An outbreak of a novel coronavirus was reported in Wuhan, China in late December, 2019, and has since become a global health emergency.1, 2 This letter describes the first Vietnamese case of coronavirus disease 2019 (COVID-19) acquired from China.
A 25-year-old Vietnamese woman who had been in Wuhan for a 2-month business trip returned to Vietnam on Jan 17, 2020. In Wuhan, she lived with two Vietnamese colleagues. They did not visit the Huanan market, which was located 20 km away, and cannot recall contact with anyone who had influenza-like symptoms. All three individuals returned to Vietnam on the same flight. On January 23, the patient presented with coughing, sneezing, fever, and chest pain. After an initial visit to a district hospital, she was transferred to Thanh Hoa General Hospital with suspected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the Thanh Hoa Provincial Center for Disease Control was informed. Her two colleagues had similar symptoms and were admitted to another hospital, where they tested positive for SARS-CoV-2. Individuals who had substantial contact with the patient and her two colleagues were quarantined and all accommodations and transit methods were decontaminated.
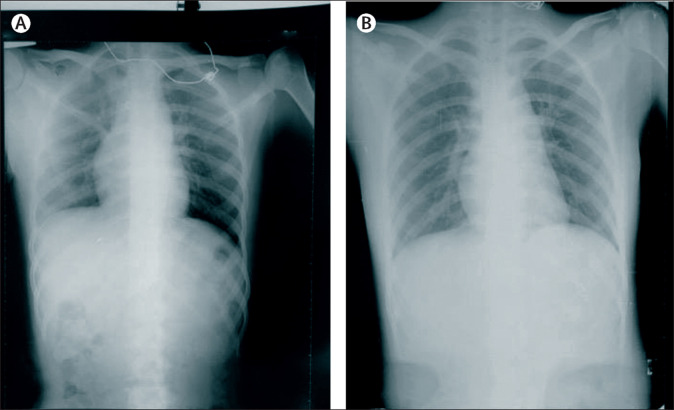
On admission to hospital, the patient was alert but exhausted, with mild chest pain, a temperature of 39·2°C, blood pressure of 120/70 mm Hg, a pulse of 100 beats per min, and a respiratory rate of 25 breaths per min. The patient had no crackles or bronchi rales on lung auscultation. All other clinical findings were normal. Initial laboratory tests showed a white blood cell count of 3·7 × 106/L, a red blood cell count of 4·28 × 109/L, a platelet count of 185 × 106/L, and a haemoglobin concentration of 127 g/L. Chest radiography showed no abnormalities (figure ). The patient's serum C-reactive protein level was 6·6 mg/dL and her serum aspartate aminotransferase and alanine transferase concentrations were 28·7 U/L and 14·6 U/L, respectively. The patient's glucose concentration was 6·8 mmol/L, her serum creatinine was 66 mmol/L, and serum urea was 3·8 mmol/L. A nasopharyngeal swab specimen was obtained to detect influenza type A and B by rapid test, as well as parainfluenza types 1–3, respiratory syncytial virus, rhinovirus, adenovirus, and human metapneumovirus by RT-PCR; results were negative for all listed pathogens. A blood sample was obtained for culture and the result was negative for bacterial infection after 5 days. Malaria testing was negative, and cardiac and pleural ultrasound results were unremarkable. The patient's nasopharyngeal specimen was tested for SARS-CoV-2 by RT-PCR at the National Institute of Hygiene and Epidemiology (Hanoi, Vietnam), and the result after 6 days was positive for SARS-CoV-2.
Figure.
Patient x-ray
(A) X-ray done at admission (January 24). (B) X-ray done 4 days after admission (January 28).
The patient was then isolated in a negative pressure room. Treatment included ceftazidime 4 g daily, paracetamol, and 1 L of intravenous saline daily. Antibiotic treatment was discontinued after 4 days. The patient had a high fever, dry cough, and chest pain for the first 2 days. On day 3, her fever subsided and her clinical condition began to improve. On day 5, the patient's body temperature returned to normal and cough and chest pain decreased substantially. No further notable findings occurred during her 9-day hospital stay.
21 people with direct contact with this patient were also isolated. Until February 6, none of these individuals had developed symptoms. As the patient's two colleagues tested positive for SARS-CoV-2,3 this suggested transmission via respiratory droplets.
Acknowledgments
We declare no competing interests. LVC and HTNG contributed equally to this work. Patient consent was obtained for publication.
References
- 1.WHO Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) Jan 30, 2019. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 2.WHO Novel Coronavirus (2019-nCoV) Situation Report—13. Feb 2, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200202-sitrep-13-ncov-v3.pdf?sfvrsn=195f4010_6
- 3.Ministry of Health COVID-19: Latest updates, constantly. https://suckhoedoisong.vn/Virus-nCoV-cap-nhat-moi-nhat-lien-tuc-n168210.html?fbclid=IwAR2VuytNA9ed9WE3N-l5wjVGDbDN8624XiKZcGoFki175-gCc_azo8AYBEI (in Vietnamese).