Highlights
-
•
An exacerbated activation of IL-33/ST2 axis was associated with severity of viral ALRI.
-
•
Detectable NPA IL-33 and sST-2 levels were risk factors for mechanical ventilation.
-
•
An increment in NPA sST2 levels was associated with longer hospital length of stay.
-
•
A systemic increase in IL-33 concentrations was associated with milder disease.
Keywords: Acute lower respiratory infection, Inflammatory cytokines, Interleukin 33, Soluble suppression of tumorigenicity 2, Respiratory viruses
Abstract
Background
Mechanisms influencing severity of acute lower respiratory infection (ALRI) in children are not established. We aimed to assess the role of inflammatory markers and respiratory viruses in ALRI severity.
Methods
Concentrations of interleukin(IL)-33, soluble suppression of tumorigenicity (sST)2, IL-1ß, tumor necrosis factor α, IL-4, IL-6 and IL- 8 and types of respiratory viruses were evaluated in children at the first and fifth days after hospital admission. Disease severity was defined as need for mechanical ventilation.
Results
Seventy-nine children <5 years-old were included; 33(41.8%) received mechanical ventilation. No associations between virus type, viral load or co-detections and severity of disease were observed. Detection of IL-33 and sST2 in nasopharyngeal aspirates (NPA) on admission were associated with higher risk for mechanical ventilation (RR = 2.89 and RR = 4.57, respectively). IL-6 and IL-8 concentrations were higher on Day 5 in mechanically ventilated children. IL-6 NPA concentrations decreased from Day 1 to Day 5 in children who did not receive mechanical ventilation. Increase in sST2 NPA concentrations from Day 1 to Day 5 was associated with longer hospital length of stay (p < 0.01).
Conclusions
An exacerbated local activation of the IL-33/ST2 axis and persistently high sST2 concentrations over time were associated with severity of viral ALRI in children.
1. Introduction
Acute lower respiratory infection (ALRI) is an important cause of morbidity and mortality in children under 5 years old worldwide [1], [2], [3], [4], [5]. Respiratory viruses represent key pathogens involved in ALRI and can cause pneumonia and acute viral bronchiolitis in infants and young children [1], [2], [3], [4], [5]. While young age, prematurity and co-morbidities have been identified as predictors of poor outcomes [6], [7], [8], [9], the role of virus types and host inflammatory responses in disease severity is not well defined, as published results are inconsistent [10], [11]. Lately, a model of immune inflammatory response in which an imbalance between T helper (Th)1, Th2 and regulatory T cells could be responsible for severe cases of ALRI in infants and young children has been suggested [10], [12], [13]. In addition, Interleukin (IL)-33, a member of the interleukin-1 family expressed in epithelium surfaces, along with its receptor, suppression of tumorigenicity (ST)2, may play an important role in the immune response associated with asthma, autoimmune disorders and infections [14], [15]. There is experimental and clinical evidence showing the involvement of the IL-33/ST2 axis in the inflammatory responses to viruses, including respiratory syncytial virus (RSV) [16], [17], influenza [18], rhinovirus [19] and metapneumovirus [20], [21].
In light of recent findings and considering the lack of efficient treatment options for viral respiratory diseases, understanding the complex interrelations between respiratory viruses and host immune responses becomes mandatory. The identification of predictors of disease severity may help to identify potential therapeutic targets. Thus, we aimed to evaluate the association between the IL-33/ST2 axis, along with other inflammatory markers (IL-1β, IL-4, IL-6, IL-8, Tumor Necrosis Factor (TNF)-α), and the severity of ALRI in young children. Patients were also assessed for viral etiology and its association with illness severity.
2. Patients and methods
This was a prospective cohort study conducted in a tertiary-care university hospital in Brazil. The study was approved by the Institutional Research Ethics Board (#17470/2014) and written informed consent was obtained from the patients’ parents. All consecutive children younger than five years attending the pediatric emergency room with ALRI who were admitted to hospital from January 1st, 2015 to December 31st, 2016 were eligible for the study. ALRI was defined by the presence of cough, tachypnea, respiratory distress with prolonged expiratory time, and wheezing or crackles on auscultation. Subjects were excluded if they had used systemic steroids for over 24 h within 4 weeks of presentation or had multiple comorbidities (more than two). Demographic, clinical and outcome data were collected from patients’ health records. Disease severity was defined as need for mechanical ventilation.
Nasopharyngeal aspirates (NPA) were obtained within 24 h of admission (Day 1) and on the fifth day (Day 5) of hospital stay. In case of hospital discharge before Day 5, the sample was collected just prior to it. NPA technique consisted of washing the nasal cavity with 3 mL of saline followed by sample collection into a mucus extractor. During the first year of the study, blood samples were also concomitantly drawn. The samples were processed, aliquoted and stored at −70 °C until analysis.
NPA were tested using real-time PCR for the presence of RSV A and B, rhinovirus, human metapneumovirus A and B, influenza virus A and B, and adenovirus, including quantification of viral load. Total RNA and DNA were extracted from 250 μL nasopharyngeal washes using TRIzol reagent (Life Technologies), following the manufacturer’s protocols. Reverse transcription was done with High Capacity Reverse cDNA transcription kit (Life Technologies, Carlsbad, CA, USA) using 1 ng of extracted RNA and random hexamers, following manufacturer’s instructions. Newly synthesized cDNA was subjected to TaqMan real-time PCR (qPCR) with specific primers and probes (Supplementary material 1) in a StepOnePlus Real-Time PCR System thermocycler (Applied Biosystems). The RNAse P gene was used as internal control for all samples. All real-time PCR assays were tested in duplicate. Reverse transcription, assembly of real-time PCR mixes, and cycling parameters were per a previously published protocol [22].
IL-33, IL-1β, IL-4, IL-6, IL-8, tumor necrosis factor (TNF)-α and the soluble receptor (sST2) were measured using a commercial enzyme-linked immunosorbent assay (ELISA; R&D systems) according to the manufacturers’ instructions. Detection limits for the assays were 0.186 pcg/ml (IL-33), 2.44 pcg/ml (sST2), 3.8 pcg/ml (IL-1β), 15.5 pcg/ml (TNF-α), 0.2 pcg/ml (IL-4), 3 pcg/ml (IL-6) and 31.1 pcg/ml (IL-8).
2.1. Statistical analysis
Analysis was made using SAS 9.4 software (SAS institute, Cary, NC). Data were expressed as median (range) or number (%). Patients were grouped according to need for mechanical ventilation. Continuous variables between groups were compared by Mann-Whitney U test and categorical variables, by Fisher’s exact test or chi-square test. Variables with repeated measures over time were analyzed after logarithmic transformation. Wilcoxon matched pairs test was used to compare concentrations of inflammatory markers at Day 1 and Day 5 in both groups. Relative risks were estimated through log-binomial regression models to compare incidences of mechanical ventilation according to the detection of IL-33 and sST2 in NPA on admission. Kaplan-Meyer estimates were calculated for hospital length of stay analysis. A Receiver Operating Characteristic (ROC) curve was constructed to assess the ability of viral loads to predict need for mechanical ventilation. For the latter, only the highest value of viral load for each individual was included. A 5% significance level was considered in all analysis.
3. Results
3.1. Study population
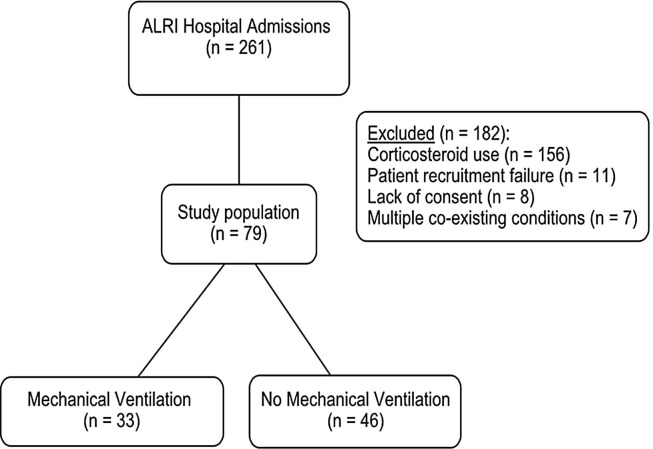
From January 1st 2015 to December 31st 2016, 261 children younger than five years were admitted to Hospital das Clínicas of Ribeirão Preto Medical School, University of São Paulo with ALRI. Seventy-nine children were included in the study. One hundred eighty two patients were excluded for the following reasons: corticosteroid use for more than 24 h in the previous 4 weeks before hospital admission (n = 156); recruitment failure (n = 11); lack of consent from parents or guardians (n = 8); multiple comorbidities (n = 7). Thirty-three patients (41.8%) received mechanical ventilation. Fig. 1 shows the flow diagram of the study.
Fig. 1.
Flow diagram of the study. ALRI, acute lower respiratory infection.
Demographic, clinical and outcome data are shown in Table 1 . Demographic data were not significantly different between groups. However, use of systemic antibiotics and the presence of atelectasis in chest radiograph were more frequent in patients who received mechanical ventilation. Moreover, duration of supplemental oxygen and hospital length of stay were longer in the mechanical ventilation group.
Table 1.
Demographic, clinical and outcome data.
| Characteristics | All (n = 79) |
Mechanical ventilation (n = 33) |
No mechanical ventilation (n = 46) |
p |
|---|---|---|---|---|
| Age (months) | 55 (10–1235) | 46 (11–1235) | 62.5 (10–651) | 0.67 |
| Weight (kg) | 4.35 (2.2–15.4) | 4.5 (2.2–15.4) | 4.4 (2.8–11) | 0.35 |
| Male gender | 48 (60.7) | 22 (66.6) | 26 (56.5) | 0.07 |
| Antibiotic use | 32 (40.5) | 24 (72.7) | 8 (17.3) | <0.0001 |
| Atelectasis in chest radiograph | 24 (30.3) | 19 (57.5) | 5 (10.8) | <0.0001 |
| White bloodcells count (/mm3) | 9900 (3100–29200) | 8500 (3100–18600) | 10,400 (5300–29200) | 0.009 |
| C-reactive protein (mg/dL) | 1.8 (0.05–10.3) | 2.11 (0.1–10.3) | 0.66 (0.05–8.2) | 0.14 |
| Comorbidities | 0.35 | |||
| Prematurity | 20 (25.3) | 13 (39.3) | 7 (15.2) | |
| Congenital heart disease | 1 (1.0) | 1 (3.0) | 0 (0) | |
| Immunosuppression | 4 (5.0) | 2 (6.0) | 2 (4.3) | |
| Cerebral palsy | 11 (13.9) | 4 (12.1) | 7 (15.2) | |
| Duration of supplemental oxygen (days) | 6 (0–248) | 11 (2–248) | 3 (0–40) | <0.0001 |
| Hospital length of stay (days) | 6 (2–248) | 14 (5–248) | 6 (2–41) | <0.0001 |
| Mortality | 1 (1.2) | 0 (0) | 1 (2.1) | 0.1 |
Data are expressed as median (range) or n (%). P values for comparison between mechanical ventilation and no mechanical ventilation groups.
3.2. Predictors of mechanical ventilation support
At least one virus was identified in the NPA of 72 (92.2%) study subjects. The most frequently detected virus was RSV (n = 60; 75.9%), followed by rhinovirus (n = 35; 31.6%), human metapneumovirus (n = 13; 16.5%), influenza (n = 5; 6.3%) and adenovirus (n = 4; 5.1%). Two or more viruses were detected in 41.7% of samples (Supplementary material 2). Neither the analysis of viral subtypes, nor the presence of co-detections reached statistical significance as risk factors for the need for mechanical ventilation support (Table 2 ).
Table 2.
Viruses detected in respiratory samples and risk for mechanical ventilation.
| Virus type | Mechanical ventilation (n = 33) |
No mechanical ventilation (n = 46) |
RR (95% CI) |
|---|---|---|---|
| RSV A | 27 (50.0) | 27 (50.0) | 2.08 (0.98–4.39) |
| RSV B | 5 (35.7) | 9 (62.3) | 0.82 (0.39–1.77) |
| Rhinovirus | 8 (32.0) | 17 (68.0) | 0.69 (0.36–1.31) |
| HMPV 1 | 2 (33.3) | 4 (66.7) | 0.78 (0.25–2.51) |
| HMPV 2 | 6 (66.7) | 3 (33.3) | 1.72 (0.79–2.99) |
| Influenza A | 3 (75.0) | 1 (25.0) | 1.87 (0.99–3.52) |
| Influenza B | 1 (100.0) | 0 (0) | |
| Adenovirus | 2 (50.0) | 2 (50.0) | 1.21 (0.44–3.34) |
| N of viruses detected | |||
| 1 virus | 15 (38.5) | 24 (61.5) | 1.35 (0.39–4.62) |
| 2 viruses | 10 (45.5) | 12 (54.5) | 1.59 (0.45–5.59) |
| 3 or 4 viruses | 6 (54.5) | 5 (45.5) | 1.91 (0.53–6.93) |
Data are expressed as number (%). RR, relative risk; CI, confidence interval. RSV, Respiratory Syncytial Virus; HMPV, Human Metapneumovirus.
Comparison of concentrations of inflammatory markers in NPA samples on the first and fifth days showed that there was no difference between groups in inflammatory markers concentrations on Day 1 (Supplementary material 3). However, IL-6 concentrations were 10.6 times higher and IL-8 concentrations were 1.4 times higher on the fifth day following hospital admission in the mechanical ventilation group (Table 3 ).
Table 3.
Comparison of concentrations (pg/ml) of interleukin (IL)-33, soluble suppression of tumorigenicity 2 (sST2) receptor, IL-1β, tumor necrosis factor (TNF)-α, IL-4, IL-6 and IL-8 in nasopharyngeal aspirates on the fifth day after hospital admission between mechanical ventilation and no mechanical ventilation groups.
| Marker | Mechanical ventilation (n = 31) | No mechanical ventilation (n = 38) | p |
|---|---|---|---|
| IL-33 | 0.186 (0.186–83.67) | 0.186 (0.186–793.3) | 0.21 |
| sST2 | 2.44 (2.44–1.105) | 2.44 (2.44–853.6) | 0.19 |
| IL-1ß | 182.1 (3.8–1.335) | 45.1 (3.8–2.195) | 0.53 |
| TNF-α | 15.5 (15.5–158.6) | 15.5 (15.5–640.5) | 0.27 |
| IL-4 | 6.9 (5.3–12.2) | 5.3 (5.3–11.1) | 0.60 |
| IL-6 | 152.6 (3–1885) | 14.4 (3–1287) | 0.001 |
| IL-8 | 1113 (31.1–4313) | 792.2 (31.1–4505) | 0.03 |
Data are expressed as median (range).
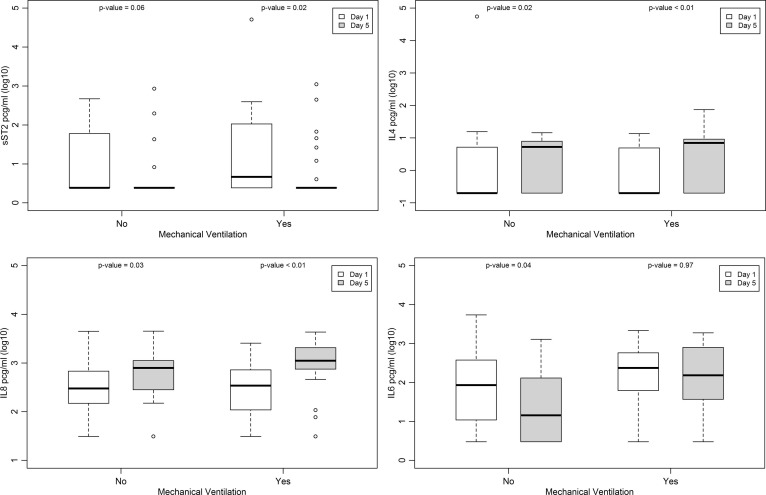
There was a significant increase in IL-4 and IL-8 NPA concentrations from Day 1 to Day 5 in both groups. Conversely, a significant reduction in sST2 NPA concentrations was observed in the mechanical ventilation group and there was a significant decrease in IL-6 NPA concentrations in children who did not receive mechanical ventilation from Day 1 to Day 5 (p < 0.05) (Fig. 2 ).
Fig. 2.
Concentrations of soluble suppression of tumorigenicity (sST)2 receptor, interleukin (IL)-4, IL-8 and IL-6 in nasopharyngeal aspirates on Day 1 and Day 5 following hospital admission in mechanical ventilation and no mechanical ventilation groups.
Blood samples were collected from 37 patients (Supplementary material 4). There was a significant increase in serum IL-33 concentrations in patients who did not receive mechanical ventilation from Day 1 (median 0.186 pcg/ml; range 0.186–840.4 pcg/ml) to Day 5 (median 28 pcg/ml; range 0.186–4253.8 pcg/ml) (p = 0.04).
3.3. Detectable levels of IL-33 and sST2 in NPA as predictors of mechanical ventilation support
On admission, patients who received mechanical ventilation had detectable NPA concentrations more frequently of both IL-33 (50% vs. 13.3%) and sST2 (87.5% vs. 40.9%) compared with patients who did not receive respiratory support, respectively (p < 0.001). Furthermore, detectable concentrations of both IL-33 and sST2 in NPA samples on admission were associated with higher risk for mechanical ventilation (RR = 2.89, 95%CI 1.83–4.57 and RR = 4.57, 95%CI 1.78–11.70, respectively). The detection of IL-33 in NPA had a sensitivity of 50% (95%CI 0.32–0.68) and specificity of 93% (95%CI 0.79–0.98) in predicting need for mechanical ventilation, with a positive likelihood ratio (LR) of 7.00 (95%CI 2.22–31.97) and a negative LR of 0.54 (95%CI 0.38–0.77). Detectable concentrations of sST2 showed sensitivity of 88% (95%CI 0.77–0.96) and specificity of 59% (95%CI 0.43–0.73) in predicting need for mechanical ventilation, with a positive LR of 2.14 (95%CI 1.46–3.12) and a negative LR of 0.21 (95%CI 0.08–0.55).
3.4. Course of sST2 concentrations and duration of hospital stay
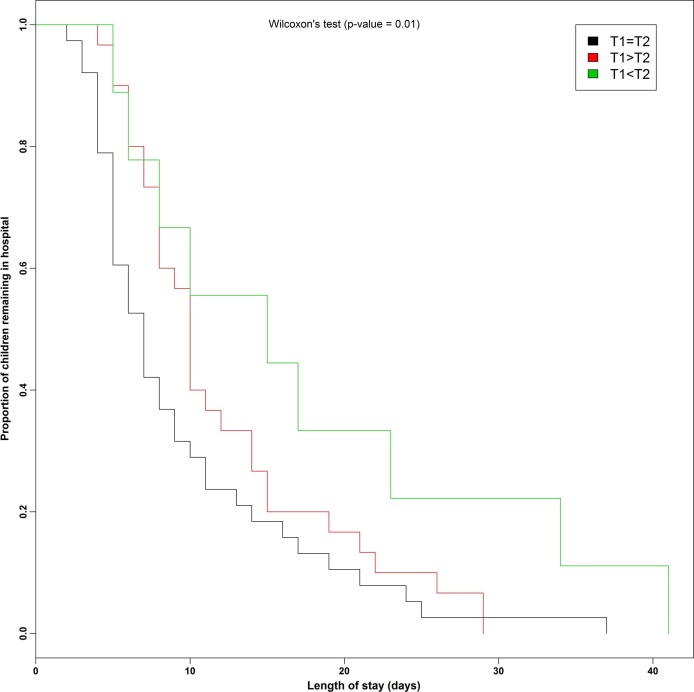
An increase in sST2 concentrations in NPA from Day 1 to Day 5 was associated with a longer hospital length of stay (p < 0.01) (Fig. 3 ).
Fig. 3.
Length of stay in hospital according to the course of soluble suppression of tumorigenicity (sST)2 concentrations in nasopharyngeal aspirates from Day 1 (T1) to Day 5 (T2).
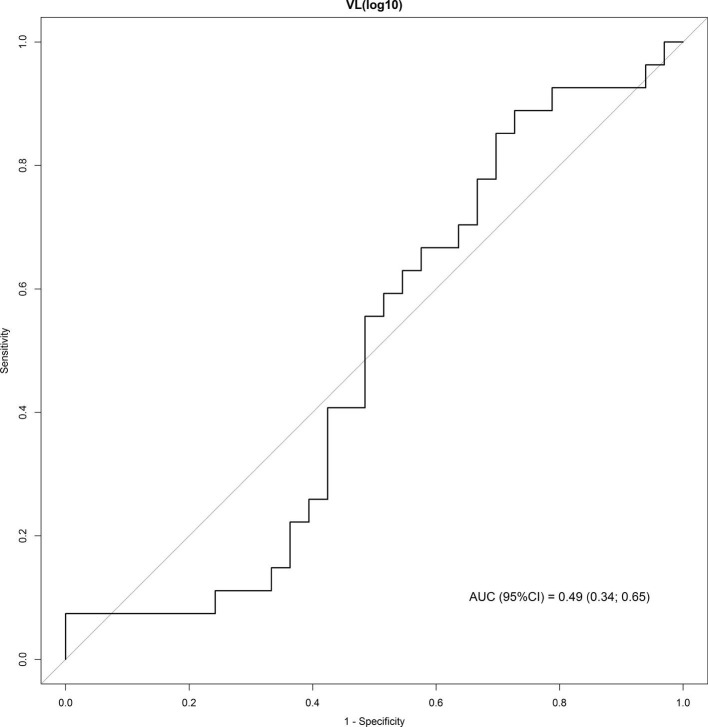
ROC curve analysis showed a poor discriminative performance of viral loads to predict need for mechanical ventilation (Supplementary material 5).
4. Discussion
To our knowledge, this is the first study to evaluate the IL-33/ST2 axis in pediatric patients with ALRI at two times during hospitalization along with other inflammatory cytokines and respiratory viruses, and their association with illness severity. Our results show an early and more prominent local activation of the IL-33/ST2 axis in children with more severe ALRI who needed ventilator support. Detectable concentrations of IL-33 and sST2 in NPA samples on the first day following hospital admission were associated with a higher risk for mechanical ventilation. Moreover, an increment in sST2 NPA concentrations from admission to hospital Day 5 was associated with a longer hospital length of stay.
In a recent review by Vázquez et al. [23], the authors highlight the importance of early identification of high-risk patients in the management of RSV disease in infants and young children, as treatment options are scarce. In this paper, IL-33 is mentioned as a potential biomarker of disease severity. However, few publications have addressed the IL-33/ST2 axis in viral respiratory diseases in children, with conflicting results. Similar to our findings, García-García et al. [13] reported higher detectable IL-33 NPA levels in infants infected by RSV and rhinovirus on hospital admission when comparing to healthy controls, although no associations between higher IL-33 detections and ICU admissions or longer hospital lengths of stay were found. Saravia et al. [16] described higher IL-33 NPA concentrations in 19 infants with RSV acute viral bronchiolitis on hospital admission when compared to IL-33 NPA concentrations 28 days later, after hospital discharge. In contrast, Christiaansen et al. [24] found that higher NPA concentrations of IL-33 on hospital admission were associated with milder disease in 23 RSV infected infants. It is worth mentioning that none of the studies evaluated the same inflammatory markers in a different moment of hospitalization other than on admission. Concentrations of these molecules on Day 5 of hospital stay enabled us to characterize the host response in the acute phase of the disease.
The soluble form of ST2, which is one of the mechanisms of IL-33 down regulation, has been identified as a reliable biomarker of poor prognosis in cardiovascular diseases, acute respiratory distress syndrome (ARDS) and other inflammatory conditions [25], [26]. Furthermore, while IL-33 plays an important role in tissue repair and restores tissue homeostasis in infectious and chronic inflammatory diseases, persistently high sST2 levels is often implicated in the emergence of tissue fibrosis. Indeed, sustained high serum concentrations of sST2 on the first three days of mechanical ventilation in adults with ARDS were associated with longer hospital length of stay, extubation failure and mortality [25]. Similarly, we have found that detection of sST2 in NPA samples on admission shows a high sensitivity and good accuracy in predicting need for mechanical ventilation in children with viral ALRI. Also, an increment of sST2 concentration over time was associated with a prolonged hospital stay in our patients. A multicenter cohort study [27] performed by Faber et al. reported high concentrations of sST2 on admission in the NPA of infants intubated for RSV bronchiolitis. Soluble ST2 concentrations were 20 times higher in the intubated group, when compared to those with milder disease. Although it is well known that the IL-33/ST2 axis takes part in eosinophilic modulation in asthma, the role of ST2 in neutrophilic inflammation of the airway, which is considered to be the main cause of ALRI severity in the pediatric population [28], is still poorly understood [29]. Schaunaman et al et al. [29] demonstrated an overexpression of ST2 in cultured normal human primary airway epithelial cells leading to neutrophilic chemoattractant IL-8 production, which was independently associated with M. pneumoniae or rhinovirus infection. Indeed, we observed an increase in IL-8 concentrations in NPA samples in both groups. IL-8 is mostly responsible for the local neutrophil afflux, which has been associated with mucus accumulation that has an important role in viral containment in upper respiratory tract, but can also lead to airway and alveolar obstruction. In fact, high concentrations of IL-8 have been often correlated to worse outcomes, such as hypoxemia and mechanical ventilation [10].
Interestingly, in our study, a significant increase in serum IL-33 concentrations from Day 1 to Day 5 of hospitalization was observed in children who did not receive mechanical ventilation, while in the mechanical ventilation group, serum IL-33 concentrations remained mostly undetected. In addition, in the group of non-ventilated patients, we also observed a decrease in IL-6 NPA concentrations from Day 1 to Day 5 following hospital admission. It has been reported that IL-33 modulates IL-6 responses [14]. Pyle et al. [30] have suggested that an early and robust production of IL-6 is necessary to the resolution of the disease by regulating Th1 responses between Day 4 and Day 7 after viral respiratory tract infection.
Despite these observations, increased concentrations of IL-6 and IL-8 found in the mechanical ventilation group in our study could be due to the inflammatory response caused by mechanical ventilation itself, as previously suggested [31]. Even though IL1-ß and TNFα have been frequently associated with disease severity [28], [32], [33], [34], our results do not corroborate these findings. We also detected an increment of IL-4 NPA concentrations in both studied groups from Day 1 to Day 5 of hospital stay. IL-4 is a typical Th2 response cytokine, frequently implicated in the emergence of recurring wheezing episodes and asthma exacerbations, especially related to rhinovirus infection [12], [19].
In our study, we did not observe any association between viral etiology, viral load or co-infections and disease severity. Viral-related risk factors for severe disease are not well established, as the pathogenesis of ALRI is the result of a complex interaction of environmental factors, viral virulence and host responses [35], [36], [37], [38]. Whereas studies in genomics have mapped transcriptome features in the host leading to inflammatory responses that are unique according to viral etiology and age of the patient [38], Cavallaro et al. [28] reported that the severity of the inflammatory response was independent of viral etiology. They also did not find an association between disease severity and co-infections, which was corroborated by a recently published meta-analysis [39]. A retrospective observational study on mechanically ventilated children with bronchiolitis admitted to the PICU showed no differences on duration of respiratory support or PICU length of stay between bronchiolitis caused by only one virus and more than one virus [40]. Hospital length of stay, however, was longer in the co-detection group. On the other hand, a recent publication reported an association between ALRI in children by coronavirus alone or in co-detection with rhinovirus C and severity of the disease, defined as need for admission to the pediatric intensive care unit in our hospital [41]. On regards to viral load, many studies have assessed the role of RSV viral load in predicting worse outcomes, with conflicting results [42]. It has been recently suggested that higher RSV viral loads at presentation elicits a more robust inflammatory response, resulting in faster viral clearance, which could be associated with milder disease [43].
The strength of this study is that we were able to analyze, concomitantly, the ALRI inflammatory modulation using a panel of seven inflammatory markers that reflects Th1, Th2 and Regulatory T cells (Tregs) responses, both in NPA and blood samples, as well as its relation to disease severity, in two distinct moments of the viral ALRI disease in children. This study, therefore, adds information to the literature, concerning the pediatric host response to respiratory infections by a multitude of viral etiologies, not exclusively by RSV and/or rhinovirus, as extensively reported before. Furthermore, no other publications known by the authors studied both IL-33 and sST2 in this context. The limitations of our study include a high rate of corticosteroid use, leading to a smaller sample size and the lack of a control group of healthy patients.
In conclusion, ALRI severity, defined by the need of mechanical ventilation, was associated with an early and more robust local activation of the IL-33/ST2 axis. During the course of the disease, an increment in sST2 local levels was correlated to a longer hospital length of stay, whereas a systemic increase in IL-33 and a local decrease of IL-6 were associated with milder disease. Finally, respiratory virus types, viral load or co-detections were not associated with disease severity in this cohort of young children with ALRI.
Funding source
This study was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
CRediT authorship contribution statement
Carolina Augusta Arantes Portugal: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing - original draft, Writing - review & editing. Ítalo Araújo Castro: Data curation. Mirela Cristina Moreira Prates: Data curation. Talita Bianca Gagliardi: Data curation, Writing - review & editing. Ronaldo Bragança Martins: Data curation. Bruna Laís Santos Jesus: Data curation. Ricardo Souza Cardoso: Data curation. Marcus Vinícius Gomes Silva: Data curation. Davi Casale Aragon: Formal analysis, Software, Validation. Eurico Arruda Neto: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing - review & editing. José Carlos Farias Alves Filho: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing - review & editing. Fernando Queiroz Cunha: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing - review & editing. Ana Paula Carvalho Panzeri Carlotti: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2019.154965.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- 1.Nair H., Nokes D.J., Gessner B.D. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet [Internet] 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhedin S., Lindstrand A., Hjelmgren A. Respiratory viruses associated with communityacquired pneumonia in children: matched case-control study. Thorax. 2015;70(9):847–853. doi: 10.1136/thoraxjnl-2015-206933. http://thorax.bmj.com/lookup/doi/10.1136/thoraxjnl-2015-206933 [cited 2018 Mar 17] [DOI] [PubMed] [Google Scholar]
- 3.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta-analysis. J. Glob. Health. 2015;5(1) doi: 10.7189/jogh.05.010408. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4593292&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheltema N.M., Gentile A., Lucion F. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob. Health. 2017;5(10):e984–e991. doi: 10.1016/S2214-109X(17)30344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi T., McAllister D.A., O’Brien K.L. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swingler G.H., Hussey G.D., Zwarenstein M. Duration of illness in ambulatory children diagnosed with bronchiolitis. Arch. Pediatr. Adolesc. Med. 2000;154(10):997–1000. doi: 10.1001/archpedi.154.10.997. http://www.ncbi.nlm.nih.gov/pubmed/11030851 [DOI] [PubMed] [Google Scholar]
- 7.Piedra P.A. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch. Pediatr. Adolesc. Med. 2012;166(8):700. doi: 10.1001/archpediatrics.2011.1669. http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpediatrics.2011.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa K., Pate B.M., Mansbach J.M. Risk factors for requiring intensive care among children admitted to ward with bronchiolitis. Acad. Pediatr. 2015;15(1):77–81. doi: 10.1016/j.acap.2014.06.008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4454380&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geoghegan S., Erviti A., Caballero M.T. Mortality due to respiratory syncytial virus. Burden and risk factors. Am. J. Respir. Crit. Care Med. 2017;195(1):96–103. doi: 10.1164/rccm.201603-0658OC. http://www.ncbi.nlm.nih.gov/pubmed/27331632 [DOI] [PubMed] [Google Scholar]
- 10.Russell C.D., Unger S.A., Walton M. The human immune response to respiratory syncytial virus infection. Clin. Microbiol. Rev. 2017;30(2):481–502. doi: 10.1128/CMR.00090-16. http://www.ncbi.nlm.nih.gov/pubmed/28179378 [cited 2018 Mar 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y., López C.B. The innate immune response to RSV: advances in our understanding of critical viral and host factors. Vaccine. 2017;35(3):481–488. doi: 10.1016/j.vaccine.2016.09.030. https://linkinghub.elsevier.com/retrieve/pii/S0264410X1630843X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert L., Sagfors A.M., Openshaw P.J.M., Culley F.J. Immunity to RSV in early-life. Front. Immunol. 2014;5(SEP):1–14. doi: 10.3389/fimmu.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-García M.L., Calvo C., Moreira A. Thymic stromal lymphopoietin, IL-33, and periostin in hospitalized infants with viral bronchiolitis. Medicine (Baltimore) 2017;96(18) doi: 10.1097/MD.0000000000006787. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed18b&NEWS=N&AN=612158793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajardo Carrasco T., Morales R.A., Pérez F. Alarmin’ immunologists: IL-33 as a putative target for modulating T cell-dependent responses. Front. Immunol. 2015;6(JUN):232. doi: 10.3389/fimmu.2015.00232. http://journal.frontiersin.org/Article/10.3389/fimmu.2015.00232/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cayrol C., Girard J.-P. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol. Rev. 2018;281(1):154–168. doi: 10.1111/imr.12619. http://doi.wiley.com/10.1111/imr.12619 [DOI] [PubMed] [Google Scholar]
- 16.Saravia J., You D., Shrestha B. Respiratory syncytial virus disease is mediated by age-variable IL-33. PLoS Pathog. 2015;11(10):1–17. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng S., Wu J., Liu J., Qi F., Liu B. IL-33 receptor (ST2) signalling is important for regulation of Th2-mediated airway inflammation in a murine model of acute respiratory syncytial virus infection. Scand. J. Immunol. 2015;81(6):494–501. doi: 10.1111/sji.12284. http://doi.wiley.com/10.1111/sji.12284 [DOI] [PubMed] [Google Scholar]
- 18.Le Goffic R., Arshad M.I., Rauch M. Infection with influenza virus induces IL-33 in murine lungs. Am. J. Respir. Cell Mol. Biol. 2011;45(6):1125–1132. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 19.Jackson D.J., Makrinioti H., Rana B.M.J. IL-33-Dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014;190(12):1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lay M.K., Céspedes P.F., Palavecino C.E. Human metapneumovirus infection activates the TSLP pathway that drives excessive pulmonary inflammation and viral replication in mice. Eur. J. Immunol. 2015;45(6):1680–1695. doi: 10.1002/eji.201445021. http://doi.wiley.com/10.1002/eji.201445021 [DOI] [PubMed] [Google Scholar]
- 21.Rostan O., Arshad M.I., Piquet-Pellorce C., Robert-Gangneux F., Gangneux J.-P., Samson M. Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases. Andrews-Polymenis HL, editor. Infect. Immun. 2015;83(5):1738–1748. doi: 10.1128/IAI.02908-14. http://iai.asm.org/lookup/doi/10.1128/IAI.02908-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proenca-Modena J.L., Pereira Valera F.C., Jacob M.G. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0042136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vázquez Y., González L., Noguera L. Cytokines in the respiratory airway as biomarkers of severity and prognosis for respiratory syncytial virus infection: an update. Front. Immunol. 2019;10(June):1154. doi: 10.3389/fimmu.2019.01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiaansen A.F., Syed M.A., Ten Eyck P.P. Altered Treg and cytokine responses in RSV-infected infants. Pediatr. Res. 2016;80(5):702–709. doi: 10.1038/pr.2016.130. http://www.nature.com/articles/pr2016130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alladina J.W., Levy S.D., Hibbert K.A. Plasma concentrations of soluble suppression of tumorigenicity-2 and interleukin-6 are predictive of successful liberation from mechanical ventilation in patients with the acute respiratory distress syndrome∗. Crit. Care Med. 2016;44(9):1735–1743. doi: 10.1097/CCM.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krychtiuk K.A., Stojkovic S., Lenz M. Predictive value of low interleukin-33 in critically ill patients. Cytokine. 2017;103(May 2017):109–113. doi: 10.1016/j.cyto.2017.09.017. http://linkinghub.elsevier.com/retrieve/pii/S1043466617302740. [DOI] [PubMed] [Google Scholar]
- 27.Faber T.E., Schuurhof A., Vonk A. IL1RL1 gene variants and nasopharyngeal IL1RL-a levels are associated with severe RSV bronchiolitis: a multicenter cohort study. PLoS ONE. 2012;7(5):19–23. doi: 10.1371/journal.pone.0034364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavallaro E.C., Liang K.-K., Lawrence M.D., Forsyth K.D., Dixon D.-L. Neutrophil infiltration and activation in bronchiolitic airways are independent of viral etiology. Pediatr. Pulmonol. 2017;52(2):238–246. doi: 10.1002/ppul.23514. http://doi.wiley.com/10.1002/ppul.23514 Available from. [DOI] [PubMed] [Google Scholar]
- 29.Schaunaman N., Sanchez A., Dimasuay K.G. Interleukin 1 receptor-like 1 (IL1RL1) promotes airway bacterial and viral infection and inflammation Ehrt S, editor. Infect. Immun. 2019;87(7):1–13. doi: 10.1128/IAI.00340-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyle C.J., Uwadiae F.I., Swieboda D.P., Harker J.A. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017;13(9) doi: 10.1371/journal.ppat.1006640. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5633202/pdf/ppat.1006640.pdf [cited 2018 May 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennus M.P., Van Vught AJ, Brabander M., Brus F., Jansen N.J., Bont L.J. Mechanical ventilation drives inflammation in severe viral bronchiolitis. PLoS ONE. 2013;8(12):8–12. doi: 10.1371/journal.pone.0083035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNamara P.S., Flanagan B.F., Selby A.M., Hart C.A., Smyth R.L. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur. Respir. J. 2004;23(1):106–112. doi: 10.1183/09031936.03.00048103. [cited 2018 May 19] Available from: http://erj.ersjournals.com/content/erj/23/1/106.full.pdf. [DOI] [PubMed] [Google Scholar]
- 33.Tabarani C.M., Bonville C.A., Suryadevara M. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2013;32(12):e437–e442. doi: 10.1097/INF.0b013e3182a14407. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-201312000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Díaz P.V., Valdivia G., Gaggero A.A. Pro-inflammatory cytokines in nasopharyngeal aspirate from hospitalized children with respiratory syncytial virus infection with or without rhinovirus bronchiolitis, and use of the cytokines as predictors of illness severity. Medicine (Baltimore) 2015;94(39) doi: 10.1097/MD.0000000000001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marguet C., Lubrano M., Gueudin M. In very young infants severity of acute bronchiolitis depends on carried viruses. Morty RE, editor. PLoS One. 2009;4(2):e4596. doi: 10.1371/journal.pone.0004596. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2644758/pdf/pone.0004596.pdf [cited 2018 May 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansbach J.M., Piedra P.A., Stevenson M.D. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130(3):e492–e500. doi: 10.1542/peds.2012-0444. http://ws003.juntadeandalucia.es:2524/content/130/3/e492.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luchsinger V., Ampuero S., Palomino M.A. Comparison of virological profiles of respiratory syncytial virus and rhinovirus in acute lower tract respiratory infections in very young Chilean infants, according to their clinical outcome. J. Clin. Virol. 2014;61(1):138–144. doi: 10.1016/j.jcv.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troy N.M., Bosco A. Respiratory viral infections and host responses; insights from genomics. Respir. Res. 2016;17(1):156. doi: 10.1186/s12931-016-0474-9. http://respiratory-research.biomedcentral.com/articles/10.1186/s12931-016-0474-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim F.J., de Klerk N., Blyth C.C., Fathima P., Moore H.C. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology. 2016;21(4):648–655. doi: 10.1111/resp.12741. http://doi.wiley.com/10.1111/resp.12741 [DOI] [PubMed] [Google Scholar]
- 40.Coleman T., Taylor A., Crothall H., Martinez F.E. Respiratory support during bronchiolitis due to one virus versus more than one virus: an observational study. J. Pediatr. Intensive Care. 2019:204–209. doi: 10.1055/s-0039-1691839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuno A.K., Gagliardi T.B., Paula F.E. Human coronavirus alone or in co-infection with rhinovirus C is a risk factor for severe respiratory disease and admission to the pediatric intensive care unit: a one-year study in Southeast Brazil. Pickles RJ, editor. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217744. http://dx.plos.org/10.1371/journal.pone.0217744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piedra F.-A., Mei M., Avadhanula V. The interdependencies of viral load, the innate immune response, and clinical outcome in children presenting to the emergency department with respiratory syncytial virus-associated bronchiolitis. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0172953. http://dx.plos.org/10.1371/journal.pone.0172953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Mauriño C., Moore-Clingenpeel M., Thomas J. Viral load dynamics and clinical disease severity in infants with respiratory syncytial virus infection. J Infect Dis [Internet]. 2019;219(8):1207–1215. doi: 10.1093/infdis/jiy655. https://academic.oup.com/jid/article/219/8/1207/5173953 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.