Abstract
The immunological assays for detection of antibodies against SARS-CoV were developed in-house and some of them are available commercially. However, the antigens used in these assays differed. In order to validate the reliability of these assays, the standard panel should be established. In this study, we have expressed and purified severe acute respiratory syndrome (SARS) structural proteins and their fragments and developed indirect enzyme-linked immunosorbent assays (ELISAs) that detect antibodies against the SARS N, N1, N2, S1, SC, S2, and M proteins as well as the human coronavirus OC43 and 229E N proteins. These assays were used to screen 58 samples from SARS convalescent patients, 40 serial serum specimens from patients at different phases of SARS infection, and 88 plasma specimens from normal blood donors. The samples from normal blood donors were also tested for antibodies against other respiratory virus. The representative samples were chosen to comprise a reference panel of SARS antibodies that may be used for the detection of SARS. The panel is composed of 25 positive samples, 25 negative samples, 7 diluted samples for anti-N antibody, 6 diluted samples for anti-S antibody, and one sample for validating precision. Comparison of detection results with different SARS antibody assays indicated that our panel should differentiate the specificity and sensitivity of different assays.
Keywords: SARS-CoV, Expression, Antigenicity, Reference panel
1. Introduction
Coronaviruses are important pathogens that mostly cause respiratory and enteric diseases. The severe acute respiratory syndrome coronavirus (SARS-CoV) is a newly identified human coronavirus that differs from all previously known human coronaviruses. Its approximately 30 kb genome is the largest among RNA viruses and encodes 19 non-structural and 4 structural proteins. The structural proteins include the spike (S), nucleocapsid (N), membrane (M) and small envelope (E) proteins. The S protein is a large membrane protein that induces neutralizing antibodies. The N protein, which is thought to participate in the replication and transcription of viral RNA, is also highly immunogenic and induces a strong antibody response. The transmembrane M protein is the most abundant glycoprotein, while the E protein is an integral membrane protein. The viral envelope is embedded with the S, M, and E proteins and surrounds a helical nucleocapsid consisting of viral RNA and the N protein [1], [2], [3], [4], [5], [6]. All are immunogenic and should induce an immune response after infection.
After the outbreak of SARS, several different assays for the laboratory diagnosis of SARS were developed. The assays included reverse transcriptase-polymerase chain reaction (RT-PCR) to detect nucleic acid as well as immunological assays to detect the N antigen and antibodies against SARS-CoV proteins. Diagnostic techniques were chosen based on time, availability of equipment, expertise, and the biological nature of the available samples. Although the detection of N antigen and RNA is very useful for early diagnosis of SARS-CoV infection, antibody detection has been widely used for diagnostic and epidemiological purposes. Therefore, the immunological assays for detection of antibodies were developed in-house and some of them are available commercially. However, the antigens used in these assays differed. Some used inactivated whole virus while others used recombinant polypeptides, even synthesized peptides. Antibodies against different viral proteins or even antigen fragments may be induced. At present, the significance of each antibody in the diagnosis, course, and prognosis of SARS is not absolutely clear. The kinetics of each antibody response and the correlation between the presence of a single antibody and antibodies against the entire virus is also unknown.
To validate the reliability of assays made with different antigens, a standard panel needs to be established. In this study, SARS N and S proteins and their fragments were expressed and purified, and samples from convalescent SARS patients and blood donors were screened with enzyme-linked immunosorbent assays (ELISAs) that were developed with these proteins. The proper samples were selected to comprise the reference panel of SARS-CoV antibodies.
2. Materials and methods
2.1. Clinical specimens and reagents
Plasma specimens were collected from patients clinically diagnosed with SARS-CoV infection who had recovered for at least 3 months in Beijing and Guangdong and Shanxi provinces. All specimens were incubated at 56 °C for one hour to inactivate the virus and stored at −20 °C. Serial serum specimens were collected from patients with clinically diagnosed SARS at 10 to 47 days after infection. All samples were stored at −20 °C and detection was carried out in a P3 laboratory. Plasma specimens (n = 88) unsuitable for transfusion were collected from the Beijing Blood Center.
The influenza A IgG, influenza B IgG, mycoplasma penumoniae IgG, parainfluenza 1/2/3 IgG, adenovirus IgG, respiratory syncytial virus IgG, measles IgG, and mumps IgG ELISAs were manufactured by IBL-Hamburg GmbH (Hamburg, Germany).
The ELISA kits to detect anti-SARS-CoV IgG antibody were manufactured by Euroimmun Medizinische Labordiagnostika AG (Lübeck, Germany) and GBI Biotechnology Company (Beijing, China). The ELISA kits to detect SARS-CoV N protein and RT-PCR kits to detect SARS CoV RNA were manufactured by Haitai Biopharmaceutical Company (Zhuhai, China) and Daan Gene Company (Shenzhen, China), respectively. The domestic kits used in this study were accredited and licensed by State Food and Drug Administration (SFDA), China. All kits were used according to the manufacturer's instructions.
2.2. Genes and vectors
The cDNA of the SARS-CoV structural proteins N and S were cloned from SARS patients from Guangdong province and stored in our laboratory [7]. The M protein was purchased from BIODESIGN International (Saco, Maine, USA). The expressed recombinant plasmids of human coronavirus (HcoV) OC43 and 229E N proteins were a gift from professor Che Xiaoyan (Zhujiang hospital, Guangzhou).
2.3. Amplification of the N and S Genes of SARS-CoV
The N gene was divided into two fragments [8], N1 (1 ∼ 761) and N2 (442 ∼ 1265), which overlap each other. The S gene was divided into three fragments [9] S1 (49 ∼ 2040), SC (1531 ∼ 2579), and S2 (2179 ∼ 3567), which also overlap one another. Moreover, 16 amino acids close to the N terminus and 66 amino acids close to the C terminus of the S protein, which were predicted to be the signal peptide and the transmembrane region, respectively, were removed.
The primers for the N, N1, N2, S1, SC, and S2 gene fragments of SARS-CoV were designed according to the sequences of SARS-CoV Urbani strain (AY278741). BamHI and NdeI sites were added at the 5′ terminus of the forward and reverse primers, respectively. Using 1 μl of cDNA as template, each PCR was carried out in a 50 μl reaction with 25 pmol of each primer and 5 U Taq polymerase. The reaction mix was inactivated at 94 °C for 3 min, followed by 30 cycles at 94 °C for 35 s, 55 °C for 35 s, and 72 °C for 90 s, with a final extension at 72°C for 10 min. The primer pairs of N1 forward and N2 reverse (amplifies N, 1269 bp), N1 forward and N1 reverse (amplifies N1, 761 bp), N2 forward and N2 reverse (amplifies N2, 824 bp), S1 forward and S1 reverse (amplifies S1, 1992 bp), SC forward and SC reverse (amplifies SC, 1449 bp), S2 forward and S2 reverse (amplifies S2, 1389 bp) as shown below (restriction sites in italics).
N1-forward:5′-GGAATTCCATATGTCTGATAATGGACCCCAATC-3′
N1-reverse:5′-CGGGATCCTCAGCAGCAGATTTCTTAGTGAC-3′
N2-forward:5′-GGAATTCCATATGGGCACCCGCAATCCTAATAAC-3′
N2-reverse:5′-CGGGATCCGCCTGAGTTGAATCAGCAGAGGC-3′
S1-forward:5′-GGAATTCCATATGGACCGGTGCACCACTTTTGAT-3′
S1-reverse:5′-CGGGATCCGACATAGTATAAGCCACAATAG-3′
SC-forward:5′-GGAATTCCATATGTGTGGACCAAAATTATCCACTG-3′
SC-reverse:5′-CGGGATCCTGTTGTGTTACATAGGTTTGAAG-3′
S2-forward:5′-GGAATTCCATATGGATTCTACTGAATGTGCTAATTT-3′
S2-reverse:5′-CGGGATCCTCATATTTTCCCAATTCTTGAAG-3′
2.4. Expression of recombinant polypeptides
All PCR products were digested with BamHI and NdeI and ligated into pET30a(+). After transformation into E. coli, positive plasmids were identified and transformed into the expression strain E. coli BL21(DE3). Bacteria were incubated at 37 °C shaking until the OD600 reached 0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG, final concentration 1 mmol/L) was then added and bacteria were further incubated at 37 °C shaking for about 4 h. The recombinant plasmids pQE30 carrying the N genes of HcoV OC43 and HcoV 229E, respectively, were expressed similarly in E. coli M15. The expression level of each protein was analyzed by using gel scanning software AlphaEaseFC 3.2.1.
2.5. Purification of recombinant polypeptides
Since the SARS N, N1, and N2 proteins, and the N proteins of HcoV OC43 and HcoV 229E were expressed in soluble form in E. coli, the bacteria were harvested after expression, lysed by sonication, and resuspended in different buffers according to their purification methods. Since the SARS S1, SC, and S2 proteins were expressed in inclusion bodies, the host bacteria were harvested after expression, lysed by sonication, washed twice with 0.1% Triton-X-100, and dissolved in 8 M urea. For the SARS N protein, the lysate was added to an anion-exchange chromatography column and the second elution peak (0.3 mol/L NaCl) was collected. After buffer exchange, the protein was added to a hydrophobic interaction chromatography column and the second elution peak (0.1 mol/L (NH4)2SO4) was collected to obtain pure SARS N protein. For the SARS N1, SC, and HcoV OC43 N proteins, the lysate was added to a Ni2+chelating affinity column and proteins were eluted with 10 mmol/L, 20 mmol/L, and 100 mmol/L imidazole. Purified proteins were obtained in the fractions eluting at 100 mmol/L imidazole. For the SARS N2, S1, and S2 proteins, the lysate was applied to a 12% sodium dodecyl sulfate (SDS) gel and put into the chamber of the Gel Eluter. After eluting at 250 mA for 60 min, the solution in each hole was collected and determined to be pure by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). For the HcoV OC43 N protein, the lysate was added to a Ni2+chelating affinity chromatography column and the peak eluting at 125 mmol/L imidazole was collected. The protein was applied to a 12% SDS-PAGE gel and eluted using the Gel Eluter as described above and determined to be pure by SDS-PAGE. The expression level of each protein was analyzed by using gel scanning software AlphaEaseFC 3.2.1.
2.6. Western blot analysis
Western blot analysis was performed to determine the antigenicity of each antigen. Purified proteins were separated by using 12% SDS-PAGE and subsequently transferred to nitrocellulose membranes. The nitrocellulose membrane was blocked with 5% skim milk overnight at room temperature and then incubated for 1.5 h with SARS-CoV antibody positive human serum (1:500 dilution) at room temperature. After washing five times with phosphate-buffered saline Tween-20 (PBST), the membrane was incubated for 1 h with alkaline phosphatase-labeled goat anti-human IgG (1:2000 dilution) at room temperature. The membrane was washed five times with PBST, transferred into NBT/BCIP solution, and incubated at room temperature until a signal was observed.
2.7. Development of indirect ELISA assay
Microtiter plates (96-well) were coated with each purified recombinant protein in 0.01 M phosphate buffer, pH 9.6, for 4 h followed by 37 °C for 2 h. The coated plates were incubated at 4 °C overnight, washed five times, blocked with 5% skim milk in PBST overnight at room temperature, and washed five times. Plasma or sera specimens were added to each well (1:500 dilution) and incubated at 37 °C for 1 h. Each well was washed 5 times, incubated with peroxidase-labeled goat anti-human antibody at 37 °C for 30 min, then washed again 5 times. The peroxidase reaction was visualized using TMB as a substrate for 15 min at 37 °C in the dark. The reaction was stopped by adding 50 μl/well of 1 N H2SO4 and absorbance was read at 450 nm. To determine a cut-off value, 30 specimens from normal blood donors were tested with each ELISA and the mean OD and standard deviation were calculated. The cut-off value was defined as the mean OD plus three standard deviations for each ELISA.
3. Results
3.1. Construction of recombinant plasmids
The target gene fragments N, N1, N2, S1, SC, and S2 of SARS-CoV were amplified from cDNA and their sizes were as expected. The positive recombinant plasmid pET-30a(+) for expressing each fragment were identified and designated as pET-SARS N, pET-SARS N1, pET-SARS N2, pET-SARS S1, pET-SARS SC, and pET-SARS S2, respectively.
3.2. Antigen expression and purification
The expressed product for each recombinant plasmid was analyzed using 12% SDS-PAGE gels ( Fig. 1). The N, N1, and N2 recombinant polypeptides of SARS-CoV were expressed in soluble form with the expected sizes of 48.3, 29.5, and 32.3 kD, respectively, while the S1, SC, and S2 polypeptides were found in inclusion bodies with sizes of 76.5, 54.8, and 53.6 kD, respectively.
Fig. 1.
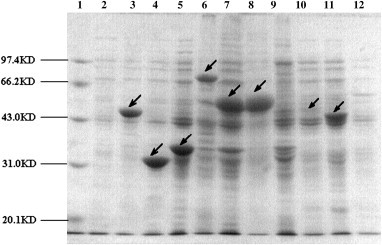
The expressed fusion proteins analyzed by SDS-PAGE. Lane 1, protein marker. Lane 2, supernate of BL-21 with pET-30a as negative control. Lane 3–5, SARS-CoV N, N1 and N2 protein expressed in supernate. Lane 6–8, SARS-CoV S1,SC and S2 protein expressed in inclusion bodies. Lane 9, pellet of BL-21 with pET-30a as negative control. Lane 10, HcoV OC43 N protein expressed in M1 supernate. Lane 11, HcoV 229E N protein expressed in M1 supernate. Lane 12, supernate of M1 with pQE30-OC43N as negative control.
The recombinant plasmids pQE30 carrying the N genes of HcoV OC43 and HcoV 229E were expressed in E. coli M15. Both N proteins could be expressed in soluble form (Fig. 1). The percentage of SARS N, N1, N2, S1, SC, and S2 proteins expressed was 36.9%, 40.1%, 23.1%, 16.9%, 24.3%, and 41.8% of total protein, respectively, while that of HcoV OC43 and HcoV 229E was 5.8% and 13.7% of total protein.
The expressed products were successfully purified using various purification methods and analyzed on a 12% SDS-PAGE gel ( Fig. 2, for purification details see Section 2). The purity of the SARS N, N1, N2, S1, SC, and S2 proteins was 98.7%, 96.8%, 95.5%, 95.0%, 97.6%, and 97.5%, respectively, while the purity of the HcoV OC43 and HcoV 229E proteins was 98.2% and 98.9%.
Fig. 2.
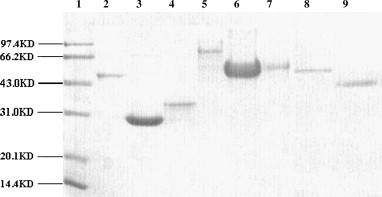
The purified proteins analyzed by SDS-PAGE. Lane 1, protein marker. Lane 2–7, purified SARS-CoV N protein (48.3 kD), SARS-CoV N1 protein (29.5 kD), N2 protein (32.3 kD), S1 protein (76.5 kD), SC protein (54.8 kD) and S2 protein (53.6 kD). Lane 8, purified HcoV OC43 N protein (50.8 kD). Lane 9, purified HcoV 229E N protein (44.7 kD).
3.3. Western blot
To test the reactivity of the SARS recombinant proteins, western blot analysis was carried out using SARS-CoV-positive human serum. Six specific bands corresponding to the molecular weights of the SARS N, N1, N2, S1, SC, and S2 proteins were observed ( Fig. 3), confirming the authenticity of the recombinant proteins.
Fig. 3.
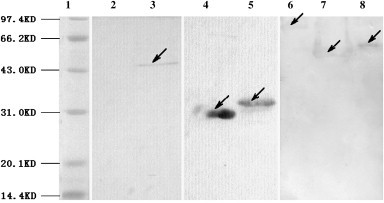
Identification of recombinant proteins by Western blot. Lane 1, protein marker. Lane 2, BL-21 with pET-30a as negative control. Lane 3–8, SARS-CoV N,N1,N2,S1,SC and S2 protein performed.
3.4. Development of indirect ELISA kits
Indirect ELISA kits to detect antibodies against different polypeptides were developed using the corresponding polypeptide. Based on reactivity with serum from uninfected individuals, the OD cutoff values were 0.503, 0.161, 0.234, 0.247, 0.168, 0.227, 0.071, 0.029, and 0.029 for SARS-CoV N, N1, N2, S1, SC, and S2 proteins, and HcoV OC43 and 229E N proteins, respectively.
Among 58 SARS convalescent plasma specimens, the percentages of IgG antibody against the SARS-CoV N, N1, N2, S1, SC, S2, and M proteins were 3.5% (2/58), 91.4% (53/58), 75.9% (44/58), 50.0% (29/58), 81.0% (52/58), 86.2% (50/58), and 96.5% (56/58), respectively ( Table 1), which shows the different antigenicities of SARS-CoV polypeptides in SARS convalescent plasma.
Table 1.
The detection results of SARS proteins and HcoV OC43, 229E N proteins (S/CO)
| Samples | N | N1 | N2 | S1 | Sc | S2 | M | OC43 | 229E |
|---|---|---|---|---|---|---|---|---|---|
| PS2 | 0.014 | 8.068 | 0.842 | 0.308 | 4.851 | 2.388 | 8.423 | 0.379 | 0.414 |
| PS3 | 0.014 | 12.07 | 8.885 | 0.607 | 1.274 | 1.137 | 8.197 | 0.310 | 0.345 |
| PS5 | 2.531 | 7.758 | 1.444 | 1.275 | 2.524 | 1.511 | 1.606 | 0.000 | 0.310 |
| PS6 | 0.165 | 2.795 | 4.278 | 1.324 | 2.220 | 1.934 | 2.676 | 0.207 | 0.448 |
| PS7 | 0.048 | 1.820 | 0.957 | 6.020 | 1.107 | 0.938 | 7.775 | 0.138 | 0.517 |
| PS8 | 0.006 | 3.522 | 1.611 | 0.943 | 3.417 | 0.987 | 2.113 | 0.241 | 0.379 |
| PS9 | 0.012 | 2.547 | 2.603 | 0.628 | 1.202 | 1.269 | 1.310 | 0.517 | 0.414 |
| PS10 | 0.080 | 3.497 | 2.538 | 1.316 | 1.494 | 2.511 | 1.535 | 0.552 | 0.414 |
| PS11 | 0.252 | 1.472 | 0.996 | 0.696 | 1.000 | 2.220 | 3.746 | 0.379 | 0.310 |
| PS13 | 0.014 | 3.099 | 1.462 | 0.684 | 1.452 | 2.529 | 1.986 | 0.483 | 0.310 |
| PS14 | 0.111 | 5.795 | 2.996 | 0.611 | 4.089 | 2.621 | 8.634 | 0.414 | 0.345 |
| PS15 | 0.010 | 2.149 | 0.974 | 1.162 | 1.232 | 1.282 | 2.746 | 0.621 | 0.379 |
| PS16 | 0.237 | 0.211 | 0.282 | 0.543 | 2.702 | 1.709 | 0.606 | 0.655 | 0.241 |
| PS17 | 0.016 | 0.503 | 0.222 | 0.534 | 2.179 | 1.273 | 4.254 | 0.621 | 0.379 |
| PS18 | 0.016 | 2.764 | 0.876 | 0.247 | 0.881 | 1.101 | 0.620 | 0.552 | 0.345 |
| PS19 | 0.016 | 3.286 | 3.115 | 0.692 | 3.000 | 2.502 | 1.296 | 0.448 | 0.345 |
| PS21 | 0.004 | 9.404 | 7.624 | 0.947 | 1.827 | 1.520 | 1.211 | 0.345 | 0.379 |
| PS22 | 0.016 | 2.957 | 3.457 | 1.312 | 5.345 | 1.850 | 2.085 | 0.621 | 0.414 |
| PS23 | 2.103 | 0.696 | 0.940 | 0.628 | 0.964 | 0.634 | 2.141 | 0.724 | 0.414 |
| PS24 | 0.030 | 2.373 | 2.248 | 0.575 | 1.054 | 0.784 | 2.169 | 0.586 | 0.379 |
| PS25 | 0.020 | 8.062 | 5.427 | 1.591 | 3.024 | 2.423 | 4.042 | 0.655 | 0.379 |
| PS26 | 0.062 | 4.727 | 2.885 | 1.538 | 2.548 | 2.366 | 2.634 | 0.655 | 0.448 |
| PS27 | 0.020 | 4.149 | 2.556 | 2.462 | 4.554 | 3.066 | 2.549 | 0.621 | 0.310 |
| PS28 | 0.020 | 2.217 | 3.274 | 0.538 | 1.452 | 1.498 | 5.859 | 0.621 | 0.379 |
| PS29 | 0.111 | 7.981 | 5.462 | 1.389 | 2.899 | 2.872 | 2.493 | 0.621 | 0.379 |
| PS12 | 0.070 | 1.143 | 2.556 | 2.611 | 4.577 | 6.242 | 3.577 | 0.552 | 0.379 |
| PS20 | 0.022 | 6.360 | 4.261 | 1.312 | 0.738 | 1.057 | 2.437 | 0.586 | 0.379 |
| PS31 | 0.028 | 7.143 | 3.000 | 5.964 | 8.333 | 6.767 | 20.660 | 0.862 | 0.552 |
Note: The sample was considered as positive if S/CO value is equal to or more than 1.
No IgG immunoglobulins were detected against the N proteins of HcoV OC43 and 229E, which indicates that no cross-reactivity occurred between the N proteins of SARS-CoV and HcoV.
IgG and IgM immunoglobulin were detected in the 58 SARS convalescent plasma specimens using an indirect ELISA developed with entire virus antigens. The prevalence of IgG immunoglobulin was 89.7% (52/58) and IgM immunoglobulin was 1.7% (1/58).
3.5. Detection of antigens in 40 SARS serial serum specimens
The SARS N protein and S1 proteins have been shown to have low antigenicity while the N1, N2, SC, S2 and M proteins have better antigenicity. Therefore, IgG immunoglobulins against the N1, N2, SC, S2 and M proteins were detected by indirect ELISA in 40 SARS serial sera taken 10 to 45 days after the onset of clinically diagnosed disease. The result showed that in the early phases of infection, the detection rates of antibody against the SARS-CoV M protein were high while the detection rates of the N and S proteins were low. However, the detection rates appeared to increase in the later phases of infection ( Fig. 4).
Fig. 4.
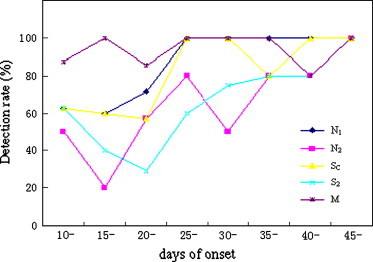
Change in detection rate of different antibodies in serial sera over the course of infection.
3.6. Candidate samples for anti-SARS antibody reference panel
3.6.1. Positive samples
Twenty-five plasma specimens from SARS convalescent patients that showed reactivity to SARS structural proteins were selected as positive references. All were positive for antibodies against SARS whole virus and negative for antibodies to both HcoV OC43 and 229E N proteins. Among them, PS2, PS21, PS16, PS9, PS22, PS20, and PS22 are positive for antibodies against entire N, N1, N2, S1, SC, S2, and M proteins respectively. Some samples such as PS3, PS21, and PS24 showed strong reactivity to the N fragment while others such as PS16 and PS17 showed strong reactivity to the S fragment, as were shown in Table 1.
3.6.2. Negative samples
Eighty-eight plasma specimens collected from Beijing Blood Center but considered unsuitable for transfusion were screened by commercial ELISA kits to IgG antibodies against influenza A virus, influenza B virus, mycoplasma pneumoniae, parainfluenza virus, adenovirus, respiratory syncytial virus, measles virus, and mumps virus. The positive rates were 100% (88/88), 95.5% (84/88), 4.5% (4/88), 97.7% (86/88), 97.7% (86/88), 100% (88/88), 71.6% (63/88), and 94.3% (83/88), respectively. Twenty-five samples of these samples were used in the panel as negative samples. All were positive for antibodies against influenza A virus, influenza B virus, parainfluenza virus, adenovirus, respiratory syncytial virus, measles virus, and mumps virus, while only 3 of 25 were positive for antibodies against mycoplasma ( Table 2).
Table 2.
The detection results of respiratory pathogen (S/CO)
| Samples | Influ A | Influ B | Mycoplasma | Parainfluenza | Adenovirus | RSV | Measles | Mumps |
|---|---|---|---|---|---|---|---|---|
| NS1 | 5.195 | 2.369 | 0.337 | 3.655 | 2.743 | 3.634 | 3.556 | 2.844 |
| NS3 | 2.913 | 3.428 | 1.379 | 2.156 | 3.052 | 3.953 | 2.941 | 2.849 |
| NS7 | 4.141 | 2.798 | 0.495 | 2.187 | 3.304 | 2.178 | 4.216 | 3.045 |
| NS18 | 5.000 | 2.828 | 0.445 | 4.635 | 4.512 | 3.281 | 1.741 | 3.366 |
| NS23 | 5.961 | 3.952 | 0.521 | 3.582 | 3.275 | 3.099 | 2.938 | 5.976 |
| NS27 | 2.964 | 2.056 | 1.274 | 2.206 | 4.696 | 4.468 | 1.335 | 5.434 |
| NS29 | 5.066 | 3.198 | 0.268 | 2.136 | 3.396 | 3.868 | 2.093 | 4.295 |
| NS30 | 6.524 | 3.428 | 0.232 | 4.557 | 3.510 | 4.087 | 1.235 | 5.840 |
| NS32 | 4.704 | 3.894 | 0.424 | 4.524 | 3.839 | 3.488 | 1.812 | 3.241 |
| NS33 | 6.254 | 4.080 | 0.295 | 1.774 | 4.822 | 3.937 | 1.713 | 2.623 |
| NS36 | 3.775 | 3.935 | 1.508 | 3.883 | 4.723 | 4.364 | 1.470 | 3.120 |
| NS40 | 4.135 | 2.311 | 0.124 | 4.538 | 4.450 | 5.397 | 4.675 | 3.241 |
| NS44 | 3.135 | 3.606 | 0.271 | 2.312 | 3.931 | 2.992 | 1.800 | 5.901 |
| NS46 | 4.766 | 2.420 | 0.166 | 5.033 | 3.381 | 2.241 | 2.857 | 5.686 |
| NS49 | 5.257 | 2.193 | 0.526 | 4.309 | 4.074 | 3.830 | 1.347 | 2.774 |
| NS51 | 4.671 | 3.789 | 0.897 | 2.713 | 3.114 | 4.818 | 1.439 | 2.934 |
| NS52 | 6.018 | 3.287 | 0.353 | 1.493 | 2.968 | 3.787 | 3.891 | 4.476 |
| NS53 | 4.317 | 2.839 | 0.547 | 2.811 | 1.710 | 4.018 | 1.779 | 5.017 |
| NS59 | 5.395 | 2.844 | 0.868 | 3.061 | 3.564 | 4.018 | 1.192 | 4.719 |
| NS64 | 4.006 | 2.483 | 0.353 | 3.382 | 3.752 | 4.508 | 1.195 | 5.000 |
| NS68 | 4.488 | 3.874 | 0.179 | 3.418 | 4.569 | 3.891 | 4.551 | 4.861 |
| NS72 | 5.844 | 3.513 | 0.289 | 4.671 | 2.844 | 3.773 | 1.475 | 3.410 |
| NS77 | 4.832 | 3.806 | 0.311 | 2.621 | 3.859 | 3.974 | 3.143 | 5.149 |
| NS79 | 5.425 | 3.035 | 0.458 | 5.173 | 4.223 | 4.053 | 2.570 | 5.580 |
| NS80 | 3.024 | 2.191 | 0.484 | 2.591 | 4.777 | 3.059 | 3.112 | 2.172 |
Note: The sample was considered as positive if S/CO value is equal to or more than 1.
3.6.3. Sensitivity samples
Sample PS12 in Table 1 reacted more strongly to the S protein while sample PS20 reacted more strongly to the N protein. Therefore, to validate the sensitivity of ELISA in detecting the N and S proteins, both PS12 and PS20 were serially diluted with defibrated plasma and used as sensitivity samples. The diluted samples for the N and S proteins were designated SN1–SN7 (diluted from PS20) and SS1–SS6, (diluted from PS12) respectively. The diluted samples were also tested for antibodies against N1, N2, SC, S2, and M recombinant proteins as well as whole virus. The results are listed in Table 3 and confirmed that diluted SN samples react strongly to N fragment proteins while diluted SS samples react strongly to S fragment proteins.
Table 3.
The detection results of sensitivity samples (S/CO)
| No. | Dilution | N1 | N2 | SC | S2 | M | WV* |
|---|---|---|---|---|---|---|---|
| SS1 | 1:1 | 0.83 | 2.52 | 8.81 | 5.35 | 5.41 | 4.70 |
| SS2 | 1:2 | 0.29 | 1.38 | 4.37 | 2.46 | 3.39 | 2.79 |
| SS3 | 1:4 | 0.12 | 0.87 | 2.11 | 1.19 | 1.24 | 1.62 |
| SS4 | 1:8 | 0.08 | 0.40 | 1.11 | 0.59 | 1.25 | 0.83 |
| SS5 | 1:16 | 0.07 | 0.26 | 0.56 | 0.26 | 0.24 | 0.41 |
| SS6 | 1:32 | 0.06 | 0.10 | 0.30 | 0.11 | 0.17 | 0.19 |
| SN1 | 1:1 | 6.76 | 5.04 | 1.21 | 1.48 | 4.06 | 8.22 |
| SN2 | 1:2 | 3.20 | 3.53 | 0.58 | 0.65 | 1.68 | 7.25 |
| SN3 | 1:4 | 1.53 | 2.30 | 0.31 | 0.30 | 1.00 | 5.65 |
| SN4 | 1:8 | 0.65 | 1.17 | 0.18 | 0.14 | 0.56 | 3.73 |
| SN5 | 1:16 | 0.28 | 0.65 | 0.04 | 0.03 | 0.15 | 2.17 |
| SN6 | 1:32 | 0.11 | 0.34 | 0.05 | 0.02 | 0.14 | 1.02 |
| SN7 | 1:64 | 0.07 | 0.15 | 0.06 | 0.02 | 0.10 | 0.49 |
Note: *WV indicates lysate of whole SARS CoV.
3.6.4. CV sample
Sample PS31 in Table 1 was diluted 1.5 times with defibrated plasma and used as the reference of coefficiency variation that validates the precision of diagnosis reagents.
3.7. Detection results of SARS CoV RNA and N antigen
All of the 25 positive samples, 13 sensitivity samples and 1 CV sample were detected for SARS CoV RNA and N antigen with accredited kits. The results indicated that none of them were positive for SARS CoV RNA and N antigen.
3.8. Detection with different assays
The samples include 25 negative samples, 25 positive samples, 7 dilution samples with strong positive reactivity for the anti-N antibody, and 6 dilution samples with strong positive reactivity for the anti-S2 antibody. Samples (0.5 ml per vial) and one sample for validation of coefficiency variation (1.5 ml per vial) were aliquoted. In total, 50 sets of the reference panel have been established. After aliquoting, the panel was validated with three different SARS antibody assays. The results indicated that different assay has different capacity to detect positive samples and diluted samples ( Table 4).
Table 4.
Comparisons of detection results using different SARS antibody assays
| Reagent | Testing No. | Negative | Positive | SS | SN | CV* (%) |
|---|---|---|---|---|---|---|
| GBI | 1 | 25/25** | 25/25 | 3/6 | 6/7 | 2.7 |
| 2 | 25/25 | 25/25 | 3/6 | 6/7 | 2.6 | |
| 3 | 25/25 | 25/25 | 3/6 | 6/7 | 2.6 | |
| 4 | 25/25 | 25/25 | 3/6 | 6/7 | 1.9 | |
| 5 | 25/25 | 25/25 | 3/6 | 6/7 | 2.8 | |
| CDC | 1 | 25/25 | 14/25 | 0/6 | 0/7 | 4.7 |
| 2 | 25/25 | 16/25 | 1/6 | 1/7 | 9.9 | |
| 3 | 25/25 | 18/25 | 2/6 | 0/7 | 3.5 | |
| Euroimmun | 1 | 25/25 | 24/25 | 2/6 | 3/7 | 4.7 |
| 2 | 25/25 | 24/25 | 3/6 | 4/7 | 6.5 | |
Note: *The CV sample was added into 10 wells in parallel at one test. CV was calculated according to mean of values and standard deviation. **The numerator means number of samples which kits give same results as indicated in reference panel while the denominator means number of examined samples.
4. Discussion
SARS has spread quickly and broken out in almost 30 countries since it was first found in Guangdong Province, China in November 2002, and became a new severe infectious disease in the 21st century that threatens the health of people all over the world. At present, the significance of each antibody in the diagnosis, course, and prognosis of SARS is unclear, as well as the kinetics of each antibody response and the correlation between the presence of antibodies against a single protein and antibodies against the entire virus.
In this study, the indirect ELISAs were developed with different fragments of SARS structure proteins and used to detect antibodies in 58 SARS plasma specimens donated by convalescent patients 3 months after onset of SARS. The results indicated that most of the expressed proteins reacted to the convalescent plasma. To our surprise, SARS N protein exhibited low antigenic activity while the activities of N1 and N2 protein, as the fragments of the whole N protein, were better. The following reasons should explain this phenomenon. Firstly, as was described in Section 2, the SARS N protein were purified with complicated poly-stepped purification methods, this may result in the change of N protein structure or property. Next, since it was expressed in E. coli, perhaps SARS whole N protein could not form its natural structure and the antigen conformational epitopes may be exposed after the protein is fragmented. Moreover, the entire SARS N protein reacted to part of negative samples, too, which revealed there may be cross-reaction between SARS and non-SARS plasma. The same result was reported by Mimoun etc. [10]. Probably there were some antigen epitopes similar to conservative N protein in some human tissue antigen or other undetected pathogen.
Meanwhile, the antigenicity of the SARS S1 protein was poor, too. Based on bioinfomatics analysis, the S1 protein is the globular domain of the S protein. The conformation of globular proteins is usually complicated and the antigen epitope may be composed of many polypeptides that are far away from one another in the linear sequence. As there is no folding and modifying function in prokaryotic expression systems, the reduction of the antigenic activity is perhaps because the conformation of the S1 protein can not be replicated in prokaryotic expression systems.
Indirect ELISA kits for IgG and IgM were applied to detect antibodies in the plasma specimens. The results are consistent with other studies [9], [11], [12], [13], [14]. The detection rate of antibody against the SARS-CoV M protein in 40 SARS serial serum specimens was high in the early phases of infection, suggesting that detection of the M protein could be helpful in early diagnosis. Although the detection rate of the N and S proteins was low in early stages of the disease, it appeared to increase in later stages. Since viruses replicate on a large scale once they infect humans, immunity initially substantially declines, but the antibody detection rate rises as immunity recovers with treatment.
Since coronaviruses are ubiquitous, infection with HcoV OC43 and HcoV 229E is common and any cross-reactivity between HcoV and SARS-CoV will increase the rates of misdiagnosis. Therefore, we developed indirect ELISA kits to detect IgG against the N proteins of HcoV OC43 and HcoV 229E and tested them on 30 SARS plasma specimens. The results showed that there was no cross-reactivity. However, the cross-reactivity between the N proteins of HcoV and SARS-CoV had been observed in other studies [10]. Probably, the samples were not sufficient to examine the cross-reactivity in this study.
The prevalence of antibodies against influenza A virus, influenza B virus, mycoplasma pneumoniae, parainfluenza virus, adenovirus, respiratory syncytial virus, measles virus, and mumps virus was also analyzed among normal blood donors. The results indicated that the prevalence of antibody against mycoplasma pneumoniae was only 4.5% while the prevalence of antibodies against influenza A virus and respiratory syncytial virus was 100%.
In this study, 25 samples positive for SARS-CoV antibodies, 25 samples positive for other respiratory virus antibodies but negative for SARS-CoV antibodies, 7 diluted samples with strong antibodies against the S protein, 6 diluted samples with strong antibodies against the N protein, and one sample for validation of coefficiency variation to validate precision were selected to comprise the reference panel of SARS-CoV antibodies. The positive samples were collected from Guangdong, Shanxi, and Beijing. The titers of antibody was different, with some strongly positive, some weak, and others in the middle. All of positive samples were negative for both SARS CoV RNA and N antigen. The negative samples were mainly selected from samples positive for antibodies against other respiratory viruses so that cross-reactivity with other viruses or specificity may be validated. We found that some samples had stronger antibodies against the N protein and some had stronger antibodies against the S protein. Although the significance of antibodies against the N or S protein is not clear for diagnostic purposes, the assay's sensitivity for both should be validated. Therefore sensitivity samples for anti-N and anti-S antibodies were included in the reference panel. In this study, 50 sets of panel had been generated with volume of 500 μl in each vial. In fact, all SARS CoV antibody assays have used indirect sandwich method with which approximately 10 μl samples would be used for each run. Meanwhile, 25 to 30 ml of plasma were left for each samples of panel and stored at −20 °C for aliquot in case of emergency. Therefore the panel should be sufficient to be used for validation of SARS antibody assays.
The candidate samples with different background were selected to comprise of reference panel and was validated with different SARS antibody assays. The results showed that different assay had different capacity to detect positive samples. This panel should be used to validate the sensitivity and specificity of SARS-CoV antibody assays and make the assays more reliable.
Footnotes
Potential conflicts of interest: None reported.
Financial support: The research was supported by the grant (NIH U19 AI51915) of National Institutes of Health and Center for Disease Control from the United States.
References
- 1.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenagle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 2.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the sars-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 3.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 4.Falsey A.R., Walsh E.E. Novel coronavirus and severe acute respiratory syndrome. Lancet. 2003;361:1312–1313. doi: 10.1016/S0140-6736(03)13084-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon L.L.M., Guan Y., Nicholls J.M., Yuen K.Y., Peiris J.S.M. The aetiology, orgins, and diagnosis of severe acute respiratory syndrome. Lancet Infect Dis. 2004;4:663–671. doi: 10.1016/S1473-3099(04)01172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ying W., Hao Y., Zhang Y., Peng W., Qin E., Cai Y. Proteomic analysis on structural proteins of Severe Acute Respiratory Syndrome coronavirus. Proteomics. 2004;4:492–504. doi: 10.1002/pmic.200300676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Wan Y.M., Xu S.H., Zhang F., Li X.H., Wang Y.C. Sequence analysis of structure genes from 5 SARS virus isolates. Clin J Microbiol Immunol. 2004;24:400–403. [Google Scholar]
- 8.He Y.X., Zhou Y.S., Wu H., Kou Z., Liu S., Jiang S. Mapping of antigenic sites on the nucleocapsid protein of the severe acute respiratory syndrome coronavorus. J Clin Microbiol. 2004;42:5309–5314. doi: 10.1128/JCM.42.11.5309-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiga O., Bernini A., Ciutti A., Chiellini S., Menciassi N., Finetti F. Molecular modeling of S1 and S2 subunits of SARS coronavirus spike glycoprotein. Biochem Biophys Res Commun. 2003;310:78–83. doi: 10.1016/j.bbrc.2003.08.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maache M., Komurian-Pradel F., Rajoharison A., Perret M., Berland J.L., Pouzol S. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid-based western blot assay Sero-epidemiological characteristic of antibody to SARS-CoV. Clin Vaccine Immunol. 2006;13:409–414. doi: 10.1128/CVI.13.3.409-414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralization antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan B.X., Liu Y.N., Xie L.X., Tian Q., Chen L.A., Chen W.J. Dynamic changes of immunoglobulin G in convalescents who have suffered from severe acute respiratory syndrome patients. Natl Med J China. 2004;84:1690–1692. [PubMed] [Google Scholar]
- 13.Woo P.C., Lau S.K., Wong B.H., Chan K.H., Chu C.M., Tsoi H.W. Longitudinal profile of immunoglobulin G (IgG), IgM, IgA, antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab. 2004;11:665–668. doi: 10.1128/CDLI.11.4.665-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D.S., Fu S.H., Meng L., Jiang J.X., Wang B.T., Wang J. Sero-epidemiological characteristic of antibody to SARS-associated coronavirus in Gansu Province. Chinese J Exp Clin Virol. 2004;18:325–327. [PubMed] [Google Scholar]


