Abstract
Feline coronavirus (FCoV) varies greatly from causing subclinical or mild enteric infections to fatal feline infectious peritonitis (FIP). The open reading frame (ORF) 7b of FCoV has been speculated to play a determining role in virulence as deletions were found to be associated with avirulent viruses. To further clarify the correlation between this gene and FIP, clinical samples from 20 cats that had succumbed to wet-type FIP and 20 clinically healthy FCoV-infected cats were analysed. The ORF7b from the peritoneal/pleural effusions of FIP cats and from the rectal swabs of healthy cats was amplified. Of the 40 FCoVs analysed, 32 were found to have an intact 7b gene whereas eight showed deletions of either three or 12 nucleotides. Surprisingly, among the eight viruses with deletions, three were from FIP diseased cats. These results show that deletions in the ORF7b gene are not constrained to low pathogenicity/enteric biotypes but also associated with pathogenicity/FIP biotypes of FCoV.
Feline coronaviruses (FCoVs) are enveloped viruses with a large, capped and polyadenylated RNA genome of about 29,190 nucleotides. 1,2 The FCoVs are group 1 coronaviruses, recently designated as members of subgroup 1a in the family Coronaviridae. Other members of this subgroup include transmissible gastroenteritis virus, canine coronavirus, raccoon dog coronavirus (RDCoV/GZ43/03), and Chinese ferret badger coronavirus (CFBCoV/DM95/03). 3
The FCoVs associated with mild enteric infections and infectious peritonitis. 4 Infection of FCoVs is worldwide; the seroprevalence varies from around 30%, 5–7 to 80%. 8,9 However, among the seropositive cats only a relatively small portion, eg, 5%, 8 11.8% 10 and 15.4%, 6 develop FIP. Currently, it is speculated that the key pathogenic feature of FCoV to induce FIP is its ability to replicate to high viral loads in monocytes and macrophages. Enteric pathotypes are able to circulate in monocytes/macrophages, but that higher rates of replication and dissemination are seen in FIP pathotypes. 11–16
The ORF7b is located at the very 3'end of the FCoV genome, which encodes a 26.5-KDa non-structural glycoprotein. The function of glycoprotein 7b in the life cycle of FCoV is not clear. 17 It has been speculated that ORF7b plays a determinative role in FCoV virulence as viruses with a truncated gene have been found to be associated with enteric infection only. 18 Field FCoV strains of FIP cats analysed thus far all contain intact ORF7b. 19–24
In order to gain a better understanding of the correlation between ORF7b and the virulence of the virus, we investigated the ORF7b of FCoVs from clinical specimens from 40 cats, half from wet-type FIP animals and half from clinically healthy cats. In contrast to the previous finding, short deletions of the 7b gene of FCoVs in body effusions were detected from some of our FIP cats.
Materials and Methods
Specimens Collection and FCoV Screening
Clinical specimens collected from cats presenting at the Animal Hospital of National Taiwan University over a 4-year period (2002–2005) were screened for FCoV by reverse transcriptase-polymerase chain reaction (RT-PCR) as described by Herrewegh et al. 25
Sample Preparation and Reverse Transcription
Rectal swab samples were suspended in 1 ml of 0.1% DEPC water. Total RNA was extracted from 300 μl of the body effusion or suspension of the rectal swab using Trizol. 26 Eleven microlitres of RNA-containing sample was added to the premix, consisting of 4 μl of 5× first strand buffer, 2.5 mmol dNTP (GeneTeks, Bioscience, Taipei), 10 pmol primer P211: 5′-CACTAGATCCAGACGTTAGCTC-3′, 25 200 mM dithiothreitol and, finally, 1 μl containing 200 unit Moloney murine leukaemia virus reverse transcriptase (Invitrogen, California), were added in a 0.6 ml reaction tube. This reaction mixture was then briefly centrifuged and incubated at 37 °C for 60 min, then at 72 °C for 15 min and finally at 94 °C for 5 min.
Seminested PCR Amplification of the 7b gene
Primers for seminested PCR were chosen from a relatively conserved region representation of the FCoV genome and an outline of the primer locations (Fig 1A). Details of the primer sequences are shown in Table 1. Following reverse transcription, 2 μl of the RT reaction mixture was added to 28 μl of the PCR mixture, which consisted of 3 μl 10× Taq buffer, each primer (5 pmol), dNTP (2.5 mmol), 1U of Taq DNA polymerase (GeneTeks, BioScience, Taipei) and 22.5 μl of 0.1% DEPC water. Thermal cycling (Mastercycler Personal, Eppendorf), consisted of 3 min of preheating at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 1 min with a final extension at 72 °C for 5 min. Seminested PCR was performed on 2 μl of the first PCR product using nested primers (7a-F1 and U-R1) under reaction conditions identical to those used in the first amplification. A negative control without DNA template was included to monitor any cross-contamination.
Fig 1.
Amplification of ORF7b of FCoV from the clinical specimens. (A) Feline coronavirus genome with enlargement of 3'-terminal region (positions and orientations of the seminested PCR primers are indicated). Amplification of 766 base pair (bp) DNA fragment from peritoneal/pleural effusions from 20 FIP cats (B), and from rectal swabs of 20 clinical healthy cats (C). M=100 bp molecular weight ladder, P=positive control, N=negative control.
Table 1.
Oligonucleotide primers used for amplification of 7b gene
| Primer | Sequence (5′to 3′) | Position | Orientation |
|---|---|---|---|
| U-R1 | ACCATTCTGTACAAGAGTAG | 8677–8696 ∗ | Antisense |
| 7a-F1 | CTGCGAGTGATCTTTCTAG | 7929–7947 ∗ | Sense |
Numerical position on the genome of FCoV/NTU2/R/′03 as determined from the 5′ATG start codon; S-gene, accession number DQ160294.
Analysis of PCR-amplified Products, Sequencing and Sequence Analysis
A 10 μl sample from each PCR mixture was analysed using a 1.5% agarose gel (Viogene, Taipei) for electrophoresis. Amplification products were visualised using UV illumination after ethidium bromide staining. The nucleotide sequences of the targeted DNA fragments were purified (Geneaid Biotech, Taipei) and determined from both orientations using an auto sequencer (ABI 3730XL, USA). The cDNA sequences of ORF7b were then compared with other FCoVs from around the world. Multiple alignments of nucleic acid sequences were performed by the Jotun Hein method using the MegAlign program (DNASTAR, Madison, WI).
Results
FCoV-infected Cats
Forty cats with positive RT-PCR results were enrolled in this study. Based on the clinical manifestation and/or necropsy findings these cats were further divided into two groups. The first group included 20 clinically healthy cats living in households where there was a history of FIP-related death. Positive RT-PCR results were obtained from the rectal swabs for this group. The age of the cats ranging from 2 month to 8 years (mean 1.49±1.96; n=19) and no sex difference (10 animals for each sex) was observed (Table 2). Another 20 cats that showed a clinical history of anorexia, weight loss, lethargy, icterus, mild antibiotic-unresponsive fever, abdominal distension and/or thoracic effusion with a low albumin to globulin ratio were included in the second group. These cats subsequently expired or were euthanased with a definite diagnosis of wet-type FIP established from positive RT-PCR for FCoV in body effusions 27,28 and post-mortem histopathological examination (when available) with typical pyogranulomatous lesions. In some cases, immunohistochemical staining for FCoV antigen was performed (data not shown). Cats in this group died between 3 months to 4 years (mean 1.15±1.15; n=20). Seven of the cats were female, 13 were male (Table 2). Details with respect to the clinical history of individual animals are listed in Table 2.
Table 2.
The clinical status of 40 cats and the corresponding FCoV analysed
| Animal | Virus | |||||
|---|---|---|---|---|---|---|
| Clinical status ∗ | Specimen | Year | Sex | Age† | Accession number (Denomination) | Denomination in brief |
| FIP | Pleural effusion | 2002 | M | 2Y | DQ648122 (FCoV/NTU1/P/'02) | NTU1P |
| FIP | Ascites | 2003 | F | 8M | DQ675414 (FCoV/NTU3/A/'03) | NTU3A |
| FIP | Pleural effusion | 2003 | F | 4M | DQ675415 (FCoV/NTU4/P/'03) | NTU4P |
| FIP | Ascites | 2003 | M | 4M | DQ675416 (FCoV/NTU5/A/'03) | NTU5A |
| FLIP | Ascites | 2003 | M | 3Y | DQ675417 (FCoV/NTU6/A/'03) | NTU6A |
| FIP | Ascites | 2003 | M | 4.5M | DQ675418 (FCoV/NTU7/A/'03) | NTU7A |
| FIP• | Pleural effusion | 2004 | M | 9M | DQ675419 (FCoV/NTU8/P/'04) | NTU8P |
| Healthy• | Rectal swab | 2004 | M | 8M | DQ675420 (FCoV/NTU9/R/'04) | NTU9R |
| Healthy• | Rectal swab | 2004 | M | 2Y | DQ675421 (FCoV/NTU10/R/'04) | NTU10R |
| Healthy• | Rectal swab | 2004 | F | 3Y | DQ675422 (FCoV/NTU11/R/'04) | NTU11R |
| Healthy• | Rectal swab | 2004 | M | 8M | DQ675423 (FCoV/NTU12/R/'04) | NTU12R |
| Healthy▴ | Rectal swab | 2004 | F | 4Y | DQ675424 (FCoV/NTU13/R/'04) | NTU13R |
| Healthy▴ | Rectal swab | 2004 | M | 6M | DQ675425 (FCoV/NTU14/R/'04) | NTU14R |
| Healthy▴ | Rectal swab | 2004 | F | 8Y | DQ675426 (FCoV/NTU15/R/'04) | NTU15R |
| Healthy▴ | Rectal swab | 2004 | F | 2.5Y | DQ675427 (FCoV/NTU16/R/'04) | NTU16R |
| FIP | Ascites | 2004 | M | 5.6M | DQ675428 (FCoV/NTU17/A/'04) | NTU17A |
| FIP | Pleural effusion | 2004 | M | 7M | DQ675429 (FCoV/NTU18/P/'04) | NTU18P |
| FIP | Ascites | 2004 | F | 4M | DQ675430 (FCoV/NTU19/A/'04) | NTU19A |
| FIP | Pleural effusion | 2004 | F | 2.5Y | DQ675431 (FCoV/NTU20/P/'04) | NTU20P |
| FIP | Ascites | 2004 | M | 8M | DQ675432 (FCoV/NTU21/A/'04) | NTU21A |
| Healthy▪ | Rectal swab | 2004 | M | 3M | DQ675433 (FCoV/NTU23/R/'04) | NTU23R |
| Healthy▪ | Rectal swab | 2004 | F | 5M | DQ675434 (FCoV/NTU24/R/'04) | NUT24R |
| Healthy▪ | Rectal swab | 2004 | M | 2.5M | DQ675435 (FCoV/NTU25/R/'04) | NTU25R |
| FIP♦ | Ascites | 2004 | M | 5M | DQ675436 (FCoV/NTU26/A/'04) | NTU26A |
| Healthy♦ | Rectal swab | 2004 | F | 2Y | DQ675437 (FCoV/NTU27/R/'04) | NTU27R |
| Healthy♦ | Rectal swab | 2004 | M | 3M | DQ675438 (FCoV/NTU28/R/'04) | NTU28R |
| Healthy♦ | Rectal swab | 2004 | F | 5M | DQ675439 (FCoV/NTU29/R/'04) | NTU29R |
| FIP | Ascites | 2004 | F | 4M | DQ675440 (FCoV/NTU31/A/'04) | NTU31A |
| Healthy | Rectal swab | 2004 | M | 1Y5M | DQ675441 (FCoV/NTU32/R/'04) | NTU32R |
| Healthy▾ | Rectal swab | 2004 | F | 3M | DQ675442 (FCoV/NTU33/R/'04) | NTU33R |
| Healthy▾ | Rectal swab | 2004 | F | 3M | DQ675443 (FCoV/NTU34/R/'04) | NTU34R |
| Healthy▾ | Rectal swab | 2004 | M | 3M | DQ675444 (FCoV/NTU35/R/'04) | NTU35R |
| FIP | Ascites | 2004 | M | 6M | DQ675445 (FCoV/NTU36/A/'04) | NTU36A |
| FIP | Ascites | 2005 | M | 3M | DQ675446 (FCoV/NTU37/A/'05) | NTU37A |
| Healthy | Rectal swab | 2005 | M | >3Y | DQ675447 (FCoV/NTU38/R/'05) | NTU38R |
| FIP | Ascites | 2005 | F | 4Y | DQ675448 (FCoV/NTU40/A/'05) | NTU40A |
| FIP | Ascites | 2005 | M | 3Y | DQ675449 (FCoV/NTU41/A/'05) | NTU41A |
| FIP | Ascites | 2005 | M | 2Y | DQ675450 (FCoV/NTU42/A/'05) | NTU42A |
| Healthy | Rectal swab | 2005 | F | 2M | DQ675451 (FCoV/NTU43/R/'05) | NTU43R |
| FIP | Ascites | 2005 | F | 7M | DQ675452 (FCoV/NTU44/A/'05) | NTU44A |
•, ▴, ▪, ♦, and ▾ each represent cats from the same household.
Age of the cat at presenting: Y=years, M=months. FIP=feline infectious peritionitis.
ORF7b Deletions Detected from both FIP and Healthy Cats
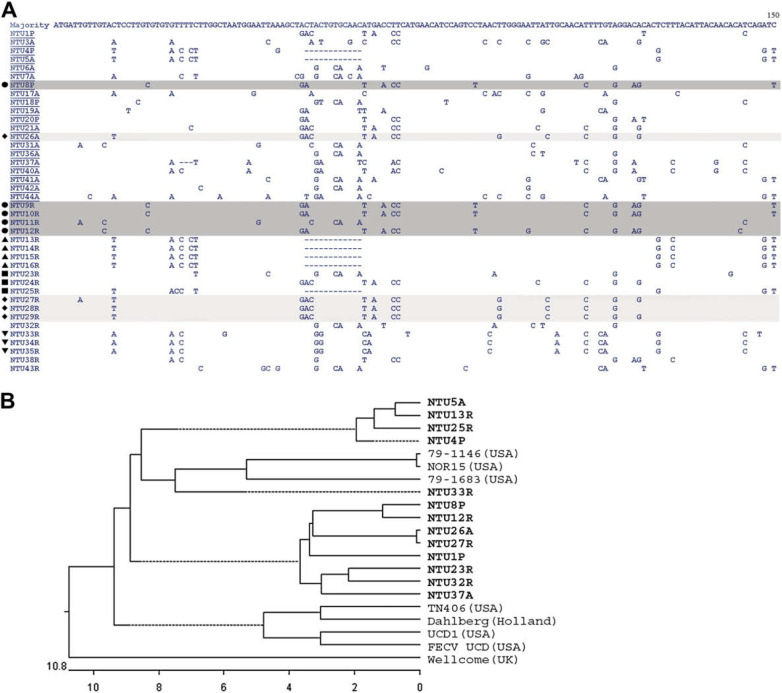
The second round RT-PCR yielded the expected 766 bp products from the body effusion of the 20 FIP cats (Fig 1B) and from the rectal swabs of 20 clinically healthy cats (Fig 1C). The complete ORF7b (621 bp) sequences for the 40 viruses were submitted to GenBank under accession numbers DQ648122 and DQ675414–DQ675452. Specimen type (P=pleural effusion, A=ascites, R=rectal swab) for virus derivation is incorporated into the viral denomination (Table 2). Sequence analysis revealed that the ORF7b of the naturally occurring FCoVs was mostly intact (32/40). However, three- and 12-nucleotide deletions were found in one (NTU37A) and seven (NTU4P, NTU5A, NTU13R, NTU14R, NTU15R, NTU16R, NTU25R) FCoVs, respectively. Four isolates with the 12-nucleotide deletion originated from the same household, whereas remaining isolates had an unrelated origin (Fig 2A). The two deletions are both located near the 5′terminus, covering positions 30–32 and 53–64, respectively; both are in-frame deletions resulting in loss of either one or four amino acids in the ORF7 protein.
Fig 2.
ORF7b gene alignment and phylogenetic analysis. (A) Multiple nucleotide sequence alignment of the sample population (n=40). The upper and lower halves are from FIP (underlined) and clinically healthy animals, respectively. ♦, •, ▴, ▾, and ▪ each represent felines from the same household. The majority of our cats show consensus strength sequences. (B) Phylogenetic tree illustrating the relationship between the local and published strains. 20 The phylogenetic trees were generated using the DNASTAR MegAlign program. The scale beneath the tree measures the distance between sequences, and the units at the bottom of the tree indicates the number of substitution events. The dotted lines indicate a negative length introduced by averaging the tree.
Of the eight FCoVs with deletions, five originated from the rectal swabs of clinically healthy cats (NTU13R, NTU14R, NTU15R, NTU16R and NTU25R), and three from the body effusions of FIP cats (NTU4P, NTU5A and NTU37A) (Fig 2A). Regardless of the clinical status of the cats, viruses originating from the same household were similar to one another (NTU9R, NTU10R, NTU11R, NTU12R and NTU8P; NTU27R, NTU28R, NTU29R and NTU26A). No sequence specificity related to the FIP occurrence could be identified throughout the ORF7b from the 40 FCoVs isolated from the FIP and clinically healthy cats (Fig 2A).
Genetic Comparison of FCoV ORF7b
An overall nucleotide sequence identity of 87.6–100% was demonstrated for the 40 local FCoVs analysed. Figure 2B shows the phylogenic relationship of the local (n=13), American (n=6), British (n=1) and Dutch (n=1) strains. Except for NTU33R, all the local FCoVs fall into two clusters genetically separated from foreign strains (Fig 2B). The NTU33R strain shows a relatively high sequence homology with two American strains, 79–1146 (91.5%) and NOR15 (91.3%). This virus originated from a clinically healthy feline. Two other cats (NTU34R & NTU35R) in the same household as NTU33R harboured viruses with nearly identical ORF7b sequences (Fig 2A).
Discussion
In 1995, Herrewegh et al, first demonstrated the association between ORF7b gene integrity and FCoV virulence in a comparative sequence analysis. 20 The gene became the focus of attention, with studies of viruses from different geographical areas of the world. The sequence findings analysed thus far, together with those from the present study, are summarised in Table 3. The full-length ORF7b gene consists of 621 nt, however, only partial sequences are available for comparison of some viruses. Of the total of 92 FCoVs analysed, deletions were identified in 13. Five of the 13 deleted viruses were laboratory strains, with eight from clinical specimens obtained in this study. On further examination, the length range of the deletions was 3–406 nt for these ORF7b-deleted FCoVs (Table 4). Five isolates with rather large deletions (56–406 nt) were all laboratory passage strains. 20 The only field viruses originating from FIP cats with deletions were the three identified in this study. The characteristics of our deletions are: (i) they are relatively short, eg, either 3 nt (NTU37A) or 12 nt (NTU4P, NTU5A); (ii) they are all in-frame deletions; and, (iii) in comparison to the downstream location for the major deletions in the laboratory strains, our deletions are all located near the 5′terminus of the gene, eg, nt 30–32 (NTU37A) and nt 53–64 (NTU4P, NTU5A).
Table 3.
Sequencing studies of 7b gene conducted around the world
| Geographical origin of virus strain | Sequencing area (nt) | Number of virus with deletion ∗ | Reference | |
| Laboratory | Field | |||
| Taiwan | 1–621 | 8/40 | ||
| Netherlands | 1–621 | 0/1† | Herrewegh et al 1995 20 | |
| USA | 1–621 | 5/11‡ | Herrewegh et al 1995 20 | |
| UK | 1–621 | 0/1§ | Herrewegh et al 1995 20 | |
| Germany | 7–146 | 0/11 | Herrewegh et al 1997 21 | |
| USA | 1–621 | 0/7∥ | 0/3 | Vennema et al 1998 24 |
| USA | 1–621 | 0/9 | Kennedy et al 2001 23 | |
| Italy | 124–613 | 0/9 | Battilani et al 2003 19 | |
| Total | 5/20 | 8/72 | ||
Number of deleted strains/total tested.
FIPV Dahlberg.
FIPV 79–1146, TN406-LP, TN406-HP, UCD1, UCD2, UCD3, UCD4, NOR15, NOR15/tsDF2, FECV UCD, 79–1683.
FIPV Wellcome.
FECV UCD, FECV RM, FIPV UCD3, UCD8, UCD9, UCD10, FIPV 79–1146.
Table 4.
Comparison of the deletions observed in ORF7b of virus strains analysed in this study and other publications
| Virus strain | Source | Deletion | ||
| Position | Nucleotide number | In frame | ||
| FCoV/NTU4/P/'03 | field/FIP ∗ | 53–64 | 12 | Yes |
| FCoV/NTU5/A/'03 | field/FIP | 53–64 | 12 | Yes |
| FCoV/NTU13/R/'04† | field/healthy | 53–64 | 12 | Yes |
| FCoV/NTU25/R/'04 | field/healthy | 53–64 | 12 | Yes |
| FCoV/NTU37/A/'05 | field/FIP | 30–32 | 3 | Yes |
| TN406-del | lab/avirulent/high-passage‡ | 118–173 | 56 | No |
| UCD4-del | lab/unclear§/6th passage | 456–512 | 57 | Yes |
| UCD2 | lab/avirulent/unknown | 96–190 and 245–339 | 95 and 95 | No |
| ts-DF2 | lab/avirulent/unknown | 120–525 | 406 | No |
| 79–1683 | lab/avirulent/unknown | 372–609 | 238 | No |
Source/animal status.
FCoV/NTU13/R/'04, FCoV/NTU14/R/'04, FCoV/NTU15/R/'04, FCoV/NTU16/R/'04 were from the same household and the sequences were identical with their genomic RNA.
Source/virulence/number of passage.
UCD4 is a virulent strain, 30 however, the virulence of this deleted mutant generated in the sixth passage during in vitro culture hasn't yet been tested.
Restriction fragment polymorphism analysis to monitor the presence of small deletions in FCoVs originating from naturally infected cats (12 FIP and four healthy cats), did not detect any deletions. 20 The data strongly suggest that ORF7b is maintained, however, the presence of nonsense codons or very small deletions (<10 bp) cannot be excluded as addressed by the authors. Our data revealed small deletions do associated with field strains. Moreover, the deletions were observed in FCoV not only from healthy cats, but also from some of the FIP animals.
The current understanding with respect to ORF7b includes the following: (i) the gene can only be found in feline and canine, but not in porcine, coronaviruses 18,29 ; (ii) the gene encodes a non-structural, soluble, secretory glycoprotein 17 ; (iii) the glycoprotein 7b is dispensable for viral replication in tissue culture; 17 and, (iv) the gene is readily lost during in vitro propagation. 20 Herein, we report identification of three FIP-related viruses with deletions in the 5′terminus of their ORF7b gene. These deletions are very small and result in no change in the reading frame, thus, the function of the deleted gp 7b is very likely to be intact in these viruses. In this study, we found that amplification of ORF7b from some FIP cats was difficult. Alteration of primer sets to target specific gene regions (data not shown), has not solved the problem of effective amplification. Is gp 7b truly indispensable in the natural FIP infection? Clarification of the presence or absence of ORF7b in FCoVs difficult to amplify may elucidate the role of gp7b in FIP pathogenesis.
In conclusion, deletion in ORF7b were previously identified in laboratory strains of variable passage number and were considered to be associated with avirulence. 20 In this study, field isolates showed small in-frame deletions in the 3′ region of ORF7b in both virulent and avirulent FCoV strains. Deletions had not previously been observed in FIP pathotypes. However, no deletions were seen in the majority of field strains. This implies that the presence of this deletion is not correlated with pathogenicity.
References
- 1.Dye C., Siddell S.G. Genomic RNA sequence of Feline coronavirus strain FIPV WSU-79/1146, J Gen Virol 86, 2005, 2249–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye C., Siddell S.G. Genomic RNA sequence of feline coronavirus strain FCoV C1Je, J Feline Med SurG 9, 2007, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijaykrishna D., Smith G.J., Zhang J.X., Peiris J.S., Chen H., Guan Y. Evolutionary insights into the ecology of coronaviruses, J Virol 81, 2007, 4012–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis, Am J Vet Res 42, 1981, 368–377. [PubMed] [Google Scholar]
- 5.Bell E.T., Toribio J.A., White J.D., Malik R., Norris J.M. Seroprevalence study of feline coronavirus in owned and feral cats in Sydney, Australia, Austral Vet J 84, 2006, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohdatsu T., Okada S., Ishizuka Y., Yamada H., Koyama H. The prevalence of types I and II feline coronavirus infections in cats, J Vet Med Sci 54, 1992, 557–562. [DOI] [PubMed] [Google Scholar]
- 7.Kiss I., Kecskemeti S., Tanyi J., Klingeborn B., Belak S. Prevalence and genetic pattern of feline coronaviruses in urban cat populations, Vet J 159, 2000, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen N.C. Serologic studies of naturally occurring feline infectious peritonitis, Am J Vet Res 37, 1976, 1449–1453. [PubMed] [Google Scholar]
- 9.Sparkes A.H., Gruffydd-Jones T.J., Howard P.E., Harbour D.A. Coronavirus serology in healthy pedigree cats, Vet Rec 131, 1992, 35–36. [DOI] [PubMed] [Google Scholar]
- 10.Sparkes A.H., Gruffydd-Jones T.J., Harbour D.A. Feline coronavirus antibodies in UK cats, Vet Rec 131, 1992, 223–224. [DOI] [PubMed] [Google Scholar]
- 11.Dewerchin H.L., Cornelissen E., Nauwynck H.J. Replication of feline coronaviruses in peripheral blood monocytes, Arch Virol 150, 2005, 2483–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kipar A., Bellmann S., Gunn-Moore D.A., et al. Histopathological alterations of lymphatic tissues in cats without feline infectious peritonitis after long-term exposure to FIP virus, Vet Microbiol 69, 1999, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kipar A., Kohler K., Leukert W., Reinacher M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection, J Compar Pathol 125, 2001, 182–191. [DOI] [PubMed] [Google Scholar]
- 14.Kipar A., Baptiste K., Barth A., Reinacher M. Natural FCoV infection: Cats with FIP exhibit significantly higher viral loads than healthy infected cats, J Feline Med Surg 8, 2006, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meli M., Kipar A., Muller C., et al. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (FCoV) type I and in naturally FCoV-infected cats, J Feline Med Surg 6, 2004, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoddart C.A., Scott F.W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence, J Virol 63, 1989, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vennema H., Heijnen L., Rottier P.J., Horzinek M.C., Spaan W.J. A novel glycoprotein of feline infectious peritonitis coronavirus contains a KDEL-like endoplasmic reticulum retention signal, J Virol 66, 1992, 4951–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vennema H., Rossen J.W., Wesseling J., Horzinek M.C., Rottier P.J. Genomic organization and expression of the 3′ end of the canine and feline enteric coronaviruses, Virol 191, 1992, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battilani M., Coradin T., Scagliarini A., et al. Quasispecies composition and phylogenetic analysis of feline coronaviruses (FCoVs) in naturally infected cats, FEMS Immunol Med Microbiol 39, 2003, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrewegh A.A.P.M., Vennema H., Horzinek M.C., Rottier P.J.M., de Groot R.J. The molecular genetics of feline coronaviruses: Comparative sequence analysis of the ORF7a/7b transcription unit of different biotypes, Virol 212, 1995, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrewegh A.A.P.M., Mahler M., Hedrich H.J., et al. Persistence and evolution of feline coronavirus in a closed cat-breeding colony, Virol 234, 1997, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy M.A., Brenneman K., Millsaps R.K., Black J., Potgieter L.N. Correlation of genomic detection of feline coronavirus with various diagnostic assays for feline infectious peritonitis, J Vet Diag Invest 10, 1998, 93–97. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy M., Boedeker N., Gibbs P., Kania S. Deletions in the 7a ORF of feline coronavirus associated with an epidemic of feline infectious peritonitis, Vet Microbiol 81, 2001, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endmic feline enteric coronaviruses, Virol 243, 1998, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrewegh A.A., de Groot R.J., Cepica A., Egberink H.F., Horzinek M.C., Rottier P.J. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR, J Clin Microbiol 33, 1995, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction, Analy Biochem 162, 1987, 156–159. [DOI] [PubMed] [Google Scholar]
- 27.Addie D.D., Paltrinieri S., Pedersen N.C. Recommendations from workshops of the second international feline coronavirus/feline infectious peritonitis symposium, J Feline Med Surg 6, 2004, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann K., Binder C., Hirschberger J., et al. Comparison of different tests to diagnose feline infectious peritonitis, J Vet Int Med 17, 2003, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Groot R.J., Andeweg A.C., Horzinek M.C., Spaan W.J. Sequence analysis of the 3′-end of the feline coronavirus FIPV 79–1146 genome: Comparison with the genome of porcine coronavirus TGEV reveals large insertions, Virology 167, 1988, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen N.C., Floyd K. Experimental studies with three new strains of feline infectious peritonitis virus: FIPV-UCD2, FIPV-UCD3, and FIPV-UCD4, Compend Contin Educ Pract Vet 7, 1985, 1001–1010. [Google Scholar]