Abstract
Molecular diagnostic techniques for viral testing have undergone rapid development in recent years. They are becoming more widely used than the classical virological assays in the majority of clinical virology laboratories, and now represent a new method for the diagnosis of human viral infections. Recently, new techniques based on multiplex RT‐PCR amplification followed by microarray analysis have been developed and evaluated. On the basis of amplification of viral genome‐specific fragments by multiplex RT‐PCR and their subsequent detection via hybridization with microorganism‐specific binding probes on solid surfaces, they allow simultaneous detection and identification of multiple viruses in a single clinical sample. The management of viral central nervous system and respiratory tract infections currently represents the two main applications of the microarrays in routine virological practice. Microarrays have shown reliable results in comparison with those of referenced (RT)‐PCR assays, and appear to be of major interest for the detection of a broad range of respiratory and neurotropic viruses, assessment of the pathogenicity of newly discovered or neglected viruses, and identification of multiple viral infections in clinical samples. Despite several limitations observed during the different studies performed, this new technology might improve the clinical management of patients by enlarging the range of the viruses detected, in particular in cases of severe infections leading to patient hospitalization in the intensive‐care unit. They might also help in the prevention of nosocomial transmission in hospital departments by contributing to the development of new epidemiological surveillance systems for viral infections.
Keywords: Central nervous system infection, DNA microarray, respiratory tract infection, virological diagnosis
Molecular diagnostic techniques for viral testing have undergone rapid development in recent years [1]. They are becoming more widely used than the classical virological assays (immunofluorescence assay and virus isolation in cell culture) in the majority of clinical virology laboratories, and now represent a new method for the diagnosis of human viral infections. More sensitive and more rapid than traditional methods, nucleic acid amplification tests have also allowed the detection of a broader panel of viruses in clinical specimens [2, 3, 4]. Recently, new techniques based on multiplex RT‐PCR amplification followed by microarray analysis have been developed and evaluated in clinical samples [5, 6, 7, 8]. Microarrays are divided into high‐density and low‐density DNA‐probe hybridization technologies. High‐density microarrays can test for thousands of potential pathogens simultaneously, allowing the detection of novel or previously uncharacterized agents, but they are not yet applicable for daily diagnosis in clinical virology practice. Only low‐density microarrays are currently CE‐marked for the in vitro diagnosis of human viral diseases. These new assays can allow rapid detection and identification, including typing and subtyping, of a broad panel of common and newly discovered human viral pathogens. The use of these microarrays might improve the clinical management of patients and the prevention of nosocomial transmission in hospital departments, and might allow the development of new epidemiological survey systems for viral infections [1, 5, 8].
Low‐density microarray technology is based on amplification of viral genome‐specific fragments, of <350 bp, by multiplex (RT‐)PCR, and their subsequent detection via hybridization with microorganism‐specific binding probes on solid surfaces, allowing simultaneous detection and identification of multiple viruses in a single clinical sample. In the commercially available microarrays, amplified products are labelled with biotin during the amplification step. They then hybridize with their respective specific probes immobilized in known sites of the microarray placed at the bottom of a single tube or of eight‐well strips. Incubation with streptavidin–peroxidase conjugate reagent leads, in the presence of the substrate, to the appearance of an insoluble product at the hybridization sites. Finally, a microarray reader piloted by specific software provided by the manufacturer allows the capture and processing of the picture obtained from the microarray [5, 6, 8, 9].
The first main application of the low‐density microarrays in routine virological practice consisted of the diagnosis of viral central nervous system (CNS) infections. Viruses are the main aetiological cause of CNS infections, ahead of bacterial and fungal causes [10]. PCR has been recognized as the reference method for the diagnosis of viral CNS infections in cerebrospinal fluid (CSF) specimens [11, 12]. The molecular tests used in routine diagnosis have to be specific and highly sensitive, allowing rapid and valuable detection of RNA and DNA viruses. At the present time, the diagnosis of viral CNS infections is usually obtained through the combination of multiple PCR and RT‐PCR assays, resulting in laboratory confirmation of c. 45% of physician‐diagnosed cases [13]. This failure can be explained by the inconsistency between the small volume of CSF available and the wide range of viruses potentially responsible for CNS infections, as well as their genetic characteristics (both DNA and RNA viruses), which also complicate rapid and large virological diagnosis using monoplex RT‐PCR and PCR assays [14]. The alternative of a multiplex PCR approach followed by microarray analysis allows optimization of the detection of neurotropic viruses. The previously published microarrays showed concordant results with single endpoint PCR tests used to assess the reliability of the method [15, 16, 17].
Recently, we evaluated the analytical and clinical performance of a commercially available multiplex RT‐PCR DNA microarray allowing rapid and simultaneous detection of nine DNA and RNA neurotropic viruses—herpes simplex virus type 1, herpes simplex virus type 2, varicella zoster virus, cytomegalovirus, Epstein–Barr virus, human herpesvirus type 6, human herpesvirus type 7 (HHV‐7), human herpesvirus type 8, and enterovirus (EV)—in a single CSF sample [9]. This evaluation was conducted in a first phase by testing proficiency samples of the 2008 and 2009 European proficiency panels (Quality Control for Molecular Diagnostics, Glasgow, UK) and, in a second phase, by testing 78 CSF specimens from patients hospitalized for CNS infections that had been previously tested with standardized commercially available PCR and RT‐PCR assays for neurotropic virus detection. The microarray demonstrated a limit of detection of <500 copies/mL for all six herpesviruses tested (no Quality Control for Molecular Diagnostics available for HHV‐7 and human herpesvirus type 8) and a lower sensitivity of >1000 copies/mL for EV detection. These results were similar to those recorded by the participants in the External Quality Assessment programmes, whatever the molecular technique used. The retrospective analysis of 68 CSF samples that initially tested positive for either herpesviruses or EV with standardized commercially available RT‐PCR and PCR assays confirmed the reliable diagnosis of the CNS infections by the microarray, as 27 of the 28 herpesvirus‐positive samples and all of the 30 EV‐positive CSF samples tested positive. Interestingly, the microarray detected 11 (37%) HHV‐7 and EV mixed infections among the 30 paediatric aseptic meningitis cases initially related to EV. Detection of HHV‐7 was confirmed by quantitative real‐time PCR assay, which demonstrated viral loads ranging from 60 to 300 genome copies per millilitre of CSF (mean value = 163 ± 96 copies/mL) [18]. Whereas EVs are well known neurotropic viruses, HHV‐7 infection remains a neglected topic [19]. Statistical analyses of the demographic, clinical and therapeutic characteristics revealed that HHV‐7 and EV mixed infection caused significantly longer lengths of stay at the hospital for children suffering from aseptic meningitis than for those infected with EV alone. The lack of correlation between HHV‐7 detection and CSF leukocyte counts suggested that the HHV‐7 DNA was from actively replicating virus and not just latent HHV‐7 DNA carried in inflammatory cells. Moreover, as the CSF samples had been routinely submitted to the virology laboratory for neurotropic virus detection from March 2002 to May 2009, no epidemiological link suggesting an outbreak of HHV‐7 infection can be established. These preliminary data led to the question of the role of HHV‐7 as an EV meningitis cofactor associated with increased severity of the disease. Finally, this first description of combined HHV‐7 and EV infection highlighted the advantage of the microarray technology for the detection of mixed viral CNS infections, as well as for the investigation of the pathogenicity of neglected viruses [8].
The second main application of microarrays is the management of respiratory tract infections (RTIs). We recently conducted two studies aimed at the evaluation of this technology in the virological diagnosis of RTIs. In the first, we prospectively tested nasal swabs or nasopharyngeal aspirates from adult and paediatric patients visiting the Reims University Medical Centre (northern France) for influenza‐like illnesses (ILIs) during the early stage of the French influenza A/H1N1 2009 pandemic. ILI can be related to A and B influenza viruses, including influenza A/H1N1 2009, but also to a large number of respiratory viruses [20]. Therefore, rapid and reliable screening of a large panel of respiratory viruses responsible for ILI is of major epidemiological and clinical interest for monitoring an influenza pandemic wave [21].
Ninety‐five respiratory samples collected in October 2009 were tested with a combination of two commercially available microarrays allowing rapid detection of influenza A virus strains, including the new A/H1N1 2009 strain and 20 other respiratory viruses [8]. Viruses were detected in 65 (68.4%) of the 95 respiratory samples tested with the microarrays, in agreement with the results of real‐time RT‐PCR assays [8]. Influenza A/H1N1 2009 was detected in only 30 (31%) samples, whereas rhinoviruses and parainfluenza viruses were important causes of ILI, with 25% and 10.5% prevalence, respectively. Moreover, the use of the microarrays revealed ten (10.5%) mixed infections, mainly influenza A/H1N1 2009 with coronavirus, human bocavirus (HBoV), human respiratory syncytial virus (hRSV), or human rhinoviruses (HRVs). The microarray technology thus appeared to be of major interest in clinical virology practice for rapid and accurate diagnosis of patients suffering from ILIs, which can be caused by a large range of respiratory viruses.
In the second study, the application of microarrays was assessed in the diagnosis and the epidemiological survey of viral infections in infants hospitalized for bronchiolitis. Bronchiolitis is an important manifestation of viral RTIs, and a large variety of viral pathogens, most notably hRSV, are implicated in the majority of hospitalized cases, with variable contributions from HRVs, Adenovirus (AdVs), human metapneumovirus A (hMPV‐A), human metapneumovirus B (hMPV‐B), influenza virus type A, influenza virus type B, parainfluenza virus type 1, parainfluenza virus type 2, and parainfluenza virus type 3 (PIV3) [22, 23]. In addition, non‐conventional pathogens (emerging or newly identified), such as the coronaviruses E‐229, NL63 and HKU1, HRV‐C and HBoV, have been associated with severe acute bronchiolitis cases [22, 23, 24].
One hundred and thirty‐eight nasopharyngeal aspirates collected from October 2007 to September 2008 were tested by direct immunofluorescence and viral culture, a combination of referenced RT‐PCRs, and a commercially available microarray allowing rapid and simultaneous detection of 17 DNA and RNA human respiratory viruses [6]. One or more viruses were detected in 126 (91%) of the specimens with the microarray, and similar results were obtained with referenced (RT)‐PCR assays [6]. As expected, the global detection rate appeared to be higher with the microarray than with classical techniques (direct immunofluorescence and virus isolation) (91% vs. 70%, p <10−3), even for common respiratory viruses such as hRSV‐A/B. Microarray analysis confirmed that hRSV‐A (52%), hRSV‐B (40%), HBoV (27%), AdVs (22%), PIV3 (15%), hMPV‐A, hMPV‐B (12%) and HRVs (8%) are the most frequently detected viruses in bronchiolitis cases [21, 22, 23, 24, 25]. The microarray also identified 85 (67%) mixed RTIs, whereas none was detected with the classical techniques. The most common associations were hRSV‐A/B with HBoV (32%), hRSV‐A/B with AdVs (30%), and hRSV‐A/B with PIV3 (23%). Furthermore, the screening of clinical specimens with the microarray appeared to be of major epidemiological interest for monitoring the circulation of viruses responsible for bronchiolitis in hospitalized infants. In addition to the classical epidemic circulation of hRSV‐A/B during the winter season, the microarray showed concomitant epidemic circulation of AdVs, PIV3, HBoV, hMPV‐A/B, HRVs and EV resulting in the majority of the mixed RTIs detected. Moreover, the epidemiological survey conducted over a period of 1 year showed a spring peak of HBoV infections that was not detected by use of the conventional techniques. With the results obtained by the microarray, the influence of the virus species and the impact of mixed viral infections on bronchiolitis severity were analysed. Statistical analyses revealed that none of the bronchiolitis severity criteria, including intensive‐care unit admission, O2 supply, O2 saturation percentage, O2 length, and length of stay at the hospital, was significantly increased in cases of mixed infections as compared with single infections, and that no specific viral combination was associated with the severity of the disease. Only infants infected with hRSV stayed longer in hospital [24]. However, given the large panel of multiple viral infections detected by the microarray, further multicentre studies will be necessary to assess whether specific viral associations could confer an elevated risk of severe bronchiolitis.
Regarding the practical aspects, the analyses performed with the microarrays could be performed with 5–10 μL of total nucleic acid extract obtained from a same aliquot of the clinical specimens. With the kits that we evaluated, the running time for amplification of multiplex RT‐PCRs was 3.30 h, and the time needed for hybridization of PCR products was 3 h. Microarray scanning and analysis were carried out within 15 min. In summary, the total time needed to complete the assay was c. 8 h from specimen extraction to microarray detection, allowing the laboratory to provide the answer to the clinician in a single working day. Moreover, for each sample analysed, the accuracy of the microarray analysis was controlled during extraction and amplification through an internal control, and during hybridization with at least three probes per amplified target detected.
Several limitations of the microarrays were observed during the different studies performed. Numerous handling steps were required during the analysis, and opportunities for automation remained limited. Moreover, the commercially available microarrays cannot be used without a microarray reader piloted by specific software provided by the manufacturers, limiting the implementation of the kits in virology laboratories. By comparison with the one‐step real‐time PCR assays frequently used in current molecular virological diagnosis, the major drawback of the technique was the handling of amplicons. Although no contamination event was observed during these evaluations, a potential risk of contamination could not be ruled out. Finally, microarray technology, which is a multiplex endpoint PCR system, did not allow quantitation of the viral load in the clinical samples. It has been assumed that quantitation of viral nucleic acid in CSF samples may be useful in monitoring the effectiveness of antiviral therapy, and for establishing the prognosis of CNS infections [26, 27, 28]. Moreover, although the diagnostic value of the viral load in the nasopharyngeal aspirates remains unclear, the quantification of respiratory viruses may provide important information about the influence of the viral load on disease severity and the role of viral pathogens in single or multiple RTIs [29]. Hence, in cases of mixed viral RTIs, specifically with viruses not detectable by classical techniques, it was not possible to determine which virus was predominant and could be considered as the aetiological agent or to demonstrate whether the virus detection could be linked to beginning, ongoing or past viral RTIs [29, 30].
The place of this new technology in virology laboratories could vary according to the aims of the analyses performed. In routine virology practice, these assays should be considered as third‐line tests, after rapid antigen tests (RATs) and monoplex (RT)‐PCR assays, to enlarge the range of the viruses detected, in particular in cases of severe infection leading to patient hospitalization in the intensive‐care unit (Fig. 1A). Another possibility could be to use them directly after the RAT, in order to confirm the first positive results obtained or, in cases of negative results with the RAT, to perform an enlarged virological screen in the same clinical sample (Fig. 1B). Finally, microarrays could be used as first‐line tests for the detection of viral pathogens; this last possibility, which does not provide rapid and cost‐efficient results to the physicians, should be limited to national centres for infectious diseases control as part of epidemiological surveys (Fig. 1C).
Figure 1.
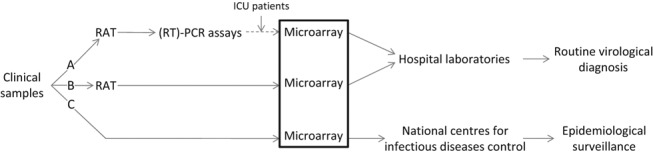
Place of microarray technology in virology laboratories: (A) After rapid antigen tests (RATs) and monoplex (RT)‐PCR assays; (B) directly after RATs; (C) as first‐line tests for the detection of viral pathogens. ICU, intensive‐care unit.
In conclusion, microarrays currently allow rapid and reliable detection of a broad range of respiratory and neurotropic viruses, as well as the identification of single and multiple viral infections in clinical samples. Despite the lack of virus quantitation, they provide rapid typing and subtyping of viral strains that can be useful in both clinical practice and epidemiological surveys.
Transparency Declaration
None of the authors of the present manuscript have a commercial or other association that might pose a conflict of interest (e.g. pharmaceutical stock ownership or consultancy). This work was supported in part by a grant for Clinical and Virological research (SFR‐CAP santé/EA‐4684) from the Medical University and School of Medicine of Reims, France.
References
- 1. Caliendo AM. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis 2011; 52 (suppl): 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahony JB, Chong S, Merante F et al. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead‐based assay. J Clin Microbiol 2007; 45: 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall DJ, Reisdorf E, Harms G et al. Evaluation of a multiplexed PCR assay for detection of respiratory viral pathogens in a public health laboratory setting. J Clin Microbiol 2007; 45: 3875–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reijans M, Dingemans G, Klaassen CH et al. RespiFinder: a new multiparameter test to differentially identify fifteen respiratory viruses. J Clin Microbiol 2008; 46: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frobert E, Escuret V, Javouhey E et al. Respiratory viruses in children admitted to hospital intensive care units: evaluating the CLART1 pneumovir DNA array. J Med Virol 2011; 83: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huguenin A, Moutte L, Renois F et al. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT‐PCR DNA microarray system. J Med Virol 2012; 84: 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, McCormac MA, Estes RW et al. Simultaneous detection and high‐throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol 2007; 45: 2105–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Renois F, Talmud D, Huguenin A et al. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza‐like illnesses by use of reverse transcription‐PCR DNA microarray systems. J Clin Microbiol 2010; 48: 3836–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leveque N, Van Haecke A, Renois F, Boutolleau D, Talmud D, Andreoletti L. Rapid virological diagnosis of central nervous system infections by use of a multiplex reverse transcription‐PCR DNA microarray. J Clin Microbiol 2011; 49: 3874–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glaser CA, Honarmand S, Anderson LJ et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis 2006; 43: 1565–1577. [DOI] [PubMed] [Google Scholar]
- 11. Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev 2004; 17: 903–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tunkel AR, Glasser CA, Bloch KC et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008; 47: 303–327. [DOI] [PubMed] [Google Scholar]
- 13. Oostenbrink R, Moons KG, Theunissen CC, Derksen‐Lubsen G, Grobbee DE, Moll HA. Signs of meningeal irritation at the emergency department: how often bacterial meningitis? Pediatr Emerg Care 2001; 17: 161–164. [DOI] [PubMed] [Google Scholar]
- 14. Huang C, Morse D, Slater B et al. Multiple‐year experience in the diagnosis of viral central nervous system infections with a panel of polymerase chain reaction assays for detection of 11 viruses. Clin Infect Dis 2004; 39: 630–635. [DOI] [PubMed] [Google Scholar]
- 15. Calvario A, Bozzi A, Scarasciulli M et al. Herpes Consensus PCR test: a useful diagnostic approach to the screening of viral diseases of the central nervous system. J Clin Virol 2002; 25 (suppl): 71–78. [DOI] [PubMed] [Google Scholar]
- 16. Jääskeläinen AJ, Piiparinen H, Lappalainen M, Vaheri A. Improved multiplex‐PCR and microarray for herpesvirus detection from CSF. J Clin Virol 2008; 42: 172–175. [DOI] [PubMed] [Google Scholar]
- 17. Boriskin YS, Rice PS, Stabler RA et al. DNA microarrays for virus detection in cases of central nervous system infection. J Clin Microbiol 2004; 42: 5811–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernandez C, Boutolleau D, Manichanh C, Mangeney N, Agut H, Gautheret‐Dejean A. Quantitation of HHV‐7 genome by real‐time polymerase chain reaction assay using MGB probe technology. J Virol Methods 2002; 106: 11–16. [DOI] [PubMed] [Google Scholar]
- 19. Ward KN. Human herpesviruses‐6 and ‐7 infections. Curr Opin Infect Dis 2005; 18: 247–252. [DOI] [PubMed] [Google Scholar]
- 20. Zambon MC, Stockton J, Clewley J, Fleming DF. Contribution of influenza and respiratory syncytial virus to community cases of influenza‐like illness: an observational study. Lancet 2001; 358: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 21. Nougairede A, Ninove L, Zandotti C et al. Point of care strategy for rapid diagnosis of novel A/H1N1 influenza virus. PLoS One 2010; 17: e9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacques J, Bouscambert‐Duchamp M, Moret H et al. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J Clin Virol 2006; 35: 463–466. [DOI] [PubMed] [Google Scholar]
- 23. Wainwright C. Acute viral bronchiolitis in children—a very common condition with few therapeutic options. Paediatr Respir Rev 2010; 11: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marguet C, Lubrano M, Gueudin M et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One 2009; 4: e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jartti T, Lehtinen P, Vuorinen T et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis 2004; 10: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalwai A, Ahmad S, Pacsa A, Al‐Nakib W. Echovirus type 9 is an important cause of viral encephalitis among infants and young children in Kuwait. J Clin Virol 2009; 44: 48–51. [DOI] [PubMed] [Google Scholar]
- 27. Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella‐zoster virus CNS disease—viral load, clinical manifestations and sequels. J Clin Virol 2009; 46: 249–253. [DOI] [PubMed] [Google Scholar]
- 28. Weinberg A, Li S, Palmer M, Tyler KL. Quantitative CSF PCR in Epstein–Barr virus infections of the central nervous system. Ann Neurol 2002; 52: 543–548. [DOI] [PubMed] [Google Scholar]
- 29. Kuypers J, Wright N, Ferrenberg J et al. Comparison of real‐time PCR assays with fluorescent‐antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44: 2382–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nokso‐Koivisto J, Kinnari T, Lindalh P, Hovi T, Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol 2002; 66: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]


