Abstract
Objective
To discuss hospital pharmacists’ role in providing pharmaceutical care for hospitalized patients with COVID-19 to promote patient care and management during the pandemic.
Method
Based on the method of evidence-based pharmacy, clinical evidence of therapeutical drugs for COVID-19 were retrieved and summarized. Based on clinical experience Chinese hospital pharmacists gained from providing pharmaceutical care services during COVID-19 pandemic, taking COVID-19 hospitalized patients’ needs into consideration, the methods and strategies hospital pharmacists shall use to provide pharmaceutical care were analyzed and summarized.
Results
Hospital pharmacists shall support pharmaceutical care services by participating in making evidence-based decisions for medication, monitoring and evaluation of medication safety and efficacy, providing strengthened care for special population and patients with combined underlying diseases, monitoring and management of convalescent plasma therapy, providing emotional counselling and psychological support, and providing scientific information about COVID-19 vaccines.
Conclusion
The need of pharmaceutical care services in COVID-19 hospitalized patients during this pandemic was quite distinguished from the past. Hospital pharmacists shall join the collaborative multidisciplinary team to improve COVID-19 patients’ outcome and reduce mortality, and to facilitate the pandemic control.
In December 2019, the Coronavirus Disease 2019 (COVID-19) epidemic caused by the novel coronavirus (SARS-CoV-2) first emerged in Wuhan, followed by a worldwide outbreak in many countries and regions in succession. On March 12, 2020, the World Health Organization (WHO) declared COVID-19 epidemic a “pandemic” worldwide.1 According to the latest updates from WHO (March 30), 693224 cases have been confirmed with COVID-19 in total worldwide, causing 33106 deaths. WHO has increased the risk assessment of COVID-19 to very high at global level.2 Currently, the COVID-19 pandemic is posing a major challenge to global public health, and seriously endangering the economy and the society.
As the forefront of the outbreak, China formulated and launched a series of emergency plans and supports in response to the COVID-19 pandemic in the first place. Effective measures have been taken to control the pandemic in a timely manner. According to the principle of “centralizing patients, centralizing experts, centralizing resources, and centralized treatment”, all confirmed cases of COVID-19 have been admitted into designated hospitals for standardized inpatient treatment,3 which has greatly improved patients’ outcomes, reduced mortality, and effectively contained further spread of the pandemic. On March 19, China reported no domestic new case for the first time, and the first prevail peak period of the pandemic was over, which was highly spoken as “an amazing achievement” by WHO.
Clinical treatment and management of COVID-19 hospitalized patients is constituting a prominent challenge worldwide. Some patients may quickly progress to severe or critical cases, with acute respiratory distress syndrome (ARDS), sepsis and multiple organ dysfunction, particular in patients with complicated underlying diseases (diabetes, cardiovascular disease, malignancy, etc.).4 , 5 Therefore, personalized, collaborative and multidisciplinary management is of great significance to improve patients’ outcomes. With the pandemic getting increasingly serious, Chinese solution of “centralizing COVID-19 patients and centralized inpatient treatment” provides certain reference for other countries to control the pandemic.
According to International Pharmaceutical Federation (FIP) guidance for pharmacists,6 as a member of the healthcare professionals, it's one of the most essential responsibilities for hospital pharmacists to join the medical collaborative team and provide pharmaceutical care services to COVID-19 patients. For COVID-19 patients receiving centralized hospitalized treatment, pharmaceutical care services are indispensable supplement for clinical treatment and management, which is of great significance for improving the level of drug therapy, improving patient's outcome and promoting the overall pandemic control. Detailed and feasible suggestions on clinical diagnosis and treatment were provided in clinical management guidance from National Health Committee (NHC) of China, WHO, and Centers for Disease Control and Prevention (CDC) of the United States.7, 8, 9 However, it's worth mentioning that, most of the available guidance did not revolve detailed suggestions on therapeutic drugs or pharmaceutical care services. Currently, community pharmacists can provide strengthened pharmaceutical care and public health services following feasible instructions.10 , 11 However, for hospital pharmacists, recommendations and guidance on providing COVID-19 hospitalized patients with pharmaceutical care is still lacking, and need to be established urgently.
Based on the Chinese perspective of “centralizing COVID-19 patients and centralized inpatient treatment”, the purpose of the paper is to establish an integrated strategy for pharmaceutical care services, which focus on COVID-19 hospitalized patients and hospital pharmacists. Finally, it aims to provide guidance for hospital pharmacists to participate in the multidisciplinary team to improve COVID-19 patients’ outcome and reduce mortality, and to facilitate the pandemic control.
Pharmaceutical care framework of COVID-19 hospitalized patients for hospital pharmacists
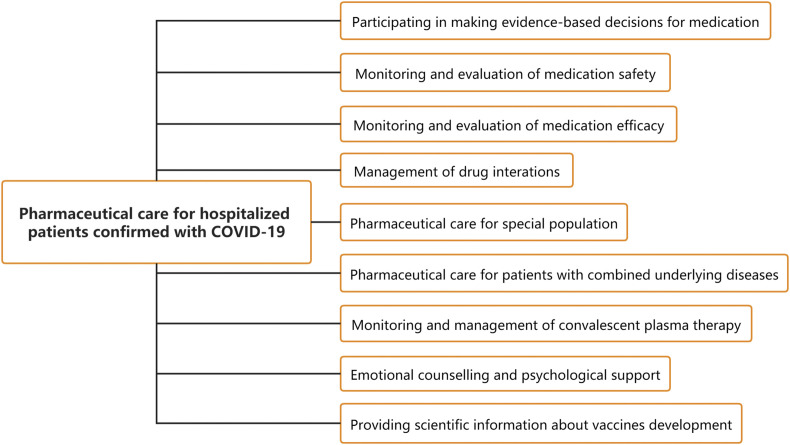
After the diagnosis of COVID-19 infection, patients need to be admitted to designated hospitals for centralized and standardized inpatient treatment. Currently, no specific drug has been confirmed to treat COVID-19, and drugs of pre-approval access are still in ongoing clinical trials. The safety and efficacy of these medications remains unclear, and some drugs (such as lopinavir-ritonavir and arbidol) may cause serious adverse reactions. Therefore, hospital pharmacists should actively participate in making evidence-based decisions for medications, and assist clinicians in formulating and adjusting drug regimens of COVID-19 patients. Meanwhile, hospital pharmacists should provide close monitoring and evaluation of medication safety and efficacy, management of drug interactions, and monitoring and management of convalescent plasma therapy. Based on the characteristics of special populations and patients with underlying diseases, pharmacists should provide strengthened pharmaceutical care services. In addition, the emotional status of COVID-19 patients is also an important factor affecting the treatment and prognosis of the disease. It's necessary for hospital pharmacists to provide COVID-19 patients with emotional counselling and psychological support. Given the importance and urgency of COVID-19 vaccine development for the control of global pandemic, hospital pharmacists should also provide vaccine-related scientific information to patients. To this end, the pharmaceutical care framework of COVID-19 hospitalized patients for hospital pharmacists was researched and constructed (see Fig. 1 for details) to help promote the pandemic control and patient management.
Fig. 1.
Framework of pharmaceutical care for hospitalized patients confirmed with COVID-19.
Recommendations and guidance on pharmaceutical care for COVID-19 hospitalized patients
Participating in making evidence-based decisions for medication
Currently, most of the COVID-19 therapeutic drugs are in ongoing clinical trials and remain pre-approval access. Combining the available evidence, clinical experience and patients’ individual characteristics, providing patients with evidence-based treatment and care is of great significance. As a member of healthcare professionals, hospital pharmacists should give full play to the specialty of pharmacy, and fully combine clinical guidance and clinical research to assist clinicians in formulating and adjusting the medication regimens for hospitalized patients with COVID-19.
Based on the method of evidence-based pharmacy, PubMed, Embase, Cochrane library and Chinese database were retrieved systematically on March 25, and clinical guidance from Chinese, American, WHO authorties were searched manually. Advances in therapeutical drugs for COVID-19 were analyzed and evaluated, and were summarized in Table 1 . In addition, the American Sosiety of Health-System Pharmacist (ASHP) provided an evidence table for COVID-19-related treatment to help practitioners better understand current treatment options.12 Based on these available evidence, hospital pharmacists should help to formulate medication regimens for hospitalized patients with COVID-19 after fully balacing clinical benefits and the risk of medications.
Table 1.
Evidence table for therapeutic drugs of COVID-19.
| Category | Therapeutic Drugs | FIP Guidance6 | Chinese Guidance7 | WHO Guidance8 | CDC Guidance9 | CPA Guidance13 | Clinical Research14, 15, 16, 17, 18, 19, 20, 21 |
|---|---|---|---|---|---|---|---|
| Antiviral | Lopinavir-Ritonavir | ●a | ● | – | – | ● | ▲c△d |
| Arbidol | ● | ● | – | – | ● | ▲△ | |
| Interferon | ● | ● | – | – | ● | – | |
| Ribavirin | ● | ● | – | – | ● | – | |
| Remdesivir | −e | – | – | – | – | – | |
| Oseltamivir | – | – | – | – | – | – | |
| Favipiravir | – | – | – | – | ● | – | |
| Chloroquine phosphate | ● | ● | – | – | ● | – | |
| Hydroxychloroquine sulfate | – | – | – | – | – | ▲△ | |
| Immunomodulator | Corticosteroids | ◯b | ◯ | ◯ | ◯ | ◯ | △ |
| Tocilizumab | ◯ | ◯ | – | – | – | – | |
| Antimicrobials | Antibiotics | ◯ | ◯ | ◯ | – | ◯ | – |
●recommend.
◯recommend only if necessary.
▲significant effect.
△no significant difference compared with control group.
−not recommend or no current clinical research results.
Antiviral drugs
Antiviral drugs recommended by the latest Chinese guidance7 include interferon-α, lopinavir-ritonavir, arbidol, chloroquine phosphate and ribavirin. And favipiravir is recommended to be included in Chinese guidance.13 Regarding to clinical researches, currently, the results of lopinavir-ritonavir, arbidol and Hydroxychloroquine sulfate on COVID-19 treatment have been reported in 6 studies.14, 15, 16, 17, 18, 19, 20, 21
For lopinavir-ritonavir: A randomized, controlled, open-label trial14 suggested, for patients with severe COVID-19, lopinavir-ritonavir treatment beyond standard care did not significantly accelerate clinical improvement or reduce mortality (P > 0.05). For patients with different clinical classifications, a retrospective study15 found that lopinavir-ritonavir combined with other antiviral drugs can shorten the time of viral nucleic acid turning negative and improve the results of lung CT imaging (P < 0.05), but the effect on clinical improvement was not significant (P > 0.05).
For arbidol: A multicenter, prospective study16 reported, compared with dual combination of lopinavir-ritonavir and interferon-α-2b, triple combination of arbidol can shorten the time of viral nucleic acid turning negative and hospitalization time (P < 0.05). In addition, a retrospective study17 showed no significant difference of the time of viral nucleic acid turning negative among arbidol treatment, lopinavir-ritonavir treatment and control group (P > 0.05).
For hydroxychloroquine sulfate: an open-label, non-randomized clinical trial18 suggested, compared with the rate of viral nucleic acid turning negative of control group (12.5%), combination of hydroxychloroquine sulfate and azithromycin (100%) and monotherapy of hydroxychloroquine sulfate (57.1%) significantly improved the rate of viral nucleic acid turning negative (P < 0.001). The effect of hydroxychloroquine sulfate is reinforced by azithromycin. And such combination may both act as an antiviral therapy against SARS-CoV-2 and prevent bacterial super-infections. However, a prospective study19 showed that, for patients with moderate COVID-19, the effect on the rate and the time of viral nucleic acid turning negative were not significant.
In addition, remdesivir, favipiravir, ribavirin, chloroquine phosphate and other antiviral drugs are still in ongoing clinical research, and the results remain unpublished. It's worth mentioning that, although clinical evidence is lacking, FDA issued an Emergency Use Authorization (EUA) for emergency use of oral formulations of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of COVID-19.20 Hospital pharmacists should combine the available evidence, fully evaluate patients' own conditions, and use antiviral drugs rationally.
Immunomodulators
According to WHO guidance,8 systemic corticosteroids are not recommended to be routinely used for treatment of viral pneumonia outside of clinical trials. CDC guidance9 recommended that corticosteroids should be avoided unless indicated for special reasons. According to guidance from FIP,6 NHC of China7 and Chinese Pharmaceutical Association(CPA),13 for patients with progressive worsening of oxygenation indicators and rapid progress in imaging, corticosteroids can be used conditionally in a short period of time. A retrospective study21 showed that for COVID-19 patients with different clinincal classification, low-to-moderate corticosteroids have no significant difference in the time of virus clearance. For moderate cases, low-to-moderate corticosteroids have no significant difference in the time of improvement of lung imaging. Therefore, before taking corticosteroids into account, pharmacists should fully evaluate the patients’ individual conditions, and carefully balance clinical benefits and the medicaiton risk.
Antimicrobials
According to WHO guidance,8 empiric antimicrobials should be used to treat all likely pathogens causing SARI and sepsis as soon as possible. Chinese guidance7 recommended that blind or inappropriate use of antibiotics should be avoided, especially the broad-spectrum antibiotics. CPA guidance13 recommended that inappropriate use of antibiotics should be avoided. Etiological tests should be taken if necessary, and antibiotics should be selected carefully based on patients' individual conditions and risk. Therefore, pharamcists should actively attend anti-infection consultations and formulate rational antimicrobial medication regimens for COVID-19 patients, combing available evidence and patients’ conditions.
Monitoring and evaluation of medication safety
In addition to current antiviral drugs recommended by the latest Chinese guidance,7 tocilizumab is recommended for severe patients with extensive lung disease and elevated IL-6 levels. For severe patients with rapid disease progression and excessive activation of inflammatory response, short-term corticosteroids could be considered to use at early stage. Some therapeutic drugs may cause serious adverse reactions, including hemolytic anemia, cardiotoxicity, infection, femoral head necrosis, and teratogenic effects. Therefore, pharmacists should cooperate with clinicians to formulate medication regimens to avoid inappropriate high-dose medications or multi-drug combinations. Besides, pharmacists should closely monitor the safety of drugs, identify adverse drug events early and adjust the medication regimens timely when attending pharmaceutical ward round and consultations.
Monitoring and evaluation of medication efficacy
No specific therapeutic drug has been proved to treat COVID-19 currently. Related antiviral drugs of pre-approval access are in ongoing clinical trials, including anti-influenza drugs, anti-HIV drugs and anti-malaria drugs. Evidence-based medical researches on medication efficacy for COVID-19 are still lacking. Therefore, pharmacists should cooperate with clinicians to closely monitor and evaluate the medication efficacy, gather evidence on drug efficacy during pharmaceutical care if possible, and keep optimizing medication regimens, to promote the COVID-19 treatment.
Management of drug interactions
Antiviral medications are related to many drug interactions. Severe COVID-19 patients tend to have underlying diseases, and the combined symptomatic drugs are more complicated. Therefore, in order to avoid symptoms aggravation due to drug interactions, pharmacists should be highly vigilant for potential drug-drug or drug-food interactions, strengthen the management of patients’ medication and diet. Taking lopinavir-ritonavir as an example, clinicians and pharmacists should avoid combining other drugs metabolized by the CYP3A enzyme (nifedipine, atorvastatin, midazolam, etc.), and educate patients not to eat foods that affect the CYP3A enzyme (grapefruit, honey, etc.). In addition, in the current clinical practice, it has been proved that integrated traditional Chinese and western medicine has a certain effect on COVID-19. Pharmacists should also strengthen the interaction management between traditional Chinese and western medicine, and recommend patients to take medicines at intervals.
Pharmaceutical care for special population
In general, human at all ages can easily be infected with COVID-19. Pharmacists should particularly strengthen pharmaceutical care for special population, such as pregnant or lactating women, children, adolescents and elderly patients. For pregnant patients, the physiological factors of pregnancy should be taken into full consideration. It is recommended to use medications with FDA Pregnancy Category B or C, and avoid using medications of D class.22 Regularly monitor vital signs of patients and fetuses during medication use, and adjust medication regimens if necessary. To prevent the spread of the SARS-CoV-2 and prevent drugs’ harm to infants through breast milk, pharmacists should advise lactating patients to suspend breastfeeding.23 As the safety and efficacy of antiviral drugs for children and adolescents remains unclear, medication regimens should be formulated carefully. For critically ill children, drugs for adults could be considered and dosage must be adjusted.24 For elderly patients with impaired immunity or complicated chronic diseases, pharmacists should adjust the dosage according to their liver and kidney function, manage medications for secondary prevention, and pay close attention to drug interactions.
Pharmaceutical care for patients with combined underlying diseases
COVID-19 patients with combined underlying diseases (diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD), malignancy, bacterial or fungal infections and organ transplantation, etc.) are prone to disease progress. Medication regimens for those patients are more complicated in general.25 There are many interactions between antiviral drugs and other symptomatic drugs, and medication efficacy and safety may be affected. Pharmacists should strengthen pharmaceutical care for those patients with combined underlying diseases. Therapeutic drug monitoring (TDM) should be strengthened, such as lopinavir-ritonavir and anti-infective drugs (voriconazole). Clinical indicators (INR, blood glucose and blood pressure) should be monitored closely and dosage should be adjusted if necessary, for example, ribavirin may reduce the anticoagulant effect of warfarin, lopinavir-ritonavir may worsen diabetes and enhance the anti-hypertensive effect of nifedipine.
Monitoring and management of convalescent plasma therapy (CPT)
For COVID-19 patients with rapid disease progression, severe or critical condition, the convalescent plasma therapy (CPT) can be applied.26 The plasma of convalescent utilizes a certain titer of virus-specific antibodies, through which patients receive the plasma infusion to obtain passive immunity and remove pathogens in the blood circulation. CPT is an effective therapy choice, and has been successfully applied to the treatment of SARS and H1N1 influenza.27 Before conducting CPT, pharmacists should assist clinicians in assessing patients' indications of CPT, combining with allergy history, contraindications of plasma transfusion, and individual clinical conditions. Also, the infusion dose should be formulated based on patients’ weight and clinical condition, usually ranging from 200 ml to 500 mL (4–5 mL/kg). When performing CPT, pharmacists need to closely record and monitor adverse effects of plasma transfusion before, during, and after transfusion, and cooperate with clinicians to manage adverse effects. Common adverse transfusion reactions include transfusion-related circulation overload, acute lung injury, dyspnea, allergic, hypotension, non-hemolytic febrile, acute hemolytic transfusion reactions, and delayed hemolytic transfusion reaction, infectious transfusion reaction, etc.
Emotional counselling and psychological support
The outbreak of COVID-19 and the change of daily life after hospitalization, together with the uncertainty about the disease progress may lead to anxiety, panic, concern and excessive pessimism in some patients confirmed with COVID-19. Pharmacists should pay full attention to the mental health and emotional management of patients during pharmaceutical care.28 Timely emotional counselling and psychological support shall be provided to help patients properly understand the COVID-19 and enhance confidence. For mild or moderate patients, pharmacists may encourage them to take adequate exercise (such as dancing) to enhance the immunity and relieve negative emotions. If the patients are considered having severe psychological problem, referral to psychiatrists shall be made. For patients with mental disorders (such as depression and anxiety disorder), pharmacists should pay close attention to their psychotropic medication compliance and assist psychiatrists in providing psychological intervention.
Providing scientific information about vaccines development
Vaccination is one of the most effective methods to halt the spread of COVID-19 at the global level. Pharmacists should provide patients with scientific information about vaccine development. At present, recombinant adenovirus vector vaccines Ad5-nCoV (Chinese Clinical Trial Registry, NO.ChiCTR2000030906) and nucleic acid vaccine mRNA-1273 (ClinicalTrials.gov, NO.NCT04283461) have entered phase 1 clinical trials in China and the US respectively. Meanwhile, 52 candidate vaccines are under pre-clinical research for evaluation of safety and efficacy.29 It is expected that by April 2020, more vaccines will enter clinical researches or emergency use.6 For subjects participating in vaccine-related clinical trials, hospital pharmacists should assist clinicians and immunologists to closely monitor their clinical manifestations and adverse reactions after vaccination. Pharmacists should also collect data on vaccines effectiveness and safety during pharmaceutical care if it is under the premise of medical ethics and promote the clinical research of the COVID-19 vaccines.
Conclusion
For hospitalized patients with COVID-19, pharmaceutical care services are indispensable supplements for clinical treatment and management. Hospital pharmacists providing targeted pharmaceutical care is of great significance for improving the level of drug therapy. Based on the Chinese perspective of “centralizing COVID-19 patients and centralized inpatient treatment”, the paper established a pharmaceutical care framework of COVID-19 hospitalized patients, including participating in making evidence-based decisions for medication, monitoring and evaluation of medication safety and efficacy, strengthened care for special population and patients with combined underlying diseases, monitoring and management of convalescent plasma therapy (CPT), providing emotional counselling and psychological support and providing scientific information about COVID-19 vaccines. The paper aims to provide reference for hospital pharmacists in providing patient care services, to improve patient's outcome and reduce the overall mortality, and finally to support hospital pharmacists' in making contributions to the control of COVID-19 pandemic.
Statement of funding source
The study is supported by grants from National Major Scientific and Technological Special Project (ZX09304012-008).
Declaration of competing interest
All authors declared that they have no conflicts of interest.
Acknowledgement
We thank the International Pharmaceutical Federation, the Chinese Pharmaceutical Association, the Peking University Third Hospital for their support to this article.
References
- 1.World Health Organization Rolling updates on coronavirus disease (COVID-19): WHO characterizes COVID-19 as a pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.World Health Organization Coronavirus disease 2019 (COVID-19) situation report –70. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200330-sitrep-70-covid-19.pdf?sfvrsn=7e0fe3f8_2
- 3.National Health Commission of the People’s Republic of China Working plan on prevention and control of novel coronavirus pneumonia. 2020. http://www.nhc.gov.cn/tigs/s7848/202001/808bbf75e5ce415aa19f74c78ddc653f.shtml [DOI] [PMC free article] [PubMed]
- 4.Zhou F., Yu T., Du R.H. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T., Wu D., Chen H.L. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. The BMJ. 2020 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Pharmaceutical Federation CORONAVIRUS SARS-CoV-2/COVID-19 PANDEMIC: information and interim guidelines for pharmacists and the pharmacy workforce. 2020. https://www.fip.org/files/content/priority-areas/coronavirus/Coronavirus-guidance-update-ENGLISH.pdf
- 7.National Health Commission of the People’s Republic of China Diagnosis and treatment protocol for COVID-19 (trial version 7) 2020. https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf [DOI] [PMC free article] [PubMed]
- 8.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 9.Centers for Disease Control and Prevention U.S. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 10.Lam Ung C.O. Community pharmacist in public health emergencies: quick to action against the coronavirus 2019-nCoV outbreak. Res Soc Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S.Q., Yang L., Zhou P.Q., Li H.B., Liu F., Zhao R.S. Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: a China perspective. Res Soc Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Society of Health-System Pharmacists Assessment of evidence for COVID-19-related treatments. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx?la=en&hash=B414CC64FD64E1AE8CA47AD753BA744EDF4FFB8C&hash=B414CC64FD64E1AE8CA47AD753BA744EDF4FFB8C; 2020
- 13.Hospital Pharmacy Professional Committee of Chinese Pharmaceutical Association. Expert Consensus on Rational Drug Use in Clinical Practice for COVID-19. (To be published).
- 14.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu A.R., Fan X., Zhao Y. Herald of Medicine; 2020. Retrospective Study of the Clinical Efficacy and Safety of Lopinavir/ritonavir Combined with Other Antiviral in the Treatment of Coronavirus Disease (COVID-19)http://kns.cnki.net/kcms/detail/42.1293.R.20200309.1902.002.html [Google Scholar]
- 16.Wei R.N., Zheng N.H., Jiang X.G. Early antiviral therapy of abidor combined with lopinavir/ritonavir and re-combinant interferonα-2b in patients with novel coronavirus pneumonia in Zhejiang: a multicenter and prospective study. Chin J Clin Infect Dis. 2020 doi: 10.3760/cma.j.cn115673-20200224-00069. [DOI] [Google Scholar]
- 17.Chen J., Ling Y., Xi X.H. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J of Infect Dis. 2020 doi: 10.3760/cma.j.cn311365-20200210-00050. [DOI] [Google Scholar]
- 18.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Chen J., Liu D.P., Liu L. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) Journal of Zhejaing University(Medical Sciences) 2020 http://kns.cnki.net/kcms/detail/33.1248.R.20200309.1507.006.html [Google Scholar]
- 20.U.S. Food & Drug Administration Letter of emergency use authorization to allow hydroxychloroquine sulfate and chloroquine phosphate to be distributed and used for certain hospitalized patients with COVID-19. 2020. https://www.fda.gov/media/136534/download
- 21.Ni Q., Ding C., Li yt. Retrospective study of low-to-moderate dose glucocorticoids on viral clearance in patients with novel coronavirus pneumonia. Chin J Clin Infect Dis. 2020 doi: 10.3760/cma.j.cn115673-20200225-00072. [DOI] [Google Scholar]
- 22.Wang X.Y., Wu J., Lu X.H. Recommendations for management of novel coronavirus (2019-nCoV) infected maternal women in Henan Province China. Journal of Zhengzhou University (Medical Sciences) 2020;55:1–3. [Google Scholar]
- 23.Maternal, Fetal Experts Committee Chinese physician society of obstetrics and gynecology, Chinese medical doctor association. Proposed management of 2019-novel coronavirus infection during pregnancy and puerperium. Chin J Prev Med. 2020;23:73–79. [Google Scholar]
- 24.Chinese Pharmaceutical Association . second ed. 2020. Coronavirus 2019-nCoV Infection: Expert Consensus on Guidance and Prevention Strategies for Hospital Pharmacists and the Pharmacy Workforce.http://www.cpa.org.cn/cpadmn/attached/file/20200216/1581854567839722.pdf [Google Scholar]
- 25.Wang R.R., Xu Q., Wang X.J., Jiang S.P., Lu X.Y. Pharmacological care strategy for antivirals in patients with COVID-19 complicated by underlying disorders. Chin J Hosp Pharm. 2020 http://kns.cnki.net/kcms/detail/42.1204.R.20200222.1810.002.html [Google Scholar]
- 26.National Health Commission of the People's Republic of China The clinical guidance of convalescent plasma therapy (trial version 2) 2020. http://www.nhc.gov.cn/yzygj/s7658/202003/61d608a7e8bf49fca418a6074c2bf5a2.shtml
- 27.Chen L., Xiong J., Bo L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao Y.P., Sun Y.K., Meng S.Q., Shi J., Lu L. 2019-nCoV epidemic: address mental health care to empower society. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020;395:e37–e38. doi: 10.1016/S0140-6736(20)30309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization DRAFT landscape of COVID-19 candidate vaccines-26 March 2020. 2020. https://www.who.int/blueprint/priority-diseases/key-action/Novel_Coronavirus_Landscape_nCoV_Mar26.PDF?ua=1