One health—an initiative designed to integrate human and veterinary health—received a boost in The Lancet Infectious Diseases. A multidisciplinary team of scientists from the Netherlands, Qatar, and the UK report the first definitive isolation of the Middle East respiratory syndrome coronavirus (MERS-CoV) in a non-human animal species, dromedary camels.1 Phylogenetic analysis of the 4·2 kb partial viral sequence obtained from one animal show the virus to be almost identical to isolates obtained from two human cases who had had contact with the camels on the affected farm. The affected smallholding is part of a much larger farm complex located about 30 km northwest of Doha, Qatar, which has been the subject of intensive epidemiological investigation since the diagnosis of the first human case on the farm 2 months ago.2 Although the virus has only been sequenced from one camel to date, some virological evidence of infection was reported in 11 of 14 camels on the farm, and serological evidence of exposure to a MERS-CoV-like virus was noted in all of them.
The close phylogenetic similarity between virus isolated from people and animals strongly suggests cross-species transmission, but present data do not inform on the direction of that transmission. Notably, the camel and human virus sequences differ by two substitutions. As the investigators note, this divergence might be the end result of a chain of transmission in animals that introduced mutations. The original source of the detected human and camel infections is unknown, although the sequences closely match an isolate from a human case of MERS-CoV reported in Hafr Al-Batin, Saudi Arabia, in June, 2013.
The detection of MERS-CoV in camels in Qatar is given greater weight by the reported, but as yet unconfirmed, detection of the virus in a camel in Saudi Arabia3 and previous findings of antibodies to MERS-CoV, or a very similar virus, in camels in the Canary Islands, Oman,4 and Egypt.5 A closely related but not identical virus has also been detected in bats.6 That MERS-CoV is a zoonosis is not surprising; assessment of the implications will nevertheless be challenging. A particular paradox is the slow rate of growth of the underlying epidemic, whether in camels, human beings, or other reservoir species. Environmental contamination is perhaps one mechanism that might explain the persistence of MERS-CoV in the absence of an explosive epidemic. This hypothesis was given recent support by a bioinformatics analysis suggesting that MERS-CoV virus has the hard outer and inner shell necessary for environmental persistence.7
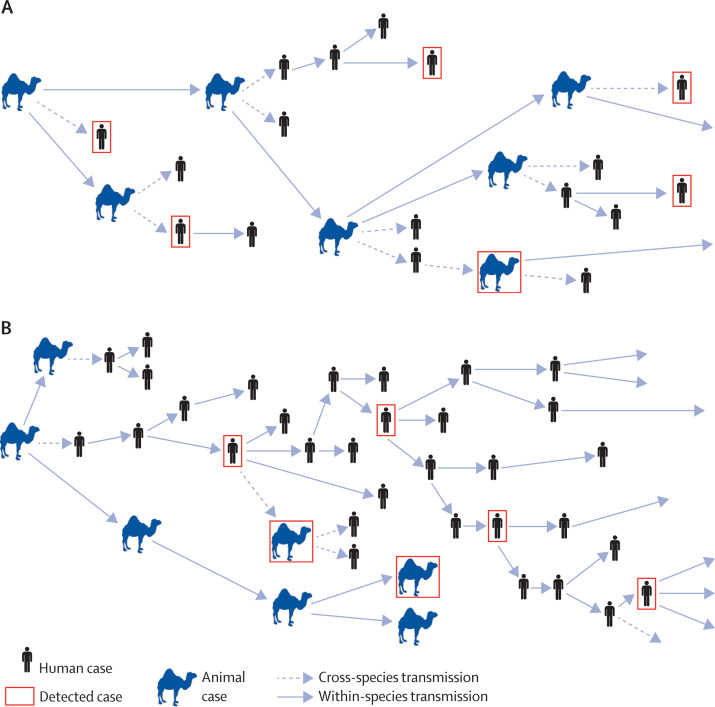
An understanding of the role of animals in the transmission of MERS-CoV is urgently needed to inform control efforts. This virus can spread from person to person, sometimes causing substantial outbreaks,8, 9 but whether the virus is capable of self-sustained (ie, epidemic) human-to-human transmission is unknown.10 If self-sustained transmission in people is not yet underway, intensive control and risk-reduction measures targeting affected animal species and their handlers might eliminate the virus from the human population. Conversely, if zoonotic exposure causes only a small fraction of human infections, then even intensive veterinary control efforts would have little effect on cases in people. The figure shows these scenarios and highlights that low case detection rates do not allow the scenarios to be distinguished from the data now available. About one in five human cases have reported exposure to animals;11 this might be an underestimate of true zoonotic exposure and growth in case incidence is slow, so there are reasons to be hopeful that animal-targeted controls might be effective.
Figure.
Possible MERS-CoV transmission scenarios
(A) Self-sustaining transmission in animals, causing spillover infections in people but no self-sustaining human-to-human transmission; control of the animal epidemic might eliminate the virus from human beings in this scenario. (B) Zoonotic exposure triggers a self-sustaining human-to-human epidemic, meaning animal-targeted controls will have only a limited effect on cases in people.
Qatar, supported by WHO and the Food and Agriculture Organization, has done an exemplary integrated outbreak investigation, with rapid collection of specimens from several animal species and the environment on the infected farm. But substantially more genetic and epidemiological data (eg, for timings of exposure events and symptom onsets) from both animal and human cases are needed to unravel the complex transmission dynamics of this virus. Passive and active surveillance in human beings and animals needs to be enhanced across the affected region, and livestock trading and movement patterns characterised and monitored. Prompt reporting of outbreaks within the frameworks provided by present human and animal health regulations is essential, but we would add the caveat that such reports need to be as comprehensive as possible to be useful. The handling of the outbreak reported here1 is a model that we hope is more widely adopted in future.
Acknowledgments
The Medical Research Council provided centre funding. The funder had no role in the writing of this Comment.
References
- 1.Haagmans BL, Al Dhahiry SHS, Reusken CBEM. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70690-X. published online Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Organisation for Animal Health (OIE) MERS-CoV, Qatar. http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=14454 (accessed Dec 10, 2013).
- 3.Ministry of Health Portal Kingdom of Saudi Arabia MOH: a new way discovered to recognize the source of novel coronavirus. http://www.moh.gov.sa/en/CoronaNew/PressReleases/Pages/mediastatemenet-2013-11-11-001.aspx (accessed Dec 11, 2013).
- 4.Reusken CBEM, Haagmans BL, Müller MA. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perera RA, Wang P, Gomaa MR. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18:20574. doi: 10.2807/1560-7917.es2013.18.36.20574. [DOI] [PubMed] [Google Scholar]
- 6.Memish ZA, Mishra N, Olival KJ. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013 doi: 10.3201/eid1911.131172. published online November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh GK, Dunker AK, Uversky V. Prediction of intrinsic disorder in MERS-CoV/HCoV-EMC supports a high oral-fecal transmission. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.22254b58675cdebc256dbe3c5aa6498b. published online Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assiri A, McGeer A, Perl TM. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijawi B, Abdallat M, Sayaydeh A. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(suppl 1):S12–S18. [PubMed] [Google Scholar]
- 10.Cauchemez S, Fraser C, Van Kerkhove MD. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14:50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The WHO MERS-CoV Research Group State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. published online Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]