Abstract
Rhinovirus is the main cause of the common cold, which remains the most frequent infection worldwide among humans. Knowledge and understanding of the rhinovirus transmission route is important to reduce morbidity as only preventive measures are effective. In this study, we investigated the potential of rhinovirus to survive on fingers. Rhinovirus-B14 was deposited on fingers for 30, 60, 90 and 120 min. Survival was defined as the ability of the virus to grow after 7 days, confirmed by immunofluorescence. Rhinovirus survival was not dependent on incubation time on fingers. Droplet disruption had no influence on survival. Survival was frequent with high rhinovirus concentrations, but rare with low-concentration droplets, which corresponded to the usual rhinovirus concentrations in mucus observed in children and adults, respectively. Our study confirms that rhinovirus infectiousness is related to the viral concentration in droplets and suggests that children represent the main transmission source, which occurs only rarely via adults. It confirms also that rhinovirus hand-related transmission is possible and supports hand hygiene as a key prevention measure.
Keywords: Fingers, hand hygiene, picornavirus, rhinovirus, survival, transmission
Introduction
Rhinoviruses are non-enveloped, positive-stranded RNA viruses belonging to the Enterovirus genus within the Picornaviridae family and the main causative agent of the common cold [1], the most frequent infection worldwide. Although usually a self-limited viral disease, it remains a source of significant morbidity in the community. Rhinovirus is associated also with asthma/wheezing and chronic obstructive pulmonary disease exacerbations, as well as several complications, such as acute otitis media, sinusitis, bronchitis and, in some cases, lower respiratory tract diseases. Pre-school children seem to be the main reservoir [2], as approximately six rhinovirus infections are observed per year and per child [3]. There are more than 150 different rhinovirus types with almost no cross-protection, which explains the frequency of rhinovirus infections and the absence of an effective vaccine or antiviral treatment. Only preventive measures are currently effective against these highly prevalent viruses and understanding their mode of transmission is important to reduce the number of infected patients.
The nasal mucosa and posterior nasopharynx have been documented as the main sites of viral replication and therefore the main shedding site [4], [5]. It is reported that person-to-person transmission is most likely due to the contamination of hands by the nasal secretions of the infected person passed to a susceptible individual, either directly to the fingers or via an environmental intermediary; infection then follows from self-inoculation to the upper nasal airways or eyes [6], [7], [8], [9]. The required infecting virus dose is below one median tissue culture infectious dose/mL (TCID50) [8], [10]. Three possible transmission routes have been described: via aerosols of respiratory droplets, direct contact by hands, or indirect contact with environmental objects (fomites).
Aerosols produced by coughing or sneezing originate mainly from saliva [11] in which the viral load is approximately 30 times lower than in nasal secretions [4], [8]. As rhinovirus transmission depends on the concentration of virus in secretions [4], this supports expert opinion that aerosol or oral transmission is a rare event [12], [13], [14]. Direct contact appears to play a major role in transmission. Rhinoviruses have been shown to transiently survive on human skin [4], [6], [13], [15], leading to the hypothesis that hand-related transmission is the main transmission mechanism [6], [13], [16]. Although less frequently than on skin [13], [17], [18], rhinovirus has been shown to survive on fomites. In an experimental study, 50% of volunteers who touched their nasal mucosa or conjunctiva after handling a contaminated fomite developed infection [15]. However, many authors consider that indirect transmission is unlikely because of the important loss of infectivity during the process [17], [19], [20]. Our study was designed to test rhinovirus stability on fingers under experimental conditions, which aimed to reproduce natural conditions as far as possible.
Materials and methods
We conducted a series of experiments to assess the duration of human rhinovirus infectiousness duration on fingers, as well as the impact of viral concentration on survival rates. Survival was defined as the ability of the virus to grow on HeLaOH cells after 7 days, confirmed by immunofluorescence. Experimental conditions aimed to reproduce natural conditions as far as possible.
Viral suspensions and cell lines
All experiments were performed using the RV-B14 strain and HeLaOH cells (kindly provided by F.H. Hayden, University of Virginia, Charlottesville, VA, USA) for viral culture. RV-B14 stock (1 × 10e8 TCID50/mL) was diluted with respiratory mucus to obtain three different concentrations: 1 × 10e5 TCID50/mL (high concentration (HC)); 1 × 10e4 TCID50/mL (average concentration (AC)); and 1 × 10e2 TCID50/mL (low concentration (LC)). Each HC and AC droplet contained 1.1 × 10e5 and 2.8 × 10e4 viral RNA copies (5.5 × 10e7 and 1.4 × 10e7 copies/mL), respectively. LC droplet viral copies were below the limit of detection by real-time RT-PCR assay, but they were expected to represent 200 viral copies given their equivalence to 100 dilutions of the AC. HC represents the average viral load of paediatric nasopharyngeal swabs in our laboratory, whereas LC corresponds to the average measured adult concentration. These values also correlate with epidemiological findings in the literature for paediatric and adult patients [6], [13], [17], [21]. Respiratory mucus was obtained by mixing clinical samples sent for routine testing that were RT-PCR and cell culture negative for the usual human respiratory viruses (influenza virus A/B, human metapneumovirus, coronavirus 229E/HKU1/OC43/NL63, respiratory syncytial virus A/B, picornavirus and parainfluenza virus 1/2/3). A further 20 min of ultraviolet radiation ensured inactivation of putative undetected viruses. To guarantee optimal growth, only mucus with a pH between 6.5 and 7 was retained.
Participants and finger contamination procedure
Six specialized laboratory collaborators (technicians and MD/PhD graduates) were recruited on a voluntary basis as previously described [22]. The protocol was approved by the institutional review board of the University Hospitals of Geneva.
Determination of infectiousness
A 2-μL drop of viral suspension of human RV-B14 mixed with respiratory secretions was deposited on the fingertips of each participant. This volume represents the mean size of a large respiratory droplet and can be easily reproduced [22]. For each subject, nine drops containing rhinovirus at different concentrations (three HC, three AC, three LC) were deposited and one negative control (mucus only). Each contaminated finger was kept untouched for a defined period of time at room temperature before testing for the presence of infectious rhinovirus. Participants' fingers were then immersed in wells (Becton Dickinson and Co., Franklin Lakes, NJ, USA) containing 1 mL of McCoy's 5A medium (1 ×) with 2% serum (Gibco, New York, NY, USA) for 60 seconds. Then, 400 μL of this eluate was used to immediately inoculate HeLaOH cells. This represents an additional 2.5-fold dilution of the viral load present in droplets before inoculation onto cell cultures (4.4 × 10e4 viral copies for HC, 1.1 × 10e4 viral copies for AC, and <100 copies for LC). After 1 h of adsorption at 33°C, 1 mL of McCoy's 5A medium (1 ×) with 2% serum (Gibco) was added and cells were incubated in 5% CO2 at 33°C for 7 days. For each 24-well plate, a negative control as well as a mock-infected control finger was included. The cytopathic effect was read daily until day 7. Cells were collected after 7 days and submitted to an immunofluorescence assay.
Immunofluorescence
A J2 mouse monoclonal antibody [23] that recognizes double-stranded RNA and an anti-mouse monoclonal IgG fluorescein isothiocyanate-conjugated antibody were used to confirm the presence of viral infection (Chemicon-Millipore, Zug, Switzerland).
Results
Based on preliminary pilot experiments, we determined that rhinovirus survival on fingertips was equivalent across different incubation times as it remained infectious on all fingers after 30, 60, 90 and 120 min. Immediately after deposition, half of the droplets were disrupted and spread on the surface of the fingertip using a pipette tip to determine whether disrupting the integrity and environment of the droplet decreased virus survival. As all intact and disrupted droplets yielded positive culture results, we decided to continue experiments with disrupted droplets only so as to reproduce real-life conditions as much as possible.
One hour after the deposit of disrupted droplets on the fingers of the six volunteers, infectious viruses could be detected by culture in all subjects contaminated with HC droplets (6/6), in four of the six volunteers with AC droplets, and none of the six volunteers with LC droplets, which confirmed the influence of concentration on survival (Fig. 1 ). Of note, when droplets were directly incubated without a passage on fingers, the virus survived in 100% (4/4) of tested fingers at HC compared with 25% (1/4) at LC, despite being below the limit of detection by PCR (data not shown). Overall, the proportion of fingers with detectable viruses was 16/18 fingers at HC, compared with 6/18 and 0/18 for the AC and LC droplets, respectively (Fig. 1). Laboratory room (mean ± standard deviation 24.6 ± 0.7°C) and hood (26.4 ± 1.8°C) temperature, as well as humidity (44.5 ± 5.6%), were similar for all experiments with all subjects.
Fig. 1.
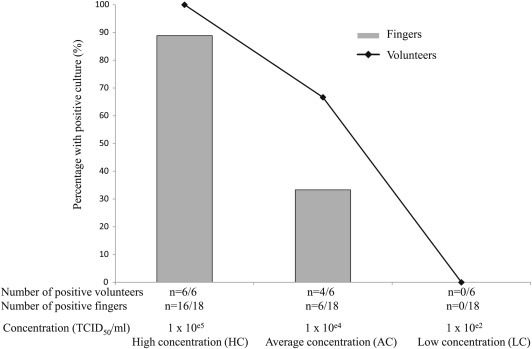
Percentage of volunteers (n = 6) and fingers (n = 18) with positive culture after 1 h.
Discussion
We aimed to investigate rhinovirus transmission by person-to-person contact, the main transmission route for the most prevalent human respiratory infection worldwide. Experiments were designed to reproduce, as much as possible, conditions that could lead to rhinovirus contamination of fingertips in the community. Our study showed that rhinovirus can survive on hands for several hours, similar to previous reports of virus survival on human skin [4], [6], [13], [15], [17], emphasizing that hand-related transmission is the main transmission route. There was no influence of drying time on virus survival under 2 h, in contrast to the study of Ansari et al. where virus survival decreased during the first hour [24].
Our study showed that virus survival, and therefore infectiousness, was related to the viral concentration in droplets. This correlates well with D'Alessio et al. who found that the secondary attack rate was related to the viral concentration in the nose [4]. Inoculum seems to be a restrictive factor for transmission, with infectiousness rapidly dropping below a given concentration. As infected children appear to have a higher viral load than adults, this may explain why children are considered to be the main transmission vector. The fact that the viral load in LC droplets was below the level of detection explains why the virus could not be recovered at these concentrations, except in one case without a passage on fingers. LC droplets correspond to the viral concentration recovered in rhinovirus-infected adults and this suggests that transmission via adults occurs rarely. A recent study investigating the transmission of cold-like illnesses between siblings showed that younger children tended to become infected first in most cases. However, the secondary attack rate was greater for older siblings, probably because of a higher viral load in younger siblings' secretions. As younger children tend also to touch nasal secretions directly with their fingers, it is probable that this enhances transmission. The viral load in the mucus of more than 1000 rhinovirus-infected children below 1 year of age was 5.79 × 10e6 TCID50/mL, which is 10 to 100 times higher than our HC of 1 × 10e5 (Regamey et al., private communication). It is very probable that the difference in virus survival between adults and children is even higher than in our results.
We showed that virus survival increased at LC when there was no passage on hands. The loss of infectiousness during interhuman contact or fomite manipulation has already been described [20], [24], highlighting again the importance of viral load for transmission. Similarly, rhinovirus was more frequently recovered on fingers from subjects with a high nasal viral load compared with a low nasal viral load [17].
Our study confirmed that droplet disruption had no influence on survival at a given concentration. We have previously shown that influenza virus survival on fingers was not related to virus concentration in a study using a similar methodology to the present experiments [22]. Survival of influenza virus on fingers declined rapidly, with less than 15% of the fingers remaining positive after 30 min [22]. The influenza envelope, which is known to be a determinant factor decreasing virus survival, may explain why survival was shorter and affected by droplet disruption, which was not the case for rhinovirus [22]. In a similar study, RV-14 in 10-μL droplets at 2.9 × 10e4 to 1.4 × 10e5 TCID50/mL survived for 1 h on almost 40% of fingers [24]. The fact that virus survival was lower compared with our results, despite the use of bigger droplets and higher viral concentrations, may be explained by the fact that our volunteers did not wash their hands or use an alcohol-based hand rub before experiments as hand rubbing has been shown to decrease virus survival even several hours after use [25]. The fact that disinfecting hands or objects with an iodine or alcohol-based solution reduces the secondary illness rate of rhinovirus infections emphasizes also the importance of hand-related transmission [8], [15], [26].
In conclusion, these laboratory results confirm that hand-related transmission of rhinovirus is possible and support hand hygiene as a key measure to prevent transmission, particularly in children who represent the main transmission source.
Transparency declaration
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank all volunteers who participated in the experiments as well as Rosemary Sudan for editorial assistance. This study was supported by grants from the Swiss National Science Foundation (ME 9580, 310030_146151 and ME 9575, 32003B_146991/1) and by the Laboratory of Virology of the University Hospitals of Geneva.
Editor: T. Avšic-Zupanc
References
- 1.Makela M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper C.M., Winther B., Mandel E.M., Doyle W.J. Temporal relationships for cold-like illnesses and otitis media in sibling pairs. Pediatr Infect Dis J. 2007;26:778–781. doi: 10.1097/INF.0b013e318124aa31. [DOI] [PubMed] [Google Scholar]
- 3.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 4.D'Alessio D.J., Peterson J.A., Dick C.R., Dick E.C. Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis. 1976;133:28–36. doi: 10.1093/infdis/133.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Winther B., Gwaltney J.M., Jr., Mygind N., Turner R.B., Hendley J.O. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–1767. [PubMed] [Google Scholar]
- 6.Gwaltney J.M., Jr., Moskalski P.B., Hendley J.O. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–467. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 7.Couch R.B., Cate T.R., Douglas R.G., Jr., Gerone P.J., Knight V. Effect of route of inoculation on experimental respiratory viral disease in volunteers and evidence for airborne transmission. Bacteriol Rev. 1966;30:517–529. doi: 10.1128/br.30.3.517-529.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendley J.O., Gwaltney J.M., Jr. Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:243–258. [PubMed] [Google Scholar]
- 9.Bynoe M.L., Hobson D., Horner J., Kipps A., Schild G.C., Tyrrell D.A. Inoculation of human volunteers with a strain of virus isolated from a common cold. Lancet. 1961;1:1194–1196. doi: 10.1016/s0140-6736(61)91941-9. [DOI] [PubMed] [Google Scholar]
- 10.Hendley J.O., Edmondson W.P., Jr., Gwaltney J.M., Jr. Relation between naturally acquired immunity and infectivity of two rhinoviruses in volunteers. J Infect Dis. 1972;125:243–248. doi: 10.1093/infdis/125.3.243. [DOI] [PubMed] [Google Scholar]
- 11.Buckland F.E., Tyrrell D.A. Experiments on the spread of colds. 1. Laboratory studies on the dispersal of nasal secretion. J Hyg (Lond) 1964;62:365–377. doi: 10.1017/s0022172400040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cate T.R., Couch R.B., Fleet W.F., Griffith W.R., Gerone P.J., Knight V. Production of tracheobronchitis in volunteers with rhinovirus in a small-particle aerosol. Am J Epidemiol. 1965;81:95–105. doi: 10.1093/oxfordjournals.aje.a120501. [DOI] [PubMed] [Google Scholar]
- 13.Hendley J.O., Wenzel R.P., Gwaltney J.M., Jr. Transmission of rhinovirus colds by self-inoculation. N Engl J Med. 1973;288:1361–1364. doi: 10.1056/NEJM197306282882601. [DOI] [PubMed] [Google Scholar]
- 14.Hendley J.O., Gwaltney J.M., Jr., Jordan W.S., Jr. Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969;89:184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 15.Gwaltney J.M., Jr., Hendley J.O. Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol. 1982;116:828–833. doi: 10.1093/oxfordjournals.aje.a113473. [DOI] [PubMed] [Google Scholar]
- 16.Gwaltney J.M., Jr., Hendley J.O. Rhinovirus transmission: one if by air, two if by hand. Am J Epidemiol. 1978;107:357–361. doi: 10.1093/oxfordjournals.aje.a112555. [DOI] [PubMed] [Google Scholar]
- 17.Reed S.E. An investigation of the possible transmission of rhinovirus colds through indirect contact. J Hyg (Lond) 1975;75:249–258. doi: 10.1017/s0022172400047288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattar S.A., Karim Y.G., Springthorpe V.S., Johnson-Lussenburg C.M. Survival of human rhinovirus type 14 dried onto nonporous inanimate surfaces: effect of relative humidity and suspending medium. Can J Microbiol. 1987;33:802–806. doi: 10.1139/m87-136. [DOI] [PubMed] [Google Scholar]
- 19.Jennings L.C., Dick E.C., Mink K.A., Wartgow C.D., Inhorn S.L. Near disappearance of rhinovirus along a fomite transmission chain. J Infect Dis. 1988;158:888–892. doi: 10.1093/infdis/158.4.888. [DOI] [PubMed] [Google Scholar]
- 20.Pancic F., Carpentier D.C., Came P.E. Role of infectious secretions in the transmission of rhinovirus. J Clin Microbiol. 1980;12:567–571. doi: 10.1128/jcm.12.4.567-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas R.G., Jr., Cate T.R., Gerone P.J., Couch R.B. Quantitative rhinovirus shedding patterns in volunteers. Am Rev Respir Dis. 1966;94:159–167. doi: 10.1164/arrd.1966.94.2.159. [DOI] [PubMed] [Google Scholar]
- 22.Thomas Y., Boquete-Suter P., Koch D., Pittet D., Kaiser L. Survival of influenza virus on human fingers. Clin Microbiol Infect. 2014;20:O58–O64. doi: 10.1111/1469-0691.12324. [DOI] [PubMed] [Google Scholar]
- 23.Jurgeit A., Moese S., Roulin P., Dorsch A., Lötzerich M., Lee W.M. An RNA replication-center assay for high content image-based quantifications of human rhinovirus and coxsackievirus infections. Virol J. 2010;7:264. doi: 10.1186/1743-422X-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansari S.A., Springthorpe V.S., Sattar S.A., Rivard S., Rahman M. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29:2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwaltney J.M., Jr., Moskalski P.B., Hendley J.O. Interruption of experimental rhinovirus transmission. J Infect Dis. 1980;142:811–815. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- 26.Turner R.B., Biedermann K.A., Morgan J.M., Keswick B., Ertel K.D., Barker M.F. Efficacy of organic acids in hand cleansers for prevention of rhinovirus infections. Antimicrob Agents Chemother. 2004;48:2595–2598. doi: 10.1128/AAC.48.7.2595-2598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


