Numerous strains of mice and rats have been used extensively in ophthalmic research, providing simulations for many different human and nonhuman ophthalmic diseases. Most ophthalmic literature and research pertaining to these species involve laboratory strains. Although this information is substantial, care needs to be taken in extrapolating the data to companion mice and rats because laboratory animals live under unnatural conditions of housing, diet, lighting, and other environmental parameters. Because of this, the information may not apply directly to mice and rats that are kept as companions. On the other hand, there is little published information about pet mice and rats. This article provides an overview of the most common conditions observed in laboratory strains of these animals, some of which are induced, occur spontaneously, or can be seen in pets. Ocular anatomy and common pathologic conditions are reviewed.
Restraint for examination
Because of the size of and difficulty in restraining mice and rats, ophthalmic examination can be challenging. Restraint is best achieved by grasping the animal in the palm of the hand with the heel of the hand cupped under the trunk and tail and by gently stabilizing the head by holding the base of the skull firmly between the thumb and forefinger. An open-ended syringe casing is helpful to facilitate examination of small rodents, such as mice. The head is allowed to extend from the end of the casing, and the tail is grasped gently through the other end. The thumb can be used as a plunger to position the animal for examination. Frequent breaks are helpful to prevent the patients from becoming too stressed. Sedation rarely is required. Mydriasis can be achieved with 1% tropicamide. Melanotic irides of some rodents may resist dilation because of the binding of the drug to uveal melanin. One percent atropine and 10% phenylephrine applied every 5 minutes for up to three applications can be used if necessary [1]. When topical anesthetic is used to perform procedures such as tonometry, only a small amount should be administered because of the potential for systemic toxicity [1].
Orbital vascular supply
The rat has an orbital venous plexus, or network of vessels, that is formed by the external dorsal ophthalmic vein, external ventral ophthalmic vein, and numerous anastomoses between these veins. In contrast, the mouse has an orbital venous sinus, or large dilated channel, comprised of these major deep orbital veins [2].
Adnexa and lacrimal systems
Eyelids in mice begin to grow on day 13 of gestation and fuse with each other by day 17. The eyelids are closed at birth and open 12 to 14 days later [3]. Eyelid separation in rats occurs between 12 and 16 days after birth [4].
Mice and rats have three lacrimal structures: the (1) intraorbital gland, (2) extraorbital gland, and (3) Harderian gland. The intraorbital gland is located deep in the retrobulbar space. The extraorbital gland is located near the base of the masseter muscle and commonly has been misinterpreted as a neoplasm because of its unusual location [5]. Gender-related differences in lacrimal gland structure have been documented in rats. The most obvious difference is that the acinar area of males is much larger than that of females [6]. In the rat, the lacrimal duct runs through a fibrous tissue cord that extends between the extraorbital lacrimal gland and the conjunctiva [7]. Because the duct is ensheathed within this dense fibrous cord, it partly is protected from external trauma, ensuring patency. This cord, which is about 2 cm long, runs mainly along the surface of the masseter muscle and courses superficially through subcutaneous tissues. The lacrimal duct extends from the extraorbital gland to the retrobulbar space and continues to the lateral canthal region where it splits into many smaller ducts that join with common ducts of the intraorbital gland. These short ducts open into the conjunctival fornix in the lateral region of the upper or the lower lid. In some adult rats, the openings appear as small white domes that are located close to the eyelid margin and are seen when the eyelids are everted manually. When the domes are present, the actual duct opening is deep within the dome, and the lower dome is usually more prominent. The ductal opening usually is associated with only one of the domes; however, visual inspection cannot determine into which dome the lacrimal duct opens.
The Harderian gland is located posterior to the globe in the orbit. It is pale pink-white, lobulated, and somewhat U-shaped [8]. A single duct extends from the gland to open at the base of the third eyelid. Microscopically, the Harderian gland tends to have a more foamy appearance compared with that of other lacrimal glands because of its high-lipid content. This gland is the only mammalian gland known to secrete lipid by the merocrine mechanism [9]. It is believed that the Harderian gland also contains pheromones that may be important in burrowing species and in facial grooming [8], [10], [11], [12], [13]. In rodents, this gland contains porphyrin, a red-brown pigment [8], [14], [15], [16], [17], [18], [19], and also secretes melatonin, which is believed to have a role in extraretinal photoreception [20]. Epiphora, or overflow of tears, usually is associated with red tear staining of the face and commonly results from ocular irritation, nasolacrimal duct obstruction, and inflammation of the salivary and lacrimal glands. Excessive production of these red tears is called chromodacryorrhea and can be confused with blood [8]. Ultraviolet light can be used to distinguish these excretions from blood; porphyrin should fluoresce, whereas blood does not [8], [20]. Ocular irritation can be caused by primary disease such as keratitis or conjunctivitis or by irritation caused by soiled bedding or high concentrations of ammonia in the housing environment [4]. Nasolacrimal duct obstruction can result from malocclusion or overgrowth of the lower incisors. The gums and nasal cavity can become inflamed secondary to trauma from the incisors. Swelling then causes obstruction of the duct. Corrective dentistry usually improves the condition [4], [20].
The most common abnormality associated with the lacrimal structures is dacryoadenitis, or inflammation of the Harderian gland. Chromodacryorrhea is the most striking clinical finding in this condition (Fig. 1 ). Dacryoadenitis usually is associated with the sialodacryoadenitis virus (SDAV), which is a coronavirus that is closely related antigenically to mouse hepatitis virus and Parker's rat corona virus [21]. SDAV is a common pathogen of rats [8], and disease can occur as an enzootic infection in breeding colonies or as epizootics in susceptible populations [21]. The virus is highly contagious and spreads rapidly by aerosol, contact, and fomite transmission [8]. Transient sniffling caused by rhinitis is observed 2 to 3 days after artificial innoculation, followed by unilateral or bilateral blepharospasm and photophobia occurring 6 to 8 days after inoculation. Intermandibular swelling caused by involvement of the salivary glands is seen frequently [21]. The most common histologic abnormalities of the acutely affected gland are diffuse edema, necrosis, and widespread infiltration with a mixed population of inflammatory cells [22]. Repair of the Harderian gland is possible, and development of new acinar structures can occur if the basement membrane is not affected severely. Secondary conditions such as keratitis, keratoconjunctivitis, uveitis, exophthalmia, and possibly multifocal retinal degeneration can be seen in association with this condition [4]. Permanent sequelae can include corneal fibrosis, anterior and posterior synechiae, cataracts, or glaucoma [4]. Some studies have reported similar infections in mice, but no cause has been identified [8], [23].
Fig. 1.
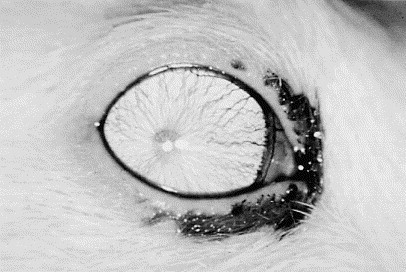
The right eye of an adult male buffalo rat with sialodacryoadenitis. The dark staining on the hair is caused by porphyrin in the tears. (Courtesy of N.C. Buyukmihci, VMD, Davis, CA.)
Acute dacryoadenitis has been reported after obtaining blood by inserting a microcapillary tube into the orbital venous sinus or plexus. Histologically, inflammation of the Harderian gland secondary to this blood-collection method is similar to that seen with SDAV; however, inflammation is localized to a few adjacent lobules, and the basement membrane in these regions usually is disrupted. Although this method has been advocated as a simple and rapid way to obtain blood from anesthetized mice and rats, this technique now is discouraged [22].
Neoplasms of the Harderian gland are fairly common in mice, but are rare in rats. Most of these neoplasms are benign, and exophthalmia is the most common finding. In one report, BALB/c strain mice were found to have the highest prevalence of these spontaneous neoplasms (about 18% of mice older than 18 months were affected). Similar tumors have been induced experimentally at early age in mice by various chemicals or radiation and tend to grow more rapidly and metastasize more frequently than do spontaneous tumors [8], [24].
Conjunctivitis occurs fairly commonly in mice and rats. Incriminated agents include Pseudomonas aeruginosa, P. pneumotropica, Salmonella, Streptobacillus moniliformis, Corynebacterium kutscheri, Lancefield group C streptococci, Mycoplasma pulmonis, mousepox or ectromelia virus, Sendai virus, and lymphocytic choriomeningitis virus. Systemic antibiotic therapy following bacterial culture and sensitivity testing may be indicated in chronic cases. In rats, conjunctivitis secondary to subclinical and clinical upper and lower respiratory tract infection with bacterial or viral agents also has been described [4].
In a study of aged rats with exophthalmia, the most common cause of conjunctivitis was inflammation of the Harderian glands. A less common cause was orbital neoplasia, such as poorly differentiated adenocarcinoma, solid carcinomas, and poorly differentiated sarcoma (Fig. 2 ) [25].
Fig. 2.
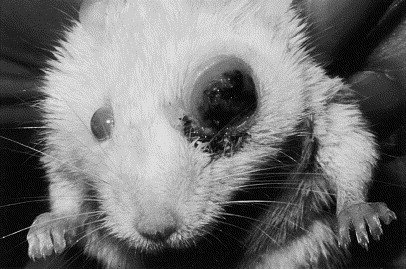
An adult mouse with left orbital neoplasia that resulted in exophthalmia. (Courtesy of N.C. Buyukmihci, VMD, Davis, CA.)
Other spontaneous neoplasms of the periocular structures have been described in aged mice and include cystadenomas and adenocarcinomas. Primary invasive squamous cell carcinoma of the conjunctiva in a rat has been reported in a carcenogenicity study [9]. In a study of a group of F3444 rats, spontaneous neoplasms included amelanotic melanomas of the eyelids [26] and intraocular and orbital malignant schwannomas [27]. Mesenchymal tumors may be seen in older animals [9]. Fibrous histiocytomas are reported to occur in rats, particularly in the head and neck region. An orbital malignant fibrous histiocytoma has been reported to invade the globe [9].
Cornea
Dystrophy is the most common abnormality of the rodent cornea and results in dense, white opacities. In one laboratory, corneal dystrophy was present in 8% to 10% of aged rats and usually appeared as a localized collection of small, white, punctate opacities. These lesions did not tend to progress after the initial observation [9]. In this same laboratory, mice showed an increase in prevalence of corneal dystrophy with age. The lesions usually were shaped geometrically and comprised a thin layer of mineralization in the anterior stroma (Fig. 3 ). The pathogenesis was not determined but may have been the result of previous keratitis [9]. Corneal degeneration may be a better term for these lesions if this is true. A study involving different strains and stocks of laboratory mice showed that manipulation of the cage environment seemed to influence the occurrence of corneal opacities. In mice living in cages that were cleaned twice weekly, the incidence of corneal opacities was 0.8%, whereas in mice living in cages that were not cleaned routinely, incidence was 23.8%. It was proposed that bacteria in fecal material split the urinary urea to form ammonia. It was theorized that the ammonia contacts the cornea and causes necrosis of the corneal epithelium, with resultant inflammation and vascularization of the stroma [28], [29]. This change eventually results in scarring and mineralization of the stroma with dysplasia of the overlying epithelium [29]. Very young mice may be more susceptible to these ammonia burns than are older mice because their eyes open before they are mobile enough to escape the high-ammonia concentrations at the bottom of the cage [29].
Fig. 3.
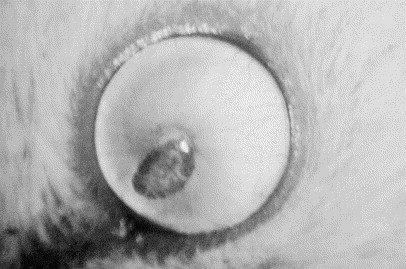
Corneal dystrophy in the left eye of an adult albino mouse. The blood vessels radiating toward the pupil are within the iris. (Courtesy of N.C. Buyukmihci, VMD, Davis, CA.)
Keratitis is seen occasionally among rats in laboratories and commonly has been attributed to two diseases [9], SDAV and purulent keratoconjunctivitis; the more common disease is SDAV. In one study, SDAV keratitis was found most commonly in young rats and showed a decreasing prevalence with age. Purulent keratoconjunctivitis has been associated with coagulase-positive staphylococci and is seen most commonly in newly weaned rats. Mice occasionally develop obvious keratitis [9]. Keratitis has been reported in mice that underwent anesthesia and was described as a white, slightly elevated plaque with an irregular surface that was located in the center of the cornea. Microscopically, the lesions ranged from mild nonspecific inflammation to severe acute inflammation with necrosis and abscess formation and did not resolve within the 2 months that they were monitored [30].
Uvea
Iris colobomas frequently appear as holes in the iris or defects in the pupillary margin. Many studies have reported young mice with a coloboma that is usually located in the ventral quadrant of the iris, resulting in a small, ventrally displaced pupil [31], [32], [33], [34]. These abnormally placed pupils usually dilate with mydriatics, but dilate less than the unaffected pupil. When the affected pupil is dilated, it typically assumes a keyhole shape. Although it is considered to be a common congenital abnormality, one study reported mice with iris colobomas that previously had been normal, raising the possibility of an inflammatory or infectious cause [34]. Histologic examination demonstrated mild infiltration of the iris and ciliary body with mononuclear inflammatory cells and occasional anterior or posterior synechiae. The rest of the eye was not affected. Similar findings have been reported in rats, usually with accompanying retinal and choroidal defects and occasional microphthalmia [34].
Synechiae have been observed in weanling and adult mice and rats. In one study, synechiae were found in about 1% of Sprague-Dawley rats of all ages [9], [35]. This condition has been seen in all individuals of some rat litters and in the dam, suggesting a heritable condition; however, an inflammatory cause cannot be excluded [9]. Synechiae and uveitis in mice are not observed commonly, although uveitis has been reported occasionally in aged mice [9]. As in any species, uveitis may result from various types of ocular trauma and possibly from systemic disease. Persistent pupillary membranes, which can be mistaken for synechiae, also are not common in either species [9].
Spontaneous intraocular neoplasms are considered relatively rare in these species. Melanoma has been reported as one of the most common neoplasms in rats [36]. Five cases of spontaneous intraocular amelanotic melanoma were identified in a group of more than 120,000 F3444 rats [37]. These tumors were white or yellow, unilateral, originated from the iris or ciliary body, and often involved the choroid.
Lens
Animal studies have contributed substantially to the understanding of lens physiology and cataract pathogenesis. Many different cataract mutations have been induced in mice and rats by irradiation or chemical mutagens to simulate mutations that are similar to those seen in human cataracts [38], [39]. Clinically and histologically, cataracts in mice and rats appear similar; however, a clear difference in the prevalence of spontaneous cataracts has been observed. In one laboratory, cataracts were shown to occur more commonly in mice than in rats, and some cases were found to be heritable [9]. Of all the mice examined at 18 months of age, 25% had some form of cataract. Rats had a prevalence of over 11% at 2 years of age. Complete cataracts were uncommon in both species, but were seen in some old Sprague-Dawley rats [9]. The pathogenesis of these spontaneous cataracts was not known. Cataracts are seen commonly in rats with retinal degeneration [40]. Lens opacities also can occur in rodents following prolonged eyelid separation, most commonly during anesthesia, and are believed to occur as a result of temperature or osmotic changes in the anterior chamber and in the lens [4]. These opacities are usually transient and resolve soon after recovery from anesthesia.
Ocular posterior segment
Mice and rats have large, spherical lenses. During ophthalmoscopy, this characteristic results in distortion of the fundus image, making the retina seem to float in the vitreous cavity [4]. Because of its small size, the mouse fundus is difficult to examine, but examination is facilitated with a +30 to 40D lens. Photographs of the mouse fundus are challenging because of the small pupillary size and the relatively flat corneal surface. In comparison, the rat fundus is relatively easier to examine and photograph [9].
Mice and rats have a rod-dominated holangiotic retina and arterioles and venules that radiate from the optic disc in a spoke-like fashion (Fig. 4, Fig. 5 ). When using a direct ophthalmoscope to view the fundus, a clear view usually requires a setting of +8D, suggesting that rats are hyperopic [41]. A persistence of the hyaloid artery is common in weanling rats and mice and often disappears with increasing age [4], [9]. When these remnants persist later into adult life, they may remain free-floating, may be associated with hemorrhage (which normally resolves completely), or may remain adherent to the posterior lens capsule [9], [42], [43]. These cataracts appear as small granular plaques and generally do not progress [9].
Fig. 4.
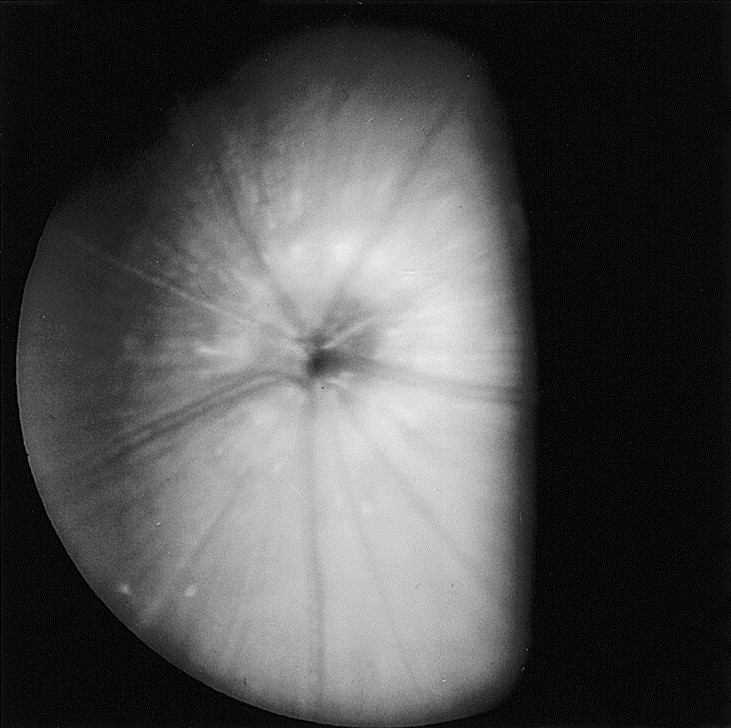
Fundus photograph of a normal male adult deer mouse (Peromyscus maniculatus), a melanotic strain. A portion of the fundus is obscured by the iris. (Courtesy of N.C. Buyukmihci, VMD, Davis, CA.)
Fig. 5.
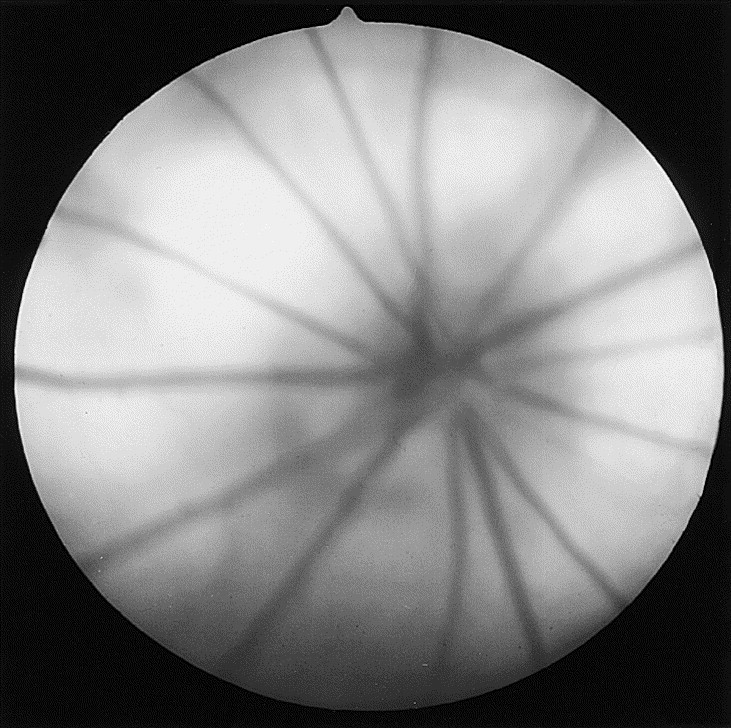
Fundus photograph of a normal adult albino rat. (Courtesy of N.C. Buyukmihci, VMD, Davis, CA.)
Retinal degeneration has been researched extensively in mice and rats. Some strains of mice and rats may have heritable retinal degeneration, whereas other strains develop degeneration secondary to other causes. Phototoxic retinopathy is the most thoroughly investigated and common cause of secondary retinal degeneration in laboratory mice and rats and has been classified into two types by Noelle [44], [45]. Type I is caused by relatively brief exposure to bright light and results in damage to the photoreceptors and the retinal pigment epithelium. Type II is caused by long-term exposure to low-intensity illumination and primarily affects the photoreceptors [44], [45]. Even moderate- or low-intensity continuous illumination has been shown to result in severe retinal damage [46]. Predisposing factors to phototoxic retinopathy include age, concentration of certain hormones, degree of ocular melanosis, diet, genetic factors, duration and intensity of light, and intermittent versus continuous light exposure [44], [46], [47], [48], [49], [50], [51], [52], [53]. Albino mice and rats are substantially more sensitive to the effects of light than are melanotic strains [51]. It would be unusual for this condition to occur in companion mice or rats that are exposed to the typical lighting conditions in home environments.
Heritable retinal degeneration has been documented in a few rat strains, including the Royal College of Surgeons' strain, in which degeneration develops within the first few weeks of life, apparently caused by an autosomal recessive condition [9], [47]. The primary defect seems to be an impaired ability of the pigment epithelium to phagocytose and prevent the accumulation of rod outer-segment fragments. The degeneration develops more slowly in darkness, indicating that other environmental factors, such as light intensity, may affect the rate of progression [9], [47], [53], [54], [55]. Ophthalmic examination usually reveals pallor of the fundus [9], [42], and retinal vessels increasingly become attenuated with time, presumably secondary to the decreased metabolic demands of the degenerating retina [55]. Heritable retinal degeneration is a more widespread in mice than in rats. Because of the widespread occurrence of degeneration in laboratory mice, it is difficult to be certain whether true senile changes exist in mice [9]. Heritable retinal degeneration would be rare in companion mice and rats.
Retinal folds may be seen occasionally in rats [9], [42]. They usually are considered to be congenital (dysplasia) but may result from focal retinal separations [9], [56]. Retinal separation, either spontaneous or congenital, rarely is seen in rats [56].
Abnormalities of the retinal vasculature are seen rarely in mice and rats; however, vessel size commonly may be altered with retinal degeneration and in spontaneously hypertensive rats. Hypertensive rats can develop associated retinal hemorrhage [9], [57]. Papilledema also can be seen in hypertensive rats [9], [57]. Saccular aneurysms of the retinal vessels have been reported sporadically in older mice and rats [58].
Optic nerve
The optic nerve head of mice and rats often appears small, cupped, or possibly colobomatous, because optic nerve fibers do not become myelinated until after leaving the eye through the poorly developed lamina cribrosa [59]. Sporadic occurrences of aplasia or hypoplasia of the optic nerve have been reported in mice and rats that have otherwise normal globes [60], [61] or in association with other anomalies of the globe, particularly microphthalmia [60], [61], [62], [63]. Colobomas of the optic nerve in rats frequently are associated with colobomas of the iris, both of which result from persistence of the fetal fissure [9], [42]. Primary neoplasms of the optic nerve are considered rare in rats, but spontaneous meningiomas of the optic nerve have been reported [64].
Microphthalmia
Many congenital ocular defects have been reported in various strains of laboratory mice and may be seen in pet mice. One of the most common defects is microphthalmia [1]. Microphthalmia alone or accompanied by cataract or retinal dysplasia has occurred sporadically in rats and is believed to be possibly of genetic origin [1]. Microphthalmia can be confused with phthisis bulbi that results from previous trauma or severe uveitis [1]. Microphthalmia can range from subtle to substantial. Only a small remnant of the eye may be present, leading to the clinical misdiagnosis of anophthalmia [65]. Complete resorption of a malformed eye may occur [66]. In the development of microphthalmia and anophthalmia, the most critical time in mice is between optic vessicle development (day 8 of gestation) and fetal fissure clossure (day 12) [65]. Interruption of this process or faulty development of the tissues results in a deformed eye. Hypervitaminosis A has been reported to increase the frequency of these defects by interfering with neural crest migration into the facial primordium [65], [67]. This migration causes a number of anomalies, such as exophthalmia and open-eyelid anomalies [65], [67]. Breeding, housing factors, and toxic environmental factors have been shown to influence the occurrence of microphthalmia [68], [69].
Glaucoma
Rats generally have the lowest intraocular pressure while lights are on and the highest while lights are off [70]. The Tono-Pen Model II tonometer (Oculab, Glendale, CA) has been shown to be a reliable method of measuring intraocular pressure in rats, even though this tonometer is calibrated with the human eye, which has marked differences from the rat eye in corneal thickness and radius of curvature [71], [72]. Glaucoma, or increased intraocular pressure, seems to be rare in the rat.
Summary
As mice and rats become more popular as pets, it is expected that they will be seen more often in general veterinary practice. It is hoped that this increase in doctor visits will be associated with an increased number of clinical reports that describe ophthalmic disorders observed in these species. Until then, clinicians must rely on extrapolation and cautious application of data that are generated in laboratory strains.
Acknowledgements
The author wishes to thank Drs. Nedim Buyukmihci, Steven Hollingsworth, and David Maggs for their review of and comments on this article. The author also thanks John Doval for his technical assistance in preparing the photographs included in this article.
References
- 1.Kern T.J. Rabbit and rodent ophthalmology. Sem Avian Exotic Pet Med. 1997;6:138–145. [Google Scholar]
- 2.Timm K. Orbital venous anatomy of the rat. Lab Anim Sci. 1979;29:636–638. [PubMed] [Google Scholar]
- 3.Fujii S., Hatakenaka N., Kaneda M. Morphogenetic study of the eyelids in NC-eob mice fetuses with an open-eyelid manlformation at birth. Lab Anim Sci. 1995;45:176–180. [PubMed] [Google Scholar]
- 4.Kern T.J. Ocular disorders of rabbits, rodents, and ferrets. In: Kirk R.W., editor. Current veterinary therapy. WB Saunders; Philadelphia: 1989. pp. 681–685. [Google Scholar]
- 5.Saunders L.Z. Ophthalmic pathology in rats and mice. In: Cotchia E., Roe F.J.C., editors. Pathology of laboratory rats and mice. Blackwell Scientific Publications; Oxford: 1967. pp. 349–371. [Google Scholar]
- 6.Corneil-Bell A.H., Sullivan D.A., Allansmith M.R. Gender-related differences in the morphology of the lacrimal gland. Invest Ophthal Vis Sci. 1985;26:1170–1175. [PubMed] [Google Scholar]
- 7.Lorber M. Regional differences within the external ‘duct’ of the rat exorbital lacrimal gland. Exp Eye Res. 1993;56:471–480. doi: 10.1006/exer.1993.1060. [DOI] [PubMed] [Google Scholar]
- 8.Seely J.C. The Harderian gland. Lab Anim. 1987;16:33–39. [Google Scholar]
- 9.Taradach C., Greaves P. Spontaneous eye lesions in laboratory animals: incidence in relation to age. Crit Rev Toxicol. 1984;12:121–147. doi: 10.3109/10408448409023759. [DOI] [PubMed] [Google Scholar]
- 10.Payne A.P. The attractiveness of Harderian gland smears to sexually naïve and experienced male golden hamsters. Anim Behav. 1979;27:897–904. doi: 10.1016/0003-3472(79)90027-7. [DOI] [PubMed] [Google Scholar]
- 11.Payne A.P., McGodey J., Moore M.R. Changes in the porphyrin content of the Harderian gland during the oestrus cycle, pregnancy, and lactation. Biochem J. 1979;178:597–604. doi: 10.1042/bj1780597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiessen D.D., Clancy A., Goodwin M. Harderian gland pheromone in the Mongolian gerbil, Meriones unguiculatus. J Chem Ecol. 1976;2:231–238. [Google Scholar]
- 13.Thiessen D.D., Pendergrass M. Harderian gland involvement in facial lesions in the Mongolian gerbil. J Am Vet Med Assoc. 1982;181:1375–1377. [PubMed] [Google Scholar]
- 14.Brownscheidle C.M., Niewenhuis R.J. Ultrastructure of the Harderian gland in male albino rats. Anat Rec. 1978;190:735–754. doi: 10.1002/ar.1091900309. [DOI] [PubMed] [Google Scholar]
- 15.Carriere R. Ultrastructural visualization of intracellular porphyrin in the rat Harderian gland. Anat Rec. 1985;213:496–504. doi: 10.1002/ar.1092130404. [DOI] [PubMed] [Google Scholar]
- 16.Hugo J., Krijt J., Vokurka M. Secretory response to light in rat Harderian gland: possible photoreceptive role of Harderian porphyrin. Gen Physiol Biophys. 1987;6:401–404. [PubMed] [Google Scholar]
- 17.Johnston H.S. The Harderian gland, its secretory duct and porphyrin content in the Mongolian gerbil. J Anat. 1983;137:615–630. [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai T. The mammalian Harderian gland: morphology, biochemistry, function, and phylogeny. Arch Histol Jap. 1981;44:299–333. doi: 10.1679/aohc1950.44.299. [DOI] [PubMed] [Google Scholar]
- 19.Sakai R., Yohro T. A histological study of the Harderian gland of Mongolian Gerbils. Anat Rec. 1981;200:259–270. doi: 10.1002/ar.1092000304. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly T.M. What's your diagnosis? Blood-caked staining around the eyes in Sprague-Dawley rats. Lab Anim. 1997;26:17–18. [Google Scholar]
- 21.Hanna P.E., Perey D.H., Paturzo F. Sialodacryoadenitis in the rat: effects of immunosuppression on the course of the disease. Am J Vet Res. 1984;45:2077–2083. [PubMed] [Google Scholar]
- 22.McGee M.A., Maronpot R.R. Harderian gland dacryoadenitis in rats resulting from orbital bleeding. Lab Anim Sci. 1979;29:639–641. [PubMed] [Google Scholar]
- 23.Maronpot R.R., Chavannes J.M. Dacryoadenitis, conjunctivitis and facial dermatitis of the mouse. Lab Anim Sci. 1977;27:277–278. [PubMed] [Google Scholar]
- 24.Holland J.M., Fry M. Neoplasms of the integumentary system and harderian gland. In: Foster H.L., Small J.D., Fox J.G., editors. Volume IV. Academic Press; New York: 1982. pp. 521–528. (The mouse in biomedical research). [Google Scholar]
- 25.Rothwell T.L.W., Everitt A.V. Exophthalmos in ageing rats with Harderian gland disease. Lab Anim. 1986;20(2):97–100. doi: 10.1258/002367786780865160. [DOI] [PubMed] [Google Scholar]
- 26.Yoshitomi K., Boorman G.A. Palpebral amelanotic melanomas in F344 rats. Vet Pathol. 1993;30:280–286. doi: 10.1177/030098589303000309. [DOI] [PubMed] [Google Scholar]
- 27.Yoshitomi K., Boorman G.A. Intraocular and orbital malignant schwannomas in F344 rats. Vet Pathol. 1991;28:457–466. doi: 10.1177/030098589102800601. [DOI] [PubMed] [Google Scholar]
- 28.Murphy J.C., Osterberg R.E., Seabaugh V.M. Ocular irritancy responses to various pHs of acids and bases with and without irrigation. Toxicology. 1982;23:281–291. doi: 10.1016/0300-483x(82)90067-1. [DOI] [PubMed] [Google Scholar]
- 29.Van Winkle T.J., Balk M.W. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci. 1986;36(3):248–255. [PubMed] [Google Scholar]
- 30.Kaplun A., Barishak R.Y. Appearance of keratitis in laboratory mice: influence of azathioprine and meticorten. Lab Anim. 1976;10:105–109. doi: 10.1258/002367776781071396. [DOI] [PubMed] [Google Scholar]
- 31.Brückner R. Spaltlampenmikroskopie und ophthalmoskopie am auge von ratte and maus. Doc Ophthalmol. 1951;5–6:452–454. doi: 10.1007/BF00143667. [DOI] [PubMed] [Google Scholar]
- 32.Enoch J.M. Vertebrate receptor optics and orientation. Doc Ophthalmol. 1979;48:373–388. doi: 10.1007/BF00141466. [DOI] [PubMed] [Google Scholar]
- 33.Muller G. Eine enwicklungsgeschectliche untersuchung über das erbliche kolobom mit mickrophthalmus bei der hausmaus. Z Mikrosk Forsch. 1950;56:520–528. [Google Scholar]
- 34.Rubin L.F., Daly I.W. Ectopic pupil in mice. Lab Anim Sci. 1982;32:64–65. [PubMed] [Google Scholar]
- 35.Heywood R. Some clinical observations on the eyes of Sprague-Dawley rats. Lab Anim. 1973;7:19–27. doi: 10.1258/002367773781005914. [DOI] [PubMed] [Google Scholar]
- 36.Magnusson G., Majeed S., Offer J.M. Intraocular melanoma in the rat. Lab Anim. 1978;12:249–252. doi: 10.1258/002367778781088503. [DOI] [PubMed] [Google Scholar]
- 37.Yoshitomi K., Boorman G.A. Spontaneous amelanotic melanomas of the uveal tract in F344 rats. Vet Pathol. 1991;28:403–409. doi: 10.1177/030098589102800508. [DOI] [PubMed] [Google Scholar]
- 38.Fumito I., Matsushima Y., Hiai H. Rupture of lens cataract: a novel hereditary recessive cataract model in the mouse. Exp Eye Res. 1997;64:107–113. doi: 10.1006/exer.1996.0192. [DOI] [PubMed] [Google Scholar]
- 39.Gorthy W.C. Cataracts in the aging rat lens: their morphological characterization and evaluation as a model for human senile cataracts. Ophthalmic Res. 1977;9:329–342. [Google Scholar]
- 40.Al-Ghoul K.J., Kuszak J.R. Anterior polar cataracts in CS rats: a predictor of mature cataract formation. Invest Opthalmol Vis Sci. 1999;40:668–679. [PubMed] [Google Scholar]
- 41.Bellhorn R.W. Laboratory animal ophthalmology. In: Gelatt K.N., editor. Veterinary ophthalmology. 2nd edition. Lea & Fibiger; Philadelphia: 1991. pp. 671–676. [Google Scholar]
- 42.Rubin L.F. Atlas of veterinary ophthalmoscopy. Les & Febiger; Philadelphia: 1974. Rat and rabbit fundus; pp. 367–397. [Google Scholar]
- 43.Saunders L.Z., Rubin L.F. Ophthalmic pathology of animals. S. Karger; Basel: 1975. [Google Scholar]
- 44.Malik S., Cohen D., Meyer E. Light damage in the developing retina of the albino rat: an electroretinographic study. Invest Ophthalmol Vis Sci. 1986;27:164–167. [PubMed] [Google Scholar]
- 45.O'Steen W.K., Kraeer S.L. Effects of hypophysectomy, pituitary gland homogenates and transplants, and prolactin on photoreceptor destruction. Invest Ophthalmol Vis Sci. 1977;16:940–946. [PubMed] [Google Scholar]
- 46.Penn J.S., Baker B.N., Howard A.G. Retinal light-damage in albino rats: lysosomal enzymes, rhodopsin, and age. Exp Eye Res. 1985;41:275–284. doi: 10.1016/s0014-4835(85)80017-8. [DOI] [PubMed] [Google Scholar]
- 47.Bourne M.C., Campbell D.A., Tansley K. Hereditary degeneration of the rat retina. Br J Ophthalmol. 1938;22:613–623. doi: 10.1136/bjo.22.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenman D.L., Bryant P., Kodell R.L. Influence of cage shelf level on retinal atrophy in mice. Lab Anim Sci. 1982;32:353–356. [PubMed] [Google Scholar]
- 49.Organisciak D.T., Jiang Y.L., Wang H.M. Retinal light damage in rats exposed to intermittent light: comparison with continuous light exposure. Invest Ophthalmolo Vis Sci. 1989;30:795–805. [PubMed] [Google Scholar]
- 50.Rapp L.M., Thum L.A., Anderson R.E. Synergism between environmental lighting and taurine depletion in causing photoreceptor cell degeneration. Exp Eye Res. 1988;46:229–238. doi: 10.1016/s0014-4835(88)80080-0. [DOI] [PubMed] [Google Scholar]
- 51.Rapp L.M., Tolman B.L., Koutz C.A. Predisposing factors to light-induced photoreceptor cell damage: retinal changes in maturing rats. Exp Eye Res. 1990;51:177–184. doi: 10.1016/0014-4835(90)90070-b. [DOI] [PubMed] [Google Scholar]
- 52.Robinson W.G., Jr., Kuwabara T., Bieri J.G. Deficiencies of vitamins E and A in the rat: retinal damage and lipofuscin accumulation. Invest Ophthalmol Vis Sci. 1980;19:1030–1037. [PubMed] [Google Scholar]
- 53.Seitz R, Weisse I, Stotzer H. Altersbedingte und lichtabhangige Netzhantveranderungen, Monatsbl Augenheilkd 1977;171:431. [PubMed]
- 54.Dowling J.E., Sidman R.L. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herron W.L., Jr., Riegel B.W., Brennan E. Retinal dystrophy in the pigmented rat. Invest Ophthalmol. 1974;13:87–94. [PubMed] [Google Scholar]
- 56.Heywood R. Retinal detachment in the rat. Lab Anim. 1976;10:389–392. doi: 10.1258/002367776780956971. [DOI] [PubMed] [Google Scholar]
- 57.Parr J.C. Retinal arterioles in the New Zealand strain of genetically hypersensitive rats. Aust J Ophthalmol. 1976;4:58–65. [PubMed] [Google Scholar]
- 58.Bellhorn R.W. Ophthalmological disorders of exotic and laboratory animals. Vet Clin North Am. 1973;3:345–356. doi: 10.1016/s0091-0279(73)50053-4. [DOI] [PubMed] [Google Scholar]
- 59.Bellhorn R.W. Retina nutritive systems in vertebrates. Sem Avian Exotic Pet Med. 1997;6:108–118. [Google Scholar]
- 60.Shibuya K., Tajima N., Nunoya T. Optic nerve dysplasia associated with meningeal defect in Sprague-Dawley rats. Vet Pathol. 1998;35:323–329. doi: 10.1177/030098589803500501. [DOI] [PubMed] [Google Scholar]
- 61.Stotzer H., Weisse I., Knappen F. Die retina-degeneration der ratte. Arzneim Forsch. 1970;20:811. [PubMed] [Google Scholar]
- 62.Shibuya K., Tajima M., Yamate J. Unilateral optic nerve aplasia in two young s/c: Wistar rats. Vet Pathol. 1989;26(6):518–520. doi: 10.1177/030098588902600611. [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi Y.K., Kitoh J., Sakai H. Aplasia of the optic nerve in microphthalmic offspring of prenatally X-irradiated rats. Congenital Anom. 1988;28:179–186. doi: 10.1111/cga.1988.28.3.179. [DOI] [PubMed] [Google Scholar]
- 64.Yoshitomi K., Everitt J.I., Boorman G.A. Primary optic nerve meningiomas in F344 rats. Vet Pathol. 1991;28:79–81. doi: 10.1177/030098589102800111. [DOI] [PubMed] [Google Scholar]
- 65.Smith R.S., Roderick T.H., Sundberg J.P. Microphthalmia and associated abnormalities in inbred black mice. Lab Anim Sci. 1994;44:551–560. [PubMed] [Google Scholar]
- 66.Kinney H.C., Klintworth G.K., Lesiewiez J. Congenital cystic microphthalmia and consequent anophthalmia in the rat: a study in abnormal ocular morphogenesis. Teratology. 1982;26:203–212. doi: 10.1002/tera.1420260213. [DOI] [PubMed] [Google Scholar]
- 67.Padmanabhan R., Singh G., Singh S. Malformations of the eye resulting from maternal hypervitaminosis A during gestation in the rat. Acta Anat. 1981;110:291–298. doi: 10.1159/000145439. [DOI] [PubMed] [Google Scholar]
- 68.Pierro L.J., Spiggle J. Congenital eye defects in the mouse: I. The influence of litter size, litter spacing, and suckling of offspring on risk of eye defect in C57BL mice. Teratology. 1969;2:337–344. doi: 10.1002/tera.1420020408. [DOI] [PubMed] [Google Scholar]
- 69.Shirai S. Eye abnormalities in mouse fetuses causes by simultaneous irradiation of X-rays and ultraound: II. Developmental abnormalities of the eye. Congenital Anom. 1978;18:269–279. [Google Scholar]
- 70.Moore C.G., Johnson E.C., Morrison J.C. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 71.Moore C.G., Milne S.T., Morrison J.C. Noninvasive measurement of rat intraocular pressure with the tono-pen. Invest Ophthalmol Vis Sci. 1993;34:363–369. [PubMed] [Google Scholar]
- 72.Carter-Dawson L., Kuwabara T., O'Brian P.J. Structural and biochemical changes in vitamin A-deficient rat retinas. Invest Ophthalmol Vis Sci. 1979;18:437–446. [PubMed] [Google Scholar]


