Abstract
Objectives
To describe the role of bacteria (including bacterial resistance), viruses (including those recently described) and mixed bacterial–viral infections in adults presenting to primary care with lower respiratory tract infection (LRTI).
Methods
In all, 3104 adults with LRTI were enrolled, of whom 141 (4.5%) had community-acquired pneumonia (CAP), and 2985 matched controls in a prospective study in 16 primary care networks in Europe, and followed patients up at 28–35 days. We detected Streptococcus pneumoniae and Haemophilus influenzae and assessed susceptibility, atypical bacteria and viruses.
Results
A potential pathogen was detected in 1844 (59%) (in 350 (11%) bacterial pathogens only, in 1190 (38%) viral pathogens only, and in 304 (10%) both bacterial and viral pathogens). The most common bacterial pathogens isolated were S. pneumoniae (5.5% overall, 9.2% in CAP patients) and H. influenzae (5.4% overall, 14.2% in CAP patients). Less than 1% of S. pneumoniae were highly resistant to penicillin and 12.6% of H. influenzae were β-lactamase positive. The most common viral pathogens detected were human rhinovirus (20.1%), influenza viruses (9.9%), and human coronavirus (7.4%). Influenza virus, human parainfluenza viruses and human respiratory syncytial virus as well as human rhinovirus, human coronavirus and human metapneumovirus were detected significantly more frequently in LRTI patients than in controls.
Conclusions
A bacterial pathogen is identified in approximately one in five adult patients with LRTI in primary care, and a viral pathogen in just under half, with mixed infections in one in ten. Penicillin-resistant pneumococci and β-lactamase-producing H. influenzae are uncommon. These new findings support a restrictive approach to antibiotic prescribing for LRTI and the use of first-line, narrow-spectrum agents in primary care.
Keywords: Aetiology, Lower respiratory tract infection, Respiratory
Introduction
Community-acquired lower respiratory tract infection (LRTI) is one of the commonest reasons for consulting in primary care and accounts for considerable antibiotic use and health-care costs. It is neither feasible nor cost-efficient to identify microbial aetiology in most patients who present with LRTI in primary care because of sampling challenges, limited access diagnostics and the limited clinical utility of receiving a result after empirical treatment decision has been made [1]. Consequently, little is known about the aetiology of LRTI in everyday primary care. In addition, detecting pathogens in both symptomatic patients and contemporaneous controls to distinguish between asymptomatic carriage and the presence of agents causing symptoms has rarely been carried out. Nevertheless, despite limited knowledge of the proportion of patients that have an identifiable bacterial aetiology and the sensitivities of these pathogens, and evidence of limited or no clinical benefit from antibiotic treatment, more than half of patients presenting to primary care with LRTI/acute cough in Europe are prescribed antibiotics [2], [3], [4]. This contributes to the selection of antimicrobial-resistant bacteria [5]. Improved knowledge of likely pathogens (at the point of care) and the likely susceptibility of bacterial pathogens, could help to guide antibiotic prescribing decisions and so help contain unnecessary antibiotic use and antimicrobial resistance. Furthermore, such information could support public health policy on prevention of respiratory illness, including vaccination.
Our primary objective was to describe the viral and bacterial aetiology in adult patients presenting to primary care with LRTI and in those with community-acquired pneumonia (CAP). Our secondary objectives were to describe the presence of resistance in bacterial infections and of mixed viral–bacterial infections.
Materials and methods
Study design and patients
The study was part of the European Union FP6 funded Network of Excellence GRACE (Genomics to combat Resistance against Antibiotics in Community-acquired LRTI in Europe Network of Excellence; www.grace-lrti.org). We recruited patients between October 2007 and April 2010 in 16 primary care networks that had a track record of conducting research based in 11 European countries: Antwerp and Ghent (Belgium); Barcelona and Mataro (Spain); Bialystok, Lodz and Szczecin (Poland); Bratislava (Slovakia); Cardiff and Southampton (UK); Jesenice (Slovenia); Jönköping (Sweden); Milan (Italy); Nice (France); Rotenburg (Germany) and Utrecht (the Netherlands).
Inclusion criteria for patients were: age ≥18 years, with an acute or worsened cough (≤28 days duration) as the main symptom, or any clinical presentation considered to be caused by LRTI by the general practitioner (GP) and consulting for the first time for this illness episode. Patients with presumed cough of non-infective origin, antibiotic consumption in the previous month, and any serious condition associated with an immunocompromised condition were excluded. For each patient, we planned to include a control patient matched for age, maximum 5 years of difference, and gender, consulting at the GP office for any other reason than acute respiratory illness within the same 2-week period. The study was approved by the local ethics committees in all participating centres and by the competent authority in each country. Written informed consent was obtained from each patient and control participant before inclusion.
Sampling and measurements
Symptomatic patients were assessed at first presentation (day 1) and between days 28 and 35. Chest radiographs were taken within 1 week after inclusion. CAP was considered present if the local radiologist reported lobar or bronchopneumonia; other diagnoses were categorized as ‘pneumonia absent’ [6].
All recruiting GPs received standardized sampling material and a protocol with detailed instructions on the sampling of the patients. Within 24 h of first presentation and inclusion, serum and EDTA blood, sputum, if available, and two nasopharyngeal flocked swabs (NPS; COPAN) were taken. At days 28–35, serum sampling and the two NPS were repeated. Controls were sampled for EDTA blood and two NPS at baseline. Sputum was not obtained from controls and the controls were not followed up. Serum, EDTA and NPS were stored frozen in the local laboratories until regular shipment to the central laboratory (University Hospital Antwerp), where specimens were stored at −80°C until analysis.
Bacterial cultures for Streptococcus pneumoniae and Haemophilus influenzae
Sputum samples were examined in the local laboratories using direct microscopy to assess the quality (ratio of white blood cells/epithelial cells ≥1 as criterion for good quality), then Gram-stained, cultured and subsequently frozen at −80°C. Streptococcus pneumoniae and H. influenzae were identified using conventional biochemical tests and isolates were frozen in microbanks until shipped in batches to the central laboratory, where NPS were cultured for S. pneumoniae and/or H. influenzae. Their susceptibility was tested at the Karolinska Institute and the Oxford University, respectively, after frozen transport. The MICs of S. pneumoniae to penicillin G, erythromycin, clindamycin, tetracycline and levofloxacin were determined. Isolates were classified as sensitive, indeterminate or resistant according to the EUCAST breakpoints for these species (www.eucast.org/antimicrobial-susceptibility-testing/breakpoints). Haemophilus influenzae isolates were tested for β-lactamase production.
PCRs for Mycoplasma pneumoniae, Chlamydia pneumoniae, Bordetella pertussis, Legionella pneumophila and respiratory viruses
Nucleic acid from NPS was extracted with the NucliSens EasyMag (bioMérieux, Marcy l'Étoile, France) in Antwerp after which aliquots were shipped to three collaborating laboratories for subsequent analysis with their in-house amplification and detection methods, which had been evaluated previously [7].
Serology for M. pneumoniae, C. pneumoniae and B. pertussis
For the detection of M. pneumoniae-specific and C. pneumoniae specific IgG or IgM antibodies, M. pneumoniae or C. pneumoniae IgG and IgM-ELISA kits (Medac GmbH, Wedel, Germany) were used according to the instructions of the manufacturer. IgG antibodies to B. pertussis toxin (Institut Virion-Serion GmbH, Würzburg, Germany) were analysed in a convalescent serum sample.
Diagnostic criteria
The isolation of S. pneumoniae and H. influenzae, and the identification of L. pneumophila or respiratory viruses by use of PCR in respiratory samples were considered to support an aetiological diagnosis. Infection with M. pneumoniae or C. pneumoniae was defined as: positive PCR in respiratory samples, the presence of IgM antibodies in the acute-phase serum and/or convalescent-phase sample, IgG seroconversion or a significant increase in IgG between acute and convalescent samples.
A patient was considered positive for an acute B. pertussis infection (infection in the last 6 months) if positive by PCR in a respiratory sample and/or the presence of an antibody titre to pertussis toxin of ≥125 IU/mL in convalescent serum (days 28–35), demonstrated previously as a cut-off with high sensitivity and specificity [8], [9].
Statistical analysis
Generalized estimating equations were used to assess differences in the proportion of potential pathogens between LRTI patients' day 1 and days 28–35 samples, and between day 1 samples of LRTI patients and controls. The case–control design was applied to assess causality between viral pathogens and LRTI (CAP). Chi-squared tests were used to assess differences in the proportion of specific viruses or bacteria between LRTI patients with and those without CAP. Student's t-test was used to assess differences in age between LRTI patients with and those without specific viral or bacterial aetiology (IBM® SPSS® Statistics, Release 20.0.0). A p value of <0.05 was considered to be statistically significant.
Results
Patient characteristics and response
A total of 3104 adult LRTI patients were included by 294 GPs from October 2007 to April 2010, 1860 (60.0%) were women (Table 1 ). The mean age was 49.8 years (range 18–92 years) and 141 were diagnosed with CAP (4.5%); among elderly patients (>65 years n = 628, 20.2%) 40 patients had a CAP (6.4%). We recruited a total of 2985 controls without symptoms of LRTI.
Table 1.
Age and gender of all patients with LRTI, LRTI with CAP, LRTI without CAP, and of matched controls
| LRTI (n = 3104) | LRTI with CAP (n = 141)a | LRTI without CAP (n = 2960)a | Matched controlsb (n = 2063) | |
|---|---|---|---|---|
| Gender | ||||
| Males, n (%) | 1244 (40.0) | 62 (44.0) | 1182 (39.9) | 820 (39.7) |
| Females, n (%) | 1860 (60.0) | 79 (56.0) | 1781 (60.1) | 1243 (60.3) |
| Age | ||||
| Mean (SD) | 49.8 (16.8) | 53.9 (15.3) | 49.4 (16.6) | 49.5 (16.6) |
| Range | 18–92 | 19–87 | 18–92 | 18–92 |
| Above 65 years, n (%) | 628 (20.2) | 40 (28.4) | 588 (19.8) | 385 (18.7) |
Abbreviations: CAP, community-acquired pneumonia; LRTI, lower respiratory tract infection.
Data missing for three patients.
Matched for age (maximum 5 years of difference) and gender, and consulting the same GP office for any other reason than LRTI within the same 2-week period.
Day 1 NPS and blood samples were available from 3085 (99.4%) and 3054 (98.4%) LRTI patients, respectively, and sputum samples from 2121 (68.3%). On days 28–35, 2673 patients (86.1%) were seen: in 2552 (95.5%) and 2575 (96.3%) of these, blood samples and NPS, respectively, could be collected. Only controls who matched with patients according to all criteria (n = 2063) were further included to estimate causality.
Aetiology in LRTI and CAP in primary care
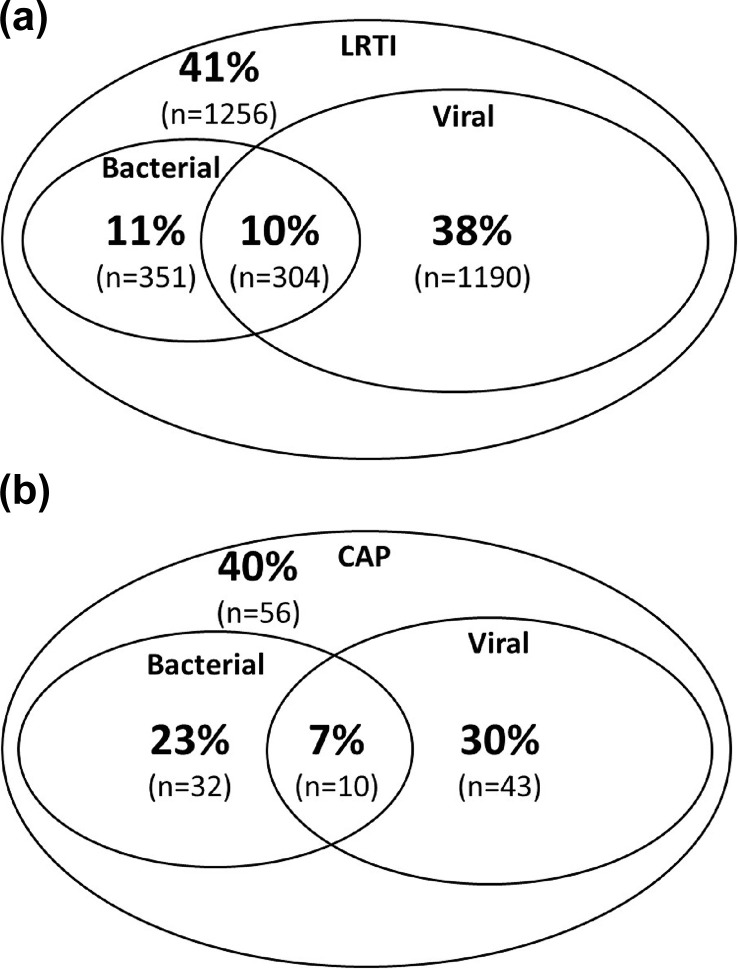
The proportion of patients with LRTI and CAP with an identified bacterial, viral or mixed aetiology is presented in Fig. 1 .
Fig. 1.
Venn diagrams of percentages (numbers) of patients with no, a bacterial, a viral or a mixed bacterial and viral aetiology detected in (a) 3104 patients with lower respiratory tract infections (LRTI) and in (b) 141 patients with community-acquired pneumonia (CAP) in primary care.
Bacterial aetiology and resistance in S. pneumoniae and H. influenzae
A potential bacterial pathogen was found in 655 (21.1%) LRTI patients on day 1, significantly more often in patients with CAP compared with those without (Fig. 1 and Table 2 ). Streptococcus pneumoniae and H. influenzae were significantly more prevalent in patients presenting with CAP. Only 9.2% of all 3104 patients and 10.6% of CAP patients were vaccinated against S. pneumoniae. Prevalence of pneumococci in these groups was 4.9% and 0%, respectively.
Table 2.
Organisms detected in patients with LRTI, LRTI with CAP and LRTI without CAP
| Organisms | LRTI (n = 3104) |
LRTI with CAP (n = 141) |
LRTI without CAP (n = 2963) |
p-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Bacteria | ||||
| Streptococcus pneumoniae | 172 (5.5) | 13 (9.2) | 159 (5.4) | 0.043 |
| Haemophilus influenzae | 167 (5.4) | 20 (14.2) | 147 (5.0) | <0.001 |
| Mycoplasma pneumoniae | 150 (4.8) | 6 (4.3) | 144 (4.9) | 0.738 |
| Chlamydia pneumoniae | 165 (5.3) | 7 (5.0) | 158 (5.3) | 0.843 |
| Bordetella pertussis | 95 (3.1) | 4 (2.8) | 91 (3.1) | 1.000a |
| Legionella pneumophila | 6 (0.2) | 1 (0.7) | 5 (0.2) | 0.236a |
| Any of the above bacteria | 655 (21.1) | 42 (29.8) | 613 (20.7) | 0.010 |
| Viruses | ||||
| Rhinovirus | 623 (20.1) | 20 (14.2) | 603 (20.4) | 0.066 |
| Influenza virus A/B | 307 (9.9) | 11 (7.8) | 296 (10.0) | 0.378 |
| Coronavirus | 231 (7.4) | 6 (4.3) | 225 (7.6) | 0.134 |
| Respiratory syncytial virus | 144 (4.6) | 4 (2.8) | 140 (4.7) | 0.289 |
| Human metapneumovirus | 138 (4.4) | 9 (6.4) | 129 (4.4) | 0.264 |
| Parainfluenza viruses 1–4 | 81 (2.6) | 4 (2.8) | 77 (2.6) | 0.786a |
| Human adenovirus | 41 (1.3) | 5 (3.5) | 36 (1.2) | 0.037a |
| Polyomaviruses | 69 (2.2) | 2 (1.4) | 69 (2.3) | 0.769a |
| Bocavirus | 18 (0.6) | 0 (0.0) | 18 (0.6) | 1.000a |
| Any of the above viruses | 1494 (48.1) | 53 (37.6) | 1441 (48.7) | 0.010 |
Abbreviations: CAP, community-acquired pneumonia; LRTI, lower respiratory tract infection.
Fisher's Exact test.
Twenty-four of 172 (14.0%) had a reduced susceptibility to penicillin G (one isolate highly resistant, 23 (13.4%) intermediate resistance). Thirty-six (20.9%) isolates were less susceptible to erythromycin/clindamycin, 78 (45.3%) had a reduced susceptibility to tetracycline and 3 (1,7%) were resistant to levofloxacin. Twenty-one of 167 (12.6%) H. influenzae isolates produced β-lactamases.
Viral aetiology
Any viral aetiology was identified in 1494 (48.1%) of LRTI patients, significantly less often in those with CAP compared with those without CAP (Fig. 1 and Table 2, Table 3 ). The commonest viruses in our cohort of patients were human rhinovirus (HRV), influenza virus and human coronavirus (HCoV). A respiratory virus was detected on days 28–35 in 336 patients (12.6%), as well as in 205 (9.9%) of the matched controls. All respiratory viruses, except for human adenovirus, human bocavirus (HBoV) and WU polyomavirus and KI polyomavirus, were significantly more frequently detected in day 1 NPS of LRTI patients than in their days 28–35 NPS or in the NPS of their matched controls (Table 3). Apart from human adenovirus, virus prevalence did not differ significantly between patients with CAP or with LRTI.
Table 3.
Viruses detected in LRTI patients and their matched controls
| Organism, n/total (%) | Patients with LRTI, |
Matched controls |
|||
|---|---|---|---|---|---|
| Day 1 (n = 3104)a | Days 28–35 (n = 2673)a | p-valueb | (n = 2063)a | p-valueb | |
| Rhinoviruses | 623 (20.1) | 113 (4.2) | <0.0001 | 72 (3.5) | <0.0001 |
| Influenza virus A/B | 307 (9.9) | 11 (0.4) | <0.0001 | 7 (0.3) | <0.0001 |
| Coronaviruses | 231 (7.4) | 71 (2.7) | <0.0001 | 29 (1.4) | <0.0001 |
| Respiratory syncytial virus | 144 (4.6) | 13 (0.5) | <0.0001 | 10 (0.5) | <0.0001 |
| Human metapneumovirus | 138 (4.4) | 7 (0.3) | <0.0001 | 3 (0.1) | <0.0001 |
| Parainfluenza viruses 1–4 | 81 (2.6) | 13 (0.5) | <0.0001 | 7 (0.3) | <0.0001 |
| Adenoviruses | 41 (1.3) | 42 (1.6) | 0.328 | 23 (1.1) | 0.831 |
| Polyomavirus | 69 (2.2) | 82 (3.1) | 0.017 | 52 (2.5) | 0.060 |
| Polyomavirus WU | 44 (1.4) | 54 (2.0) | 36 (1.7) | ||
| Polyomavirus KI | 27 (0.9) | 28 (1.0) | 17 (0.8) | ||
| Bocavirus | 18 (0.6) | 11 (0.4) | 0.433 | 16 (0.8) | 0.161 |
Abbreviations: CAP, community-acquired pneumonia; LRTI, lower respiratory tract infection.
Denominator varies per aetiological agent due to ‘not tested’ in max 0.6% on days 1 and 3.7% of samples on days 28–35 and missing data in controls.
The Generalized Estimating Equations took clustering of Day 1 and Days 28–35 samples within the same patients and clustering of Day 1 samples of patients and their matched controls into account.
In all, 23.6% of all LRTI patients and 29.1% of CAP patients were vaccinated against influenza virus. Prevalence of influenza virus in these groups was 5.3% and 4.9%, respectively.
Detection of atypical bacterial agents or viruses at follow up within the same patient
Casewise analysis of atypical bacterial agents or viruses detected during illness compared with subsequent detection at follow up is presented in Table 4 . None of the patients who were initially PCR positive for M. pneumoniae, B. pertussis, influenza virus or human parainfluenza viruses 1–4 remained positive for these aetiologies at follow up. Very few patients positive for HRV, HCoV, respiratory syncytial virus, human metapneumovirus (HMPV), polyomaviruses (WU+KI) and HBoV had the same pathogen detected at follow up.
Table 4.
Viruses and atypical bacteria detected at baseline (acute phase of illness) and at follow up within the same patient
| Virus or atypical detection by PCR | Baseline (acute illness) n (%) of total (n = 3104) | At follow up n (%) of positives during the acute phase |
|---|---|---|
| Bacteria | ||
| Mycoplasma pneumoniae | 31 (1.0) | 0 (0.0) |
| Chlamydia pneumoniae | 26 (0.8) | 1 (3.8) |
| Bordetella pertussis | 39 (1.3) | 0 (0.0) |
| Viruses | ||
| Rhinovirus | 623 (20.1) | 27 (4.3) |
| Influenza virus A/B | 307 (9.9) | 0 (0.0) |
| Coronaviruses | 231 (7.4) | 4 (1.7) |
| Respiratory syncytial virus | 144 (4.6) | 1 (0.7) |
| Human metapneumovirus | 138 (4.4) | 1 (0.7) |
| Parainfluenza viruses 1–4 | 81 (2.6) | 0 (0.0) |
| Adenoviruses | 41 (1.3) | 2 (4.9) |
| Polyomaviruses (WU+KI) | 69 (2.2) | 2 (2.9) |
| Bocavirus | 18 (0.6) | 1 (5.6) |
Mixed infections
Among all 3104 LRTI patients, a mixed bacterial, mixed viral or mixed bacterial–viral infection was detected in 51 (1.6%), 118 (3.8%) and 304 (9.8%) patients, respectively. The pathogens involved are described in more detail in the Supplementary material.
Discussion
This is the only prospective, large international, case–control study using standardized sampling and comprehensive microbiological work up to provide accurate estimates of the prevalence of both bacterial and viral aetiology in patients consulting with LRTI in primary care. The overall microbiological yield was high, mainly due to the high prevalence of viruses. A potential bacterial pathogen was isolated in only one in five patients, and antibiotic-resistant pathogens were rare.
Comparison with literature
Previous studies have mainly studied more severely ill patients hospitalized with CAP rather than LRTI in primary care [1], [10], [11], [12], and few of those studies used comprehensive diagnostic methods, including PCR, to detect respiratory viruses [10], [12], [13]. We identified a potential pathogen in about 60% of CAP patients. However, comparisons are difficult in that our study is unique in terms of study design, the broad inclusion criteria, the high numbers of patients sampled at baseline and follow up, the inclusion of matched controls, and the comprehensive conventional and molecular microbiological diagnostics used.
Bacterial aetiology and resistance in CAP
The prevalence of S. pneumoniae and H. influenzae in our CAP subgroup was significantly higher than in the non-CAP patients, but lower in comparison to most previous studies. We do not consider that the implementation of pneumococcal vaccine influenced our findings because of the small number of CAP patients who had been vaccinated. However, only 5% of patients in the most comprehensive aetiological study of adult patients hospitalized with CAP in the USA had pneumococcal pneumonia [14]. High-level penicillin resistance in pneumococci remains very low in all European countries in this setting, which supports the recommendation that if antibiotics are to be prescribed, amoxicillin should be the first-line agent for LRTI [1]. Mycoplasma pneumoniae infections occur in epidemics every 4–5 years: we included patients in our study between two epidemic waves, possibly explaining the low M. pneumoniae prevalence observed [15], [16]. This is also the first large European prospective study on the prevalence of pertussis in adults consulting primary care physicians for acute cough [17].
Importance of respiratory viruses, including newly detected viruses
We detected at least one respiratory viral pathogen in almost 50% of patients. NPS sampling may have yielded significantly more infected respiratory epithelial cells [18], with sensitive PCR-based diagnostic techniques augmenting specifically for viruses.
Influenza virus, human parainfluenza viruses 1–4 and respiratory syncytial virus are recognized causes of CAP in hospitalized patients and in the elderly [13] Influenza vaccination resulted in lower prevalence of influenza virus in the elderly (data not shown). HRV, HCoV and HMPV are rarely detected in CAP and other LRTI in outpatients.
HRV has been associated with outbreaks of severe respiratory disease, including CAP, in older people [19], [20], [21] and has been isolated in hospitalized patients with CAP [10], but a prevalence of 14.2% in CAP in outpatients is high and a novel finding. HCoV have recently been identified in small numbers of adults with severe pneumonia [10], [21], but is not routinely tested for in adult outpatients with CAP or LRTI. We may have underestimated the prevalence of HCoV as HKU-1 testing was not performed and HKU-1 is generally as prevalent as NL63 and OC43 [22]. Infections due to HMPV are mainly described in long-term care facilities [10]. We found HMPV more prevalent in outpatients with CAP compared with those with other LRTIs, with even greater prevalence in CAP patients than respiratory syncytial virus, and similar to the 3%–7% HMPV infection prevalence found in hospitalized adults [23]. Although numbers are small, human adenovirus was significantly more prevalent in CAP compared with other LRTI, a unique finding in immunocompetent outpatients. The high rates of viral detection in outpatients with LRTI and CAP suggests that comprehensive microbiological assessment is important to guide management and may explain the limited average benefit from antibiotic treatment in the placebo-controlled study that we conducted in a large subset of patients included in the present analysis [2].
Our study is the first that compared the prevalence of respiratory viruses in symptomatic adults with that in matched controls without respiratory symptoms. Influenza virus, human parainfluenza viruses 1–4 and respiratory syncytial virus were never, or rarely, detected in controls or at follow up in symptomatic patients. This strongly implicates these agents as causative pathogens. Similarly, the significantly lower prevalence of HRV, HMPV and HCoV in patients at follow up and in controls suggests that asymptomatic carriage of these viruses is uncommon in adults, and indicates that these viruses should also be regarded as causative agents in CAP [11], [14], [23].
For HRV, the rates of prolonged shedding (same genotype in 35%) versus re-infection (other genotype) in the GRACE study have been further investigated [24].
Human bocavirus was detected in CAP and in <1% of LRTI patients at baseline, with similar findings among controls and at follow up of patients. HBoV was identified in respiratory specimens from 1.5% of hospitalized adults with no alternative viral aetiology, but controls were not included in that study [25]. As HBoV is often found in the presence of other pathogens in respiratory specimens we agree that HBoV probably has no relevance or primary role as a causative agent in LRTI in primary care [26]. There may be an association between high HBoV viral loads and HBoV being the only virus detected [27], suggesting that a quantitative approach should be considered [26].
This also applies to KI polyomavirus and WU polyomavirus. Although it is not yet possible to draw firm conclusions on their role in human pathology [28], [29], [30], our data show no evidence for a causative role in outpatient CAP or LRTI—assessment of the viral loads could potentially help to further clarify their significance.
Limitations
Sputum was not obtained from all patients, and sputa and follow-up serology were not obtained from control patients. Consequently, a valid estimation of the prevalence of bacterial pathogens in controls was not always possible. Although the most important elements of this study are the descriptive results, we also performed multiple statistical tests so the finding of statistical significance may reflect type I error. However this is much less likely when supporting prior work on aetiology (e.g. bacterial causes of CAP) or when the p-value is very small (e.g. the case–control comparisons of viral aetiology).
Conclusions and implications for future management of LRTI
This unique comprehensive prospective study using modern microbiological methods suggests that the traditional view of aetiology in CAP and outpatient LRTI should be revised. We have found that viral CAP and LRTI are also caused by HRV, HCoV and HMPV. Our high viral detection rates should also inform clinical decision making. Better diagnostics are needed to distinguish viral from bacterial CAP or LRTI at the point of care.
The current study provides microbiological evidence for why antibiotics do not help patients with LRTI. Only approximately one in five LRTI patients have a bacterial pathogen isolated and so could conceivably benefit from antibiotic treatment. This evidence should support primary care clinicians' restrictive approach to antibiotic prescribing for LRTI. If they consider antibiotics are indeed indicated, the low resistance levels in S. pneumoniae and H. influenzae should support the prescription of narrow-spectrum antibiotics.
Transparency declaration
We declare that we have no conflict of interest. Part of this work was presented at the ECCMID, Berlin, Germany, 27–30 September 2013 (S-557).
Contributors
The larger GRACE observational study was designed by CCB, TV, PL, SC and HG, and sampling protocols by MI, CL, KL and HG. MI, CL, PL, TV and HG supervised the day-to-day management at study sites. PCR and serological analyses were performed by KL, AV, CL, KH, KZ, EC, FC and AVL. Data were analysed by MI, KL and CL. Statistical analysis was performed by SC.The manuscript was drafted by MI, KL, SC, HG and CCB and was reviewed by all authors.
Acknowledgements
We thank the GPs, the GRACE study team, and the patients for taking part in this study.
Editor: L. Leibovici
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cmi.2018.02.004.
Funding
GRACE (Genomics to combat Resistance against Antibiotics in CA-LRTI in Europe, www.grace-lrti.org) was supported by the Research Foundation Flanders (Belgium) (G·0274·08N) and the 6th Framework Program of the European Commission, contract no. LSHM-CT-2005-518226). The work reported in this publication has been financially supported through the European Science Foundation (ESF), in the framework of the Research Networking Programme TRACE (http://archives.esf.org/trace; 09-RNP-053). The funding sources were not involved in the design, conduct, analysis and interpretation of the data, nor in the writing and decision to submit the paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Woodhead M., Blasi F., Ewig S., Garau J., Huchon G., Ieven M. Guidelines for the management of adult lower respiratory tract infections–full version. Clin Microbiol Infect. 2011;17(Suppl. 6):E1–E59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little P., Stuart B., Moore M., Coenen S., Butler C.C., Godycki-Cwirko M. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis. 2013;13:123–129. doi: 10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 3.Moore M., Stuart B., Coenen S., Butler C.C., Goossens H., Verheij T.J. Amoxicillin for acute lower respiratory tract infection in primary care: subgroup analysis of potential high-risk groups. Br J Gen Pract. 2014;64:e75–80. doi: 10.3399/bjgp14X677121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler C.C., Hood K., Verheij T., Little P., Melbye H., Nuttall J. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malhotra-Kumar S., Van Heirstraeten L., Coenen S., Lammens C., Adriaenssens N., Kowalczyk A. Impact of amoxicillin therapy on resistance selection in patients with community-acquired lower respiratory tract infections: a randomized, placebo-controlled study. J Antimicrob Chemother. 2016;71:3258–3267. doi: 10.1093/jac/dkw234. [DOI] [PubMed] [Google Scholar]
- 6.van Vugt S.F., Broekhuizen B.D., Lammens C., Zuithoff N.P., de Jong P.A., Coenen S. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ. 2013;346:f2450. doi: 10.1136/bmj.f2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loens K., van Loon A.M., Coenjaerts F., van A.Y., Goossens H., Wallace P. Performance of different mono- and multiplex nucleic acid amplification tests on a multipathogen external quality assessment panel. J Clin Microbiol. 2012;50:977–987. doi: 10.1128/JCM.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Melker H.E., Versteegh F.G., Conyn-Van Spaendonck M.A., Elvers L.H., Berbers G.A., van der Z.A. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol. 2000;38:800–806. doi: 10.1128/jcm.38.2.800-806.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huygen K., Rodeghiero C., Govaerts D., Leroux-Roels I., Melin P., Reynders M. Bordetella pertussis seroprevalence in Belgian adults aged 20–39 years. Epidemiol Infect. 2012;2014(142):724–728. doi: 10.1017/S0950268813002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creer D.D., Dilworth J.P., Gillespie S.H., Johnston A.R., Johnston S.L., Ling C. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax. 2006;61:75–79. doi: 10.1136/thx.2004.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson N., Kalin M., Tiveljung-Lindell A., Giske C.G., Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50:202–209. doi: 10.1086/648678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieberman D., Shimoni A., Shemer-Avni Y., Keren-Naos A., Shtainberg R., Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010;138:811–816. doi: 10.1378/chest.09-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalker V.J., Stocki T., Mentasti M., Fleming D., Sadler C., Ellis J. Mycoplasma pneumoniae infection in primary care investigated by real-time PCR in England and Wales. Eur J Clin Microbiol Infect Dis. 2011;30:915–921. doi: 10.1007/s10096-011-1176-3. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen J.N., Voldstedlund M., Andersen R.L., Ellermann-Eriksen S., Jensen T.G., Johansen H.K. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010;15(45) pii:19708. [PubMed] [Google Scholar]
- 17.Teepe J., Broekhuizen B., Ieven M., Loens K., Huygen K., Kretzschmar M. Prevalence, diagnosis, and disease course of pertussis in adults with acute cough in primary care. Br J Gen Pract. 2015;65:e662–e667. doi: 10.3399/bjgp15X686917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernes S.S., Quarsten H., Hagen E., Lyngroth A.L., Pripp A.H., Bjorvatn B. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011;30:159–165. doi: 10.1007/s10096-010-1064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg S.B. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2007;28:182–192. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- 20.Hicks L.A., Shepard C.W., Britz P.H., Erdman D.D., Fischer M., Flannery B.L. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geriatr Soc. 2006;54:284–289. doi: 10.1111/j.1532-5415.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 21.Falsey A.R., McElhaney J.E., Beran J., van Essen G.A., Duval X., Esen M. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209:1873–1881. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlateva K.T., Crusio K.M., Leontovich A.M., Lauber C., Claas E., Kravchenko A.A. Design and validation of consensus-degenerate hybrid oligonucleotide primers for broad and sensitive detection of corona- and toroviruses. J Virol Methods. 2011;177:174–183. doi: 10.1016/j.jviromet.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falsey A.R., Criddle M.C., Walsh E.E. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol. 2006;35:46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Zlateva K.T., de Vries J.J., Coenjaerts F.E., van Loon A.M., Verheij T., Little P. Prolonged shedding of rhinovirus and re-infection in adults with respiratory tract illness. Eur Respir J. 2014;44:169–177. doi: 10.1183/09031936.00172113. [DOI] [PubMed] [Google Scholar]
- 25.Chow B.D., Huang Y.T., Esper F.P. Evidence of human bocavirus circulating in children and adults, Cleveland, Ohio. J Clin Virol. 2008;43:302–306. doi: 10.1016/j.jcv.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghietto L.M., Majul D., Ferreyra S.P., Baumeister E., Avaro M., Insfran C. Comorbidity and high viral load linked to clinical presentation of respiratory human bocavirus infection. Arch Virol. 2015;160:117–127. doi: 10.1007/s00705-014-2238-5. [DOI] [PubMed] [Google Scholar]
- 27.Schildgen O., Muller A., Allander T., Mackay I.M., Volz S., Kupfer B. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babakir-Mina M., Ciccozzi M., Perno C.F., Ciotti M. The novel KI, WU, MC polyomaviruses: possible human pathogens? New Microbiol. 2011;34:1–8. [PubMed] [Google Scholar]
- 29.Norja P., Ubillos I., Templeton K., Simmonds P. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40:307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babakir-Mina M., Ciccozzi M., Perno C.F., Ciotti M. The human polyomaviruses KI and WU: virological background and clinical implications. APMIS. 2013;121:746–754. doi: 10.1111/apm.12091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.