Abstract
Two thousand, two hundred and seven cats from 14 shelters of a major UK cat charity were blood tested for feline coronavirus (FCoV) antibodies. Data was collated on breed, sex, age, number of cats at original location, outdoor access, health status, and time spent in the shelter prior to sampling (range 0 to 4 years). Some cats were also tested for feline leukaemia virus antigen, feline immunodeficiency virus, and Toxoplasma gondii antibodies. The effect of these variables on FCoV seropositivity was explored by multivariable logistic regression. FCoV seropositivity in cats that had spent 5 days or less in a shelter at sampling was significantly associated with a multi-cat origin, cats aged 3 years or less, and Persian breed. Whether pet, stray or feral, health status, indoor/outdoor access, and sex had no significant effect. Overall FCoV seropositivity was associated with time spent in a shelter but this association was not linear. Cats that had spent more than 60 days in a shelter were over five times as likely to be seropositive. This may be the result of a change in husbandry from solitary to communal housing for cats remaining in shelters long term. Rescue of cats for less than 60 days was not associated with a significant increasing risk of seropositivity. Significant variation existed in seropositivity between individual shelters overall and in cats rescued for less than 5 days. These findings may reflect inter-shelter variation in cat husbandry and variation in seropositivity of shelter intake respectively.
Introduction
Feline infectious peritonitis (FIP) is the leading infectious cause of cat death (Vennema et al., 1998) and occurs in up to 10% of cats infected with feline coronavirus (FCoV). No FIP vaccine is available in the UK and attempts to control FIP centre on keeping FCoV infected cats separate from uninfected cats (Addie and Jarrett, 1992). Whilst rescue shelters were not the primary source of FCoV for many relinquished cats in the United States, exposure to the shelter environment resulted in amplification of shedding and spread of infection to susceptible individuals (Pedersen et al., 2004). This is not necessarily the case in the United Kingdom.
Identification of FCoV infection is complicated by the fact that the virus can be shed intermittently. Establishment of an individual cat'sinfection status by RT–PCR requires testing of multiple faecal samples (Addie and Jarrett, 2001; Herrewegh et al., 1997). Serological detection of anti-FCoV antibodies is an alternative to detection of virus in faeces by RT–PCR. At any point in time approximately one in three seropositive cats sheds FCoV (Addie and Jarrett, 1992); the higher the antibody titre, the more likely the cat is to be shedding virus (Addie and Jarrett, 2001).
The purpose of this study is to identify risk factors associated with FCoV seropositivity in cats relinquished to rescue shelters in the UK and to evaluate the role of exposure to the rescue shelter environment.
Materials and methods
Cats
Two thousand, two hundred and fourteen cats relinquished to 14 British cat charity shelters were tested for FCoV antibodies. A questionnaire was completed for each cat either by the relinquishing owner or shelter manager giving details of the cat's sex, breed, age, health status at the time of sampling, whether originating from a multi-cat or single cat household, whether free-ranging or indoor, whether pet, stray or feral, and how long the cat had been in the rescue shelter at the time of sampling.
Antibody testing
Antibody testing was performed by immunofluorescence (IF) (Addie and Jarrett, 1992). Immunofluorescent antibody titres of 20 or more were considered seropositive (SP) and titres of 10 or less were considered to be seronegative (SN). Cats were tested once, rather than sequentially throughout their stay in the shelter. In some cases cats were also tested for feline leukaemia virus (FeLV) antigen, feline immunodeficiency virus (FIV) antibodies, and Toxoplasmosa gondii antibodies.
Statistics
Data was cross-classified according to the following categories: sex, neutering status, breed, health status, number of cats at original location, free-ranging or indoor, shelter, FeLV infection, FIV and T. gondii seropositivity. The resulting contingency tables were analysed by calculation of the chi-square statistic, odds ratios, and 95% confidence intervals. Age and time spent in the shelter prior to sampling were assessed as continuous variables by the Mann–Whitney U test.
Multivariable logistic regression modeling (Statistix 7.1 software; Analytical software; USA) was used to control for confounding variables. Variables entered the model at P<0.25 in the univariable analysis and were retained in the model at P<0.05 as assessed by log likelihood ratio tests. The linearity of continuous variables in the logit was explored through the use of fractional polynomials and ordered categorical design variables. Finally, interaction terms were introduced and retained if the model fit improved.
The effect of some factors could be expected to change with prolonged exposure to a shelter environment: for example, outdoor access has ceased, number of cats at original location, life-style as a pet, stray or feral cat has changed. As a result these variables were excluded from the analysis of the entire data set. Analysis was repeated on a data subset containing cats that had been in the shelter for 5 days or less prior to sampling and including all variables.
Results
Population
Overall, 566 (25.6%) of cats tested were seropositive for FCoV antibodies.
Cats were tested from 14 shelters: median 141.5 cats per shelter (range 24–371 cats per shelter). Data on submitting shelter was unavailable for 13 cats (0.6%). The median age of the cats was 2 years (range 5 days to 18 years). Data on age was unavailable for 415 (18.7%) of cats. Cats had spent a median of 5 days in the shelter prior to sampling (range 0 to 1460 days). Data on time in shelter was unavailable for 96 (4.3%) cats. Categorical population description is shown in Table 1. One thousand, one hundred and seventy-three cats had spent 5 days or less in a shelter at the time of sampling and were included in the data subset for analysis; 24.3% of these cats tested seropositive for FCoV antibodies.
Table 1.
Population by factor categories
| Factor | Category | |||
|---|---|---|---|---|
| Sex | Male | Female | No data | |
| 826 (37.3%) | 1242 (56.1%) | 146 (6.6%) | ||
| Neutering status | Entire | Neutered | No data | |
| 66 (3.0%) | 1956 (88.3%) | 192 (8.7%) | ||
| Breed | Domestic | Pedigree a | No data | |
| 2113 (95.4%) | 48 (2.2%) | 53 (2.4%) | ||
| Type | Pet | Stray | Feral | No data |
| 1431 (64.6%) | 533 (24.1%) | 174 (7.9%) | 76 (3.4%) | |
| Cat's in original location | Single cat | Multi-cat b | No data | |
| 546 (24.7%) | 1507 (68.0%) | 161 (7.3%) | ||
| Outdoor access | Free roaming | Indoor | No data | |
| 1897 (85.7%) | 179 (8.1%) | 138 (6.2%) | ||
| Health status | Healthy | Sick | No data | |
| 1930 (87.2%) | 122 (5.5%) | 162 (7.3%) | ||
| FeLV | Positive | Negative | No data | |
| 12 (0.5%) | 2150 (97.1%) | 52 (2.4%) | ||
| FIV | Positive | Negative | No data | |
| 91 (4.1%) | 2065 (93.3%) | 58 (2.6%) | ||
| Toxoplasmosis | Positive | Negative | No data | |
| 499 (22.5%) | 1640 (74.1%) | 75 (3.4%) | ||
Nine different pedigree breeds were reported: median 4 cats of each breed (range 2–15); the most commonly sampled pedigree breed was Persian.
The precise number of cats in a cat's original location was available for 67 cats from a multi-cat household: median 2 cats per multicat household (range 2 to 39).
Risk factors—overall
Univariable analysis suggested that the following variables may have been associated with seropositivity to FCoV (P<0.25): pedigree breed, age, sick health status, seronegativity for FIV, shelter, and time spent in shelter prior to sampling. Sex, neutering, and FeLV infection status and T. gondii seropositivity all had no significant effect.
When multivariable logistic regression was used to control for confounding factors breed, health status, and FIV seropositivity were excluded from the model as confounding factors (Table 2). Time in shelter prior to sampling was not linear in the logit. Model fit was significantly improved when time in shelter prior to sampling was treated as the binary categorical variable: less than or equal to 60 days or greater than 60 days. Age was not linear in the logit. Model fit was improved when age was treated as the binary categorical variable: less than or equal to 3 years or greater than 3 years. No interaction terms significantly improved model fit.
Table 2.
Multivariable odds ratios and 95% confidence intervals for the association between risk factors and FCoV seropositivity (not including interaction terms)
| Characteristic | Seropositive (no.) | Seronegative (no.) | Odds ratio | 95% CI |
|---|---|---|---|---|
| Age | ||||
| Less than or equal to 3 years | 326 | 780 | 1.0 | |
| Greater than 3 years | 106 | 438 | 0.56 | 0.47, 0.72 |
| Time in shelter prior to sampling | ||||
| Less than or equal to 60 days | 382 | 1181 | 1.0 | |
| Greater than 60 days | 50 | 41 | 5.44 | 3.39, 8.72 |
| Shelter | ||||
| A | 104 | 167 | 1.0 | |
| B | 27 | 102 | 0.56 | 0.43, 0.71 |
| C | 39 | 121 | 0.54 | 0.35, 0.83 |
| D | 3 | 30 | 0.15 | 0.04, 0.49 |
| E | 60 | 142 | 0.76 | 0.51, 1.13 |
| F | 29 | 55 | 0.75 | 0.46, 1.22 |
| G | 29 | 230 | 0.14 | 0.09, 0.23 |
| H | 5 | 16 | 0.51 | 0.20, 1.32 |
| I | 18 | 37 | 0.72 | 0.39, 1.35 |
| L | 34 | 95 | 0.56 | 0.36, 0.89 |
| M | 21 | 79 | 0.42 | 0.24, 0.71 |
| N | 40 | 68 | 0.77 | 0.48, 1.23 |
| O | 19 | 60 | 0.52 | 0.29, 0.92 |
| P | 4 | 16 | 0.55 | 0.20, 1.56 |
Results in italics are not significantly different from shelter A.
Cats relinquished because of the cat's illness were not significantly more likely to be seropositive to FCoV. Only 9 cats were given up for this reason and only one had an illness that could be related to FCoV status (the cat had FIP). The remaining 8 cats had non-FCoV related conditions (such as roadaccidents).
Risk factors—time in shelter up to 5 days
Univariable analysis suggested that the following variables may have been associated with seropositivity to FCoV (P<0.25): pedigree breed, Persian breed, age, outdoor access, sick health status, multi-cat household, status as a pet, stray, or feral cat, seropositivity for toxoplasmosis, shelter, and time spent in shelter prior to sampling. Sex, neutering, and FeLV and FIV status all had nosignificant effect.
The results of multivariable logistic regression to control for confounding factors are shown in Table 3. Outdoor access, health status, status as a pet, stray, or feral cat, time spent in shelter, and toxoplasmosis seropositivity were excluded from the model as confounding factors. Age was not linear in the logit. Model fit was improved when age was treated as the binary categorical variable: less than or equal to 3 years or greater than 3 years. The number of cats within a multi-cat household had no significant effect on FCoV seropositivity. However, there was limited data available (see Table 1).
Table 3.
Multivariable odds ratios and 95% confidence intervals for the association between risk factors and FCoV seropositivity up to 5 days time spent in shelter
| Characteristic | Seropositive (no.) | Seronegative (no.) | Odds ratio | 95% CI |
|---|---|---|---|---|
| Age | ||||
| Less than or equal to 3 yr | 127 | 425 | 1.0 | |
| Greater than 3 yr | 47 | 248 | 0.57 | 0.38, 0.85 |
| Cats in original location | ||||
| Single | 31 | 221 | 1.0 | |
| Multiple | 143 | 452 | 2.34 | 1.46, 3.74 |
| Breed | ||||
| Other breed | 170 | 671 | 1.0 | |
| Persian | 4 | 2 | 11.29 | 1.76, 72.62 |
| Shelter | ||||
| A | 12 | 40 | 1.0 | |
| B | 20 | 82 | 1.06 | 0.46, 2.43 |
| C | 27 | 86 | 1.01 | 0.46, 2.22 |
| D | 3 | 24 | 0.40 | 0.10, 1.60 |
| E | 38 | 63 | 2.21 | 1.01, 4.80 |
| F | 9 | 21 | 1.18 | 0.42, 3.28 |
| G | 12 | 144 | 0.31 | 0.13, 0.75 |
| H | 1 | 6 | 0.69 | 0.07, 6.60 |
| I | 9 | 14 | 3.69 | 1.20, 11.37 |
| L | 14 | 52 | 0.98 | 0.39, 2.41 |
| M | 7 | 47 | 0.54 | 0.19, 1.53 |
| N | 11 | 51 | 0.84 | 0.33, 2.15 |
| O | 7 | 32 | 0.85 | 0.29, 2.46 |
| P | 4 | 11 | 1.31 | 0.34, 4.96 |
Results in italics are not significant.
Discussion
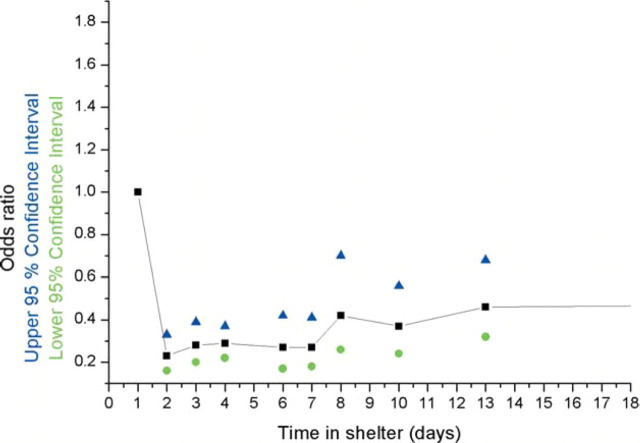
In order to evaluate risk factors likely to be altered by exposure to the shelter environment we chose to analyse a subset of data from cats that had spent minimal time in the shelter. Our intention was to exclude data from cases that had seroconverted as a result of exposure to the shelter environment. The interval between primary FCoV infection and seroconversion is between 7 and 18 days (Stoddart et al., 1988). The time for an anamnestic antibody response to develop in a cat that has previously been infected is likely to be less but remains unknown. As a result, we chose to analyse data from cats that had spent 5 days or less in the shelter. This decision was supported by the appearance of time in shelter prior to sampling in the logit (Fig. 1). The risk of seropositivity remained constant in cats that had spent up to 6 days in the shelter at the time of sampling.
Figure 1.
Risk of FCoV seropositivity vs time in shelter (0–18 days).
Cats originating from multi-cat households were over twice as likely to be FCoV seropositive as cats from single-cat households. This finding is consistent with the faecal oral transmission of FCoV.
Outdoor access could also be expected to affect FCoV seropositivity. In this population it did not. Outside access allows cats to bury faeces often in distinct territories and so minimise faecal oral contact and FCoV transmission. However, outdoor access does not mean cats will not make use of communal litter trays; 50% (50/101) of UK pet cats from multi-cat households with outdoor access still make use of communal litter trays (Cave, unpublished data). Even when cats exclusively bury faeces outdoors, urban environments with high cat population densities may lead to communal use of soiling areas such as gardens.
Feral cats were not associated with a reduced risk of seropositivity. This was surprising because feral nature is generally associated with cats living in isolation and so at reduced exposure to FCoV (Legendre et al., 2002). However, in this population of cats relinquished to cat shelters, 87.5% (91/104) feral cats that had spent 5 days or less in a shelter were recorded as originating from a multi-cat household. Feral cats relinquished to cat shelters would have to be trapped. This is more cost effective in areas where multiple feral cats are residing and may explain this finding.
In common with previous reports, we demonstrated an association between young age and increased FCoV seropositivity (Pedersen, 1995). However, this relationship was not linear and was better described in this population by considering age as less or more than 3 years old.
Pedigree breeds have previously been reported to be at increased risk of FCoV positivity (Pedersen, 1995). Analysis of the data from cats that had spent 5 days or less in a shelter did reveal a significant association between pedigree breed and FCoV seropositivity. The majority of pedigree cats were Persians and modelling for Persian breed instead of pedigree breed improved model fit. The effect of breed would not be expected to alter with time spent in shelter. However, analysis of the entire dataset did not reveal any association with breed. The inconsistency of this association suggests that it may be the result of very small numbers of pedigree cats in the dataset. Most cats relinquished to rescue shelters are domestics. Further studies of data containing more pedigree cats is required before firm conclusions can be drawn on thisassociation.
FCoV seropositivity was not associated withbeing sick at the time of sampling, being infected with FeLV, FIV, or T. gondii, or relinquishment because of cat illness. These findings were consistent with previous reports that acute FCoV infection is usually asymptomatic (Pedersen, 1995).
FCoV seropositivity is not indicative of current infection unless seroconversion can be demonstrated (Addie and Jarrett, 1992). In the current study cats were only sampled once making demonstration of seroconversion impossible; FCoV seropositivity thus reflects past exposure. Therefore documentation of increasing seropositivity in the population with increasing time spent in the shelter cannot be assumed to represent seroconversion following exposure to the shelter environment. It could also represent a trend for FCoV seropositive cats to remain in the shelter for longer. Increasing time spent in a shelter was associated with increased risk of FCoV seropositivity but this association was not linear. Model fit was significantly improved by considering time in shelter as a binary factor: 60 days or less or more than 60 days.
It is very unlikely that seroconversion in a shelter takes 60 days to occur. A second possibility is that seropositivity may be associated with a reason for shelter staff to retain a cat for longer before re-homing, for example because FCoV infection had induced diarrhoea. However, this population had not been previously tested for anti-FCoV antibodies, so we cannot know when seroconversion occurred. Acute FCoV infection is usually asymptomatic or associated with only mild diarrhoea or upper respiratory signs, and in this study, FCoV seropositive cats were not more likely to be sick at the time of sampling. There was no significant association between FCoV infection and serological results for other infectious diseases. Finally another risk factor for seropositivity may change with increasing time in shelters. When cats enter shelters it is recommended that they be keptisolated from other cats. However, if they remain in a shelter for a prolonged period such as 60 days they are likely to be transferred to group housing and so would transfer to a multi-cat environment which would probably include cats with which they had not previously been in contact. As already demonstrated multi-cat environments are associated with transmission of FCoV. In our opinion this is the most likely explanation for the association of FCoV seropositivity with time spent in a shelter of more than 60 days. A further study following a cohort of cats from the time of shelter entry would be required to ascertain these findings. The reasons for prolonged retention in the shelter and the timing of movement to group housing would also need to be documented.
FCoV seropositivity significantly varied between individual shelters. Variation in cats that have spent 5 days or less in a shelter probably represents variation in FCoV seropositivity within the cat population from which the shelter rescues cats. As Table 3shows shelters B, C, D, G, L, M, and O admit cats with significantly lower rates of seropositivity than shelter A. The other shelters do no differ significantly from shelter A. Further assessment of areas from which these shelters rescue cats is required to determine why this is so.
Variation in the entire dataset would, in addition, be affected by husbandry practices within the individual shelters that promote or retard FCoV seroconversion. Examination of Table 2reveals that when cats that have spent more than 5 days in a shelter are included in the analysis only shelter G remains at a significantly lower rate of seropositivity than shelter A. Shelters E and I both become associated with greater seropositivity than shelter A. The remaining shelters do not differ significantly from shelter A. This may mean that care of cats in shelter G is more successful at preventing FCoV transmission whereas that in shelters E and I promotes it. Further assessment of cat management within individual shelters would be required to ascertain this.
This study confirms young age and multi-cat households as risk factors for FCoV seropositivity in cats relinquished to UK rescue shelters. There is significant variation in rates of seropositivity of cats admitted to UK rescue shelters; the reasons for this are unclear. Increased time spent in UK shelters is associated with increased risk of seropositivity but the relationship is not linear. UK shelters appear to be able to successfully rescue cats for up to 60 days without significantly increasing their risk of seropositivity. Increasing risk of seropositivity in cats that remain in shelters for more than 60 days may be associated with a change in management to communal housing.
Acknowledgments
This work was funded by Cats Protection (CP) and Companion Animal Diagnostics. TAC was funded by the RCVS Trust (Clarke and Sparrow). We would like to thank the then CP veterinary surgeon, Margaret Roberts, for instigating this survey. The authors are grateful to the CP workers for completing the forms and to the veterinary surgeons attending the CP shelters for taking blood samples. We are grateful to Michael McDonald, Alex Muller and Kate Park for technical assistance and to Maria Williams and Janet McGrane for administering the samples and Lesley Dinning and Morag Wallace for secretarial assistance.
References
- Addie D.D., Jarrett J.O. A study of naturally occurring feline coronavirus infection in kittens, Veterinary Record, 130, 1992, 133–137. [DOI] [PubMed] [Google Scholar]
- Addie D.D., Jarrett J.O. Use of a reverse-transcriptase polymerase chain reaction for monitoring feline coronavirus shedding by healthy cats, Veterinary Record, 148, 2001, 649–653. [DOI] [PubMed] [Google Scholar]
- Herrewegh A., Mahler M., Hedrich H.J., Haagmans B.L., Egberink H.F., Horzinek M.C., Rottier P.J.M., de Groot R.J. Persistence and evolution of feline coronavirus in a closed cat-breeding colony, Virology, 234, 1997, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre A.M., Luria B.J., Gorman S.P., Lee I.T., Levy J.K., 2002. Prevalence of coronavirus antibodies in feral cats in Gainesville, Florida. Second International Feline Coronavirus/Feline Infectious Peritonitis Symposium, Glasgow, Scotland.
- Pedersen N.C., Sato R., Foley J.E., Poland A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on Feline Enteric Coronavirus, Journal of Feline Medicine and Surgery, 6, 2004, 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. An overview of feline enteric coronavirus and infectious peritonitis virus infection, Feline Practice, 23, 1995, 7–20. [Google Scholar]
- Stoddart M.E., Gaskell R.M., Harbour D.A., Gaskell C.J. Virus shedding and immune responses in cats inoculated with cell culture-adapted feline infectious peritonitis virus, Veterinary Microbiology, 16, 1988, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Poland A., Foley J., Pedersen N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses, Virology, 243, 1998, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]