Abstract
Objective
To examine data in support of the viral hypothesis of atopic disease.
Data Sources
We retrieved review articles and original research from MEDLINE, OVID, and PubMed (1950-June 2009) that addressed our topic of interest, using the terms respiratory virus, asthma, IgE, atopy, and viral-induced wheeze.
Study Selection
Articles were selected for their relevance to viruses and their role in asthma.
Results
Much of the data in support of the viral role in asthma focuses on rhinovirus and respiratory syncytial virus. Epidemiologic studies have used factors such as day-care and family size as surrogates for infection in studies that support and negate the role of viruses in the development of asthma. A large volume of literature supports the theory that virus exacerbates preexisting asthma by setting off the inflammatory cascade. No mechanistic studies fully explain how viral infections can translate or exacerbate atopic disease. We provide information from our mouse model that suggests that dendritic cells, IgE, and FcɛRI are critical to the induction of atopy. Studies of patients taking antiviral agents (eg, ribavirin or palvizumab) support the notion that interfering with respiratory viral infections may decrease the development of atopy.
Conclusions
Many studies suggest strongly that viral infections may predispose patients to the development of asthma and other atopic diseases. Further, mechanistic studies are necessary to allow for the development of targeted therapeutics to prevent the translation of viral into atopic disease.
Off-label disclosure: Drs Kumar and Grayson have indicated that this article does not include the discussion of unapproved/investigative use of a commercial product/device.
Financial disclosure: Dr Grayson has received research grants from Genentech and the National Institutes of Health.
Instructions for CME credit
-
1.
Read the CME review article in this issue carefully and complete the activity by answering the self-assessment examination questions on the form on page 188.
-
2.
To receive CME credit, complete the entire form and submit it to the ACAAI office within 1 year after receipt of this issue of the Annals.
INTRODUCTION
Antiviral responses are interferon mediated and often viewed as prototypical TH1 immune responses. Yet, we know that viral infections can aggravate asthma, cause symptoms similar to allergic rhinitis, and may induce development of asthma and atopic disease—all TH2 hallmark diseases. Much of our focus determining the role of infection in the development of atopic disease stems from studies exploring the hygiene hypothesis. This theory postulates that the relative increase in the incidence of atopic disease in western society is due to increased cleanliness.1 However, the hygiene hypothesis does not fully explain increased atopy and fails to acknowledge the role viral infections play in the development of atopic disease. How viral infections have this ability to be a TH1/TH2 “double-edged sword” is not well understood. In this review we explore the data supporting and refuting a role for viruses in the development of asthma and atopic disease. We also provide a paradigm, developed from our studies that suggest IgE and viral infections together lead to the development of atopic disease. We retrieved review articles and original research from MEDLINE, OVID, and PubMed (1950-June 2009) that addressed our topic of interest, using the terms respiratory virus, asthma, IgE, atopy, and viral-induced wheeze. Articles were selected for their relevance to viruses and their role in asthma. In reviewing the literature, we noted that there are essentially 3 avenues of viral asthma research: data supporting a role for viruses in the development of asthma, data focused on the role of viruses in asthma exacerbations, and data supporting a role of viruses in causing atopy.2
VIRUSES AND CAUSATION OF ASTHMA
Evidence favoring a causative role for viral infections in asthma development includes studies focused on specific viruses, as well as several epidemiologic studies that have documented a role for viral exposure factors, such as day-care and family size. The strongest evidence comes from studies exploring the role of respiratory syncytial virus (RSV).
RSV is a single-stranded RNA paramyxovirus that infects most children by 2 years of age.3 Although most have a rather mild course, a subset of infants (primarily in the 2- to 6-month age group) develop severe bronchiolitis requiring hospitalization. Sigurs and coworkers4 showed that after the viral infection these individuals were left with a markedly increased risk for developing asthma (odds ratio [OR], 12.7), as well as allergic sensitization (OR, 2.4). The risk remained present through at least 7½ years of age.
The Tucson Children’s Respiratory Study includes more than 800 children with documented RSV infection in infancy. However, unlike the study by Sigurs et al, these infections were not severe enough to require hospitalization.5 These children have been followed up longitudinally for the development of asthma and atopic disease. RSV infection was shown to increase the risk of wheezing early in life, but this risk was transient, disappearing between 10 and 13 years of age, suggesting a possible effect of puberty.
Although RSV is frequently associated with progression to asthma, other viruses have also been implicated. In a study performed in Greece of 119 infants with bronchiolitis, 74% had viruses detected in their nasopharyngeal washings. Of this group, 29% were found by polymerase chain reaction (PCR) to have rhinovirus isolates.6 On the basis of the sheer number of viral exposures, rhinovirus is an important pathogen in the development of acute bronchiolitis in infants. Other studies have documented an association between rhinovirus infection and the development of wheezing later in life. The Childhood Origins of Asthma (COAST) study examined suburban children from birth to 3 years of age who were at high risk for asthma (1 parent with asthma or allergy).7 This study showed that the most important risk factor for development of wheeze by the third year of life was a symptomatic rhinovirus infection.8 The association of wheezing with prior rhinovirus infection was stronger than that with RSV or any other viral pathogen. The COAST study involved a multivariate analysis that attempted to correct for other factors, such as pets, older siblings, participation in day-care, breastfeeding, atopic dermatitis, and the presence of food specific IgE. Taking these factors into account, rhinovirus was found to be most associated with later wheeze. Further analysis of cytokine profiles in this cohort suggested that children with impaired TH1 responses (decreased release of interferon γ from cord blood monocytes) had increased numbers of viral infections in the first year of life.9
Other studies have made similar conclusions regarding the role of rhinoviruses in the development of asthma. A recent study of 259 children found that wheezing with rhinovirus led to a strong risk (OR, 25.6) of developing asthma by 6 years of age.10 Rhinovirus has been looked at in mouse models of asthma as well and has been found to worsen underlying asthmatic inflammation and airway hyperresponsiveness.11
A study by Kusel et al12 in 2007 looked at the risk of wheezing at 5 years of age in 198 patients at high risk of developing asthma. These patients were initially enrolled as infants and monitored for respiratory infections, as well as with periodic checks for asthma and atopy. At 5 years of age, the patients with current wheeze and asthma had higher rates of respiratory infections with rhinovirus or RSV. Interestingly, the association between viral infection and wheeze or asthma was limited to the subgroup of patients who had developed allergic sensitization at younger than 2 years. This finding suggests a role between viral infection and development of allergic sensitization.
Parainfluenza viruses may also be implicated in the development of asthma. Parainfluenza viruses are a group of RNA viruses that have been associated with croup, pneumonia, and bronchiolitis in infants.13 The Canadian Asthma Primary Prevention Study studied 455 children from atopic families and performed serial viral PCR samples at 2, 4, 8, and 12 months.14 They found that children with parainfluenza infection in the first year of life had higher odds of developing asthma in their second year of life. A study in Sweden examined cytokine profiles of children infected with RSV, influenza, or parainfluenza and found comparable increases in TH2 cytokine profiles in all infected children younger than 3 months.15 Thus, similarities exist between RNA respiratory viruses in their ability to skew toward an atopic predisposition.
Epidemiologic data have contributed to the idea that viruses may cause asthma and atopic disease. These studies have looked at exposure to day-care and increased family size as surrogates for viral exposure. A study from Sweden in 2006 enrolled more than 10,000 children and used a questionnaire to document multiple airway infections and day-care attendance, which were both found to be risk factors for asthma.16 Recently, a Tennessee cohort of 90,000 children was examined to see what percentage of children with asthma at 4 to 5½ years of age had bronchiolitis as infants and in what season the bronchiolitis occurred.17 An increased risk of asthma was found in children with episodes of bronchiolitis during nonwinter months. In addition, those with bronchiolitis during a month associated with higher rhinovirus exposure had a 25% greater risk of developing childhood asthma than those who developed infections during months where RSV was the primary cause of bronchiolitis. They also found that patients born 4 months before the winter virus peak had an increased risk of developing asthma, thus emphasizing that timing of the virus plays a crucial role in the development of atopy.18
The role of day-care as a risk factor for asthma is controversial. Although some studies suggest a day-care–imparted risk for asthma, others show a protective effect. Some have argued that the underlying issue is that day-care by itself may not be a risk factor, but when actual diagnosed infections are taken into account, the association in the data becomes more robust.19 A study from 2003 in which 453 children with a parental history of atopy were followed up for the development of asthma, wheezing, and eczema showed that although most day-care participants had less asthma, eczema, or wheezing, a subgroup—those with a maternal history of asthma—had a higher rate of the disease.20 This emphasizes once again that applying general assumptions about a population as a whole may complicate the perceptions regarding development of atopy.
Another analysis from the Tucson Children’s Respiratory Study analyzed the presence of asthma in relation to day-care attendance and number of siblings at home in 1,035 children.21 This study suggested a protective role for day-care and having older siblings at home, at least in terms of development or presence of asthma from ages 6 through 13 years.
In 2007, the medical records of more than 500,000 Israeli military personnel were searched for an association between family size and asthma development.22 In this study, asthma prevalence increased in families of up to 3 siblings, but 4 or more siblings was protective of asthma development. The presumption in these studies is that sibling number would be a surrogate for viral infection. How more than 3 siblings would reduce the number of viral infections or the risk of asthma remains unexplained. Although this suggests that increasing viral infections may increase the risk of asthma, there is a tipping-point at which enough exposures (assuming larger families have more viral infections) lead to an overwhelming TH1 environment that trumps the TH2 proatopic/asthmatic response. This is an interesting hypothesis; however, many more studies will need to be performed to determine if this is indeed the case.
VIRUSES AND THE EXACERBATION OF ASTHMA
Viruses have been known for awhile to induce exacerbation of preexisting asthma in both adults and children. Past studies have found a seasonal pattern of asthma admissions that correlates with the seasonal pattern of viral infections.23 Several studies have used PCR analysis to identify viruses in patients with asthma exacerbations. Johnston and coworkers24 studied 108 children aged 9 to 11 years who had decreases in peak flow rates and an asthma exacerbation and found that 80% or more had detectable viruses in nasal aspirates. The most predominant virus identified was rhinovirus. In a study of 138 adults, similar results indicated that subjective symptoms of upper respiratory tract infections were seen in 80% of patients with asthma exacerbations. Further, peak flows were reduced in conjunction with viral infections. In this study, 44% of patients with asthma exacerbations (identified as peak flow decreases of >50 L/min) had documented viral infections.25 In inner-city adults with asthma, 44% of asthma exacerbations were secondary to respiratory tract viral infections.26 This study also documented that 55% of acute emergency department visits for asthma exacerbations in adults were secondary to a viral respiratory illness. Thus, viruses clearly exacerbate preexisting asthma. How viral infections exacerbate asthma is not understood. Theories have been proposed to explain how viral infections are able to exacerbate asthma, with most relying on the obvious: antiviral-induced inflammation drives bronchial hyperreponsiveness and instigates the underlying inflammatory pathways of asthma.27 However, this is somewhat of a circular argument and does not get to the underlying mechanistic connections between antiviral responses and the exacerbation of TH2-mediated diseases, such as asthma. Indeed, whether this inflammatory response is TH2 mediated remains unclear because the mechanism through which viral infections exacerbate or induce asthma may be via non–T-cell–mediated pathways. For example, when asthmatic patients were experimentally inoculated with rhinovirus, an increase in nasal granulocyte colony-stimulating factor and interleukin-8 was seen.28 These cytokines correlated with a subsequent increase in neutrophils in the airways, suggesting that neutrophils may play a critical role in viral-induced asthma exacerbation. It remains to be seen if other innate immune components have critical roles in the translation of viral to asthmatic disease.
Although we have been presenting data supporting the role of viruses in potentially inducing asthma, some studies have argued that atopic individuals are predisposed to develop viral infections. For example, in a study of 8,280 pairs of Danish twins born between 1994 and 2000 the relationship between RSV infection and asthma was explored with various mathematical models.29 The best-fit model of the data supported the hypothesis that asthma was a risk for RSV infection and not the other way around. Solving this chicken and egg argument is a major focus of research, and we are hopeful that a clearer relationship between RSV and asthma will emerge in the near future.
As shown in Figure 1 , the timing of viral infection, as well as the actual viral pathogen, may be critically important in determining whether a given infection will lead to disease progression, regression, or aggravation. Awaiting additional studies are the mechanistic underpinnings that will explain these temporal differences in disease expression.
Figure 1.
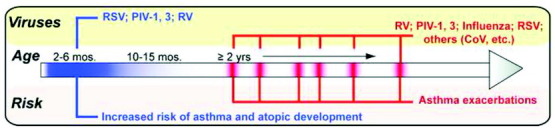
Viral infections and the age and risk they impart for the development of atopic disease. Respiratory viral infections early in life (blue bar) are capable of imparting an increased risk for the development of asthma and atopic disease. Later in life (red bars), viral infections are associated primarily with exacerbations of preexisting asthma. RSV indicates respiratory syncytial virus; PIV, parainfluenza virus; RV, rhinovirus; CoV, coronavirus.
VIRUSES AND DEVELOPMENT OF ATOPY
Since the early 1970s it has been recognized that viral infections lead to increased IgE—both viral specific and nonspecific. The reason for this increase and the role for IgE in viral illnesses are not known. Nonetheless, the association of IgE elevation with viral infections and the similarity of upper respiratory tract viral infection symptoms with those from allergic rhinitis has suggested a possible causative link. Evidence of this possible link was first reported in 1979, when Frick and coworkers30 studied 13 children from atopic families to see if and when they developed atopic disease. In this small study, 11 of the children were noted to have upper respiratory tract infection 1 to 2 months before the initial development of allergic sensitization. This interesting association led the authors to hypothesize that viral infections might precipitate the development of atopic disease. Of course, the chicken and egg argument also applies here—are atopic individuals more susceptible to viral infection or could viral infections drive atopic disease?
We have used a mouse model to explore the role of viruses in the development of postviral atopic disease. In this model, mice infected with the mouse parainfluenza virus, Sendai virus, develop airway hyperreactivity and mucous cell metaplasia after clearance of the virus. The airway changes are long-lasting and are still present at least 1 year after the initial infection. Interestingly, we found that the high-affinity receptor for IgE, FcɛRI, was expressed by lung dendritic cells during the viral infection, and IgE against Sendai virus was produced during the infection.31 Cross-linking the dendritic cell–expressed FcɛRI led to production of a TH2 cell chemoattractant (CCL28) that was necessary for development of the postviral mucous cell metaplasia (through an interleukin 13–dependent process). These data provide some mechanistic insight into how a viral infection might translate into asthma.
More recently, we have begun exploring whether exposure to a nonviral antigen during a viral infection would be sufficient to drive IgE production against the nonviral antigen. Indeed, in our early studies, we noted that the total IgE level remained elevated after clearance of the virus, whereas the viral specific IgE levels rapidly decreased. This finding suggested that it might be possible to produce IgE against environmental antigens as a consequence of a viral infection. Our preliminary data have indicated that indeed exposure to a nonviral antigen at a critical point during the antiviral response may be all that is necessary to generate IgE against the nonviral antigen.32, 33 These data have begun to elucidate the potential mechanisms underlying the intriguing results reported by Frick and coworkers in 1979. Further, as shown in Figure 2 , these data suggest that viral infections may be sufficient to induce an atopic cycle, a self-perpetuating loop leading to increased atopic sensitizations as a result of an initial infection. We should stress that the atopic cycle is based on data in mouse models, and whether similar mechanisms operate in humans remains to be determined but is an avenue of active research.
Figure 2.
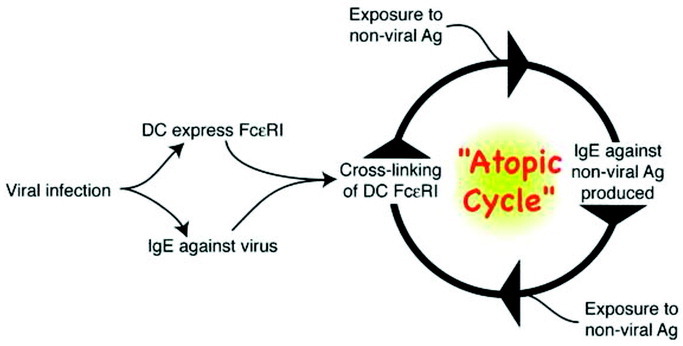
The atopic cycle. On the basis of studies in the mouse, which have not yet been validated in humans, viral infections are capable of inducing the high-affinity receptor for IgE (FcɛRI) on dendritic cells (DCs), as well as the production of IgE against the virus. Exposure to a nonviral antigen (Ag) after cross-linking of FcɛRI on the DCs can lead to production of IgE against the nonviral Ag (see text for details). This allows the new IgE to bind to the DC-expressed FcɛRI and, with subsequent exposure to the same nonviral Ag, restarts the cycle, potentially explaining the development of multiple sensitivities as a result of an initial viral infection.
IMPLICATIONS FOR THERAPY
If viral infections lead to the development of atopic disease, then it should be possible to intervene and prevent allergies and asthma. Clearly, the first target would be preventing viral infections. This is becoming more plausible given newly developed vaccine strategies and antiviral agents. For example, the therapeutic monoclonal antibody against RSV, palvizumab (Synagis), is one option. This monoclonal antibody is directed against an epitope in the A antigenic site of the F protein of RSV. Currently, the American Academy of Pediatrics recommends its use for high-risk infants during RSV season.34 In studies examining the long-term outcome of asthma in this particular patient population, it was found that patients who received palvizumab had lower rates of recurrent wheezing compared with those who did not.35
Giving antivirals for RSV has been shown in other studies to lead to a trend toward less atopy. In a recent study,36 175 patients who had acute RSV were studied—some received ribavirin and some did not. In the group that received ribavirin, rates of physician-diagnosed wheezing were decreased, along with decreased rates of allergic sensitization. These intriguing data suggest that the prevention or early treatment of viral infections may be able to prevent subsequent development of asthma and atopic disease.
Other potential targets in the viral cascade include the NFκB pathway, which is believed to have a role in both asthma and chronic obstructive pulmonary disease.37 The use of small interfering RNA technology (in which double-stranded RNA is introduced that can interfere and silence expression of specific genes) is an area of active research, with the targets being genes, such as those involved in the NFκB pathway.
On the basis of our work in the mouse model, it appears that targeting IgE during a viral infection would be beneficial in reducing development of postviral atopic disease. If this is indeed true, then omaluzimab (Xolair), the humanized anti-IgE monoclonal antibody, could be useful in breaking the virus-IgE-dendritic cell atopic cycle. Interfering with chemokines involved in this response (such as CCL28) would also be predicted to be productive targets for blocking the virus to atopy translation.38 The actual effectiveness of these strategies awaits large-scale clinical trials; we are hopeful that in the near future interventions will be developed to stem the tide of atopic disease development. However, because the role of IgE (or CCL28) in the normal antiviral response is not understood, we remain cautious about the effects of depleting these mediators.
CONCLUSION
Although the jury remains undecided on the actual role viruses play in the development of asthma and atopic disease, much data support a role at least in focused developmental windows. It is highly likely that the underlying genetic makeup of the individual will modulate the response to the viral infection, complicating studies exploring the risk of atopic disease and viral infection. Nonetheless, the studies that have been performed and those that are ongoing have provided insights that will help to shape future therapeutic interventions. Our hope is that in the relatively near future, treatments will be developed and used that will lessen or prevent the translation of viral diseases into asthma and atopy. Two important questions come from all of these studies—why is IgE produced as part of the antiviral response, and could the development of atopic disease be a hereditary outcropping of the antiviral role of IgE? We look forward to future studies that will answer these interesting and vexing questions.
Objectives: After reading this article, participants should be able to demonstrate an increased understanding of their knowledge of allergy/asthma/immunology clinical treatment and how this new information can be applied to their own practices.
Participants: This program is designed for physicians who are involved in providing patient care and who wish to advance their current knowledge in the field of allergy/asthma/immunology.
Credits: ACAAI designates each Annals CME Review Article for a maximum of 2 category 1 credits toward the AMA Physician’s Recognition Award. Each physician should claim only those credits that he/she actually spent in the activity. The American College of Allergy, Asthma and Immunology is accredited by the Accreditation Council for Continuing Medical Education to sponsor continuing medical education for physicians.
Footnotes
This educational activity is supported by an educational grant from GlaxoSmithKline.
CME Examination
1–10, Kumar A. 2009;103:181–187.
CME Test Questions
-
1.Data from the Tucson Children’s Respiratory Study showed that respiratory syncytial virus (RSV) was a risk for wheezing early in life; however, this effect subsided by what age?
-
a.12 to 18 months
-
b.18 months to 3 years
-
c.3 to 5 years
-
d.7 to 10 years
-
e.10 to 13 years
-
a.
-
2.The Childhood Origins of Asthma (COAST) study found that the most important risk factor for asthma was which of the following?
-
a.RSV infection
-
b.rhinovirus infection
-
c.parainfluenza infection
-
d.bacterial infection
-
e.parasitic infection
-
a.
-
3.The COAST study found that patients with decreased interferon γ responses in cord blood monocytes had:
-
a.increased numbers of viral infections
-
b.decreased numbers of viral infections
-
c.increased numbers of bacterial infections
-
d.decreased numbers of bacterial infections
-
e.no change in the number of overall infections
-
a.
-
4.In a study of military personnel in Israel examining sibling number as risk for asthma, rates for asthma decreased when the number of siblings was greater than:
-
a.1
-
b.2
-
c.3
-
d.4
-
e.5
-
a.
-
5.In a mouse model of asthma, infection with Sendai virus led to which of the following?
-
a.decreased FcɛR1 on lung dendritic cells
-
b.increased FcɛR1 on lung dendritic cells
-
c.decreased IgE on lung mast cells
-
d.decreased IgE on lung basophils
-
e.decrease in free IgE
-
a.
-
6.Data from studies using palvizumab in high-risk infants led to:
-
a.higher rates of atopic dermatitis
-
b.higher rates of allergic rhinitis
-
c.lower rates of recurrent wheezing
-
d.lower rates of drug allergy
-
e.lower rates of food allergy
-
a.
-
7.Antiviral agents used for acute RSV led to the following:
-
a.increased rates of physician-diagnosed wheezing
-
b.increased rates of adult asthma
-
c.decreased rates of physician-diagnosed wheezing
-
d.decreased rates of allergic sensitization
-
e.both c and d
-
a.
-
8.Studies that support the role of viruses in the exacerbation of asthma have been shown in:
-
a.adults
-
b.children
-
c.both adults and children
-
d.neither
-
a.
-
9.In a study looking at the role of viral seasons in bronchiolitis, which of the following occurred?
-
a.a decreased risk of asthma during nonwinter virus months
-
b.bronchiolitis during months with rhinovirus led to a 25% increased risk of asthma compared with RSV
-
c.RSV-associated bronchiolitis led to a decrease in the risk of asthma
-
d.asthma rates stayed the same no matter in what season the child developed bronchiolitis
-
e.asthma rates decreased after bronchiolitis infections
-
a.
-
10.In epidemiologic studies of asthma, the following have been used as surrogates of viral infections:
-
a.obesity
-
b.pets
-
c.day-care
-
d.family size
-
e.both c and d
-
a.
Answers to CME examination—Annals of Allergy, Asthma & Immunology, September 2000 Kumar A: The role of viruses in the development and exacerbation of atopic disease. Ann Allergy Asthma Immunol. 2009;103:181–187.
-
1.
e
-
2.
b
-
3.
a
-
4.
d
-
5.
b
-
6.
c
-
7.
e
-
8.
c
-
9.
b
-
10.
e
REFERENCES
- 1.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemanske RF., Jr Viruses and asthma: inception, exacerbation, and possible prevention. J Pediatr. 2003;142(2 suppl):S3–S7. doi: 10.1067/mpd.2003.19. [DOI] [PubMed] [Google Scholar]
- 3.Meissner HC. Reducing the impact of viral respiratory infections in children. Pediatr Clin North Am. 2005;52:695–710. doi: 10.1016/j.pcl.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 5.Stein RT, Sherrill D, Morgan WJ. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos NG, Moustaki M, Tsolia M. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 7.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13(suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemanske RF, Jr, Jackson DJ, Gangnon RE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Friedlander SL, Jackson DJ, Gangnon RE. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J. 2005;24(11 suppl):S170–S176. doi: 10.1097/01.inf.0000187273.47390.01. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DJ, Gangnon RE, Evans MD. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett NW, Walton RP, Edwards MR. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusel MM, de Klerk NH, Kebadze T. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanock RM, Murphy BR, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Field Virology. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. p. 1341. [Google Scholar]
- 14.Lee KK, Hegele RG, Manfreda J. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42:290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 15.Kristjansson S, Bjarnarson SP, Wennergren G. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–811. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Hagerhed-Engman L, Bornehag CG, Sundell J, Aberg N. Day-care attendance and increased risk for respiratory and allergic symptoms in preschool age. Allergy. 2006;61:447–453. doi: 10.1111/j.1398-9995.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- 17.Carroll KN, Wu P, Gebretsadik T. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P, Dupont WD, Griffin MR. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallia P, Johnston SL. Respiratory viruses: do they protect from or induce asthma? Allergy. 2002;57:1118–1129. doi: 10.1034/j.1398-9995.2002.02169.x. [DOI] [PubMed] [Google Scholar]
- 20.Celedon JC, Wright RJ, Litonjua AA. Day care attendance in early life, maternal history of asthma, and asthma at the age of 6 years. Am J Respir Crit Care Med. 2003;167:1239–1243. doi: 10.1164/rccm.200209-1063OC. [DOI] [PubMed] [Google Scholar]
- 21.Ball TM, Castro-Rodriguez JA, Griffith KA. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg S, Israeli E, Schwartz S. Asthma prevalence, family size, and birth order. Chest. 2007;131:1747–1752. doi: 10.1378/chest.06-2818. [DOI] [PubMed] [Google Scholar]
- 23.Johnston SL, Pattemore PK, Sanderson G. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154(3 pt 1):654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atmar RL, Guy E, Guntupalli KK. Respiratory tract viral infections in inner-city asthmatic adults. Arch Intern Med. 1998;158:2453–2459. doi: 10.1001/archinte.158.22.2453. [DOI] [PubMed] [Google Scholar]
- 27.Schwarze J, Gelfand EW. The role of viruses in development or exacerbation of atopic asthma. Clin Chest Med. 2000;21:279–287. doi: 10.1016/S0272-5231(05)70266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarjour NN, Gern JE, Kelly EA. The effect of an experimental rhinovirus 16 infection on bronchial lavage neutrophils. J Allergy Clin Immunol. 2000;105(6 pt 1):1169–1177. doi: 10.1067/mai.2000.106376. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen SF, van der Sluis S, Stensballe LG. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 30.Frick OL, German DF, Mills J. Development of allergy in children, I: association with virus infections. J Allergy Clin Immunol. 1979;63:228–241. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayson MH, Cheung D, Rohlfing MM. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SS, Kitchens RT, Grayson MH. Single non-viral antigen exposure during a paramyxoviral respiratory infection is sufficient to drive specific IgE production [abstract] J Allergy Clin Immunol. 2008;121:S139. [Google Scholar]
- 33.Ehlenbach SJ, Kitchens RT, Swanson S. Exposure to non-viral antigen during severe paramyxoviral respiratory infections is sufficient to generate airway hyper-reactivity upon subsequent non-viral antigen challenge [abstract] J Allergy Clin Immunol. 2009;123:S257. [Google Scholar]
- 34.Meissner HC, Long SS. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112(6 pt 1):1447–1452. doi: 10.1542/peds.112.6.1447. [DOI] [PubMed] [Google Scholar]
- 35.Simoes EA, Groothuis JR, Carbonell-Estrany X. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Chen CH, Lin YT, Yang YH. Ribavirin for respiratory syncytial virus bronchiolitis reduced the risk of asthma and allergen sensitization. Pediatr Allergy Immunol. 2008;19:166–172. doi: 10.1111/j.1399-3038.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 37.Edwards MR, Bartlett NW, Clarke D. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan SH, Park SS, Sirajuddin IA. Respiratory virus and asthma: the role of immunoglobulin E. Clin Ther. 2008;30:1017–1024. doi: 10.1016/j.clinthera.2008.06.002. [DOI] [PubMed] [Google Scholar]


