Abstract
Medical records and magnetic resonance (MR) images of 14 cats with inflammatory diseases affecting the central nervous system (CNS) were reviewed retrospectively. Cases included eight cats with feline infectious peritonitis and two cats with toxoplasmosis. Abnormalities affecting the CNS were observed in MR images in 10 (71%) cats. Intracranial lesions appeared as slightly hypointense foci in T1-weighted images in two (14%) cats, as hyperintense foci in T2-weighted images in seven (50%) cats and as hyperintense foci after intravenous administration of a gadolinium-based contrast medium in 10 (71%) cats. In six cats with lesions in T1- and/or T2-weighted images, additional lesions were visible in T1-weighted images obtained after gadolinium-based contrast medium administration. In three cats, lesions were visible only after contrast medium administration. In our study, MR imaging (MRI) did not appear to detect all cases of CNS inflammation in the population of cats with inflammatory cerebrospinal fluid (CSF); however, MRI adds information about the sites and morphology of intracranial lesions that should help to distinguish between neoplasia and inflammatory conditions and, possibly, between different inflammatory conditions.
Meningoencephalitis in cats is most frequently associated with infections, including feline infectious peritonitis (FIP) and toxoplasmosis (Foley et al 1998, Gunn-Moore 2005). In a recent histological review of tissues from 286 cats with neurological disorders, 92 (32%) had inflammatory and/or infectious lesions affecting the central nervous system (CNS) (Bradshaw et al 2004). Of these, 47 (51%) had FIP, 33 (36%) had non-specific encephalitis or meningitis, eight (9%) had protozoal tissue cysts (presumed to represent toxoplasmosis) and one (1%) had cryptococcosis (Bradshaw et al 2004). Other reported causes of meningoencephalitis include feline immunodeficiency virus (Gunn-Moore et al 1996), mycosis (Lavely and Lipsitz 2005), immune-mediated (Rand et al 1994b) and extension of bacterial infection from the inner or middle ear (Foley et al 1998).
Antemortem diagnosis of CNS inflammatory diseases can be challenging. Cerebrospinal fluid (CSF) analysis is historically the principal test used for the diagnosis of intracranial inflammatory conditions (Rand et al 1994b, Tipold 1995, Singh et al 2005). Although increased CSF white cell count and protein concentration can occur secondary to other pathological conditions, such as neoplasia (Rand et al 1994a), animals with confirmed CNS inflammatory disease rarely have normal CSF, hence it is a sensitive but non-specific test (Rand et al 1994b).
Although computed tomography (CT) may be preferred when it is necessary to examine in detail the calvaria or when acute intracranial haemorrhage is suspected, magnetic resonance imaging (MRI) is generally considered the imaging modality of choice for examining the intracranial structures. In animals with CNS signs, MRI may be used before CSF collection to identify any intracranial mass that may be considered a contraindication for subarachnoid puncture, or to gain more information in animals with non-specific CSF results. Potential MRI findings in cats with inflammatory conditions include hydrocephalus, ependymal, neural or meningeal enhancement in T1-weighted images after gadolinium-based contrast medium intravenous administration and focal, diffuse or multifocal hyperintensity in T2-weighted images (Foley et al 1998, Mellema et al 2002, Cherubini et al 2005, Pfohl and Dewey 2005); however, this is a lightly documented subject. The aim of the present study was to describe the MRI findings in a series of cats with inflammatory CNS conditions.
Materials and methods
Selection criteria
Medical records at the Queen Mother Hospital for Animals, The Royal Veterinary College, between January 1999 and July 2005 were searched for cats that had MRI of the head and inflammatory CSF or a histological diagnosis of an inflammatory CNS condition. Cats with a history of receiving corticosteroids before referral were excluded from the study.
CSF analysis
All CSF samples were collected from the cerebellomedullary cistern and analysed within 20 min. In case of minor blood contamination, the leukocyte count was adjusted by subtracting one white blood cell (WBC) mm−3 for every 100 red blood cells (RBC) mm−3. Although the CSF protein concentration formula was considered to adjust the CSF protein level by subtracting 1 mg/dl for every 1000 RBC/μl (Wamsley and Alleman 2004), however no cases presented RBC over 1000/μl. CSF results were suggestive of inflammation if the protein level was >0.25 g/l or the white cell count was >5 mm−3. In cats in which an infectious aetiology was suspected on the basis of history, clinical signs and/or CSF protein level or leukocyte count, feline coronavirus (FCoV) and Toxoplasma gondii antibody titres were tested in serum and CSF.
MRI protocol and review
MRI was performed using a 1.5 T magnet (Gyroscan 1.5 T, Philips). In all cats, transverse T1- and T2-weighted images, sagittal T2-weighted images, and transverse T1-weighted images after contrast administration (intravenous administration of 28 mg/kg gadoteric acid (Dotarem; Guerbet Laboratories)) were acquired. To facilitate unbiased interpretation, magnetic resonance (MR) images of cats with inflammatory diseases were mixed with images of 24 cats with neurological signs not thought to be associated with inflammatory CNS conditions and the images were reviewed by one observer (CRL) working without any clinical information. Only the interpretations of MR images of cats that satisfied the inclusion criteria for inflammatory CNS disease were used in this study. Abnormalities observed in MR images were categorised according to the following subjective criteria: signal intensity compared to cerebral grey matter; evidence of increased signal intensity in T1-weighted images after gadolinium contrast medium administration; lesion site (telencephalon, diencephalon, mesencephalon, metencephalon, myelencephalon, meninges, ependyma); distribution (focal, multifocal, diffuse); margins (distinct or indistinct); presence of ventricular dilatation (mild, moderate, marked); and presence of cerebral or cerebellar herniation. Any abnormalities affecting extracranial structures were also recorded.
Pathology
Postmortem specimens of various organs including the brain were collected and processed using standard methods. Samples for histology were fixed in neutral buffered 10% formalin, embedded in paraffin and stained with haematoxylin and eosin (HE) and Luxol fast blue.
Results
Case selection
A total of 251 records were examined and 14 cats were found to satisfy the inclusion criteria. Ten cats were domestic shorthairs; there were more males (11) than females (3); the median age was 3 years (range 4 months–9 years). Eight cats (57%) had the neurological form of FIP. Cats with FIP tended to be younger (median age 16 months; range 4 months–4 years) than cats with other inflammatory CNS conditions. The clinical signs and results of haematology and plasma protein concentration in 14 cats with inflammatory CNS conditions are summarised in Table 1. Hyperglobulinaemia was observed in four (50%) cats affected by FIP.
Table 1.
Major clinical signs, neurological localisation, haematology and plasma protein results in 14 cats with inflammatory conditions affecting the CNS
| Cat | Clinical signs | Neurological localisation | Haematology | Plasma protein |
|---|---|---|---|---|
| 1 | Depression, decreased menace response, ascites | Right forebrain | Neutrophilia | Hyperglobulinemia |
| 2 | Ataxia, tetraparesis, mydriasis | Brainstem | WNL | Hyperglobulinemia |
| 3 | Tetraparesis, vertical nystagmus, hypermetria | CVS and cerebellum | WNL | WNL |
| 4 | Stupor, anisocoria, positional nystagmus, seizures | Multifocal | WNL | Hyperglobulinemia |
| 5 | CP deficits, generalised hyperaesthesia | Forebrain | WNL | WNL |
| 6 | Hypermetria, intentional head tremor | Cerebellum | WNL | WNL |
| 7 | Seizures, CP deficits | Forebrain | WNL | NP |
| 8 | Depression, CP deficits, neck pain, seizures | Forebrain | WNL | Hyperglobulinemia |
| 9 | Seizures, right circling, hemiparesis, depression | Right forebrain | WBC | WNL |
| 10 | Opisthotonus, anisocoria, depression, no PLR | Brainstem | NP | NP |
| 11 | Compulsive gait, circling, hypermetria | Multifocal | WNL | WNL |
| 12 | Head tilt, generalised ataxia | PVS | WNL | Hyperglobulinemia |
| 13 | Head tilt, anisocoria, depression, facial paralysis | CVS | WNL | WNL |
| 14 | Head tilt, depression, proprioceptive deficits | CVS | WNL | WNL |
CVS=central vestibular system, PVS=peripheral vestibular system, WNL=within normal limits, NP=not performed, CP=conscious proprioception, PLR=pupil light reflex.
Clinical pathology
CSF was collected from 12 cats and data are reported in Table 2. Total protein level in CSF was increased in all cases (median 6.3 g/l, range 0.37–28.3 g/l) and was more markedly increased in the cats with FIP compared to cats with other inflammatory/infectious conditions. CSF WBC count was increased in nine cats (75%), including six with FIP, one with toxoplasmosis, one with neutrophilic meningoencephalitis and one with histiocytic encephalitis. The median CSF WBC count in these nine cats was 138 mm−3 (range 9–523 mm−3), with equal numbers of cats with predominantly neutrophilic versus mixed pleocytosis. In three cats the total WBC count was normal, but differential cell count revealed non-degenerate neutrophils in one cat and activated leukocytes in two cats. FCoV titres in CSF were positive in three (50%) cats with FIP whereas serum titres levels were positive in five (83%) cats. Both cats with toxoplasmosis had positive CSF titres.
Table 2.
Results of CSF analysis in 12 cats with inflammatory conditions affecting the CNS
| Cat | Age, sex | Diagnosis | CSF | Serum | PM | ||
|---|---|---|---|---|---|---|---|
| WBC count/cytology | Total protein (g/l) | Ig titres | Ig titres | ||||
| 1 | 3y, FN | FIP, effusive | 17 mm−3; MP | 1.03 | Negative | 1:10,240 | Yes |
| 2 | 11m, M | FIP, non-effusive | 78 mm−3; MP | 28.2 | 1:1000 | 1:3200 | Yes |
| 3 | 4y, FN | FIP, non-effusive | 302 mm−3; NeP | 7.9 | 1:100 | Negative | Yes |
| 4 | 8m, M | FIP, non-effusive | 4 mm−3; non-degenerate neutrophils | 7.29 | 1:100 | 1:1000 | Yes |
| 5 | 2y, FN | FIP, non-effusive | 13 mm−3; MP | 0.93 | Negative | Negative | Yes |
| 6 | 8m, MN | FIP, non-effusive | 523 mm−3; MP | 20.1 | Negative | Negative | Yes |
| 7 | 4m, M | FIP, non-effusive | NP | NP | NP | 1:1280 | Yes |
| 8 | 6m, M | FIP, non-effusive | 189 mm−3; MP | 7.2 | Negative | Negative | Yes |
| 9 | 4y, MN | Toxoplasmosis | 3 mm−3; activated macrophages | 0.45 | 1:16 | Negative | Yes |
| 10 | 9y, MN | Toxoplasmosis | 23 mm−3; NeP | 0.37 | 1:256 | 1:256 | Yes |
| 11 | 4y, MN | Neutrophilic meningoencephalitis | 9 mm−3; NeP | 0.71 | Negative | Negative | NP |
| 12 | 5y, M | Lymphocytic meningoencephalitis | 3 mm−3; reactive lymphocytes | 0.40 | Negative | Negative | Yes |
| 13 | 7y, MN | Bacterial meningoencephalitis | NP | NP | NP | NP | Yes |
| 14 | 2y, MN | Histiocytic encephalitis | 83 mm−3; NeP | 0.73 | Negative | Negative | Yes |
PM=post mortem examination, F=female, M=male, N=neutered, MP=mixed pleocytosis, NeP=neutrophilic pleocytosis, NP=not performed, FIP=feline infectious peritonitis.
Magnetic resonance imaging
Intracranial lesions were observed in MR images in 10 of 14 cats (71%) (Table 3). In three cats with FIP and one cat with histiocytic encephalitis the MR images were considered normal.
Table 3.
Results of MR imaging in 14 cats with inflammatory conditions affecting the CNS
| Cat | Site | Distribution | Margins | Cerebellar herniation | Dilated ventricles | Signal intensity in | Gad-based contrast uptake | Additional lesions visible after Gad IV administration | |
|---|---|---|---|---|---|---|---|---|---|
| T1-WI | T2-WI | ||||||||
| 1 | - | - | - | - | - | - | - | - | - |
| 2 | - | - | - | - | - | - | - | - | - |
| 3 | 4,6 | Focal | Indistinct | - | + | Mild hypo | Hyper | ++ | - |
| 4 | 1,7 | Diffuse | Distinct | + | ++ | Iso | Iso | +++ | Yes |
| 5 | - | - | - | - | - | - | - | - | - |
| 6 | 6 | Diffuse | Distinct | - | - | Iso | Hyper | ++ | Yes |
| 7 | 3,7 | Diffuse | Distinct | ++ | ++ | Iso | Iso | +++ | Yes |
| 8 | 6,7 | Diffuse | Distinct | + | + | Iso | Iso | ++ | Yes |
| 9 | 1 | Multifocal | Indistinct | - | - | Mild hypo | Hyper | ++ | Yes |
| 10 | 4,5,6 | Multifocal | Indistinct | - | - | Iso | Hyper | ++ | Yes |
| 11 | 3 | Focal | Indistinct | - | - | Iso | Hyper | +++ | Yes |
| 12 | 6 | Focal | Distinct | - | - | Iso | Mild hyper | ++ | Yes |
| 13 | 4,6 | Focal | Indistinct | - | + | Iso | Mild hyper | ++ | Yes |
| 14 | - | - | - | - | - | - | - | - | - |
Sites: 1=telencephalon, 2=diencephalons, 3=mesencephalon, 4=pons and/or cerebellum, 5=medulla oblongata, 6=meninges, 7=ependyma. Iso=isointense signal, hyper=hyperintense signal, hypo=hypointense signal. −=absent, +=mild, ++=moderate, +++=marked. LV=lateral ventricles. Gad=gadolinium, IV=intravenous, WI=weighted images.
Intracranial lesions were observed as slightly hypointense foci in T1-weighted images in two cats (14%), as hyperintense foci in T2-weighted images in seven cats (50%) and as hyperintense foci after gadolinium contrast administration in 10 cats (71%). In six cats with lesions in T1- and/or T2-weighted images, additional lesions were visible in T1-weighted images obtained after gadolinium contrast medium administration. In three cats, lesions were visible only in T1-weighted images obtained after gadolinium contrast administration (Fig 1). There was a trend for lesions in cats with FIP to have distinct margins more frequently than other aetiologies; both cats with toxoplasmosis had lesions with indistinct margins. Lesion distribution was considered to be diffuse in four cats (40%), focal in four cats (40%) and multifocal in two cats (20%). Both cats with toxoplasmosis had multifocal lesions (Fig 2). Ventricular dilation was observed in five cats (36%), including all three with lesions affecting the ependyma. No predilection sites were identified, although there was a trend for meningeal and ependymal lesion to be more frequently observed in cats with FIP (Fig 3). There was evidence of cerebellar herniation in three cats with FIP.
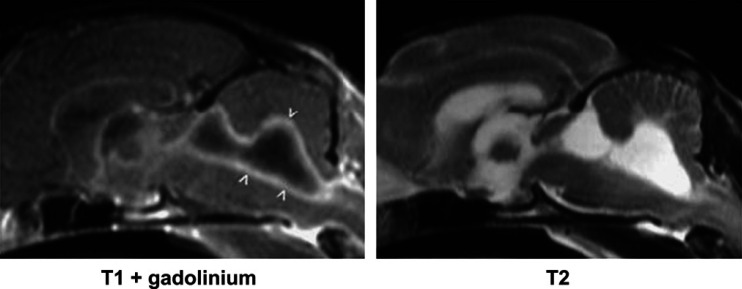
Fig 1.
Example of lesions that are visible only in T1-weighted images obtained after gadolinium contrast medium administration. Relatively uniform, diffuse enhancement is evident affecting the meninges (arrowheads) of this cat with FIP. The T1- and T2-weighted images appear normal.
Fig 2.
Example of images from a cat with toxoplasmosis showing multifocal lesions. The initial T1-weighted image (left) appears normal, but hyperintense foci (arrowheads) are visible affecting the cerebrum after gadolinium contrast medium administration. The T2-weighted image shows hyperintense foci, representing oedema, in the same sites.
Fig 3.
Sagittal images of a cat with FIP in which there is marked dilation of the fourth ventricle and moderate dilation of the third ventricle. The ependymal lining of the ventricle has enhanced signal after gadolinium contrast medium intravenous administration (arrowheads). Meningeal and ependymal lesions tended to be observed more frequently in cats with FIP.
Extracranial lesions were identified in three cats. Two cats had material in the tympanic cavity compatible with otitis media; one of these had no intracranial signs, but the other had a focal lesion affecting the metencephalon and adjacent meninges and a diagnosis of bacterial meningitis. It is likely that the otitis and meningoencephalitis were related in this cat (Fig 4). The remaining cat had thickening of the lining of the frontal sinuses compatible with sinusitis and no intracranial signs.
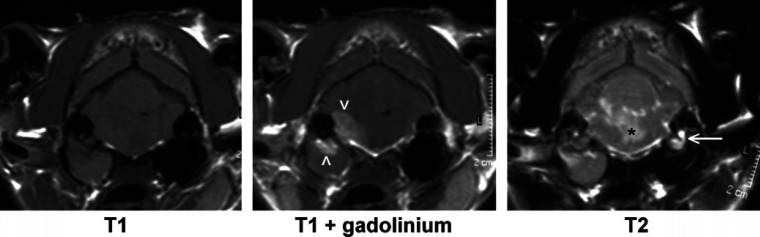
Fig 4.
MR images of a cat with otitis externa, media and interna and associated meningoencephalitis. Isoechoic material fills the right bulla and horizontal auditory canal. Focal enhancement after gadolinium contrast medium administration is visible affecting the lining and the dorsal aspect of the right bulla and adjacent pons (arrowheads). In the T2-weighted image there is evidence of oedema affecting the pons (*) and a lack of a signal from the right inner ear. (The normal signal from perilymph in the left inner ear is arrowed.)
Discussion
In this study, CSF was suggestive of inflammatory conditions in all cats in which analysis was performed. Three cats had a normal WBC count with abnormal cytological findings, as reported in certain inflammatory conditions, such as protozoan infection (Rand et al 1994b, Singh et al 2005), and non-inflammatory diseases, such as neoplasia or vascular conditions (Rand et al 1994a). Steroid administration decreases the WBC count and/or total protein levels (Wamsley and Alleman 2004); therefore, in order to avoid any false negative results we excluded from the present study any cats with previous steroid treatment.
Normal cats can have positive serum titres because of cross reaction with different strains of FCoV (Sparkes et al 1994, Foley et al 1998, Green 2006). For this reason, it has been suggested that only positive serum titres >1:1600 should be considered indicative of FIP (Hartmann et al 2003). The dry form of FIP is said to have relatively high FCoV antibody titres and to be rarely seronegative (Foley et al 1998, Green 2006); however, cats with neurological FIP can have negative serum titres (Kline et al 1994, Baroni and Heinold 1995, Foley et al 1998). This was observed in four of seven (57%) cats with histologically confirmed FIP in the present study. One reason for negative titres in cats with FIP may be that immune complexes already conjugated with the body's antibodies cannot be recognised by immunological tests (Green 2006). Positive CSF antibody titres were found in both cats with toxoplasmosis.
Lesions were observed in MR images in 10 of 14 (71%) cats affected by an inflammatory CNS condition. Although the number of patients is insufficient for firm conclusions about the sensitivity of MRI, it is apparent that a negative MR scan cannot rule out presence of neurological FIP, as only half the cats with FIP had lesions in MR images. MRI did not appear to detect all cases of CNS inflammation in the population of cats in our study with CSF findings suggestive of CNS inflammation; however, MRI adds information about the sites and morphology of intracranial lesions that helps to distinguish both between neoplasia and inflammatory conditions and, to some extent, between different inflammatory conditions. Compared to computed tomography (CT) (Plummer et al 1992), MRI is thought to have slightly higher overall sensitivity (Lamb et al 2005). Increased sensitivity for multiple lesions and meningeal enhancement makes MR more likely than CT to enable identification of the signs that aid differentiation of intracranial inflammation from neoplasia.
In this series, inflammatory lesions of the CNS were visible as slightly hypointense foci in T1-weighted images in two cats (14%) and as hyperintense foci in T2-weighted images in seven cats (50%). In six cats with lesions in T1- and/or T2-weighted images, additional lesions were visible in T1-weighted images obtained after gadolinium administration. In three cats, lesions were visible only in T1-weighted images obtained after gadolinium contrast medium administration. Hence, as reported for dogs (Lamb et al 2005), use of gadolinium contrast medium seems to increase the sensitivity of MR for intracranial inflammatory lesions.
In the present study, more cats had a single focal lesion than had multifocal lesions on MRI. This result contrasts with previous studies in which multifocal lesions were associated with non-neoplastic conditions, including inflammatory disease (Cherubini et al 2005, Lamb et al 2005). For example, in a recent study of 25 dogs with inflammatory CSF, abnormalities were found by MRI in 19 (76%) dogs with inflammatory CSF. Two dogs had focal lesions, 10 had multifocal lesions and seven had diffuse lesions (Lamb et al 2005). Both cats with toxoplasmosis in the present study had multifocal lesions on MRI, a finding that has not been reported previously. Variable MR findings have been reported in humans with toxoplasmosis (Brightbill et al 1996, Dietrich et al 2000, Chon-Han et al 2003) including multiple or solitary masses.
Three cats with FIP had ventricular dilation and enhancement of the ependyma after gadolinium contrast medium administration (Fig 3). This observation reflects the typical histological distribution of FIP lesions, which principally involve the inner and outer surfaces of the brain (Summers et al 1995). Ventricular dilation in cats with FIP may reflect abnormal CSF drainage, as dilated ventricles have been always observed in association with meningeal and/or ependymal involvement, two structures largely involved in CSF flow regulation (Summers et al 1995). Ventricular dilation may lead to increased intracranial pressure and cerebellar herniation through the foramen magnum. In MR images the cerebellum is usually considered herniated when the caudal tip of the vermis is within the foramen magnum; this is best assessed in sagittal images. Cerebellar herniation was observed in three of five cats with ventricular dilation in the present study. Anecdotal evidence suggests that there is marked variability in the shape of the cerebellar vermis in cats, which may complicate the assessment of herniation. Further studies, with larger numbers, are necessary to validate the MR assessment of the cerebellum in cats.
On the basis of this small series and other reports, it appears that CSF analysis can detect a higher number of cats with CNS inflammation than MRI; however, MRI adds more specific information about intracranial lesions that should help to distinguish both between cats with neoplasia and inflammatory conditions and, possibly, between different inflammatory conditions. Further studies involving larger numbers are required to investigate specific MRI characteristics in different feline inflammatory CNS diseases.
Acknowledgment
Authors would like to acknowledge Ms Linda Slater for her invaluable technical support.
References
- Baroni M., Heinold Y. A review of the clinical diagnosis of feline infectious peritonitis viral meningoencephalomyelitis, Progress in Veterinary Neurology 6, 1995, 88–94. [Google Scholar]
- Bradshaw J.M., Pearson G.R., Gruffydd-Jones T.J. A retrospective study of 286 cases of neurological disorders of the cat, Journal of Comparative Pathology 131, 2004, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightbill T.C., Post M.J., Hensley G.T., Ruiz A. MR of Toxoplasma encephalitis: signal characteristics on T2-weighted images and pathological correlation, Journal of Computer Assisted Tomography 20, 1996, 417–422. [DOI] [PubMed] [Google Scholar]
- Cherubini G.B., Mantis P., Martinez T., Lamb C.R., Cappello R. Utility of magnetic resonance imaging for distinguishing neoplastic from non-neoplastic brain lesions in dogs and cats, Veterinary Radiology and Ultrasound 46, 2005, 384–387. [DOI] [PubMed] [Google Scholar]
- Chon-Han C.H., Cortez S.C., Tung G.A. Diffusion weighted MRI of cerebral Toxoplasma abscess, American Journal of Roentgenology 181, 2003, 1711–1714. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Maschke M., Dorfler A., Prumbaum M., Forsting M. MRI of intracranial toxoplasmosis after bone marrow transplantation, Neuroradiology 42, 2000, 14–18. [DOI] [PubMed] [Google Scholar]
- Foley J.E., Lapointe J.M., Koblik P., Poland A., Pedersen N.C. Diagnostic features of clinical neurologic feline infectious peritonitis, Journal of Veterinary Internal Medicine 12, 1998, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.E. Infectious Diseases in the Dog and Cat, 3rd edn., 2006, Elsevier Inc; pp. 88–102 [Google Scholar]
- Gunn-Moore D. Infectious diseases of the central nervous system, Veterinary Clinics of North America: Small Animal Practice 35, 2005, 103–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn-Moore D.A., Pearson G.R., Harbour D.A., Whiting C.V. Encephalitis associated with giant cells in a cat with naturally occurring feline immunodeficiency virus infection demonstrated by in situ hybridization, Veterinary Pathology 33, 1996, 699–703. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Binder C., Hirschberger J., Cole D., Reinacher M., Schroo S., Frost J., Egberink H., Lutz H., Hermanns W. Comparison of different tests to diagnose feline infectious peritonitis, Journal of Veterinary Internal Medicine 17 (6), 2003, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline K.L., Joseph R.J., Averill D.R. Feline infectious peritonitis with neurologic involvement: clinical and pathological findings in 24 cats, Journal of the American Animal Hospital Association 30, 1994, 111–118. [Google Scholar]
- Lamb C.R., Croson P.J., Cappello R., Cherubini G.B. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid, Veterinary Radiology and Ultrasound 46, 2005, 17–22. [DOI] [PubMed] [Google Scholar]
- Lavely J., Lipsitz D. Fungal infections of the central nervous system in the dog and cat, Clinical Techniques in Small Animal Practice 20, 2005, 212–219. [DOI] [PubMed] [Google Scholar]
- Mellema L.M., Samii V.F., Vernau K.M., LeCouteur R.A. Meningeal enhancement on magnetic resonance imaging in 15 dogs and 3 cats, Veterinary Radiology and Ultrasound 43, 2002, 10–15. [DOI] [PubMed] [Google Scholar]
- Pfohl J.C., Dewey C.W. Intracranial Toxoplasma gondii granuloma in a cat, Journal of Feline Medicine and Surgery 7, 2005, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer S.B., Wheeler S.J., Thrall D.E., Kornegay J.N. Computed tomography of primary inflammatory brain disorders in dogs and cats, Veterinary Radiology and Ultrasound 33, 1992, 307–312. [Google Scholar]
- Rand J.S., Parent J., Percy D., Jacobs R. Clinical, cerebrospinal fluid and histological data from 34 cats with primary non-inflammatory disease of the central nervous system, Canadian Veterinary Journal 35, 1994a, 257–292. [PMC free article] [PubMed] [Google Scholar]
- Rand J.S., Parent J., Percy D., Jacobs R. Clinical, cerebrospinal fluid and histological data from 34 cats with primary inflammatory disease of the central nervous system, Canadian Veterinary Journal 35, 1994b, 103–110. [PMC free article] [PubMed] [Google Scholar]
- Singh M., Foster D.J., Child G., Lamb W.A. Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis and outcome, Journal of Feline Medicine and Surgery 7, 2005, 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes A.H., Gruffydd-Jones T.J., Harbour D.A. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis, Journal of the American Animal Hospital Association 30, 1994, 345–350. [Google Scholar]
- Summers B.A., Cummings J.F., de Lahunta A. Veterinary Neuropathology, 1995, Mosby-Year Book Inc: St Louis, Missouri, pp. 300–307 [Google Scholar]
- Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: a retrospective study, Journal of Veterinary Internal Medicine 9, 1995, 304–314. [DOI] [PubMed] [Google Scholar]
- Wamsley H., Alleman A.R. Clinical pathology. Platt S., Olby N. BSAVA Manual of Canine and Feline Neurology, 3rd edn, 2004, British Small Animal Veterinary Association: Gloucester, 35–53. [Google Scholar]