In a paper published 2 years ago in this journal, some of us described the potentially therapeutic benefits of the quinoline antimalarial chloroquine in viral diseases such as HIV-1/AIDS and severe acute respiratory syndrome (SARS).1 Chloroquine/hydroxychloroquine has since been adopted to treat HIV-1-infected patients in clinical trials, and new insights into its antiviral activity have been obtained from in-vitro studies.
On the HIV/AIDS front, chloroquine (250 mg twice daily) has been administered to HIV-1-infected patients with baseline viral loads over 50 000 copies per mL, in combination with lamivudine (150 mg twice daily) and hydroxyurea (500 mg twice daily) in an ongoing clinical trial in India.2 Ten out of 18 volunteers had an undetectable viral load at week 24.2 The median drop in viral load was more than 2·0 log,2 more than the median 1·5 log drop seen with a nucleoside reverse transcriptase inhibitor (NRTI) and hydroxyurea alone.3
These results are different from those of another trial in Singapore using didanosine (125–250 mg twice daily), hydroxyurea (500 mg twice daily), and hydroxychloroquine (200 mg twice daily, corresponding to 125 mg of chloroquine).1 The median drop in viral load was 1·3 log, similar to that induced by a NRTI plus hydroxyurea. Follow-up of these patients at week 144 suggests that the value of hydroxychloroquine may lie in the maintenance of the effects of didanosine/hydroxyurea.4
The discrepancy between the two studies, besides differences in the design and patients enrolled, probably reflects the different dosages of chloroquine/hydroxychloroquine. Drops in viral load are reported to occur using daily doses of 800 mg of hydroxychloroquine,1 corresponding to 500 mg of chloroquine (as used in the Indian study), but not using 250 mg of chloroquine daily,5 corresponding to 400 mg of hydroxychloroquine (as adopted in the Singapore study). Chloroquine/hydroxychloroquine might thus be a valuable option to be tested in low-cost antiretroviral combinations, but correct dosages should be used, considering that the study participants should be regularly monitored to prevent retinopathy. Prospective randomised double-blind placebo studies are also needed to assess the contribution of chloroquine/hydroxychloroquine as part of an antiretroviral regimen. According to new in-vitro results, the antiretroviral effects of chloroquine are attributable to the inhibition of viral particle glycosylation.6 These effects appeared to be specific, since the chloroquine concentrations effective in vitro neither affected any other step in HIV-1 replication nor were cytotoxic.6
Our hypothesis that chloroquine might inhibit replication of the SARS coronavirus1 has been confirmed in two independent in-vitro studies.7, 8 Researchers at the Belgian Catholic University of Leuven found that chloroquine inhibited SARS coronavirus replication with a 50% effective concentration of 8·8 (SE 1·2) μmol/L, within the range of blood concentrations achievable during antimalarial treatment.7 The dose inducing 50% cytostatic activity was much higher (261·3 [14·5] μmol/L). Time-of-addition experiments indicated that chloroquine affected an early stage of SARS coronavirus replication.7 Researchers at the Centers for Disease Control and Prevention (Atlanta, GA, USA) reported potent anti-SARS coronavirus effects of chloroquine in vitro, attributable to a deficit in the glycosylation of the SARS coronavirus receptor ACE2.8 Again, the antiviral drug concentrations were not cytotoxic. If animal models confirm these results, chloroquine might represent a valuable therapeutic option if SARS re-emerges.
The broad spectrum antiviral effects of chloroquine deserve particular attention in a time in which the world is threatened by the possibility of a new influenza pandemic, and the availability of effective drugs would be fundamental during evaluation of an effective vaccine. The effect of chloroquine against replication of Orthomyxoviridae has long been known.9, 10 Inhibitory effects of chloroquine on both type A and B influenza viruses have been described.9, 10 We are currently investigating the inhibitory effect of chloroquine on the H5N9/A/chicken/Italy/9097/97 avian influenza virus, recently isolated from poultry in Italy.11 Depending on the viral challenging doses and the methods adopted to detect the antiviral effects, the inhibitory concentrations fell within the 0·5–10 μmol/L range—ie, clinically achievable in plasma during malaria treatment (LDT, AS, ID, RC, and AC, unpublished data). If these effects are confirmed, chloroquine would deserve to be tested against the H5N1 type A avian influenza virus, currently a matter of serious concern for public health.
As discussed above, glycosylation inhibition might represent a major mechanism for the antiviral effects of chloroquine, suggesting that specific interactions of chloroquine with sugar-modifying enzymes or glycosyltransferases may occur within human cells (figure ). Chloroquine was recently shown to inhibit quinone reductase 2,13 a structural neighbour of UDP-N-acetylglucosamine 2-epimerases,14 which are involved in sialic acid biosynthesis. If chloroquine should indeed inhibit the biosynthesis of sialic acid, this effect could explain not only the effects of chloroquine on HIV and SARS coronavirus (sialic acid moieties are present in HIV-1 glycoproteins and SARS coronavirus receptor ACE2), but also the in-vitro effects on orthomyxoviruses (which use sialic acid moieties as receptors15). These effects deserve further investigation, in that they may lead to new strategies controlling the replication of several viruses.
Figure.
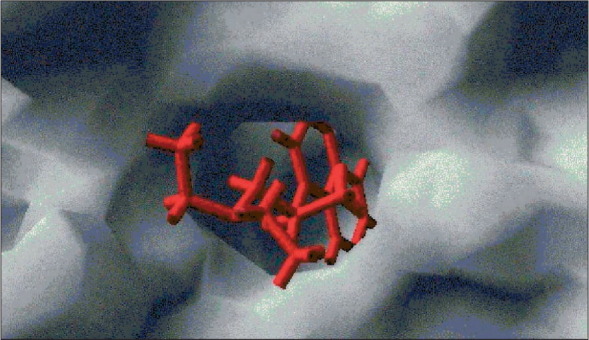
Can chloroquine interact with sugar-modifying enzymes?
This computer-assisted simulation of ligand/protein docking by use of the program GOLD12 indicates that chloroquine (red) fits to the active site of UDP-N-acetylglucosamine 2-epimerase (grey). This evidence suggests that chloroquine could inhibit the enzyme that catalyses the rate-determining step in the sialic acid biosynthetic pathway.
References
- 1.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi SR, Butala N, Patwardhan MR, Daver NG, Kelkar D. Low cost anti-retroviral options: chloroquine based ARV regimen combined with hydroxyurea and lamivudine: a new economical triple therapy. J Assoc Phys India. 2004;52:597–598. [PubMed] [Google Scholar]
- 3.Lori F, Foli A, Groff A. Optimal suppression of HIV replication by low-dose hydroxyurea through the combination of antiviral and cytostatic (‘virostatic’) mechanisms. AIDS. 2005;19:1173–1181. doi: 10.1097/01.aids.0000176217.02743.d1. [DOI] [PubMed] [Google Scholar]
- 4.Paton NI, Aboulhab J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med. 2005;6:13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 5.Luchters SMF, Veldhuijzen NJ, Nsanzabera D. et al. A phase I/II randomised placebo controlled study to evaluate chloroquine administration to reduce HIV-1 RNA in breast milk in an HIV-1 infected breastfeeding population: the CHARGE Study. XV International Conference on AIDS; Bangkok, Thailand; July 11–16, 2004. Abstract TuPeB4499.
- 6.Savarino A, Lucia MB, Rastrelli E. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr. 1996;35:223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent MJ, Bergeron E, Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DK, Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci USA. 1981;78:3605–3609. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata M, Aoki H, Tsurumi T. Mechanism of uncoating of influenza B virus in MDCK cells: action of chloroquine. J Gen Virol. 1983;64:1149–1156. doi: 10.1099/0022-1317-64-5-1149. [DOI] [PubMed] [Google Scholar]
- 11.Donatelli I, Campitelli L, Di Trani L. Characterization of H5N2 influenza viruses from Italian poultry. J Gen Virol. 2001;82:623–630. doi: 10.1099/0022-1317-82-3-623. [DOI] [PubMed] [Google Scholar]
- 12.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 13.Kwiek JJ, Haystead TA, Rudolph J. Kinetic mechanism of quinone oxidoreductase 2 and its inhibition by the antimalarial quinolines. Biochemistry. 2004;43:4538–4547. doi: 10.1021/bi035923w. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Biotechnology Information MMDB—Entrez's Structure Database. http://www.ncbi.nlm.nih.gov/Structure/MMDB/mmdb.shtml (accessed Dec 14, 2005)
- 15.Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005;5:184–188. doi: 10.1016/S1473-3099(05)01311-3. [DOI] [PubMed] [Google Scholar]


