Graphical abstract
Keywords: Lamicaeae, Herbs, Antiviral, Antiretroviral, Anti-HIV
Abstract
Constant search for new drugs with antiviral properties often extends to products of natural origin. Lamiaceae is one of the most important herbal families, well known for various biological and medicinal effects of a variety of aromatic spices, including thyme, mint, oregano, basil, sage, savory, rosemary, self-heal, hyssop, lemon balm and many others. The paper provides a review of antiviral potential of previously mentioned plants which has been demonstrated so far, with special emphasis on anti-HIV properties. Relevant articles were compiled by searching plant names combined with keywords describing antiviral activity. The antiviral effect is direct, with prominent activity against enveloped viral species. Initial stages of the viral life cycle are the most affected, as these plants appear to be targeting mainly viral structures responsible for attachment to target cells. In case of HIV, there is some activity against key enzymes in the viral life cycle. Even in the case of drug resistance, there is an equal susceptibility to applied herbal preparations. Some in vivo experiments suggest that use of Lamiaceae representatives could help in prevention and treatment of some viral diseases. A possible reduction of side effects of diseases and conventional drug therapy are also some aspects worth further investigations.
1. Introduction
Modern therapy of human immunodeficiency virus (HIV) infections and acquired immunodeficiency syndrome (AIDS) is called combined antiretroviral therapy (cART). As the name suggests, the therapy combines at least three drugs from which at least two are classified in different groups (Table 1 ) based on their mechanism of activity (M-o-A) [1].
Table 1.
Groups of antiretroviral drugs based on the mechanism of activity.
| Name of the group | Mechanism of activity (M-o-A) | Some representatives of the drug group | |
|---|---|---|---|
| 1. | nucleoside-analog reverse transcriptase inhibitors (NRTIs)–analogues of nucleoside substrates | drugs targeting reverse transcriptase (RT) of HIV via active site needed for transcription of RNA into DNA | abacavir, didanosine, emtricitabine, lamivudine, stavudine, zalcitabine, zidovudine, tenofovir |
| 2. | non-nucleoside reverse transcriptase inhibitors (NNRTIs) | drugs targeting RT via allosteric modifications of a selected group of enzymes (affecting HIV-1, and not affecting HIV-2) | etravirine, delavirdine, efavirenz, nevirapine |
| 3. | integrase inhibitors (IIs) | drugs targeting integrase, the enzyme responsible for integration of viral DNA into host DNA | raltegravir, elvitegravir |
| 4. | protease inhibitors (PIs) | the target is an enzyme responsible for virion maturation | amprenavir, atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, tipranavir |
| 5. | fusion inhibitors (FIs) | inhibitors of six helix bundle of gp41, a subunit of protein responsible for fusion of HIV particles with host cells | enfuvirtide |
| 6. | co-receptor antagonists | drugs targeting interaction of HIV gp120 and chemokine receptors via allosteric modification | maraviroc |
Unfortunately, cART is associated with numerous and various adverse effects in patients, ranging from mild to life-threatening [2]. Furthermore, resistance to almost all groups of drugs could occur in both HIV-1 and HIV-2 [3]. Finally, the therapy is not available to everyone, reaching an estimated 46% of infected people [4]. Bearing all of that in mind, it is clear why a constant search for safe, efficient and cheap drugs with significant anti-HIV effects is required.
So far, numerous studies have been conducted in order to evaluate antiviral properties of mainly medicinal and aromatic herbs, but also plants not used in traditional medicine [5]. Different plant-based products and isolated compounds of natural origin exhibited antiviral effects against various viruses, including HIV [6]. Different classes of bioactive compounds from plants, including flavonoids, coumarins, polyphenols, tannins, terpenoids, essential oils, alkaloids, polysaccharides and proteins were tested and proven to be active against a wide range of viral species [7].
The use of medicinal plants has numerous advantages. It was determined that HIV patients who opt for herbal treatment along side the conventional one have lower mortality, longer life expectancy and better life quality than the patients using only conventional therapy [8,9]. Plants also have the potential to reduce the harmful effects of drugs [10]. Furthermore, there are some reports of case studies of people using only plants as therapy for HIV, who managed to keep the viral load undetectable during or after applied phytotherapy [11,12]. Although plants and compounds discovered so far cannot replace conventional drugs, these reports provide a starting point for further examination of activity of some plants and their possible aplication in adjunctive therapy.
However, there are not many published papers that comprehensively present the data related to antiretroviral effects of particular groups of plants. Therefore, in this paper, the results of conducted studies on antiviral properties of selected genera and species of Lamiaceae family are summarized. The focus is set specifically on anti-HIV properties with possible or confirmed M-o-A, but the activities against other viruses are also described, with the purpose of providing supplementary data on antiviral properties.
Relevant articles were searched for through the PubMed database and Google Scholar, by using a combination of Latin or English names of selected plants with words “antiviral”, “antiretroviral”, “anti-HIV”, “HIV” and “antimicrobial”. The content of discovered articles was evaluated to determine their suitability for our topic. Afterwards, the references of the selected articles were examined for additional relevant literature. Only articles in the English language, with full text available were included.
2. Lamiaceae family
Lamiaceae (Labiatae) plant family is the sixth largest plant family, with >200 genera and >7000 species. Plants belonging to this family are dispersed worldwide and considered as some of the least difficult plants to cultivate. Coupled with easiness of cultivation, their aromatic, spicy properties make them an important part of pharmaceutical, food and cosmetic industries [13]. Plants discussed in this paper are herbaceous, so the parts most frequently used are leaves and herba, upper parts of the segment of the plant, mostly collected in full blossom. They can be used as fresh or dried material (as it is the case in culinary and food industries), but also in dosage forms specially prepared for external and internal use. Traditional forms of application for medicinal purposes include infusum (commonly called tea- form obtained by pouring nearly boiling water over the plant material), syrup, tincture, gargle (mouthwash), drops and many others. Besides drinking and using as mouthwash, these forms can be applied by rubbing and inhaling as well [[14], [15], [16]]. Some of the mainly used Lamiaceae plants are presented in Table 2 . Beside their traditional use, many of them are also accepted in conventional medicine. For this reason, the World Health Organization suggests that plants such as some Lamiaceae family representatives, with a long history of use and confirmed therapeutic effects should be further evaluated for new activities and properties that could be useful in treatment and prevention of different diseases [17].
Table 2.
Some of the Lamiaceae plants discussed in the paper and their use in traditional and conventional medicine.
| Latin name | Common name | Some of traditional uses | Reference | Use in conventional medicine, approved by relevant institutions | Reference |
|---|---|---|---|---|---|
| Thymus vulgaris | garden thyme | antiseptic, carminative, eupeptic | [18] | bronchoantispasmodic, expectorant, antibacterial | [19,20,21] |
| Thymus serpyllum | wild thyme | antiseptic, spasmolytic, carminative, expectorant, sedative | [15] | antimicrobial | [19] |
| Mentha × piperita | peppermint | eupeptic, cholagogue, carminative, spasmolytic | [22] | antispasmodic, carminative, cholagogue, secretolytic, eupeptic (internally), cooling agent for myalgia and headache (externally) | [19,23,24] |
| Rosmarinus officinalis | rosemary | cholagogue, eupeptic, irritant, food additive | [16] | eupeptic, antispasmodic (internally), in rheumatic diseases and circulatory problems (externally) | [19,25,26] |
| Melissa officinalis | lemon balm | mild sedative, cholagogue, carminative | [27] | sedative, hypnotic, carminative (internally), symptomatic treatment of herpes labialis (externally) | [19,24,28] |
| Origanum vulgare | oregano | antiparasitic, antihelmintic, cholagogue, carminative, spasmolytic | [29] | – | |
| Origanum majorana | marjoram | antispasmodic (internally), relief of irritated skin around nostrils (externally) | [30] | ||
| Ocimum basilicum | sweet basil | spasmolytic, eupeptic, carminative | [31] | – | |
| Prunella vulgaris | self-heal | antifebrile, immunoregulator, anti-inflamatory, treatment of breast disorders | [32] | – | |
| Hyssopus officinalis | hyssop | antiseptic, expectorant | [33] | – | |
| Salvia officinalis | common sage | antiseptic, astringent, gastroprotective, anti-inflammatory, spasmolytic | [34] | eupeptic, inhibition of excessive perspiration (internally), antibacterial, virustatic, astringent (externally) | [19,35] |
| Satureja hortensis | summer savory | antiseptic, eupeptic, spasmolytic | [36] | – | |
| Satureja montana | winter savory | – |
- Data not found in revised literature.
Different plants belonging to this family are classified into two subfamilies based on the presence of rosmarinic acid (Nepetoideae) or iridoids (Lamioideae). However, both of the plant groups of plants have various levels of essential oil (EO), with higher percent usually found in Nepetoideae subfamily [37,38]. Plants from this family are well known for their antibacterial, antifungal and antioxidant properties [[39], [40], [41], [42]] and are used for extraction and isolation of a wide range of bioactive compounds [[43], [44], [45]]. This review included ten genera of Lamiaceae family, which are, to the best of our knowledge, the most widely known representatives, with distribution all over the world and a well-established tradition of use.
3. Anti(retro)viral properties of Lamiaceae
Antiviral effects of Lamiaceae plants have mainly been proven in vitro, with some effects also having been confirmed in other types of experiments, such as indirect effects in healthy volunteers, or in patients. The main findings are displayed in Table 3 - activity against HIV and Table 4 - activity against other viruses. The activity against HIV in Table 3 refers to HIV-1, unless otherwise stated.
Table 3.
Some representatives of Lamiaceae family with anti-HIV activity.
| Plant species | Type of extract | EC (μg/ml) | SI | Additional comments | References |
|---|---|---|---|---|---|
| Thymussp. | |||||
| T. quinquecostatus | AE | 31 | 4 |
|
[46] |
| T. serpyllum | AE | 31 | 4 |
|
[46] |
| T. vulgaris | AE | 62 | 4 | [46] | |
| ME | >500 | <3.00 | [47] | ||
| T. daenensis subspecies daenensis | ME | 300 | 5.26 |
|
[47] |
| T. daenensis subspecies lancifolius | ME | >500 | <3.16 | [47] | |
| T. kotschyanus | ME | >500 | <3.18 | [47] | |
| T. carmanicus | ME | >500 | <3.11 | [47] | |
| Menthasp. | |||||
| Mentha × piperita var. citrata | AE | 16 | 16 |
|
[46] |
| Mentha × piperita s.l.–“grapefruit mint” | AE | 16 | 8 |
|
[46] |
| Mentha × piperita | AE | 62 | 4 | [46] | |
| AE | – | – |
|
[48] | |
| EE | 125 | 2 | [46] | ||
| M. spicata | AE | 31 | 8 |
|
[46] |
| M. pulegium | AE | 62 | 2 | [46] | |
| EE | 125 | 4 | |||
| M. suaveolens | AE | 250 | 4 | [46] | |
| EE | 62 | 4 | [46] | ||
| M. haplocalyx | AE | – | – |
|
[49] |
| ME | – | – |
|
[49] | |
| Rosmarinussp. | |||||
| R. officinalis | AE | 62 | 4 | [46] | |
| EE | 250 | 2 | [46] | ||
| EE | – | – |
|
[50] | |
| EE | – | – |
|
[51] | |
| Melissasp. | |||||
| M. officinalis | AE | 16 | 4 |
|
[46] |
| AE | – | – |
|
[48] | |
| Origanumsp. | |||||
| O. majorana | AE | 31 | 8 |
|
[46] |
| O. vulgare | AE | 31 | 8 |
|
[46] |
| Ocimumsp. | |||||
| O. basilicum | AE | 31 | 4 |
|
[46] |
| EE | 125 | >8 | [46] | ||
| O. basilicum cultivar ”cinnamon” | AE | 16 | 8 |
|
[46] |
| EE | 1000 | >1 | [46] | ||
| O. gratissimum | AE | 10 | 110 |
|
[52] |
| Prunellasp. | |||||
| P. vulgaris | AE | – | – |
|
[46,[53], [54], [55], [56], [57]] |
| AE | – | – |
|
[46,54,55,57] | |
| AE | – | – |
|
[54,58] | |
| AE | – | – |
|
[46,58] | |
| AE | – | – |
|
[55] | |
| AE | – | – |
|
[49] | |
| AE | – | – |
|
[59] | |
| AE | – | – |
|
[60] | |
| EE |
|
[56] | |||
| EE | – | – |
|
[57] | |
| ME | – | – |
|
[49] | |
| EAE | – | – |
|
[55] | |
| Hyssopussp. | |||||
| H. officinalis | AE | – | – |
|
[61] |
| EE | – | – |
|
[50] | |
| Salviasp. | |||||
| S. officinalis | AE | 62 | 4 |
|
[46] |
| AE | – | – |
|
[48] | |
| S. elegans | AE | 31 | 2 |
|
[46] |
| EE | 31 | 2 | [46] | ||
| S. sclarea | AE | 250 | 2 | [46] | |
| S. miltiorrhiza | AE | – | – |
|
[58] |
| EE | – | – |
|
[57] | |
| S. triloba | EE | – | – |
|
[50] |
| Saturejasp. | |||||
| S. hortensis | AE | 62 | 1 | [46] | |
| S. montana | AE | 16 | 4 |
|
[46] |
- Assay was not conducted/not applicable.
EC- effective concentration, which displays activity against HIV.
SI- selectivity index, ratio between toxic and effective concentration, parameter of (non) toxicity.
AE- aqueous extract.
EE- ethanolic extract.
ME- methanolic extract.
EAE- ethyl acetate extract.
RT- reverse transcriptase.
PBMC- peripheral blood mononuclear cells.
Table 4.
Some representatives of Lamiaceae family with antiviral activities against other viruses.
| Plant species | Type of extract (with main compound(s)) | Additional comments | References |
|---|---|---|---|
| DNA Viruses With Envelopes | |||
| herpes simplex virus (HSV) | |||
| thyme | EO (p-cymen and thymol) |
|
[62] |
| Thymus vulgaris | EO (thymol and carvacrol) |
|
[63] |
| EO (thymol and p-cymen) |
|
[64] | |
| AE |
|
[65] | |
| Thymus capitata | AE (derivate of apigenin), EE (thymol) and EO (thymol) |
|
[66] |
| Thymus linearis | ME |
|
[67] |
| Mentha × piperita | EO (menthol and menthon) |
|
[68] |
| Mentha suaveolens | EO (piperitenon oxide) |
|
[69] |
| Rosmarinus officinalis | AE |
|
[64] |
| Melissa officinalis | EO (β-cubebene and β-caryophyllene) |
|
[70] |
| EO (geranial, β-caryophyllen and neral) |
|
[71] | |
| AE, EE |
|
[72] | |
| AE |
|
[73] | |
| AE |
|
[74] | |
| EE |
|
[75] | |
| cream containing extract |
|
[76] | |
| Ocimum basilicum | AE, EE |
|
[77] |
| Hyssopus officinalis | EO (isopinocamphone and 1-pinocamphone) |
|
[78] |
| EO (isopinocamphone and 1-pinocamphone) |
|
[64] | |
| Salvia officinalis | AE, EE |
|
[71] |
| AE |
|
[64] | |
| EO (1,8-cineol and α-thujone) |
|
[79] | |
| creams containing extract |
|
[80] | |
| Satureja boliviana | AE, EE |
|
[81] |
| Satureja thymbra | EO (p-cymene, α-pinene and thymol) |
|
[79] |
| hepatitis B virus | |||
| Ocimum basilicum | AE, EE |
|
[77] |
| RNA Viruses With Envelopes | |||
| influenza virus | |||
| Thymus linearis | ME |
|
[67] |
| Melissa officinalis | EO (geranial and neral) |
|
[82] |
| EE |
|
[83] | |
| Ocimum sanctum | ME |
|
[84] |
| Prunella vulgaris | AE |
|
[85] |
| mumps, measles, vesicular stomatitis virus (VSV) | |||
| Thymus vulgaris | AE |
|
[64] |
| Rosmarinus officinalis | AE |
|
[64] |
| Salvia officinalis | AE |
|
[64] |
| Satureja boliviana | AE,EE |
|
[81] |
| Newcastle Disease virus | |||
| Thymus vulgaris | EE |
|
[86] |
| SARS coronavirus | |||
| Salvia officinalis | EO (1,8-cineol and α-thujone) | [79] | |
| Satureja thymbra | EO (p-cymene, α-pinene and thymol) |
|
[79] |
| bovine viral diarrhoea virus | |||
| Ocimum basilicum | EO |
|
[87] |
| yellow fever virus | |||
| Origanum vulgare | EO (trans sabinene) | [88] | |
| dengue virus | |||
| Ocimum sanctum | ME |
|
[89] |
| ebola virus | |||
| Prunella vulgaris | AE | [90] | |
| equine infectious anemia virus | |||
| Prunella vulgaris | AE, EE |
|
[91] |
| Japanese encephalitis virus, Sindbis | |||
| Salvia miltiorrhiza | AE, EAE |
|
[92] |
| DNA Viruses Without Envelopes | |||
| adenovirus | |||
| Thymus mastichina | EO |
|
[93] |
| Ocimum basilicum | AE, EE |
|
[77] |
| Hyssopus officinalis | EO |
|
[93] |
| RNA Viruses Without Envelopes | |||
| echovirus 9 | |||
| Salvia miltiorrhiza | AE, EAE |
|
[92] |
| polio virus | |||
| Satureja boliviana | AE |
|
[81] |
| norovirus | |||
| Thymus mastichina | EO |
|
[93] |
| Origanum vulgare | EO |
|
[94] |
| Hyssopus officinalis | EO |
|
[93] |
| coxsackie virus | |||
| Mentha haplocalyx | AE |
|
[94] |
| Ocimum basilicum | AE, EE |
|
[77] |
| Salvia miltiorrhiza | AE, EAE |
|
[92] |
| enterovirus | |||
| Mentha haplocalyx | AE |
|
[94] |
| Ocimum basilicum | AE, EE |
|
[77] |
| Salvia miltiorrhiza | AE, EAE |
|
[92] |
EO- essential oil.
AE- aqueous extract.
EE- ethanolic extract.
ME- methanolic extract.
4. Natural products and considerations of adequate form
An investigated substance (IS) can be:
-
1.
a plant extract obtained by using different solvents e.g. aqueous (AE), ethanolic (EE), methanolic (ME), ethyl acetate (ЕАE), etc.,
-
2.
an EO isolated from raw plant material mainly by hydro- or stem distillation and
-
3.
a single chemical compound, either of natural origin or (semi)synthesized.
The majority of tested plants were prepared in the form of AE. This type of extraction is probably the simplest for preparation, as it resembles the preparation of previously mentioned infusum (tea) which is used traditionally and convenient for use at patient’s home [95]. Two other frequent types of extracts are EE and EO. The first type, EE can also be prepared at home (e.g. by macerating fresh or dried plants with diluted ethanol); and while ethanol can enhance the extraction of active compounds, higher concentrations of ethanol can show interactions with antiretroviral and other drugs. On the other hand, EOs are available as commercial products, and can be bought at different stores. Of course, only adequate application of EOs in patients (e.g. diluted in a lipid carrier) will prevent possible direct toxicity and allergic reactions [96]. Other types of extracts, obtained by using different solvents (e.g. chloroform, hexane…) have not been used for assessment of anti(retro)viral properties, probably due to their toxicity.
It is interesting to notice that conducted researches drew a comparison between several extracts. Yamasaki et al. [46] compared AE and EE for several plants, generally proving AE as more active against HIV. Other research generally supports this claim. In the case of self-heal, AE was also found to be superior in comparison to EE [56], while EAE exhibited no effects [55]. The latter paper also provided proof that AE can be absorbed from the intestine into blood, further giving potential for in vivo activity [55]. All of these data suggest that AEs are the most interesting types of extracts, which should be further studied for potential application in patients.
5. Active compounds in plants
Usually, the first step in investigating of complex natural products’ biological activity (including antiviral) is to determine the most abundant compound in any type of extract. Several of revised papers had this approach, and detection of some compounds led to further investigation of their activity against viruses.
5.1. Compounds with anti-HIV activity
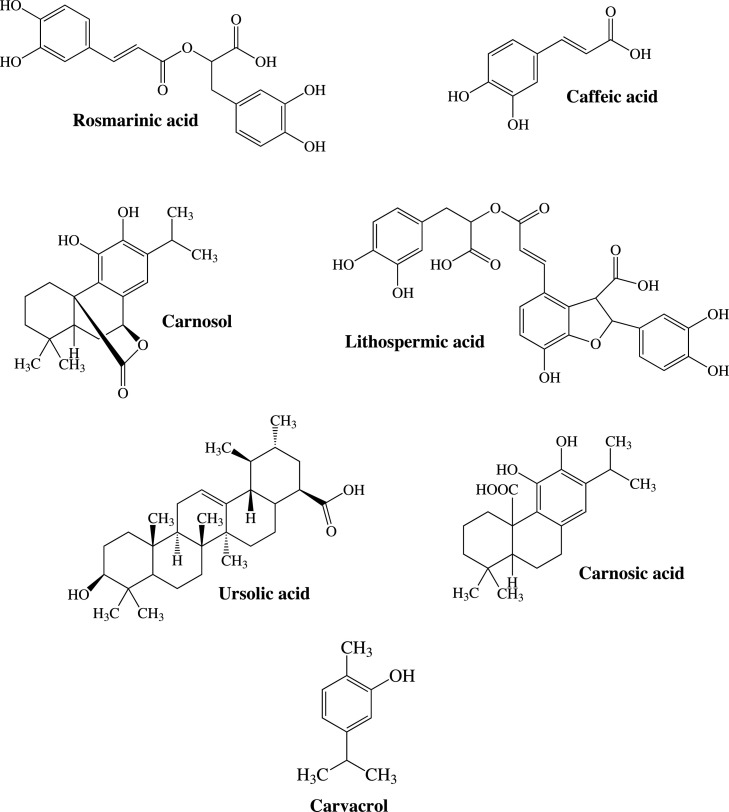
Out of all of the compounds found in rosemary, only carnosol (Fig. 1 ) exhibited inhibition of HIV replication with acceptable toxicity [51]. A chemical analysis of hyssop AE indicated a high concentration of caffeic acid (Fig. 1) whose anti-HIV activity was later confirmed [61] and further investigation led to discovery of substituted polysaccharide, named MAR-10, another substance from hyssop with notable in vitro anti-HIV activity [97]. This is not the only polysaccharide with detected anti-HIV properties. Investigating inflorescence of self-heal, researchers were able to isolate sulfated polysaccharide named prunellin, with excellent activity against HIV, without detected cytotoxicity [54,98]. Furthermore, various active compounds were isolated from different representatives of genus Salvia and tested for antiviral properties. Semi-synthesized caffeoyl-coumarin derivatives of sagecoumarin (isolated from S. officinalis) were proven to be extremely potent IIs, exhibiting activity in concentrations of 1–2 μmol l−1. Unfortunately, they exhibited poor activity against viral replication [99]. Lithospermic acid (Fig. 1) and lithospermic acid B (polycyclic phenolic carboxylic acids) were isolated from S. miltiorrhiza (Danshen, red sage) root extract and were shown to be potent and selective IIs [100]. Molecular docking calculations predicted that these compounds would be effective even in the case of HIV strains resistant to conventional therapy [101]. Using root of S. yunnanensis (another biological source of Danshen), researchers isolated polyphenol salvianolic acid N, which exhibited notable anti-HIV-1 activity and RT and integrase inhibition in vitro [102]. Five more tested compounds: salvianolic acid A, methyl salvianolate A, ethyl salvianolate A, lithospermic acid and cis-lithospermic acid showed activity against HIV-1 replication in concentrations 2.04–6.11 μg ml−1. They were also good IIs, but weak inhibitors of RT and protease [103].
Fig. 1.
Some compounds presumed to be responsible for anti(retro)viral effects.
5.2. Compounds with activity against other viruses
Various compounds from Lamiaceae plants have been studied for their activity against DNA and RNA viruses, both with and without envelopes. A polysaccharide similar to prunellin demonstrated activity against HSV-1 (both acyclovir susceptible and resistant) and HSV-2. This activity appears to be specific against HSV, as this compound was found to be ineffective against DNA enveloped cytomegalovirus, RNA enveloped human influenza virus types A and B and VSV and against RNA non-enveloped poliovirus type 1 [104]. It was later demonstrated that another extracted polysaccharide acts against HSV antigen expression [105]. A third isolated polysaccharide, from the same plant, self-heal, also expressed anti-HSV activity, and its effectiveness was confirmed in skin and genital lesions in guinea pigs and mice, respectively [106]. Other types of compounds, diterpenes safficinolide and sageone, isolated from the common sage (S. officinalis) also exhibited anti-HSV-1 effects [107]. From the 2,5-dihydroxybenzoic acid (phenolic acid) and several glycosides of acacetin only weak activity against HSV-1 has been recorded [108]. Protocatehuic aldehide isolated from species S. miltiorrhiza expressed activity against duck HBV both in vitro (cell cultures) and in vivo (ducklings) [109].
Diterpene carnosic acid (Fig. 1) was found to be effective against RNA enveloped respiratory syncytial virus (RSV) with acceptable toxicity [110]. Apigenin (flavonoid aglycone) and acacetin glycosides showed weak to moderate activity against human RSV Long strain [108]. The previously mentioned safficinolide and sageone exhibited effects against another RNA virus, VSV [107].
It is interesting to point out that the research even detected some compounds with activity against RNA non-enveloped viruses, generally viewed as more challenging for therapy. Rosmarinic acid (Fig. 1) and lithospermate B, as single compounds from S. miltiorrhiza, were effective against enterovirus 71 [111]. Carvacrol (Fig. 1) (commonly found as a major compound of O. vulgare EO) displayed activity against non-enveloped murine norovirus [112]. Ursolic acid (Fig. 1) had excellent activity against RNA non-enveloped viruses such as coxackievirus B1 and enterovirus 71, but also against DNA non-enveloped adenoviruses [77]. In silico molecular studies of various compounds from O. sanctum also confirmed good antiviral activity of ursolic acid [113].
6. Activity versus toxicity
The most important parameter that needs to be determined in antiviral testing is the minimum concentration needed for inhibition of viral replication. It is suggested that these values should be ≤100 μg mL−1 for extracts and EOs, and as it can be seen in Table 3, majority of plants with activity against HIV follow this rule. However, this is not the only parameter that should be used for assessment of antiviral effectiveness of different plant based products. Due to the fact that studies of antiviral activity are performed in living systems (usually cell cultures), a minimum concentration that displays (cyto)toxicity should also be reported [114]. Selectivity index (SI) represents the ratio of determined cytotoxic concentration and effective concentration. SI is a valuable indicator of effectiveness of the tested substance, and, if the compound or mixture exhibits activity in low concentrations, it is not considered useful and applicable if its SI is also low. Only values of SI higher than 4 are considered appropriate [114,115]. For example, carnosic acid was toxic for tested cells, even though it displayed potent activity against HIV integrase in vitro [116]. Compounds salvianolic acids D and E, isolated from different Salvia species, were proven to be extremely effective in vitro against HIV-1 integrase. However, they were not further evaluated for antiviral effects due to their toxicity [117]. Some plants’ extracts e.g. EE of Salvia triloba [50], EE of Satureja boliviana [81], ME of Ocimum sanctum [89], commercially available rosemary extract and a mixture of Provençal Herbs (containing rosemary, sage, thyme and oregano) [51] were proven as toxic, so estimation of their anti(retro)viral activity was not possible.
7. Studying mechanism of antiviral activity
7.1. Time-of-addition (T-o-A) studies
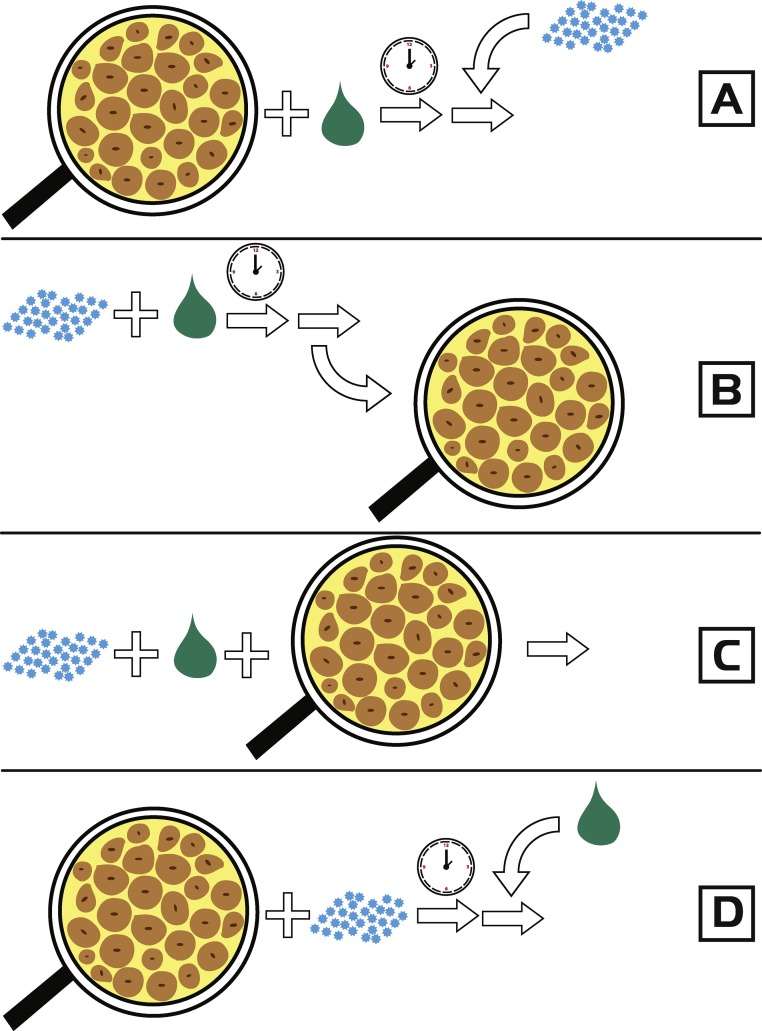
A notable number of studies reviewed in this paper have also tried to detect the exact M-o-A of IS. This was achieved through the time-of-addition (T-o-A) study, which is comprised of assays with different procedures of addition of IS, viral suspension and cell cultures [48,[54], [55], [56],[62], [63], [64],66,68,69,71,73,74,77,82,87,88,91,92,94,104,106,112]. Based on the highest activity in a certain assay, a conclusion about the exact type of M-o-A can be derived (Fig. 2 ). Generally, four assays for investigating antiviral effects of different mixtures and substances are in use:
-
1.
Pretreatment of the cell culture- IS is incubated with a cell culture, and after a certain time period, a viral suspension is added. In this case, the results show whether the IS was able to penetrate inside the cells and manifest its activity.
-
2.
Pretreatment of the virus- A viral suspension is incubated together with the IS. After the incubation, the suspension is rinsed of substance and inoculated into the cell culture. Activity in this assay indicates direct activity towards the virus and its structures, e.g. the envelope.
-
3.
Adsorption- The viral suspension and IS are added simultaneously into cell cultures. This assay indicates activity towards structures responsible for viral attachment and/or penetration of virus into the cells.
-
4.
Intracellular replication- IS is added during viral replication inside the cells. Substances showing activity in this assay are able to affect later stages of the viral life cycle [71].
Fig. 2.
Scheme of time-of-addition assays: A. pretreatment of cells (cells are treated with substance), B. pretreatment of virus (virus is treated with substance), C. adsorption (virus and substance are simultaneously added) and D. intracellular replication (substance is added during viral replication). Adapted and modified from Schnitzler et al. [71]. The figure was color-styled to be understandable by all readers, including colorblind, according to Roskoski [118].
In the case of different Lamiaceae species, the majority of IS, both extracts and single compounds, displayed activity in cases of pretreatment of viral particles. This resulted in strong and dose-dependent inhibition of viral infectivity. Several experiments suggested that viral envelope is the target site for ISs, as it is confirmed by comparing antiviral activities against enveloped and non-enveloped viral species. This is not a strict rule- some IS demonstrated activity against non-enveloped viruses [112] and there was no activity against some enveloped viruses [94].
Activity in cases of virus pretreatment does not exclude activity in the assay of adsorption inhibition; as it is possible that IS displays effects in a short period of time during simultaneous addition of IS and of viral particles into cell cultures. However, minor activity recorded in adsorption inhibition demonstrates that IS can affect both the viral envelope and other structures responsible for viral attachment and/or penetration into target cells.
Some research provided evidence that ISs had no lytic effects towards viral particles. These papers demonstrated that the integrity of particles was mantained after treatment with AEs of peppermint, lemon balm, common sage [48] and self-heal [56,91] and EO of oregano and carvacrol [112].
Interestingly, a drastic change in HIV virion density after exposure to AEs of lemon balm and peppermint was detected [48]. Researchers presumed that this was the consequence of ISs interactions with macromolecules in viral structures, responsible for normal viral integrity and infectivity, although this was not confirmed with experiments. The change of density was not recorded in the case of HIV [56] or in the equine infectious anemia virus [91] treated with self-heal.
As it can be seen in Table 4, EOs with different main compounds were also investigated for antiviral effects. Usually, only two or three compounds are present in higher concentrations and considered responsible for the activity. Main compounds in EO are usually determined by genetics, but different ecological factors could affect their concentration rather than the method used for isolation [119]. The effect of different main compounds in EO can be illustrated in the example of lemon balm. First EO with β-cubebene and β-caryophyllene as major compounds exhibited effects in cases of cell pretreatment with IS [70], while EO containing mainly geranial, β-caryophyllene and neral achieved effects when HSV-1 and HSV-2 viruses were pretreated and effects were absent in other T-o-A studies [71]. This leads to a conclusion that different compounds affect different stages of viral life cycle and achieve effect through different mechanisms.
7.2. Comparison with conventional drugs
Researchers often compare natural products with conventional drugs, commonly included as positive control- this provides information on strength of activity of a natural product. Another possible reason for these assays is to determine whether IS has the same M-o-A.
In the case of HIV, there is frequent comparison of activity against RT (Table 3). This unique and specific HIV enzyme is one of the key targets for conventional therapy, so it is no surprise that researchers tried to detect plants with activity against this enzyme. However, in HIV’s life cycle, several other structures can also be targets, so natural products are also compared with drugs displaying other M-o-A. This can be ilustrated in the example of Prunella vulgaris (Xia Ku Cao, self-heal), a plant frequently used in Traditional Chinese Medicine. Besides activity against RT (which resembles the M-o-A of nucleoside RT inhibitors), AE demonstrated activity against enzymes integrase and protease, and inhibition of interaction of HIV gp120 and gp41 with corresponding structures on target cells, similar to activities of integrase inhibitors, protease inhibitors, co-receptor antagonists and fusion inhibitors, respectively (Table 1, Table 3), thus mimicking M-o-A of antiretroviral drugs. As it was previously mentioned, some isolated compounds were also found to have a M-o-A similar to conventional drugs.
8. Studying activity in cases of chronic HIV infection
Besides simulating acute infection, in the case of HIV it is also important to investigate the activity of compounds and natural products in chronic infection. This is achieved through simulation in an in vitro assay with non-infected cells and cells chronically infected with HIV (with cell lines being of the same or different origin). Techniques of these assays are versatile, but the general principle is to co-culture both cell cultures in the presence of IS, determining whether IS can prevent virus spreading to non-infected cells [120]. Some of the examined Lamiaceae species (lemon balm, self-heal, and some representatives from genera Thymus, Mentha, Origanum, Ocimum, Salvia and Satureja) exhibited this activity (Table 3). However, the lack of effect may correspond with previously detected substance activity only in early stages of viral life cycle.
9. Potential position in the therapy
Extracts and EOs can also be compared for their activity against different viral strains, both susceptible and resistant to conventional drugs. This was most frequently tested on herpes simplex viral strains, as resistance to acyclovir is a therapeutical problem. Several Lamiaceae representatives (thyme, peppermint, lemon balm, self-heal and hyssop) were tested simultaneously against acyclovir resistant and acyclovir susceptible strains of HSV. Interestingly, they were equally susceptible to used extracts [63,68,74]. Besides this, lemon balm was proven to be efficient in cases of HIV strains resistant to enfuviride, fusion inhibitor [48].
Potential synergism with conventional drugs could result in new recommendations of plant application with antiviral therapy. In revised literature, EO of Mentha suaveolens showed a synergistic effect with acyclovir against HSV-1 [69] and EO of lemon balm showed synergism with oseltamivir against avian H9N2 influenza virus [82]. In both cases, sub-optimal doses of drugs were enhanced with EOs, resulting in good antiviral activities.
Both of the previous cases (comparison of strains and synergism) have led to the conclusion that natural products can exhibit different M-o-A than conventional drugs. Still, idea of combining drugs (both conventional and herbal) with different M-o-A could be a good therapeutical approach. This is in accordance with the main idea of cART- use of several drugs results in good activity against virus, with decreased side effects and toxic properties of drugs.
10. The “non- in vitro” studies and future considerations
As previously stated, the majority of studies are conducted in vitro. This is the main type of investigation due to convenience and lower cost of studies. However, concerning HIV, it is completely understandable from an ethical point of view why these in vitro studies are the only type of conducted studies, to the best of our knowledge. Still, some other types of investigations are being attempted, in order to get valid results, possibly applicable to patients.
Some papers investigated activity in ovo [84,86]. This approach is presumably conducted in order to simulate natural living cells (rather than cell cultures), giving a more accurate answer to the question- Does IS show activity and lack of toxicity at the same time? Animal studies are not widely accepted, and only study by Dimitrova et al. [72] demonstrated a lack of activity of lemon balm against HSV-1 when it was tested in rabbits, in provoked eye infection.
Thyme and hyssop preparations were estimated for their ability to increase the number and/or viability of peripheral blood mononuclear cells (PBMCs) of healthy donors [47,97]. A study by Feng et al. [57] included healthy volunteers who had taken self-heal for a week. These individuals had a reduced level of chemokine co-receptors in T cells, one of key structures in the HIV life cycle, and one of therapeutic targets. The positive outcome in these ex vivo and in vivo studies suggest modulatory effects of these plants towards immunity. This also suggest that antiviral activity can be indirect, rather than having direct effects (e.g. targeting viral envelope) as it is seen in in vitro studies.
Finally, some plants were studied for their activity in patients, in assays testing pharmaceutical formulations for local application in cases of recurrent herpes labialis. Commercially available cream containing extract of lemon balm was proven to be efficient in the treatment of this sometimes complex problem. The cream was able to reduce the intensity of complaints, number of blisters and affected area size in patients, especially if the application is started immediately after the onset of prodromes [76]. Also, creams containing sage extract, as well as sage and rhubarb mixture were proven to be efficient in the treatment of herpes labialis in patients. Application of these creams reduced the symptoms and duration of viral episodes [80]. These investigations of antiviral activity in some patients and their positive outcomes suggest that application of adequate formulations confirmed antiviral activity which was previously noted only in vitro. Local application is an effective type of treatment which may be advised to patients in addition to conventional therapy, reducing side effects of viral diseases.
As Lamiaceae plants are demonstrated to be effective in vitro, possible ideas for future researches would be to test these plants in patients with viral infections. The starting points would be to select a plant (possibly by using informations of effective concentrations and SIs) and adequate form (as it is previously explained, AE appears to be the most promising). Use of the most abundant compounds in products (some suggested in the previous text) is an even more precise approach to investigation. Application of plants should, however, be carefully monitored. Although the plants generally have many potential beneficial effects, they also have potential to cause interactions with antiretroviral and other drugs, resulting in a drastic increase or decrease of drug concentrations in patients, leading to toxicity or therapeutic failure. However, in cases of HIV, it is believed that prolonged exposure to drugs (not exceeding therapeutic concentrations) can be positive for patients, as it prevents selection of resistant strains and possibly decreases doses of conventional drugs, together with their side effects [121]. With careful monitoring of drug concentration, management of simultaneous use of conventional therapy and phytotherapy should not be a problem.
11. Conclusions
Numerous in vitro screenings pointed to notable anti(retro)viral properties of different species of the Lamiaceae family. The effect is generally strong and dose-dependent, and the most likely mechanism of activity is direct effect against outer structures (primarily viral envelop) consequentially disabling viral attachment to target cells. Several other experiments with local therapeutic forms gave encouraging results, demonstrating potential of Lamiaceae plants as adjunctive therapy. Further clinical trials could give final conclusions on antiviral activity and the application on patients.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by The Provincial Secretariat for Science and Technological Development of Vojvodina (grant number 114-451-2730/2016-03) and The Ministry of Education, Science and Technological Development, Republic of Serbia (grant number OI 172058).
References
- 1.Arts E.J., Hazuda D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012;2(4):a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res. 2010;85(1):201–209. doi: 10.1016/j.antiviral.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Cortez K.J., Maldarelli F. Clinical management of HIV drug resistance. Viruses. 2014;3(4):347–378. doi: 10.3390/v3040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS (Joint United Nations Programme on HIV/AIDS) 2016. Global AIDS Update 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. (Accessed 5 March 2017) [Google Scholar]
- 5.Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95(3):412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 6.Mukhtar M., Arshad M., Ahmad M., Pomerantz R.J., Wigdahl B., Parveen Z. Antiviral potentials of medicinal plants. Virus Res. 2008;131(2):111–120. doi: 10.1016/j.virusres.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D.L., Mehta R.G., Moriarty R.M. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev. Med. Chem. 2008;8(11):1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y., Wang X., Li Z., Jiang Z., Guo H., Liu Z., Xu L. Survival of AIDS patients treated with traditional Chinese medicine in rural central China: a retrospective cohort study, 2004-2012. Evid. Based Complement. Altern. Med. 2015;2015:282819. doi: 10.1155/2015/282819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z.B., Yang J.P., Xu L.R. Effectiveness and safety of traditional Chinese medicine in treating acquired immune deficiency syndrome: 2004-2014. Infect. Dis. Poverty. 2015;4:59. doi: 10.1186/s40249-015-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W., Wang J., Liu Y. Effect of traditional Chinese medicine for treating human immunodeficiency virus infections and acquired immune deficiency syndrome: boosting immune and alleviating symptoms. Chin. J. Integr. Med. 2016;22(1):3–8. doi: 10.1007/s11655-015-2122-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Jin F., Wang Q., Suo Z. Long-term survival of AIDS patients treated with only traditional Chinese medicine. AIDS Res. Hum. Retroviruses. 2017;33(2):90–92. doi: 10.1089/aid.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H.L., Sun C.Z., Jiang W.P., Dai Z.K., Shi W.X., Yang K.K., Mu X.J., Zhang X.X., Sui Y. Eight-year survival of AIDS patients treated with Chinese herbal medicine. Am. J. Chin. Med. 2014;42(2):261–274. doi: 10.1142/S0192415X14500177. [DOI] [PubMed] [Google Scholar]
- 13.Raja R. Ramasubramania. Medicinally potential plants of Labiatae (Lamiaceae) family: an overview. Res. J. Med. Plants. 2012;6(3):203–213. [Google Scholar]
- 14.Amber R., Adnan M., Tariq A., Mussarat S. A review on antiviral activity of the Himalayan medicinalplants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017;69(2):109–122. doi: 10.1111/jphp.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarić S., Mitrović M., Pavlović P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid. Based Complement. Altern. Med. 2015;2015:101978. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begum A., Sandhya S., Shaffath Ali S., Vinod K.R., Reddy S., Banji D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae) Acta Sci. Pol. Technol. Aliment. 2013;12(1):61–73. [PubMed] [Google Scholar]
- 17.World Health Organisation (WHO) 2013. WHO Traditional Medicine Strategy: 2014-2023. http://apps.who.int/iris/bitstream/10665/92455/1/9789241506090_eng.pdf?ua=1. (Accessed 5 November 2017) [Google Scholar]
- 18.Prasanth Reddy V., Ravi Vital K., Varsha P.V., Satyam S. Review on Thymus vulgaris traditional uses and pharmacological properties. Med. Aromat. Plants. 2014;3(3):164. [Google Scholar]
- 19.Blumenthal M., editor. The Complete German Commision E Monographs, Therapeutic Guide to Herbal Medicines. American Botanical Council; Austin, Texas: 1998. [Google Scholar]
- 20.European Medicines Agency (EMA), Thyme Thymus vulgaris L. and Thymus zygis L., Herba, EMA/307113/2016. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Summary_of_assessment_report_for_the_public/2016/10/WC500214266.pdf/>. (Accessed 6 November 2017).
- 21.World Health Organization (WHO) vol. 1. 1999. (WHO Monographs on Selected Medicinal Plants). [Google Scholar]
- 22.McKay D.L., Blumberg J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother. Res. 2006;20(8):619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 23.European Medicines Agency (EMA), European Union Herbal Monograph on Mentha x piperita L., Folium, EMA/HMPC/572705/2014. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2017/02/WC500222195.pdf/>. (Accessed 6 November 2017).
- 24.World Health Organization (WHO) vol. 2. 2004. (WHO Monographs on Selected Medicinal Plants). [Google Scholar]
- 25.European Medicines Agency (EMA), Community Herbal Monograph on Rosmarinus officinalis L., Aetheroleum, EMEA/HMPC/235453/2009. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2011/01/WC500101493.pdf/>. (Accessed 6 November 2017).
- 26.World Health Organization (WHO) vol. 4. 2009. (WHO Monographs on Selected Medicinal Plants). [Google Scholar]
- 27.Shakeri A., Sahebkar A., Javadi B. Melissa officinalis L.–a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 28.European Medicines Agency (EMA), Melissa Leaf Melissa officinalis L., Folium, EMA/HMPC/310761/2013. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Summary_of_assessment_report_for_the_public/2014/06/WC500168590.pdf/>. (Accessed 6 November 2017).
- 29.Chishti S., Kaloo Z.A., Sultan P. Medicinal importance of genus Origanum: a review. J. Pharmacogn. Phytother. 2013;5(10):170–177. [Google Scholar]
- 30.European Medicines Agency (EMA), European Union Herbal Monograph on Origanum majorana L., Herba, EMA/HMPC/166517/2015. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Herbal_monograph/2016/02/WC500201951.pdf/>. (Accessed 6 November 2017).
- 31.Pandey A.K., Singh P., Tripathi N.N. Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pac. J. Trop. Biomed. 2014;4(9):682–694. [Google Scholar]
- 32.Bai Y., Xia B., Xie W., Zhou Y., Xie J., Li H., Liao D., Lin L., Li C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016;204:483–496. doi: 10.1016/j.foodchem.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 33.Fathiazad F., Hamedeyazdan S. A review on Hyssopus officinalis L.: composition and biological activities. Afr. J. Pharm. Pharmacol. 2011;5(8):1959–1966. [Google Scholar]
- 34.Carović-Stanko K., Petek M., Grdiša M., Pintar J., Bedeković D., Herak Ćustić M., Satovic Z. Medicinal plants of the family Lamiaceae as functional foods–a review. Czech J. Food Sci. 2016;34(5):377–390. [Google Scholar]
- 35.European Medicines Agency (EMA), Community Herbal Monograph on Salvia officinalis L., Folium, EMA/HMPC/331653/2008. <http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2010/02/WC500070852.pdf/>. (Accessed 6 November 2017).
- 36.Jafari F., Ghavidel F., Zarshenas M.M. A critical overview on the pharmacological and clinical aspects of popular Satureja species. J. Acupunct. Meridian Stud. 2016;9(3):118–127. doi: 10.1016/j.jams.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Mimica-Dukic N., Bozin B. Essential oils from Lamiaceae species as promising antioxidant and antimicrobial agents. Nat. Prod. Commun. 2007;2(4):445–452. [Google Scholar]
- 38.Mimica-Dukic N., Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr. Pharm. Des. 2008;14(29):3141–3150. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- 39.Bozin B., Mimica-Dukic N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54(5):1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- 40.Bozin B., Mimica-Dukic N., Samojlik I., Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007;55(19):7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 41.Mimica-Dukić N., Bozin B., Soković M., Mihajlović B., Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003;69(5):413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 42.Mimica-Dukic N., Bozin B., Sokovic M., Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004;52(9):2485–2489. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- 43.Gavarić N., Kladar N., Mišan A., Nikolić A., Samojlik I., Mimica-Dukić N., Božin B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crops Prod. 2015;74:457–464. [Google Scholar]
- 44.Nabavi S.M. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402–414. doi: 10.1016/j.foodchem.2016.04.111. [DOI] [PubMed] [Google Scholar]
- 45.Friedman M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014;62(31):7652–7670. doi: 10.1021/jf5023862. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki K., Nakano M., Kawahata T., Mori H., Otake T., Ueba N., Oishi I., Inami R., Yamane M., Nakamura M., Murata H., Nakanishi T. Anti-HIV-1 activity of herbs in Labiatae. Biol. Pharm. Bull. 1998;21(8):829–833. doi: 10.1248/bpb.21.829. [DOI] [PubMed] [Google Scholar]
- 47.Soleimani-Farsani M., Behbahani M., Isfahani H.Z. The effect of root, shoot and seed extracts of the iranian Thymus L. (Family: Lamiaceae) species on HIV-1 replication and CD4 expression. Cell J. 2016;18(2):255–261. doi: 10.22074/cellj.2016.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geuenich S., Goffinet C., Venzke S., Nolkemper S., Baumann I., Plinkert P., Reichling J., Keppler O.T. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology. 2008;5:27. doi: 10.1186/1742-4690-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam T.L., Lam M.L., Au T.K., Ip D.T., Ng T.B., Fong W.P., Wan D.C.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000;67(23):2889–2896. doi: 10.1016/s0024-3205(00)00864-x. [DOI] [PubMed] [Google Scholar]
- 50.Bedoya L.M., Palomino S.S., Abad M.J., Bermejo P., Alcami J. Screening of selected plant extracts for in vitro inhibitory activity on human immunodeficiency virus. Phytother. Res. 2002;16(6):550–554. doi: 10.1002/ptr.992. [DOI] [PubMed] [Google Scholar]
- 51.Aruoma O.I., Spencer J.P., Rossi R., Aeschbach R., Khan A., Mahmood N., Munoz A., Murcia A., Butler J., Halliwell B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provençal herbs. Food Chem. Toxicol. 1996;34(5):449–456. doi: 10.1016/0278-6915(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 52.Ayisi N.K., Nyadedzor C. Comparative in vitro effects of AZT and extracts of Ocimum gratissimum, Ficus polita, Clausena anisata, Alchornea cordifolia, and Elaeophorbia drupifera against HIV-1 and HIV-2 infections. Antiviral Res. 2003;58(1):25–33. doi: 10.1016/s0166-3542(02)00166-3. [DOI] [PubMed] [Google Scholar]
- 53.Chang R.S., Yeung H.W. Inhibition of growth of human immunodeficiency virus in vitro by crude extracts of Chinese medicinal herbs. Antiviral Res. 1988;9(3):163–175. doi: 10.1016/0166-3542(88)90001-0. [DOI] [PubMed] [Google Scholar]
- 54.Yao X.J., Wainberg M.A., Parniak M.A. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology. 1992;187(1):56–62. doi: 10.1016/0042-6822(92)90294-y. [DOI] [PubMed] [Google Scholar]
- 55.Kageyama S., Kurokawa M., Shiraki K. Extract of Prunella vulgaris spikes inhibits HIV replication at reverse transcription in vitro and can be absorbed from intestine in vivo. Antiviral Chem. Chemother. 2000;11(2):157–164. doi: 10.1177/095632020001100207. [DOI] [PubMed] [Google Scholar]
- 56.Oh C.S., Price J., Brindley M.A., Widrlechner M.P., Qu L., McCoy J.A., Murphy P., Hauck C., Maury W. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol. J. 2011;8:188. doi: 10.1186/1743-422X-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng L., Wang L., Ma Y.Y., Li M., Zhao G.Q. A potential in vitro and in vivo anti-HIV drug screening system for Chinese herbal medicines. Phytother. Res. 2012;26(6):899–907. doi: 10.1002/ptr.3658. [DOI] [PubMed] [Google Scholar]
- 58.Collins R.A., Ng T.B., Fong W.P., Wan C.C., Yeung H.W. A comparison of human immunodeficiency virus type 1 inhibition by partially purified aqueous extracts of Chinese medicinal herbs. Life Sci. 1997;60(23):PL345–351. doi: 10.1016/s0024-3205(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 59.Au T.K., Lam T.L., Ng T.B., Fong W.P., Wan D.C. A comparison of HIV-1 integrase inhibition by aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2001;68(14):1687–1694. doi: 10.1016/s0024-3205(01)00945-6. [DOI] [PubMed] [Google Scholar]
- 60.Liu S., Jiang S., Wu Z., Lv L., Zhang J., Zhu Z., Wu S. Identification of inhibitors of the HIV-1 gp41 six-helix bundle formation from extracts of Chinese medicinal herbs Prunella vulgaris and Rhizoma cibotte. Life Sci. 2002;71(15):1779–1791. doi: 10.1016/s0024-3205(02)01939-2. [DOI] [PubMed] [Google Scholar]
- 61.Kreis W., Kaplan M.H., Freeman J., Sun D.K., Sarin P.S. Inhibition of HIV replication by Hyssop officinalis extracts. Antiviral Res. 1990;14(6):323–337. doi: 10.1016/0166-3542(90)90051-8. [DOI] [PubMed] [Google Scholar]
- 62.Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010;24(5):673–679. doi: 10.1002/ptr.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schnitzler P., Koch C., Reichling J. Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob. Agents Chemother. 2007;51(5):1859–1862. doi: 10.1128/AAC.00426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch C., Reichling J., Schneele J., Schnitzler P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine. 2008;15(1-2):71–78. doi: 10.1016/j.phymed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 65.El-Awady S.I., Essam T., Hashem A., Boseila A.H.A., Mohmmed A.F. Assessment of antiviral activity for Lamiaceae family members against Rna and Dna virus models using cell -culture: in vitro study. World J. Med. Sci. 2014;11(1):111–119. [Google Scholar]
- 66.Boubaker-Elandalousi R., Mekni-Toujani M., Kaabi B., Larbi I., Diouani M.F., Gharbi M., Akkari H., B’chir F., Ghram A. Non-cytotoxic Thymus capitata extracts prevent Bovine herpesvirus-1 infection in cell cultures. BMC Vet. Res. 2014;10:231. doi: 10.1186/s12917-014-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajbhandari M., Mentel R., Jha P.K., Chaudhary R.P., Bhattarai S., Gewali M.B., Karmacharya N., Hipper M., Lindequist U. Antiviral activity of some plants used in Nepalese traditional medicine. Evid. Based Complement. Altern. Med. 2009;6(4):517–522. doi: 10.1093/ecam/nem156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10(6–7):504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- 69.Civitelli L., Panella S., Marcocci M.E., De Petris A., Garzoli S., Pepi F., Vavala E., Ragno R., Nencioni L., Palamara A.T., Angiolella L. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine. 2014;21(6):857–865. doi: 10.1016/j.phymed.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 70.Allahverdiyev A., Duran N., Ozguven M., Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine. 2004;11(7–8):657–661. doi: 10.1016/j.phymed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Schnitzler P., Schuhmacher A., Astani A., Reichling J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine. 2008;15(9):734–740. doi: 10.1016/j.phymed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 72.Dimitrova Z., Dimov B., Manolova N., Pancheva S., Ilieva D., Shishkov S. Antiherpes effect of Melissa officinalis L. extracts. Acta Microbiol. Bulg. 1993;29:65–72. [PubMed] [Google Scholar]
- 73.Astani A., Reichling J., Schnitzler P. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro. Chemotherapy. 2012;58(1):70–77. doi: 10.1159/000335590. [DOI] [PubMed] [Google Scholar]
- 74.Astani A., Navid M.H., Schnitzler P. Attachment and penetration of acyclovir-resistant herpes simplex virus are inhibited by Melissa officinalis extract. Phytother. Res. 2014;28(10):1547–1552. doi: 10.1002/ptr.5166. [DOI] [PubMed] [Google Scholar]
- 75.Mazzanti G., Battinelli L., Pompeo C., Serrilli A.M., Rossi R., Sauzullo I., Mengoni F., Vullo V. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008;22(16):1433–1440. doi: 10.1080/14786410802075939. [DOI] [PubMed] [Google Scholar]
- 76.Koytchev R., Alken R.G., Dundarov S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine. 1999;6(4):225–230. doi: 10.1016/S0944-7113(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 77.Chiang L.C., Ng L.T., Cheng P.W., Chiang W., Lin C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32(10):811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 78.Schnitzler P., Nolkemper S., Stintzing F.C., Reichling J. Comparative in vitro study on the anti-herpetic effect of phytochemically characterized aqueous and ethanolic extracts of Salvia officinalis grown at two different locations. Phytomedicine. 2008;15(1–2):62–70. doi: 10.1016/j.phymed.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saller R., Büechi S., Meyrat R., Schmidhauser C. Combined herbal preparation for topical treatment of Herpes labialis. Forsch. Komplementarmed. Klass. Naturheilkd. 2001;8(6):373–382. doi: 10.1159/000057255. [DOI] [PubMed] [Google Scholar]
- 81.Abad M.J., Bermejo P., Gonzales E., Iglesias I., Irurzun A., Carrasco L. Antiviral activity of Bolivian plant extracts. Gen. Pharmacol. 1999;32(4):499–503. doi: 10.1016/s0306-3623(98)00214-6. [DOI] [PubMed] [Google Scholar]
- 82.Pourghanbari G., Nili H., Moattari A., Mohammadi A., Iraji A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2) VirusDisease. 2016;27(2):170–178. doi: 10.1007/s13337-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jalali P., Moattari A., Mohammadi A., Ghazanfari N., Pourghanbari G. Melissa offcinalis effcacy against human influenza virus (New H1N1) in comparison with oseltamivir. Asian Pac. J. Trop. Dis. 2016;6(9):714–717. [Google Scholar]
- 84.Jadhav P., Lal H., Kshirsagar N. Assessment of potency of PC-complexed Ocimum sanctum methanol extract in embryonated eggs against Influenza virus (H1N1) Pharmacogn. Mag. 2014;10(Suppl. 1):S86–91. doi: 10.4103/0973-1296.127352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rezatofighi S.E., Seydabadi A., Seyyed Nejad S.M. Evaluating the efficacy of Achillea millefolium and Thymus vulgaris extracts against newcastle disease virus in ovo. Jundishapur J. Microbiol. 2014;7(2):e9016. doi: 10.5812/jjm.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian L., Wang Z., Wu H., Wang S., Wang Y., Wang Y., Xu J., Wang L., Qi F., Fang M., Yu D., Fang X. Evaluation of the anti-neuraminidase activity of the traditional Chinese medicines and determination of the anti-influenza A virus effects of the neuraminidase inhibitory TCMs in vitro and in vivo. J. Ethnopharmacol. 2011;137(1):534–542. doi: 10.1016/j.jep.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Kubiça T.F., Alves S.H., Weiblen R., Lovato L.T. In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014;45(1):209–214. doi: 10.1590/S1517-83822014005000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meneses R., Ocazionez R.E., Martínez J.R., Stashenko E.E. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2009;8:8. doi: 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang L.I., Ling A.P., Koh R.Y., Chye S.M., Voon K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012;12:3. doi: 10.1186/1472-6882-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang X., Ao Z., Bello A., Ran X., Liu S., Wigle J., Kobinger G., Yao X. Characterization of the inhibitory effect of an extract of Prunella vulgaris on Ebola virus glycoprotein (GP)-mediated virus entry and infection. Antiviral Res. 2016;127:20–31. doi: 10.1016/j.antiviral.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brindley M.A., Widrlechner M.P., McCoy J.A., Murphy P., Hauck C., Rizshsky L., Nicolau B., Maury W. Inhibition of lentivirus replication by aqueous extracts of Prunella vulgaris. Virol. J. 2009;6:8. doi: 10.1186/1743-422X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu B.W., Pan T.L., Leu Y.L., Chang Y.K., Tai P.J., Lin K.H., Horng J.T. Antiviral effects of Salvia miltiorrhiza (Danshen) against enterovirus 71. Am. J. Chin. Med. 2007;35(1):153–168. doi: 10.1142/S0192415X07004709. [DOI] [PubMed] [Google Scholar]
- 93.Kovač K., Diez-Valcarce M., Raspor P., Hernández M., Rodríguez-Lázaro D. Natural plant essential oils do not inactivate non-enveloped enteric viruses. Food Environ. Virol. 2012;4(4):209–212. doi: 10.1007/s12560-012-9088-7. [DOI] [PubMed] [Google Scholar]
- 94.Chen X., Wang C., Xu L., Chen X., Wang W., Yang G., Tan R.X., Li E., Jin Y. A laboratory evaluation of medicinal herbs used in China for the treatment of hand, foot, and mouth disease. Evid. Based Complement. Altern. Med. 2013;2013:504563. doi: 10.1155/2013/504563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ncube N.S., Afolayan A.J., Okoh A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 2008;7(12):1797–1806. [Google Scholar]
- 96.Edris A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 97.Gollapudi S., Sharma H.A., Aggarwal S., Byers L.D., Ensley H.E., Gupta S. Isolation of a previously unidentified polysaccharide (MAR-10) from Hyssop officinalis that exhibits strong activity against human immunodeficiency virus type 1. Biochem. Biophys. Res. Commun. 1995;210(1):145–151. doi: 10.1006/bbrc.1995.1639. [DOI] [PubMed] [Google Scholar]
- 98.Tabba H.D., Chang R.S., Smith K.M. Isolation, purification, and partial characterization of prunellin, an anti-HIV component from aqueous extracts of Prunella vulgaris. Antiviral Res. 1989;11(5–6):263–273. doi: 10.1016/0166-3542(89)90036-3. [DOI] [PubMed] [Google Scholar]
- 99.Bailly F., Queffelec C., Mbemba G., Mouscadet J.F., Cotelle P. Synthesis and HIV-1 integrase inhibitory activities of caffeic acid dimers derived from Salvia officinalis. Bioorg. Med. Chem. Lett. 2005;15(22):5053–5056. doi: 10.1016/j.bmcl.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 100.Abd-Elazem I.S., Chen H.S., Bates R.B., Huang R.C. Isolation of two highly potent and non-toxic inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase from Salvia miltiorrhiza. Antiviral Res. 2002;55(1):91–106. doi: 10.1016/s0166-3542(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 101.Nunthaboot N., Lugsanangarm K., Kokpol S., Abd-Elazem I.S. Binding mode prediction of biologically active compounds from plant Salvia Miltiorrhiza as integrase inhibitor. Bioinformation. 2013;9(8):426–431. doi: 10.6026/97320630009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Z.F., Chen H.S., Peng Z.G., Li Z.R., Jiang J.D. A potent anti-HIV polyphenol from Salvia yunnanensis. J. Asian Nat. Prod. Res. 2008;10(3–4):273–277. doi: 10.1080/10286020701605075. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Z.F., Peng Z.G., Gao L., Dong B., Li J.R., Li Z.Y., Chen H.S. Three new derivatives of anti-HIV-1 polyphenols isolated from Salvia yunnanensis. J. Asian Nat. Prod. Res. 2008;10(5):391–396. doi: 10.1080/10286020801966591. [DOI] [PubMed] [Google Scholar]
- 104.Xu H.X., Lee S.H., Lee S.F., White R.L., Blay J. Isolation and characterization of an anti-HSV polysaccharide from Prunella vulgaris. Antiviral Res. 1999;44(1):43–54. doi: 10.1016/s0166-3542(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 105.Chiu L.C., Zhu W., Ooi V.E. A polysaccharide fraction from medicinal herb Prunella vulgaris downregulates the expression of herpes simplex virus antigen in Vero cells. J. Ethnopharmacol. 2004;93(1):63–68. doi: 10.1016/j.jep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y., But P.P., Ooi V.E., Xu H.X., Delaney G.D., Lee S.H., Lee S.F. Chemical properties, mode of action, and in vivo anti-herpes activities of a lignin-carbohydrate complex from Prunella vulgaris. Antiviral Res. 2007;75(3):242–249. doi: 10.1016/j.antiviral.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Tada M., Okuno K., Chiba K., Ohnishi E., Yoshii T. Antiviral diterpenes from Salvia officinalis. Phytochemistry. 1994;35(2):539–541. [Google Scholar]
- 108.Zhang X.L., Guo Y.S., Wang C.H., Li G.Q., Xu J.J., Chung H.Y., Ye W.C., Li Y.L., Wang G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014;152:300–306. doi: 10.1016/j.foodchem.2013.11.153. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Z., Zhang Y., Ding X.R., Chen S.H., Yang J., Wang X.J., Jia G.L., Chen H.S., Bo X.C., Wang S.Q. Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antiviral Res. 2007;74(1):59–64. doi: 10.1016/j.antiviral.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Shin H.B., Choi M.S., Ryu B., Lee N.R., Kim H.I., Choi H.E., Chang J., Lee K.T., Jang D.S., Inn K.S. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013;10:303. doi: 10.1186/1743-422X-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chung Y.C., Hsieh F.C., Lin Y.J., Wu T.Y., Lin C.W., Lin C.T., Tang N.Y., Jinn T.R. Magnesium lithospermate B and rosmarinic acid two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Eur. J. Pharmacol. 2015;755:127–133. doi: 10.1016/j.ejphar.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 112.Gilling D.H., Kitajima M., Torrey J.R., Bright K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014;116(5):1149–1163. doi: 10.1111/jam.12453. [DOI] [PubMed] [Google Scholar]
- 113.Alhazmi M.I. Molecular docking of selected phytocompounds with H1N1 proteins. Bioinformation. 2015;11(4):196–202. doi: 10.6026/97320630011196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006;106(3):290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 115.Amoros M., Simões C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J. Nat. Prod. 1992;55(12):1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 116.Pariš A., Štrukelj B., Renko M., Turk V., Pukl M., Umek A., Korant B.D. Inhibitory effect of carnosic acid on HIV-1 protease in cell-free assays. J. Nat. Prod. 1993;56(8):1426–1430. doi: 10.1021/np50098a031. Erratum in: J. Nat. Prod. 57 (4) (1994) 552. [DOI] [PubMed] [Google Scholar]
- 117.Queffélec C., Bailly F., Mbemba G., Mouscadet J.F., Hayes S., Debyser Z., Witvrouw M., Cotelle P. Synthesis and antiviral properties of some polyphenols related to Salvia genus. Bioorg. Med. Chem. Lett. 2008;18(16):4736–4740. doi: 10.1016/j.bmcl.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 118.Roskoski R., Jr. Guidelines for preparing color figures for everyone including the colorblind. Pharmacol. Res. 2017;119:240–241. doi: 10.1016/j.phrs.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Islam M.T., da Mata A.M., de Aguiar R.P., Paz M.F., de Alencar M.V., Ferreira P.M., de Carvalho Melo-Cavalcante A.A. Therapeutic potential of essential oils focusing on diterpenes. Phytother. Res. 2016;30(9):1420–1444. doi: 10.1002/ptr.5652. [DOI] [PubMed] [Google Scholar]
- 120.Daelemans D., Pauwels R., De Clercq E., Pannecouque C. A time-of-drug addition approach to target identification of antiviral compounds. Nat. Protoc. 2011;6(6):925–933. doi: 10.1038/nprot.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lamorde M., Byakika-Kibwika P., Merry C. Pharmacokinetic interactions between antiretroviral drugs and herbal medicines. Br. J. Hosp. Med. (Lond.) 2012;73(3):132–136. doi: 10.12968/hmed.2012.73.3.132. [DOI] [PubMed] [Google Scholar]