Summary
Background
Current diagnostic tests are inadequate to detect typhoid cases, as well as the chronic carrier state, the sole reservoir of Salmonella enterica serovar Typhi. The current study was conducted to find new molecular signatures of pathogen/disease to understand the mechanism behind the host–pathogen interaction in enteric fever.
Methods
Proteomics-based studies were done to determine the expression of differentially expressed proteins in the plasma of controls, acute typhoid cases, and chronic typhoid carriers. Further, transcriptome-based analysis using reverse-transcriptase PCR (RT-PCR) was done in controls, acute typhoid cases, and chronic typhoid carriers.
Results
Results showed the upregulation of proprotein convertase subtilisin, furin, haptoglobin, and albumin in the plasma of chronic typhoid carriers. The elevation in mRNA expression of four differentially expressed proteins confirms the changes at the transcriptional level. Further, the increase in albumin and haptoglobin in chronic typhoid carriers shows their role in free radical generation, inflammation, and monocyte cell signaling.
Conclusion
Through proteomics techniques, this study identified four proteins in the chronic typhoid carrier host that may have a role in the disease pathogenesis of enteric fever.
Keywords: 2D gel electrophoresis, Chronic typhoid carrier, Haptoglobin, Transcriptomics
1. Introduction
Enteric fever is a serious systemic disease caused by the bacterium Salmonella enterica serovar Typhi (S. typhi) and other S. enterica serovars, including Salmonella enterica Paratyphi A, B, and C.1 These organisms are facultative intracellular pathogens and cause systemic infections following ingestion of the organism, colonization of the small intestine, invasion of the gastrointestinal mucosal surface, and dissemination throughout the body in the reticuloendothelial system including the liver, spleen, and bone marrow.3, 4 The complete pathogenesis of enteric fever is unknown, and complications of the disease become more severe when it is harbored in the gall bladder, resulting in a chronic carrier state through which the bacteria are disseminated via frequent shedding.5 The disease is prevalent in underdeveloped countries, where clean drinking water is lacking and hygiene standards are compromised.1 In developed countries, the incidence is lower and the disease is mainly associated with travel to endemic locations.2 The long-term persistence of S. typhi in carriers explains why typhoid fever remains endemic in regions of the world with poor quality drinking water and defective sewage disposal.5
Retrospective studies carried out in endemic regions have suggested the association of risk factors with carriage. Middle-aged adults are more likely to become carriers, while children are usually short-term carriers.3, 6 S. typhi carriers are more likely to be female and suffering from gall bladder problems.7, 8 The carrier state is thought to be related to bacterial biofilm formation on the surface of a gall stone.9 The pathogenesis of enteric fever remains to be characterized; the host–pathogen interaction involves many intracellular events that prevent the identification and eradication of the pathogen by the host.10, 11 There is no animal model for S. typhi and much of our understanding is derived from the Salmonella typhimurium mouse infection model and cell culture experiments.11
The identification of carriers is the best way to prevent the spread of the bacterium; however isolation of S. typhi requires multiple fecal samples shed over the course of a year by the carrier, because of the poor sensitivity of the existing methods of isolation. The test is based on the detection of antibodies against the Salmonella lipopolysaccharide (LPS) O antigen; flagellar H antigen is more indicative of past and current Salmonella infection and not the carrier state.12 The PCR for typhoid has yielded a sensitivity of >90% using nested primers, but on other hand the DNA from uncultured and dead bacteria can also be amplified by PCR, therefore colony-forming units cannot be specifically correlated with the PCR diagnosis. Moreover molecular methods for the detection of S. typhi are very technically demanding.13
Proteomics-based methods are currently being employed to increase our understanding of the pathogenesis of enteric fever and to explore the peripheral signatures of enteric fever.13 The molecular signature can be of host or bacterial origin, or a combination of both, produced during the process of infection.13 Biomarkers for intracellular pathogens, such as tuberculosis, have been developed using proteomics and mass spectrometry.14, 15 The severe acute respiratory syndrome (SARS) protein biomarker has been identified using surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF).15 The present study was conducted to investigate the expression of differentially expressed proteins in asymptomatic chronic typhoid carriers, acute typhoid cases, and healthy controls. Two-dimensional (2D) gel electrophoresis and mass spectrometry techniques were used to identify newer proteins that may provide an insight into the host–pathogen interactions after Salmonella infection and in further colonization of the host leading to the chronic typhoid carrier state. A transcriptomics-based analysis was further conducted to correlate the changes occurring at the mRNA level translated exponentially at the protein level. The results of the present study will provide a better understanding and insight into the mechanism of the pathogenesis of Salmonella and allow the development of better diagnostic and therapeutic approaches to enteric fever.
2. Materials and methods
2.1. Materials
Bovine serum albumin (BSA), Tris-base, acrylamide, N,N′-methylene bisacrylamide, urea, agarose, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), sodium dodecyl sulfate (SDS), dithioerythreitol (DTE), dithiothreitol (DTT), bromophenol blue, ethylenediaminetetraacetic acid (EDTA), and protease inhibitor cocktail were purchased from Sigma–Aldrich, USA. Bradford reagent was procured from Bio-Rad, USA, and Immobiline pH gradient strips, Immobiline pH gradient buffer, and cover fluid were purchased from Bio-Rad, USA. Glycerol, sodium thiosulfate, potassium ferricyanide, silver nitrate, sodium carbonate, formaldehyde, and methanol were procured from Merck Ltd, India. Thiourea, acetonitrile, acetic acid, ammonium bicarbonate, trifluoroacetic acid (TFA), and other chemicals required for the study were procured locally from Sisco Research Laboratory, India. MacConkey agar and deoxycholate citrate agar were from Hi-Media, India, and TGF-β and IL-10 ELISA kits were from Invitrogen Corp., USA.
2.2. Selection of subjects
The study was designed to collect blood samples from outpatients admitted to Sir Sundar Lal Hospital, Institute of Medical Science, Banaras Hindu University, Varanasi. Patients were examined by an expert clinician, and those with symptoms resembling enteric fever were directed for routine clinical laboratory tests, including Typhidot () and culture. Patients were aged between 16 and 65 years and were of both sexes, i.e., male and female. The patients’ past clinical histories and treatments were also recorded. Blood was collected in an anticoagulant-coated vial by expert clinicians. The controls were selected from healthy volunteers who were apparently healthy and without any symptoms of visible disease. Written consent was obtained from each participant. The study design was approved by the institutional ethics committee.
2.3. Sample preparation
Blood samples were collected from controls, acute typhoid cases, and chronic typhoid carriers, through venipuncture, into EDTA-coated commercially available vials. The samples were processed immediately for separation of plasma. The Typhidot assay (AB Diagnopath Pvt Ltd, New Delhi, India) was performed as per the manufacturer's instructions and results were later confirmed by culture and PCR-based methods. The blood of S. typhi-positive subjects was centrifuged at 250 × g for 20 min at 20 °C; platelet-rich plasma was obtained, which was centrifuged at 1800 × g for 10 min to obtain platelet-free plasma. The plasma was mixed with protease inhibitor cocktail and stored at −70 °C until further use.
2.4. Estimation of protein content
The protein concentration was measured by Bradford's method16 using BSA as standard.
2.5. 2D-polyacrylamide gel electrophoresis (2D-PAGE)
2D-PAGE was performed using the standard procedure.17, 18 The rehydration reaction was done by using equal amounts of protein of controls and typhoid patients, about 200 μg, and 150 μl commercial isoelectric focusing rehydration buffer containing 8 M urea, 2% CHAPS, 0.5% immobilized pH gradient (IPG) buffer, 0.02% bromophenol blue, and 15 mM DTT, along with IPG strip (pH 4–7); the reaction was performed overnight at room temperature and isoelectric focusing was performed in a Multiphor II electrophoresis unit (Bio-Rad) for 14000 volt h (500 V for 30 min, 1000 V for 10 min, 2000 V for 10 min, 5000 V for 10 min, and 8000 V for the remaining period). IPG strips were equilibrated for 20 min in equilibration buffer containing 6 M urea, 30% glycerol, 2% SDS, 0.05 M Tris–HCl pH 8.8, and 0.002% bromophenol blue. Second-dimension electrophoresis was performed using 12.5% resolving polyacrylamide gel. A broad range of molecular weight markers were run in parallel to the strips during the second dimension to calculate the molecular weight of the proteins of interest. The resulting gels were stained with silver nitrate using the standard procedure.19 In brief, gels were fixed for 2 h in fixing solution (methanol:acetic acid:water, 50:5:45), transferred into water for 1 h, sensitized in sodium thiosulfate (0.02%) for 2 min, rinsed with water, incubated in silver nitrate (0.1%) for 30 min, and finally rinsed with water. The protein spots in the gels were developed in developing solution containing 2% sodium carbonate and 100 μl of 37% formaldehyde. Removal of the developer and addition of 1% glacial acetic acid was used to stop the staining.
2.6. 2D gel analysis
The 2D gels of controls, acute typhoid cases, and chronic typhoid carriers were compared using ImageMaster 2D Platinum software. The contrast for background and spots was normalized for every gel. Each gel was assigned landmarks following spot matching. The normalized percentage volumes of the spots from individual gels were compared between groups to calculate the percentage change in volume in the acute typhoid and chronic typhoid groups vs. control. The change in percentage volume of spots in the 2D gels of controls and acute typhoid cases and chronic typhoid carriers was calculated, and 2D spots with a significant change in spot volume were used for further analysis.
2.7. Sample preparation for mass spectrometry
The sample for mass spectrometry was made using the standard procedure,20 with slight modifications. In brief, silver-stained spots found to be differentially expressed were excised, cut into pieces, and washed with 500 μl water with agitation on a vortex mixer. To remove silver stains, excised spots were incubated with 250 μl of 50 mM sodium thiosulfate and 15 mM potassium ferricyanide for 5 min and washed twice with 500 μl water to remove reducing agents. The excised spots were incubated at 60 °C for 30 min with 500 μl of 100 mM NH4HCO3 and 45 mM DTT, followed by cooling; 500 μl of 100 mM iodoacetamide was then added and further incubation carried out for 30 min in the dark at room temperature. Excised spots were sliced and equilibrated with 500 μl of 50 mM ammonium bicarbonate in 50% acetonitrile, dehydrated with 500 μl of 100% acetonitrile for 20 min, and air-dried. Sliced spots were rehydrated in 30 μl of a solution containing 0.02 μg/μl trypsin and 50 mM ammonium bicarbonate at 4 °C for 60 min. The supernatant was discarded and 30–50 μl of 50 mM ammonium bicarbonate was added to the excised spots; these were incubated at 37 °C for 16–18 h and the supernatant removed. Twenty-five to fifty microliters of 1% TFA in 60% acetonitrile was added to the pellet and this was sonicated for 10 min. The supernatant was removed following centrifugation at 12000 × g for 30 s. Both the supernatants were mixed, freeze-dried, and concentrated by centrifugal evaporation to dryness.
2.8. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) and liquid chromatography mass spectrometry (LC-MS)
Mass spectrometric analysis of samples was done at The Centre for Genomic Applications (TCGA), New Delhi. In brief, matrix, namely α-cyano-4-hydroxycinnamic acid (CHCA) matrix, was mixed with the dissolved trypsinized peptide samples. After drying, the peptides were spotted on a ground steel plate and subjected to Bruker Ultraflex MALDI-TOF/TOF and 2D Nano LC-ESI-Trap (Agilent) for mass spectrometric identification. The instrument was equipped with a pulsed nitrogen laser.
2.9. Analysis of peptide sequences
Data acquisition and analysis was performed using Flex Control and Flex Analysis/Biotools version 2.2 software, respectively. Data were acquired in reflectron-positive mode using 15–18% laser power. The MALDI and tandem spectra used for protein identification from tryptic fragments were searched against the MASCOT search engine. Mass tolerance and monoisotopic values (100 ppm for peptide mass fingerprint and peptide mass tolerance of 2 Da for MS/MS spectra) were used for searching. The probability-based MOWSE score was calculated in terms of ion score –10*log (p), where p is the probability and the observed match was considered a random event. Protein scores were derived from ions as a non-probabilistic basis for ranking protein hits, and proteins identified by MALDI-TOF and LC-MS were of the expected size based on their position in the gel.
2.10. Reverse-transcriptase PCR (RT-PCR)
RNA was isolated from whole blood using TRIzol reagent. Polyadenylated RNA was reverse-transcribed using oligo-dT primers and the RT-PCR was performed using an RT-PCR kit (Thermo-Scientific) in accordance with the manufacturer's protocol. The cDNA was synthesized from isolated RNA using RevertAid Minus Mu-LV Reverse Transcriptase under standard conditions, as supplied by the manufacturer. The primer sequences used for this study are given in Table 3 . The gene expression of β-actin was evaluated concurrently with haptoglobin, proprotein convertase subtilisin (PC5), furin, and albumin. A total 2-μl aliquot of cDNA was added to a final volume of 25 μl of PCR mixture (10 mM Tris HCl, 50 mM KCl, 1.5 mM MgCl2, 0.5 mM dNTP, 0.4 μM primers, 2.5 U Taq DNA polymerase). The reaction conditions and cycles for amplification of the respective genes are given in Table 2 . The PCR products were visualized using agarose gel electrophoresis. The band density was analyzed using a computerized densitometry system (Alpha Imager System, Alpha Innotech Corporation, South Africa).
Table 3.
Sequences of the PCR primers used, PCR product length, and reference source
| Gene | NIH GenBank accession No. | Product length (bp) | Primer sequence, 5′–3′ |
|---|---|---|---|
| Haptoglobin | NM005143 | 338 | F = CCTGAATGTGAAGCAGTATGTa R = TTCTGTTTGAGTTTGATGAGCa |
| β-Actin |
AY141970 |
226 | F = CGTGGGCCGCCCTAGGCACCA R = GGGGGCCTCGGTCAGCAGCAC |
| Proprotein convertase subtilisin | NM001190482 | 109 | F = CCTGGAAGAGAGGCTACACG R = CAACTTGCCAGAGCATCGTA |
| Furin |
XM003919409 |
112 | F = GTACAGTGGCTGGAACAGCA R = GCTGAGTGACACCAGACAGG |
| Albumin | M12523 | 230 | F = GTAATCGGTTGGCAGCCAATG R = CACTCTTGTGTGCATCTCG |
F, forward; R, reverse.
Nucleotide primer sequence.
Table 2.
Temperature and time conditions for the PCRs
| h-Hp |
β-Actin |
PC5 |
Furin |
Albumin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-denaturation | 95 °C | 1 min | 95 °C | 1 min | 95 °C | 1 min | 95 °C | 1 min | 94 °C | 5 min |
| Denaturation | 94 °C | 40 s | 94 °C | 30 s | 95 °C | 30 s | 95 °C | 30 s | 94 °C | 1 min |
| Primer annealing | 55 °C | 30 s | 55 °C | 30 s | 60 °C | 30 s | 60 °C | 30 s | 62 °C | 1 min |
| Extension | 72 °C | 40 s | 72 °C | 1 min | 72 °C | 60 s | 72 °C | 1 min | 72 °C | 1 min |
| Number of cycles | 34 | 30 | 40 | 40 | 35 | |||||
| Final extension | 72 °C for 5 min | 72 °C for 5 min | 72 °C for 5 min | 72 °C for 5 min | 72 °C for 10 min | |||||
| Cooling | 4 °C | 4 °C | 4 °C | 4 °C | 4 °C | |||||
h-Hp, human haptoglobin; PC5, proprotein convertase subtilisin.
2.11. Statistical analysis
The Student's t-test was used for comparisons between the different groups; the data are expressed as the mean ± standard error. The differences were considered statistically significant when the p-value was less than 0.05.
3. Results
3.1. Classification of subjects
The subjects who underwent a clinical examination and who had a previous history of enteric fever were subjected to routine clinical laboratory tests. A total of 50 acute typhoid cases (age 323 ± 19.42 months, 65% female, 27/50 (54%) culture-positive) and 50 chronic typhoid carriers (age 353 ± 27.18 months, 78% female, 18/50 (36%) culture-positive) were suspected positive for enteric fever after serological and culture tests. Typhidot IgG positivity was found in 33/50 (66%) with a history of fever of more than 12 ± 3.0 days and a past history of typhoid fever; these cases were assigned to the chronic typhoid carriers group. Typhidot IgM positivity was found in 43/50 (86%) cases with a history of fever of 6 ± 3.0 days and with no past history of enteric fever; these cases were assigned to the acute typhoid group. Fifty healthy volunteers aged 304 ± 20.12 months (50% female) with no signs of any type of disease and found negative by culture and serological tests were assigned to the control group. Details of the patient and control groups are given in Table 1 .
Table 1.
Details of patients and controls, with clinical laboratory investigations
| Control (Group 1) |
Acute typhoid (Group 2) |
Chronic typhoid carrier (Group 3) |
|
|---|---|---|---|
| Number | 50 | 50 | 50 |
| Age, months | 304 ± 20.12 | 323 ± 19.42 | 353 ± 27.18 |
| Sex, % female | 50 | 65 | 78 |
| Duration of fever, days | No fever | 6 ± 3.0 | 12 ± 3.0 |
| Culture test positivity, % | Negative | 54 | 36 |
| Typhidot IgG positivity, % | Negative | 14 | 66 |
| Typhidot IgM positivity, % | Negative | 86 | 10 |
3.2. 2D gel electrophoresis of plasma proteins in enteric fever patients
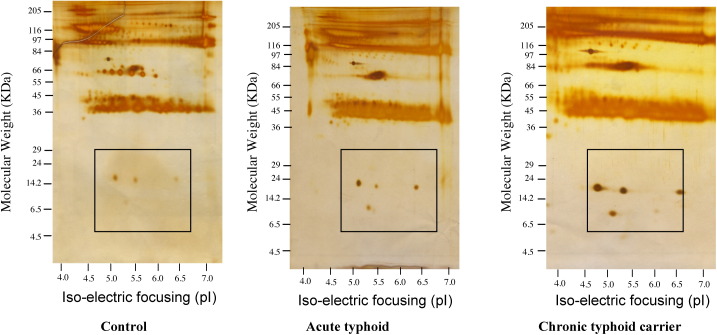
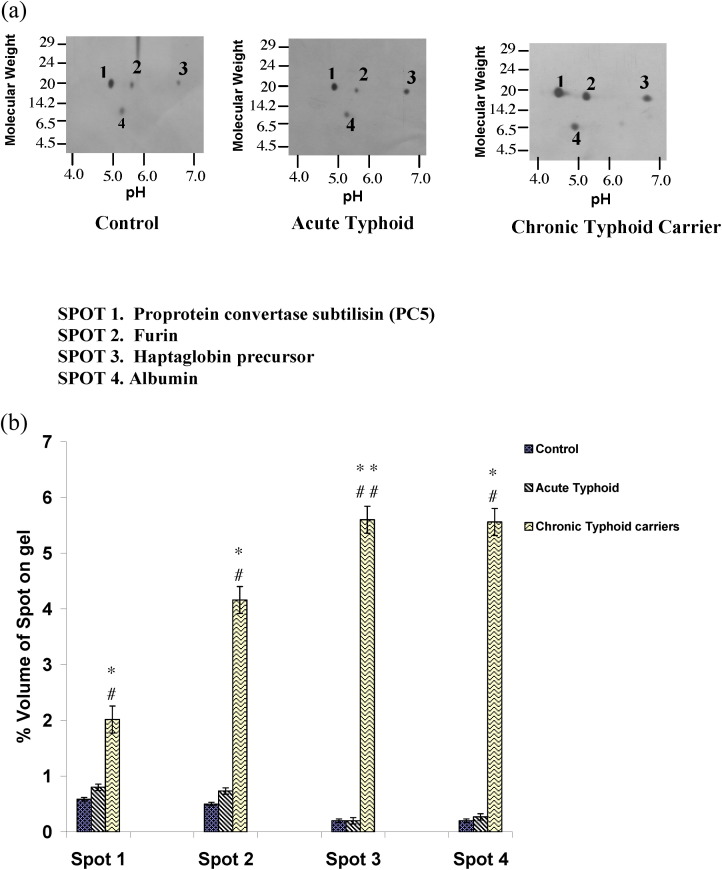
The plasma proteins from 50 controls, 50 acute typhoid cases, and 50 chronic typhoid carriers were subjected to 2D gel electrophoresis. Each set of experiments was reproduced twice and the data analyzed by ImageMaster 2D Platinum software. Figure 1 shows the complete resolution of the proteins on 2D gels. A percentage volume change of spots for the typhoid groups greater than two-fold that of the controls was taken into consideration. The expression of four protein spots was found to be significantly upregulated in chronic typhoid carriers as compared to controls and acute typhoid cases, however very little change was observed between the expression of the four spots in acute typhoid cases as compared to controls. Further, the differences in expression of all four spots showed a significant upregulation in cases of chronic typhoid carriers as compared to acute typhoid cases (Figure 2 ). Mass spectrometric analysis using MALDI-TOF and LC-MS identified these proteins as proprotein convertase subtilisin, furin, haptoglobin, and albumin (Figure 2). MALDI mass spectra and the probability plot corresponding to proprotein convertase subtilisin, furin, haptoglobin, and albumin, provided m/z values for each protein; these were used to search proteins in the available protein database. With regard to the ion score (−10*log (p), where p is the probability and the observed match was random), protein scores greater than 33 for proprotein convertase subtilisin, furin, and haptoglobin, and greater than 34 for albumin, were considered significant (p < 0.05). The probability-based MOWSE score obtained for albumin was 157 (p < 0.05); the score was 143 for haptoglobin, 58 for furin, and 38 for proprotein convertase subtilisin. Details of the peptide summary report are given in Table 4 , as per the minimum information about proteomics experiment (MIAPE) standard.
Figure 1.
Complete 2D gel electrophoretogram showing the resolution of plasma proteins isolated from controls, acute typhoid cases, and chronic typhoid carriers.
Figure 2.
a 2D gel electrophoretogram of plasma proteins from controls, acute typhoid cases, and chronic typhoid carriers after silver staining. (b) Graphical representation of the percentage volume changes of spot expression in the control, acute typhoid cases, and chronic typhoid carriers groups; *p < 0.05 for controls vs. chronic typhoid carriers, and #p < 0.05 for acute typhoid cases vs. chronic typhoid carriers in the case of spot 1 (proprotein convertase subtilisin); *p < 0.05 for controls vs. chronic typhoid carriers, and #p < 0.05 for acute typhoid cases vs. chronic typhoid carriers in the case of spot 2 (furin); **p < 0.01 for controls vs. chronic typhoid carriers, and ##p < 0.01 for acute typhoid cases vs. chronic typhoid carriers in the case of spot 3 (haptoglobin); *p < 0.05 for controls vs. chronic typhoid carriers, and #p < 0.05 for acute typhoid cases vs. chronic typhoid carriers in the case of spot 4 (albumin).
Table 4.
Details of the peptide summary reporta
| Query | Observed | Mr(expt) | Mr(calc) | Delta | Miss | Score | Expect | Rank | Peptide |
|---|---|---|---|---|---|---|---|---|---|
| P00739 (HPTR_HUMAN)b | |||||||||
| 385 | 490.12 | 978.22 | 977.50 | 0.72 | 0 | (38) | 0.027 | 1 | K.NPANPVQR.I |
| 386 | 490.15 | 978.29 | 977.50 | 0.79 | 0 | 42 | 0.012 | 1 | K.HPVDQVQR.I |
| 456 | 538.17 | 1074.33 | 1074.58 | −0.25 | 1 | 3 | 95 | 5 | R.MLCVRLGAR.N |
| 651 | 712.57 | 1423.12 | 1422.76 | 0.36 | 1 | (31) | 0.1 | 1 | K.NDVTDISDDRFPK.C |
| 652 | 475.75 | 1424.24 | 1422.76 | 1.48 | 1 | (31) | 0.098 | 1 | K.NDVTDISDDRFPK .C |
| 653 | 475.77 | 1424.29 | 1422.76 | 1.53 | 1 | 41 | 0.0091 | 1 | K.NDVTDISDDRFPK.C |
| 665 | 485.05 | 1452.14 | 1451.70 | 0.44 | 1 | 4 | 51 | 10 | K.DYVAPGRMLCVR.L + oxidation (M) |
| 784 | 834.45 | 1666.88 | 1666.80 | 0.08 | 0 | 58 | 0.00018 | 1 | K.LTLYVGKKQLVEIEK.Q |
| P09958 (FURIN_HUMAN)c | |||||||||
| 504 | 319.87 | 956.60 | 957.51 | −0.91 | 0 | 3 | 93 | 10 | K.LLAADAIR.M |
| 698 | 738.83 | 1475.64 | 1474.78 | 0.86 | 0 | 58 | 0.00022 | 1 | R.TSEANNYGTLTK.W |
| Q6UW60 (PCSK4_HUMAN)d | |||||||||
| 319 | 445.28 | 444.27 | 444.23 | 0.04 | 0 | 8 | 1.5 | 3 | K.GPGSK.N |
| 491 | 320.13 | 957.37 | 958.58 | −1.21 | 1 | 13 | 7.9 | 1 | K.TAAPALRV.Q |
| 516 | 337.47 | 1009.39 | 1009.41 | −0.01 | 0 | 17 | 2.9 | 1 | -.MDLPLYAWLSR.C + oxidation (M) |
| P02768 (ALBUMIN_HUMAN)e | |||||||||
| 246 | 712.38 | 711.37 | 711.37 | 0.01 | 0 | (9) | 16 | 2 | K.SEIAHR.F |
| 247 | 356.94 | 711.86 | 711.37 | 0.50 | 0 | 31 | 0.11 | 1 | K.SEVAHR.F |
| 300 | 805.42 | 804.41 | 803.40 | 1.01 | 0 | 3 | 1.1e+002 | 2 | K.ATEDQLK.T |
| 384 | 450.17 | 898.32 | 897.47 | 0.85 | 0 | (23) | 0.74 | 1 | R.LCVLHEK.T |
| 385 | 899.37 | 898.36 | 897.47 | 0.89 | 0 | (16) | 3.8 | 9 | R.LCVLHEK.T |
| 387 | 450.32 | 898.62 | 897.47 | 1.15 | 0 | (17) | 3.2 | 3 | R.LCVLHEK.T |
| 388 | 450.43 | 898.85 | 897.47 | 1.38 | 0 | 27 | 0.37 | 1 | R.LCTVATL.T |
| 501 | 367.10 | 1098.29 | 1099.66 | −1.37 | 1 | 9 | 18 | 9 | K.KQTALAELVK.H |
| 517 | 558.66 | 1115.31 | 1115.65 | −0.34 | 1 | 5 | 43 | 6 | K.LATDLTKINK.E |
| 685 | 1479.61 | 1478.60 | 1478.79 | −0.19 | 0 | (0) | 1.1e+002 | 4 | K.LGEYGFQNAILVR.Y |
| 686 | 1479.68 | 1478.67 | 1478.79 | −0.12 | 0 | (24) | 0.45 | 1 | K.LGEYKFQNALLVR.Y |
| 687 | 1479.77 | 1478.76 | 1478.79 | −0.03 | 0 | (16) | 2.9 | 1 | K.LGEYKFQNALLVR.Y |
| 688 | 740.67 | 1479.32 | 1478.79 | 0.53 | 0 | 70 | 1.1e−005 | 1 | K.LGEYKFQNALLVR.Y |
| 689 | 1480.71 | 1479.70 | 1478.79 | 0.91 | 0 | (29) | 0.16 | 1 | K.LGEYKFQNALLVR.Y |
| 690 | 740.87 | 1479.72 | 1478.79 | 0.93 | 0 | (52) | 0.00077 | 1 | K.LGEYKFQNALLVR.Y |
| 691 | 741.39 | 1480.76 | 1478.79 | 1.97 | 0 | (14) | 5.6 | 1 | K.LGEYKFQNALLVR.Y |
| 823 | 941.90 | 1881.78 | 1881.93 | −0.15 | 0 | 17 | 1.8 | 1 | R.RPCFSALEVDETYVPK.E |
Search parameters: Type of search: MS/MS ion search; enzyme: trypsin; fixed modifications: carbamidomethyl (C); variable modifications: oxidation (M); mass values: monoisotopic; protein mass: unrestricted; peptide mass tolerance: ±2 Da; fragment mass tolerance: ±0.8 Da; max missed cleavages: 1; instrument type: ESI-TRAP.
P00739 (HPTR_HUMAN), haptoglobin-related protein – human; mass 39020, score 143, query matched 8.
P09958 (FURIN_HUMAN), furin protein – human; mass 86778, score 58, query matched 2.
Q6UW60 (PCSK4_HUMAN), proprotein convertase subtilisin/kexin type 4; mass 83115, score 38, query matched 3.
P02768 (ALBUMIN_HUMAN), albumin; mass 70710, score 157, query matched 18.
3.3. Reverse transcription and expression analysis
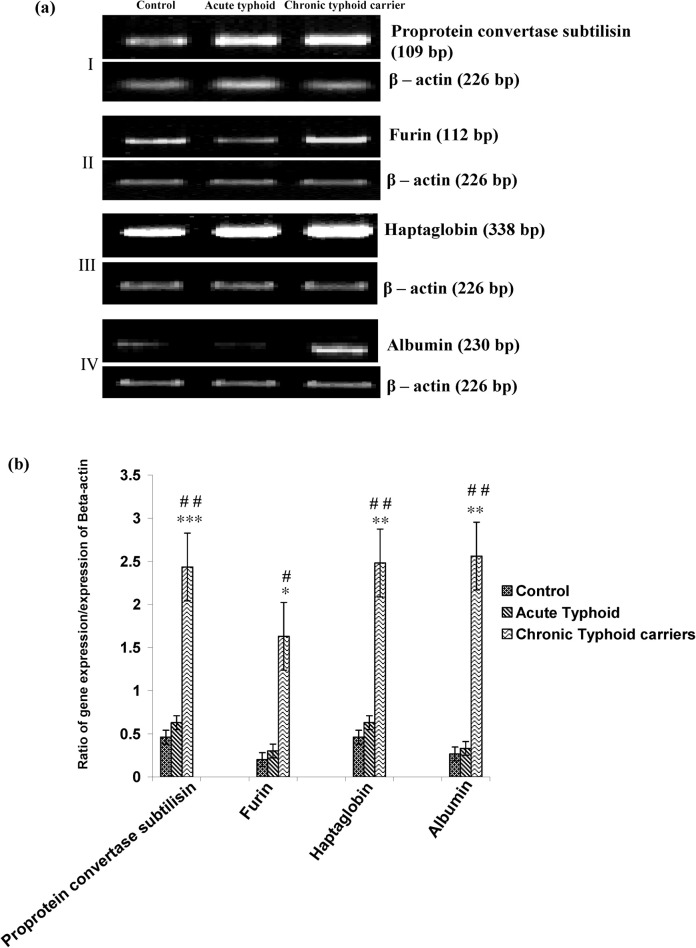
Changes found at the protein level in the controls, acute typhoid cases, and chronic typhoid carriers were further evaluated at the mRNA level. The expression of proprotein convertase subtilisin, haptoglobin, and albumin in chronic typhoid carriers was significantly higher compared to those of control and acute typhoid cases (p < 0.01 and p < 0.01, respectively; Figure 3 ). Further, the expression of furin in cases of chronic typhoid was significantly higher as compared with control and acute typhoid cases (p < 0.05 and p < 0.05, respectively; Figure 3). However, no significant changes were observed between the acute typhoid cases and the control group in the expression analysis of all genes (Figure 3).
Figure 3.
a Agarose gel electrophoretogram of proprotein convertase subtilisin, furin, haptoglobin, and albumin on 1.5% agarose gel electrophoresis and the corresponding β-actin expression in the control, acute typhoid cases, and chronic typhoid carriers groups, respectively. (b) Graphical representation of the ratio of gene expression with corresponding β-actin expression; ***p < 0.001 for controls vs. chronic typhoid carriers, and ##p < 0.01 for acute typhoid cases vs. chronic typhoid carriers in the case of proprotein convertase subtilisin; *p < 0.05 for controls vs. chronic typhoid carriers, and #p < 0.05 for acute typhoid cases vs. chronic typhoid carriers in the case of furin; **p < 0.01 for controls vs. chronic typhoid carriers, and ##p < 0.01 for acute typhoid cases vs. chronic typhoid carriers in the case of haptoglobin; **p < 0.01 for controls vs. chronic typhoid carriers, and ##p < 0.01 for acute typhoid cases vs. chronic typhoid carriers in the case of albumin.
4. Discussion
The diagnosis of S. typhi and S. paratyphi A in chronic typhoid carriers requires special consideration as they are a silent threat to others in the population.21, 22 There is no gold standard test for the detection of chronic typhoid carriers, but the least invasive and most acceptable is stool culture.23 Although molecular methods such as PCR have been used extensively in the identification of acute typhoid cases, its application in chronic typhoid cases is limited due to the low level of S. typhi bacteremia in blood.24, 25, 26, 28 Bacteriological screening of S. typhi carriers is expensive and logistically difficult to perform, therefore a serological means is of practical importance.25, 26 In the current study we tried to screen for S. typhi infection in enteric fever cases using bacteriological and serological methods and used 2D gel electrophoresis and mass spectrometry as an alternative method to differentiate chronic typhoid carriers based on alterations in the expression of proteins.
When analyzed, the plasma proteome of controls, acute typhoid cases, and chronic typhoid carriers showed significant differences in the expression of four protein spots. Mass spectrometry data revealed these spots to be proprotein convertase subtilisin, furin, haptoglobin, and albumin. Proprotein convertase subtilisin and furin are enzymes that cause specific proteolysis in a regulatory mechanism for the generation of biologically active proteins.27 The process of activation is performed by subtilisin and/or kexin-related enzymes known as proprotein convertases (PCs). These recognize and process precursor proteins at the consensus motif RXR/RR.29, 30
The role of PCs and furin has been reported in the pathogenesis of Gram-negative bacilli and viruses.31 The release of LPS from Gram-negative bacteria results in the induction of proprotein convertase subtilisin, which affects the level of lipid and lipoprotein metabolism through regulation of lipid receptors on hepatic cells.31 The chronic carrier stage and gall stone formation are highly associated, because a cholesterol-rich and lithogenic diet allow biofilm formation.32 In the present study, an increase in proprotein convertase subtilisin in chronic carriers shows the involvement of chronic inflammation induced by the pathogen in the host and an alteration in lipid metabolism and lipid concentration that might lead to gall stone formation. The formation of gall stones in chronic typhoid carriers and the intake of a cholesterol-rich diet were observed in most of the chronic typhoid carriers in our study.
The exploitation of host furin in the activation of bacterial toxin, allowing entry into the host cell, has also been well reported.48 Proteolytic processing by furin is an important determinant of pathogenicity for viruses and bacterial toxins.34 The presence of furin in the trans-Golgi network requires a minimum recognition motif for the optimal processing of various bacterial and viral endotoxins in the disease pathogenesis.34 The cell-specific type expression of furin is not clear; T-cells predominantly express furin, which activates T-cell-activated genes that modulate an important immunosuppressive cytokine.48 The increase in furin expression in our study might have some role in the induction of immunosuppressive cytokines. Transforming growth factor beta (TGF-β) upregulates the expression of the fur gene, which in turn increases pro-TGFβ1 maturation.35
The roles of proprotein convertase subtilisin and furin in the activation of matrix metalloproteinases (MT-MMP), which are involved in extracellular matrix degradation, are known.33 An increase in TGF-β upregulates the activity of MT-MMPs, and their involvement in different types of cancer is well established.33 A large cohort study of a typhoid outbreak in 1964 showed a markedly increased risk of gall bladder cancer in chronic carriers – as high as 167 times that of non-carriers.35 The expression of MT-MMP in the stromal component may be essential for the malignant potential of gall bladder cancer.36 The increase in furin and proprotein convertase subtilisin indicates that after infection with Salmonella, these proteins may induce the expression of MT-MMPs, which play an important role in cancer invasiveness in gall bladder cancer.37
Typhoid fever can cause anemia by a variety of mechanisms.38 In acute infection or hepatic dysfunction, there is usually very little change in serum albumin levels, because albumin has a long biological half-life.39, 40 The level of albumin was upregulated in chronic typhoid cases in our study, and this might be due to an increase in free radicals during Salmonella infection; the release of hydroxyl radicals and nitric oxide in cases of Salmonella has already been reported.41, 42 The increase in albumin found mainly in chronic typhoid carriers in the current study might occur in order to counteract the generation of free radicals, because albumin is known to have antioxidant properties during free radical generation in the case of infection.43, 44, 45
The antagonistic effect of haptoglobin on endotoxin was first demonstrated by Baseler and Burrell.46 The effect of haptoglobin on endotoxin-induced cytokine release is not yet known.47 However, it is evident from past research that haptoglobin enters monocytes and neutrophils and interrupts the intracellular function triggered by intracellular LPS.47 The role of haptoglobin in the modulation of proinflammatory cytokines and anti-inflammatory cytokines is debatable and needs further investigation.47 The present study showed an increase in haptoglobin, which is an acute phase protein, in chronic typhoid carriers, and this might have a significant role in triggering the intracellular signaling of monocytes and neutrophils during typhoid carriage. The role of haptoglobin as a modulator of inflammation in pathological conditions such as respiratory infections and endotoxic shock is already known.47 Thus, increased levels of haptoglobin during typhoid carriage provide the environment for S. typhi to modulate the release of cytokines during infection and to sustain its survival in the host. The data from the present study clearly indicate the role of the proteins identified in chronic typhoid carriers, which have a potential effect in perturbing the host inflammatory response triggered by S. typhi during its carriage. Further, the role of these proteins in the intracellular cell signaling of monocytes in chronic typhoid carriers needs further investigation in order to understand the mechanism of the host–pathogen interaction and to develop diagnostic kits and therapeutic drug targets.
Acknowledgement
The authors acknowledge the University Grants Commission (UGC), India, for financial help (Abhai Kumar) and the Ministry of Food Science and Technology for a research fellowship (Smita Singh).
Ethical approval: Written consent was obtained from each participant. The study design was approved by the institutional ethics committee.
Conflict of interest: The authors declare that they have no conflicts of interest.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
References
- 1.Bhan M.K., Bahl R., Bhatnagar S. Typhoid and paratyphoid fever. Lancet. 2005;366:749–762. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 2.Connor B.A., Schwartz E. Typhoid and paratyphoid fever in travellers. Lancet Infect Dis. 2005;5:623–628. doi: 10.1016/S1473-3099(05)70239-5. [DOI] [PubMed] [Google Scholar]
- 3.Levine M.M., Black R.E., Lanata C. Precise estimation of the number of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 4.Khatri N.S., Maskey P., Pudel S. Gallbladder carriage of Salmonella Paratyphi A may be an important factor in the increasing incidence of this infection in South Asia. Ann Intern Med. 2009;150:567–568. doi: 10.7326/0003-4819-150-8-200904210-00017. [DOI] [PubMed] [Google Scholar]
- 5.Roumagnac P., Weill F.X., Dolecek C., Baker S., Brisse S., Chinh N.T. Evolutionary history of Salmonella Typhi. Science. 2006;314:1301–1304. doi: 10.1126/science.1134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames W.R., Robins M. Age and sex as factors in the development of the typhoid carrier state, and a method for estimating carrier prevalence. Am J Public Health. 1943;33:221–230. doi: 10.2105/ajph.33.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merselis J.G., Jr., Kaye D., Connolly C.S., Hook E.W. Quantitative bacteriology of the typhoid carrier state. Am J Trop Med Hyg. 1964;13:425–429. doi: 10.4269/ajtmh.1964.13.425. [DOI] [PubMed] [Google Scholar]
- 8.Nath G., Mauryal P., Gulati A.K., Singh T.B., Srivastava R., Kumar K., Tripathi S.K. Comparison of Vi serology and nested PCR in diagnosis of chronic typhoid carriers in two different study populations in typhoid endemic area of India. Southeast Asian J Trop Med Public Health. 2010;41:636–640. [PubMed] [Google Scholar]
- 9.Gonzalez-Escobedo G., Marshall J.M., Gunn J.S. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol. 2010;9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsolis R.M., Young G.M., Solnick J.V., Bäumler A.J. From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol. 2008;6:883–892. doi: 10.1038/nrmicro2012. [DOI] [PubMed] [Google Scholar]
- 11.Andrews-Polymenis H.L., Bäumler A., McCormick B.A., Fang F.C. Taming the elephant: Salmonella biology, pathogenesis and prevention. Infect Immun. 2010;78:2356–2369. doi: 10.1128/IAI.00096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olopoenia L.A., King A.L. Widal agglutination test—100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–84. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker S, Favorov M, Dougan G. Searching for the elusive typhoid diagnostic. BMC Infect Dis 2010; 10: 45. [DOI] [PMC free article] [PubMed]
- 14.Hodgetts A., Levin M., Kroll J.S., Langford P.R. Biomarker discovery in infectious diseases using SELDI. Future Microbiol. 2007;2:35–49. doi: 10.2217/17460913.2.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Mazzulli T., Low D.E., Poutanen S.M. Proteomics and severe acute respiratory syndrome (SARS): emerging technology meets emerging pathogen. Clin Chem. 2005;51:6–7. doi: 10.1373/clinchem.2004.041574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.O’Farrell P.H. High-resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 18.Klose J. Protein mapping by combined iso-electric focusing and electrophoresis of mouse tissues. A novel approach to testing for induced point mutation in mammals. Humangenetik. 1975;26:231–243. doi: 10.1007/BF00281458. [DOI] [PubMed] [Google Scholar]
- 19.Shevchenko M., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 20.Thongboonkerd V., Gozal E., Schleben L.R., Jr., Arthur J.M., Pierce W.M., Cai J. Proteomic analysis reveals alterations in renal Kallikrein pathway during hypoxia induced hypertension. J Biol Chem. 2002;277:34708–34716. doi: 10.1074/jbc.M203799200. [DOI] [PubMed] [Google Scholar]
- 21.Levine M.M., Black R.E., Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 22.Khatri N.S., Maskey P., Poudel S., Jaiswal V.K., Karkey A., Koirala S. Gallbladder carriage of Salmonella paratyphi A may be an important factor in the increasing incidence of this infection in South Asia. Ann Intern Med. 2009;150:567–568. doi: 10.7326/0003-4819-150-8-200904210-00017. [DOI] [PubMed] [Google Scholar]
- 23.McCall C.E., Martin W.T., Boring J.R. Efficiency of cultures of rectal swabs and faecal specimens in detecting Salmonella carriers: correlation with numbers of Salmonellas excreted. J Hyg (Lond) 1966;64:261–269. doi: 10.1017/s0022172400040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell J., Doyle L., Addison R., Reller L., Hall G., Procop G. Broad range (pan) Salmonella and Salmonella serotype Typhi-specific real-time PCR assays: potential tools for the clinical microbiologist. Am J Clin Pathol. 2005;123:339–345. doi: 10.1309/DP0H-Y5UT-10HQ-W9YM. [DOI] [PubMed] [Google Scholar]
- 25.Massi M.N., Shirakawa T., Gotoh A., Bishnu A., Hatta M., Kawabata M. Rapid diagnosis of typhoid fever by PCR assay using one pair of primers from flagellin gene of Salmonella Typhi. J Infect Chemother. 2003;9:233–237. doi: 10.1007/s10156-003-0256-4. [DOI] [PubMed] [Google Scholar]
- 26.Prakash P., Mishra O.P., Singh A.K., Gulati A.K., Nath G. Evaluation of nested PCR in diagnosis of typhoid fever. J Clin Microbiol. 2005;43:431–432. doi: 10.1128/JCM.43.1.431-432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassi D.E., Fu J., Lopez de Cicco R., Klein-Szanto A.J. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez M.M., Cardona-Castro N.M. Development and evaluation of a PCR method for diagnosis of Salmonella enteric fever, based on DNA sequences of the hilA gene. Biomedica. 2004;24:194–199. [PubMed] [Google Scholar]
- 29.Steiner D.F. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhong M., Munzer J.S., Basak A. The prosegments of furin and PC7 as potent inhibitors of proprotein convertases. In vitro and ex vivo assessment of their efficacy and selectivity. J Biol Chem. 1999;274:33913–33920. doi: 10.1074/jbc.274.48.33913. [DOI] [PubMed] [Google Scholar]
- 31.Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun 2008;19;374:341–4. [DOI] [PMC free article] [PubMed]
- 32.Crawford R.W., Rosales-Reyes R., Ramírez-Aguilar Mde L., Chapa-Azuela O., Alpuche-Aranda C., Gunn J.S. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 2010;107:4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galdiero M., Marcatili A., Cipollaro de l’Ero G., Nuzzo I., Bentivoglio C., Romano Carratelli C. Effect of transforming growth factor beta on experimental Salmonella Typhimurium infection in mice. Infect Immun. 1999;67:1432–1438. doi: 10.1128/iai.67.3.1432-1438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiryaev SA, Remacle AG, Ratnikov BI, Nelson NA, Savinov AY, Wei G, et al. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J Biol Chem 2007;20;282:20847–53. [DOI] [PubMed]
- 35.Blanchette F, Day R, Dong W, Laprise MH, Dubois CM. TGFbeta1 regulates gene expression of its own converting enzyme furin. J Clin Invest 1997;15 99:1974–83. [DOI] [PMC free article] [PubMed]
- 36.Caygill C.P., Hill M.J., Braddick M., Sharp J.C. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet. 1994;343:83–84. doi: 10.1016/s0140-6736(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 37.Karadag N., Kirimlioglu H., Isik B., Yilmaz S., Kirimlioglu V. Expression of matrix metalloproteinases in gallbladder carcinoma and their significance in carcinogenesis. Appl Immunohistochem Mol Morphol. 2008;16:148–152. doi: 10.1097/PAI.0b013e318061b748. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Kumar S, Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol 2006; 15 93:633–9. [DOI] [PubMed]
- 39.Seedat Y.K., Nathoo B.C. Acute renal failure in Blacks and Indians in South Africa—comparison after ten years. Nephron. 1993;64:198–201. doi: 10.1159/000187314. [DOI] [PubMed] [Google Scholar]
- 40.Granthan J.J. Acute renal failure. In: Wyngaarden J.B., Smith L.H. Jr., editors. 18th ed. Vol. 1. W.B. Saunders; Philadelphia: 1988. pp. 558–572. (Cecil text book of medicine.). [Google Scholar]
- 41.Chudhury V.P., Sing B.M., Sinclair S. Salmonella nephritis. Indian Pediatr. 1977;14:857–859. [PubMed] [Google Scholar]
- 42.Palmer R.M., Ashton D.S., Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 43.Maeda H., Akaike T., Wu J., Noguchi Y., Sakata Y. Bradykinin and nitric oxide in infectious disease and cancer. Immunopharmacology. 1996;33:222–230. doi: 10.1016/0162-3109(96)00063-x. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez B., Ferrer-Sueta G., Freeman B.A., Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 45.Halliwell B., Gutteridge J.M. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:18. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 46.Baseler M.W., Burrell R. Purification of haptoglobin and its effects on lymphocyte and alveolar macrophage responses. Inflammation. 1983;7:387–400. doi: 10.1007/BF00916303. [DOI] [PubMed] [Google Scholar]
- 47.Arredouani M.S., Kasran A.J., Vanoirbeek J.A., Berger F.G., Baumann H., Ceuppens J.L. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263–271. doi: 10.1111/j.1365-2567.2004.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesu M., Watford W.T., Wei L., Xu L., Fuss I., Strober W. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]