Abstract
Pneumonia has been a leading cause of death in both developed and developing countries as long as health indicators have been available. Yet the issues of concern for this syndrome are far from static. Improvements in access to health services have lowered infant mortality rates, benefiting children around the world and lowering the fraction of child deaths caused by pneumonia. However, progress has been interrupted repeatedly by the emergence of new pathogens. Landmark randomized controlled trials have now demonstrated the effectiveness of Haemophilus influenzae type b (Hib) and multivalent pneumococcal conjugate vaccines against childhood pneumonia, as well as meningitis and bacteremic disease. Momentum has gathered to tackle long-standing economic obstacles to expand access to new vaccines and programs for the poorest countries of the world. A pressing challenge for the control of pneumonia in developing countries is to identify better metrics for pneumonia. Surveillance tools are needed that will bridge studies of interventions, establish preventable disease burden, and serve as indicators for monitoring new programs.
Burden of pneumonia in children
Deaths
Pneumonia and influenza rank among the top 10 causes of deaths worldwide.1 Based on advances in access, pediatric care, and changing population demographics, relatively few pediatric pneumonia deaths still occur in industrialized countries, where deaths from pneumonia now are concentrated among older adults. Recent efforts led by the World Health Organization (WHO) to standardize estimates of burden of disease across program areas identify acute respiratory infections (ARIs) as a leading cause of death among children in developing countries; however, the proportion of all deaths attributable to ARIs declines as childhood mortality rates fall.2 WHO has determined that ARI is the most common infectious cause of death in children younger than 5 years, with an estimated 1.89 million (95% confidence limit, 1.58–2.19 million) deaths occurring in children younger than 5 years olds.2 Although the proportion of deaths attributable to ARI among European children younger than 5 years of age was 11 percent, ARI caused 22 and 19 percent of such deaths in Africa and Asia, respectively. The vast majority of deaths attributable to ARI are caused by pneumonia, with bronchiolitis accounting for most of the remaining deaths. Although upper respiratory infections are extremely common occurrences, they typically are mild. We propose to use the term “pneumonia” in the broader sense to refer to severe acute infections of the lungs, including infections by viral, bacterial, and other pathogens, and involving the alveoli, bronchioles, bronchi, and occasionally pleura and other tissues. The United Nation’s Millennial Development Goal (http://www.unmilleniumproject.org) is to reduce mortality rates by two-thirds by 2015 among children younger than 5 years old. However, the magnitude of illness attributable to pneumonia is such that countries seeking to achieve that goal will not be successful unless they give attention to pneumonia prevention and control.
Beyond mortality
Between 1970 and 2000, the number of countries with infant mortality rates greater than 90 per 1000 fell from 79 to 23 (UN Environment Program, 2003). As mortality rates for both infants and children younger than 5 years old decline in developing countries, child survival data alone no longer are sufficiently sensitive or specific to guide policy makers in decision-making regarding health program investments. Comparing disease burden trends and evaluating the impact of programs between countries and/or regions also is difficult without considering major influences such as the prevalence of human immunodeficiency virus (HIV). Newer surveillance tools are needed that measure pneumonia morbidity, in addition to mortality rates. Regional comparisons of morbidity rates must consider the impact of important factors driving overall child health status, such as neonatal health, prevalence of acquired immunodeficiency syndrome (AIDS) and malaria, and population coverage with vaccines, as well as case management interventions.3
Beyond children
Much surveillance and modeling have focused on pneumonia or ARI in children. However, pneumonia also is a major problem in adults, and children can be important reservoirs for respiratory pathogens, including pneumococcus and influenza. Thus, important links exist between children and adults for risks related to the development of pneumonia, as well as opportunities for prevention, and future surveillance may benefit from linking these concerns.
History repeats itself: The past as prelude
Trends in pediatric pneumonia mortality rates in the United States may provide lessons for global public health challenges in the 21st century. During the second half of the 20th century, pneumonia deaths in children in the United States declined by 97 percent, from 24,637 in 1939 to 800 in 1996 (Fig 1). 4 The first steep declines occurred from 1944 to 1950, likely influenced by the availability of penicillin (Fig 2). Declines were reversed swiftly with the emergence of a new pathogen—the 1957 pandemic influenza virus (influenza A H2N2). Further declines from 1966 to 1982 appear to be attributable to expanded access to medical care, notably the Medicaid legislation (Title XIX, enacted in 1965), and increased availability of treatment services for lower-income populations. Expanded access to care, such as case management referral strategies, and antimicrobial agents for symptomatic children have measurable effects on mortality rates in many developing countries,5 but the emergence of new pathogens, in particular the HIV and antimicrobial resistant-bacteria, has slowed the progress or fully reversed it in some areas.
Figure 1.
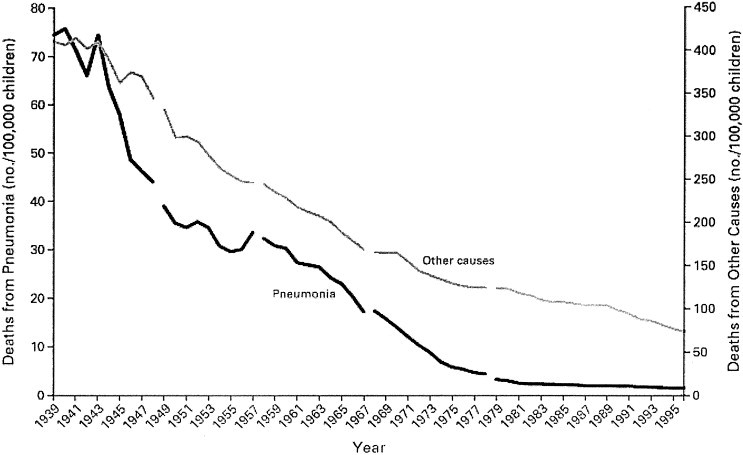
Deaths from pneumonia and from other causes in childhood from 1939 through 1996 in the United States. Gaps in the curves indicate changes in the International Classification of Diseases codes. Reprinted with permission.4
Figure 2.
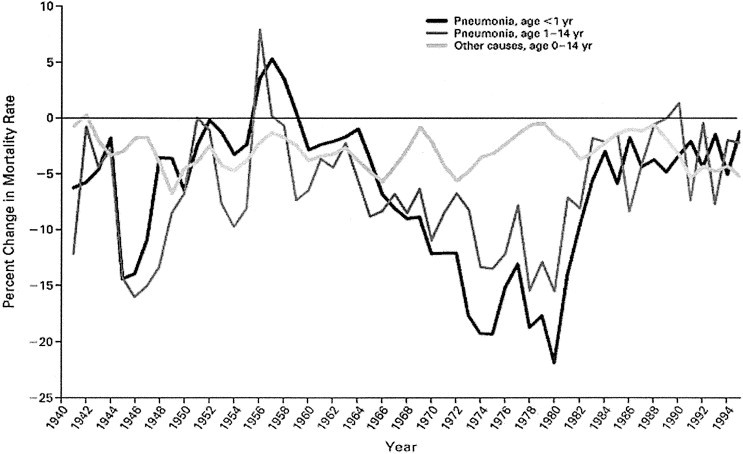
Annual percent change in rates of mortality from pneumonia and from other causes in infants and older children, from 1939 through 1996. The curves were smoothed by using a 3-year moving average (ie, the percent change for each year is an average of the changes for that year and for the previous and subsequent years). Reprinted with permission.4
Etiologic agents and diagnosis of pneumonia
Rates of pneumonia are considerably higher in developing countries, where a larger proportion of disease burden is attributed to bacterial causes. Establishing the etiologic agent responsible for pneumonia is difficult. Intensive studies have failed to identify a pathogen in a substantial proportion of episodes, and both bacteria and viruses can be detected from upper respiratory specimens of controls. Coinfection with multiple organisms, particularly viruses together with bacteria, also complicates interpreting data from etiology studies.
Perhaps the clearest evidence for causal agents in episodes of pneumonia comes from those studies that have included transthoracic aspiration from areas of pulmonary consolidation. Lung aspirate studies conducted between 1971 and 1988 and reviewed by the WHO found bacterial etiologies in 54.6 percent4, 5, 6 of 835 children6; the same review found blood cultures were positive for bacterial agents in 13.4 to 26.1 percent of children younger than 5 years old. Combined data from lung aspirate and blood culture evaluations revealed that Streptococcus pneumoniae (37.9%), Haemophilus influenzae (33.2%), and Staphylococcus aureus (7.9%) were the bacteria most commonly isolated.6 In young children, respiratory syncytial virus (RSV) is the leading viral agent associated with pneumonia, although its frequency in a community fluctuates with epidemics.7, 8 Whereas pneumococcus and H. influenzae predominate among infants and toddlers, atypical bacteria (Mycoplasma pneumoniae, Chlamydophila pneumoniae)9 and viruses appear more often in older children. The contribution of certain pathogens, such as atypical bacteria and influenza viruses, to the total burden of lower respiratory infections in tropical regions has not been studied well.
Ideally, clinical management of pneumonia would be based on knowledge of the pathogen causing infection, but the etiologic agent responsible for individual pneumonia episodes is surprisingly elusive for a large proportion of cases, even when extensive efforts are made. Most treatment guidelines use empiric approaches to assess whether the illness is bacterial and thus would benefit from treatment with antimicrobial agents, and they incorporate knowledge regarding likely pathogens and recent resistance patterns to further target selection of therapy. Our understanding of which pathogens are major causes of pneumonia may be biased by the sensitivity and specificity of existing diagnostic tests, including those dependent on classic microbiology and newer molecular approaches. Making a pathogen-specific diagnosis in cases of pneumonia is challenging in developed countries, even when the cost of testing is not a concern; in developing countries, limited access to radiology and laboratory testing further constrains obtaining information on the diagnosis of pneumonia and of etiologic agents.
Prevention studies also benefit from diagnostic precision on the etiology of episodes of pneumonia.10 A randomized controlled trial of Hib conjugate vaccine in Gambia found 10 cases of Hib pneumonia proven by blood culture or lung aspirate among recipients of control vaccine versus no cases among recipients of the Hib conjugate (vaccine efficacy of 100%); the same trial estimated that 50 cases of radiographically proven pneumonia were prevented, with a vaccine efficacy against x-ray pneumonia of 21 percent.11 A randomized controlled trial of 7-valent pneumococcal conjugate vaccine in children in Northern California found 97.4 percent effectiveness against invasive pneumococcal disease and 20.5 percent effectiveness against pneumonia documented by x-ray; yet the difference in incidence between vaccine recipients and controls in these end points was 165 invasive disease cases per 100,000 compared with 240 episodes of x-ray pneumonia per 100,000, respectively.12 The burden of all pneumonia that could be prevented by one of these vaccines might be greatly underestimated, with an emphasis on laboratory-confirmed, invasive disease alone.
Attempts to improve the ability to diagnose pneumonia have included evaluations of clinical signs and symptoms, which led the WHO to recommend use of age-specific cutoffs for rapid respiratory rate and the presence of chest wall in-drawing as signs of severe pneumonia.6 This focus had a principal goal of enhanced sensitivity, to identify as many possible true cases to offer potentially life-saving therapy. Other studies have focused on enhancing the specificity of diagnosis, typically a goal for vaccine evaluations, so that one can measure the efficacy of a pathogen-specific intervention (eg, 7-valent pneumococcal vaccine) against only those pneumonias the vaccine was designed to prevent. Even differentiating bacterial from viral pneumonias remains challenging, although recent studies show that serologic markers such as procalcitonin may hold some promise.13 Confirming pathogen-specific, let alone serotype-specific, etiologies for pneumonia remains difficult unless lung aspirates are performed, and they are appropriate only when certain radiographic patterns are evident. Substantial efforts recently were devoted to standardizing the interpretation of x-rays from children suspected of having pneumonia, so that a common language for interpreting trials of different pneumococcal and Hib vaccines could be established. The WHO has posted the resulting standard tool on its web site14; the tool focuses on identification of alveolar consolidation and/or effusions. Researchers are evaluating improvements in this tool, and some experts have raised the concern that consolidation, with or without effusions, is an insensitive indicator of bacterial pneumonia in HIV-infected children.15
Seasonal variations in the incidence of pneumonia pathogens
Most pathogens causing pneumonia vary in incidence on a regularly repeating annual cycle; this pattern is most prominent with the major viral pathogens but also is evident with important bacterial pathogens (Fig 3). Influenza exhibits a distinct seasonality, with winter peaks in temperate regions occurring with sufficiently regular onset and with bands of latitude around the globe having sufficiently similar timing to allow for advance planning and preparation of the annual trivalent vaccine.16 Similarly, outbreaks of RSV occur with regularity each winter and spring in temperate climates, and the predicted timing of these outbreaks is critical for making recommendations for prophylaxis with several months of RSV-specific monoclonal antibodies for high-risk premature infants and others.17 The timing of influenza and other respiratory viruses in tropical regions is described poorly, providing an impediment to control of these diseases in much of the developing world.
Figure 3.
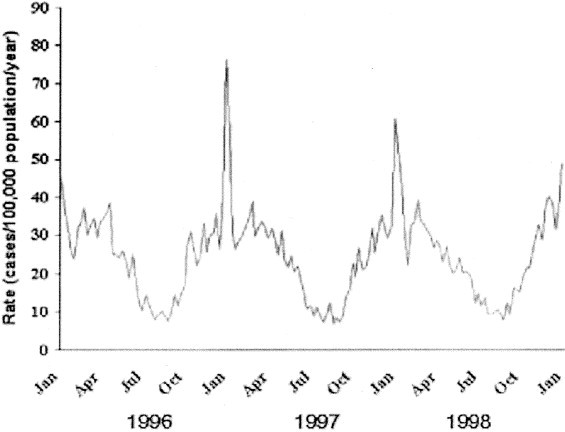
Weekly rates of invasive pneumococcal disease in the United States, January 1996 to December 1998. Weekly numbers of cases from active surveillance areas in California, Connecticut, Georgia, Maryland, Minnesota, Oregon, and Tennessee were divided by the population under surveillance that year and multiplied by 52 to give annualized weekly rates. Reprinted with permission.18
Even less is understood about the underlying reasons for seasonal variation in respiratory pathogens, and this lack of understanding impairs our ability to anticipate and respond to future outbreaks, as highlighted by the disappearance of severe acute respiratory syndrome (SARS) coronavirus and the uncertainty over when or if it will return.18 What causes seasonal respiratory pathogens to reappear each winter, and perhaps more importantly, what causes them to disappear? Is it the cold? Dry air? Do the pathogens migrate across the equator each year and return, or do they remain in a persistent or latent state within the mammalian hosts until some seasonal cue signals the time is right for re-emergence? The answers to such questions are an important area for future investigations and may hold practical implications for control of pneumonia in temperate as well as tropical regions in the future.
Risk factors for pneumonia in the 21st century
Both host and environment contribute to the risk that a child will develop pneumonia in the first few years of life. Numerous study designs, including case-control, cohort, and longitudinal surveillance approaches, have been applied to identifying risk factors for acquiring pneumonia in the developing world. Rates of pneumonia vary with age19, 20; rates are higher in those younger than 2 years of age compared with older children. Nutritional factors also influence risk of developing disease. Malnourished children are at higher risk of developing pneumonia and are more likely to die. Breastfeeding reduces the risk of the occurrence of episodes of pneumonia and death from ARIs.21, 22, 23 Comorbidities, in particular HIV infection, are major risk factors for the acquisition of pneumonia in developing countries.24 Recent studies in South Africa conducted in the context of a placebo-controlled vaccine trial estimated that hospitalization for pneumococcal bacteremic lower respiratory tract infection among HIV-infected infants who were 2 to 24 months old occurred at a rate of 1233/100,000 compared with a rate of 29/100,000 among uninfected children.24
In addition to host factors, the environment influences the risk of developing pneumonia by its effect on exposure to pathogens and to irritants that might increase susceptibility. Indoor air quality, as indicated by type of cooking fuel used, ventilation, and passive cigarette smoke, appears to be related to the frequency of episodes of pneumonia in children in developing countries. Both family size and household crowding have been linked to the risk of developing pneumonia; whereas the former also may be influenced by birth spacing and nutritional factors, the latter is a consistent finding in developed and developing countries. Poverty is linked with higher rates of pneumonia, but this rate may reflect a combination of nutritional, environmental, and behavioral influences. Sanitation influences many health outcomes and might affect the risk of developing pneumonia directly or indirectly. Interventions promoting handwashing have resulted in reductions in respiratory illness in children, although studies have been too small to identify impact on lower respiratory infections, and handwashing may be more effective in reducing viruses compared with respiratory bacteria. However, preceding viral infection increases the risk of developing some types of bacterial pneumonia; this connection has been well-illustrated for relationships between influenza and other respiratory virus infection and secondary pneumonia caused by pneumococcus, Staphylococcus aureus, and group A streptococcus.
Because rates of pneumonia in children are lower in developed countries, many features of development and increased standards of living likely promote reduced risk of developing respiratory infections, including pneumonia.
Emerging pathogens
The emergence of new pathogens has been a dominant theme in infectious diseases for centuries, and the 21st century already has seen emergence of major new respiratory pathogens including the SARS coronavirus, H3N2 Fujian strain influenza virus, and H5N1 avian influenza. Although children were relatively spared SARS CoV compared with adults,25 children have dominated in reports of severe outcomes associated with the 2003 to 2004 H3N2 Fujian strain in the United States26 and 2004 avian influenza in countries in Asia.
The 2004 epidemic of influenza A (H5N1), known as avian influenza, illustrates the potential for new pneumonia pathogens to affect children and the difficulties in measuring the burden of disease and guiding control measures in much of rural Asia and elsewhere in the developing world. The 1997 outbreak of influenza A (H5N1) in Hong Kong established for the first time that avian influenza viruses could infect humans directly, with a predilection for children and a resulting illness that was fatal in 6 of 18 patients.27, 28 The 2004 avian outbreak is far more widespread, with poultry disease reported across much of east and southeast Asia. Direct infection of humans was confirmed in patients hospitalized at major referral hospitals in Hanoi and Bangkok, raising concerns among many experts about the potential for pandemic spread (MMWR, accepted for publication). Given the wide geographic distribution of avian disease, the likelihood exists that human infections might also be more widespread, but surveillance for pneumonia in much of the region is passive or nonexistent, and laboratory capacity to identify routine pneumonia pathogens, especially less newly emergent ones, is quite limited.
The emergence of HIV-infection in the latter part of the 20th century has had a profound influence on pneumonia in children. HIV infection is estimated to increase the risk of developing pneumonia by 40- to 50-fold.15, 24 The clinical presentation of pneumonia may vary, with less-predictable radiographic patterns and a wider spectrum of pathogens associated with disease.15 HIV-infected children are at risk of acquiring opportunistic pathogens such as Pneumocystis carinii pneumonia, but they also have substantially higher risk of acquiring common bacterial agents such as S. pneumoniae and H. influenzae. Clinical algorithms for management of severe ARIs in children, produced for the developing world, are undergoing re-evaluation to address the reality that in many countries with high prevalence of AIDS, most episodes of severe respiratory disease requiring hospitalization will be associated with HIV infection. In Soweto, where an estimated 5 percent of the birth cohort was infected with HIV in 1997 and 1998, approximately 46 percent of hospitalized children who required x-rays for acute respiratory tract infection were infected with HIV.15 Empiric management may need to be altered in these circumstances, and offering counseling and testing for HIV to children with pneumonia may be a reasonable standard of care, particularly in locales where referrals for HIV support and treatment (eg, trimethoprim-sulfamethoxazole prophylaxis, antiretroviral treatment) are becoming available.
In addition to the emergence of previously unrecognized pathogens are the concerns about familiar pathogens that have continued to evolve and the emergence of resistance to commonly used drugs that now challenges empiric treatment approaches for many syndromes. As malaria, tuberculosis, and AIDS clearly show, resistance is not a problem limited to developed countries with easy access to antibiotics. Worldwide, antimicrobial resistance has emerged in several respiratory pathogens, including resistance to penicillin and cotrimoxazole within S. pneumoniae,29 beta-lactam-resistant H. influenzae,30 methicillin-resistant S. aureus,31 and amantadine-resistant influenza virus, to name a few. Development of programs to track resistance in pathogens of major importance in developing countries is challenging due to limited availability of laboratories and/or reagents needed for accurate susceptibility testing. A new manual aimed at enhancing detection of resistance in bacteria of major public health importance, including the respiratory pathogens S. pneumoniae and H. influenzae, now is available for reference laboratories in developing countries.32 Note: The manual can be ordered without charge through WHO (http://www.who.int/csr/resources/publications/drugresist/WHO-CDS-CSR-RMD-2003-6/en/).
Emerging vaccines
Vaccines had an enormous influence on morbidity and mortality in the 20th century.33 Increased global access to childhood vaccines through the Expanded Program on Immunization has reduced the incidence of respiratory illness associated with measles, pertussis, and diphtheria. In 1990, infants in developed countries began to benefit from introduction of Hib conjugate vaccines, with resulting declines in meningitis and bacteremic pneumonia. The 21st century already holds substantial promise that developing countries will benefit from recently developed vaccines, such as Hib conjugate, and even newer products targeted at pneumococcus, RSV, and influenza.
Haemophilus influenzae b vaccines
Hib conjugate vaccines were developed to counter childhood meningitis, which in developing countries can be fatal in 20 to 30 percent of episodes, with additional risk to survivors of suffering long-term sequelae (eg, hearing loss, learning disabilities). Hib conjugate vaccines are highly effective against meningitis and other invasive diseases caused by Hib,34, 35, 36 including bacteremic pneumonia, and proved to have important herd effects by reducing acquisition of oropharyngeal colonization, thereby decreasing spread of the organism and protecting unimmunized persons.37, 38 This herd effect resulted in greater than anticipated benefits of vaccine introduction and had important implications for developing countries, where partial immunization may be a frequent finding. Vaccine efficacy trials performed in the Gambia11 and Chile39, 40 demonstrated that in addition to excellent vaccine efficacy being achieved against meningitis and other invasive disease, vaccinated children had 21 to 26 percent less severe pneumonia, defined by consolidation and/or effusions on radiographs, than did placebo recipients. Although the proportion of episodes of pneumonia that could be attributed to Hib had been difficult to determine from etiology studies, these vaccine trials suggested that as much as one-fourth of severe pneumonia in young children was preventable via Hib conjugate vaccine. The trials essentially used the Hib vaccine as a ‘probe’ to uncover the proportion of disease attributable to the pathogen and preventable via the specific vaccine.
To help overcome the traditional delay between adoption of vaccines in industrialized countries and use in developing countries, the Global Alliance for Vaccines and Immunizations (GAVI) and the Global Vaccine Fund have catalyzed a series of activities to promote accelerated uptake of new and underutilized vaccines, including Hib vaccine. As of December 2003, GAVI has committed to provide Hib-containing vaccines to 16 countries (http://www.vaccinealliance.org/home/Support_to_Country/Country_Status/index.php). Although cost traditionally has been a barrier to broader implementation, appreciation for disease burden, particularly in Asia, also has limited uptake of Hib vaccine regionally. A Hib rapid assessment tool has been developed to assist national authorities in developing countries with assessment of local disease burden and decision-making about introduction of vaccine (even when cost is no barrier).41 (available at http://www.who.int/vaccines-documents/DocsPDF01/www625.pdf.)
To address major uncertainty about the burden of Hib disease in Indonesia, a community randomized evaluation of the vaccine-preventable burden of pneumonia and meningitis was performed among more than 55,000 children in Lombok42 (accessible at http://www.icaac.org/43ICAAC/Latebreakerabstracts.pdf, abstract G-2054a). Communities were randomized to receive Hib conjugate vaccine combined with DTP or DTP alone, through the routine immunization program. Active surveillance targeted radiographically defined pneumonia as well as meningitis, defined by both clinical and microbiologic endpoints. Preliminary results of the trial suggest that a greater than expected burden of vaccine-preventable clinically defined meningitis exists in children in Lombok (rates 154/100,000 children <2 years), but limited or no benefit of introduction of a vaccine on respiratory end points. The findings are consistent with overall benefit to the population from Hib vaccine, but the lack of benefit for respiratory end points was challenging to interpret. Hib may not be an important cause of pneumonia in Indonesian children, or it may cause disease in children too young to have been vaccinated during the trial, or antibodies induced by Hib conjugate vaccine may have been sufficient to prevent the acquisition of invasive disease, including meningitis, but not sufficient to protect against the development of nonbacteremic pneumonia. Differential impact on pneumonias among children who did not present to healthcare facilities also might have influenced trial results. Data regarding colonization studies and additional analysis of the trial should be forthcoming. Additional evaluations of Hib vaccine in Asia also are likely to occur during the next few years.
Although controversy remains regarding Hib vaccine in Asian children, high disease burden and clear-cut benefits for meningitis and pneumonia have been demonstrated for children in numerous countries in Africa. The GAVI offered the 94 poorest countries of the world funds for strengthening immunization, including access to 5 years of Hib vaccines; 16 countries are introducing Hib vaccine through this program. Current priorities are to monitor the impact of introduction of vaccine, identify lessons learned from new adopters, and apply these lessons to future introduction programs for vaccines still under investigation, including rotavirus vaccine, meningococcal A conjugate, and pneumococcal conjugate vaccines.
Pneumococcal conjugate vaccines
Children in the United States already have benefited from introduction of 7-valent pneumococcal conjugate vaccine, which was licensed in 2000 for routine use. Through 2001, invasive pneumococcal disease in the population younger than 2 years old had dropped by 69 percent, and evidence of significant herd effects was already available for the 20- to 39-year-old age group (32% decline) and for those 65 years and older (18% decline).43 Declines have continued to occur in 2002 and beyond. Although serotype distribution in many other parts of the world suggests that higher valency formulations will be needed,44 9- and 11-valent pneumococcal conjugate vaccines are in development stages. The 9-valent formulation from Wyeth has been studied in nearly 40,000 South African children and was 83 percent effective in reducing the incidence of invasive disease caused by vaccine-serotypes in non-HIV-infected children and 20 to 25 percent effective against radiographically defined pneumonia with alveolar consolidation in the same population (intent-to-treat and per protocol analyses, respectively).45 Efficacy was 65 percent for vaccine-type invasive disease in HIV infected patients but only 13 percent (with 95% confidence interval -7 to 29%, ie, not significantly different from zero) in HIV infected. Other large clinical trials are ongoing with 9-valent vaccine in the Gambia and an 11-valent formulation in the Philippines; both of these studies include pneumonia and invasive disease end points. Although efficacy against invasive disease caused by vaccine serotypes consistently has been demonstrated for conjugate pneumococcal vaccines, the ultimate effectiveness of this kind of vaccine for noninvasive syndromes, including pneumonia, has been challenging to measure. The standardization of pneumonia endpoints determined by radiology has been a focus of WHO,14 and standard tools for measuring colonization across clinical trials also have been developed.46 A new challenge will be to bridge from the results of efficacy trials of radiographic pneumonia to end points that can be tracked more easily in diverse developing countries. Thus, trial investigators and other surveillance efforts also are tracking potentially preventable respiratory disease burden measured through more accessible indicators, such as hospital admissions for pneumonia. To accelerate development of pneumococcal conjugate vaccines, the Gates Foundation is supporting the PNEUMO-ADIP (Accelerated development and introduction plan) (www.preventpneumonia.com), which has in turn funded regional surveillance networks and enhanced research activities performed at vaccine field trial sites and elsewhere. Numerous activities also are focused on vaccine financing and marketing to facilitate optimal interplay between vaccine demand and supply for public markets.
Although pneumococcal conjugate vaccines already have been shown to have efficacy against invasive disease and pneumonia, conjugate vaccines do not lead to reduced pneumococcal colonization of the nasopharynx; instead, vaccine-serotypes become less common but are replaced by other serotypes.47 This problem was not observed in Hib colonization studies after use of Hib conjugate vaccine and may reflect the wide diversity of pneumococcal types that colonize the nasopharynx. Whether replacement with nonvaccine serotypes eventually will blunt the impact of conjugate vaccines on pneumococcal invasive disease and pneumonia is not known yet.
Future pneumococcal vaccines
In addition to evaluating higher valency conjugate vaccines, researchers and industry are studying the use of proteins that are common to all pneumococci as antigens for candidate vaccines. PspA, PsaA, and pneumolysin all have been studied in animals, and additional efforts are being made to explore the value of this approach to pneumococcal prevention.48, 49 Serotype diversity and possible replacement disease should not be an issue with common proteins, and some authorities also consider that manufacturing costs would be lower, potentially translating to lower cost and products that are more affordable for developing country markets. Common protein vaccines also are promising as potential tools for prevention of noninvasive pneumonia in adults, which is a major cause of illness and death in developed countries.
Influenza vaccines
During the past several years, a growing appreciation for the substantial burden of influenza among young children has resulted in expanded recommendations for routine immunization in this group. In 2002, the American Academy of Pediatrics and Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices formally encouraged the immunization of children 6 through 23 months of age, with the ultimate goal being universal immunization among young children.50, 51 The burden of influenza in tropical and developing regions is not well appreciated, but recent evidence implies that, contrary to predictions based on the attenuation of influenza by hot weather, the burden among children in tropical and subtropical regions may be as great or greater than that in temperate zones.52 Better information about the occurrence of influenza in the tropics, seasonal patterns, and the burden of disease in young children in rural areas will be important for guiding future efforts to control pneumonia associated with influenza infection in developing countries.
Novel approaches to pneumonia surveillance
If control of pneumonia among children in the developing world is to move from the case-management approach advocated since the early 1980s to an approach based on the prevention of infection with specific pathogens, surveillance systems capable of measuring and monitoring the burden of disease from these pathogens will be required to inform decisions on major control efforts such as the introduction of expensive new vaccines. One example of an approach to such surveillance systems was implemented in 2002 by the Thai Ministry of Public Health, with support from the International Emerging Infections Program (IEIP) of the U.S. Centers for Disease Control and Prevention. The IEIP surveillance is a population-based system conducted throughout two provinces that focuses on complete ascertainment of severe pneumonia, digitalization of radiographic images, and comprehensive testing for a battery of pneumonia pathogens.53 Although the system focuses on the causes and burden of severe pneumonia requiring hospitalization, periodic community surveys measure the burden of less severe pneumonia and other respiratory illnesses. A focus on laboratory testing is aimed at permitting estimation of disease caused by potentially available vaccines, such as influenza vaccines and the conjugate Hib and pneumococcal vaccines. A second IEIP in Kenya will expand and adapt the surveillance to the African setting in 2004 and may help to provide rigorous data on the burden of disease caused by specific pneumonia pathogens to help guide control measures. Strengthened surveillance of pneumonia in other countries will be needed in the future as new vaccines and other tools to control pneumonia become more widely available.
Conclusions
Pneumonia continues to be a major health problem in children in developing countries, despite strides made through increased access to case-management strategies. Expanded access to Hib conjugate vaccine and continued development of pneumococcal and RSV vaccines are needed to have substantial impact on continued disease burden. New pathogens pose ongoing threat to control of pneumonia, either by causing pneumonia (eg, human metapneumovirus, SARS coronavirus, or avian influenza) directly or by drastically modifying host susceptibility to pneumonia agents (as is the case with HIV). The pneumonia research field has suffered from fragmentation between those evaluating improvements in treatment (eg, integrated management of childhood illness, vitamin A and zinc supplementation, short course antibiotics) and those investigating prevention (pneumococcal, Hib, RSV vaccines). Both groups will benefit from having common tools for diagnosis and surveillance of pneumonia and from collaborating on achieving real progress in prevention and control of pneumonia.
References
- 1.Murray C., Lopez A. Harvard School of Public Health; Boston: 1996. Global Health Statistics: A Compendium of Incidence, Prevalence and Mortality Estimates for Over 200 Conditions. [Google Scholar]
- 2.Williams B.G., Gouws E., Boschi-Pinto C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 3.Jones G., Steketee R.W., Black R.E. Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 4.Dowell S.F., Kupronis B.A., Zell E.R. Mortality from pneumonia in children in the United States, 1939 through 1996. N Engl J Med. 2000;342:1399–1407. doi: 10.1056/NEJM200005113421904. [DOI] [PubMed] [Google Scholar]
- 5.Sazawal S., Black R.E. Meta-analysis of intervention trials on case-management of pneumonia in community settings. Lancet. 1992;340:528–533. doi: 10.1016/0140-6736(92)91720-s. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO; Geneva: 1991. Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. Report No.: WHO/ARI/91.20. [Google Scholar]
- 7.Weber M.W., Mulholland E.K., Greenwood B.M. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Weber M.W., Dackour R., Usen S. The clinical spectrum of respiratory syncytial virus disease in The Gambia. Pediatr Infect Dis J. 1998;17:224–230. doi: 10.1097/00006454-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Principi N., Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2000;1:334–344. doi: 10.1016/S1473-3099(01)00147-5. [DOI] [PubMed] [Google Scholar]
- 10.Mulholland K., Levine O., Nohynek H. Evaluation of vaccines for the prevention of pneumonia in children in developing countries. Epidemiol Rev. 1999;21:43–55. doi: 10.1093/oxfordjournals.epirev.a017987. [DOI] [PubMed] [Google Scholar]
- 11.Mulholland K., Hilton S., Adegbola R. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 12.Black S., Shinefield H., Fireman B. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Moulin F., Raymond J., Lorrot M. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child. 2001;84:332–336. doi: 10.1136/adc.84.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Pneumonia Vaccine Trial Investigators’ Group . WHO; Geneva: 2001. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Report No.: WHO/V&B/01.35. [Google Scholar]
- 15.Madhi S., Cumin E., Klugman K.P. Defining the potential impact of bacterial polysaccharide-protein vaccines in reducing the burden of pneumonia in HIV-1 infected and un-infected children. Pediatr Infect Dis J. 2002;21:393–399. doi: 10.1097/00006454-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Cox N., Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 17.Stensballe L.G., Devasundaram J.K., Simoes E.A. Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(suppl 2):S21–S32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 18.Dowell S. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaman K., Baqui A.H., Yunus M. Acute respiratory infections in children: a community-based longitudinal study in rural Bangladesh. J Tropical Pediatr. 1997;43:133–137. doi: 10.1093/tropej/43.3.133. [DOI] [PubMed] [Google Scholar]
- 20.Usen S., Adegbola R., Mulholland K. Epidemiology of invasive pneumococcal disease in the Western Region, The Gambia. Pediatr Infect Dis J. 1998;17:23–28. doi: 10.1097/00006454-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Koch A., Molbak K., Homoe P. Risk factors for acute respiratory tract infections in young Greenlandic children. Am J Epidemiol. 2003;158:374–384. doi: 10.1093/aje/kwg143. [DOI] [PubMed] [Google Scholar]
- 22.Arifeen S., Black R.E., Antelman G. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:E67. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 23.Yoon P.W., Black R.E., Moulton L.H. Effect of not breastfeeding on the risk of diarrheal and respiratory mortality in children under 2 years of age in Metro Cebu, The Philippines. Am J Epidemiol. 1996;143:1142–1148. doi: 10.1093/oxfordjournals.aje.a008692. [DOI] [PubMed] [Google Scholar]
- 24.Madhi S.A., Petersen K., Madhi A. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–176. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 25.Hon K.L., Leung C.W., Cheng W.T. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Update: influenza-associated deaths reported among children aged <18 Years—United States, 2003–04 influenza season. MMWR. 2004;52:1286–1288. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Isolation of avian influenza A(H5N1) viruses from humans-Hong Kong, May-December 1997. MMWR. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 28.Yuen K.Y., Chan P.K., Peiris M. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 29.Schrag S.J., Beall B., Dowell S.F. Limiting the spread of resistant pneumococci: biological and epidemiologic evidence for the effectiveness of alternative interventions. Clin Microbiol Rev. 2000;13:588–601. doi: 10.1128/cmr.13.4.588-601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorgensen J.H., Doern G.V., Maher L.A. Antimicrobial resistance among respiratory isolates of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the United States. Antimicrob Agents Chemother. 1990;34:2075–2080. doi: 10.1128/aac.34.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Four Pediatric Deaths from Community-Acquired Methicillin-Resistant Staphylococcus aureus-Minnesota and North Dakota, 1997–1999. MMWR. 1999;48:707–710. [PubMed] [Google Scholar]
- 32.World Health Organization . WHO, CDC, USAID; Geneva: 2003. Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health concern in the developing world. Report No.: WHO/CDS/CSR/RMD/2003.6. [Google Scholar]
- 33.Centers for Disease Control and Prevention Achievements in public health, 1900–1999 impact of vaccines universally recommended for children-United States, 1990–1998. MMWR. 1999;48:243–248. [PubMed] [Google Scholar]
- 34.Peltola H., Kilpi T., Antila M. Rapid disappearance of Haemophilus influenzae type b meningitis after routine childhood immunisation with conjugate vaccines. Lancet. 1992;340:592–594. doi: 10.1016/0140-6736(92)92117-x. [DOI] [PubMed] [Google Scholar]
- 35.Santosham M., Wolff M., Reid R. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med. 1991;324:1767–1772. doi: 10.1056/NEJM199106203242503. [DOI] [PubMed] [Google Scholar]
- 36.Black S.B., Shinefeld H.R., Fireman B. Efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine in a United States population of 61,080 children. Pediatr Infect Dis J. 1991;10:97–104. doi: 10.1097/00006454-199102000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Adams W.G., Deaver K.A., Cochi S.L. Decline of childhood Haemophilus influenzae type b disease in the Hib vaccine era. JAMA. 1993;269:221–226. [PubMed] [Google Scholar]
- 38.Takala A.K., Eskola J., Leinonen M. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–986. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 39.Lagos R., Horwitz I., Toro J. Large scale, postlicensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: Practicality and effectiveness in preventing invasive Haemophilus influenzae type b infections. Pediatr Infect Dis J. 1996;15:216–222. doi: 10.1097/00006454-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Levine O.S., Lagos R., Munoz A. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization . WHO; Geneva: 2001. Estimating the local burden of Hib disease preventable by vaccination: a rapid assessment tool. Report No.: WHO/V&B/01.27. [Google Scholar]
- 42.Gessner B.D., Group L.H.S. Interscience Conference on Antimicrobial Agents and Chemotherapeutics; 2003 September 2003. American Society of Microbiology; Chicago: 2003. A randomized controlled clinical trial of the effect of Hib vaccine on pneumonia and meningitis in children living on Lombok Island, Indonesia. [Google Scholar]
- 43.Whitney C.G., Farley M.M., Hadler J. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 44.Haussdorff W.P., Bryant J., Paradiso P.R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 45.Klugman K.P., Madhi S.A., Huebner R.E. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization: Pneumococcal Vaccine Trials Carriage Working Group Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–e11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien K.L., Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine. 2003;21:1815–1825. doi: 10.1016/s0264-410x(02)00807-1. [DOI] [PubMed] [Google Scholar]
- 48.Briles D.E., Hollingshead S.K., Nabors G.S. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine. 2000;19:S87–S95. doi: 10.1016/s0264-410x(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 49.Romero-Steiner S., Pilishvili T., Sampson J.S. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin Diagn Lab Immunol. 2003;10:246–251. doi: 10.1128/CDLI.10.2.246-251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson W.W., Shay D.K., Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 51.Bridges C.B., Harper S.A., Fukuda K. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2003;52(RR 8):1–34. [PubMed] [Google Scholar]
- 52.Chiu S.S., Lau Y.L., Chan K.H. Influenza-related hospitalizations among children in Hong Kong. N Engl J Med. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 53.Dowell S., Chunsuttiwat S., Olsen S. The International Emerging Infections Program, Thailand—An Early Report. In: Scheld W.M., Hughs J.M., Murray B.E., editors. Emerging Infections 6. ASM Press; Washington, DC: 2004. [Google Scholar]


