Abstract
Clin Microbiol Infect 2012; 18: 74–80
Abstract
Nasopharyngeal aspirates were collected from 813 children ≤14 years old with acute lower respiratory tract infections in Lanzhou, China, from December 2006 to November 2009. PCR or RT‐PCR was used to screen for the presence of 10 respiratory viruses. Viral agents were identified in 73.92% (601/813) of specimens, including RSV in 40.71%, hMPV in 6.15%, IFVA in 7.13%, IFVB in 0.98%, PIV1‐3 in 7.87%, HCoV‐HKU1 in 2.21%, HCoV‐NL63 in 3.81%, HRV in 19.93%, AdV in 7.50% and HBoV in 11.56%. Two or more viruses were detected in 34.44% (280/813) of cases. The newly identified respiratory viruses, HBoV, hMPV, HCoV‐HKU1 and HCoV‐NL63, accounted for 22.01% of the detected viral pathogens. RSV and HRV were frequently detected in patients with bronchiolitis, and hMPV was frequently associated with pneumonia. HCoV‐NL63 was found to be one of the causative agents of acute respiratory wheezing in young children. No seasonal variation was found in the incidence of detection of HCoV‐HKU1, HCoV‐NL63 or HBoV. This 3‐year study demonstrated that viral pathogens play an important role in children with ALRTIs, and more attention should be paid to these newly identified viral agents.
Keywords: Acute lower respiratory tract infection, child, China, respiratory viruses
Introduction
Acute lower respiratory tract infections (ALRTIs) are the leading cause of morbidity and mortality in children worldwide, with the greatest number of deaths occurring in developing countries [1]. In China, pneumonia is the leading cause of death in children under 5 years old (>30 000 per annum [2]). Viruses account for 30–90% of ALRTIs in young children [3, 4]. Respiratory syncytial virus (RSV), parainfluenza viruses (PIV1‐3) and influenza viruses A (IFVA) and B (IFVB) are the most common viral agents responsible, being detected in 63.1% of Chinese children hospitalized with acute respiratory infections [5]. However, many new respiratory viruses have recently been identified as causative agents of lower respiratory illnesses in children (e.g. human coronaviruses (HCoV)–NL63 and HCoV–HKU1, human metapneumovirus (hMPV) and human bocavirus (HBoV)) [6, 7, 8]. Moreover, rhinovirus (HRV), which has generally been considered a cause of mild upper respiratory illnesses in children and adults, is now considered to be a major cause of ALRTIs and asthmatic exacerbations [9, 10]. However, these viral agents of ALRTIs are rarely investigated or reported in China. To further understand the epidemiology of ALRTIs in children, we conducted a 3‐year viral aetiology study to assess the contribution of 10 respiratory viruses to childhood ALRTIs in Lanzhou City, China.
Materials and Methods
Study participants and samples
From December 2006 to November 2009, nasopharyngeal aspirates (NPAs) were collected from 813 children <14 years of age who had been admitted to the First Hospital of Lanzhou University with ALRTIs on one fixed day (Tuesday) per week. ALRTIs were classified according to World Health Organization definitions. Informed consent was obtained from the subjects’ parents/guardians. The subjects’ age distribution was 10 days to 14 years (22.58 ± 28.20 months; mean ± SD), the male:female ratio was 1.9:1, and the outpatient:inpatient ratio was 1:3. The study protocol was approved by the hospital ethics committee. NPAs collected from each subject were transported immediately to the laboratory at the National Institute for Viral Disease Control and Prevention, China Centre for Disease Control, and stored at −80°C until further processing.
RNA and DNA extraction
Viral DNA and RNA were extracted from 140 μL of each NPA specimen using the QIAamp viral DNA and QIAamp Viral RNA Mini kits (Qiagen, Shanghai, China) according to the manufacturer’s instructions. cDNA was synthesized using random hexamer primers with Superscript II RH‐reverse transcriptase (Invitrogen, Carlsbad, CA, USA).
Viral detection
Identification of RSV, HRV, hMPV, IFVA, IFVB, HCoV‐HKU1, HCoV‐NL63 and PIV1‐3 viruses was performed by RT‐PCR using eight sets of primers [11, 12]. Two sets of primers were used to amplify AdV and HBoV DNA by PCR [13, 14].
Statistical analysis
The statistical significance of differences between groups was compared using the chi‐square or Fisher’s exact test; p <0.05 was considered significant.
Results
Respiratory virus prevalence
Of a total of 813 NPAs collected from December 2006 to November 2009, 601(73.92%) were positive for at least one respiratory virus (detection rates of 71.25%, 76.49% and 62.82% in the 2006–2007, 2007–2008 and 2008–2009 seasons, respectively). In total, 877 positive PCR results were observed. Co‐infection with at least two types of virus was detected in 280 (46.59%) samples. The most frequently detected virus was RSV (40.71%), followed by HRV (19.93%), HBoV (11.56%), PIV1‐3 (7.87%), AdV (7.50%), IFVA (7.13%), HMPV (6.15%), NL63 (3.81%), HKU1 (2.21%) and IFVB (0.98%).
Patient characteristics
The male:female ratio of positive specimens was 1.8:1, but this difference was not significant (p 0.375; chi‐square test). No significant effect of sex on the likelihood of isolation of any of the seven viruses assayed was detected (Table 1; p 0.284; chi‐square test). The frequencies of virus detection in each of the six age groups were 60.71%, 77.08%, 80.43%, 76.62%, 76.70% and 61.94%, respectively (Table 2; p 0.001; chi‐square test). The highest frequency of viral isolation occurred in subjects 7–12 months of age. Significant differences in the rate of RSV infection among the six age groups were detected (p 0.000; chi‐square test). However, equivalent differences for AdV, HRV, HMPV, PIV3, IFVA, IFVB, HKU1, NL63 and HBoV did not reach significance. RSV‐associated ALTRIs predominated in children <1 year old (Table 3).
Table 1.
Comparison of the characteristics of patients mono‐infected with one of the six viruses
| Characteristic | RSV (n = 153) | hMPV (n = 19) | HRV (n = 59) | PIV1‐3 (n = 20) | IFVA (n = 14) | AdV (n = 23) | HBoV (n = 19) | p |
|---|---|---|---|---|---|---|---|---|
| No. (%) of patients with coinfectiona | 178 (55.78%) | 31 (62%) | 103 (63.59%) | 44 (68.75%) | 44 (75.86%) | 38 (62.29%) | 75 (79.79%) | <0.01b,c |
| Age (months) | ||||||||
| Median (range) | 9 (0.03–144) | 11 (0.58–108) | 8 (0.2–132) | 9.5 (0.6–96) | 9 (0.03–168) | 18 (0.03–108) | 14.5 (0.1–108) | |
| Sex | ||||||||
| Male:female | 94:59 | 13:6 | 33:26 | 12:8 | 7:7 | 19:4 | 14:5 | >0.01c |
| Clinical diagnosis | ||||||||
| Pneumonia | 77 (50.3) | 13 (68.4) | 38 (61.0) | 8 (40) | 11 (78.6) | 18 (78.3) | 10 (52.6) | |
| Bronchitis | 8 (5.2) | 1 (5.3) | 1 (1.7) | 4 (20) | 3 (21.4) | 1 (4.3) | 4 (21.1) | |
| Bronchiolitis | 68 (44.4) | 5 (26.3) | 20 (33.9) | 8 (40) | 0 (0) | 4 (17.4) | 5 (26.3) | |
aEpisodes of coinfection with >1 virus were excluded from the analysis of demographic and clinical diagnosis.
bCoinfection rates of HBoV and IFVA were higher than those of RSV.
cCalculated using the Chi‐square test.
Table 2.
Number of positive specimens in different age groups
| Age, months | ||||||
|---|---|---|---|---|---|---|
| 0–1 | −6 | −12 | −36 | −60 | >60 | |
| Specimens tested | 112 | 192 | 138 | 201 | 103 | 67 |
| No. of positive specimens | 68 | 148 | 111 | 154 | 79 | 41 |
| % positive | 60.71 | 77.08 | 80.43 | 76.6 2 | 76.70 | 61.94 |
Table 3.
Frequency of virus isolation in children of different ages with ALRTIs
| Age, months | Total | p value | ||||||
|---|---|---|---|---|---|---|---|---|
| −1 (%) | −6 (%) | −12 (%) | −36 (%) | −60 (%) | >60 (%) | |||
| (n = 112) | (n = 192) | (n = 138) | (n = 201) | (n = 103) | (n = 67) | |||
| RSV | 27 (24.1) | 101(52.6) | 69 (50) | 79 (39.3) | 42 (40.8) | 13 (19.4) | 331 | 0.000 |
| HRV | 18 (16.1) | 52 (27.1) | 27 (19.6) | 39 (19.4) | 13 (12.6) | 13 (19.4) | 162 | 0.057 |
| HMPV | 2 (1.8) | 14 (7.3) | 10 (7.2) | 10 (5.0) | 10 (9.7) | 4 (6.0) | 50 | 0.207 |
| PIV3 | 4 (3.6) | 18 (9.4) | 13 (9.4) | 18 (9.0) | 7 (6.8) | 4 (6.0) | 64 | 0.449 |
| IFVA | 9 (8.0) | 14 (7.3) | 9 (6.5) | 17 (8.5) | 5 (4.9) | 4 (6.0) | 58 | 0.889 |
| IFVB | 0 | 1 (0.5) | 1 (0.7) | 3 (1.5) | 2 (1.9) | 1 (1.5) | 8 | 0.652 |
| HKU1 | 3 (2.7) | 4 (2.1) | 4 (2.9) | 4 (2.0) | 0 | 3 (4.5) | 18 | 0.495 |
| NL63 | 0 | 7 (3.6) | 6 (4.3) | 11 (5.5) | 3 (2.9) | 4 (6.0) | 31 | 0.210 |
| AdV | 4 (3.6) | 9 (4.7) | 11 (8.0) | 18 (9.0) | 12 (11.7) | 7 (10.4) | 61 | 0.120 |
| HBoV | 10 (8.9) | 16 (8.3) | 18 (13.0) | 28 (13.9) | 16 (15.5) | 6 (9.0) | 94 | 0.292 |
| Total | 77 | 236 | 168 | 227 | 110 | 59 | 877 | |
Seasonal distribution
The seasonal distribution of respiratory virus detection is shown in Fig. 1. During the study period, RSV, hMPV, PIV and HRV showed a fixed seasonal rhythm: the majority of those viruses were detected from November to April. IFVA also exhibited seasonal variation and reached a peak from December 2008 to February 2009. Additionally, IFVA was not detected after April 2009, a time when an H1N1 pandemic was occurring. Detection of IFVB was temporally scattered, being isolated only during April and December 2007, March 2008 and February 2009. Coronavirus HKU1 was also mainly detected during the winter/spring months, with a peak in January 2008. HCoV‐Nl63 did not exhibit any noticeable seasonal rhythm and was not detected during 2008; peak detection occurred during July and October 2009. Notably, HBoV was identified throughout each year of the study period, but exhibited a complex prevalence pattern. No seasonal fluctuation in HBoV prevalence was apparent from December 2006 to November 2008. However, a peak (56% of the total isolates) was reached between December 2008 and April 2009; after that, HBoV was not detected until November 2009. AdV was detected with a relatively constant frequency throughout the study period, although no clear seasonal distribution could be identified.
Figure 1.
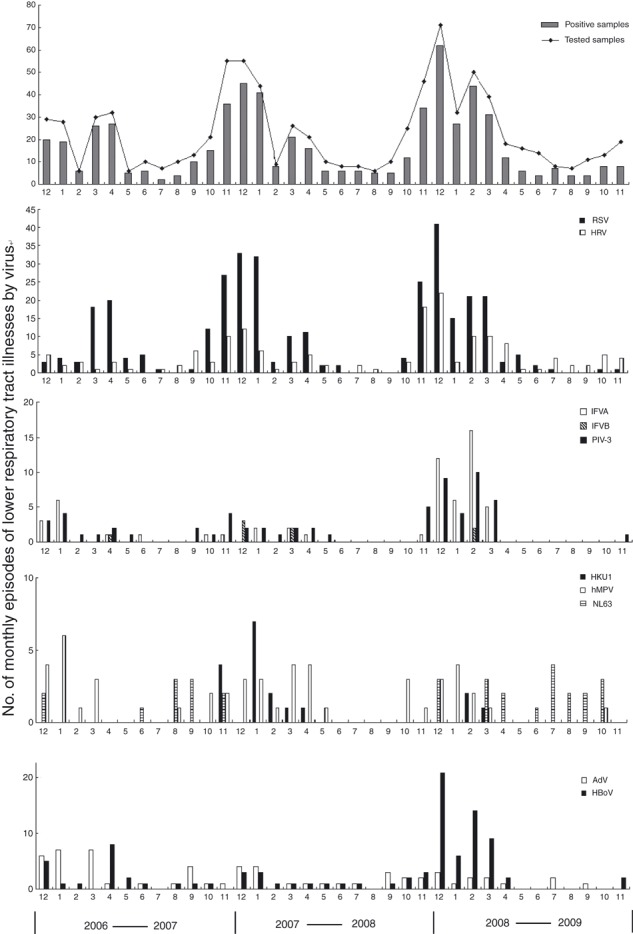
Monthly occurrence of ALRTIs associated with 10 respiratory viruses isolated from children between 2006 and 2009.
Clinical characteristics and co‐infections
The clinical characteristics of 307 subjects in whom only one virus was detected are summarized in Table 1 (HKU1, NL63 and IFVB were excluded from the analysis of demographic and clinical characteristics because fewer than five subjects in each group were infected with only one of these viruses). The rate of co‐infection (detection of more than one type of virus in a single subject) among the six virus types exhibited marked differences. HBoV showed the highest co‐infection rate; 79.8% (75/94) of HBoV‐positive samples contained at least one other virus, most commonly RSV (37 cases), followed by HRV (21), ADV (12), IFVA (12), PIV3 (10), HMPV (4), HKU1 (3) and NL63 (2). IFVA was more frequently detected in patients with mixed viral infections (44/58 IFVA‐positive samples) than were the other viruses, with the exception of HBoV.
Of the 49 HCoV‐HKU1 and HCoV‐NL63‐positive samples, 36 (73.47%) were co‐infected with at least one other virus, most commonly HRV (14 cases) and RSV (11). Thirty‐one of 50 (62%) hMPV‐positive samples were co‐infected, most frequently with RSV (data not shown).
Subjects who were positive for RSV, HRV, PIV1‐3 and AdV frequently suffered from pneumonia. RSV and HRV were frequently detected in patients who were diagnosed with bronchiolitis. Of the 31 NL63‐positive subjects (data not shown), 10 (32.26%) had experienced wheezing. The difference in the frequency of wheezing between subjects infected with NL63 and RSV (35.95%) was not significant (chi‐square test; p 0.685).
Discussion
ALRTIs are one of the most common paediatric diseases; indeed, pneumonia is the main cause of death in children under 5 years of age in developing countries [15]. In China, 24.5–65.2% of hospitalized paediatric patients are admitted due to an ALRTI [16]. In this study, we evaluated the overall prevalence of 10 respiratory viruses identified by PCR or RT‐PCR in children with ALRTIs from 2006 to 2009. Of the 813 samples collected, respiratory viruses were detected in 601 (73.92%), which is in agreement with other reports suggesting that 30–90% of ALRTs have a viral aetiology [3, 4, 17]. However, the rate of respiratory virus detection in our study was higher than in some other reports [5, 18], which may have been due to differences in detection methods. Direct immunoflourescence and viral culture are the most often applied methods; in contrast, we used RT‐PCR and PCR, which are highly sensitive [18]. Another possibility is attempted detection of different viruses. Ten respiratory viruses were included in this study, including the novel viruses HBoV, hMPV, HCoV‐HKU1 and HCoV‐NL63. Note that seasonal differences in the rates of detection of respiratory viruses correlated with the occurrence of ALRTI epidemics (Fig. 1).
RSV was the most frequently detected respiratory virus in children with ALRTIs in our study (40.71% of the ALRTIs and 37.74% of total viral pathogens), in agreement with previous reports [19, 20]. HRV has generally been considered a cause of mild upper respiratory illnesses in children and adults. However, HRV was second only to RSV in this study and was detected in 19.93% of samples. This rate was similar to that reported by Juven et al. [21]. The annual incidence of hMPV‐associated ALRTIs was relatively stable during the study period. This is not consistent with previous reports, which suggested that hMPV‐associated ALRTIs vary annually [18, 22]. This suggests that hMPV may exhibit geographically variable circulation patterns. Detection rates of 0.7–8.8% for HCoV‐NL63 and 1.6–6% for HCoV‐HKU1 in children with acute respiratory tract infections have been reported [18, 23]. In this study, HCoV‐NL63 and HCoV‐HKU1 accounted for 5.66% of the total cases, which suggests that these viruses are important causes of ALRTIs in children in Lanzhou City. The co‐infection rate in HCoV‐NL63 and HCoV‐HKU1‐positive samples was 73.47% (36/49) in this study, which is in line with previous reports [23]. Since its discovery in 2005, HBoV has been frequently detected worldwide in NPAs, with incidences varying from 1.5% to 18.9% [24, 25, 26]. The present study showed a relatively high HBoV infection rate (11.56%) and a higher frequency of co‐infection (79.8%, 75/94) than the other viral agents; RSV and HRV were most frequently detected in conjunction with HBoV. Given this high co‐infection rate, the exact role of HBoV in ALRTIs needs to be investigated further [18, 27]. The newly identified respiratory viruses (HBoV, hMPV, HCoV‐NL63 and, HKU1) accounted for 22.01% of the total in this study. Thus these data suggest that viral pathogens are important causes of ALRTIs in Lanzhou City and that the novel respiratory viruses play an important role in children with ALRTIs. The rate of mixed infection, 46.59% (280/601), was significantly higher than in other reports (8.6–11.5%) [17, 19], suggesting marked geographical variation. As to whether the mixed infection compared with single infection with viruses may increase the severity of disease, there have been some conflicting data. In our study, due to limited clinical information, we can’t reach a conclusion about this issue. Additional study is needed to better define the role of mixed infection in the severity of disease.
RSV, hMPV, PIV1‐3 and HRV exhibited fixed seasonal epidemic patterns, but no seasonal variation was noted for AdV, which is in agreement with most previous reports [5, 18, 20]. Early reports suggested that epidemics of influenza occur in the winter months in temperate climates, whereas influenza may be seen year‐round in the tropics. In our study, influenza occurred most frequently from November to April. However, influenza virus incidence did not peak in 2009, the time of the H1N1 pandemic, suggesting that the H1NI epidemic did not spread to Lanzhou City prior to November 2009. Previous reports suggested that HBoV infection occurred predominantly during the winter season or that it increased in spring and summer [18, 28]. In our study, HBoV was identified throughout each of the first 2 years of the study period, without any noticeable seasonal fluctuation. However, detection of this virus peaked (56% of total isolates) between December 2008 and April 2009, and then it was not detected again until November 2009. This suggests that HBoV may exhibit a geographically variable prevalence pattern. HCoV‐NL63 and HCoV‐HKU1 have been in continuous circulation since they were first identified and cause numerous infections annually [23]. In our study, HCoV‐HKU1 was detected mainly during winter and spring, with a peak in January 2008. However, HCoV‐Nl63 did not exhibit any fixed seasonal rhythm; indeed this virus was not detected in 2008 and peaked during July and October 2009. HCoV‐OC43 and HCoV‐229E predominantly circulate during the winter and early spring with outbreaks occurring every 2–4 years [23]. Whether HCoV‐HKU1 and HCoV‐Nl63 infections follow a similar prevalence pattern is not clear. Thus, further study, and particularly long‐term surveillance, is needed to address these issues.
Subjects 7–12 months of age showed the highest frequency of viral detection. However, no significant differences in infection rates in the six age groups for each viral pathogen were detected, the sole exception being RSV (2, 3). RSV predominated in subjects <1 year of age. This differs from other reports [18], which suggested that RSV predominated among younger infants, but that AdV, hMPV and IFVA were more frequently detected in children over 24 months of age. Viral ALRTIs have been suggested to occur more frequently in boys [17, 29]. In this study, although the male:female ratio of positive specimens was 1.8:1, this was not significant (p 0.375; chi‐square test). Further investigation is needed to elucidate the precise effect of age and sex on the risk of viral infection in this patient group.
RSV and HRV were frequently detected in patients with bronchiolitis, and AdV and IFVA were common in subjects with pneumonia. HBoV was more frequently observed in those with pneumonia and bronchiolitis in this study, which suggests that HBoV is an important viral agent in children with ALRTIs, as reported elsewhere [8, 30]. HCoV‐NL63 has been suggested to be a causative agent of acute respiratory wheezing in young children [23]. In this study, HCoV‐NL63 patients also had a high frequency of wheezing (32.26%), which was not significantly different from that of RSV (35.95%). Our data suggested that hMPV was frequently associated with pneumonia, but other studies have suggested that hMPV is more often associated with bronchiolitis or wheezy bronchitis [6]. Furthermore, HRV is generally considered to be a common cause of mild upper respiratory illnesses in children. However, here, HRV was the most common viral pathogen in young patients with ALRTIs. More data are needed to address the exact clinical role of these newly identified respiratory viruses.
As ALRTIs cause a high disease burden in China, strengthening research into the pathogens that cause these infections is very important in developing new diagnostic and therapeutic tools. This 3‐year study demonstrated that at least three‐quarters of ALRTIs in children were associated with known viral pathogens. RSV and HRV were the two most common viruses detected. Novel viral agents, including HBoV, HCoV‐HKU1, HCoV‐NL63 and hMPV, accounted for a high proportion of ALRTIs. More attention should therefore be paid to these agents. Additional study is required to define the exact role and molecular and epidemiological characteristics of these newly identified viral agents in ALRTIs in different geographical regions.
Transparency Declaration
None of the authors has a conflict of interest.
Acknowledgements
This work was partly supported by the ‘973’ National Key Basic Research Program of China (Grant No.2007CB310500) and China Mega‐Project for Infectious Disease (2009ZX10004‐001 and 2009ZX10004‐101).
References
- 1. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2: 25–32. [DOI] [PubMed] [Google Scholar]
- 2. Mulholland K, Temple B. Causes of death in children younger than 5 years in China in 2008. Lancet 2010; 376: 89–90. [DOI] [PubMed] [Google Scholar]
- 3. Mulholland EK, Ogunlesi OO, Adegbola RA et al. Etiology of serious infections in young Gambian infants. Pediatr Infect Dis J 1999; 18: S35–S41. [DOI] [PubMed] [Google Scholar]
- 4. Manjarrez ME, Rosete DP, Rincon M, Villalba J, Cravioto A, Cabrera R. Comparative viral frequency in Mexican children under 5 years of age with and without upper respiratory symptoms. J Med Microbiol 2003; 52: 579–583. [DOI] [PubMed] [Google Scholar]
- 5. Zhang HY, Li ZM, Zhang GL, Diao TT, Cao CX, Sun HQ. Respiratory viruses in hospitalized children with acute lower respiratory tract infections in harbin, China. Jpn J Infect Dis 2009; 62: 458–460. [PubMed] [Google Scholar]
- 6. Williams JV, Harris PA, Tollefson SJ et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004; 350: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Hoek L, Pyrc K, Jebbink MF et al. Identification of a new human coronavirus. Nat Med 2004; 10: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fry AM, Lu X, Chittaganpitch M et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 2007; 195: 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calvo Rey C, Garcia Garcia ML, Casas Flecha I et al. Role of rhinovirus in respiratory tract infections in hospitalized children. An Pediatr (Barc) 2006; 65: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol 2005; 116: 267–273. [DOI] [PubMed] [Google Scholar]
- 11. Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL‐63 infections in children: a 1‐year study. J Clin Microbiol 2005; 43: 4567–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellau‐Pujol S, Vabret A, Legrand L et al. Development of three multiplex RT‐PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 2005; 126: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hierholzer JC, Halonen PE, Dahlen PO, Bingham PG, McDonough MM. Detection of adenovirus in clinical specimens by polymerase chain reaction and liquid‐phase hybridization quantitated by time‐resolved fluorometry. J Clin Microbiol 1993; 31: 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 2005; 102: 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis 1990; 12 (suppl 8): S870–S888. [DOI] [PubMed] [Google Scholar]
- 16. Min L, Mei Z, Quan L et al. The epidemiologic feature of viral infection in the children with acute lower respiratory tract infections in Shanghai. Chin J Infect Chemother 2005; 5: 152–155. [Google Scholar]
- 17. Yun BY, Kim MR, Park JY, Choi EH, Lee HJ, Yun CK. Viral etiology and epidemiology of acute lower respiratory tract infections in Korean children. Pediatr Infect Dis J 1995; 14: 1054–1059. [DOI] [PubMed] [Google Scholar]
- 18. Choi EH, Lee HJ, Kim SJ et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis 2006; 43: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. J Infect 2000; 41: 152–158. [DOI] [PubMed] [Google Scholar]
- 20. Lin TY, Huang YC, Ning HC, Tsao KC. Surveillance of respiratory viral infections among pediatric outpatients in northern Taiwan. J Clin Virol 2004; 30: 81–85. [DOI] [PubMed] [Google Scholar]
- 21. Juven T, Mertsola J, Waris M et al. Etiology of community‐acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 2000; 19: 293–298. [DOI] [PubMed] [Google Scholar]
- 22. Williams JV, Wang CK, Yang CF et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20‐year experience. J Infect Dis 2006; 193: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Principi N, Bosis S, Esposito S. Effects of coronavirus infections in children. Emerg Infect Dis 2010; 16: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allander T, Jartti T, Gupta S et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastien N, Brandt K, Dust K, Ward D, Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis 2006; 12: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng LS, Yuan XH, Xie ZP et al. Human bocavirus infection in young children with acute respiratory tract infection in Lanzhou, China. J Med Virol 2010; 82: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol 2006; 35: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children’s hospital. Clin Infect Dis 2006; 43: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr 1986; 108: 635–646. [DOI] [PubMed] [Google Scholar]
- 30. Ma X, Endo R, Ishiguro N et al. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol 2006; 44: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]


