Abstract
In order to mitigate human and financial losses as a result of future global pandemics, we must plan now. As the Ebola virus pandemic declines, we must reflect on how we have mismanaged this recent international crisis and how we can better prepare for the next global pandemic. Of great concern is the increasing frequency of pandemics occurring over the last few decades. Clearly, the window of opportunity to act is closing. This editorial discusses many issues including priority emerging and re-emerging infectious diseases; the challenges of meeting international health regulations; the strengthening of global health systems; global pandemic funding; and the One Health approach to future pandemic planning. We recommend that the global health community unites to urgently address these issues in order to avoid the next humanitarian crisis.
Keywords: Pandemic, epidemics, planning, emerging infectious diseases, health systems
1. Introduction
The West African Ebola virus pandemic has shown us yet again that the world is ill prepared to respond to a global health emergency. This follows similar statements that were made after the H1N1outbreak in 2009 that “The world is ill prepared to respond to a severe influenza pandemic or to any similar global, sustained and threatening public health emergency”.1 Our response to the Ebola zoonotic ‘spillover’ was delayed and as a result 11,158 people lost their lives in nine countries.2 The direct financial cost of the Ebola pandemic was estimated to be in the vicinity of six billion US dollars and global economic losses over 15 billion dollars.3 Clearly there are lessons to be learnt from the Ebola outbreak.
In 2005, following the Severe Acute Respiratory Syndrome (SARS) pandemic, the International Health Regulations (IHR) were modified. While two thirds of the 194 World Health Assembly countries have failed to comply with the regulations as of 2015, and for the one third who say they did, there are serious concerns about the reliability of their self-assessment.4 Now, with Liberia declared free of Ebola and declining incidence in Sierra Leone and Guinea, these same regulations are once again being revisited after more than a decade.4 Is this a futile exercise and should the IHRs be abandoned if they cannot be enforced by WHO and fulfilled by the World Health Assembly (WHA) member nations? The national health systems in West Africa, and for most low and middle income countries (LMICs), would not meet IHR standards (despite claims by some member WHA nations) and it is unlikely that following the Ebola pandemic much will change.
Many have stated that WHO failed to respond to the current Ebola epidemic in a timely manner4 but even if they did, would the outcome have been really that different? There were no drugs or vaccines available to treat and prevent the disease, thus quarantine, isolation and safe burials were the primary methods utilized to halt the spread of disease and were initiated by the afflicted nations themselves.5 It typically takes years if not decades to develop a vaccine or drug that will have public health impact. One only has to look at the countless billions that have been spent on trying to develop a vaccine for HIV, thus far without success. Moreover, weak, malnourished, immunosuppressed populations living in poverty with little or no hygiene, sanitation or running water will always be highly susceptible to new emerging or re-emerging infectious diseases.6 At ‘ground zero’ of the Ebola epidemic it was believed that in 2013, hungry children living in the remote Guinean village of Meliandou killed and ate infected fruit bats.7, 8 Thus, what can realistically be done to prevent and contain future national epidemics from becoming global pandemics? We discuss a number of issues that urgently need to be addressed in order to plan, and possibly prevent, the next global pandemic.
2. Emerging and Re-emerging Infectious Diseases
If one looks at the history of emerging or re-emerging infectious disease pandemics globally, on average they have appeared every decade but now, worryingly, the frequency between pandemics seems to be disturbingly shorter as evident with Severe Acute Respiratory Syndrome (SARS) in 2003, Influenza A H1N5 (bird flu) in 2007, H1N1 (swine flu) in 2009, Middle East Respiratory Syndrome (MERS) in 2012 and Ebola in 2014.9 Overpopulation and poverty are the primary contributing factors that have brought about this change and are strongly linked with global warming, environmental degradation, habitat destruction, and increased human/host/reservoir interaction.10 Weak malnourished populations in LMICs serve as the breeding grounds for future pandemics (Figure 1 ). For example, in metro Manila, the most densely populated city in the world, approximately six million people live in slums with no piped water or toilets. According to WHO, 137 million people in urban centres have no access to safe drinking water and over 600 million lack sanitation.10 The UN predicts that the world's urban population will double to over six billion by 2050 and most of the increase in density will occur in LMICs.10 Population density is directly correlated with the rate of transmission of respiratory and faecal-oral pathogens (e.g. Mycobacterium tuberculosis, influenza, cholera, rotavirus, helminths).10
Figure 1.
The breeding grounds for the next global pandemic: left panel illustrates slums in Metro-Manila, The Philippines; the middle panel shows slums in Dhaka, Bangladesh, and the right panel displays slums in Kibera, Kenya. Note photographs are available on public domain.
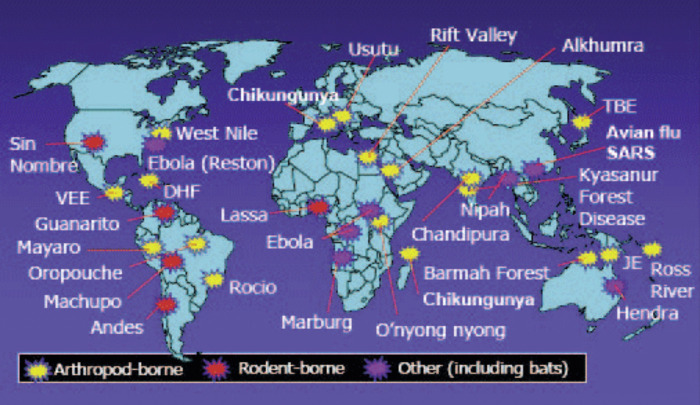
Between 1940 and 2004 there were 335 emerging infectious disease (EID) origins reported globally.9 Figure 2 illustrates some of the most recent EID epidemics. EIDs are primarily zoonotic (60%), originating in wildlife populations (e.g. HIV, SARS, Ebola, West Nile Virus, Lyme Disease) but bacterial pathogens have become increasingly of concern due to antibiotic resistance especially in the developing world.9, 11 Multidrug-resistance (MDR) to Mycobacterium tuberculosis, Streptococcus pneumoniae and Staphylococcus aureus are a global concern and gram-negative bacteria resistance to β-lactams is widespread.11 Drug resistance to enteropathogens has also become a major global health challenge. MDR Salmonella enterica Typhi and S. enterica Paratyphi are common in Asia and sub-Saharan Africa, and there are increasing reports of reduced susceptibility to fluoroquinolones.12 Campylobacter jejuni resistance to fluoroquinolones has become a concern in Southeast Asia, with rates of resistance of 80% reported from Thailand.12 Viral pathogens (e.g. Ebola, Makona variant (EBOV), MERS-CoV, H1N1) are also of concern due to their high rates of nucleotide substitution, poor mutation error-correction rate ability and capacity to quickly adapt to human hosts.9
Figure 2.
The map shows the location of recent emerging infectious diseases caused primarily by zoonotic diseases transmitted to humans via insect vectors, or animals. Note the ‘one-health’ map is available on public domain.
Table 1 displays some potentially pandemic pathogens that should be under active global surveillance. The current outbreak of MERS-CoV in South Korea is of grave concern given the case fatality rate is over 10%. Surveillance of zoonotic diseases is largely based on detecting illnesses in humans who often serve as the sentinel species and dead-end hosts.13 Apart from rabies, most national surveillance systems in the world do not monitor zoonotic diseases appearing in wildlife, yet 72% of zoonotic EIDs (e.g. Anthrax, Nipah virus, Hantavirus, type A influenza, SARS, MERS-CoV, Ebola) come from this source.9, 13 Many RNA viruses have emerged and dispersed globally such as Chikungunya virus, West Nile virus and dengue virus. These three arboviruses alone have morbidity and mortality rates that far exceed those of the combined rates of SARS, Ebola and MERS-CoV.14, 15 Thus, EID discovery efforts need to be directed toward reservoirs and vectors at the human-animal interface.16 The integration of human, veterinary, and agricultural medicine, as proposed by the ‘One Health’ approach, should result in earlier warning of EIDs and provide us with a better opportunity to respond to potential spill-over threats.13, 17 Moreover, targeting surveillance to regional hotspots of EIDs provides an evidence-based rationale for more appropriate allocation of global resources.16
Table 1.
Potential pathogens of a future global pandemic.
| Pathogen | Areas of High Risk | Modes of Transmission | Incubation | Common | Vaccine | Treatment | |
|---|---|---|---|---|---|---|---|
| Period | Symptoms | ||||||
| Influenza A |  |
Asia, South East Asia, | Wild birds, poultry, pigs, | 1-4 days | Productive cough, sore throat, | Fluvax®, inactivated split virion; | Oseltamivir (Tamiflu) 30-75mg |
| e.g. H1N1, H5N1, | Middle East | humans (respiratory) | fever, malaise, myalgia, | LAIV, live attenuated nasal spray | twice daily for 5 days; | ||
| H3N2 | rhinitis | Zanamivir (Relenza) 10 mg | |||||
| inhaled every 12 hr for 5 days | |||||||
| MERS-CoV |  |
Middle East, Asia | Bats, camels, | 2-14 days | As above | No vaccine available | No antiviral treatment |
| humans contact | |||||||
| Ebola |  |
Central Africa, West Africa | Bats, human body fluids | 2-21 days | Haemorrhage, fever, sore throat | No vaccine available | No antiviral treatment |
| vomiting, diarrohea, muscular | |||||||
| pain, headache, rash | |||||||
| MDR-Malaria |  |
South East Asia, East Africa | Anopheles mosquito | 9-14 days | Fever, headache, chills, vomiting | No vaccine available | ACTs† recommended |
| e.g. P. falciparum | South America | e.g. Artemether, 40 mg + | |||||
| lumefantrine, 240 mg twice a | |||||||
| day for 3 days | |||||||
| Chikungunya |  |
Africa, Southeast Asia, Asia, | Aedes mosquito | 2-12 days | Biphasic fever, joint pain, | No vaccine available | No antiviral treatment |
| Caribbean, Venezuela, USA, | maculopapular rash, uveitis, | ||||||
| France, Italy, Australia | headache, vomiting, insomnia | ||||||
| Campylobacteria |  |
South Asia, | Poultry, milk, | 1-4 days | Acute watery diarrhea, fever | No vaccine available | Azithromycin, 500 mg |
| South-east Asia | drinking water | once a day for 3 days | |||||
| Salmonella |  |
South Asia, Africa | Human contact, food, | 5-14 days | Fever, headache, malaise, | Attenuated strain Ty21a | Ciprofloxacin, 20 mg/kg/day |
| Serovar typhi | South East Asia, Oceania | drinking water | abdominal pain, diarrhea | typhoid vaccine; Vi capsular | for 7 days; or Azithromycin, | ||
| polysacchride typhoid vaccine; | 20 mg/kg/day for 7 days | ||||||
| Nontyphoidal | Poutry, eggs, meat | 8-24 hr | Killed whole-cell typhoid vaccine | ||||
Note: All figures were obtained from public domain. †ACT = Artemisinin-based Combination Therapy.
3. International Health Regulations (IHR)
The 2014 outbreak of Ebola once again tested the revised 2005 IHR. According to Gostin and Friedman (2015) “WHO fell short of its leadership responsibilities, and the IHR – the governing legal framework – displayed deficiencies”.3 The three West African countries involved (Guinea, Liberia, Sierra Leone) in the pandemic failed to comply with the IHRs capacity-building mandate and, to date, two thirds of WHA member countries have failed to comply with the same regulations.3, 4 Of the one third of the WHA member nations that said they did comply, there has been no evaluation to verify their claims.3, 4 Like the outbreak of H1N1 in 2009, the response raises questions regarding the extent to which the IHR can serve as a framework for global pandemic responses.3, 4, 18
If the WHA member nations (194) do not take the IHR core capacity-building requirements of disease surveillance, reporting, and response seriously, then why continue to use them as an international framework? In reality WHA member nations from LMICs see the regulations as an enormous obligation primarily developed to protect the health and welfare of developed nations.3, 18 During the Ebola outbreak, controversy arose when American and Spanish nationals were preferentially chosen to receive the experimental drug ZMapp over West African nationals.19 Moreover, when foreign medical staff became infected they were flown home for what was deemed superior medical care. Clearly these ethical issues, which are well known by the WHA member representatives, will impact on future IHR compliance. Furthermore, member nations from LMICs do not have the national capacity to adhere to IHR, given they have very weak infrastructure and poorly financed health systems.3 LMICs must be given considerable financial and capacity building assistance or they will be unable to comply. These massive inequities must be addressed if we are to plan appropriately for the next pandemic.
4. Global Health System Strengthening
For most countries in the developing world it is difficult to improve their health systems to a standard that is similar to that of high-income countries. Moreover, as mentioned, most LMIC countries will not be able to establish core IHR capabilities without considerable donor support and international assistance for training, creating the necessary laboratory infrastructure for prompt diagnosis, and the technology required for ‘real-time’ reporting of epidemics.20 Point of care screening tests for use in community health posts are increasingly available for rapid diagnosis of emerging pathogens and will shorten the time from presentation to treatment. However improvements and access to diagnostic technologies will need to be supported by the capacity to interpret and act on the findings. Presently limited health-care dollars are spent on running tertiary national hospitals with little, or none, spent on preventive services, disease control or epidemic preparedness. However, most countries do have offices or departments for communicable disease control with the number of staff engaged in such full-time activities varying considerably. At the district/municipal level most developing countries have medical health officers and at the community level a considerable human resource of community health workers (CHWs).
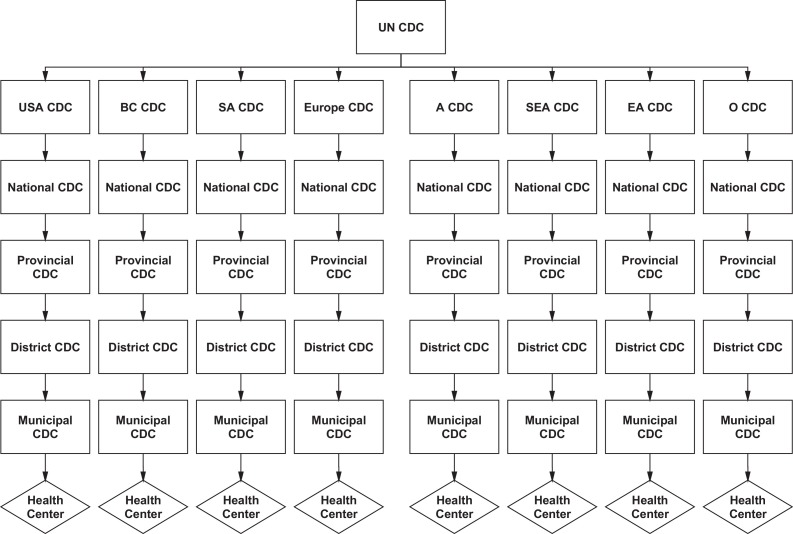
Gostin and Friedman (2015) have proposed a new global health framework with robust national health systems at its foundation and an empowered WHO at its apex.3 However, WHO has failed to provide the necessary leadership to coordinate global health emergencies on the ground and adequately support WHA member nations to develop core IHR capacities. In September 2014, the UN assumed leadership of the Ebola response and created the UN Mission for Emergency Ebola Response (UNMEER), the first UN mission to respond to public health emergencies.3 In contrast with IHR recommendations, Security Council resolutions are legally binding for member countries.3 We now propose a new UN Centre for Disease Control (UN CDC), potentially based in New York, to serve at the apex of a new global health framework with a number of new and existing regional CDCs reporting directly to it (Figure 3 ). A proposed structure might be: National CDC departments reporting to their regional CDCs, and Provincial/District/Municipal CDC departments reporting to their National CDCs with Community Health Workers at local health centres reporting to their municipal health officers. In sum, at the apex of our proposed global health framework would sit a new UN CDC with Security Council authority and at the foundation, CHWs in local health centres. CHWs have transformed the health-care systems of many developing nations including Bangladesh, India, Ethiopia, and Malawi and are absolutely crucial for future global security.21
Figure 3.
A proposed global health security hierarchy: the UN Center for Disease Control (UN CDC) will serve as the command headquarters for global health security and pandemic response. The regional CDCs in South America (SA CDC), Stockholm, Europe (European CDC), Ethiopia, Africa (A CDC), Southeast Asia (SEA CDC), East Asia (EA CDC), and Oceania (O CDC) will report directly to the UN CDC along with the USA CDC and the BC CDC in Canada. National CDC departments will report to their regional CDCs, and Provincial, District, and Municipal CDC departments will report to the National CDC. All health centers at the local village level will report to their municipal/district offices.
5. Global Pandemic Funding
On October 10th 2014, World Bank President, Dr Jim Yong Kim, has proposed a new pandemic emergency facility (PEF).22 As stated on their website “The World Bank Group is playing a lead role in conceptualizing the facility, working in coordination with international organizations, including the WHO, the private sector and other development partners. PEF is a global financing facility that would channel funds swiftly to governments, multilateral agencies, NGOs and others, to finance efforts to contain dangerous epidemic outbreaks before they turn into pandemics. Financing from the PEF will be linked to strong country-level epidemic and pandemic emergency preparedness plans, thereby incentivizing recipient governments and the international community to introduce greater rigor and discipline into crisis preparedness and reduce the potential for moral hazard. The PEF is expected to cover a range of response activities such as: (i) rapid deployment of a trained and ready health care work force; (ii) medical equipment, pharmaceuticals and diagnostic supplies; (iii) logistics and food supplies; and (iv) coordination and communication. The PEF would not cover pandemic preparedness or reconstruction efforts. A total of $1 billion is available for all of the 77 poorest countries through June 2017.”22
If the WHO contingency fund (100 million US dollars) and the World Bank pandemic emergency facility cannot be utilised to strengthen national health systems in LMICs in order to meet IHRs core capabilities, then how can this be achieved? A multi-billion US dollar International Health System fund has been proposed3 but considerable funding from both the private and public sector will need to be secured if the fund is to be successfully launched. The G20, the European Union, and philanthropic organizations will need to contribute. The implementation and monitoring of such funds at the national level will have to be carefully scrutinised and audited if the core capacities of the IHRs are to be achieved and maintained. Ultimately LMIC nations themselves will need to allocate health care dollars toward health prevention and epidemic planning. For many LMICs this is not a priority and they are ill prepared to respond to epidemics on their own soils. Building national capacity is the rate limiting step for global health security. If the international community fails to support this capacity-building initiative then this puts the world in a precarious situation with regard to future pandemics.
6. Conclusions
It is well known in management circles that ‘if one fails to plan then one should plan to fail’. With regard to pandemic planning, if we fail to build national epidemic capacities in LMICs then we should plan to deal with a global pandemic in the not too distant future. However, in order to build such national capacity it will take considerable international political will that at the moment seems to be lacking. Instead of allocating huge resources that ‘react’ to pandemics, funds must be earmarked to ‘prevent’ pandemics. This would include building national capacities of LMICs and smart surveillance of EIDs in identified hotspots in the tropical and subtropical world. What are the likely organisms to cause a future pandemic and where will they originate from? Zoonosis from wildlife represents the most significant global health threat of our time yet little funds are spent monitoring and identifying new zoonotic pathogens originating in wildlife. Clearly a ‘One Health’ approach is the way forward.
Acknowledgments: We thank the Australian National Health and Medical Research Council for providing financial support for our research. Professor Crowe would like to acknowledge the Victorian Operational Infrastructure Support Program received by the Burnet Institute.
Financial support: Our work is supported by the Australian National Health and Medical Research Council.
Potential conflicts of interest: All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential.
Conflicts of Interes:. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
References
- 1.Fineberg H.V. Pandemic preparedness and response- lessons from the H1N1 influenza of 2009. NEJM. 2014;370:13351342. doi: 10.1056/NEJMra1208802. [DOI] [PubMed] [Google Scholar]
- 2.WHO Ebola Situation Report – 10 June 2015. http://apps.who.int/ebola/en/current-situation/ebola-situation-report-10-june-2015.
- 3.Gostin L.O., Friedman E.A. A retrospective and prospective analysis of the West African Ebola virus disease epidemic: robust national health systems at the foundation and an empowerd WHO at the apex. Lancet. 2015;385:1902–1909. doi: 10.1016/S0140-6736(15)60644-4. [DOI] [PubMed] [Google Scholar]
- 4.Panel of independent experts. Report of the Ebola Interim Assessment Panel. WHO reference number: A68/25 http://www.who.int/csr/resources/publications/ebola/ebola-interim-assessment/en/.
- 5.Ross A.G., Olveda R.M., Li Y.S. Are we ready for a global pandemic of Ebola virus? International Journal Infectious Diseases. 2014;28:217–218. doi: 10.1016/j.ijid.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Ross A.G., Li Y.S., Olveda R.M. An audacious goal: the elimination of schistosomiasis in our lifetime through mass drug administration. Lancet. 2015;385(9983):2220–2221. doi: 10.1016/S0140-6736(14)61417-3. [DOI] [PubMed] [Google Scholar]
- 7.Vogel G. Bat-filled tree may have been ground zero for the Ebola epidemic. Science. 2014 http://news.sciencemag.org/africa/2014/12/bat-filled-tree-may-have-been-ground-zero-ebola-epidemic [Google Scholar]
- 8.Bausch D.G., Schwarz L. Outbreak of Ebola Virus Disease in Guinea: Where Ecology Meets Economy. Plos Neglected Tropical Diseases. 2014;8:1–5. doi: 10.1371/journal.pntd.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alirol E., Getaz L., Stoll B., Chappuis F., Loutan L. Urbanisation and infectious diseases in a global world. Lancet Infectious Diseases. 2010;10:131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernet G., Mary C., Altmann D.N., Doumbo O., Morpeth S., Bhutta Z.A., Klugman K.P. Surveillance for Antimicrobial Drug Resistance in Under-Resourced Countries. Emerging Infectious Diseases. 2014;20:434–441. doi: 10.3201/eid2003.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross A.G., Olds G.R., Farrar J., Cripps A.W., McManus D.P. Enteropathogens and chronic illness in returning travellers. NEJM. 2013;368:1817–1825. doi: 10.1056/NEJMra1207777. [DOI] [PubMed] [Google Scholar]
- 13.Childs J.E., Gordon E.R. Surveillance and control of zoonotic agents prior to disease detection in humans. Mount Sinai Journal of Medicine. 2009;76:421–428. doi: 10.1002/msj.20133. [DOI] [PubMed] [Google Scholar]
- 14.Liang G., Gao X., Gould E.A. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerging Microbes and Infections. 2015;4:1–5. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karesh W.B., Dobson A., Lloyd-Smith J.A., Lubroth J., Dixon M.A., Bennett M. Ecology of zoonoses: natural and unnatural histories. Lancet. 2012;380:19361944. doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse S.S., Mazet J.A., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B., Zambrana-Torrelio C., Lipkin W.I., Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasler B., Gilbert W., jones B.A., Pfeiffer D.U., Rushton J., Otte M.J. The economic value of One Health in relation to the mitigation of zoonotic disease risks. Current Topics in Microbiology and Immunology. 2012;365:127–151. doi: 10.1007/82_2012_239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz R., Fischer J. The revised international health regulations: a framework for global pandemic response. Global Health Governance. 2010;III(2) http://www.ghgj.org [Google Scholar]
- 19.Petherick A. Ebola in West Africa: learning the lessons. Lancet. 2015;385:591–592. doi: 10.1016/S0140-6736(15)60075-7. [DOI] [PubMed] [Google Scholar]
- 20.Heymann D.L., Chen L., Takemi K., Fidler D.P., Tapperp J.W., Thomas M.J. Global health security: the wider lessons from the West African Ebola virus disease epidemic. Lancet. 2015;385:1184–1901. doi: 10.1016/S0140-6736(15)60858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn S.C., Kumar S. Health inequalities and infectious disease epidemics: a challenge for global health security. Biosecur Bioterror. 2014;12:263–273. doi: 10.1089/bsp.2014.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The World Bank. Global Pandemic Emergency Facility. http://www.worldbank.org/en/news/press-release/2014/10/10/world-bank-group-president-calls-new-global-pandemic-emergency-facility.