Abstract
This study set out to investigate the cytokines and acute phase proteins (APPs) associated with the acute stages of experimentally-induced swine influenza virus (SIV) infection in 3-week-old, colostrum-deprived, caesarean-derived piglets. The piglets were inoculated intratracheally with 107.5 50% egg infective dose [EID50] Swine/Belgium/1/98 (H1N1) SIV and were euthanased at time-points between 0 and 120 h post-inoculation (PI). Broncho-alveolar lavage fluid (BALF), lung homogenates and sera were examined for inflammatory mediators by bioassay or ELISA. Interferon (IFN)-α, interleukin (IL)-6, IL-1 and tumour necrosis factor (TNF)-α peaked in BALF 24–30 h PI, when virus titres and the severity of clinical signs were maximal.
Whereas IFN-γ and IL-12, but not IL-18, increased in tandem in BALF, serum cytokine concentrations were either undetectable or were up to 100-fold lower. The APP C-reactive protein (CRP) and haptoglobin peaked 24 h later than the cytokines and reached higher levels in serum than in BALF. In contrast, lipopolysaccharide (LPS)-binding protein (LBP) only increased in BALF. Lung virus titres tightly correlated with BALF IFN-α, IL-6, IL-1, TNF-α, IFN-γ and IL-12, as well as with serum IL-6, IFN-α and IFN-γ. Signs of disease correlated with the same cytokines in BALF and serum, as well as with BALF LBP and serum CRP. The findings suggest that IFN-γ and IL-12 play a role in the pathogenesis of SIV and that APPs are induced by cytokines. This influenza infection model may have value in assessing the therapeutic potential of cytokine antagonists.
Keywords: Acute phase proteins, Broncho-alveolar lavage, Cytokines, Pathogenesis, Swine influenza
Introduction
Swine influenza virus (SIV) is a major cause of acute respiratory disease in pigs (Loeffen et al., 1999). Clinical signs include high fever, depression, anorexia and laboured abdominal breathing (Shope, 1931, Olsen et al., 2006), and the virus replicates in epithelial cells of the nasal mucosa, tonsils, trachea and the lungs, the major target organ. Infected susceptible pigs exhibit extensive gross lung lesions with microscopic evidence of necrosis, desquamation of bronchiolar epithelium and neutrophil infiltration (Olsen et al., 2006). Infection is generally limited to the respiratory tract and attempts to demonstrate viraemia or virus replication outside the tract have been largely unsuccessful.
Swine influenza can be reproduced experimentally by intratracheal (IT) inoculation of naïve pigs with a high dose of virus (107.5 50% egg infective dose [EID50]). This results in respiratory and more systemic clinical signs, high lung titres of virus (⩾108.0 50% tissue culture infective dose [TCID50]/g), the activation of several pro-inflammatory cytokines and in neutrophils making up >50% of the broncho-alveolar lavage fluid (BALF) cells 1 day post-inoculation (PI). Experimental infection with representative H1N1, H3N2 and H1N2 SIV strains demonstrated highly significant correlations between clinical signs and the levels of interferon (IFN)-α, interleukin (IL)-6 and tumour necrosis factor (TNF)-α in BALF (Van Reeth et al., 2002). In contrast, correlation between signs of disease and IL-8 or IL-1 was much weaker.
The clinical signs and pathology of influenza in pigs are similar to those in humans and the cytokine profile in the BALF of affected pigs is similar to that found in nasal lavage fluids of experimentally-infected human volunteers (Hayden et al., 1998). Given that pigs are naturally susceptible to the same influenza A virus sub-types (H1N1, H3N2, H1N2) as humans, and that they have similar physiological features, this species may form a valuable experimental model to study the pathogenesis of influenza (Kuiken and Taubenberger, 2008).
IL-12, IL-18 and IFN-γ may also play a role in the pathogenesis of influenza; IL-12 is a potent regulator of cell-mediated immune responses such as proliferation of and IFN-γ production by T- and NK-cells (Gately et al., 1991, Monteiro et al., 1998). IFN-γ has numerous immunological functions such as enhancement of MHC expression and anti-viral activity and IL-18 is an IFN-γ-inducing factor (Konishi et al., 1997).
Intranasal inoculation of human volunteers with A/Texas/36/91 (H1N1) resulted in an increase in IFN-γ in nasal lavage fluid 2–5 days PI (Fritz et al., 1999). In mice inoculated with A/PuertoRico/8/34 (H1N1), both IFN-γ and IL-12 were found in BALF 3–7 days PI (Monteiro et al., 1998) and a study in IL-18 ‘knock-out’ mice suggested a role for IL-18 in viral clearance from the lungs and in the induction of IFN-γ (Denton et al., 2007). However, the activities of these cytokines in a swine influenza infection model have not been studied (Jung et al., 2004).
Acute phase proteins (APPs) such as haptoglobin (HG), lipopolysaccharide (LPS)-binding protein (LBP) and C-reactive protein (CRP) are produced by the liver in response to cytokine activity and play a role in the pathogenesis of respiratory disease (Moshage et al., 1988). Haptoglobin binds free haemoglobin thus removing it from the circulation, LBP binds and neutralises LPS and CRP has roles in macrophage activation and opsonisation. The cytokines IL-1, IL-6 and TNF-α appear to be the main inducers of APPs (Petersen et al., 2004). Following experimental influenza virus infection of human volunteers, CRP in sera peaked 3 days PI (Whicher et al., 1985), and in natural infection CRP is significantly higher in acute relative to convalescent sera (Falsey et al., 2001, Melbye et al., 2004). Haptoglobin concentrations peaked 7–10 days PI in horses following experimental infection with influenza virus (Kent and Goodall, 1991) and in horses naturally infected with equine influenza virus, serum amyloid A was elevated during the acute stage of the disease (Hulten et al., 1999). To our knowledge the activities of APPs have not been studied in pigs with influenza.
The objectives of this study were to investigate the cytokine and APP responses in the lungs and circulation of SIV-infected pigs and to correlate these responses with lung virus titres and inflammation. The findings will enhance our understanding of the pathogenesis of influenza virus infection in pigs and other species.
Materials and methods
Preparation of virus inoculum
A SIV Swine/Belgium/1/98 (H1N1) strain had been isolated from the lungs of fattening pigs during an outbreak of acute respiratory disease. The stock used for inoculation represented the third passage in eggs. Inoculations of 107.5 EID50 of virus in 3 mL of phosphate-buffered saline (PBS) were given IT, using a 20-gauge needle attached to a syringe inserted through the skin cranial to the thoracic inlet.
Experimental design and BALF cell analysis
All experimental procedures were approved by The Local Ethical Committee of The Faculty of Veterinary Medicine, Ghent University (authorisation reference number EC 2005/88).
Sixteen, 3-week-old caesarean-derived, colostrum-deprived pigs were used. The pigs were from two sows and were housed in Horsefall-type isolation units with positive-pressure ventilation and were fed ultra-high-temperature-treated cow’s milk supplemented with antibiotics. Two pigs were sham-inoculated with PBS and euthanased the next day. The 14 remaining animals were inoculated IT with SIV and euthanased at 24 h (n = 3), 30 h (n = 2), 48 h (n = 3), 72 h (n = 3) and 120 h (n = 3) PI. Euthanasia was by intravenous injection of sodium pentobarbital (Natrium Pentobarbital 20%, Kela). A ‘score’ of clinical disease severity was attributed to each animal just prior to euthanasia based on each of the following clinical signs: anorexia, depression and coughing whereby one point was assigned for the presence of each of these signs. Where the respiration rate was 60–90/min and >90/min, one and two additional points were added to the clinical score, respectively.
At euthanasia, blood samples were collected, the whole lung was excised and the right lung lavaged with cold PBS to obtain BALF (Van Reeth et al., 1999). Samples of the left lung lobes were pooled and tissue homogenates prepared for virus titration and cytokine quantification. The BALF was separated into cells and cell-free fluids by centrifugation. Cell-free BALF was concentrated 20-fold by dialysis against polyethylene glycol, was cleared of residual virus by centrifugation and was used to measure cytokine and APP concentrations (see below). Total numbers of BALF cells were counted using a Türk chamber. Cytocentrifuge preparations were stained using Diff-Quik (Medion Diagnostics) to determine neutrophil numbers.
Virological examination and quantification of cytokines
Virus titration of lung homogenates was carried out in Madin–Darby canine kidney cells (Van Reeth et al., 2002) and the virus titres calculated as previously described (Reed and Muench, 1938). IFN-α, TNF-α, IL-1 and IL-6 were quantified in sera, BALF and lung homogenates by bioassay as previously described (Van Reeth et al., 1999, Van Reeth et al., 2002). In summary, IFN-α was quantified using a cytopathic effect reduction test with Madin-Darby bovine kidney cells and vesicular stomatitis virus. TNF-α concentrations were determined using a cytotoxicity test in porcine kidney sub-clone 15 (PK 15) cells and IL-1 and IL-6 activity was measured using proliferation assays in D10(N4)M and B9 cells, respectively. All bioassays were repeated twice or thrice and geometric means of the concentrations were calculated.
Assay specificity was demonstrated by neutralising the samples with specific antibodies for IFN-α, TNF-α and IL-6 or by pre-incubation of the D10(N4)M cells with IL-1 receptor antagonist. Various ELISAs were used to determine the concentrations of IFN-γ (Swine IFN-γ ELISA, Biosource), IL-18 (Pig IL-18 ELISA, BenderMed Systems) and IL-12 (Porcine IL-12/IL-23 p40 ELISA, R&D Systems). The detection limits of these assays were 2, 23 and 18 pg/mL, respectively. All samples were tested in duplicate according to manufacturers’ instructions.
Quantification of acute phase proteins
C-reactive protein, HG and LBP were measured in serum and BALF and the concentrations of CRP (Phase Range Porcine C-reactive Protein Assay, Tridelta Development Ltd.) and LBP (LBP ELISA, Hycult Biotechnology) were determined by ELISA. A colorimetric assay was used to measure HG (Phase Range Porcine Haptoglobin Assay, Tridelta Development Ltd.). The detection limits for CRP, LBP and HG were 47 ng/mL, 1.6 ng/mL and 50 μg/mL, respectively. All samples were tested in duplicate according to manufacturers’ instructions.
Statistical analysis
Spearman rank correlation coefficients (ρ) were calculated to compare individual cytokine and APP levels in the lungs and circulation, lung virus titres, neutrophil numbers in BALF and clinical scores. A P-value <0.01 was taken as significant. Standard Mann–Whitney tests were used to compare cytokine or APP concentrations between SIV-inoculated and control pigs.
Results
Clinical signs, virus titres and lung inflammation
The PBS-inoculated control pigs did not exhibit clinical signs, did not have virus in their lungs and had negligible numbers of neutrophils (1 × 106) in BALF. In SIV-inoculated animals, clinical signs consisted of depression and tachypnoea with abdominal breathing. These signs peaked 24–30 h PI and had completely resolved 72 h PI (Table 1 ). Lung virus titres were maximal 24–30 h PI (109.6 TCID50/g) and had decreased substantially by 5 days PI (105.8 TCID50/g). Increased numbers of neutrophils were noted in BALF 24–30 h PI whereas numbers of mononuclear cells remained constant (Table 1). Neutrophil numbers had returned to baseline values by 5 days PI (8–20 × 106).
Table 1.
Details of clinical scores at euthanasia, lung virus titres and inflammatory changes in broncho-alveolar lavage fluid (BALF) following intratracheal inoculation of pigs with swine influenza virus. PI, post-inoculation.
| PI (h) | n | Mean clinical score ± SD | Mean virus titre ± SD (log10 TCID50/g) | Mean cell numbers in BALF (x106) ± SD |
||
|---|---|---|---|---|---|---|
| Total | Neutrophils | Mononuclear cells | ||||
| 0 | 2 | 0.0 ± 0.0 | <1.7 ± 0 | 131 ± 18 | 1 ± 0 | 130 ± 18 |
| 24 | 3 | 1.7 ± 1.5 | 9.6 ± 0.3 | 458 ± 310 | 332 ± 336 | 126 ± 50 |
| 30 | 2 | 3.5 ± 0.7 | 9.2 ± 0.2 | 614 ± 303 | 481 ± 211 | 133 ± 92 |
| 48 | 3 | 1.7 ± 1.2 | 8.7 ± 0.3 | 331 ± 131 | 114 ± 113 | 217 ± 122 |
| 72 | 3 | 0.0 ± 0.0 | 7.5 ± 0.7 | 118 ± 24 | 11 ± 3 | 107 ± 26 |
| 120 | 3 | 0.0 ± 0.0 | 5.8 ± 0.6 | 228 ± 18 | 15 ± 5 | 213 ± 20 |
Cytokine profile in BALF, serum and lung tissue
The concentrations of bioactive IFN-α, IL-6, TNF-α and IL-1 in BALF, serum and lung tissue are illustrated in Fig. 1 . IFN-α, TNF-α, IL-1 and IL-6 were undetectable in controls but elevated at 24 h and 30 h PI in the BALF of infected animals. At later time-points PI, the concentration of these cytokines was either >20-fold lower, or at the limit of detection. The maximum concentrations of IFN-α and IL-6 in BALF (72,542 U/mL and 104,439 U/mL, respectively), were approximately 100-fold greater than those of TNF-α and IL-1 (706 U/mL and 213 U/mL, respectively) (Fig. 1A). In comparison, the cytokine profiles in the lung (Fig. 1B) and serum (Fig. 1C) were similar but approximately 10 and 100 times lower, respectively. Only the levels of IFN-α and IL-6 were substantially increased in lung tissue and serum.
Fig. 1.
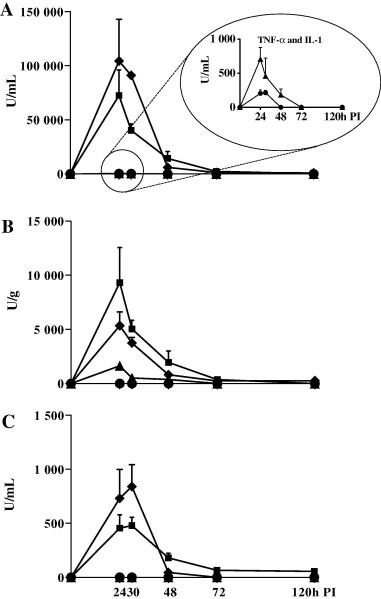
Temporal profiles of IFN-α (■), IL-6 (♦), TNF-α (▴) and IL-1 (●) in BALF (A), lung tissue (B) and serum (C) post-inoculation (PI) with swine influenza virus. Values represent means of 2 or 3 pigs (+standard error of mean [SEM]), and are expressed as biological units (U)/mL (for BALF and serum) or U/g (lung tissue).
The concentrations of IL-12, IFN-γ and IL-18 in BALF, serum and lung are illustrated in Fig. 2 . Only IL-18 was detectable at all three locations in the controls. In BALF, the concentrations of IL-12 and IFN-γ peaked at 30 h PI (14,678 pg/mL and 3230 pg/mL, respectively) before gradually declining. IL-18 levels, in contrast, were lower at 24 and 30 h PI than at time of experimental infection, but increased after this time. The cytokine profile in lung tissue was similar to that in BALF, although IFN-γ and IL-18 concentrations were 2 and 10 times higher, respectively. IL-12 was not detected in serum at any time-point, and the concentrations of IFN-γ and IL-18 in serum were approximately 10 and 2 times lower than in BALF, respectively. The concentration of IL-18 dropped below the detection limit in serum at 30 h PI. At this time the concentration of IL-18 decreased in BALF and lung. Given the small number of pigs under study, cytokine concentrations PI did not differ significantly from control values.
Fig. 2.
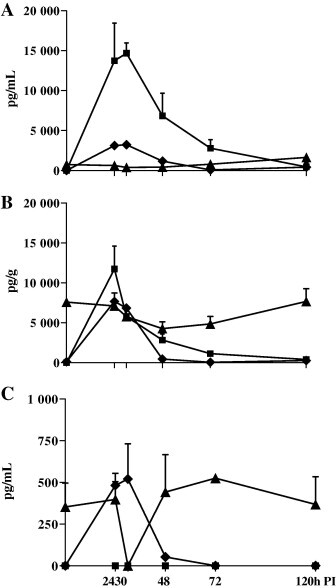
Temporal profiles of IL-12 (■), IFN-γ (♦) and IL-18 (▴) in BALF (A), lung tissue (B) and serum (C) post-inoculation (PI) with swine influenza virus. Values represent means of 2 or 3 pigs (+standard error of mean [SEM]), and are expressed as pg/mL (for BALF and serum) or pg/g (lung tissue).
Acute phase protein profile in BALF and serum
The concentrations of LBP, HG and CRP in BALF and serum are detailed in Fig. 3 . Control pigs had low HG (63 μg/mL) and LBP (387 ng/mL) and undetectable CRP (<47 ng/mL) concentrations in BALF. Serum from controls had low or undetectable concentrations of CRP and HG, but elevated levels of LBP (10,042 ng/mL). In infected pigs, the concentrations of APP in BALF peaked 30 h (for LBP) or 48 h (HG and CRP), PI and then decreased. While the concentrations of CRP and HG peaked at 48 h PI in serum, the level of LBP remained relatively constant. In general, the concentrations of APP in serum were approximately 4-fold higher than in BALF. Given the small number of pigs under study, APP concentrations PI did not differ significantly from control values.
Fig. 3.
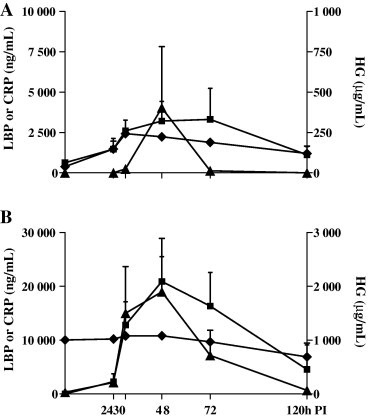
Temporal profiles of HG (■), LBP (♦) and CRP (▴) in BALF (A) and serum (B) post-inoculation (PI) with swine influenza virus. Values represent means of 2 or 3 pigs (+standard error of mean [SEM]), and are expressed as μg/mL (HG) or ng/mL (LBP and CRP).
Statistical analysis
Correlations between lung virus titres, neutrophil infiltration in BALF and clinical scores and the cytokines and APP in BALF and serum are detailed in Table 2, Table 3 . Virus titres and neutrophil percentages in BALF correlated significantly with each other and with the BALF cytokines, excluding IL-18. Clinical score correlated significantly with lung virus titres and with neutrophil infiltration and cytokine concentrations in BALF, although there was a negative correlation with the IL-18 concentration. Of the APP in BALF only LBP correlated with signs of disease. The strongest correlations were found between virus titres and IFN-α (ρ = 0.962) or IL-6 (ρ = 0.940). Cytokines in BALF (except IL-18) also significantly correlated with each other (ρ > 0.717). IL-18 was negatively but not significantly correlated with all other parameters. Of the APP, only LBP correlated significantly with IL-6 and IL-12 concentrations and with neutrophil infiltration in BALF.
Table 2.
Details of correlations using Spearman’s ρ correlation coefficient between cytokines and acute phase proteins in broncho-alveolar lavage fluid, lung virus titres (VT), neutrophil (neutro) infiltration in BALF and clinical score (CS).
| IFN-α | IL-6 | IL-1 | TNF-α | IFN-γ | IL-18 | IL-12 | HG | CRP | LBP | VT | Neutro | CS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-α | 1 | 0.920a | 0.812a | 0.899a | 0.856a | −0.530 | 0.925a | 0.386 | 0.275 | 0.608 | 0.962a | 0.861a | 0.738a |
| IL-6 | – | 1 | 0.777a | 0.918a | 0.932a | −0.533 | 0.893a | 0.274 | 0.178 | 0.670a | 0.940a | 0.855a | 0.819a |
| IL-1 | – | – | 1 | 0.787a | 0.766a | −0.390 | 0.717a | −0.069 | −0.016 | 0.243 | 0.788a | 0.733a | 0.689a |
| TNF-α | – | – | – | 1 | 0.901a | −0.573 | 0.870a | 0.193 | 0.164 | 0.534 | 0.912a | 0.801a | 0.815a |
| IFN-γ | – | – | – | – | 1 | −0.486 | 0.814a | 0.119 | 0.137 | 0.561 | 0.884a | 0.799a | 0.717a |
| IL-18 | – | – | – | – | – | 1 | −0.603 | −0.332 | −0.591 | −0.506 | −0.549 | −0.541 | −0.565a |
| IL-12 | – | – | – | – | – | – | 1 | 0.355 | 0.430 | 0.766a | 0.889a | 0.920a | 0.843a |
| HG | – | – | – | – | – | – | – | 1 | 0.558 | 0.437 | 0.314 | 0.218 | 0.571 |
| CRP | – | – | – | – | – | – | – | – | 1 | 0.481 | 0.160 | 0.354 | 0.463 |
| LBP | – | – | – | – | – | – | – | – | – | 1 | 0.600 | 0.759a | 0.689a |
| VT | – | – | – | – | – | – | – | – | – | – | 1 | 0.860a | 0.691a |
| Neutro | – | – | – | – | – | – | – | – | – | – | – | 1 | 0.704a |
| CS | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
IFN, interferon; IL, interleukin; TNF, tumour necrosis factor; HG, haptoglobin; LPS, lipopolysaccharide; LBP, LPS-binding protein; CRP, C-reactive protein.
Correlation is significant at the 0.01 level.
Table 3.
Details of correlations using Spearman’s ρ correlation coefficient between cytokines and acute phase proteins in serum, lung virus titres (VT), neutrophil (neutro) infiltration in broncho-alveolar lavage fluid and clinical score (CS).
| IFN-α | IL-6 | IL-1 | TNF-α | IFN-γ | IL-18 | IL-12 | HG | CRP | LBP | VT | Neutro | CS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-α | 1 | 0.821a | NA | NA | 0.894a | −0.287 | NA | 0.444 | 0.524 | 0.258 | 0.892a | 0.823a | 0.744a |
| IL-6 | – | 1 | NA | NA | 0.914a | −0.504 | NA | 0.308 | 0.380 | 0.198 | 0.871a | 0.895a | 0.811a |
| IL-1 | – | – | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| TNF-α | – | – | – | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| IFN-γ | – | – | – | – | 1 | −0.408 | NA | 0.258 | 0.424 | 0.280 | 0.906a | 0.770a | 0.767a |
| IL-18 | – | – | – | – | – | 1 | NA | −0.221 | 0.000 | −0.022 | −0.188 | −0.388 | −0.456 |
| IL-12 | – | – | – | – | – | – | NA | NA | NA | NA | NA | NA | NA |
| HG | – | – | – | – | – | – | – | 1 | 0.637a | −0.203 | 0.339 | 0.412 | 0.429 |
| CRP | – | – | – | – | – | – | – | – | 1 | 0.136 | 0.476 | 0.357 | 0.629a |
| LBP | – | – | – | – | – | – | – | – | – | 1 | 0.270 | 0.271 | 0.251 |
| VT | – | – | – | – | – | – | – | – | – | – | 1 | 0.860a | 0.691a |
| Neutro | – | – | – | – | – | – | – | – | – | – | – | 1 | 0.704a |
| CS | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
NA, not applicable; IFN, interferon; IL, interleukin; TNF, tumour necrosis factor; HG, haptoglobin; LPS, lipopolysaccharide; LBP, LPS-binding protein; CRP, C-reactive protein.
Correlation is significant at the 0.01 level.
IL-1, TNF-α and IL-12 concentrations were below the detection limit in serum so that correlation coefficients could not be calculated. With respect to BALF, there were strong correlations between lung virus titres, neutrophil infiltration and clinical scores and with IFN-α, IFN-γ and IL-6 (ρ ⩾ 0.770). The only significant correlation for serum APPs was between HG and CRP and between CRP and clinical score. Correlations between cytokine concentrations in BALF and APPs in serum were also calculated (data not shown). The only significant finding was a negative correlation between IL-18 in BALF and CRP in serum (ρ = −0.681).
Discussion
The findings of the present study build on previous work investigating the cytokine profile in BALF during experimental SIV infection. Previous studies demonstrated significant increases in IFN-α, TNF-α, IL-6 and IL-1 following infection, and elevations in the concentrations of the first three cytokines in particular strongly correlated with virus replication and disease (Van Reeth et al., 2002). The current study found similar cytokine response profiles and correlations for IL-12 and IFN-γ, but not for IL-18. Elevations in HG, LBP and CRP, were, unlike the cytokine responses, higher in serum than in BALF and peaked 24 h later. Furthermore, APP did not directly correlate with viral load.
The finding of IFN-γ in both BALF and serum 24 h PI, contrasts with previous studies. Infection with a Korean H1N1 SIV isolate resulted in a non-significant increase in IFN-γ concentration in BALF 7 days PI (Jung et al., 2004) and no change was found in the serum IFN-γ concentration PI with a North American H3N2 SIV isolate (Wesley et al., 2006). Factors that may have attributed to the greater, more rapid IFN-γ response in our study were the larger inoculation dose used and the IT rather than the intranasal route of inoculation, which resulted in a higher pulmonary viral load. Unlike Jung et al. (2004), we assayed concentrated BALF, which increases the sensitivity of cytokine detection, and also focused on earlier time-points PI. Furthermore, the profiles of IFN-γ and IL-12 in BALF in our infection model are similar to those in BALB/c mice and to nasal lavage fluid from infected human volunteers (Monteiro et al., 1998, Fritz et al., 1999).
The finding of IL-18 in BALF, lung tissue and serum of controls and the decrease in its concentration following infection, are difficult to explain. The cytokine is expressed in precursor form within cells prior to its enzymatic cleavage by IL-1β converting enzyme and release (Ghayur et al., 1997). The ELISA used in our study detects this extra-cellular, mature form of IL-18. High concentrations of IL-18 were found in rat lung homogenates by ELISA (Jordan et al., 2001) and both pro and mature IL-18 have been detected in human peripheral blood mononuclear cells (Puren et al., 1999). Low IL-18 concentrations in the BALF of un-infected 1-day-old gnotobiotic pigs had increased significantly by 2 and 4 weeks following infection with Mycoplasma hyopneumoniae (Muneta et al., 2008). Similar elevations were found 3 days PI in mice inoculated with herpes simplex virus type 1 (Reading et al., 2007) and human macrophages secrete IL-18 after inoculation with H3N2 influenza virus (Pirhonen et al., 1999).
Poxvirus and papillomavirus proteins bind IL-18 (Xiang and Moss, 1999, Cho et al., 2001) as does the IL-18-binding-protein, induced by IFN-α (Kaser et al., 2002). It is possible that the concentration of IL-18 is affected by changes in the concentration of IL-1β converting enzyme, also involved in the secretion of IL-1β and in apoptosis (Lynch et al., 1997). The reduced IL-18 response found in our study does not fit with the role attributed to this cytokine in the clearance of influenza virus in a murine infection model (Denton et al., 2007).
Most of the cytokines produced in response to SIV infection were found at higher concentrations in BALF than in lung tissue; only TNF-α and IFN-γ were found in similar amounts in both sample types. Importantly, only the interferons and IL-6 reached detectable levels in serum and these concentrations were 5- to 100-fold lower than in BALF, findings which fit with observations in humans (Hayden et al., 1998). Increased plasma concentrations of various cytokines have also been reported in, hospitalised human patients with H1N1 (Lee et al., 2007) and highly pathogenic avian H5N1 (Peiris et al., 2004, Gambotto et al., 2008) infection. In these patients, plasma cytokine concentrations tightly correlated with viral RNA levels in nasopharyngeal or throat swabs, as well as with clinical severity ‘scores’.
The respiratory tract is the major site of influenza virus replication suggesting that this is the initial site of cytokine production with a subsequent ‘spill-over’ into the circulation. It is therefore likely that serum levels of these mediators are only elevated in severely affected patients, a suggestion supported by the cytokine profiles identified in BALF, lung tissue and serum in our study. Openshaw (2004) has stated that the site of sampling, the stage of infection and the method used to assay cytokines in patients, critically determine the significance of their interpretation.
The APPs peaked later in the infection than did the cytokines and their serum concentrations were higher than in BALF. Serum CRP and HG increased more than did the concentration of these proteins in BALF. Increased serum CRP has been reported in humans with influenza (Whicher et al., 1985) and increased serum HG in horses experimentally infected with influenza virus (Kent and Goodall, 1991). LBP was detected at relatively high concentrations in control sera, as previously described by Sachse et al. (2004). Furthermore, infection with SIV did not alter the serum LBP concentrations but resulted in a rise in this protein in BALF.
The response profiles of both LBP and HG resembled those in pigs experimentally infected with porcine respiratory coronavirus (PRCV) (Van Gucht et al., 2006). These PRCV-infected animals also had unchanged serum and elevated BALF LBP concentrations, whilst serum HG levels peaked later than in SIV-infected pigs. The finding that LBP increased in BALF and not in serum suggests local pulmonary production of LBP, a hypothesis supported by the finding that A549 human epithelial cells produce LBP in response to IL-1, IL-6 and TNF-α (Dentener et al., 2000). While an exact role for APPs in viral infections remains unclear, our findings suggest their induction may represent a non-specific hepatic response to circulating cytokines.
Conclusions
Infection with influenza viruses induces the production of similar profiles of cytokines and inflammatory mediators in pigs and humans. Although murine ‘knock-out’ models are particularly useful in elucidating the role of specific cytokines in the pathogenesis of influenza, this porcine model system can also provide useful data, given that pigs are a natural influenza host and anatomically and physiologically resemble humans.
Conflict of interest statement
None of the authors of this paper has any financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors wish to thank Lieve Sys, Nele Dennequin, Fernand De Backer and Geert Opsomer for excellent technical assistance.
References
- Cho Y.S., Kang J.W., Cho M., Cho C.W., Lee S., Choe Y.K., Kim Y., Choi I., Park S.N., Kim S., Dinarello C.A., Yoon D.Y. Down modulation of IL-18 expression by human papillomavirus type 16 E6 oncogene via binding to IL-18. FEBS Letters. 2001;501:139–145. doi: 10.1016/s0014-5793(01)02652-7. [DOI] [PubMed] [Google Scholar]
- Dentener M.A., Vreugdenhil A.C.E., Hoet P.H.M., Vernooy J.H.J., Nieman F.H.M., Heumann D., Janssen Y.M.W., Buurman W.A., Wouters E.F.M. Production of the acute-phase protein lipopolysaccharide-binding protein by respiratory type II epithelial cells – implications for local defense to bacterial endotoxins. American Journal of Respiratory Cell and Molecular Biology. 2000;23:146–153. doi: 10.1165/ajrcmb.23.2.3855. [DOI] [PubMed] [Google Scholar]
- Denton A.E., Doherty P.C., Turner S.J., La Gruta N.L. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. European Journal of Immunology. 2007;37:368–375. doi: 10.1002/eji.200636766. [DOI] [PubMed] [Google Scholar]
- Falsey A.R., Walsh E.E., Francis C.W., Looney R.J., Kolassa J.E., Hall W.J., Abraham G.N. Response of C-reactive protein and serum amyloid A infection in older adults. Journal of Infectious Diseases. 2001;183:995–999. doi: 10.1086/319275. [DOI] [PubMed] [Google Scholar]
- Fritz R.S., Hayden F.G., Calfee D.P., Cass L.M.R., Peng A.W., Alvord W.G., Strober W., Straus S.E. Nasal cytokine and chemokine responses in experimental influenza A virus infection: results of a placebo-controlled trial of intravenous zanamivir treatment. Journal of Infectious Diseases. 1999;180:586–593. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- Gambotto A., Barratt-Boyes S.M., de Jong M.D., Neumann G., Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- Gately M.K., Desai B.B., Wolitzky A.G., Quinn P.M., Dwyer C.M., Podlaski F.J., Familletti P.C., Sinigaglia F., Chizonnite R., Gubler U., Stern A.S. Regulation of human lymphocyte-proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) Journal of Immunology. 1991;147:874–882. [PubMed] [Google Scholar]
- Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M., Wong W., Kamen R., Tracey D., Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Fritz R.S., Lobo M.C., Alvord W.G., Strober W., Straus S.E. Local and systemic cytokine responses during experimental human influenza A virus infection – relation to symptom formation and host defense. Journal of Clinical Investigation. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulten C., Sandgren B., Skioldebrand E., Klingeborn B., Marhaug G., Forsberg M. The acute phase protein serum amyloid A (SAA) as an inflammatory marker in equine influenza virus infection. Acta Veterinaria Scandinavica. 1999;40:323–333. doi: 10.1186/BF03547012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan J.A., Guo R.F., Yun E.C., Sarma V., Warner R.L., Crouch L.D., Senaldi G., Ulich T.R., Ward P.A. Role of IL-18 in acute lung inflammation. Journal of Immunology. 2001;167:7060–7068. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- Jung K., Ha Y., Ha S.K., Han D.U., Kim D.W., Moon W.K., Chae C. Antiviral effect of Saccharomyces cerevisiae β-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. Journal of Veterinary Medicine Series B – Infectious Diseases and Veterinary Public Health. 2004;51:72–76. doi: 10.1111/j.1439-0450.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- Kaser A., Novick D., Rubinstein M., Siegmund B., Enrich B., Koch R.O., Vogel W., Kim S.H., Dinarello C.A., Tilg H. Interferon-α induces interleukin-18 binding protein in chronic hepatitis C patients. Clinical and Experimental Immunology. 2002;129:332–338. doi: 10.1046/j.1365-2249.2002.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J.E., Goodall J. Assessment of an immunoturbidimetric method for measuring equine serum haptoglobin concentrations. Equine Veterinary Journal. 1991;23:59–66. doi: 10.1111/j.2042-3306.1991.tb02716.x. [DOI] [PubMed] [Google Scholar]
- Konishi K., Tanabe F., Taniguchi M., Yamauchi H., Tanimoto T., Ikeda M., Orita K., Kurimoto M. A simple and sensitive bioassay for the detection of human interleukin-18/interferon-γ-inducing factor using human myelomonocytic KG-1 cells. Journal of Immunological Methods. 1997;209:187–191. doi: 10.1016/s0022-1759(97)00164-6. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Taubenberger J.K. Pathology of human influenza revisited. Vaccine. 2008;26:D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Wong C.K., Chan P.K.S., Lun S.W.M., Lui G., Wong B., Hui D.S.C., Lam C.W.K., Cockram C.S., Choi K.W., Yeung A.C.M., Tang J.W., Sung J.J.Y. Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza a virus infection. Clinical Infectious Diseases. 2007;45:723–731. doi: 10.1086/520981. [DOI] [PubMed] [Google Scholar]
- Loeffen W.L.A., Kamp E.M., Stockhofe-Zurwieden N., van Nieuwstadt A., Bongers J.H., Hunneman W.A., Elbers A.R.W., Baars J., Nell T., van Zuderveld F.G. Survey of infectious agents involved in acute respiratory disease in finishing pigs. Veterinary Record. 1999;145:123–129. doi: 10.1136/vr.145.5.123. [DOI] [PubMed] [Google Scholar]
- Lynch T., Vasilakos J.P., Raser K., Keane K.M., Shivers B.D. Inhibition of the interleukin-1β converting enzyme family rescues neurons from apoptotic death. Molecular Psychiatry. 1997;2:227–238. doi: 10.1038/sj.mp.4000242. [DOI] [PubMed] [Google Scholar]
- Melbye H., Hvidsten D., Holm A., Nordbo S.A., Brox J. The course of C-reactive protein response in untreated upper respiratory tract infection. British Journal of General Practice. 2004;54:653–658. [PMC free article] [PubMed] [Google Scholar]
- Monteiro J.M., Harvey C., Trinchieri G. Role of interleukin-12 in primary influenza virus infection. Journal of Virology. 1998;72:4825–4831. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshage H.J., Roelofs H.M.J., Vanpelt J.F., Hazenberg B.P.C., Vanleeuwen M.A., Limburg P.C., Aarden L.A., Yap S.H. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochemical and Biophysical Research Communications. 1988;155:112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- Muneta Y., Minagawa Y., Shimoji Y., Ogawa Y., Hikono H., Mori Y. Immune response of gnotobiotic piglets against Mycoplasma hyopneumoniae. Journal of Veterinary Medical Science. 2008;70:1065–1070. doi: 10.1292/jvms.70.1065. [DOI] [PubMed] [Google Scholar]
- Olsen C.W., Brown I., Easterday B.C., Van Reeth K. In: Diseases of Swine. Straw B.E., Zimmerman J.J., D’Allaire S., Taylor D.J., editors. Iowa State University Press; Ames, IA, USA: 2006. Swine influenza; pp. 469–482. [Google Scholar]
- Openshaw P.J.M. What does the peripheral blood tell you in SARS ? Clinical and Experimental Immunology. 2004;136:11–12. doi: 10.1111/j.1365-2249.2004.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Yu W.C., Leung C.W., Cheung C.Y., Ng W.F., Nicholls J.M., Ng T.K., Chan K.H., Lai S.T., Lim W.L., Yuen K.Y., Guan Y. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Veterinary Research. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pirhonen J., Sareneva T., Kurimoto M., Julkunen I., Matikainen S. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. Journal of Immunology. 1999;162:7322–7329. [PubMed] [Google Scholar]
- Puren A.J., Fantuzzi G., Dinarello C.A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading P.C., Whitney P.G., Barr D.P., Wojtasiak M., Mintern J.D., Waithman J., Brooks A.G. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type I infection. Journal of Immunology. 2007;179:3214–3221. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Sachse K., Grossmann E., Berndt A., Schutt C., Henning K., Theegarten D., Anhenn O., Reinhold P. Respiratory chlamydial infection based on experimental aerosol challenge of pigs with Chlamydia suis. Comparative Immunology, Microbiology and Infectious Diseases. 2004;27:7–23. doi: 10.1016/S0147-9571(02)00079-6. [DOI] [PubMed] [Google Scholar]
- Shope R. Swine influenza: I. Experimental transmission and pathology. Journal of Experimental Medicine. 1931;54:349–359. doi: 10.1084/jem.54.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gucht S., Atanasova K., Barbé F., Cox E., Pensaert M., Van Reeth K. Effect of porcine respiratory coronavirus infection on lipopolysaccharide recognition proteins and haptoglobin levels in the lungs. Microbes and Infection. 2006;8:1492–1501. doi: 10.1016/j.micinf.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Research in Veterinary Science. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Van Gucht S., Pensaert M. Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza virus challenge of vaccination-immune pigs. Viral Immunology. 2002;15:583–594. doi: 10.1089/088282402320914520. [DOI] [PubMed] [Google Scholar]
- Wesley R.D., Lager K.M., Kehrli M.E. Infection with porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Canadian Journal of Veterinary Research. 2006;70:176–182. [PMC free article] [PubMed] [Google Scholar]
- Whicher J.T., Chambers R.E., Higginson J., Nashef L., Higgins P.G. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. Journal of Clinical Pathology. 1985;38:312–316. doi: 10.1136/jcp.38.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Moss B. IL-18 binding and inhibition of interferon-γ induction by human poxvirus-encoded proteins. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11537–11542. doi: 10.1073/pnas.96.20.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]


