372: 398–405. [DOI] [PubMed] [Google Scholar]
- 519. Jefferson T, Di PC, Al‐Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; 2: CD004876. [DOI] [PubMed] [Google Scholar]
- 520. Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double‐blind placebo‐controlled trial. JAMA 1994; 272: 1661–1665. [PubMed] [Google Scholar]
- 521. Fiore AE, Shay DK, Haber P et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56 (RR‐6): 1–54. [PubMed] [Google Scholar]
- 522. Wang Z, Tobler S, Roayaei J, Eick A. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009; 301: 945–953. [DOI] [PubMed] [Google Scholar]
- 523. Monto AS, Ohmit SE, Petrie JG et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 2009; 361: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 524. Nichol K, D’Heilly S, Ehlinger EP. Influenza vaccination among college and university students: impact on influenzalike illness, health care use, and impaired school performance. Arch Pediatr Adolesc Med 2008; 162: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 525. Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2004; 3: CD001269. [DOI] [PubMed] [Google Scholar]
- 526. Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2006; 3: CD005187. [DOI] [PubMed] [Google Scholar]
- 527. Thomas RE, Jefferson T, Lasserson TJ. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev 2010; 2: CD005187. [DOI] [PubMed] [Google Scholar]
- 528. Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit‐adjuvanted influenza vaccine in elderly subjects. Vaccine 2003; 21: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 529. van Kessel DA, van Velzen‐Blad H, van den Bosch JM, Rijkers GT. Impaired pneumococcal antibody response in bronchiectasis of unknown aetiology. Eur Respir J 2005; 25: 482–489. [DOI] [PubMed] [Google Scholar]
- 530. Ortqvist A, Henckaerts I, Hedlund J, Poolman J. Non‐response to specific serotypes likely cause for failure to 23‐valent pneumococcal polysaccharide vaccine in the elderly. Vaccine 2007; 25: 2445–2450. [DOI] [PubMed] [Google Scholar]
- 531. Abraham‐Van Parijs B. Review of pneumococcal conjugate vaccine in adults: implications on clinical development. Vaccine 2004; 22: 1362–1371. [DOI] [PubMed] [Google Scholar]
- 532. Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez‐Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein‐conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 2008; 198: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. de Roux A, Schmole‐Thoma B, Siber GR et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 534. Kyaw MH, Lynfield R, Schaffner W et al. Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae . N Engl J Med 2006; 354: 1455–1463. [DOI] [PubMed] [Google Scholar]
- 535. Whitney CG, Farley MM, Hadler J et al. Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 536. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time‐series analysis. Lancet 2007; 369: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 537. Nelson JC, Jackson M, Yu O et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults 211. Vaccine 2008; 26: 4947–4954. [DOI] [PubMed] [Google Scholar]
- 538. Melegaro A, Edmunds WJ. The 23‐valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta‐analyses. Eur J Epidemiol 2004; 19: 353–363. [DOI] [PubMed] [Google Scholar]
- 539. Dear K, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2003; 4: CD000422. [DOI] [PubMed] [Google Scholar]
- 540. Conaty S, Watson L, Dinnes J, Waugh N. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 2004; 22: 3214–3224. [DOI] [PubMed] [Google Scholar]
- 541. Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; 1: CD000422. [DOI] [PubMed] [Google Scholar]
- 542. Alfageme I, Vazquez R, Reyes N et al. Clinical efficacy of anti‐pneumococcal vaccination in patients with COPD. Thorax 2006; 61: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Jackson LA, Neuzil KM, Yu O et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 544. Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J 2004; 23: 363–368. [DOI] [PubMed] [Google Scholar]
- 545. Vila‐Corcoles A, Ochoa‐Gondar O, Hospital I et al. Protective effects of the 23‐valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN‐65 study. Clin Infect Dis 2006; 43: 860–868. [DOI] [PubMed] [Google Scholar]
- 546. Maruyama T, Taguchi O, Niederman MS et al. Efficacy of 23‐valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 2010; 340: c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547. Christenson B, Pauksen K, Sylvan SP. Effect of influenza and pneumococcal vaccines in elderly persons in years of low influenza activity. Virol J 2008; 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Ochoa‐Gondar O, Vila‐Corcoles A, Ansa X et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN‐65 study. Vaccine 2008; 26: 1955–1962. [DOI] [PubMed] [Google Scholar]
- 549. Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007; 22: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Skull SA, Andrews RM, Byrnes GB et al. Prevention of community‐acquired pneumonia among a cohort of hospitalized elderly: benefit due to influenza and pneumococcal vaccination not demonstrated. Vaccine 2007; 25: 4631–4640. [DOI] [PubMed] [Google Scholar]
- 551. Spindler C, Hedlund J, Jasir A, Normark BH, Ortqvist A. Effects of a large‐scale introduction of the pneumococcal polysaccharide vaccine among elderly persons in Stockholm, Sweden. Vaccine 2008; 26: 5541–5546. [DOI] [PubMed] [Google Scholar]
- 552. Mooney JD, Weir A, McMenamin J et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008; 8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community‐acquired pneumonia. Clin Infect Dis 2006; 42: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 554. Mykietiuk A, Carratala J, Dominguez A et al. Effect of prior pneumococcal vaccination on clinical outcome of hospitalized adults with community‐acquired pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis 2006; 25: 457–462. [DOI] [PubMed] [Google Scholar]
- 555. Melegaro A, Edmunds WJ. The 23‐valent pneumococcal polysaccharide vaccine. Part II. A cost‐effectiveness analysis for invasive disease in the elderly in England and Wales. Eur J Epidemiol 2004; 19: 365–375. [DOI] [PubMed] [Google Scholar]
- 556. Mangtani P, Roberts JA, Hall AJ, Cutts FT. An economic analysis of a pneumococcal vaccine programme in people aged over 64 years in a developed country setting. Int J Epidemiol 2005; 34: 565–574. [DOI] [PubMed] [Google Scholar]
- 557. McIntosh ED, Conway P, Willingham J, Hollingsworth R, Lloyd A. Pneumococcal pneumonia in the UK – how herd immunity affects the cost‐effectiveness of 7‐valent pneumococcal conjugate vaccine (PCV). Vaccine 2005; 23: 1739–1745. [DOI] [PubMed] [Google Scholar]
- 558. Jackson LA, Neuzil KM, Whitney CG et al. Safety of varying dosages of 7‐valent pneumococcal protein conjugate vaccine in seniors previously vaccinated with 23‐valent pneumococcal polysaccharide vaccine. Vaccine 2005; 23: 3697–3703. [DOI] [PubMed] [Google Scholar]
- 559. Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23‐valent pneumococcal polysaccharide vaccine in middle‐aged and elderly persons previously treated for pneumonia. Vaccine 2003; 22: 96–103. [DOI] [PubMed] [Google Scholar]
- 560. Waites KB, Canupp KC, Chen YY, DeVivo MJ, Nahm MH. Revaccination of adults with spinal cord injury using the 23‐valent pneumococcal polysaccharide vaccine. J Spinal Cord Med 2008; 31: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community‐acquired pneumonia. Arch Intern Med 2007; 167: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 562. Dexter PR, Perkins SM, Maharry KS, Jones K, McDonald CJ. Inpatient computer‐based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA 2004; 292: 2366–2371. [DOI] [PubMed] [Google Scholar]
- 563. Jacobson VJ, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev 2005; 3: CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. deHart MP, Salinas SK, Barnette LJ Jr et al. Project protect: pneumococcal vaccination in Washington State nursing homes. J Am Med Dir Assoc 2005; 6: 91–96. [DOI] [PubMed] [Google Scholar]
- 565. Jha AK, Wright SM, Perlin JB. Performance measures, vaccinations, and pneumonia rates among high‐risk patients in Veterans Administration health care. Am J Public Health 2007; 97: 2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


