Abstract
During the 2015 Korean MERS outbreak, we experienced atypical presentations of MERS-CoV infections in three immunocompromised hosts that warranted exceptional management. Case 1 showed delayed symptom development after a four-day asymptomatic period, Case 2 experienced a 20-day incubation period, and Case 3 exhibited persistent viral shedding without clinical deterioration. Recognizing these exceptions is extremely important in the management of MERS-CoV-exposed or -infected patients and for control of potential MERS outbreaks.
Keywords: Middle East respiratory syndrome coronavirus, Immunocompromised host, Incubation period, Asymptomatic period, Corticosteroid
1. Introduction
Middle East respiratory syndrome virus (MERS-CoV), a novel beta coronavirus, is notorious for its high fatality of up to 60% in comorbid patients [1]. After exposure to the virus, MERS-CoV-infected patients typically present with fever and respiratory symptoms within 14-day incubation period, and the diagnosis is confirmed by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay of respiratory specimen. Severe patients rapidly develop pneumonia during the first week of illness, and progress to respiratory failure during the second week [1], [2]. During the 2015 Korean MERS outbreak, we experienced atypical presentations of MERS-CoV infections in three immunocompromised hosts, which suggest exceptional management for these hosts should be considered. All three patients were infected from the same super-spreading MERS patient at our emergency department (ED), which has been previously described [3], [4]. The clinical courses of the three patients are concisely depicted in Fig. 1 in addition to the case description below.
Fig. 1.
Clinical courses of MERS-CoV infection in three immunocompromised hosts with atypical presentation. (a) Case 1: A 42-year-old female with MDS showed delayed symptom onset after a four-day asymptomatic period. (b) Case 2: A 49-year-old female, who had received an auto-PBSCT for recurred DLBCL experienced a long incubation period of 20 days. (c) Case 3: A 34-year-old male with PTCL exhibited persistent viral shedding without clinical deterioration while using corticosteroid to control lymphoma and hemolytic anemia. Abbreviations: MERS-CoV, Middle East respiratory syndrome coronavirus; MDS, myelodysplastic syndrome; auto-PBSCT, autologous peripheral blood stem cell transplantation; DLBCL, diffuse large B-cell lymphoma; PTCL, peripheral T-cell lymphoma; rRT-PCR, real-time reverse transcriptase polymerase chain reaction; CXR, chest X-ray; BT, body temperature; Ct, threshold cycle; ICU, intensive care unit; TB, tuberculosis; Pd, prednisolone.
2. Case reports
2.1. Case 1: delayed symptom development after a four-day asymptomatic period
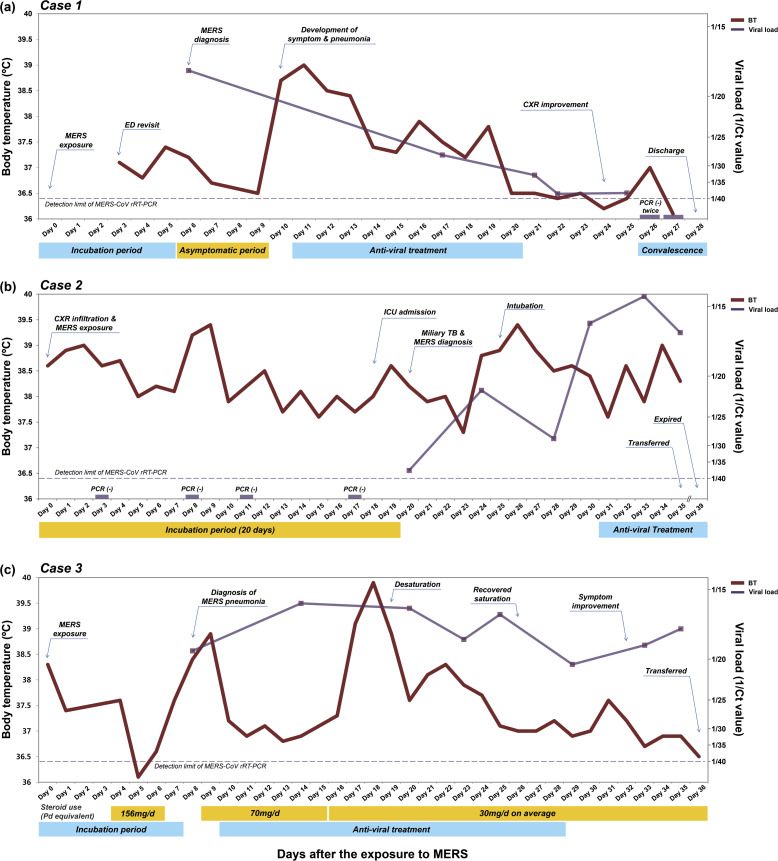
A 42-year-old female with known myelodysplastic syndrome (MDS) visited our ED with complaint of abdominal pain and menorrhagia on May 26, 2015. She had anemia (hemoglobin 7.3 g/dL), thrombocytopenia (platelet count 21,000/μL), and lymphopenia (absolute lymphocyte count 470/μL) at the ED visit. She was discharged from the ED on the next day after supportive care including red blood cell and platelet transfusion. She visited the ED again with the same symptoms on June 1, and was admitted to an isolation room because she had stayed with the MERS patient at a 223.9 m2-sized room of ED for 23 h on May 29 [3]. Although she did not have any MERS-related symptoms, she was screened for MERS-CoV infection by sputum rRT-PCR on June 4 [5]; the result was positive with a cycle threshold (Ct) value of 17.8. She remained in an asymptomatic status for four more days and received only supportive care and close monitoring. On June 8, she suddenly developed fever, myalgia, cough, and diarrhea, and her chest X-ray (CXR) showed pneumonic infiltration in left upper lobe (Fig. 2 -a). On the next day, she was treated with a combination antiviral regimen of ribavirin, lopinavir/ritonavir, and interferon alpha 2a, as the follow-up CXR demonstrated aggravated infiltrates (Fig. 2-b). After 10 days of antiviral treatment, her symptoms and CXR were markedly improved (Fig. 2-c), and she was discharged on June 26, after two consecutive negative tests by MERS-CoV rRT-PCR.
Fig. 2.
Serial chest X-ray images of Case 1. (a) Chest X-ray taken on dpex 10 showed pneumonic infiltration in left upper lobe (arrow). (b) New infiltration appeared in left lower lobe on dpex 17 (arrow). (c) Pneumonic infiltrations were markedly improved on dpex 24. Abbreviations: dpex, days post exposure.
2.2. Case 2: delayed development of MERS after a 20 day incubation period
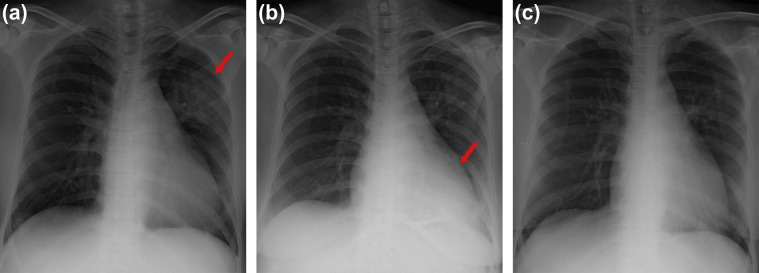
A 49-year-old female, who had received autologous peripheral blood stem cell transplantation (auto-PBSCT) two months prior for recurred diffuse large B-cell lymphoma (DLBCL), visited our ED with complaint of fever and dry cough on May 27. As her initial laboratory tests showed pancytopenia (white blood cell count 1430/μL with neutrophil 56.6% and lymphocyte 34.3%, hemoglobin 7.6 g/dL, and platelet count 32,000/μL), she was empirically treated with piperacillin/tazobactam for febrile neutropenia. On May 29, her CXR showed bilateral haziness with micro-nodular infiltrations, which was not previously observed. On the same day, she was also exposed to the MERS patient at a 223.9 m2-sized room of ED for 23 h. Her fever persisted and infiltration on CXR worsened despite escalation of antibiotics (Fig. 3 -a). On suspicion of MERS-CoV infection, her sputa were repeatedly tested by MERS-CoV rRT-PCR four times from June 1 to June 15, all of which were negative. On June 16, she was admitted to the intensive care unit (ICU) due to severe dyspnea, metabolic acidosis and mental change (Fig. 3-b). On June 18, she was diagnosed with miliary tuberculosis by chest computed tomography (CT) and sputum PCR for Mycobacterium tuberculosis complex, and anti-tuberculosis medication was started. Meanwhile, her follow-up sputum rRT-PCR for MERS-CoV was equivocal (Ct value of 37.1, with Ct values below 35 considered positive [5]) on the same day, converting to positive with a Ct value of 21.6 on June 22. She was intubated for mechanical ventilation the next day (Fig. 3-c). Despite worsening CXR and oxygenation, combination antiviral treatment of ribavirin, lopinavir/ritonavir, and interferon alpha 2a could not be started until June 29 due to pancytopenia and hepatic dysfunction. On June 30, mycobacterial culture of her sputum reported growth of M. tuberculosis. She was transferred to a national hospital designated for the treatment of infectious disease on July 3 [4], and expired on July 7.
Fig. 3.
Serial chest X-ray images of Case 2. (a) Chest X-ray taken on dpex 14. Bilateral micro-nodular infiltration worsened despite antibiotic treatment. (b) On dpex 18, the patient was admitted to ICU due to severe dyspnea. Chest X-ray showed aggravated infiltration in left upper and lower lobe (arrow). (c) Chest X-ray taken on dpex 25 after endotracheal intubation. Diffuse infiltration of left lung was markedly aggravated (arrow). Abbreviations: dpex, days post exposure; ICU, intensive care unit.
2.3. Case 3: persistent viral shedding without clinical deterioration
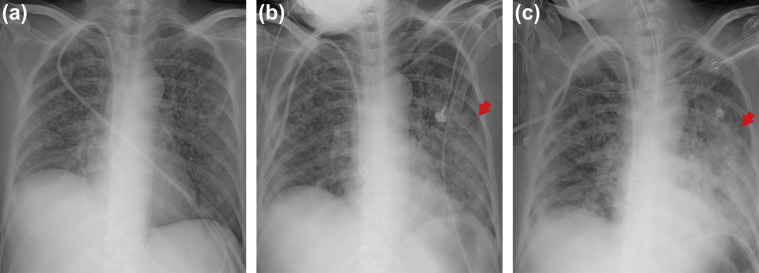
A 34-year-old male, who had received an auto-PBSCT six months prior due to peripheral T cell lymphoma (PTCL) visited our ED with complaint of cough, sputum and mild fever on May 27, and shared the same radiology room at the same time period (30 min before and 2 h after) with the MERS patient on the next day. His chest CT revealed mild infiltration of both lower lobes that was not apparent on CXR. On the diagnosis of community-acquired pneumonia, he was discharged with oral antibiotics after two days of intravenous antibiotic treatment. However, his fever persisted and lymphoma-related fever was suspected. On June 1, he was admitted to the general ward to evaluate the status of his lymphoma, including PET-CT and bone marrow examination, which revealed recurrence of PTCL. To suppress lymphoma activity, 125 mg/day of methylprednisolone was administered for three days, and his fever subsided. After steroids were stopped, fever, cough and sputum were aggravated, and CXR showed newly developed infiltration of both upper lobes (Fig. 4 -a). Based on his contact history with the MERS patient at the ED, he was tested for MERS-CoV rRT-PCR on June 5, which was positive with a Ct value of 19.2. From June 6, 70 mg/day of oral prednisone was re-administered for one week to control autoimmune hemolytic anemia; and then tapered to 30 mg/day on average thereafter. The combination antiviral agents of ribavirin, lopinavir/ritonavir, and interferon alpha 2a were started on June 7, and maintained for 20 days. Although he experienced symptom aggravation during the initial two weeks followed by relief, pneumonic infiltration on CXRs was sustained (Fig. 4-b and c), and viral shedding continued with high viral loads for one month. He was transferred to a national hospital designated for the treatment of infectious disease on July 3.
Fig. 4.
Serial chest X-ray images of Case 3. (a) On dpex 8, new infiltrations of both upper lobes were observed (arrows). (b) Chest X-ray taken on dpex 23. Both upper lobe lesions were markedly aggravated. (c) Chest X-ray taken dpex 32. Although symptom and vital signs improved after antiviral treatment, pneumonic infiltration on chest X-ray was sustained. Abbreviations: dpex, days post exposure.
3. Discussion
Although many clinical studies about MERS-CoV infection have been reported to date, it is not well understood whether viral shedding of MERS-CoV precedes symptom onset. Likewise, the likelihood of progression of asymptomatic rRT-PCR-positive case to symptomatic illness is also unclear [6]. During the 2015 Korean outbreak, most patients were diagnosed with MERS-CoV infection in the presence of symptoms, while asymptomatic rRT-PCR-positive persons did not progress to symptomatic illness [2], [4]. However, Case 1, who had underlying MDS, progressed to MERS pneumonia after a four-day asymptomatic period. Whereas MERS-CoV infections generally show step-wise progression, i.e., exposure, symptom development with viral shedding, pneumonia development, and respiratory failure [2], Case 1 showed pneumonic infiltration on CXR together with symptom development. This simultaneous development of symptoms and pneumonia suggests that only symptom expression might be delayed or masked rather than shown as a lag in overall disease progression. Case 1 implies that MERS-exposed immunocompromised hosts should be properly isolated and closely monitored even if they do not initially complain of symptoms.
The incubation period of MERS-CoV is known to be within 14 days after exposure, and contact investigation and monitoring have typically been performed based on this standard [1], [4], [7]. However, Case 2 showed a longer incubation period of 20 days, probably owing to suppressed immune status due to multiple courses of chemotherapy and auto-PBSCT. This case suggests that the incubation period in immunocompromised hosts could be longer than 14 days. Although the patient experienced persistent fever and progressive consolidation on CXR due to existing tuberculosis, her sputum rRT-PCRs for MERS-CoV were consecutively negative until post exposure day (dpex) 17. Moreover, her first non-negative rRT-PCR on dpex 20 showed only an equivocal result, while follow-up tests revealed increasing viral loads. This progression implies that viral shedding started around dpex 20, indicating an incubation period of 20 days. Even though the possibility of false negativity of earlier tests due to inadequate specimens cannot be excluded, recognizing the potential for such exceptionally long incubation period in immunocompromised hosts is extremely important in epidemiologic investigations and infection control in real world. These patients should be monitored for at least three weeks after exposure to MERS, and contact tracing should be based on exposures within three weeks.
Based on the experience of other severe acute respiratory infections, including severe acute respiratory syndrome coronavirus (SARS-CoV), high-dose corticosteroids are recommended to be avoided in the management of MERS-CoV infection [8]. Case 3 used prolonged corticosteroid therapy to control lymphoma activity and hemolytic anemia, which probably contributed to persistent viral shedding without clinical progression of the disease. The inevitable use of corticosteroid hampered not only recovery of the patient, but also control of transmission. His viral shedding persisted with Ct values under 20, which is far below the mean value of the spreaders of 2015 Korean MERS outbreak [9]. As high viral load is a risk factor for transmission [9], appropriate airborne infection isolation room and careful wearing and removal of PPE is required in managing such patients. Considering these aspects, patients who cannot avoid corticosteroid therapy should be aggressively managed from symptom onset, including experimental therapeutic measures such as antiviral therapy and convalescent plasma infusion therapy [10]. Likewise, use of other immunosuppressive agents should also be cautious, and aggressive management of the patient should be considered if such use is unavoidable.
We presented atypical presentations of MERS-CoV infection in three immunocompromised hosts. Recognizing these exceptions is extremely important in the management of MERS-CoV-exposed or -infected patients and for control of potential MERS outbreak in the future.
Funding
This work was supported by a Samsung Biomedical Research Institute (SBRI) grant [#SMX1161321].
Conflicts of interest
None.
Acknowledgements
We would like to express our sincerest condolences to the patients and families who suffered from the MERS outbreak. We also greatly appreciate the efforts of the health care personnel and staff members at Samsung Medical Center and all other hospitals who worked together to overcome the MERS outbreak.
References
- 1.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko J.H., Park G.E., Lee J.Y., Lee J.Y., Cho S.Y., Ha Y.E., et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016 Nov;73(5):468–475. doi: 10.1016/j.jinf.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho S.Y., Kang J.M., Ha Y.E., Park G.E., Lee J.Y., Ko J.H., et al. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park G.E., Ko J.H., Peck K.R., Lee J.Y., Lee J.Y., Cho S.Y., et al. Control of an outbreak of Middle East respiratory syndrome in a tertiary hospital in Korea. Ann Intern Med. 2016;165:87–93. doi: 10.7326/M15-2495. [DOI] [PubMed] [Google Scholar]
- 5.Huh H.J., Ko J.H., Kim Y.E., Park C.H., Hong G., Choi R., et al. Importance of specimen type and quality in diagnosing Middle East respiratory syndrome. Ann Lab Med. 2017;37:81–83. doi: 10.3343/alm.2017.37.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Management of asymptomatic persons who are RTPCR positive for Middle East respiratory syndrome coronavirus (MERS-CoV) Interim Guidance. WHO; 27 July 2015. http://www.who.int/csr/disease/coronavirus_infections/management_of_asymptomatic_patients/en/ Accessed 23 November 2016. [Google Scholar]
- 7.Investigation of cases of human infection with Middle East respiratory syndrome coronavirus (MERS-CoV) Interim Guidance. WHO; 3 July 2015. http://www.who.int/csr/disease/coronavirus_infections/mers-investigation-cases/en/ Accessed 23 November 2016. [Google Scholar]
- 8.Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus (MERS-CoV) infection is suspected, Interim guidance. WHO; July 2015. http://www.who.int/csr/disease/coronavirus_infections/case-management-ipc/en/ Accessed 23 November 2016. [Google Scholar]
- 9.Kim S.W., Park J.W., Jung H.D., Yang J.S., Park Y.S., Lee C., et al. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin Infect Dis. 2016 Dec 10 doi: 10.1093/cid/ciw768. pii: ciw768 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo Y., Fisher D. A review of treatment modalities for Middle East respiratory syndrome. J Antimicrob Chemother. 2016;71:3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]