Abstract
In cats, primary or secondary immune-mediated thrombocytopenia have rarely been described or characterised. The objective of this study was to determine platelet-bound antibodies (PBA) by a flow cytometric assay in both healthy and thrombocytopenic cats. Direct PBA testing was performed in 42 thrombocytopenic cats (platelet counts 6–179×109/l, median 56×109/l). Of these 42 cats, 19 had positive PBA test results, 17 of which were considered to have secondary immune-mediated thrombocytopenia (sITP). Underlying diseases included fat necroses (four cases), feline infectious peritonitis (three), feline leukaemia virus (two) or feline immunodeficiency virus (two) infections, lymphoma (two), leukaemia (one), hepatitis (one), pyelonephritis (one), or hyperthyroidism (one). In two cats, no underlying disease was found suggesting a primary immune-mediated thrombocytopenia (pITP). The PBA test was negative in 23 cats diagnosed with varying underlying diseases and in 47 healthy control cats with platelet values within the reference range. Only seven of the 42 cats with thrombocytopenia (platelet count 10–57×109/l, median 34×109/l) had spontaneous bleeding. This study suggests that immune-mediated destruction of platelets might be an important pathological mechanism for feline thrombocytopenia caused by various underlying diseases. In cats, pITP appears to be rarely diagnosed.
In cats, decreased platelet counts are a common laboratory finding. This pseudothrombocytopenia (incorrect low platelet values) occurs with automated counting of platelets as there is a tendency for feline platelets to aggregate and difficulty in differentiating between the similar size of some feline platelets and red blood cells (Zelmanovic and Hetherington 1998). Manual counting or slide evaluation must always be undertaken to confirm the thrombocytopenia.
A reduced production, increased destruction or increased utilisation of platelets, sequestration in the spleen, or a combination of these pathomechanisms can cause thrombocytopenia. In dogs, underlying diseases which may lead to platelet decreases have been described in detail and more frequently in dogs than in cats (Grindem et al 1991, Lewis and Meyers 1996a, Kohn et al 2000a). Immune-mediated thrombocytopenia (ITP) and Evans' syndrome have been described frequently in dogs, while there are only occasional case reports for cats (Joshi et al 1979, Harvey and Gaskin 1980, Cain et al 1988, Tyler et al 1991, Jordan et al 1993, Garon et al 1999, Tasker et al 1999). ITP occurs as a primary or idiopathic (pITP) and as a secondary (sITP) form (Reagan and Rebar 1995, Lewis et al 1995a). In secondary ITP, infections, other immune-mediated diseases (eg, systemic lupus erythematosus), drugs, neoplasias, blood transfusions, or vaccination may trigger an increased production of antibodies which may adhere to or cross-react with platelet receptors causing an increased destruction of platelets by the mononuclear phagocytic system (Lewis et al 1995a). The diagnosis of a primary ITP is based on the exclusion of underlying diseases, the presence of a mostly severe thrombocytopenia, the response to immunosuppressive therapy, and the presence of platelet-bound antibodies (PBA) (Lewis and Meyers 1996b, Baldwin and Cowell 2001). While direct positive detection of PBA by flow cytometry is an established diagnostic tool in dogs (Lewis et al 1995a, 1996b, Kohn et al 2000a), little information is available describing the use of this tool in cats.
The objective of this study was to evaluate underlying diseases in cats with thrombocytopenia and to establish possible causes of platelet deprivation. In these thrombocytopenic cats, a flow cytometric assay was used to detect whether PBA was present and what role this might play in the thrombocytopenia.
Material and Methods
Patients
Cats with platelet numbers less than 180×109/l were included in the study (Mischke 1999). They were presented at the Clinic for Small Animals, Free University of Berlin between January 1999 and June 2000, and an exhaustive diagnostic evaluation including determination of PBA was performed. Healthy cats showing no clinical signs and normal laboratory results with platelet counts within the reference range were used as controls.
In all cats a complete blood count, a differential cell count and clinical chemistry were performed. The initial 1–2 ml of blood was collected in lithium-heparin tubes for clinical chemistry (Electrolyte-14+-Analyzer, Nova Biomedicals, Rödermark; Cobas Mira Plus, Roche Diagnostica, Grenzach-Wyhlen). Another 1 ml of blood was collected in K-EDTA tubes for haematological evaluation (Cell-Dyn 3500, Abbott, Wiesbaden). Attention was paid to complete the examination of the blood samples, especially the counting of thrombocytes, within 30 min, and the EDTA-tubes were carefully checked for small blood clots. For all cats, the platelets were counted microscopically using Thrombo Plus-tubes (Sarstedt, Nümbrecht) and a Neubauer counting chamber (Moritz and Hoffmann 1997). Further testing included coagulation testing (prothrombin time by Hepato Quick, Boehringer, Mannheim; partial thromboplastin time by Pathromtin, Dade Behring, Marburg) and serological tests (eg, feline immunodeficiency virus (FIV) antibodies, feline leukaemia virus (FeLV) antigen, coronavirus antibodies). In certain cases, a direct Coombs’ test (n=21) and anti-nuclear antibody testing (one case) were performed, and cytological (15) and histological (nine) tests were done. In 39 cats, radiography of thorax and abdomen and/or abdominal ultrasound were performed.
Platelet-bound Antibodies
Detection of PBA by flow cytometry (FACScan, Becton Dickinson, New Jersey, USA) was performed at the Institute of Immunology, University of Veterinary Medicine, Hannover. Separated and washed platelets were labelled by a platelet specific monoclonal mouse antibody followed by phycoerythrin-conjugated goat anti-mouse IgG antibodies (Dianova, Hamburg). Thus, particles having the same size as platelets (eg, fragments of red blood cells) can be distinguished from thrombocytes and excluded from the analysis. Fluorescein-conjugated polyclonal goat anti-feline IgG antibodies (heavy and light chains) (Dianova, Hamburg) were used to detect those platelets that were carrying antibodies. Along with each blood sample of a patient (1.0–1.5 ml K-EDTA-blood) a control sample (K-EDTA-blood of a healthy cat) was sent overnight without cooling and analysed simultaneously, ie, within 18–24 h after harvest in order to avoid artefacts mimicking false-positive platelet reactions.
Statistics
SPSS 11.5 for Windows (Microsoft) was used for non-parametric statistical analysis. Ranges, mean with standard deviations, and median values are reported. A Mann–Whitney U-test was used for group comparison (significant at P<0.05). Box plots were used for graphical representation of platelet numbers.
Results
Over a time period of 18 months, 42 thrombocytopenic cats were included in the study. PBA testing was established for these 42 patients (platelet counts 6–179×109/l, mean 74±55×109/l, median 56×109/l) and for 47 healthy control cats (platelet counts 182–564×109/l, mean 310±127×109/l, median 256×109/l).
The PBA test applied here was negative in all healthy and 23 thrombocytopenic cats (platelet values 15–179×109/l, mean 89±59×109/l, median 82×109/l). These 23 cats with negative test results suffered from viral or bacterial infectious diseases, inflammatory diseases, neoplasias, immunological disorders, and various other diseases (Table 1). Spontaneous haemorrhage was found only in two of these 23 cats. One cat diagnosed with FIV had bled into the abdomen (platelet count 22×109/l). The other cat, which was diagnosed with erythrocytic-megakaryocytic bone marrow aplasia, had bleeding of the gums and petechiae and ecchymoses on the abdomen, ear, retina, and sclera (platelet count 34×109/l).
Table 1.
Results of platelet bound antibody (PBA) tests in 42 cats with thrombocytopenia
| Diseases | PBA test | |
|---|---|---|
| Positive | Negative | |
| Infectious diseases (viral) | ||
| Feline infectious peritonitis | 3 | 4 |
| Feline leukaemia virus | 2 | 1 |
| Feline immunodeficiency virus | 2 | 2 |
| Inflammatory diseases | ||
| Fat necrosis | 4 | |
| Pancreatitis | 2 | |
| Feline lower urinary tract disease | 1 | |
| Hepatitis | 1 | |
| Cholangiohepatitis | 1 | |
| Nasal polyp | 1 | |
| Ulcerative gastritis | 1 | |
| Infectious diseases (bacterial) | ||
| Pneumonia | 1 | |
| Pyelonephritis | 1 | |
| Abscess | 1 | |
| Neoplasia | ||
| Lymphoma | 2 | 3 |
| Leukaemia | 1 | 1 |
| Immune-mediated diseases | ||
| Evans' syndrome | 1 | |
| Primary immune-mediated thrombocytopenia | 1 | |
| Immune-mediated haemolytic anaemia | 1 | |
| Various diseases | ||
| Erythrocytic-megakaryocytic bone marrow aplasia | 1 | |
| Hepatopathy | 1 | |
| Hyperthyroidism | 1 | |
| Peritoneopericardial hernia | 1 | |
Nineteen thrombocytopenic cats (platelet values 6–179×109/l, mean 60±47×109/l, median 42×109/l) tested positive for PBA, suggesting ITP. Seventeen of these 19 cats were diagnosed with the following underlying diseases: viral or bacterial infectious diseases, inflammatory diseases, neoplasias, and various other disorders (Table 1). In two of the cats, an underlying disease could not be found: cat 1, a 9-year-old female-spayed British shorthair, with anaemia and thrombocytopenia had IgG antibodies bound to erythrocytes and platelets suggesting an Evans' syndrome (ITP and immune-mediated haemolytic anaemia) and cat 2, a 9-year-old male-castrated domestic shorthair, was thought to have a primary ITP (Table 1).
Cat 1 (possible Evans' syndrome) suffered from anaemia, which was non-regenerative initially (haematocrit 0.22 l/l, absolute numbers of aggregated reticulocytes 8380/μl); the leukocyte count was 4.8×109/l. The direct differentiated Coombs' test was positive (class IgG anti-erythrocytic antibodies) and anti-nuclear antibody-titre was negative. Apart from splenomegaly, radiography and ultrasonography were unremarkable. The first two cytometric tests (initially and after 6 months) were positive for PBA (platelet values 6 and 38×109/l). Follow-up after 11 months yielded a negative test result (platelet count 101×109/l).
A regenerative anaemia was present in cat 2 (haematocrit 0.16l/l, absolute aggregated reticulocyte count 208,560/μl); the white blood cell count was 6.3×109/l. The PBA test was positive initially and after 14 days (platelet counts 41 and 100×109/l). It was negative after 3 months (platelet count 172×109/l). The direct Coombs' test was negative.
The differential cell count, parameters of clinical chemistry and the coagulation panel (prothrombin time and partial thromboplastin time) were within the reference range in both cats. Both cats were started on an immunosuppressive dosage of prednisolone (2 mg/kg twice daily); cats 1 and 2 received whole blood transfusions at day 5 and at days 1 and 4, respectively. Both patients received follow-ups and checks of their blood parameters during a time period of 19 and 3 months, respectively. In cat 1, platelet counts reached levels over 100×109/l only twice (out of 22 checks) in 19 months; platelet values ranged from 6 to 106×109/l. After 10 days of therapy, cat 2 showed a constant increase in thrombocytes to levels >100×109/l.
These two cats had clinical signs that indicated spontaneous bleeding and included epistaxis and petechiae in the soft palate and ear (cat 1, platelet value 10×109/l) or gum bleeding (cat 2, platelet count 46×109/l). Three other PBA test positive cats also displayed spontaneous bleeding. These three cats had underlying diseases, two were FIV positive cats and one had leukaemia. One of the FIV positive cats (platelet value 57×109/l) had haematomas in the cheek, petechiae in the ears, soft palate, and around the anus; the second cat suffering from FIV (platelet count 16×109/l) had gum bleeding. The cat with leukaemia bled from the gums, had a haematoma above the eye, and petechiae in the oral mucosa, the abdomen and the ear at platelet counts of 42×109/l.
In this study, only seven of the 42 thrombocytopenic cats displayed spontaneous bleeding. Platelet counts ranged from 10 to 57×109/l (mean 24.3±17.1×109/l, median 34×109/l). In those cats showing thrombocytopenia but not bleeding, numbers of thrombocytes ranged from 15 to 179×109/l (mean 82.2±55.8×109/l, median 74×109/l) (Fig 1). Three out of seven bleeding cats had platelet values <30×109/l. However, another 10 of the 42 thrombocytopenic cats also displayed platelet counts <30×109/l without spontaneous bleeding. Of the seven cats with a positive PBA test and platelet values <30×109/l, two cats displayed bleeding. Of the six cats with negative PBA test results and thrombocyte counts <30×109/l, bleeding occurred in one cat.
Fig 1.
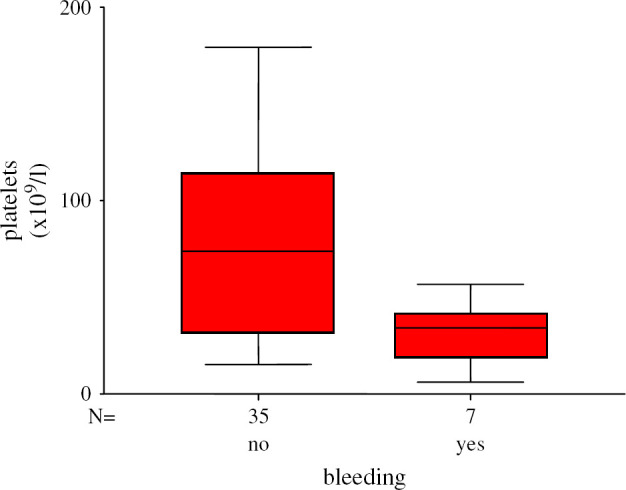
Forty-two cats with thrombocytopenia; number of platelets in cats with and without spontaneous bleeding. The number of platelets was significantly lower in cats with spontaneous bleeding than in cats without spontaneous bleeding (P=0.018).
Discussion
Direct platelet-bound antibody testing was performed in 42 thrombocytopenic cats through the use of flow cytometry. Clinical evaluations along with laboratory testing, radiology, serology and other ancillary procedures were carried out to check for underlying diseases in these cats and to look for possible causes of thrombocytopenia. All automatically determined thrombocytopenias were corroborated by microscopic evaluation using counting chambers to exclude pseudothrombocytopenia.
For cats, little information is available on the application of direct or indirect methods to detect platelet-bound or anti-megakaryocytic antibodies to evaluate immunological factors in the pathogenesis of thrombocytopenia. Joshi et al (1979) used the platelet-factor-3-test (indirect method to detect PBA in serum). For detection of anti-megakaryocytic antibodies direct and indirect immunofluorescence and immunohistochemistry have been described (Joshi et al 1979, Tasker et al 1999). In this study, flow cytometry was used for direct PBA testing in cats. The advantage of flow cytometry over other methodologies is the small amount of blood required and even in severely thrombocytopenic animals enough platelets can be recovered for reliable evaluation. Little denaturation of antigens and antibodies on the platelet membrane occur as there is minimal sample handling and manipulation.
Platelet-bound antibodies may be anti-platelet auto-antibodies, but could also represent ‘secondary’ antibodies (Lewis et al 1995b). The latter include immune complexes, which are bound to platelet Fc receptors, antibodies against platelet antigens, which are formed by modification of the antigen (eg, caused by disease), or antibodies which bind to antigens, which were adsorbed by the surface of thrombocytes (eg, tumour antigens, drug metabolites) (Karpatkin et al 1992).
Glycoprotein IIb/IIIa was identified as target antigen for auto-antibodies in humans and in one of four dogs suffering from ITP, glycoprotein IIb alone was present in three out of these four dogs (Lewis and Meyers 1996a). Target antigens for platelet-antibodies have not been studied in cats. In human medicine but not in veterinary medicine, an enzyme immune test specific for glycoprotein (‘Monoclonal antibody immobilisation of platelet antigens’ assay) is used for confirmation of auto-antibodies (Kiefel and Santoso 1996).
A positive PBA test suggests that immunological processes are involved in the destruction of platelets. However, positive test results are not specific for primary ITP and other causes of ITP may be more common. According to Lewis et al (1995a), false-positive results of the test can be caused by storage at room temperature. In one study, Lewis et al (1995a) compared mean fluorescence of platelets of healthy dogs (n=5) after 0, 24, 48, and 72 h after storage on ice or at room temperatures. Concentrations of PBA were increased significantly after storage at room temperatures, while concentrations of IgG on thrombocytes that had been stored on ice were not increased significantly. In our semi-blind study (the investigating laboratory had no knowledge of the clinical situation of the blood donor), none of the healthy control cats, the blood of which was treated in the same fashion as that of the sick cats, tested positive. False-negative results can be caused by the antibodies decreasing due to immunosuppressive therapy, by antibodies being too low at the onset, and due to technical problems (eg, using wrong antibodies) (Campbell et al 1984, Kristensen et al 1994a).
Clinical studies evaluating the causes for thrombocytopenia in cats are infrequently found in the literature (Jordan et al 1993, Peterson et al 1995, Thomas and Green 1998). In our group of 42 cats, 33.3% (14/42) of the thrombocytopenic cats suffered from viral infectious diseases: 16.6% suffered from FIP, 7.1% from FeLV, and 9.5% from FIV. In Jordan et al (1993) only 4.9% cats had FIP, but 14.6% had FeLV infection. The mechanisms associated with thrombocytopenia during a viral infection may be multifactorial and vary with the agent (Weiss 2000). According to Axthelm and Krakowa (1987), virus induced thrombocytopenia may be caused by inhabiting precursor cells, thus reducing megakaryocytopoiesis, direct platelet damage or lysis by the virus itself, removal of platelets by the mononuclear phagocytic system, or by disseminated intravascular coagulation. Three of the seven cats suffering from feline infectious peritonitis (FIP) were PBA test positive, suggesting immune-mediated destruction of the platelets as a contributing factor. However, these cats also suffered from anaemia and leukopenia (pancytopenia), indicating a decreased bone marrow production as well. Two of the three cats infected with FeLV and two out of four cats infected with FIV had positive PBA test results, and an immune-mediated destruction of thrombocytes was considered a possible pathological mechanism.
Twenty-six percent of the cats suffered from an inflammatory disease, such as fat necrosis or pancreatitis. Five of the 11 cats tested PBA positive and six tested negative. Strikingly, all of the four cats suffering from fat necrosis were positive for PBA. Seven percent of the cats suffered from a bacterial infection. In inflammatory disease states interactions of platelets with altered or damaged endothelial surfaces cause extensive platelet activation, clumping, and removal of platelets by the mononuclear phagocytic system. Platelet destruction in bacterial infections can occur as a result of platelet adherence or aggregation to activated monocytes or neutrophils. Exotoxins may directly damage platelets and contribute to thrombocytopenia (Bithell 1993, Russell and Grindem 2000). A positive direct PBA test was also described in dogs suffering from ehrlichiosis, babesiosis, leishmaniasis, and various bacterial infections (abscess, prostatitis) (Lewis et al 1995a, Kohn et al 2000a). Therefore, in dogs as well as in cats immune-mediated destruction might contribute to thrombocytopenia in different infectious diseases.
In this study, 17 % (7/42) of the thrombocytopenic cats suffered from neoplastic diseases (five with lymphoma, two with leukaemia). In Jordan et al (1993), lymphoma or leukaemia were present in 31.7% of the cats. There is a wide variety of pathomechanisms that may cause thrombocytopenia in the course of a neoplastic disease: Platelets may be sequestered in the spleen, liver, or the tumour as such; consumption of platelets may be increased (eg, due to disseminated intravascular coagulation); they may be destroyed by immune reactions, and production may be reduced because of bone marrow involvement (Helfand 1988). Some cats suffering from lymphoma or leukaemia tested PBA negative while others tested positive. Thrombocytopenic dogs suffering from neoplastic diseases, such as lymphoma or tumours of the liver or spleen had positive PBA test results. Thus, there are indications for an immune-mediated destruction of platelets in both dogs and cats with neoplastic diseases (Kohn et al 2000b).
In this study, only one out of 42 thrombocytopenic cats was diagnosed with pITP. In another cat, a Coombs' test positive anaemia was diagnosed in addition to ITP. In these two cats, PBA detection was performed several times. A study on dogs showed that in seven dogs suffering from pITP PBA tests were positive at first, but negative after having been treated with prednisolone for 7–32 days (Kohn et al 2000b). It is not clear, why in this study PBA was detected over a longer period of time in cats with pITP and Evans' syndrome than in dogs. However, an evaluation of this feature was not possible as there were too few cases. Moreover, both cats received blood transfusions which can be a trigger of (secondary) ITP by itself (Kohn et al 2000a).
Primary ITP has been suggested only occasionally as an independent disease in cats (Harvey and Gaskin 1980, Jordan et al 1993, Garon et al 1999, Tasker et al 1999). In a few cases, ITP has been described in association with immune-mediated haemolytic anaemia (Joshi et al 1979, Cain et al 1988, Tyler et al 1991) or systemic lupus erythematosus (Gabbert 1983). Indication of immune-mediated platelet destruction has been suggested in these studies by detection of anti-megakaryocytic auto-antibodies (direct immunofluorescence or immunohistochemistry) (Joshi et al 1979, Tasker et al 1999) and the platelet-factor-3-test (Joshi et al 1979).
In humans and small animals, spontaneous bleeding occurs rarely at platelet values of more than 20–30×109/l if they do not also suffer from thrombocytopathia or a coagulopathy (Couto 2003, Retzlaff and Mesters 2004). In this study, seven out of 42 cats displayed spontaneous bleeding. In a study by Jordan et al (1993), nine out of 41 cats showed spontaneous haemorrhage. Bleeding was related to platelet values below 30×109/l, and occurred in cats suffering from neoplasia (n=5), infections with FeLV (2), pITP (n=1), or associated to an Eisenmenger's syndrome (n=1). In our study, 10 of 13 cats with platelet counts <30×109/l displayed no clinical signs of haemorrhage. Whether bleeding occurs or not is not only dependent on the number of platelets but also on their age and functionality and on the vascular integrity. According to Jordan et al (1993) cats seem to be able to tolerate very low platelet counts without showing spontaneous bleeding. This could be explained by thrombocytes of cats being activated more easily than those of dogs. Feline platelets are stimulated more readily by aggregation inductors, such as collagen or thrombin, than those of dogs (Hart and Nolte 1991). Activation of thrombocytes results in the release of serotonin. Meyers et al (1982) showed that dense-granules of cats contain 3 or 1.5 times the amount of serotonin compared to dense-granules of humans or dogs, respectively. Serotonin is a potent vasoconstrictor and induces primary aggregation of platelets. In addition, serotonin will potentiate the aggregation effect of other agonists (Meyers et al 1982).
Of the seven cats suffering from spontaneous bleeding examined in our study, five were PBA test positive; three of five had platelet counts of more than 30×109/l. Colman et al (1983) showed that PBA may alter the shape, volume, and morphology of human thrombocytes, which may interfere with their function. The effect of anti-platelet antibodies on in vitro platelet function was investigated in 15 dogs with ITP. In 13 of these dogs, maximal platelet aggregation was significantly inhibited in response to adenosine diphosphate, thrombin, or collagen/epinephrine suggesting platelet dysfunction (Kristensen et al 1994b). Similar studies in cats have not been performed.
In our study, PBA test was positive in cats suffering from various diseases. Nineteen out of 42 thrombocytopenic cats had positive test results. In 17 of these 19 cats, a secondary ITP was suggested because of the confirmation of an underlying disease. Two cats had probable primary ITP which appears to be rare in cats. Secondary ITP may play an important role in the pathogenesis and presentation of other disease syndromes.
Acknowledgments
We would like to thank the laboratory staff of the Clinic for Small Animals, Free University of Berlin, and the Institute of Immunology, University of Veterinary Medicine, Hannover, in particular Silke Schoeneberg, Sonja Kordex and Udo Rabe.
References
- Axthelm M.K., Krakowa S. Canine distemper virus-induced thrombocytopenia, American Journal of Veterinary Research 48, 1987, 1269–1275. [PubMed] [Google Scholar]
- Baldwin C.J., Cowell R.L. Thrombocytopenia. August J.R. Consultations in Feline Internal Medicine, 4th edn, 2001, WB Saunders Company: Philadelphia, 468–478. [Google Scholar]
- Bithell T.C. Thrombotic thrombocytopenic purpura and other forms of non-immunologic platelet destruction. Lee G.R., Bithell T.C., Foerster J., Athens J.W., Lukens J.N. Wintrobe's Clinical Hematology, 9th edn, 1993, Lea and Fiebiger: Philadelphia, 1356–1362. [Google Scholar]
- Cain G.R., Cain J.L., Turrel J.M., Theilen G., Jain N.C. Immune-mediated hemolytic anemia and thrombocytopenia in a cat after bone marrow transplantation, Veterinary Pathology 25, 1988, 161–162. [DOI] [PubMed] [Google Scholar]
- Campbell K.L., George J.W., Greene C.E. Application of the enzyme-linked immunosorbent assay for the detection of platelet antibodies in dogs, American Journal of Veterinary Research 45, 1984, 2561–2564. [PubMed] [Google Scholar]
- Colman R.W., Nachmias V.T., Cines D.B., Schreiber A.D. Effect of antiplatelet antibody on platelet shape change, volume, and morphology, American Journal of Physiology 244, 1983, 357–361. [DOI] [PubMed] [Google Scholar]
- Couto C.G. Disorders of hemostasis. Nelson R.W., Couto C.G. Small Animal Internal Medicine, 3rd edn, 2003, Mosby: St. Louis, 1185–1199. [Google Scholar]
- Gabbert N.H. Systemic lupus erythematosus in a cat with thrombocytopenia, Veterinary Medicine, Small Animal Clinician January, 1983, 77–79.
- Garon C.L., Scott M.A., Selting K.A., Cohn L.A. Idiopathic thrombocytopenic purpura in a cat, Journal of the American Animal Hospital Association 35, 1999, 464–470. [DOI] [PubMed] [Google Scholar]
- Grindem C.B., Breitschwerdt E.B., Corbett W.T., Jans H.E. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases, Veterinary Clinical Pathology 20, 1991, 38–43. [DOI] [PubMed] [Google Scholar]
- Hart S., Nolte I. Zur Thrombozytenaggregation bei der Katze, Tierärztliche Praxis 19, 1991, 413–418. [PubMed] [Google Scholar]
- Harvey J.W., Gaskin J.M. Idiopathic thrombocytopenia and hemorrhage followed by thrombocytosis in a cat, Feline Practice 10, 1980, 25–31. [Google Scholar]
- Helfand S.C. Platelets and neoplasia, Veterinary Clinics of North America Small Animal Practice 18, 1988, 131–156. [DOI] [PubMed] [Google Scholar]
- Jordan H.L., Grindem C.B., Breitschwerdt E.B. Thrombocytopenia in cats: a retrospective study of 41 cases, Journal of Veterinary Internal Medicine 7, 1993, 261–265. [DOI] [PubMed] [Google Scholar]
- Joshi B.C., Raplee R.G., Powell A.L., Hancock F. Autoimmune thrombocytopenia in a cat, Journal of American Animal Hospital Association 15, 1979, 585–588. [Google Scholar]
- Karpatkin S., Xia J., Patel J., Thorbecke G.J. Serum platelet-reactive IgG of autoimmune thrombocytopenic purpura patients is not F(ab')2 mediated and a function of storage, Blood 80, 1992, 3164–3172. [PubMed] [Google Scholar]
- Kiefel V., Santoso S. Nachweis von thrombozytären Antigenen und Antiköpern. Mueller-Eckhardt C. Transfusionsmedizin, 2nd edn, 1996, Springer Verlag: Berlin, 597–602. [Google Scholar]
- Kohn B., Engelbrecht R., Leibold W., Giger U. Klinische Befunde, Diagnostik und Behandlungserfolge bei der primären und sekundären immunbedingten Thrombozytopenie beim Hund, Kleintierpraxis 45, 2000a, 893–907. [Google Scholar]
- Kohn B., Engelbrecht R., Giger U., Leibold W. Platelet-bound antibodies in dogs with thrombocytopenia and change with treatment, Journal of Veterinary Internal Medicine 14, 2000b, 134, (abstract)10772483 [Google Scholar]
- Kristensen A.T., Weiss D.J., Klausner J.S., Laber J., Christie D.J. Detection of antiplatelet antibody with a platelet immunofluorescence assay, Journal of Veterinary Internal Medicine 8, 1994a, 36–39. [DOI] [PubMed] [Google Scholar]
- Kristensen A.T., Weiss D.J., Klausner J.S. Platelet dysfunction associated with immune-mediated thrombocytopenia in dogs, Journal of Veterinary Internal Medicine 8, 1994b, 323–327. [DOI] [PubMed] [Google Scholar]
- Lewis D.C., Meyers K.M., Callan M.B., Bucheler J., Giger U. Detection of platelet-bound and serum platelet-bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs, Journal of the American Veterinary Medical Association 206, 1995a, 47–52. [PubMed] [Google Scholar]
- Lewis D.C., McVey D.S., Shuman W.S., Muller W.B. Development and characterization of a flow cytometric assay for detection of platelet-bound immunoglobulin G in dogs, American Journal of Veterinary Research 56, 1995b, 1555–1558. [PubMed] [Google Scholar]
- Lewis D.C., Meyers H.M. Studies of platelet-bound and serum platelet-bindable immunoglobulins in dogs with idiopathic thrombocytopenic purpura, Experimental Hematology 24, 1996a, 696–701. [PubMed] [Google Scholar]
- Lewis D.C., Meyers K.M. Canine idiopathic thrombocytopenic purpura, Journal of Veterinary Internal Medicine 10, 1996b, 207–218. [DOI] [PubMed] [Google Scholar]
- Meyers K.M., Holmsen H., Seachord C.L. Comparative study of platelet dense granule constituents, American Journal of Physiology 243, 1982, 454–461. [DOI] [PubMed] [Google Scholar]
- Mischke R. Hämostase. Kraft W., Dürr U.M. Klinische Labordiagnostik in der Tiermedizin, 5th edn, 1999, Schattauer Verlagsgesellschaft: Stuttgart, 92–111. [Google Scholar]
- Moritz A., Hoffmann C. Thrombozytenzählung bei der Katze, Tierärztliche Praxis 25, 1997, 695–700. [PubMed] [Google Scholar]
- Peterson J.L., Couto C.G., Wellman M.L. Hemostatic disorders in cats: A retrospective study and review of the literature, Journal of Veterinary Internal Medicine 9, 1995, 298–303. [DOI] [PubMed] [Google Scholar]
- Reagan W.J., Rebar A.H. Platelet disorders. Ettinger S.J. Textbook of Veterinary Internal Medicine, 4th edn, 1995, W.B. Saunders Company: Philadelphia, 1967–1976. [Google Scholar]
- Retzlaff S., Mesters R.M. Hämostasestörungen. Berdel W.E., Böhm M., Classen M., Diehl V., Kochsiek K., Schmiegel W. Innere Medizin, 5th edn, 2004, Urban und Fischer Verlag: München, 797–810. [Google Scholar]
- Russell K.E., Grindem C.B. Secondary thrombocytopenia. Feldman B., Zinkl J., Jain N. Schalm's Veterinary Hematology, 5th edn, 2000, Lippincott, Williams and Williams: Baltimore, 487–495. [Google Scholar]
- Tasker S., Mackin A.J., Day M.J. Primary immune-mediated thrombocytopenia in a cat, Journal of Small Animal Practice 40, 1999, 127–131. [DOI] [PubMed] [Google Scholar]
- Thomas J.S., Green R.A. Clotting times and antithrombin III activity in cats with naturally developing diseases: 85 cases (1984–1994), Journal of the American Veterinary Medical Association 9, 1998, 1290–1295. [PubMed] [Google Scholar]
- Tyler R.D., Cowell R.L., Loar A.S. Tests for autoimmune diseases. August J.R. Consultations in Feline Internal Medicine, 1991, WB Saunders Company: Philadelphia, 359–365. [Google Scholar]
- Weiss D.J. Platelet production defects. Feldman B., Zinkl J., Jain N. Schalm's Veterinary Hematology, 5th edn, 2000, Lippincott, Williams and Williams: Baltimore, 469–471. [Google Scholar]
- Zelmanovic D., Hetherington E.J. Automated analysis of feline platelets in whole blood, including platelet count, mean platelet volume, and activation state, Veterinary Clinical Pathology 27, 1998, 2–9. [DOI] [PubMed] [Google Scholar]


