Abstract
Chronic caudal stomatitis with alveolar/buccal mucositis in calicivirus-positive cats is the most severe presentation of feline chronic gingivostomatitis. Refractory cases are helped by antibiotic and anti-inflammatory treatments often including glucocorticoids. In order to evaluate the comparative efficacy of oromucosal administration of recombinant feline interferon omega (rFeIFN-ω) versus oral administration of glucocorticoids, a randomised, multi-centre, controlled, double-blind study was performed in 39 cats. The progression of behavioural, clinical and lesional scores was assessed over 90 days. Daily oromucosal treatment with 0.1 MU of rFeIFN-ω was associated with a significant improvement of clinical lesions (caudal stomatitis and alveolar/buccal mucositis) and a decrease of pain scores from D0 to D90. Although no such statistical improvement was noticed in the prednisolone group, there was, however, no significant difference between the two groups for most of the parameters, except pain at D60 and D90.
Feline chronic gingivostomatitis (FCGS) is an ill-defined syndrome characterised by inflammatory lesions, mostly ulcerative or ulcero-proliferative in nature, affecting gingival and non-gingival oral mucosa. 1,2 Apart from inflammation associated with periodontal disease, which has been recognised with a very high prevalence in cats, 3 inflammatory lesions have been identified in cats in two specific unusual anatomical sites: the mucosa lateral to the palatoglossal arches and the alveolar mucosa in the premolar/molar area, sometimes extending to the buccal mucosa. 1–5 These inflammatory conditions have been recently respectively re-termed as caudal stomatitis and alveolar/buccal mucositis and are the main features in cats treated for this painful condition. 5,6 Because FCGS syndrome may relate to various oral inflammatory lesions among which gingivitis/periodontitis is predominant, this broad term should be used with caution. It should always be specified whether or not FCGS is associated with caudal stomatitis, as the significant factor seems to be the presence or absence of this type of lesion. 7
It is thought that this clinical entity is a multifactorial condition where the host's immune system is responding inappropriately to chronic oral antigenic stimulation of various origins; dental related conditions such as periodontal disease and dental resorptions, as well as the influence of viral infections or carriage have been suggested to play a role. 7–10 Calicivirus has been known for a long time to cause acute focal or multifocal ulcerative glossitis and palatitis as well as acute upper respiratory disorders. 11–13 The prevalence of calicivirus carriage associated with ‘chronic gingivitis/stomatitis’ has also been reported to be higher than in the random population. 13 More specifically, it has been shown that acute caudal stomatitis can be experimentally induced with calicivirus strains sampled from the oropharynx of cats suffering from chronic stomatitis. 14 However, this experimentally induced acute caudal stomatitis did not result in a chronic disease in the experimental setting. Recently, it was shown using polymerase chain reaction (PCR) technology that almost all cats presenting with caudal stomatitis were chronic oral calicivirus carriers whereas only 30% of cats with chronic gingivitis/stomatitis but without caudal stomatitis were. 5,15 Specific feline calicivirus (FCV) biotypes responsible for stomatitis have not been observed. However, the chronic carrier state in cats has been shown to be related to the emergence of antigenically distant viruses and it has been suggested that antigenic variations, resulting from a series of mutations, are induced by the immunological pressure during chronic infection and constitute an escape mechanism for the virus. 16
Medical treatment of gingivostomatitis cats with caudal stomatitis has been unrewarding with no specific treatment showing superiority. 8 Extraction of teeth in the vicinity of alveolar mucositis and caudal stomatitis, as well as teeth suffering from periodontitis or resorptive lesions, in order to suppress any chronic oral antigenic stimulation has shown the best results with 50–60% of the cats being clinically cured and 30–40% significantly improved. 2,17 Cats showing no or little improvement, refractory cases, require constant medical therapy such as antibiotics and anti-inflammatory drugs. Corticosteroids are often used by clinicians in order to decrease oral inflammation and stimulate appetite.
Interferons (IFNs) are a family of cytokines which were first recognised for their ability to impede viral replication, a function that is indeed critical for host survival in response to viral infection. They bind to specific receptors on the cell surface and induce a signal which, through the synthesis of a certain number of enzymes (such as protein kinase, 2′,5′-oligoadenylate synthetase and Mx protein GTPases) interferes with cellular and viral processes. 18 IFNs do not directly protect cells against viral infection, but rather render cells less suitable as an environment for viral replication. There are two different types of IFN with distinct immunological properties. Type I IFNs, which include a number of IFN-α subtypes, can be produced by almost all cells under appropriate conditions. 19 IFN-ω, also a type I IFN, was described in 1992. 20 A recombinant feline interferon omega (rFeIFN-ω) has been marketed for parvovirus infection in dogs and retroviruses in cats and has been shown to share the typical characteristics of type I human IFN and to have antiviral activity against feline herpesvirus, FCV and feline coronavirus. 21,22 A recent study has shown that the antiviral activities of rFeIFN-ω against various viruses were higher than those of FeIFN-α. 23 Human IFN-α has been used in cats by parenteral administration (IV, IM, SC) but this results in the production of neutralising antibodies with inhibition of the therapeutic effects of the active principle. The administration of a species-specific feline IFN (rFeIFN) has been shown to prevent this event. 24 Mucosal administration of IFN has also been investigated. The therapeutic effect of IFN after oromucosal administration is due to immunomodulary activity through the oropharyngeal lymphoid tissues and via paracrine activity as this glycoprotein is destroyed during transit through the digestive tract. 25,26 The biological activity of IFN after mucosal application has been detected in cats in a dose-dependent fashion by evaluation of Mx protein expression in white blood cells. 18 Based on these data, rFeIFN-ω has been tried in the field as a topical ophthalmological medication in cases of herpetic conjunctivitis and keratitis as well as a local therapy (local infiltration and oromucosal administration) for FCGS syndrome. The use of rFeIFN-ω by oromucosal administration in FCGS has not been substantiated by any published study although there has been a single published case report. 27
The purpose of this study was to compare the efficacy of glucocorticoid treatment versus oromucosal administration of rFeIFN-ω in calicivirus-positive cats suffering from chronic caudal stomatitis/alveolar mucositis refractory to dental extraction treatment.
Materials and methods
Animals
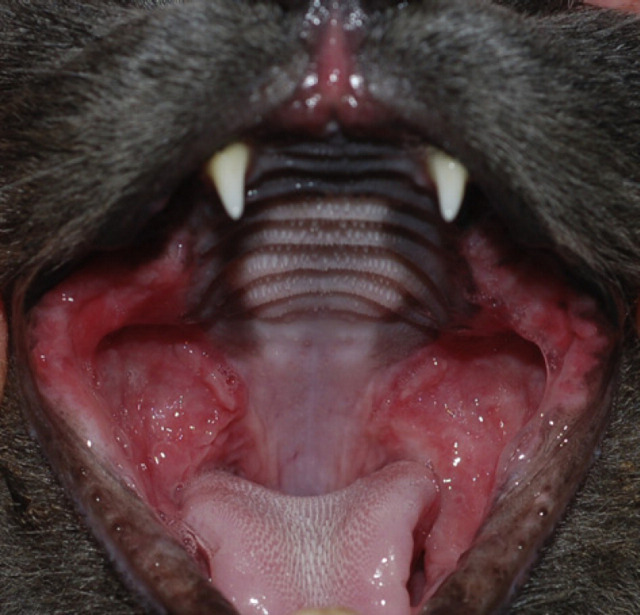
Forty-three cats with FCGS were recruited from the clinic population, presented to 13 veterinary practices, specialised in dentistry located in five European countries (Belgium, France, Germany, Spain and Switzerland) (Fig 1). Among the 43 included cases, four were lost to follow-up immediately after inclusion day or withdrawn from statistical analysis because of protocol deviations. Amongst the remaining 39 cats, various breeds were represented (British Shorthair (one), domestic shorthair (DSH) (five), European (26), Maine Coon (three), Persian (one), Siamese (two) and cross breed (one)). Animal characteristics at baseline are summarised in Table 1. All included animals were calicivirus-positive cats presenting with refractory gingivostomatitis defined by the inclusion criteria summarised in Table 2. Samples from clinical cases were submitted to the Analysis Department of Scanelis Laboratory, France to detect FCV RNA by real-time reverse transcriptase-PCR (RT-PCR). A real-time RT-PCR designed in the conserved 5′ region of the viral genome was used to detect FCV RNA as described previously. 28
Fig 1.
Feline chronic gingivostomatitis.
Table 1.
Characteristics of cat population in both groups.
| Characteristics | IFN | Prednisolone | P value | |
|---|---|---|---|---|
| Age: | Mean (SD) | 6.9 (3.6) | 8.2 (4.3) | 0.49 (Wilcoxon test) |
| Min–max | 0.8–13 | 1.1–15 | ||
| Weight: | Mean (SD) | 4.4 (0.9) | 5.1 (1.1) | 0.07 (Wilcoxon test) |
| Min–max | 2.5–5.7 | 3.6–7 | ||
| Sex: | M/F | 15/9 | 9/6 | 0.91 (Kendall's tau b) |
| Previous episodes FCGS | 18 (86%) | 12 (92%) | 0.82 (Fischer test) | |
Table 2.
Inclusion criteria.
Cats included in the trial should meet three conditions:
|
| The day of inclusion is noted as D0. |
Cats presenting only periodontal lesions and/or alveolar/buccal mucositis without caudal stomatitis, those having received immunomodulatory therapy (cyclosporin A or cytokine-based protocol such as IFN) within the 4 previous weeks, those having received long-acting glucocorticoid or progestagen therapy within the 4 previous weeks and those suffering from diabetes mellitus, hyperadrenocorticism, hepatic failure or chronic renal failure were not enrolled in the study. Exclusion from the study applied to animals whose condition needed the administration of another medical treatment in case of unsatisfactory results during the course of the study (these animals were considered as cases of treatment failure), animals developing severe unrelated disease, animals presenting significant signs of intolerance to the product, animals that did not receive the whole treatment and animals that received forbidden treatment such as corticosteroids other than those tested in the study, non-steroidal anti-inflammatory drugs (NSAIDs) other than those authorised in the frame of the study, products with progesterone activity, immunomodulating or immunosuppressive products or oro-dental treatments in the course of the study (scaling, cleaning, polishing, selective extraction).
Treatments
On day of inclusion, the cats were allocated at random (one randomisation plan per site) into two groups. Each site was provided with vials of powder and diluents as well as tablets, either identified as products A (rFeIFN-ω powder/diluent + placebo tablets) or B (placebo powder/diluent + prednisolone tablets), and with four identical envelopes marked from 1 to 4 containing a label with the letter A or B corresponding to the vials and tablets to be used. For each study site, randomisation was balanced by groups of four cats; two cats were treated with products A and two cats with products B. For every new cat meeting the inclusion criteria, an incremental case number was attributed and a corresponding envelope containing the group label was drawn in numerical order (from 1 to 4). Group anonymity was maintained throughout the study and was only revealed at the time of the statistical analysis, which was performed by an independent statistician.
IFN group
Twenty-four cats received daily administrations of rFeIFN-ω (Virbagen Omega; Virbac, Carros, France) 0.1 MU per day for 90 days by topical oromucosal administration. On D0 the rFeIFN-ω solution was reconstituted by mixing the freeze-dried pellet with the diluent as supplied by the manufacturer to produce a 10 MU/ml solution. Three insulin syringes each containing 0.3 ml (3 MU) of the reconstituted IFN solution were prepared. Two of those syringes were frozen. A 1 in 50 solution was prepared from the third syringe by diluting the 0.3 ml of IFN in 15 ml of sterile isotonic saline. Then, this 1/50 dilution was aliquoted into 30 syringes of 0.5 ml (0.1 MU). One syringe of those 30 syringes was administered to the cat on D0, and the other 29 syringes were given to the owner and stored in the fridge at +4°C. The pet owner administered daily one syringe of 0.5 ml of diluted IFN solution by oromucosal administration until D30. This was performed by positioning the syringe caudally to the canines in the oral commissure under the cheek. The solution was slowly dispensed under the cheek while maintaining the cat with its mouth closed for 30 s to 1 min to maximise mucosal contact by allowing slow swallowing and to avoid spilling of the solution. On D30 and D60, the owner came back to the clinic. On this occasion, the investigator defrosted a frozen insulin syringe containing 0.3 ml of the IFN solution and prepared a fresh 1 in 50 dilution in normal saline which he or she aliquoted into 30 syringes as described above.
Prednisolone group
Fifteen cats were given prednisolone (Megasolone 5; Merial, Lyon, France) orally for 3 weeks at the decreasing dose rate of 1 mg/kg/day for the first 7 days, followed by 1 mg/kg/day every other day for the 7 following days, and then followed by 0.5 mg/kg/day every other day for the 7 last days.
Antibiotics
All cats included in the study received 3 weeks of antibiotic therapy with clindamycin (Antirobe; Pfizer) 25 mg oral solution at the dose of 11 mg/kg, once a day for 3 weeks.
Blinding
To respect blindness, placebo tablets were given to cats in the IFN group using the same protocol as described for the prednisolone group. Placebo oral administrations were given to cats in the prednisolone group using the same protocol as described for the IFN group.
During the course of the study, cats presenting with acute pain or oral clinical condition requiring additional therapy (‘rescue medications’) were allowed to be treated with repeated injections of butorphanol (Alvegesic; Virbac) at the dose of 0.2–0.4 mg/kg SC and/or with daily administrations of meloxicam (Metacam; Boehringer Ingelheim) oral solution 0.5 mg/ml at the dose of 3 drops/kg for less than 7 days after D21 and/or with oral administration of clindamycin hydrochloride (Antirobe; Pfizer) at the dose of 11 mg/kg/day for 21 days.
Clinical follow-up
Cats were observed during a 90-day period. All animals were examined five times: on D0, D15, D30, D60 and D90. When possible, the cat's condition was checked by phone call on D120. At each visit, the cat's body weight was registered and the following clinical examinations were performed by investigators for efficacy assessment. The behaviour was evaluated on four parameters scored on a scale of 0–3: inappetence, pain on food intake or when yawning, hypersalivation and reduced activity. The signs associated with FCGS were assessed by scoring three parameters: pain on opening of cat's mouth by the veterinarian (0–3 scale), halitosis and/or thickening of the saliva (0–2 scale) as well as enlargement of mandibular lymph nodes (0–2 scale). For all these parameters, a score of 0 was normal and a higher score was synonymous with worsening of the condition. The specific inflammatory lesions of FCGS were evaluated by scoring three parameters: caudal stomatitis intensity (0–4 scale), alveolar/buccal mucositis (0–4 scale) and area of the caudal stomatitis lesions (0–6 scale). Scoring systems used for efficacy parameters are presented in Table 3. Response to treatment, classified as worsening, lacking response, moderate improvement, marked improvement or complete cure, as well as general and local tolerance of treatments, were assessed by both investigators and owners. Overall treatment compliance was also evaluated by owners in both treatment groups by reporting at the end of the study whether the oral administration of the solution was easily handled or not.
Table 3.
Scoring systems used for evaluation of lesions.
| Caudal stomatitis intensity score | |
| The investigator scores the intensity of the inflammatory lesions on both sides, ie, the right and the left separately. | |
| Score | Description |
| 0 | Absence of lesion |
| 1 | Slight inflammation, no ulceration, no proliferation, no spontaneous bleeding, no bleeding induced by gentle pressure |
| 2 | Mild inflammation, no ulceration, no or slight proliferation, no spontaneous bleeding, no bleeding induced by gentle pressure |
| 3 | Moderate inflammation, ulcerative or ulcero-proliferative lesion may be observed, no spontaneous bleeding but bleeding induced by gentle pressure on the lesions |
| 4 | Severe inflammation, ulcerative or ulcero-proliferative lesion may be observed, spontaneous bleeding |
| Alveolar/buccal mucositis score | |
| Score | Description |
| 0 | Absence of lesion |
| 1 | Slight inflammation, no ulceration, no proliferation, no spontaneous bleeding, no bleeding induced by gentle pressure |
| 2 | Mild inflammation, no ulceration, no or slight proliferation, no spontaneous bleeding, no bleeding induced by gentle pressure |
| 3 | Moderate inflammation, ulcerative or ulcero-proliferative lesion may be observed, no spontaneous bleeding but bleeding induced by gentle pressure on the lesions |
| 4 | Severe inflammation, ulcerative or ulcero-proliferative lesion may be observed, spontaneous bleeding |
| Area of the caudal stomatitis lesions | |
| The investigator will score the area of the inflammatory lesions on both sides, ie, the right and the left separately. | |
| Score | Description * |
| 0 | No lesion area |
| 1 | Total area from 0 up to 0.5 cm2 |
| 2 | Total area from 0.5 up to 1 cm2 |
| 3 | Total area from 1 up to 2 cm2 |
| 4 | Total area from 2 up to 3 cm2 |
| 5 | Total area from 3 up to 4 cm2 |
| 6 | Total area >4 cm2 |
A colour template was printed and distributed to investigator in order to better evaluate surface area.
Data analysis
The efficacy of rFeIFN-ω was primarily assessed on the basis of the progression over time of the scores of specific inflammatory lesions and lesion area. All other parameters were considered as secondary. As distributions of all observed continuous data were not normal, a non-parametric Wilcoxon Mann–Whitney test was used for treatment group comparison at each time point (D0, D15, D30, D60 and D90), and pair-wise comparisons were performed within each treatment group between every on-treatment time point (D15, D30, D60 and D90) and D0 using a non-parametric sign test. Furthermore, the effect of rescue medications on the mean scores of registered parameters was studied using a Kruskal–Wallis test, which is a non-parametric alternative to the one-way ANOVA for data not normally distributed. This test allowed us to compare, in the same treatment group, the median of the scores of cats receiving rescue medications and the median of the scores of cats not receiving rescue medications. Categorical data were compared between treatment groups using a χ 2 test or a Fisher's exact test. The LOCF (last observation carried forward) method was used for missing data: the last observation was retained until the next record. The threshold of significance for all statistical analyses was fixed at α = 0.05 (5%).
Results
Efficacy assessment
Body weight and behaviour (Fig 2)
Fig 2.
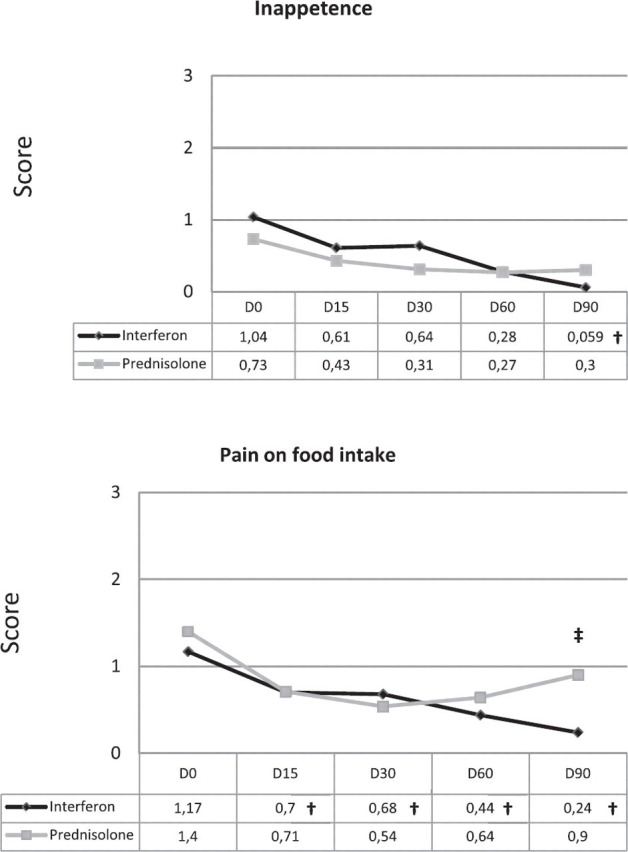
Progression of the four behavioural scores. †Statistically significant intragroup difference from D0. ‡Statistically significant difference between groups.
The curves of the mean animal body weight over time were relatively flat in both treatment groups, with no significant intergroup or intragroup differences (4.40 kg +/−0.87 (D0) and 4.32 +/−0.78 (D90) in IFN group versus 5.05 kg +/−1.15 (D0) and 4.91 +/−1.11 (D90) in the prednisolone group). The progression over time of the four behavioural parameters showed an overall improvement (lower scores) between D0 and D90 in both treatment groups (Fig 1). The mean scores of pain on food intake or when yawning on D90 and of reduced activity on D60 and D90 were significantly improved in the IFN group (P = 0.009, P = 0.017 and P = 0.006, respectively) compared to the prednisolone group. Significant intragroup differences were also shown within the IFN group for all behavioural parameters, on D90 for inappetence (P = 0.004), at all time points for pain on food intake or when yawning (0.002 ≤ P≤ 0.004), on D15 and D90 for hypersalivation (P = 0.009 and P = 0.003, respectively), and from D30 to D90 for reduced activity (0.004 ≤ P ≤ 0.03). No improvement (decrease in scores) in the prednisolone group was noticed for any of the behavioural parameters except for hypersalivation scores which were lower on D30, D60 and D90 than on D0 (0.02 ≤ P ≤ 0.04).
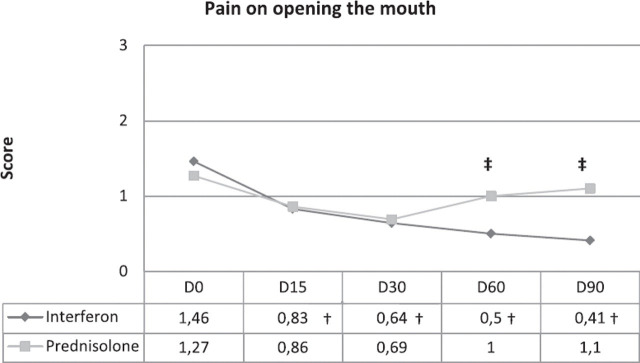
Signs associated with FCGS syndrome (Fig 3)
Fig 3.
Progression of pain on opening the mouth score. †Statistically significant intragroup difference from D0. ‡Statistically significant difference between group.
An overall tendency of improvement was observed in both treatment groups between D0 and D90. The mean score of pain on opening cat's mouth was lower in the IFN group on D60 and D90 (P = 0.017 and P = 0.007, respectively) compared to the prednisolone group (Fig 2). Within-group differences were shown for pain on all time points (P = 0.002) in the IFN group but not in the prednisolone group. Halitosis only decreased on D15 (P = 0.046) in the IFN group and on D90 (P = 0.023) in the prednisolone group. Mandibular lymph nodes were only decreased in size on D30 (P = 0.040) in the prednisolone group.
Specific inflammatory lesions of FCGS
A continuous and steady decrease of scores was observed for caudal stomatitis intensity and for area of the caudal stomatitis in both treatment groups, and for alveolar/buccal mucositis in the IFN group only (Fig 4). The improvement was significant at all time points except D15 for area of caudal stomatitis in the IFN group, whereas it was only significant on D30 for area of caudal stomatitis in the prednisolone group (Table 4). However, intergroup differences were not significant for all three scores. Further analysis was made by combining caudal stomatitis scores into a global score. The significant decrease of lesional scores at all time points compared to D0 in the IFN group was confirmed whereas a significant decrease in prednisolone group was only observed at D15 compared to D0.
Fig 4.
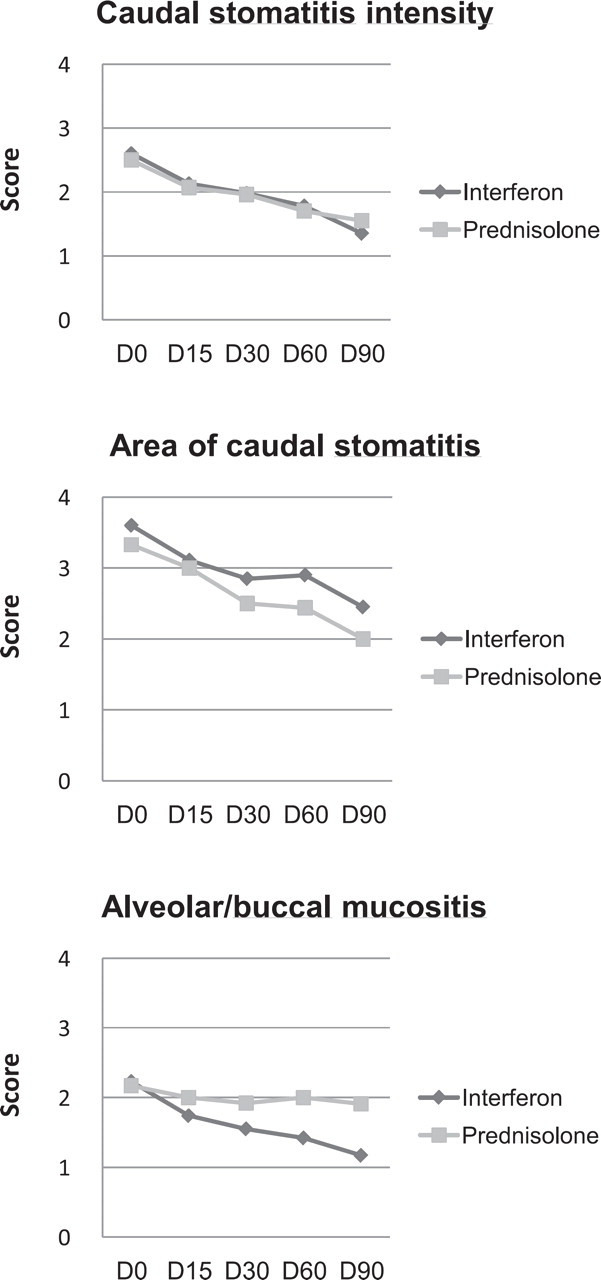
Progression of lesional scores.
Table 4.
Progression of lesional scores in each group.
| Lesions | Caudal stomatitis intensity | Area of caudal stomatitis | Alveolar/buccal mucositis score | |||
|---|---|---|---|---|---|---|
| Group | Prednisolone | IFN | Prednisolone | IFN | Prednisolone | IFN |
| D15/D0 | 0.077 | 0.0094* | 0.077 | 0.21 | 0.29 | 0.0098* |
| D30/D0 | 0.0455* | 0.0098* | 0.0455* | 0.016* | 0.45 | 0.0033* |
| D60/D0 | 0.077 | 0.0098* | 0.077 | 0.027* | 0.45 | 0.0098* |
| D90/D0 | 0.077 | 0.0012* | 0.077 | 0.0094* | 0.50 | 0.0019* |
Statistically different from D0 (P < 0.05).
Response to treatment
The repartition of the five categories used to assess response to treatment by investigators and owners is presented in Table 5. An excellent correlation was observed between investigators’ and owners’ evaluations. Complete cure and marked improvement occurred in 45% of cats in the IFN group and in 23% of cats in the prednisolone group when assessment was made by investigators, and in 39% of cats in the IFN group and in 23% of cats in the prednisolone group when assessment was made by owners. However, these differences between the two groups were not significant.
Table 5.
Response to treatment evaluated by investigators and owners at the end of the study while the groups where still blinded.
| Response to treatment evaluated | Investigator | Owner | ||
|---|---|---|---|---|
| Prednisolone | IFN | Prednisolone | IFN | |
| Complete cure | 7.7% | 10.0% | 7.6% | 11.1% |
| Marked improvement | 15.4% | 35.0% | 15.4% | 27.8% |
| Moderate improvement | 30.8% | 10.0% | 30.8% | 16.7% |
| Lacking response | 23.1% | 35.0% | 23.1% | 38.9% |
| Worsening | 23.1% | 10.0% | 23.1% | 5.6% |
No significant difference between groups and between investigator and owners assessments.
Need for rescue medications
Eight cats (33.3%) in the IFN group versus nine cats (60.0%) in the prednisolone group needed rescue medications during the course of the study. Differences between treatment groups were, however, not significant. The effect of rescue medications was assessed by comparing in each treatment group the median of the scores of the animals receiving rescue medications and the median of the scores of the animals not receiving rescue medications. A potential effect of rescue medications on the mean scores of reduced activity in the IFN group was pointed out. There were statistical differences in the IFN group at day 60 (P = 0.0005) and day 90 (P = 0.0025) but not in the prednisolone group for reduced activity scores between the median of the scores of the animals receiving rescue medications and the median of the scores of the animals not receiving rescue medications. There was no significant influence of the presence or absence of rescue medications on the score of pain on opening cat's mouth at any time in both treatment groups. There was no influence in the IFN group regarding food intake on D90, pain on food intake or when yawning at all time points, hypersalivation on D15 and D90, and reduced activity on D30. In the prednisolone group, there were also no significant differences, rescue medications or not, for hypersalivation on D30, D60 and D90.
Withdrawals due to lack of efficacy
Five cats (21.0%) in the IFN group versus four cats (27.0%) in the prednisolone group were withdrawn from the study for unsatisfactory clinical results. Differences between treatment groups were not significant.
Treatment tolerance
In the investigator's opinion, the treatment was well tolerated in 22 cats (96%) in the IFN group versus 13 cats (100%) in the prednisolone group. Two adverse events were noted during the study, one in each treatment group (one case of dehydration and anorexia in the IFN group, and one case of polydipsia in the prednisolone group). No treatment had to be stopped because of treatment intolerance.
Treatment compliance
Both groups of cats were receiving oromucosal treatment (either IFN or placebo) daily for 90 days as well as tablets (either prednisolone or placebo) daily or every other day for 21 days. Owners found that treatments were easy to administer in 17 cats (89%) in the IFN group and in 12 cats (92%) in the prednisolone group. There was no statistical difference between the groups.
Discussion
Dental extraction has been shown to provide the best long-term improvement in cats suffering from FCGS with caudal stomatitis, with 50–60% of the cats being clinically cured. 2,17,29 However, about 10% of cats are not improved by this treatment and about 30% still require medications in order to eat without experiencing too much pain. 2,17 Apart from numerous anecdotal reports and presentations during congresses, there are no published studies on the treatment of cats refractory to dental extraction treatment. This study is the first randomised, controlled, double-blind study on this frustrating condition. Though no medical treatment can be recognised as the gold standard for this condition, corticosteroids were used as a positive control because of their wide and frequent use by practitioners in such cases. Prednisolone was selected because of its better bioavailability and tolerance compared to prednisone or dexamethasone. 30,31 A tapering anti-inflammatory dose was chosen in order to lessen adrenocortical suppression and to avoid stimulation of virus replication in chronic virus-carriers. 32
Both young and old cats may be affected by this condition which is reflected by the wide range of ages observed in the study (10 months to 15 years). The mean age of cats was not different in both groups (6.9+/−3.6 vs 8.2+/−4.) and was middle-age which is consistent with cats which often have been affected early and have been continuously suffering for a long period of time and is also consistent with the population in previous reports on dental treatment of such cats. 2,29 There was no sex predilection and most of the cats were DSH cats. Due to the low prevalence of other breeds in the study and without the knowledge of the breeds’ prevalence in the different practices involved in the study, no breed predilection could be determined. Individual's characteristics (breed, sex, age, body weight and FCGS history) of cats in both treatment groups were not significantly different (P < 0.05).
Mean body weight of cats at D0 was within normal range for a population mainly composed of DSH cats, which is consistent with mean general condition and behavioural scores (inappetence, pain on opening the mouth or when yawning, hypersalivation and reduced activity) only slightly to moderately altered at D0 in both groups. This may be reflecting the positive effect of medications which were given to the cats prior to inclusion in the study. Body weight was maintained throughout the study without any intragroup or intergroup differences. No deterioration in any of the general condition/behavioural scores was noticed after the start of the study, when previous treatments were withdrawn, indicating that, in both groups, treatments were able to sufficiently control pain and enable the cat to eat. This is a very important point as, for humane reasons, when dealing with such a debilitating condition, a positive control group has to be used and one has to ensure that cats in both groups are appropriately managed.
In the IFN group, pain scores (pain on food intake, pain when the cat's mouth was opened) showed a progressive and significant decrease (=less pain) along time from D0 to D90. Reduced activity scores also showed a significant decrease (=more active) starting at day 30 but a potential effect of rescue medications was noted at D60 and D90 which prevents us from concluding that IFN was solely responsible for the improved activity in the IFN group at these two time points, though it would seem logical that a decrease in pain would result in more active cats. Pain and reduced activity scores also decreased from D0 to D30 in the prednisolone group, and subsequently increased from D30 to D90, however, these changes were not significant. It would have seemed logical that the initial 3-week course of glucocorticoids (D0–D21) would have affected these parameters. However, the lack of significance might be due to a smaller number of cats in the prednisolone group compared to the IFN group and subsequent lack of statistical power. Hypersalivation, on the other hand, showed a progressive decrease from D30 to D90 in the prednisolone group whereas it was only significantly improved at D90 in the IFN group. Mean hypersalivation scores were statistically significantly lower on D30, D60 and D90 than on D0 (0.02 ≤ P ≤ 0.04) in the prednisolone group with no statistically significant influence of rescue medications. This may indicate that though the prednisolone was stopped at D21 a carry-over effect may have been observed throughout the following study period. In the field, clinical improvement usually lasts a few to several days after glucocorticoid treatment is stopped. Because of the concern not to favour viral activity, we elected to give only a 3-week course of prednisolone, expecting that a possible carry-over effect would extend the improvement up until the time rescue therapy would be needed. Because IFN does not possess direct anti-inflammatory nor pain killing effects and was expected to progressively modify the immune balance in chronically-diseased cats, the treatment was extended over the whole 90-day study period.
When comparing both groups for behavioural parameters and clinical signs associated with FCGS, the mean pain score on food intake or yawning was significantly lower (less pain) in the IFN group compared to the prednisolone group on D90 (P = 0.0085) and the mean pain score on opening of the cat's mouth was also significantly lower (less pain) in the IFN group on D60 (P = 0.017) and D90 (P = 0.0066). This indicates that though IFN does not possess anti-inflammatory, nor analgesic effects, it might have been able to trigger sufficient improvement of the oral cavity condition to result in less oral pain.
In the IFN group, all lesional scores (intensity and surface area of caudal stomatitis as well as intensity of alveolar/buccal mucositis) showed a significant decrease (0.0012 < P < 0.027) over time from D0 to D90 (except for surface area at D15 − P = 0.21). In comparison, no significant decrease of these parameters was noticed in cats receiving prednisolone. When comparing both groups together, no significant difference was observed between the two treatments. Further analysis was made by combining caudal stomatitis scores into a global score (intensity + surface area or intensity × surface area). The significant decrease of lesion scores at all time points compared to D0 in the IFN group was confirmed whereas a significant decrease in prednisolone group was only observed at D15 compared to D0. The latter might be due to the direct anti-inflammatory effect during the first 2 weeks. However, no intergroup difference was noticed either. This apparent discrepancy between the significant improvement in the IFN group and the lack of significant difference in the prednisolone group together with lack of difference between the two treatment regimens might be due to the low number of cats in the prednisolone group and lack of statistical power. The significant decrease over time of lesion scores in cats treated with IFN is a clear indication of the effect of the molecule on the pathogenic process leading to oral inflammatory lesions.
Because cats which experienced sufficient pain during the study to affect their ability to eat were allowed to receive additional NSAIDs and antibiotics (rescue medications), differences between groups might also have been masked by rescue medications given to the cats after D21. Eight cats (33.3%) in the IFN group and nine cats (60.0%) in the prednisolone group needed rescue medication during the course of the study. The difference between treatment groups was however not significant (Fisher's exact test, P = 0.46) thus the use of rescue medications is unlikely to have affected one treatment group more than the other one. The effect of rescue medications was assessed in more detail on behavioural scores, which are the scores more likely to be modified by these additional treatments. There was no significant influence of rescue medications in the IFN group regarding pain on opening of the mouth and pain on food intake or when yawning at all time points, on inappetence on D90, hypersalivation on D90, and reduced activity on D30, indicating that the significant improvements of pain scores and of hypersalivation scores in that group are likely to be due to treatment effect. However, there was a statistically significant difference of mean reduced activity scores in the IFN group (P = 0.0005 on D60 and P = 0.0025 on D90) but not in the prednisolone group at D60 and D90 between the animals receiving rescue medications or not. The significantly improved mean reduced activity scores in the IFN group at D60 (P = 0.017) and at D90 (P = 0.006) might have been due to rescue medications received by some of the cats. Because pain scores were not affected by rescue medications given in both prednisolone and IFN groups, and because pain is related to intensity and extent of the lesions, it is unlikely that rescue medications could have significantly affected the assessment of lesional scores.
Overall treatment efficacy was assessed by both the owners and the investigators while the groups where still blinded; good statistical agreement was reached between clinicians’ and owners’ assessments. Treatment efficacy was classified in four categories from totally effective (complete clinical cure) to partially effective (marked or moderate improvement) to ineffective (lack of improvement or worsening). There was no statistical difference in any of these categories between IFN and prednisolone groups. Clinical cure was achieved in only 10% or less of the cats whatever the treatment was. This is not surprising considering the study population; only refractory cases of FCGS with caudal stomatitis, which had not improved after dental extractions and medical treatment, were selected. This clinical entity is a multifactorial condition and we were not expecting that any of the treatments would be a magic bullet. Nevertheless, 55% of cats in IFN group and 54% in the prednisolone group were either clinically cured or their condition was moderately to markedly improved over the study period. When considering only cats that were clinically cured or markedly improved, investigators found 45% cats classified as such in the IFN group versus 23% in the prednisolone group. Though these results are very encouraging for the IFN group there was no statistical difference between groups and this is likely to be due to the low number of cats in each group and the subsequent lack of statistical power.
This study has several limitations. Identifying and selecting cats following the strict inclusion/exclusion criteria of the study was a difficult task and resulted in fewer cats being incorporated in this multi-centre study compared to what we originally expected. This is likely to have resulted in a lack of statistical power and might account for the lack of statistical significance between the IFN and the prednisolone groups in some situations where there was a statistically significant improvement along time in the IFN group but not in the prednisolone one. Nevertheless, we consider that strict definition of the condition studied and strict case selection is essential when studying FCGS as so many different clinical conditions are usually mixed in study panels that results become just impossible to interpret. Any study has to be limited in time but it might be that 3 months was not long enough to be able to show an improvement with IFN in some cats. This seems to be confirmed by anecdotal reports and clinical experience. In some studies on feline infectious peritonitis (FIP), feline leukaemia virus (FeLV) or feline immunodeficiency virus (FIV) where survival time and general health parameters were monitored, the study period was much longer, up to a year. 33,34 Because FCGS with caudal stomatitis is such a debilitating condition, we feared that it would be difficult to follow the cats over a long period of time without taking the risk of losing many cats for follow-up and/or having them being given unauthorised treatments. With such a condition, the need for adjunct treatments (rescue medications) has to be expected and properly planned in the protocol to avoid bias in the study. Rescue medications may artificially improve the results in one group compared to the other one. It might have been the case on reduced activity score in the IFN group on D60 and D90. However, it did not influence the different oral pain scores (pain on food intake and when yawning and pain when the investigator opened the mouth) at all time points nor inappetence at D90 and subsequently the improvement of the clinical lesions in the IFN group and response to treatment in both groups was not biased. Last, we selected as a positive control a 3-week course of tapered prednisolone treatment instead of a continuous administration over the 3-month study period. Though the latter might have been seen as a better option, there were several reasons for that decision. Long-term glucocorticoid treatment might be associated with various side effects, especially in older debilitated cats as well as in chronic oral calicivirus-carriers. In the field, glucocorticoids are often administered to effect with a tapering dose over a few weeks. Because of a carry-over effect, the glucocorticoid treatment, which is only symptomatic, is only resumed when needed. The significant decrease in hypersalivation in the prednisolone group after D30 might have been due to that effect.
In conclusion, daily oromucosal administration of 0.1 MU rFeIFN-ω was associated with a significant improvement of clinical lesions (caudal stomatitis and alveolar/buccal mucositis) and a decrease of pain scores from D0 to D90. Though no such statistical improvement was noticed in the prednisolone group, there was, however, no significant difference between the two groups for most of the parameters, except pain at D60 and D90. Within the limitation of this study, oromucosal administration of rFeIFN-ω was shown to result in significant improvement and was found to be at least as good as short-term prednisolone therapy in the treatment of calicivirus-positive cats presenting with FCGS with caudal stomatitis refractory to dental extractions.
Conflict of interest
This study was sponsored by Virbac SAS, France.
Acknowledgements
Thanks go to Scanelis (Toulouse, France) which performed the PCR testings as well as to the investigators involved in this study: Drs Boissier, Boulet, Camy, Dufour, Grimberg, Hennet, Kaspar, Latil, Lhomme, Palmero, Roes, Roux and Schreyer.
References
- 1. Gaskell R.M., Gruffydd-Jones T. Intractable feline stomatitis, Vet Annu 17, 1977, 195–199. [Google Scholar]
- 2. Hennet P. Chronic gingivo-stomatitis in cats: long-term follow-up of 30 cases treated by dental extractions, J Vet Dent 14, 1997, 15–21. [Google Scholar]
- 3. Girard N., Servet E., Biourge V., Hennet P. Periodontal health status in a colony of 109 cats, J Vet Dent 26, 2009, 149–155. [DOI] [PubMed] [Google Scholar]
- 4. Pedersen N.C. Inflammatory oral cavity diseases of the cat, Vet Clin North Am Small Anim Pract 22, 1992, 1323–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lommer M., Verstraete F.J. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis, Oral Microbiol Immunol 18, 2003, 131–134. [DOI] [PubMed] [Google Scholar]
- 6.Oropharyngeal inflammation. AVDC Nomenclature committee. 2009. http://www.avdc.org/?q=node/29(accessed Feb 15, 2010).
- 7. Hennet P. Stomatites chroniques felines: une meilleure compréhension, Médecin vétérinaire du Québec 36, 2006–2007, 133–137. [Google Scholar]
- 8. Harley R., Helps C.R., Harbour D.A., Gruffydd-Jones T.J., Day M.J. Cytokine mRNA expression in lesions in cats with chronic gingivostomatitis, Clin Diagn Lab Immunol 6, 1999, 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sims T.J., Moncla B.J., Page R.C. Serum antibody response to antigens of oral gram-negative bacteria by cats with plasma-cell gingivitis-pharyngitis, J Dent Res 69, 1990, 877–882. [DOI] [PubMed] [Google Scholar]
- 10. Dowers K.L., Hawley J.R., Brewer M.M., Morris A.K., Radecki S.V., Lappin M.R. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1infection with gingivostomatitis in cats, J Feline Med Surg 12, 2010, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Povey C. Viral diseases of cats: current concepts, Vet Rec 98, 1976, 293–299. [DOI] [PubMed] [Google Scholar]
- 12. Harbour D.A., Howard P.E., Gaskell R.M. Isolation of feline calicivirus and feline herpesvirus from domestic cats 1980–1989, Vet Rec 128, 1991, 77–80. [DOI] [PubMed] [Google Scholar]
- 13. ABCD guidelines on feline calicivirus, European Advisory Board on cat diseases; March 2007. http://www.abcd-vets.org/guidelines/pdf/abcd_fcv_guidelines_0703.pdf. (accessed Feb 15, 2010). [Google Scholar]
- 14. Reubel G.H., Hoffmann D.E., Pedersen N.C. Acute and chronic faucitis of domestic cats: a feline Calicivirus-Induced disease, Vet Clin North Am Small Anim Pract 22, 1992, 1347–1360. [DOI] [PubMed] [Google Scholar]
- 15. Hennet P, Boucraut-Baralon C. Relationship between oral calicivirus and herpesvirus carriage and ‘palatoglossitis’ lesions. In: Proceedings of the 19th annual Veterinary Dental Forum; 2005 Oct 13–16; Orlando, USA.
- 16. Poulet H., Brunet S., Soulier M., Leroy V., Goutebroze S., Chappuis G. Comparison between acute/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: pathogenicity, antigenic profile and cross-neutralisation studies, Arch Virol 145, 2000, 243–261. [DOI] [PubMed] [Google Scholar]
- 17. Girard N, Hennet P. Retrospective study of dental extraction for treatment of chronic caudal stomatitis in 60 calicivirus-positive cats. Proceedings of the 19th annual Veterinary Dental Forum; 2005 Oct 13–16; Orlando, USA.
- 18. Braclein T., Theise S., Metzler A., Spiess B.M., Richter M. Activity of feline interferon-omega after ocular or oral administration in cats as indicated by Mx protein expression in conjunctival and white blood cells, Am J Vet Res 67, 2006, 1025–1032. [DOI] [PubMed] [Google Scholar]
- 19. Decker T., Stockinger S., Karaghiosoff M., Muller M., Koravik P. IFNs and STATs in innate immunity to microorganisms, J Clin Invest 109, 2002, 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura N., Sudo T., Matsuda S., Yanai A. Molecular cloning of feline interferon cDNA by direct expression, Biosci Biotechnol Biochem 56, 1992, 211–214. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto J.K., Okuda T., Yanai A. Anti feline herpesvirus and calicivirus effects of feline interferon, J Interfer Res 10, 1990, S114. [Google Scholar]
- 22. Ueda Y., Sakurai T., Kasama K., et al. Pharmacokinetic properties of recombinant feline interferon and its stimulatory effect on 2′,5′ oligoadenylate synthetase activity in the cat, J Vet Med Sci 55, 1993, 1–6. [DOI] [PubMed] [Google Scholar]
- 23. Wang H., Jia X., Yang L., Sun L., Liu W. Comparison of antiviral activity between FeIFN-omega and FeIFN-alpha, Sheng Wu Gong Cheng Xue Bao 24, 2008, 1556–1560. [PubMed] [Google Scholar]
- 24. Zeidner N.S., Myles M.H., Mathiason-DuBard C.K., Dreitz M.J., Mullins J.I., Hoover E.A. Alpha interferon (2b) in combination with zidovudine for the treatment of presymptomatic feline leukemia virus-induced immunodeficiency syndrome, Antimicrob Agents Chemother 34, 1990, 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummins J.M., Krakowka S., Thompson C.D. Systemic effects of interferons after oral administration in animals and humans, Am J Vet Res 66, 2005, 164–176. [DOI] [PubMed] [Google Scholar]
- 26. Tovey M., Maury C. Oromucosal interferon therapy: marked antiviral and antitumor activity, J Interferon Cytokines Res 19, 1999, 145–155. [DOI] [PubMed] [Google Scholar]
- 27. Southerdern P., Gorrel C. Treatment of a case of refractory feline chronic gingivostomatitis with feline recombinant interferon omega, J Small Anim Pract 48, 2007, 104–106. [DOI] [PubMed] [Google Scholar]
- 28. Reynolds B.S., Poulet H., Pingret J.L., et al. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France, J Feline Med Surg 11, 2009, 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellei E., Dalla F., Masetti L., Pisoni L., Joechler M. Surgical therapy in chronic feline gingivostomatitis (FCGS), Vet Res Commun 32 (suppl 1), 2008, S231–S234. [DOI] [PubMed] [Google Scholar]
- 30. Graham-Mize C.A., Rosser E.J. Bioavailability and activity of prednisone and prednisolone in the feline patient, Vet Dermatol 15, 2004, 7–10. [Google Scholar]
- 31. Lowe A.D., Graves T.K., Campbell K.L., Schaeffer D.J. A pilot study comparing the diabetogenic effects of dexamethasone and prednisolone in cats, J Am Anim Hosp Assoc 45, 2009, 215–224. [DOI] [PubMed] [Google Scholar]
- 32. Middleton D.J., Watson A.D., Howe C.J., Caterson I.D. Suppression of cortisol responses to exogenous adrenocorticotrophic hormone, and the occurrence of side effects attributable to glucocorticoid excess, in cats during therapy with megestrol acetate and prednisolone, Can J Vet Res 5, 1987, 60–65. [PMC free article] [PubMed] [Google Scholar]
- 33. de Mari K., Maynard L., Sanquer A., Lebreux B., Eun H.-M. Therapeutic effects of recombinant feline interferon-v on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats, J Vet Intern Med 18, 2004, 477–482. [DOI] [PubMed] [Google Scholar]
- 34. Ritz S., Egberink H., Hartmann K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis, J Vet Intern Med 21, 2007, 1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]