Abstract
Viral myocarditis has been suggested as an etiology for cardiomyopathy in several mammalian species. Myocarditis and idiopathic cardiomyopathy have been reported in the domestic cat, although a viral etiology has not been demonstrated. Because of the continuing interest in the potential relationship between viral myocarditis and cardiomyopathy, we evaluated hearts from cats with spontaneous, idiopathic cardiomyopathy for viral genomic material within myocytes by polymerase chain reaction, and for the presence of myocarditis by light microscopy. Thirty-one (31) formalin-fixed hearts from domestic cats who died of idiopathic cardiomyopathy were randomly selected from pathology archives. Seventeen (17) formalin-fixed hearts from healthy cats were similarly selected as normal controls. The polymerase chain reaction (PCR) was used to evaluate myocardial tissue for the presence of viral genome from feline panleukopenia virus, herpes virus, calici virus, and corona virus. Hearts were examined using light microscopy for histologic evidence of myocarditis according to the Dallas criteria. Panleukopenia virus was identified by PCR in 10 of 31 cats with cardiomyopathy but in none of the controls. Neither cardiomyopathic or control cats tested positive by PCR for herpes virus, calici virus, and corona virus. Myocarditis was detected by histologic examination in 18 of 31 cardiomyopathic cats and in none of 17 control cats. Myocarditis and or feline panleukopenia virus genome was detected in felines with idiopathic hypertrophic, dilated, and restrictive cardiomyopathy, suggesting a possible role of viral infection and inflammation in the pathogenesis of cardiomyopathy in this species.
Introduction
While the capability of viruses to infect and injure cardiovascular tissue is well established, a causal association between viral infection and myocardial cell injury has been difficult to prove, and the diagnosis of viral myocarditis is often elusive. Accordingly, an animal model of myocarditis and cardiomyopathy could be useful to explore relationships between myocardial inflammation and myocardial injury. The domestic cat provides a model of spontaneous cardiomyopathy whose histopathologic features include myocyte injury, necrosis, and in some cases, myocarditis 1, 2, 3, 4, 5. Feline cardiomyopathy is characterized by myocardial dysfunction and structural derangement and is an important cause of arrhythmias, heart failure, and death 1, 2, 3, 6, 7. The etiology of feline cardiomyopathy is typically unknown although taurine deficiency has been associated with the development of dilated cardiomyopathy and some cases of hypertrophic cardiomyopathy have been demonstrated to be familial 8, 9. While a relationship between viral myocarditis and feline myocardial disease has been suspected 1, 2, the pathogenesis is usually unknown, and these disorders have been diagnosed as idiopathic cardiomyopathy, by definition a heart muscle disease of unknown etiology. Recently, the polymerase chain reaction (PCR) has been used to amplify viral genetic material and allow identification of minute amounts of target DNA 10, 11, 12. Since viral myocarditis has been implicated in chronic myocardial injury and dysfunction 13, 14, 15, 16, 17, 18, PCR could potentially be used to identify viral infection in this model of feline cardiomyopathy. The purpose of this study was to evaluate archival myocardial tissues for cardiomyopathy and histopathologic lesions of myocarditis and use PCR to detect viral genome in hearts from these animals.
Materials and Methods
The cardiac pathology archive of the Caspary Research Institute was reviewed for cats that died of idiopathic cardiomyopathy. This search identified formalin-fixed hearts from 120 cases between 1994 and 1997. One-quarter of these were randomly selected for study and represented 18 cats with hypertrophic cardiomyopathy (HCM), 6 with restrictive cardiomyopathy (RCM), and 7 with dilated cardiomyopathy (DCM). For comparison, formalin-fixed, age- and gender-matched cats were selected from the pathology archives of The Ohio State University Veterinary Teaching Hospital who died for noncardiac conditions. Between 1995 and 1997, 17 such cases were identified and comprised the control group. We screened for feline panleukopenia (a parvovirus), calici, herpes, and corona virus, agents that are prevalent in the domestic cat (19), and reported to cause myocarditis in humans, dogs, rabbits, and pigs 10, 16, 20, 21, 22, 23.
Virus Control Sample Preparation
DNA/RNA pellets were extracted from cell cultures of panleukopenia, herpes, calici, and corona virus to be used as positive controls. Genomic material was extracted by adding 1.0 ml of the cell suspension to EDTA (10 mM) and 2% sodium dodecyl sulfate (SDS), extracting with two extractions of a phenol:cholorform:isoamyl alcohol mix (25:24:1, pH 8), and one extraction with pure chloroform (24). The DNA pellet was washed with 75% ethanol, and resuspended in 50 ul of TE buffer (10 mM Tris-HCl, 1mM EDTA, pH 7.5). The final RNA pellet was washed with 75% ethanol and resuspended in 50 ul of DEPC treated distilled water.
Myocardial Tissue Selection and Preparation
From each heart, cross sectional slices of myocardial tissue weighing approximately 100 mg were excised from the left and right ventricles and interventricular septum at the level of the chordae tendinae. Each tissue sample was pulverized using a 99.5% alumina, non-porous mortar bowl and pestle. A modified RNazol procedure was used to simultaneously isolate total RNA and genomic DNA (24). Samples were analyzed by spectrophotometry to determine the concentration of the final nucleic acid product. Mortar/pestles were cleaned and autoclaved for one hour between homogenizations.
To verify that genomic DNA was extracted from formalin fixed myocardial tissue, a highly conserved region of the feline β-myosin heavy chain gene was amplified by PCR from each extraction (Genebank Accession # AF003767). Fifteen microliters (15 ml) of each reaction mixture was analyzed by electrophoresis on a 1% agarose gel stained with ethidium bromide.
Virus Primer Design and Synthesis
Primers were designed to amplify conserved regions of each virus based on published sequence. For panleukopenia, primers were designed to amplify a 397 base pair (bp) region of the nonstructural protein 1 (25). Herpes virus primers were designed to amplify a 295 bp region of glycoprotein I, located within the unique short region of the viral genome (26). Calici virus primers were designed to amplify a 121 bp segment of a nonstructural protein located near the 5′ end (27). For corona virus, primers were designed from previously published sequences to amplify a 177 bp region from the highly conserved 3′ untranslated region of the feline corona virus genome (28). All primers were synthesized by an automated oligonucleotide synthesizer.
Polymerase Chain Reaction
For panleukopenia and herpes virus, 250 ug of extracted nucleic acid was combined with 2.5 ul of each primer (20 uM), 2.5 ul of 10× PCR Buffer (260 mM Tris-HCl, pH 8.8, 26 mM MgCl2), 2.5 ul of each dNTP (10 mM), and the appropriate amount of distilled water making a 250 ul reaction. After five minute incubation at 95°C, 0.2 ul (1U) Taq DNA polymerase (Amersham Life Science Inc. Arlington Heights, IL, USA) was added and 45 cycles at the optimal conditions were performed (Table 1) .
Table 1.
Polymerase Chain Reaction Conditions for Viral Amplification
| Virus | Cell conditions | Number of cycles |
|---|---|---|
| Panleukopenia | 94° C × 30 seconds, 53° C × 35 seconds, 72° C × 35 seconds. 72° C × 10 minutes | 45 |
| Herpes | 94° C for 60 seconds, 55° C for 60 seconds, 72 °C for 60 seconds. 72° C × 10 minutes | 45 |
| Calici | 94° C × 30 seconds, 53° C × 35 seconds, 72° C × 35 seconds. 72° C × 10 minutes | 40 |
| Corona | 94° C × 50 seconds, 55° C × 60 seconds, 72° C × 60 seconds. 72° C × 10 minutes | 35 |
Reverse transcriptase-PCR (RT-PCR) was used to evaluate both RNA viruses, calici and corona virus. Calici virus was evaluated by generating a first-strand cDNA from 0.5 ug of extracted total nucleic acid in the presence of 1 ul (40 U) RNasin RNase inhibitor, 4 ul of 5× reverse transcriptase buffer (200 mM Tris-HCl, pH 7.5, 30 mM MgCl2, 10 mM spermidine, 50 mM NaCl), 5 ul (10 mM) of dNTP, 1 ul (20 uM) reverse primer, 1 ul (10 U) AMV reverse transcriptase (Promega, Madison, WI, USA), and DEPC treated distilled water to a total reaction volume of 20 ul. The cocktail was incubated at 37°C for 90 minutes. Five microliters of first-strand cDNA was combined with 2.5 ul of each virus primer, 2.5 ul 10× PCR buffer, 2.5 ul dNTP, and 4.8 ul distilled water. After an initial five-minute incubation at 95°C, 0.2 ul (1U) Taq DNA polymerase (Amersham Live Science Inc. Arlington Heights, IL, USA) was added and 40 cycles performed. Corona virus was evaluated by using a previously published protocol (Table 1) (28). Fifteen microliters (15 ml) of each reaction was analyzed on a 1% agarose gel containing 0.5 ug/ml of ethidium bromide. The gels were placed under ultraviolet light for the visualization of amplified products. A positive control extracted from cell cultures of each virus, and negative control (no template) was run simultaneously with the samples being tested for each particular virus. Samples that amplified the correct sized product in duplicate were considered positive for that particular virus. All samples were analyzed without prior knowledge of clinical data.
The PCR product of the panleukopenia reaction (397 bp fragment) generated from one of the feline myocardial samples was isolated, purified, and sequenced using an automated sequencer. Both panleukopenia primers were used to generate forward and reverse sequence data.
Histopathology
Following a complete necropsy examination in each cat, myocardium was sampled from basal, mid, and apical ventricular levels, right and left atria. Transmural tissue blocks were cut perpendicular to the long-axis of the right ventricular wall (RVW), left ventricular free wall (LVPW), and ventricular septum (IVS). Hearts were fixed in 10% phosphate-buffered formalin, embedded in paraffin, sliced in 5-micron-thick sections, and stained with hematoxylin-eosin (H&E) and Masson trichrome stains. Specimens were viewed initially without knowledge of clinical history. The histologic diagnosis of active myocarditis was based upon the Dallas criteria (29).
Statistical Analysis
Statistical analysis was performed using a commercial software program (SigmaStat® 2.0, Jandel Corp. San Rafael, CA, USA). Categorical data are presented as absolute number or percentage. Quantitative data are listed as mean ± standard deviation. The two-tailed Fisher's exact test was used for comparisons between proportions and between groups. Median values of quantitative data were compared using the Mann-Whitney Rank sum test. Differences were considered statistically significant when the p value was <0.05.
Results
Study Animals
Age (mean ± standard deviation), gender, and breed of cats included: cats with HCM were 9.2 ± 5.3 years old; 13 were male and 5 were female. Representative breeds included 11 domestic short hair (DSH), 2 domestic long hair (DLH), 2 Maine coon cats, 2 Rex, and 1 European short hair. Cats with RCM were 6.4 ± 1.7 years old; 5 were male and 1 was female. Breeds included 5 DSH and 1 Birman. Cats with DCM were 7.5 ± 3.1 years old; 2 were male and 5 were female. Breeds included 6 DSH and one Abyssinian. The age for this group of 31 cats was 8.4 ± 4.5 years. The age of the 17 normal control cats was 6.1 ± 5.3 years; 11 were male and 6 were female. Breeds included 8 DSH, 4 DLH, and 1 case each of Persian, Himalayan, Siamese, Manx, and oriental short hair.
Polymerase Chain Reaction
PCR amplification of panleukopenia genome occurred in myocardial tissue specimens from 10 of 31 cats (32.2%) with cardiomyopathy (Figure 1) . These included 5 with HCM, 2 with RCM, and 3 with DCM. Amplification occurred from different ventricular sites as follows: LVPW only in 4 cats; IVS only in 1 cat; RVW only in 1 cat; both LVPW and RVW in 2 cats; and in 2 cats, from IVS, LVPW, and RVW. In contrast, feline panleukopenia virus was not amplified in any of the control cats. The genetic sequence of the purified PCR product detected in cardiomyopathy tissues matched the sequence of the panleukopenia (parvovirus) clone obtained through Genebank. No cats (affected or control) amplified calici, herpes, or corona virus.
Figure 1.
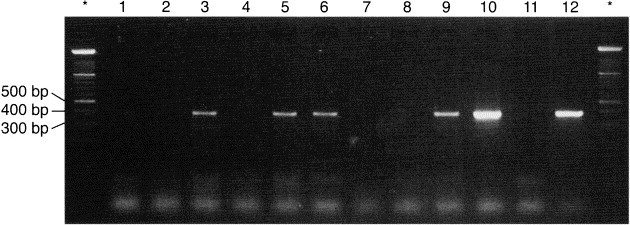
Agarose gel electrophoresis of the 397 base pair region of the nonstructural protein 1 of panleukopenia. Lanes 1, 2, 4, 7, 8 contain negative samples from cardiomyopathic cats. Lane 11 is a negative control, lane 12 is a positive control. The initial lane (identified by an asterisk) contains the DNA ladder
Histopathology Analysis
Active myocarditis was detected in 18 of 31 cats (58%) with idiopathic cardiomyopathy. These included 10 of 18 cats (55%) with HCM, 4 of 6 cats (66.6%) with RCM, and 4 of 7 cats (57.1%) with DCM. Inflammatory infiltrates were always located adjacent to myocyte injury or necrosis and were observed in left atrial myocardium from 13 cats, right atrial myocardium from 11, LVPW from 5, IVS from 3, and RVW from 4 cats. Myocarditis was observed in more than one cardiac chamber in 10 of the 18 cases. In the 8 cats with one chamber affected, these included left atrial myocardium in 4 cats, right atrial myocardium in 2 cats, and in LVPW in 2 cats. The distribution of inflammatory infiltrate was focal in 13 cats and in these cases, infiltrates were most commonly subendocardial in location. In one cat myocarditis was diffuse. In 4 cats inflammatory infiltrates were diffuse and were present confluently between endocardial or epicardium and subjacent myocardium. Inflammatory cells were characterized as predominantly mild lymphocytic infiltration with occasional plasmacytes or neutrophils in 15 cats (Figure 2 , top), and by predominantly mild to moderate neutrophilic infiltration with occasional lymphocytes and plasma cells in 3 cats (Figure 2, bottom). All inflammatory infiltrates were associated with degenerating or necrotic myocytes, often in the presence of interstitial or replacement fibrosis.
Figure 2.

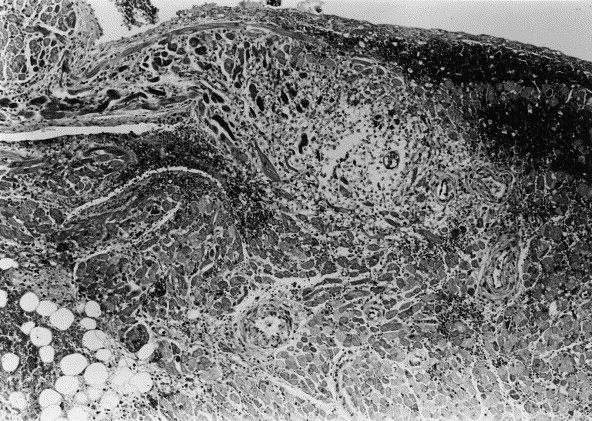
Photomicrographs of representative myocardial sections from cats with cardiomyopathy and myocarditis. (Top) Section from the left ventricular free wall. Extensive mixed inflammatory infiltrate is located predominantly in the subendocardial region consisting of lymphocytes, neutrophils, and plasmacytes. Adjacent myocyte necrosis is severe. Hematoxylin and eosin stain (H&E); magnification ×200. (Bottom) Section from the right auricle. Diffuse neutrophilic infiltrate is associated with extensive myocardial necrosis which is particularly prominent in the subendocardial region. Subendocardial hemorrhage is present. Hematoxylin and eosin stain (H&E); magnification ×100
Relationship Between PCR and Histopathology
Of the 31 cats with cardiomyopathy, myocardial tissues from 8 had panleukopenia virus DNA amplified by PCR as well as histopathologic evidence of myocarditis; 2 cats had panleukopenia DNA amplified but no evidence of myocarditis; 10 cats had histopathologic evidence of myocarditis without PCR detection of virus; and 11 had neither PCR detection of virus or myocarditis. Of the 17 normal control cats, none had panleukopenia virus DNA amplified by PCR or histopathologic evidence of myocarditis.
Results of Statistical Analysis
There was no statistical difference in gender (p = 0.824) or median age (p = 0.052) between the group of control cats and the group of cats with cardiomyopathy. There was no statistical difference (p = 0.601) in median age between: cats that were PCR positive for panleukopenia virus genome with myocarditis (6.5 years) or without myocarditis (9 years); cats that were PCR negative with myocarditis (9.5 years); and cats that were PCR negative without myocarditis (5.7 years). There was a statistically significant association (p = 0.003) between the presence or absence of myocarditis and PCR status of panleukopenia viral genome.
Discussion
There is mounting evidence to suggest that viruses are a cause of myocarditis 10, 11, 13, 14, 15, 16, 30, 31, 32, 33. Traditional diagnostic methods have been insensitive for establishing viral etiology (10), while PCR has been sensitive in detecting viral DNA or RNA from endomyocardial biopsies and postmortem cardiac tissues, thereby providing new insights into infectious heart disease 11, 14, 32, 33. An animal model of cardiomyopathy and myocarditis could help advance the understanding of viral heart disease. Domestic cats are noted for naturally occurring cardiomyopathy 3, 5, 7, 34, and viral myocarditis has been suspected on the basis of histopathologic abnormalities 1, 2. In this report, PCR was used to screen feline myocardial tissues for viruses, and we report the first association of panleukopenia virus genome from feline hearts with spontaneous cardiomyopathy and myocarditis.
There is accumulating evidence linking viral myocarditis to the development of cardiomyopathy 30, 33, 35, 36. Morphologic disruption of the cytoskeleton caused by viral infection may contribute to the pathogenesis of acquired dilated cardiomyopathy (37), and cardiomyopathy characterized by diffuse thickening of the left ventricular endocardium and diastolic dysfunction has been associated with mumps virus (32). It is also conceivable that myocarditis contributes to the pathology of hypertrophic cardiomyopathy or its final common pathway, given the cascade of events which affect sarcomeric protein, cardiac function, cause myocardial injury, or promote heart failure 36, 38, 39, 40, 41.
Our study further supports the concept that a molecular mechanism involving viral myocarditis may be involved in the development or progression of cardiomyopathy. We detected feline panleukopenia virus genome in a high percentage of feline hearts with idiopathic dilated, hypertrophic, and restrictive cardiomyopathy, while these findings were absent in hearts from normal cats. Myocardial inflammatory infiltrates ranging from neutrophils to lymphocytes were detected in 55% of the cats with HCM, and both myocarditis and panleukopenia virus genome were identified in 11% of these hearts. Myocarditis has been documented in young competitive athletes experiencing sudden death with HCM and with other from of myocardial disease (42), and is common histopathologic feature of arrhythmogenic right ventricular cardiomyopathy (ARVC) 43, 44. Although ARVC has been associated with coxsackievirus in humans, we are not aware of an association between viral myocarditis and HCM (45). The detection of feline panleukopenia virus from a high proportion of hearts from cats with HCM is intriguing and somewhat unexpected. Similar to human beings, a familial association has been demonstrated in some breeds of cats with HCM 8, 46. However, there are many cases in which there is no evidence of a familial nature, and an etiology is not detected (8). Although a cause and effect relationship between viral myocarditis and HCM has not been proven, it may be speculated that a virus may have a direct effect on a sarcomeric gene or sarcomeric protein leading to the development of HCM (37). This type of relationship has been previously observed in the development of DCM secondary to enteroviral myocarditis and would be consistent with the final common pathway theory that has been proposed for HCM (38).
Myocarditis caused by parvovirus has been reported in humans 10, 30, dogs (47), and pigs (16), and feline panleukopenia virus is a member of the genus Parvovirus. Species-specific strains of parvovirus are characterized by strong conservation of a central DNA sequence and feline panleukopenia virus shares substantial sequence similarity with canine parvovirus (25). Because panleukopenia virus preferentially affects cells undergoing rapid mitosis and is comparable in this respect to canine parvovirus 48, 49, myocardial infections might be expected to occur similarly, that is, soon after birth. Thus, as with dogs infected with parvovirus, cats surviving panleukopenia viral myocarditis might later develop cardiomyopathy. Chronic myocardial inflammation and persistence of viral myocarditis may have played a role in the development of myocardial injury or cardiomyopathy in our study, and similar pathogenesis has been reported in human patients 12, 29, 47, 50 and in a murine model of myocarditis (51). Alternatively, since hypertrophic growth is also accompanied by DNA synthesis, viral infection might have occurred after cardiac hypertrophy was initiated by an unrelated process (52).
Although myocarditis was not detected in some cats in which panleukopenia virus genome was amplified by PCR, the insensitivity of light microscopy for establishing a diagnosis of myocarditis according to the Dallas classification has been demonstrated 15, 53, 54. Moreover, histopathologic lesions of myocarditis correlate poorly with PCR (10), even though the long term sequelae of viral myocarditis with persistence of viral genome has been documented 36, 37, 55.
Limitation of Study
We selected to screen cats for the presence of herpes, calici, parvovirus, and corona viruses by PCR because of the prevalence and importance of these agents in the feline population (19). While histopathologic myocardial lesions have been reported only rarely from cats infected with panleukopenia virus 56, 57, 58, our data suggests that this virus has substantial cardiotropic activity in cats. Neither herpes, calici, nor corona virus genome was detected in any of the cardiac tissues. Although herpes virus myocarditis has been reported in humans and canines 10, 59, it is possible that herpes virus, as well as corona and calici virus are non-cardiotropic in the cat. Technical factors in PCR could have limited our ability to detect viral myocardial infection, but since PCR consistently amplified positive controls from cell cultures, this would seem unlikely. It is conceivable that cats with cardiomyopathy were infected by viral strains, which were substantially different from our primer sequences and positive control, and therefore, could not be amplified using our methodologies. However, primers were carefully selected from conserved regions of the virus, and would be expected to be consistent between strains. Sample selection or handling can affect the ability to detect myocarditis 13, 53, 54. We sampled multiple regions of myocardium to improve sampling efficacy. Nevertheless, focal regions of infection may have been missed. Although successful PCR amplification of viral genome from long-term formalin fixed biopsy tissues have been reported 10, 11, nucleic acid degradation over time may have negatively influenced PCR because archival samples were maintained in buffered formalin until time of analysis.
In the present study, the precise relationship between inflammation, cardiomyopathy, and the molecular detection of panleukopenia virus DNA in formalin-fixed myocardial tissue is uncertain. Our data derives exclusively from a retrospective evaluation of such factors. Therefore, additional studies are required to better define these relationships.
Summary
In summary, we have demonstrated an association between myocarditis and panleukopenia virus genome in the myocardium of cats with idiopathic hypertrophic, dilated and restrictive cardiomyopathy, and lack of these findings in normal feline hearts. Thus, we infer a potential link between inflammation, infection, and myocardial disease in this feline model. The precise relationship between myocardial inflammation and the molecular detection of feline panleukopenia virus in formalin-fixed myocardial tissue is uncertain, and further studies are required to elucidate the role of these factors in the pathogenesis of acquired cardiomyopathy.
References
- 1.Stalis I.H., Bossbaly M.J., Van Winkle T.J. Feline endomyocarditis and left ventricular endocardial fibrosis. Vet Pathol. 1995;32:122–126. doi: 10.1177/030098589503200204. [DOI] [PubMed] [Google Scholar]
- 2.Lui S.K. Myocarditis and cardiomyopathy in the dog and cat. Heart and Vessels. 1985;1(Suppl I):I-122–I-126. doi: 10.1007/BF02072377. [DOI] [PubMed] [Google Scholar]
- 3.Liu S.K., Roberts W.C., Maron B.J. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiol. 1993;72:944–951. doi: 10.1016/0002-9149(93)91112-u. [DOI] [PubMed] [Google Scholar]
- 4.Fox P.R., Basso C., Maron B.J. Spontaneous occurrence of arrhythmogenic right ventricular cardiomyopathy in the domestic cat: A new animal model. Circulation. 1998;17(Suppl):S297–S298. doi: 10.1161/01.cir.102.15.1863. (Abstract.) [DOI] [PubMed] [Google Scholar]
- 5.Tilley L.P., Liu S.-K., Gilbertson S.R., Wagner B.M., Lord P.F. Primary myocardial disease in the cat. A model for human cardiomyopathy. Am J Pathol. 1977;87:493–513. [PMC free article] [PubMed] [Google Scholar]
- 6.Atkins C.E., Gallo A.M., Kurzman I.D., Cowen P. Risk factors, clinical signs and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985–1989) J Am Vet Med Asoc. 1992;201:613–618. [PubMed] [Google Scholar]
- 7.Fox P.R., Liu S.-K., Maron B.J. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645–2651. doi: 10.1161/01.cir.92.9.2645. [DOI] [PubMed] [Google Scholar]
- 8.Kittleson M.D., Meurs K.M., Munro M.J., Kittleson J.A., Liu S.K., Pion P.D., Towbin J.A. Familial hypertrophic cardiomyopathy in Maine Coon cats: An animal model of human disease. Circulation. 1999;99:3172–3180. doi: 10.1161/01.cir.99.24.3172. [DOI] [PubMed] [Google Scholar]
- 9.Pion P.D., Kittleson M.D., Rogers Q.R., Morris J.G. Myocardial failure in cats associated with low plasma taurine: A reversible cardiomyopathy. Science. 1985;237:764–768. doi: 10.1126/science.3616607. [DOI] [PubMed] [Google Scholar]
- 10.Martin A.B., Webber S., Fricker F.J., Jaffe R., Demmler G., Kearney D., Zhang Y.-H., Bodurtha J., Gelb B., Hi J., Bricker T., Towbin J.A. Acute Myocarditis: Rapid diagnosis by PCR in children. Circulation. 1994;90:330–339. doi: 10.1161/01.cir.90.1.330. [DOI] [PubMed] [Google Scholar]
- 11.Griffin L., Kearney D., Ni J., Jaffe R., Fricker F.J., Webber S., Demmler G., Gelb B., Towbin J. Analysis of formalin-fixed and frozen myocardial autopsy samples for viral genome in childhood myocarditis and dilated cardiomyopathy with endocardial fibroelastosis using polymerase chain reaction (PCR) Cardiovasc Pathol. 1995;4:3–11. doi: 10.1016/1054-8807(94)00025-m. [DOI] [PubMed] [Google Scholar]
- 12.Stiles J., McDermott M., Bigsby D., Willis M., Martin C., Roberts W., Greene C. Use of nested polymerase chain reaction to identify feline herpes virus in ocular tissue from clinically normal cats and cats with corneal sequestra or conjunctivitis. Am J Vet Res. 1997;58:338–342. [PubMed] [Google Scholar]
- 13.Fujioka S., Koide H., Kitaura Y., Deguchi H., Kawamura K., Hirai K. Molecular detection and differentiation of enteroviruses in endomyocardial biopsies and pericardial effusions from dilated cardiomyopathy and myocarditis. Am Heart J. 1996;131:760–765. doi: 10.1016/s0002-8703(96)90284-7. [DOI] [PubMed] [Google Scholar]
- 14.Jin O., Sole M.J., Butany J.W., Chia W.-K., McLaughlin P.R., Liu P., Liew C.C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990;82:8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl U., Noutsias M., Seeberg B., Schannwell M., Welp L.P., Schultheiss H.P. Chronic inflammation in the myocardium of patients with clinically suspected dilated cardiomyopathy. J Card Fail. 1994;1:13–25. doi: 10.1016/1071-9164(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 16.Bolt D.M., Hani H., Muller E., Waldvogel A.S. Non-suppurative myocarditis in piglets associated with porcine parvovirus infection. J Comp Pathol. 1997;117:107–118. doi: 10.1016/s0021-9975(97)80027-8. [DOI] [PubMed] [Google Scholar]
- 17.Kolbeck P.C., Steenbergen C., Wolfe J.A., SanFilippo F., Jennings R.B. The correlation of mononuclear cell phenotype in endomyocardial biopsies with clinical history and cardiac dysfunction. Am J Clin Pathol. 1989;91:37–44. doi: 10.1093/ajcp/91.1.37. [DOI] [PubMed] [Google Scholar]
- 18.Quigley PJ, Richardson PJ, Meany BT, Olsen EG, Monaghan MJ, Jackson G, Jewitt DE. Long-term follow-up in biopsy proven myocarditis. Progression to dilated cardiomyopathy. (Abstract.) Circulation 1986;74(suppl II):II-142.
- 19.Fenner F., Bachman P.A., Gibbs E.P.J., Murphy F.A., Studdert M.J., White D.O. Veterinary Virology. Academic Press; Orlando, FL: 1987. pp. 632–633. [Google Scholar]
- 20.Meunier P.C., Cooper B.J., Appel M.J.G., Slauson D.O. Experimental viral myocarditis: Parvoviral infection of neonatal pups. Vet Pathol. 1984;21:509–514. doi: 10.1177/030098588402100510. [DOI] [PubMed] [Google Scholar]
- 21.Lenghaus C., Studdert M.J. Animal model of human disease: Acute and chronic viral myocarditis. Acute diffuse nonsuppurative myocarditis and residual myocardial scarring following infection with canine parvovirus. Am J Pathol. 1984;115:316–319. [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander L.K., Keene B.W., Small J.D., Yount B., Baric R.S. Electrocardiographic changes following rabbit coronavirus-induced myocarditis and dilated cardiomyopathy. Ad Exper Med & Biol. 1993;342:365–370. doi: 10.1007/978-1-4615-2996-5_56. [DOI] [PubMed] [Google Scholar]
- 23.Hesselton R.M., Yang W.C., Medveczky P., Sullivan J.L. Pathogenesis of herpesvirus sylvilagus infection in cottontail rabbits. Am J Pathol. 1988;133:639–647. [PMC free article] [PubMed] [Google Scholar]
- 24.Chomczynski P., Sacchi N. Single step method of RNA isolation by guanidine thiocyante-phenol-cholorform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Martyn J.C., Davidson B.E., Studdert M.J. Nucleotide sequence of feline panleukopenia virus: Comparison with canine parvovirus identifies host-specific differences. J Gen Virol. 1990;71:2747–2753. doi: 10.1099/0022-1317-71-11-2747. [DOI] [PubMed] [Google Scholar]
- 26.Maeda K., Kawaguchi Y., Ono M., Tajima T., Mikami T. Restriction endonuclease analysis of field isolates of feline herpesvirus type 1 and identification of heterogeneous regions. J Clin Microbiol. 1995;33:217–221. doi: 10.1128/jcm.33.1.217-221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter M.J., Milton I.D., Meanger J., Bennett M., Gaskell R.M., Turner P.C. The complete nucleotide sequence of a feline calicivirus. Virology. 1992;190:443–448. doi: 10.1016/0042-6822(92)91231-i. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Scott F.W. Detection of feline coronaviruses in cell cultures and in fresh and fixed feline tissues using polymerase chain reaction. Vet Micro. 1994;42:65–77. doi: 10.1016/0378-1135(94)90078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artez H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J.T., Fenoglio J.J., Olsen E.G.J., Schoen F.J. Myocarditis: A histopathologic definition and classification. Am J Cardiovasc Pathol. 1986;1:3–14. [PubMed] [Google Scholar]
- 30.Schowengerdt K.O., Ni J., Denfield S.W., Gajarski R.J., Bowles N.E., Rosenthal G., Kearney D.L., Price J.K., Rogers B.B., Schauer G.M., Chinnock R.E., Towbin J.A. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: Diagnosis using the polymerase chain reaction. Circulation. 1997;96:3549–3554. doi: 10.1161/01.cir.96.10.3549. [DOI] [PubMed] [Google Scholar]
- 31.Sole M.J., Liu P. Viral myocarditis: A paradigm for understanding the pathogenesis and treatment of dilated cardiomyopathy. J Am Coll Cardiol. 1993;22(Suppl A):99A–105A. doi: 10.1016/0735-1097(93)90470-l. [DOI] [PubMed] [Google Scholar]
- 32.Ni J., Bowles N.E., Kim Y.H., Demmler G., Kearney D., Bricker J.T., Towbin J.A. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95:133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 33.Bowles N., Rose M.L., Taylor P., Banner N.R., Morgan-Capner P., Cunningham L., Archard L.C., Yacoub M.H. End-stage dilated cardiomyopathy. Persistence of enterovirus RNA myocardium at cardiac transplantation and lack of immune response. Circulation. 1989;80:1128–1136. doi: 10.1161/01.cir.80.5.1128. [DOI] [PubMed] [Google Scholar]
- 34.Pion P.D., Kittleson M.D., Rogers Q.R., Morris J.G. Myocardial failure in cats associated with low plasma taurine: A reversible cardiomyopathy. Science. 1987;237:764–768. doi: 10.1126/science.3616607. [DOI] [PubMed] [Google Scholar]
- 35.Matsumori A., Sasayama S. Newer aspects of pathogenesis of heart failure: hepatitis C virus infection in myocarditis and cardiomyopathy. J Card Fail. 1996;2(4 Suppl):S187–S194. doi: 10.1016/s1071-9164(96)80076-5. [DOI] [PubMed] [Google Scholar]
- 36.Towbin J.A., Bowles K.R., Bowles N.E. Etiologies of cardiomyopathy and heart failure. Nature Med. 1999;5:266–267. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- 37.Badorff C., Lee G.H., Lamphear B.J., Martone M.E., Campbell K.P., Rhoads R.E., Knowton K.U. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nature Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 38.Towbin J.A. The role of cytoskeletal proteins in cardiomyopathies. Curr Opin Cell Biol. 1998;10:131–139. doi: 10.1016/s0955-0674(98)80096-3. [DOI] [PubMed] [Google Scholar]
- 39.Lechin M., Quiñones M.A., Omran A., Hill R., Yu Q.-T., Rakowski H., Wigle D., Liew C.C., Sole M., Roberts R., Marian A.J. Angiotensin-I converting enzyme genotypes and left ventricular hypertrophy in patients with hypertrophic cardiomyopathy. Circulation. 1995;92:1808–1812. doi: 10.1161/01.cir.92.7.1808. [DOI] [PubMed] [Google Scholar]
- 40.Georgakopoulos D., Christe M.E., Giewat M., Seidman C.M., Seidman J.G., Kass D.A. The pathogenesis of familial hypertrophic cardiomyopathy: Early and evolving effects from an α-cardiac myosin heavy chain missense mutation. Nature Med. 1999;5:327–330. doi: 10.1038/6549. [DOI] [PubMed] [Google Scholar]
- 41.Lim H.W., Molkentin J.D. Calcineurin and human heart failure. Nature Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- 42.Maron B.J., Shirani J., Poliac L.C., Mathenge R., Roberts W.C., Mueller F.O. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. J Am Med Assoc. 1996;276:199–204. [PubMed] [Google Scholar]
- 43.Corrado D., Thiene G., Nava A., Rossi L., Pennelli N. Sudden death in young competitive athletes: Clinicopathologic correlation in 22 cases. Am J Med. 1990;89:588–596. doi: 10.1016/0002-9343(90)90176-e. [DOI] [PubMed] [Google Scholar]
- 44.Daliento L., Turrini P., Nava A., Rizzoli G., Angelini A., Buja G., Scognamiglio R., Thiene G. Arrhythmogenic right ventricular cardiomyopathy in young versus adult patients: Similarities and differences. J Am Coll Cardiol. 1995;25:655–664. doi: 10.1016/0735-1097(94)00433-Q. [DOI] [PubMed] [Google Scholar]
- 45.Grumbach I.M., Heim A., Vonhof S., Stille-Siegener M., Mall G., Gonska B.D., Kreuzer H., Andreas S., Figulla H.R. Coxsackievirus genome in myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Cardiology. 1998;89:241–245. doi: 10.1159/000006794. [DOI] [PubMed] [Google Scholar]
- 46.Meurs K.M., Kittleson M.D., Towbin J.A., Ware W.A. Familial systolic anterior motion of the mitral valve and/or hypertrophic cardiomyopathy is apparently inherited as an autosomal dominant trait in a family of American Shorthair cats. J Vet Intern Med. 1997;11:138. [Google Scholar]
- 47.Atwell R.B., Kelly W.R. Canine Parvovirus: A cause of chronic myocardial fibrosis and adolescent congestive heart failure. J Small Anim Prac. 1980;21:609–620. doi: 10.1111/j.1748-5827.1980.tb01363.x. [DOI] [PubMed] [Google Scholar]
- 48.Carlson J.H., Scott F.W., Duncan J.R. Feline panleukopenia. II. The relationship of intestinal mucosal cell proliferation rates to viral infection and development of lesions. Vet Pathol. 1977;14:173–181. doi: 10.1177/030098587701400209. [DOI] [PubMed] [Google Scholar]
- 49.Johnson R.H. Feline panleukopenia virus. IV. Methods for obtaining reproducible in vitro results. Res Vet Sci. 1967;8:256–264. [PubMed] [Google Scholar]
- 50.Lecomte D., Fornes P., Fouret P., Nicholas G. Isolated myocardial fibrosis as a cause of sudden cardiac death and its possible relation to myocarditis. J Forensic Sci. 1993;38:617–621. [PubMed] [Google Scholar]
- 51.Wee L., Liu P., Penn L., Butany J.W., McLaughlin P.R., Sole M.J., Liew C.C. Persistence of viral genome into late stages of murine myocarditis detected by polymerase chain reaction. Circulation. 1992;85:1605–1614. doi: 10.1161/01.cir.86.5.1605. [DOI] [PubMed] [Google Scholar]
- 52.Kollek K., Tseng B.Y., Goulian M. DNA polymerase requirements for parvovirus H-1 DNA replication in vitro. J Virol. 1982;41:982–989. doi: 10.1128/jvi.41.3.982-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauck A.J., Kearney D.L., Edwards W.D. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 54.Chow L.H., Radio S.J., Sears T.D., McManus B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J Am Coll Cardiol. 1989;14:915–920. doi: 10.1016/0735-1097(89)90465-8. [DOI] [PubMed] [Google Scholar]
- 55.Pauschinger M., Bowles N.E., Fuentes-Garcia F.J., Pham V., Kuhl U., Schwimmbeck P.L., Schultheiss H.P., Towbin J.A. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation. 1999;99:1348–1354. doi: 10.1161/01.cir.99.10.1348. [DOI] [PubMed] [Google Scholar]
- 56.Langheinrich K.A., Nielsen S.W. Histopathology of feline panleukopenia: A report of 65 cases. J Am Vet Med Assoc. 1971;158:863–872. [PubMed] [Google Scholar]
- 57.Carlson J.H., Scott F.W., Duncan J.R. Feline panleukopenia. I. Pathogenesis in germfree and specific pathogen-free cats. Vet Pathol. 1977;14:79–88. doi: 10.1177/030098587701400110. [DOI] [PubMed] [Google Scholar]
- 58.Rise W.H. The histopathology of panleukopenia (agranulocytosis) in the domestic cat. Am J Vet Res. 1946;7:455–465. [PubMed] [Google Scholar]
- 59.Hashimoto A., Hirai K., Suzuki Y., Fujimoto Y. Experimental transplacental transmission of canine herpesvirus in pregnant bitches during the second trimester of gestation. Am J Vet Res. 1983;44:610–614. [PubMed] [Google Scholar]


